Abstract
Background
Radiographic progression-free survival (rPFS) is associated with overall survival (OS) in chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) patients. Using readily assessable baseline clinical and laboratory parameters, we developed a prognostic index model for rPFS in chemotherapy-naïve mCRPC patients without visceral disease who were treated with abiraterone acetate plus prednisone.
Methods
Data from the abiraterone acetate plus prednisone arm of COU-AA-302 were used. rPFS was defined based on modified Prostate Cancer Working Group 2 criteria. Baseline variables were assessed for association with rPFS through univariate Cox modeling. The lower (LLN) and upper (ULN) limits of laboratory normal were used to dichotomize most laboratory parameters; baseline median was used to dichotomize prostate-specific antigen (PSA). Prognostic factors for rPFS were identified by multivariate Cox modeling. Model accuracy was estimated by the C-index.
Results
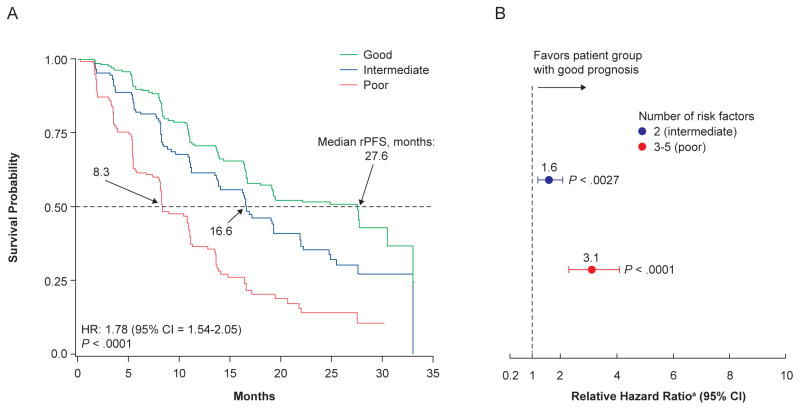
Presence of lymph node metastasis (hazard ratio [HR]=1.76, P<.0001), lactate dehydrogenase >ULN (234 IU/L) (HR=1.71, P=.0001), ≥ 10 bone metastases (HR=1.71, P=.0015), hemoglobin ≤LLN (12.7 g/dL) (HR=1.47, P=.0030) and PSA >39.5 ng/mL (HR=1.42, P=.0078) were associated with poor outcome. Patients were categorized into 3 prognostic groups (good, n=230; intermediate, n=152; poor, n=164) based on number of risk factors. Median rPFS was calculated (27.6, 16.6, and 8.3 months for good, intermediate and poor, respectively). The C-index was 0.83 (95% confidence interval = 0.73–0.91).
Conclusions
The prognostic index model for rPFS reveals differential outcomes based on factors readily available in clinical practice. If validated, this model can be integrated into clinical practice and design of risk-stratified trials.
Keywords (MeSH): Cox models, Laboratory marker, Metastasis, Risk group, Statistical regression
Introduction
Metastatic castration-resistant prostate cancer (mCRPC) exhibits a broad-spectrum of disease burden as determined by radiographic imaging and laboratory studies (eg, prostate-specific antigen [PSA], lactate dehydrogenase [LDH], and alkaline phosphate). Clinical conditions range from minimal disease with no symptoms to extensive disease with moderate to severe symptoms. The availability of several life-prolonging agents for mCRPC1–7 presents dual challenges of therapy choice and timing of use. With a goal of understanding the patient’s prognosis across the full clinical spectrum, in particular during the time of evolving treatment options, models for the likelihood of disease progression and progression-free survival may aid in clinical judgment, risk assignment for future clinical trials, and even for potential future regulatory strategies. The recent development of a number of models for overall survival (OS) in men with mCRPC after first- or second-line chemotherapy can be utilized in the assessment of overall prognosis.8–12 However, to date, no model has been developed for the prediction of the intermediate outcome of progression-free survival in the clinically diverse chemotherapy-naïve mCRPC setting. Guidance on the accurate assessment of prognosis of such patients is needed.
COU-AA-302 was a phase III trial in asymptomatic/minimally symptomatic, chemotherapy-naïve mCRPC patients without visceral disease treated with abiraterone acetate (hereafter abiraterone) plus prednisone. In the final analysis, abiraterone plus prednisone significantly improved OS and radiographic progression-free survival (rPFS) compared with placebo plus prednisone (hereafter prednisone alone).5–7 Because of the broad spectrum of disease burden among patients enrolled, the COU-AA-302 trial provides an opportunity for new insights into the spectrum of prostate cancer associated with clinically meaningful improvements in rPFS in response to inhibition of persistent androgen receptor signaling.1,5–7
Models to predict clinically significant end points in addition to OS are necessary to address the need for an intermediate end point in mCRPC trials. Using data from COU-AA-302, we previously demonstrated that rPFS was positively associated with OS in patients with chemotherapy-naïve asymptomatic or mildly symptomatic mCRPC (Spearman’s correlation coefficient = 0.72; Kendall’s tau statistic = 0.53; maximum likelihood values for Clayton, Hougaard, and Plackett copulas were 3796.11, 3796.99, and 3797.79, respectively), providing evidence for further development of rPFS as an intermediate end point in mCRPC trials.13 Given the association between rPFS and OS and the need for prognostic models that can predict clinically significant end points in the chemotherapy-naïve setting, we developed a prognostic index model for rPFS in chemotherapy-naïve patients with mCRPC based on data from COU-AA-302 using factors that would be readily evaluable in routine clinical practice.
Materials and Methods
Patient Population
COU-AA-302 (NCT00887198) was a multinational, randomized, double-blind, placebo controlled phase III trial of abiraterone plus prednisone versus prednisone alone in asymptomatic or mildly symptomatic, chemotherapy-naïve patients with mCRPC. In collaboration with US and European regulatory agencies, the COU-AA-302 study was designed with two co-primary end points—rPFS and OS. Patients with visceral metastases or who had received previous therapy with ketoconazole for > 7 days were excluded. A total of 1088 patients were included in this study (abiraterone plus prednisone = 546; prednisone alone = 542). The study design, safety, and efficacy results are published elsewhere.5–7 The review boards at all participating institutions approved the study, which was conducted according to the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation. All patients provided written informed consent prior to participation.
Clinical and Laboratory Factors
Sixteen routinely available and readily assessable clinical, pathologic, and baseline laboratory factors were identified and included. The 546 patients in the abiraterone plus prednisone arm were evaluated for the presence or absence of these relevant baseline demographic and disease characteristics, and this cohort formed the basis for the modeling. The prognostic model for rPFS based on centrally reviewed imaging data was developed using the second interim analysis dataset, which was performed after 333 (43% of 773) events had occurred.6 The rPFS end point was adapted from Prostate Cancer Working Group criteria14,15 and modified Response Evaluation Criteria In Solid Tumors (RECIST). The imaging schedule was based on sponsor and FDA discussions. It is defined as the time from randomization to the occurrence of progression per bone scan (first bone scan with ≥ 2 new lesions compared with baseline observed < 12 weeks from randomization and confirmed by a second bone scan ≥ 6 weeks later demonstrating ≥ 2 additional new lesions or first bone scan with ≥ 2 new lesions compared with baseline observed ≥ 12 weeks from randomization with new lesions verified on the next scan ≥ 6 weeks later); progression of soft tissue lesions measured by computed tomography or magnetic resonance imaging as defined in modified RECIST; or death from any cause.
Statistical Methods
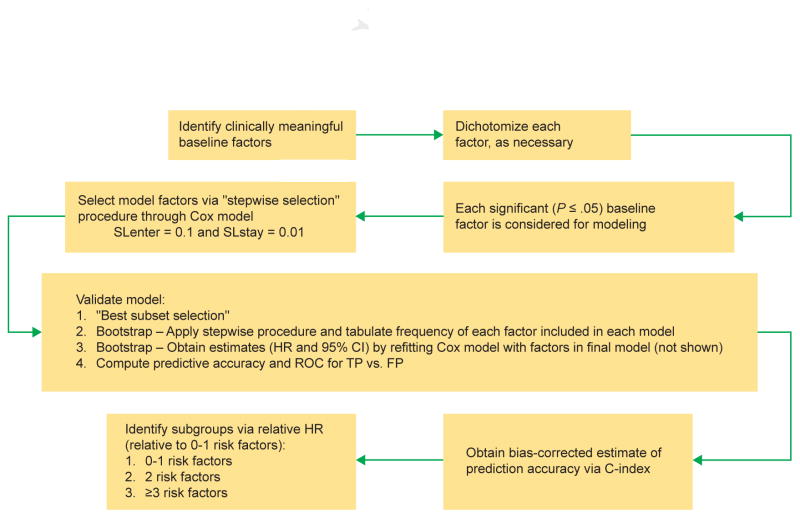
The modeling involved multiple steps (Figure 1). Laboratory factors were dichotomized into high or low risk according to median values or the lower and upper limits of normal (LLN and ULN, respectively). Laboratory analyses were performed by a central reference laboratory. For nonlaboratory parameters, median values were used due to the skewed distribution observed. First, selected clinically relevant baseline factors were assessed for significant association with rPFS using a univariate Cox regression model. A P value ≤ .05 was required for inclusion in the subsequent stepwise selection procedure. A multivariate Cox model was then used with a stepwise selection procedure to identify the prognostic factors for rPFS with a significance level of 0.1 for entry into the model and 0.01 for removal of each factor from the model. The final model was determined based on the Akaike Information Criterion (AIC) and the model chi-squared score. The combination of the significance levels for entry and removal and the AIC/chi-squared score were used to derive a model that is limited to the factors that contribute most to the model.
Figure 1. Schematic Representation of the Modeling Process.
Abbreviations: CI = confidence interval; HR = hazard ratio; SLenter = significance level for entry in the model; SLstay = significance level for retention in the model.
Internal validation of the predictive performance of the final model was assessed by a bootstrap resampling procedure.16 Five hundred samples were generated randomly with replacement from the original data (N = 546), and the stepwise Cox regression was applied with the same selection criteria as described above for the original model. The frequency with which each factor was selected in the resulting model was then tabulated. Factors selected most frequently were deemed most impactful and informed the model selection process. Parameter estimates (hazard ratio [HR] and 95% confidence interval [CI]) for the final model were obtained by refitting the Cox regression model with the same factors selected in the final model. After the final model was established, patients were categorized into 3 risk groups based on the number of baseline risk factors, and the median rPFS was calculated for each risk group. The concordance index (C-index) was computed to estimate the predictive accuracy of the model.17 A C-index of 0.5 suggests no predictive discrimination, while an index of 1.0 indicates perfect discriminatory power. The distribution of rPFS was calculated using the Kaplan–Meier method. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) and the receiver operating characteristic analysis was performed using R v.2.15.3 (Comprehensive R Archive Network).
Results
Nine of 16 baseline clinical and laboratory factors were found to be significantly associated with rPFS (P ≤ .05) through a univariate Cox model and were advanced forward (Table 1) to the next step of the modeling. A multivariate Cox regression model with a stepwise procedure identified the following 5 of 9 adverse prognostic factors to be the strongest predictors of rPFS, and these were included in the final model (Table 2): LDH (> ULN [234 IU/L] vs. ≤ ULN), bone metastases (≥10 vs. < 10), PSA (> 39.5 vs. ≤ 39.5 ng/mL), lymph node metastases (present vs. absent), and hemoglobin (≤ LLN [12.7 g/dL] vs. > LLN). The C-index was 0.83 (95% CI = 0.73–0.91).
Table 1.
Baseline Clinical and Laboratory Parameters Considered for Inclusion in the Model (Univariate Analysis)
| Baseline Risk Factors | P Value | HR (95% CI) |
|---|---|---|
| LDH (> ULN [234 IU/L] vs. ≤ ULN) | <.0001 | 2.03 (1.50–2.75) |
| Bone metastases (≥10 vs. < 10)a | <.0001 | 1.95 (1.53–2.48) |
| ALP (> ULN [131 IU/L] vs. ≤ ULN) | <.0001 | 1.76 (1.37–2.26) |
| PSA (> 39.5 vs. ≤ 39.5 ng/mL) | <.0001 | 1.76 (1.39–2.23) |
| Lymph node metastases (present vs. absent) | <.0001 | 1.72 (1.36–2.17) |
| Hemoglobin (≤ LLN [12.7 g/dL] vs. > LLN) | <.0001 | 1.63 (1.28–2.03) |
| BPI (2–3 vs. 0–1) | .0015 | 1.56 (1.18–2.04) |
| Albumin (≤ 4 vs. > 4 g/dL) | .0074 | 1.38 (1.09–1.74) |
| Gleason score (≥ 8 vs. < 8) | .22 | 1.17 (0.91–1.49) |
| Time from start of androgen deprivation therapy to initiation of abiraterone acetate (≤ 36 vs. > 36 months) | .22 | 1.16 (0.92–1.46) |
| Prior prostatectomy (yes vs. no) | .52 | 1.08 (0.86–1.36) |
| Age (≥ 70 vs. < 70 years) | .57 | 1.07 (0.85–1.35) |
| M1 disease at diagnosis (yes vs. no) | .57 | 0.93 (0.70–1.22) |
| Prior radiation therapy (yes vs. no) | .47 | 0.92 (0.73–1.16) |
| ECOG PS (1 vs. 0) | .34 | 0.87 (0.65–1.16) |
| Testosterone (> 0.4 vs. ≤0.4 ng/mL)b | .04 | 1.67 (1.03–2.70) |
Note: Shaded factors were excluded from modeling.
Abbreviations: ALP = alkaline phosphatase; BPI = Brief Pain Inventory; CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; HR = hazard ratio; IU = international units; LDH = lactate dehydrogenase; LLN = lower limit of normal; PSA = prostate-specific antigen; rPFS = radiographic progression-free survival; ULN = upper limit of normal.
Individual bone lesions were counted up to 10, and then grouped as > 10 to 20 and > 20.
Measured by radioimmunoassay.
Table 2.
Results From Stepwise Selection Final Model (Multivariate Analysis)
| Baseline Risk Factors | P Value | HR | 95% CI |
|---|---|---|---|
| LDH (> ULN [234 IU/L] vs. ≤ ULN) | .0015 | 1.71 | 1.23–2.38 |
| Bone metastases (≥ 10 vs. < 10) | .0001 | 1.71 | 1.30–2.23 |
| PSA (> 39.5 vs. ≤ 39.5 ng/mL) | .0078 | 1.42 | 1.10–1.85 |
| Lymph node metastases (present vs. absent) | <.0001 | 1.76 | 1.37–2.27 |
| Hemoglobin (≤ LLN [12.7 g/dL vs. > LLN) | .003 | 1.47 | 1.14–1.89 |
Abbreviations: CI = confidence interval; HR = hazard ratio; IU = international units; LDH = lactate dehydrogenase; LLN = lower limit of normal; PSA = prostate-specific antigen; ULN = upper limit of normal.
Model Checking and Bootstrap Validation
To avoid overfitting, the independent factors were limited to those contributing most to the final model based on the AIC and the model chi-squared score (Supplementary Figure 1). Results from the best subset selection indicated that inclusion of additional risk factors beyond these 5 factors did not improve the model’s prognostic value (Supplementary Figure 1). From the 500 bootstrap samples, these 5 risk factors were also selected most frequently via application of the stepwise Cox regression procedure, suggesting robust internal consistency (Supplementary Figure 2).
Risk Grouping (Table 3 and Figure 2)
Table 3.
Patients Categorized Into Risk Groups by Number of Baseline Risk Factors and Similar Relative Hazard Ratios
| No. of Baseline Risk Factors | Patients, n (%) | HR (Relative to Patients With 0 Risk Factors) | 95% CI | Median rPFS, Months | |
|---|---|---|---|---|---|
| Good prognosis (n = 230) | 0 | 85 (15.6) | – | 27.6 | |
| 1 | 145 (26.6) | 1.71 | 1.10–2.64 | ||
|
| |||||
| Intermediate prognosis (n = 152) | 2 | 152 (27.8) | 2.19 | 1.42–3.38 | 16.6 |
|
| |||||
| Poor prognosis (n = 164) | 3 | 112 (20.5) | 3.56 | 2.28–5.56 | 8.3 |
| 4 | 40 (7.3) | 5.46 | 3.16–9.44 | ||
| 5 | 12 (2.2) | 9.19 | 4.18–20.23 | ||
Abbreviations: CI = confidence interval; HR = hazard ratio; rPFS = radiographic progression-free survival.
Figure 2. Kaplan–Meier Curves for rPFS by Risk Group (a) and Relative Hazard Ratio by Risk Group (b), as Estimated by the Stepwise Final Model for Patients Treated With Abiraterone Plus Prednisone.
Abbreviations: CI = confidence interval; HR = hazard ratio; rPFS = radiographic progression-free survival.
aVersus patients with good prognosis.
Patients were categorized into 3 risk groups (good, intermediate, and poor prognostic groups) based on the number of baseline risk factors significantly associated with rPFS and similar relative HRs. Each risk group had distinct rates of reduction in radiographic progression. HRs for the intermediate and poor prognosis groups relative to the good prognosis group were determined. Most patients had 0 to 1 risk factor and were considered to have good prognosis (n = 230; 42.1%), for which median rPFS was 27.6 months. Patients with 2 risk factors were considered to have intermediate prognosis (n = 152; 27.8%), for which median rPFS was 16.6 months (HR = 2.19 relative to the good prognosis group), and patients with 3 to 5 risk factors were considered to have poor prognosis (n = 164; 30.0%), for which median rPFS was 8.3 months (HR = 3.1).
Discussion
A prognostic model for rPFS was developed for chemotherapy-naïve patients with mCRPC with no visceral metastases who were treated with abiraterone plus prednisone. The model was developed using 5 factors that are available during routine patient treatment and that are strongly associated with rPFS. Other prognostic factors that contributed to poor prognosis in chemotherapy-naïve patients with mCRPC were identified, but the model was limited to 5 factors because the additional factors did not contribute to the prognostic information in any meaningful way, according to the AIC and chi-squared statistics. The model categorized patients into 3 distinct risk groups (poor, intermediate, and good prognoses), and the C-index (0.83 [95% CI = 0.73–0.91]) suggests good predictive accuracy of the model.
It is worth noting that approximately 42% of patients entering into the trial fell into the “good risk” category of this model, and this is encouraging with respect to the interpretation of the study overall, as it was designed specifically for patients without visceral metastases and with little to no symptoms from their cancer. Indirectly, it may indicate that as these risk factors increase in an individual patient, he is more likely to become symptomatic. The true relationship of symptoms to disease status cannot be determined from this analysis.
Our model has a few limitations. This model was developed based on a phase III clinical trial with rigorous inclusion criteria, which may not reflect everyday clinical practice. Progression-free survival in COU-AA-302 was evaluated primarily by radiographic imaging (computed tomography and bone scan), and this approach may also differ from routine practice, in which PSA levels and symptoms might influence treatment decisions. Furthermore, bone scans and other imaging studies were performed more often than in routine practice, and suspected lesions by bone scan required a second, confirmatory scan at least 6 weeks later. In addition, patients with visceral metastases at baseline were excluded. Another limitation of our model is that patients who discontinued therapy due to clinical progression (such as need for chronic opioid use, a change in treatment to chemotherapy, or a significant decline in performance status) were not included as rPFS events in this study. Patients who discontinued therapy due to clinical progression are the subject of a separate analysis. Together, the rigorous inclusion criteria and method of determination of radiographic progression in COU-AA-302 made it difficult to identify an independent dataset for external validation of our model. Therefore, satisfactory discrimination and prognostic value of this model have not yet been demonstrated in an independent dataset, which is required before it can be applied in standard clinical practice.
Although the model is prognostic in nature, it does speak to the wide spectrum of baseline clinical factors seen in the chemotherapy-naïve patient population. The profound 3-fold difference in rPFS between the good versus poor risk group in this model suggests that further work should be done to clarify whether these markers are predictors of treatment response and to stratify by these or similar risk groups. For the treating clinician, there may be implications for the timing of therapy initiation. In a hypothesis-generating analysis of COU-AA-302, initiation of treatment with abiraterone plus prednisone was associated with greater clinical benefit among patients without significant pain (BPI 0-1 vs. ≥ 2) or pronounced PSA elevation (< 80 vs. ≥ 80 ng/mL).18 It is plausible that a patient in the good risk group who is observed and does not receive treatment, but then progresses to the intermediate or poor risk group, may receive less benefit when therapy is initiated. Such a concept would need to be evaluated prospectively.
The adverse prognostic value of the presence of lymph node metastases in this analysis requires further study as lymph node-only disease in men with mCRPC has typically been associated with a better prognosis.19 These findings may reflect the fact that such patients were more likely to develop RECIST-defined progression of disease, which may occur earlier than the development of new bone lesions, and which was the marker of rPFS for those with bone-only disease. An alternative explanation may arise from the recent observations that lymph nodes may harbor small-cell or neuroendocrine histologies that are associated with a poor prognosis.20
To the best of our knowledge, the prognostic index model described herein is the first contemporary model for rPFS in asymptomatic/minimally symptomatic mCRPC patients treated with abiraterone plus prednisone. Our findings suggest that there is a broad clinical spectrum of chemotherapy-naïve mCRPC, which could warrant a tailored approach to therapy for each respective group of patients. Future studies should (1) explore the performance of subsequent chemotherapy in patients, especially those in the poor prognosis risk group who should receive closer follow-up, and (2) characterize the molecular profile of the tumors of such individuals with respect to androgen receptor variants, tumor histology, and genomic aberrations.21,22 With prospective validation, this model may allow more accurate assessment of risk in patients with earlier stage mCRPC using factors that are routinely assessed in everyday clinical practice, and may aid in clinical decision-making, patient selection or stratification for clinical trials, clinical trial design, and modification of clinical guidelines to approaches that are more individualized for patient needs.
Conclusions
The prognostic index model for rPFS developed from chemotherapy-naïve patients reveals differential outcomes with abiraterone plus prednisone therapy based on factors readily available in clinical practice. The over 3-fold difference in rPFS based on these factors suggests that, with validation, they may be useful in clinical trial stratification as well as routine clinical care.
Supplementary Material
Clinical Practice Points.
Prognostic models for earlier intermediate end points in addition to OS are necessary to assess risk in patients with earlier stage of mCRPC
We developed a prognostic index model for rPFS in chemotherapy-naïve mCRPC patients treated with abiraterone acetate plus prednisone. Data are from COU-AA-302, a phase III trial of abiraterone acetate plus prednisone versus prednisone alone in asymptomatic/minimally symptomatic patients with mCRPC and no visceral metastases
The model uses 5 factors readily available in clinical practice: LDH (>ULN [234 IU/L] vs. ≤ULN), bone metastases (≥10 vs. <10), PSA (>39.5 vs. ≤39.5 ng/mL), lymph node metastases (present vs. absent), and hemoglobin (≤LLN [12.7 g/dL] vs. >LLN)
Patients were categorized into 3 prognostic groups (good, intermediate, and poor) based on the number of risk factors
There was a profound 3-fold difference in rPFS between the good versus poor risk group in this model. Median rPFS was 27.6, 16.6, and 8.3 months for patients in the good, intermediate, and poor prognostic groups, respectively. The C-index was 0.83 (95% CI = 0.73–0.91)
Our findings suggest that there is a broad clinical spectrum of chemotherapy-naïve mCRPC
The prognostic value of this model should be demonstrated in an independent dataset. If validated, this model may allow assessment of risk in patients with earlier stage mCRPC using factors that are routinely assessed in everyday clinical practice, and may aid in clinical decision-making, patient selection or stratification for clinical trials, clinical trial design, and modification of clinical guidelines to approaches that are more individualized for patient needs
Acknowledgments
Writing assistance was provided by Lashon Pringle, PhD, of PAREXEL and was funded by Janssen Global Services, LLC.
Footnotes
Disclosure
C.J.R. has received honoraria from Janssen Research & Development. T.K., J.L., and P.D.P. are employed by Janssen Research & Development and own stock or hold an ownership interest with Johnson & Johnson. A.M. was an employee of Janssen Research & Development during the time of this analysis and has owned stock or held an ownership interest with Johnson & Johnson. J.C. has served as consultant/advisor to and received honoraria from Janssen Research & Development. E.E. has served as a consultant/advisor for AstraZeneca, Janssen Research & Development, Sanofi, and Tokai Pharmaceuticals, received institutional research funding from Janssen Research & Development, and received honoraria from AstraZeneca, Janssen Research & Development, Sanofi, and Takeda. P.W.K. and K.N.C. serve on the advisory board for and receive clinical research funding from Janssen Research & Development. P.F.A.M. has no direct or indirect commercial incentive associated with publishing this article. F.S. is a consultant/advisor to and has received honoraria and research funding from Astellas and Janssen Research & Development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:1755–6. doi: 10.1056/NEJMc1410239. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 5.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–25. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castraton-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 8.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 9.Halabi S, Lin CY, Small EJ, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013;105:1729–37. doi: 10.1093/jnci/djt280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–7. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravi P, Mateo J, Lorente D, et al. External validation of a prognostic model predicting overall survival in metastatic castrate-resistant prostate cancer patients treated with abiraterone. Eur Urol. 2014;66:8–11. doi: 10.1016/j.eururo.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–82. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Morris MJ, Molina A, Small EJ, et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol. 2015;33:1356–63. doi: 10.1200/JCO.2014.55.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH, George SL. The bootstrap and identification of prognostic factors via Cox’s proportional hazards regression model. Stat Med. 1985;4:39–46. doi: 10.1002/sim.4780040107. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, Carles J, Gschwend H, et al. The phase 3 COU-AA-302 study of abiraterone acetate (AA) in men with chemotherapy (CT)-naïve metastatic castration-resistant prostate cancer (mCRPC): stratified analysis based on pain, prostate-specific antigen (PSA) and Gleason score (GS). The 31st Annual EAU Congress; March 11–15, 2016; Munich, Germany. Abstract 775. [DOI] [PubMed] [Google Scholar]
- 19.Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;10:1652–9. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okoye E, Choi EK, Divatia M, Miles BJ, Ayala AG, Ro JY. De novo large cell neuroendocrine carcinoma of the prostate gland with pelvic lymph node metastasis: a case report with review of literature. Int J Clin Exp Pathol. 2014;7:9061–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–24. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.