Abstract
Cancer mortality rates in the United States continue to decline. Reductions in tobacco use, uptake of preventive measures, adoption of early detection methods, and better treatments have resulted in improved cancer outcomes for men and women. Despite this progress, some population groups continue to experience an excessive cancer burden when compared with other population groups. One of the most prominent cancer health disparities exists in prostate cancer. Prostate cancer mortality rates are highest among men of African ancestry when compared with other men, both in the United States and globally. This disparity and other cancer health disparities are largely explained by differences in access to health care, diet, lifestyle, cultural barriers, and disparate exposures to carcinogens and pathogens. Dietary and lifestyle factors, pathogens, and ancestry-related factors can modify tumor biology and induce a more aggressive disease. There are numerous examples of how environmental exposures, like tobacco, chronic stress, or dietary factors, induce an adverse tumor biology, leading to a more aggressive disease and decreased patient survival. Because of population differences in the exposure to these risk factors, they can be the cause of cancer disparities. In this review, we will summarize recent advances in our understanding of prostate and breast cancer disparities in the United States and discuss how the analysis of tumor biology can advance health disparity research.
Cancer mortality rates in the United States have been declining since the 1990s because of reduced tobacco use among adults, more widespread cancer screening and testing, and improved cancer therapies.1, 2 Despite this progress, disparities continue to persist across different racial and ethnic groups.2, 3 In the United States, African Americans disproportionately bear the cancer burden and have the highest death rates for many cancer types of any racial or ethnic group.4
Breast cancer and prostate cancer are the two most common invasive cancers in women and men, respectively. Advancements in detection and cancer therapy have improved cancer care and disease outcomes for these two cancer types. Although incidence rates for prostate cancer are decreasing in the United States, they are still increasing in many parts of the world. The latter can be attributed to population aging and adaptation of a Western lifestyle. Likewise, breast cancer incidence rates are also increasing globally. In the United States, these rates have been stable for many years; however, for African-American women, breast cancer incidence increased (0.7% per year), whereas the disease incidence decreased (1.0% per year) among European-American women between 2000 and 2009.5 Although breast cancer mortality has decreased annually by an average of 2%, likely because of improved diagnosis and treatment, African-American women still have the highest mortality when compared with other racial and ethnic groups.4, 5, 6 The reasons for the observed disparities are multifactorial and include access to health care, socioeconomic status, health literacy, and discrimination. Nonetheless, these socioeconomic and sociobehavioral factors do not fully explain these health disparities. Therefore, health disparity research has begun to explore biological factors. This approach is further supported by the observation that disease characteristics of prostate and breast cancer show significant geographic and ethnic variations.7, 8 Research efforts have begun to concentrate on molecular mechanisms in tumor biology and ancestry-related aspects that may contribute to cancer disparities (Figure 1). This review will focus on bringing to light the molecular and epidemiologic work that has gone into understanding the tumor biological differences that may contribute to cancer health disparities.
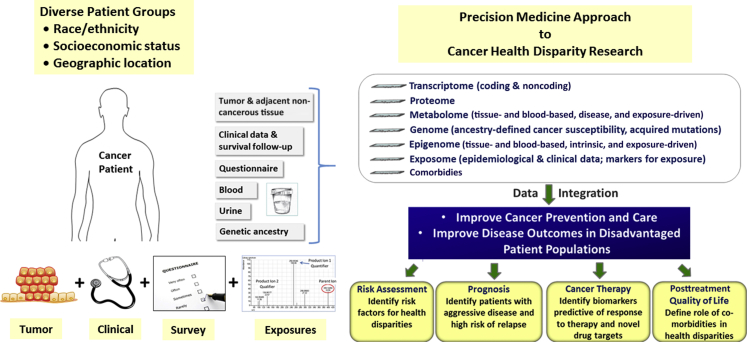
Figure 1.
Analysis of tumor biology to advance cancer health disparity research. A precision medicine approach is shown for cancer health disparity research that uses an integrated analysis of tumor tissues and other biospecimens, clinical data, survey and exposure data (eg, diet and lifestyle, environmental exposures, family and sexual history, occupation, social isolation, and discrimination), and patient characteristics (eg, socioeconomic status, body mass index, comorbidities, and genetic ancestry).
Survival Health Disparities in Breast and Prostate Cancer in the United States
Survival Health Disparities in Prostate Cancer
Prostate cancer is the second leading cause of cancer-related deaths in US men and exhibits a pronounced racial disparity. Well-established risk factors for the disease are age, family history of the disease, race/ethnicity, and inherited genetic factors that include approximately 100 disease susceptibility loci that are known.9 African-American men have a mortality rate more than double that of European-American men, in part because of their increased risk of developing the disease.5 Surveillance, epidemiology, and end results data for the period from 1975 to 2012 show that African-American men have an earlier mean age of a prostate cancer diagnosis (69.1 versus 71.1 years) and a shorter mean survival period (44.4 versus 48.4 months) when compared with European-American men.10 This survival disparity has been observed for >20 years, despite the approximately equal prevalence of prostate-specific antigen testing among African-American and European-American men.11, 12 The higher disease mortality of African-American men is caused by multiple factors, including access to care, biological/genetic differences, geography, and patient characteristics and behaviors.12 Interestingly, men of West-African ancestry from the Caribbean and South America exhibit incidence and mortality rates similar to African-American men, suggesting a possible ancestral basis for some of these common outcomes.13
The higher mortality rates among African-American men may also occur because these men are prone to a more aggressive type of prostate cancer. A study conducted by Tyson and Castle14 examined racial disparities in survival for patients with clinically localized prostate cancer. Even though the investigators adjusted for clinical and socioeconomic factors, African-American men still had the worst survival compared with European-American, Hispanic, or Asian men. Powell et al15 analyzed prostate autopsy material from 1056 African-American and European-American men who did not have a prior diagnosis of prostate cancer. They compared autopsy findings with results from the analysis of resected tumors. Although the disease presentation was similar between the two patient groups at the subclinical stage, among the patients with clinically detected prostate tumors, African-American men tended to have a more aggressive disease than European-American men. This finding is consistent with an accelerated disease progression in African-American men, indicating that differences in tumor biology may exist between the two patient groups.
Survival Health Disparities in Breast Cancer
The US health disparity in breast cancer presents itself different from the disparity in prostate cancer because it is mostly a survival health disparity, with a disproportionately high mortality among African-American, Latina, and Native American women.6 Although advancements in cancer treatment, screening, and diagnosis are helping women overall, breast cancer incidence rates in African-American women are increasing. Currently, the 5-year relative survival rate is 80% for African-American women compared with 91% among European-American women.4 It is believed that nonbiological and biological factors play a major role in this survival disparity.16 Nonbiological factors include socioeconomic factors, access to health care, cultural factors, and comorbidities.
A poorer survival of African-American breast cancer patients has also been observed in equal access health care systems and clinical trial settings.17, 18 It was thought that the increased prevalence of early-onset estrogen receptor (ER)–negative tumors and basal-like/triple-negative tumors in women of African ancestry is largely responsible for the excessive mortality. Although this argument is plausible, one has to be cautious because the breast cancer survival disparity occurs irrespective of the tumor's ER status.19 A study by Hershman et al20 found that, after controlling for stage, demographics, socioeconomic variables, tumor characteristics, and treatment factors, an excessive mortality remained among both pre and postmenopausal African-American women who were diagnosed with early-stage breast cancer. These findings are consistent with the more recent observations by Silber et al.16 Therefore, still poorly understood molecular and genetic differences in tumor biology may promote a more aggressive disease in women of African descent. A better understanding of these biological factors is critical for addressing the high disease mortality observed in African-American women.
Biological Determinants of Cancer Health Disparities
Observations from migration studies that immigrants tend to acquire cancer rates of their new home country within two generations indicate the importance of modifiable exposures as major risk factors for common cancers in the United States.21, 22 Yet, breast and prostate tumors tend to be more aggressive at diagnosis in African Americans than they are in European-Americans. This disparity has not been eliminated with the equal use of cancer screening and has generated a heightened interest in understanding the biology of these tumors. Subsequently, this research led to the discovery of differences in both genetic predisposition to cancer and molecular subtypes between these population groups, which we will discuss later in separate sections. Apart from these observations, additional differences in the tumor biology between African-American and European-American patients were described by investigators who used gene expression, mutational, and immunohistochemical analyses of tumors.
In prostate cancer, the epidermal growth factor receptor was found to be more commonly expressed in tumors from African-American patients when compared with European-American patients.23 Using a differential display analysis, a novel prostate-specific gene, termed PCGEM1, was identified that encodes a long noncoding RNA with a cell growth–promoting function.24 This transcript is most highly expressed in prostate tumors of African-American patients. Others described ZBTB33 (alias KAISO) and motor neuron and pancreas homeobox 1 (MNX1) as oncogenically up-regulated genes in tumors of these patients. KAISO is regulated by epidermal growth factor receptor signaling and is a key transcription factor that induces epithelial-to-mesenchymal transition, whereas MNX1 regulates lipid synthesis.25, 26 Last, a recurrent deletion on chromosome 3q13.31 was found to occur in prostate tumors of African-American patients that associates with the loss of the ZBTB20-LSAMP locus and with an aggressive disease.27
Few studies have examined the biology of breast tumors in African-American women beyond the concept of molecular subtypes. Several used gene expression profiling to study the biology.28, 29 A race/ethnicity-associated gene signature of poor outcome was described for luminal A breast tumors.30 This signature comprises six genes that are differentially expressed between African-American and European-American patients. Terunuma et al31 reported a high prevalence of a MYC signaling signature in tumors of African-American patients that was associated with the accumulation of the oncometabolite, 2-hydroxyglutarate. Earlier studies described race/ethnicity differences in the expression of cell cycle regulatory proteins, including p53, which is encoded by the TP53 tumor suppressor gene.32 These findings were confirmed when it was shown that tumors from African-American patients were more likely to carry a TP53 mutation.28, 33 The high prevalence of triple-negative tumors in women of African descent accounts for much of this race/ethnicity difference in the TP53 mutation's frequency because TP53 mutations are most common in this disease subtype.33, 34 Interestingly, the tumor TP53 status has been associated with socioeconomic deprivation.35, 36 Thus, an environmental factor related to socioeconomic status may promote the development of TP53 mutations in breast tumors. Alternatively, socioeconomic deprivation may specifically increase the risk of triple-negative breast cancer.
Disparities in Molecular Subtypes of Prostate and Breast Cancer
Disparities in Molecular Subtypes of Prostate Cancer
Prostate cancer evolves as a multistage disease with an accumulation of genomic aberrations during disease progression.37 Recurrent genomic rearrangements at cancer-related loci define prostate cancer subtypes.38 TMPRSS2:ERG gene fusion events, which produce fusion transcripts and oncogenic overexpression of ERG, an oncogene that encodes a member of the E26 transformation–specific (ETS) family of transcription factors, are signature mutations of a subset of prostate tumors and have important biological implications.39 Tumors carrying these gene fusions fall into a distinct molecular subtype. Other subtypes are negative for ETS-fusion gene arrangements but may overexpress the SPINK1 oncogene, or carry a SPOP mutation, and have a poor prognosis.38, 40 Conversely, localized prostate cancer contains few recurrent mutations in oncogenes or tumor suppressor.41 Instead, local prostate cancer is characterized by ETS-fusion gene arrangements and allelic gains of the MYC (alias c-myc) gene and deletions of the PTEN, TP53, and NKX3-1 tumor suppressors, with additional common changes in DNA methylation that increase aggressiveness.42, 43 In stark contrast, nearly half of the metastatic castration-resistant prostate cancers harbor mutations in the androgen receptor, TP53, and PTEN genes and additional mutations in BRCA1, BRCA2, and ATM, among others.44 However, because most studies included only patients of European descent, the mutational landscape of prostate cancer may not have been fully captured.
Multiple reports have shown that prostate tumors from patients of either European, African, or Asian descent exhibit notable differences in acquired chromosomal aberrations.40, 45, 46, 47 These studies examined the frequency of recurrent ERG gene fusions and PTEN loss in diverse patient populations. Although common in European and European-American patients (40% to 50% frequency), TMPRSS2:ERG gene fusion events were found to be significantly less frequent in African-American patients (20% to 30%) and were rather uncommon in patients from Asia in these studies (approximately 10%).48 A similar pattern was observed for PTEN loss, with rates being significantly lower in Asian and African-American patients.47, 48 These findings indicate robust race/ethnic differences in disease cause and mutational events affecting these patients.
Disparities in Molecular Subtypes of Breast Cancer
Breast cancer is a heterogeneous disease that encompasses at least four major molecular subtypes. Large-scale gene expression studies showed that breast tumors can be classified into subtypes with distinct gene expression.34 Molecular signatures characterize two luminal subtypes (A and B), the HER2 signaling-enriched tumors, and basal-like tumors. Among the subtypes, basal-like and HER2-positive tumors that are ER negative tend to yield the most aggressive disease. Basal-like tumors overlap largely with a group of tumors referred to as triple negative, which are negative for ER, HER2, and progesterone receptor expression. Research into novel therapies of breast cancer has focused on triple-negative tumors because these tumors are not treatable by current endocrine therapies, such as tamoxifen and aromatase inhibitors, or by HER2-targeting therapies, such as trastuzumab (Herceptin; Genentech/Roche, South San Francisco, CA). It was recognized starting in 2006 that the prevalence of these subtypes varies among women in the United States, with the greatest difference observed between women of African and European descent.33, 49 Investigations in West and Central Africa have provided further corroboration that women of African descent tend to develop early-onset, high-grade, ER-negative tumors more frequently than women of European descent.8 Early-onset ER-negative tumors also develop more frequently in women from India and Pakistan.50 Finally, cancer epidemiology showed that the rates of ER-negative breast tumors can change in a population over time, pointing to an influence of environmental and reproductive factors in the development of these tumors.51
Immune-Inflammation Signatures in Breast and Prostate Tumors of African-American Patients
Immune-Inflammation Signatures in Prostate Cancer
Inflammation and neoangiogenesis are key biological processes in cancer biology.52 Inflammation is also a putative risk factor for prostate cancer and has been associated with aggressive disease.53, 54 We and others have previously described a distinct immune-inflammation signature in prostate tumors of African-American patients,55, 56, 57, 58 which is absent in most European-American patients (Table 1). Moreover, race/ethnicity differences in prostatic inflammation have been described in benign prostate tissues59, 60 (Table 1), but their relationship to disease risk and aggressiveness remains unclear, because the findings in these two studies were not consistent. Because of the observations that tumors of African-American men commonly contain an immune-inflammation signature, we investigated the link between the regular use of aspirin, a nonsteroidal anti-inflammatory drug, and prostate cancer. Regular aspirin use significantly reduces the risk of both advanced prostate cancer and disease recurrence in African-American men.61 Our observation that regular aspirin use increases disease-free survival among these men confirms a similar observation in a previous study.62
Table 1.
Reports of Race/Ethnic Differences in Tumor-Associated Markers of Inflammation in Prostate Tumors
| Tumor marker | Observation | Reference |
|---|---|---|
| Immune-inflammation signatures | Gene expression differences between tumors from AA and EA men related to immune response and inflammation. Prominent immune-inflammation signature and a distinct interferon-related gene signature in AA tumors. | 55 |
| Increased expression of cytokines in AA tumors. | 56 | |
| Distinct DNA copy number alterations in AA tumors. Alterations at the gene expression and DNA copy number levels point to differences in immune response between AA and EA men. | 57 | |
| Increased expression of genes associated with immune response and inflammation in AA tumors. A prominent interferon signature is present. | 58 | |
| Tissue inflammation | Pathology-based scoring of inflammation in benign prostate tissue from AA and EA men. Inflammation was more prevalent in AA men. | 59 |
| Pathology-based scoring of acute and chronic inflammation in benign prostate tissue from Asian, AA, EA, and Hispanic men, all participants of the REDUCE trial. No race/ethnic differences in chronic inflammation but more acute inflammation in Asian men, less in AA men, when compared with EA men. Acute inflammation was associated with a lower prostate cancer risk. | 60 |
AA, African-American; EA, European-American; REDUCE, A Clinical Research Study to Reduce the Incidence of Prostate Cancer in Men Who Are at Increased Risk.
Currently, no study has assessed whether men of African descent are affected by a systemic inflammatory process that increases the risk of lethal prostate cancer. However, there is evidence from the measurement of blood C-reactive protein levels that African Americans tend to have higher levels of this inflammation marker than European-Americans,61, 63 indicating increased systemic inflammation. Although environmental exposures, including infections, promote systemic inflammation, ancestral factors may also influence inflammatory processes. For example, allele frequencies of genetic variants in inflammation-related genes can markedly differ among population groups.64 Therefore, ancestral factors may be a cause of the immune-inflammation signature in African-American prostate cancer patients.
In summary, there is evidence that prostate cancer patients of African descent experience an increased occurrence of a low-grade chronic inflammation in their tumors. These observations suggest that disease progression and recurrence can be prevented, at least to some extent, with regular use of anti-inflammatory drugs, such as aspirin, in this high-risk group of patients.
Immune-Inflammation Signatures in Breast Cancer
In breast cancer, an immune-inflammation signature describes a subset of triple-negative breast tumors.65 Inflammation in tumors includes the recruitment of tumor-associated macrophages (TAMs). Notably, their numbers were found to be elevated in breast tumors of African and African-American women by several investigators28, 66, 67, 68 (Table 2), suggesting an opportunity of targeting this immune environment as a therapeutic intervention. Moreover, Martin et al28 observed an increased microvessel density in these tumors. An elevated tumor vascularization in African-American breast cancer patients was also reported by Lindner et al.69 Tumor angiogenesis correlates with breast cancer metastasis and poor survival.70 Several mediators of inflammation contribute to increased angiogenesis and breast cancer progression.71 Aberrant expression of inflammation mediators, such as cyclooxygenase-2 and inducible nitric oxide synthase, occurs in breast tumors.72, 73 The significance of TAMs and an altered tumor microenvironment in the promotion of breast cancer progression is well documented.52, 71 Thus, chronic inflammatory stimuli may have a crucial role in disease progression through inflammation-induced angiogenesis and tissue remodeling. Although solid evidence is still missing, current data suggest that inflammation-induced breast cancer progression may be more prevalent in patients of African descent and may relate to increased inflammatory cytokine levels in these women.74
Table 2.
Reports of Race/Ethnic Differences in Immune Cell Numbers in Breast Tumors
| Cancer type | Observation | Reference |
|---|---|---|
| Tumor-associated macrophages | Number of CD68-positive macrophages is higher in breast tumors of AA patients than EA patients. Chemotaxis-related gene signature in AA tumors. | 28 |
| Proliferating cellular nuclear antigen–positive macrophages are increased in breast tumors of Latina and AA women when compared with EA women. | 66 | |
| High numbers of tumor-associated macrophages in breast tumors of Nigerian women. | 67 | |
| High immune cell infiltration, including CD163-positive macrophages, in breast tumors of Kenyan women. | 68 |
AA, African-American; EA, European-American.
Differences in Cancer Epigenetics among Population Groups
DNA methylation regulates gene expression and cell differentiation and is partly inherited.75 Environmental exposures and obesity can modify DNA methylation patterns and alter the tumor epigenome, as shown for prostate and breast cancer.76, 77, 78 Changes to DNA methylation are present in all cancers and cooperate with acquired genetic alterations to promote the cancer phenotype.79 Alterations in DNA methylation are among the earliest somatic changes that can be detected in cancerous lesions and occur at the stage of proliferative inflammatory atrophy, a precursor lesion in prostate cancer development.80 An aberrant increase in DNA methylation can lead to numerous downstream effects, including inactivation of PTEN, a bona fide tumor suppressor gene in prostate cancer, or a loss of GSTP1 expression, which is observed in 90% of prostate cancers. Epigenome-wide DNA methylation patterns have been linked to aggressive and lethal prostate cancer42, 43 and to breast cancer recurrence, metastasis, and survival.81, 82 Others have described molecular subgroups in breast cancer based on a global DNA methylation pattern.83 Hence, therapies that target the cancer epigenome are being developed.79
Race/ethnicity-related differences in DNA methylation have been observed in peripheral blood mononuclear cells of newborns.84 This study observed significant differences in DNA methylation affecting cancer-related loci, indicating that early life exposures may induce a DNA methylation pattern that later associates with disease susceptibility. Others reported a relationship between African ancestry and global DNA hypomethylation in blood leukocytes of cancer patients.85 Therefore, ancestry-related factors may influence the regulation of DNA methylation. Given the importance of epigenetic alterations in the development and progression of cancer, multiple investigators examined and compared DNA methylation in prostate and breast tumors of African-American, European-American, and Asian patients using targeted and genome-wide approaches.
Race/Ethnic Differences in Prostate Cancer Epigenetics
In the prostate cancer studies, a pattern emerged consistent with an increased prevalence of DNA hypermethylation at several disease-related loci in African-American tumors when compared with similar stage tumors from European-American men86, 87 (Table 3). Some of these alterations may relate to disease progression. Recently, the transcriptional regulator, KAISO, was found to be overexpressed in prostate tumors from African-American patients.25 KAISO recognizes clusters of methylated CpG nucleotides and enhances invasion of prostate cancer cells. This suggests that aberrant KAISO expression in tumors from African-American men may enhance disease aggressiveness by mechanisms that include aberrant DNA methylation of cancer-related genomic loci.
Table 3.
Reports of Race/Ethnic Differences in Tumor DNA Methylation Patterns
| Cancer type | Observation | Reference |
|---|---|---|
| Prostate cancer | CpG hypermethylation of the GSTP1 promoter is more prevalent in prostate tumors of AA men than EA or Asian men. | 86 |
| Globally higher DNA methylation in AA prostate tumors when compared with EA tumors in a genome-wide DNA methylation analysis. | 87 | |
| Breast cancer | Prevalence of DNA methylation–defined tumor subtypes is different between AA and EA patients. Using a genome-wide DNA methylation analysis, the authors identified a poor survival tumor subtype with distinct DNA methylation, high tissue 2-hydroxyglutarate levels, and heightened occurrence in AA patients. | 31 |
| Greater DNA methylation differences in ER-negative than ER-positive breast cancer, comparing AA with EA women in a genome-wide DNA methylation analysis. Globally, the number of differentially methylated CpG sites was low. | 88 | |
| Few differences in promoter methylation between AA and EA breast cancer patients in the tumors and in peripheral blood monocytes of these patients. More methylation differences in ER-negative tumors. | 89 | |
| In ER-negative breast cancer, AA patients had higher DNA methylation in four gene promoters of a five-gene panel when compared with EA patients. Differences were only observed among women with early-onset disease. No racial differences in ER-positive breast cancer. | 90 |
AA, African-American; EA, European-American; ER, estrogen receptor.
Race/Ethnic Differences in Breast Cancer Epigenetics
In breast cancer studies, investigations focused on the differences in DNA methylation between African-American and European-American patients by analyzing tumors with both candidate gene and genome-wide approaches31, 88, 89, 90 (Table 3). Differences in methylation between the two patient groups were reported for the ER-negative disease, but less so for the ER-positive disease. In general, the observed differences were not widespread but rather restricted to a few loci, and may be partially related to differences in MYC signaling among these patients.31 There is also evidence that some of the reported DNA methylation differences may not be tumor specific because similar patterns have been found in normal breast tissues and peripheral blood monocytes.89
To date, the mechanisms that lead to race/ethnicity differences in DNA methylation patterns of tumors are not fully understood. Whether these patterns are induced by ancestral genetic factors, the mutational landscape, environmental exposures, intrinsic differences in tumor biology, including cancer metabolism and metabolism-induced epigenetic alterations, or a combination of these factors also remains unknown. However, population differences in genome-wide DNA methylation have been described by Heyn et al.91 The study showed that two-thirds of the population-specific CpG sites were directly associated with the underlying genetic background of the populations. This finding indicates that inheritance and genetic ancestry are key drivers of epigenetic differences between populations that not only contribute to physical appearance, but may also contribute to traits, such as behavior, disease susceptibility, and disease aggressiveness.
Stress Signaling–Mediated Effects in Cancer Biology and Disease Progression
Stress Signaling–Mediated Effects in Prostate Cancer
Cancer epidemiology has linked stressful life events and discrimination to poor disease survival.92 Several investigations into the effects of stress exposures on tumor biology have also focused on prostate cancer. It was discovered that catecholamine signaling increases the metastatic phenotype of prostate cancer cells.93 Recently, a stress-related signaling signature was found to occur in human prostate tumors,94 suggesting that environmentally induced stress signaling may adversely affect prostate cancer patients. Although there is no direct evidence that stress exposures affect prostate cancer patients from minority or disadvantaged backgrounds more so than other patients, a functional annotation of copy number and gene expression differences comparing tumors from African-American and European-American patients showed that these differences not only relate to immune function but also to neurotransmitter transport and catecholamine metabolic processes.57 Although the significance of these findings is unclear, the data suggest that differences in stress-induced catecholamine signaling may exist between the two patient groups.
Stress Signaling–Mediated Effects in Breast Cancer
Stressful life events and perceived experiences of racism have a significant association with adverse health behaviors and breast cancer,95, 96 whereas stress because of a cancer diagnosis may disproportionally affect minority populations.97 Social class, social isolation, and related stress affect immune cell function and tumor biology. In mice, chronic restraint stress decreased levels and function of p53 and promoted tumorigenesis.98 In women, Baker et al,35 and Starks et al36 observed a relationship between socioeconomic deprivation and an increased frequency of TP53 mutations in breast tumors. Other studies reported that social adversity in early life leads to resistance in glucocorticoid signaling and increased proinflammatory signaling.99 Social stress also affects the response to infections, telomere length, and immune cell function, and elevates the tumor catecholamine content.100, 101 Thus, stress exposures have a potentially oncogenic function in tumor biology and may have a disproportionally high adverse impact in socially deprived and minority populations. Specifically, in breast cancer biology, social isolation can lead to reprogramming of tumor biology.102, 103
Chronic stress influences tumor biology through two major pathways involving catecholamines and glucocorticoids.104 Stress-induced β-adrenergic signaling has been shown to promote cancer progression and metastasis in animal models of breast cancer.105, 106 The mechanism by which this pathway promotes metastasis has been described and includes recruitment of TAMs and interactions between TAMs and cancer cells that cause increased tumor angiogenesis and distant metastasis.105, 106 Recently, social isolation was found to be associated with elevated tumor catecholamine levels in human ovarian tumors and with a tumor gene signature, albeit this study was rather small.107 In 2010, it was first reported that β-blocker use after a disease diagnosis reduces disease recurrence and improves cancer-specific survival of breast cancer patients.108 In another study, users of the β-blocker, propranolol, were found to be significantly less likely to develop advanced breast cancer and have reduced breast cancer–specific mortality.109 β-blocker use has also been associated with improved recurrence-free survival in triple-negative breast cancer.110 Jointly, these data indicate that stress may influence human breast cancer biology through activation of the prometastatic catecholamine pathway, leading to a poor outcome phenotype in a subpopulation of patients who would benefit from β-blocker use and stress management.
Our group observed that breast tumors from African-American patients contained a higher microvessel density and more TAMs than tumors from European-American patients, independent of disease stage and tumor ER status.28 Because the phenotype is consistent with characteristics that are induced by catecholamine signaling in experimental models,105 our findings may indicate that increased signaling by this pathway occurs in tumors of these patients. Thus, inhibitors of catecholamine signaling may improve outcomes specifically among African-American patients, which has not been examined thus far.
Contribution of Population Genetics and Ancestry to Health Disparities
Genetic ancestral factors not only characterize population groups but also participate in selection and can define phenotypic traits within populations.111 Several studies have shown that gene expression differences exist among populations that are attributable to common genetic variations and ancestral background. Two of these studies investigated gene expression variations between individuals of European and African ancestry (Nigeria) using lymphoblastoid cell lines.112, 113 The researchers observed that these variations can cluster in disease-related pathways, raising the possibility that differences in common genetic variations among population groups cause population-specific susceptibilities for common diseases, like cancer, because of their effect on the transcriptome.
Contribution of Ancestry to Prostate Cancer Health Disparities
Prostate cancer is a genetically heterogeneous disease, in which inherited factors may account for approximately 40% to 50% of the cases.114 Several familial susceptibility genes have been described, including RNASEL, BRCA1, BRCA2, and HOXB13. Germline mutations in these genes account for 5% to 10% of all prostate cancer cases. Most of the inherited risk for prostate cancer arises from common genetic variants. Approximately 100 disease susceptibility loci are currently known,9 but not all of them confer risk in men of African ancestry.115 Numerous studies have examined the possibility of low-penetrance genes contributing to the excessive burden of prostate cancer in men of African ancestry. To date, the best characterized risk locus for prostate cancer is located at 8q24. This locus likely affects tumor biology because it encodes an enhancer region that regulates MYC in an allele-specific manner.116 Multiple common variants within this locus increase the risk of prostate cancer in diverse populations.117, 118, 119 As shown by admixture mapping and genome-wide association studies, 8q24 confers an even higher risk for prostate cancer in men of West African ancestry, when compared with men of European and Asian ancestry, partly explained by variants that were only found in men of African ancestry.117, 118, 120 Thus, the 8q24 region accounts for at least some of the excessive disease risk among men of African ancestry. A susceptibility locus at 17q21 has been identified as another genetic risk factor that appears to be restricted to men of African ancestry.121 However, few common genetic variations have been associated with prostate cancer aggressiveness, mortality, or response to therapy,122 and none has been associated with increased mortality among men of African ancestry.
Contribution of Ancestry to Breast Cancer Health Disparities
Genome-wide association studies and admixture mapping have also been used to examine the contribution of inherited risk to breast cancer and to the excessive risk of breast cancer mortality among women of African and Latina descent. Numerous genome-wide association studies showed that a large set of common genetic variants defines the risk of breast cancer, the ER- and triple-negative disease, and breast cancer survival.123, 124, 125, 126 The most strongly associated risk locus has been mapped to the region containing fibroblast growth factor receptor 2.123 However, an analysis within the Multiethnic Cohort Study observed significant population heterogeneity for the association of 12 highly replicated single-nucleotide polymorphisms with breast cancer and did not find an association of these single-nucleotide polymorphisms with breast cancer in African Americans.127 Additional studies did not replicate genome-wide association study findings from European populations in populations of African ancestry.128 Instead, different low-penetrance risk loci for breast cancer may exist in populations of African ancestry.129, 130, 131 One study suggested that a common variant at the TERT-CLPTM1L locus may be an underlying cause for the high prevalence of ER-negative tumors among women of African ancestry.132 Admixture scans in African-American and Latina women with breast cancer further support the concept of ancestry-related risk factors in breast cancer. Two studies of Latina women found that European and indigenous American ancestry are significantly associated with breast cancer risk and survival, respectively, in this population.133 Admixture mapping of African-American women identified new breast cancer risk loci for this population.134
Together, these data show that population genetics and ancestry likely contribute to existing health disparities and partly explain the increased risk of prostate cancer among men of African ancestry, whereas these factors may also promote an excessive breast cancer mortality among African-American and Latina women.
Conclusions
Not all segments of the US population have equally benefited from the advances in our knowledge and treatment of cancer. Minority, immigrant, and disadvantaged populations continue to experience an excessive cancer burden. This burden is not only attributable to barriers in access to health care and cultural obstacles, but is also attributable to distinct carcinogen and pathogen exposures, environmentally induced stress, comorbidities, and ancestry-related risk factors. These factors, singularly or in combination, are the likely causes of the existing cancer health disparities in the United States and globally. There is strong evidence from migration studies that the environment defines cancer risk, but there is also evidence that population differences in genetic ancestry can lead to population differences in cancer susceptibility. One mechanism by which environmental and ancestry-related factors affect health outcomes is by inducing an adverse tumor biology. Multiple studies have identified differences in tumor biology and epigenetic factors among diverse patient populations. For example, the analysis of tumor markers revealed the existence of tumor biological and mutational differences in prostate cancer among patients of African, European, and Asian descent. These differences indicate disparities in disease cause and may arise from pathogenic mechanisms that are different between population groups. Environmental factors have also been shown to define the cause of many cancer types, and both environmentally induced stress and toxins from tobacco smoke can induce a tumor biology that increases the odds of a metastatic disease.105, 135 Changes in tumor biology attributable to modifiable risk factors may also affect early disease detection and the response to therapy. These changes may contribute to a distinct disease presentation of prostate cancer among men of African descent, including the excessive mortality in this patient group in the United States and globally. Thus, gaining an understanding of tumor biology in diverse patient populations can advance cancer health disparity research and provide new insights that can be harnessed to improve cancer prevention and care and to reduce or eliminate outcome disparities in cancer.
Footnotes
Race in Cancer Health Disparities Theme Issue
Supported by the Intramural Research Program of the NIH, the National Cancer Institute, and the Center for Cancer Research grants ZIA BC 010499, ZIA BC 010624, and ZIA BC 010887.
Disclosures: None declared.
This article is part of a review series on understanding the complex role of race in cancer health disparities.
References
- 1.Byers T. Two decades of declining cancer mortality: progress with disparity. Annu Rev Public Health. 2010;31:121–132. doi: 10.1146/annurev.publhealth.121208.131047. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis C.E., Siegel R.L., Sauer A.G., Miller K.D., Fedewa S.A., Alcaraz K.I., Jemal A. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe E.B., Meltzer J.P., Bethea T.N. Health disparities and cancer: racial disparities in cancer mortality in the United States, 2000-2010. Front Public Health. 2015;3:51. doi: 10.3389/fpubh.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desantis C., Siegel R., Bandi P., Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 7.Zeigler-Johnson C.M., Rennert H., Mittal R.D., Jalloh M., Sachdeva R., Malkowicz S.B., Mandhani A., Mittal B., Gueye S.M., Rebbeck T.R. Evaluation of prostate cancer characteristics in four populations worldwide. Can J Urol. 2008;15:4056–4064. [PMC free article] [PubMed] [Google Scholar]
- 8.Huo D., Ikpatt F., Khramtsov A., Dangou J.M., Nanda R., Dignam J., Zhang B., Grushko T., Zhang C., Oluwasola O., Malaka D., Malami S., Odetunde A., Adeoye A.O., Iyare F., Falusi A., Perou C.M., Olopade O.I. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Olama A.A., Kote-Jarai Z., Berndt S.I., Conti D.V., Schumacher F., Han Y. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46:1103–1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietro G.D., Chornokur G., Kumar N.B., Davis C., Park J.Y. Racial differences in the diagnosis and treatment of prostate cancer. Int Neurourol J. 2016;20:S112–S119. doi: 10.5213/inj.1632722.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariotto A.B., Etzioni R., Krapcho M., Feuer E.J. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877–1886. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 12.Aizer A.A., Wilhite T.J., Chen M.H., Graham P.L., Choueiri T.K., Hoffman K.E., Martin N.E., Trinh Q.D., Hu J.C., Nguyen P.L. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 13.Odedina F.T., Akinremi T.O., Chinegwundoh F., Roberts R., Yu D., Reams R.R., Freedman M.L., Rivers B., Green B.L., Kumar N. Prostate cancer disparities in black men of African descent: a comparative literature review of prostate cancer burden among black men in the United States, Caribbean, United Kingdom, and West Africa. Infect Agent Cancer. 2009;4 Suppl 1:S2. doi: 10.1186/1750-9378-4-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyson M.D., 2nd, Castle E.P. Racial disparities in survival for patients with clinically localized prostate cancer adjusted for treatment effects. Mayo Clin Proc. 2014;89:300–307. doi: 10.1016/j.mayocp.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Powell I.J., Bock C.H., Ruterbusch J.J., Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183:1792–1796. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silber J.H., Rosenbaum P.R., Clark A.S., Giantonio B.J., Ross R.N., Teng Y., Wang M., Niknam B.A., Ludwig J.M., Wang W., Even-Shoshan O., Fox K.R. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 17.Jatoi I., Becher H., Leake C.R. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98:894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 18.Albain K.S., Unger J.M., Crowley J.J., Coltman C.A., Jr., Hershman D.L. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menashe I., Anderson W.F., Jatoi I., Rosenberg P.S. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hershman D., McBride R., Jacobson J.S., Lamerato L., Roberts K., Grann V.R., Neugut A.I. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 21.Thomas D.B., Karagas M.R. Cancer in first and second generation Americans. Cancer Res. 1987;47:5771–5776. [PubMed] [Google Scholar]
- 22.Maskarinec G., Noh J.J. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis. 2004;14:431–439. [PubMed] [Google Scholar]
- 23.Shuch B., Mikhail M., Satagopan J., Lee P., Yee H., Chang C., Cordon-Cardo C., Taneja S.S., Osman I. Racial disparity of epidermal growth factor receptor expression in prostate cancer. J Clin Oncol. 2004;22:4673–4677. doi: 10.1200/JCO.2004.06.134. [DOI] [PubMed] [Google Scholar]
- 24.Petrovics G., Zhang W., Makarem M., Street J.P., Connelly R., Sun L., Sesterhenn I.A., Srikantan V., Moul J.W., Srivastava S. Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene. 2004;23:605–611. doi: 10.1038/sj.onc.1207069. [DOI] [PubMed] [Google Scholar]
- 25.Jones J., Wang H., Zhou J., Hardy S., Turner T., Austin D., He Q., Wells A., Grizzle W.E., Yates C. Nuclear kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am J Pathol. 2012;181:1836–1846. doi: 10.1016/j.ajpath.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L., Wang J., Wang Y., Zhang Y., Castro P., Shao L., Sreekumar A., Putluri N., Guha N., Deepak S., Padmanaban A., Creighton C.J., Ittmann M. MNX1 is oncogenically upregulated in African-American prostate cancer. Cancer Res. 2016;76:6290–6298. doi: 10.1158/0008-5472.CAN-16-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrovics G., Li H., Stumpel T., Tan S.H., Young D., Katta S., Li Q., Ying K., Klocke B., Ravindranath L., Kohaar I., Chen Y., Ribli D., Grote K., Zou H., Cheng J., Dalgard C.L., Zhang S., Csabai I., Kagan J., Takeda D., Loda M., Srivastava S., Scherf M., Seifert M., Gaiser T., McLeod D.G., Szallasi Z., Ebner R., Werner T., Sesterhenn I.A., Freedman M., Dobi A., Srivastava S. A novel genomic alteration of LSAMP associates with aggressive prostate cancer in African American men. EBioMedicine. 2015;2:1957–1964. doi: 10.1016/j.ebiom.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin D.N., Boersma B.J., Yi M., Reimers M., Howe T.M., Yfantis H.G., Tsai Y.C., Williams E.H., Lee D.H., Stephens R.M., Weissman A.M., Ambs S. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One. 2009;4:e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Field L.A., Love B., Deyarmin B., Hooke J.A., Shriver C.D., Ellsworth R.E. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. 2012;118:1334–1344. doi: 10.1002/cncr.26405. [DOI] [PubMed] [Google Scholar]
- 30.D'Arcy M., Fleming J., Robinson W.R., Kirk E.L., Perou C.M., Troester M.A. Race-associated biological differences among luminal A breast tumors. Breast Cancer Res Treat. 2015;152:437–448. doi: 10.1007/s10549-015-3474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terunuma A., Putluri N., Mishra P., Mathe E.A., Dorsey T.H., Yi M., Wallace T.A., Issaq H.J., Zhou M., Killian J.K., Stevenson H.S., Karoly E.D., Chan K., Samanta S., Prieto D., Hsu T.Y., Kurley S.J., Putluri V., Sonavane R., Edelman D.C., Wulff J., Starks A.M., Yang Y., Kittles R.A., Yfantis H.G., Lee D.H., Ioffe O.B., Schiff R., Stephens R.M., Meltzer P.S., Veenstra T.D., Westbrook T.F., Sreekumar A., Ambs S. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Invest. 2014;124:398–412. doi: 10.1172/JCI71180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones B.A., Kasl S.V., Howe C.L., Lachman M., Dubrow R., Curnen M.M., Soler-Vila H., Beeghly A., Duan F., Owens P. African-American/white differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2004;101:1293–1301. doi: 10.1002/cncr.20500. [DOI] [PubMed] [Google Scholar]
- 33.Keenan T., Moy B., Mroz E.A., Ross K., Niemierko A., Rocco J.W., Isakoff S., Ellisen L.W., Bardia A. Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol. 2015;33:3621–3627. doi: 10.1200/JCO.2015.62.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker L., Quinlan P.R., Patten N., Ashfield A., Birse-Stewart-Bell L.J., McCowan C., Bourdon J.C., Purdie C.A., Jordan L.B., Dewar J.A., Wu L., Thompson A.M. p53 Mutation, deprivation and poor prognosis in primary breast cancer. Br J Cancer. 2010;102:719–726. doi: 10.1038/sj.bjc.6605540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starks A.M., Martin D.N., Dorsey T.H., Boersma B.J., Wallace T.A., Ambs S. Household income is associated with the p53 mutation frequency in human breast tumors. PLoS One. 2013;8:e57361. doi: 10.1371/journal.pone.0057361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abate-Shen C., Shen M.M. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 38.Attard G., Parker C., Eeles R.A., Schroder F., Tomlins S.A., Tannock I., Drake C.G., de Bono J.S. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 39.Rosen P., Sesterhenn I.A., Brassell S.A., McLeod D.G., Srivastava S., Dobi A. Clinical potential of the ERG oncoprotein in prostate cancer. Nat Rev Urol. 2012;9:131–137. doi: 10.1038/nrurol.2012.10. [DOI] [PubMed] [Google Scholar]
- 40.Faisal F.A., Sundi D., Tosoian J.J., Choeurng V., Alshalalfa M., Ross A.E., Klein E., Den R., Dicker A., Erho N., Davicioni E., Lotan T.L., Schaeffer E.M. Racial variations in prostate cancer molecular subtypes and androgen receptor signaling reflect anatomic tumor location. Eur Urol. 2016;70:14–17. doi: 10.1016/j.eururo.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser M., Sabelnykova V.Y., Yamaguchi T.N., Heisler L.E., Livingstone J., Huang V. Genomic hallmarks of localized, non-indolent prostate cancer. Nature. 2017;541:359–364. doi: 10.1038/nature20788. [DOI] [PubMed] [Google Scholar]
- 42.Zhao S., Geybels M.S., Leonardson A., Rubicz R., Kolb S., Yan Q., Klotzle B., Bibikova M., Hurtado-Coll A., Troyer D., Lance R., Lin D.W., Wright J.L., Ostrander E.A., Fan J.B., Feng Z., Stanford J.L. Epigenome-wide tumor DNA methylation profiling identifies novel prognostic biomarkers of metastatic-lethal progression in men diagnosed with clinically localized prostate cancer. Clin Cancer Res. 2017;23:311–319. doi: 10.1158/1078-0432.CCR-16-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mundbjerg K., Chopra S., Alemozaffar M., Duymich C., Lakshminarasimhan R., Nichols P.W., Aron M., Siegmund K.D., Ukimura O., Aron M., Stern M., Gill P., Carpten J.D., Orntoft T.F., Sorensen K.D., Weisenberger D.J., Jones P.A., Duddalwar V., Gill I., Liang G. Identifying aggressive prostate cancer foci using a DNA methylation classifier. Genome Biol. 2017;18:3. doi: 10.1186/s13059-016-1129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson D., Van Allen E.M., Wu Y.M., Schultz N., Lonigro R.J., Mosquera J.M. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magi-Galluzzi C., Tsusuki T., Elson P., Simmerman K., Lafargue C., Esgueva R., Klein E., Rubin M.A., Zhou M. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71:489–497. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 46.Khani F., Mosquera J.M., Park K., Blattner M., O'Reilly C., MacDonald T.Y., Chen Z., Srivastava A., Tewari A.K., Barbieri C.E., Rubin M.A., Robinson B.D. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20:4925–4934. doi: 10.1158/1078-0432.CCR-13-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tosoian J.J., Almutairi F., Morais C.L., Glavaris S., Hicks J., Sundi D., Humphreys E., Han M., De Marzo A.M., Ross A.E., Tomlins S.A., Schaeffer E.M., Trock B.J., Lotan T.L. Prevalence and prognostic significance of PTEN loss in African-American and European-American men undergoing radical prostatectomy. Eur Urol. 2017;71:697–700. doi: 10.1016/j.eururo.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin D.N., Starks A.M., Ambs S. Biological determinants of health disparities in prostate cancer. Curr Opin Oncol. 2013;25:235–241. doi: 10.1097/CCO.0b013e32835eb5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carey L.A., Perou C.M., Livasy C.A., Dressler L.G., Cowan D., Conway K., Karaca G., Troester M.A., Tse C.K., Edmiston S., Deming S.L., Geradts J., Cheang M.C., Nielsen T.O., Moorman P.G., Earp H.S., Millikan R.C. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 50.Kakarala M., Rozek L., Cote M., Liyanage S., Brenner D.E. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S.: a SEER analysis. BMC Cancer. 2010;10:191. doi: 10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hausauer A.K., Keegan T.H., Chang E.T., Clarke C.A. Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res. 2007;9:R90. doi: 10.1186/bcr1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurel B., Lucia M.S., Thompson I.M., Jr., Goodman P.J., Tangen C.M., Kristal A.R., Parnes H.L., Hoque A., Lippman S.M., Sutcliffe S., Peskoe S.B., Drake C.G., Nelson W.G., De Marzo A.M., Platz E.A. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23:847–856. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klink J.C., Banez L.L., Gerber L., Lark A., Vollmer R.T., Freedland S.J. Intratumoral inflammation is associated with more aggressive prostate cancer. World J Urol. 2013;31:1497–1503. doi: 10.1007/s00345-013-1065-8. [DOI] [PubMed] [Google Scholar]
- 55.Wallace T.A., Prueitt R.L., Yi M., Howe T.M., Gillespie J.W., Yfantis H.G., Stephens R.M., Caporaso N.E., Loffredo C.A., Ambs S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008;68:927–936. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 56.Powell I.J., Dyson G., Land S., Ruterbusch J., Bock C.H., Lenk S., Herawi M., Everson R., Giroux C.N., Schwartz A.G., Bollig-Fischer A. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prev. 2013;22:891–897. doi: 10.1158/1055-9965.EPI-12-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose A.E., Satagopan J.M., Oddoux C., Zhou Q., Xu R., Olshen A.B., Yu J.Z., Dash A., Jean-Gilles J., Reuter V., Gerald W.L., Lee P., Osman I. Copy number and gene expression differences between African American and Caucasian American prostate cancer. J Transl Med. 2010;8:70. doi: 10.1186/1479-5876-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hardiman G., Savage S.J., Hazard E.S., Wilson R.C., Courtney S.M., Smith M.T., Hollis B.W., Halbert C.H., Gattoni-Celli S. Systems analysis of the prostate transcriptome in African-American men compared with European-American men. Pharmacogenomics. 2016;17:1129–1143. doi: 10.2217/pgs-2016-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eastham J.A., May R.A., Whatley T., Crow A., Venable D.D., Sartor O. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. J Natl Cancer Inst. 1998;90:756–760. doi: 10.1093/jnci/90.10.756. [DOI] [PubMed] [Google Scholar]
- 60.Vidal A.C., Chen Z., Howard L.E., Moreira D.M., Castro-Santamaria R., Andriole G.L., Taioli E., Fowke J.H., Knudsen B., Drake C.G., Nickel J.C., Freedland S.J. Racial differences in prostate inflammation: results from the REDUCE study. Oncotarget. 2016;8:71393–71399. doi: 10.18632/oncotarget.10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith C.J., Dorsey T.H., Tang W., Jordan S.V., Loffredo C.A., Ambs S. Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American men. Cancer Epidemiol Biomarkers Prev. 2017;26:845–853. doi: 10.1158/1055-9965.EPI-16-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osborn V.W., Chen S.C., Weiner J., Schwartz D., Schreiber D. Impact of aspirin on clinical outcomes for African American men with prostate cancer undergoing radiation. Tumori. 2016;102:65–70. doi: 10.5301/tj.5000424. [DOI] [PubMed] [Google Scholar]
- 63.Khera A., McGuire D.K., Murphy S.A., Stanek H.G., Das S.R., Vongpatanasin W., Wians F.H., Jr., Grundy S.M., de Lemos J.A. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 64.Van Dyke A.L., Cote M.L., Wenzlaff A.S., Land S., Schwartz A.G. Cytokine SNPs: comparison of allele frequencies by race and implications for future studies. Cytokine. 2009;46:236–244. doi: 10.1016/j.cyto.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehmann B.D., Bauer J.A., Chen X., Sanders M.E., Chakravarthy A.B., Shyr Y., Pietenpol J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukhtar R.A., Moore A.P., Nseyo O., Baehner F.L., Au A., Moore D.H., Twomey P., Campbell M.J., Esserman L.J. Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res Treat. 2011;130:635–644. doi: 10.1007/s10549-011-1646-4. [DOI] [PubMed] [Google Scholar]
- 67.Adisa C.A., Eleweke N., Alfred A.A., Campbell M.J., Sharma R., Nseyo O., Tandon V., Mukhtar R., Greninger A., Risi J.D., Esserman L.J. Biology of breast cancer in Nigerian women: a pilot study. Ann Afr Med. 2012;11:169–175. doi: 10.4103/1596-3519.96880. [DOI] [PubMed] [Google Scholar]
- 68.Sawe R.T., Kerper M., Badve S., Li J., Sandoval-Cooper M., Xie J., Shi Z., Patel K., Chumba D., Ofulla A., Prosperi J., Taylor K., Stack M.S., Mining S., Littlepage L.E. Aggressive breast cancer in western Kenya has early onset, high proliferation, and immune cell infiltration. BMC Cancer. 2016;16:204. doi: 10.1186/s12885-016-2204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindner R., Sullivan C., Offor O., Lezon-Geyda K., Halligan K., Fischbach N., Shah M., Bossuyt V., Schulz V., Tuck D.P., Harris L.N. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One. 2013;8:e71915. doi: 10.1371/journal.pone.0071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis C.E., Leek R., Harris A., McGee J.O. Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol. 1995;57:747–751. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- 72.Glynn S.A., Prueitt R.L., Ridnour L.A., Boersma B.J., Dorsey T.M., Wink D.A., Goodman J.E., Yfantis H.G., Lee D.H., Ambs S. COX-2 activation is associated with Akt phosphorylation and poor survival in ER-negative, HER2-positive breast cancer. BMC Cancer. 2010;10:626. doi: 10.1186/1471-2407-10-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glynn S.A., Boersma B.J., Dorsey T.H., Yi M., Yfantis H.G., Ridnour L.A., Martin D.N., Switzer C.H., Hudson R.S., Wink D.A., Lee D.H., Stephens R.M., Ambs S. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest. 2010;120:3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park N.J., Kang D.H. Inflammatory cytokine levels and breast cancer risk factors: racial differences of healthy Caucasian and African American women. Oncol Nurs Forum. 2013;40:490–500. doi: 10.1188/13.ONF.40-05AP. [DOI] [PubMed] [Google Scholar]
- 75.Jones P.A., Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805–811. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Enokida H., Shiina H., Urakami S., Terashima M., Ogishima T., Li L.C., Kawahara M., Nakagawa M., Kane C.J., Carroll P.R., Igawa M., Dahiya R. Smoking influences aberrant CpG hypermethylation of multiple genes in human prostate carcinoma. Cancer. 2006;106:79–86. doi: 10.1002/cncr.21577. [DOI] [PubMed] [Google Scholar]
- 77.Christensen B.C., Kelsey K.T., Zheng S., Houseman E.A., Marsit C.J., Wrensch M.R., Wiemels J.L., Nelson H.H., Karagas M.R., Kushi L.H., Kwan M.L., Wiencke J.K. Breast cancer DNA methylation profiles are associated with tumor size and alcohol and folate intake. PLoS Genet. 2010;6:e1001043. doi: 10.1371/journal.pgen.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hair B.Y., Troester M.A., Edmiston S.N., Parrish E.A., Robinson W.R., Wu M.C., Olshan A.F., Swift-Scanlan T., Conway K. Body mass index is associated with gene methylation in estrogen receptor-positive breast tumors. Cancer Epidemiol Biomarkers Prev. 2015;24:580–586. doi: 10.1158/1055-9965.EPI-14-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baylin S.B., Jones P.A. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a019505. a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson W.G., De Marzo A.M., Yegnasubramanian S. Epigenetic alterations in human prostate cancers. Endocrinology. 2009;150:3991–4002. doi: 10.1210/en.2009-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fackler M.J., Umbricht C.B., Williams D., Argani P., Cruz L.A., Merino V.F., Teo W.W., Zhang Z., Huang P., Visvananthan K., Marks J., Ethier S., Gray J.W., Wolff A.C., Cope L.M., Sukumar S. Genome-wide methylation analysis identifies genes specific to breast cancer hormone receptor status and risk of recurrence. Cancer Res. 2011;71:6195–6207. doi: 10.1158/0008-5472.CAN-11-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang F., Turcan S., Rimner A., Kaufman A., Giri D., Morris L.G., Shen R., Seshan V., Mo Q., Heguy A., Baylin S.B., Ahuja N., Viale A., Massague J., Norton L., Vahdat L.T., Moynahan M.E., Chan T.A. Breast cancer methylomes establish an epigenomic foundation for metastasis. Sci Transl Med. 2011;3:75ra25. doi: 10.1126/scitranslmed.3001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dedeurwaerder S., Desmedt C., Calonne E., Singhal S.K., Haibe-Kains B., Defrance M., Michiels S., Volkmar M., Deplus R., Luciani J., Lallemand F., Larsimont D., Toussaint J., Haussy S., Rothe F., Rouas G., Metzger O., Majjaj S., Saini K., Putmans P., Hames G., van Baren N., Coulie P.G., Piccart M., Sotiriou C., Fuks F. DNA methylation profiling reveals a predominant immune component in breast cancers. EMBO Mol Med. 2011;3:726–741. doi: 10.1002/emmm.201100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adkins R.M., Krushkal J., Tylavsky F.A., Thomas F. Racial differences in gene-specific DNA methylation levels are present at birth. Birth Defects Res A Clin Mol Teratol. 2011;91:728–736. doi: 10.1002/bdra.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cappetta M., Berdasco M., Hochmann J., Bonilla C., Sans M., Hidalgo P.C., Artagaveytia N., Kittles R., Martinez M., Esteller M., Bertoni B. Effect of genetic ancestry on leukocyte global DNA methylation in cancer patients. BMC Cancer. 2015;15:434. doi: 10.1186/s12885-015-1461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enokida H., Shiina H., Urakami S., Igawa M., Ogishima T., Pookot D., Li L.C., Tabatabai Z.L., Kawahara M., Nakagawa M., Kane C.J., Carroll P.R., Dahiya R. Ethnic group-related differences in CpG hypermethylation of the GSTP1 gene promoter among African-American, Caucasian and Asian patients with prostate cancer. Int J Cancer. 2005;116:174–181. doi: 10.1002/ijc.21017. [DOI] [PubMed] [Google Scholar]
- 87.Devaney J.M., Wang S., Furbert-Harris P., Apprey V., Ittmann M., Wang B.D., Olender J., Lee N.H., Kwabi-Addo B. Genome-wide differentially methylated genes in prostate cancer tissues from African-American and Caucasian men. Epigenetics. 2015;10:319–328. doi: 10.1080/15592294.2015.1022019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ambrosone C.B., Young A.C., Sucheston L.E., Wang D., Yan L., Liu S., Tang L., Hu Q., Freudenheim J.L., Shields P.G., Morrison C.D., Demissie K., Higgins M.J. Genome-wide methylation patterns provide insight into differences in breast tumor biology between American women of African and European ancestry. Oncotarget. 2013;5:237–248. doi: 10.18632/oncotarget.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Conway K., Edmiston S.N., Tse C.K., Bryant C., Kuan P.F., Hair B.Y., Parrish E.A., May R., Swift-Scanlan T. Racial variation in breast tumor promoter methylation in the Carolina Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2015;24:921–930. doi: 10.1158/1055-9965.EPI-14-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehrotra J., Ganpat M.M., Kanaan Y., Fackler M.J., McVeigh M., Lahti-Domenici J., Polyak K., Argani P., Naab T., Garrett E., Parmigiani G., Broome C., Sukumar S. Estrogen receptor/progesterone receptor-negative breast cancers of young African-American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin Cancer Res. 2004;10:2052–2057. doi: 10.1158/1078-0432.ccr-03-0514. [DOI] [PubMed] [Google Scholar]
- 91.Heyn H., Moran S., Hernando-Herraez I., Sayols S., Gomez A., Sandoval J., Monk D., Hata K., Marques-Bonet T., Wang L., Esteller M. DNA methylation contributes to natural human variation. Genome Res. 2013;23:1363–1372. doi: 10.1101/gr.154187.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chida Y., Hamer M., Wardle J., Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 93.Palm D., Lang K., Niggemann B., Drell T.L., Masur K., Zaenker K.S., Entschladen F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 94.Lu D., Sinnott J.A., Valdimarsdottir U., Fang F., Gerke T., Tyekucheva S., Fiorentino M., Lambe M., Sesso H.D., Sweeney C.J., Wilson K.M., Giovannucci E.L., Loda M., Mucci L.A., Fall K. Stress-related signaling pathways in lethal and nonlethal prostate cancer. Clin Cancer Res. 2016;22:765–772. doi: 10.1158/1078-0432.CCR-15-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor T.R., Williams C.D., Makambi K.H., Mouton C., Harrell J.P., Cozier Y., Palmer J.R., Rosenberg L., Adams-Campbell L.L. Racial discrimination and breast cancer incidence in US black women: the Black Women's Health Study. Am J Epidemiol. 2007;166:46–54. doi: 10.1093/aje/kwm056. [DOI] [PubMed] [Google Scholar]
- 96.Shariff-Marco S., Klassen A.C., Bowie J.V. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010;100:364–374. doi: 10.2105/AJPH.2009.163899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vin-Raviv N., Hillyer G.C., Hershman D.L., Galea S., Leoce N., Bovbjerg D.H., Kushi L.H., Kroenke C., Lamerato L., Ambrosone C.B., Valdimorsdottir H., Jandorf L., Mandelblatt J.S., Tsai W.Y., Neugut A.I. Racial disparities in posttraumatic stress after diagnosis of localized breast cancer: the BQUAL study. J Natl Cancer Inst. 2013;105:563–572. doi: 10.1093/jnci/djt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Z., Liu L., Zhang C., Zheng T., Wang J., Lin M., Zhao Y., Wang X., Levine A.J., Hu W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:7013–7018. doi: 10.1073/pnas.1203930109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller G.E., Chen E., Fok A.K., Walker H., Lim A., Nicholls E.F., Cole S., Kobor M.S. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cole S.W., Hawkley L.C., Arevalo J.M., Cacioppo J.T. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Entringer S., Epel E.S., Kumsta R., Lin J., Hellhammer D.H., Blackburn E.H., Wust S., Wadhwa P.D. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108:E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams J.B., Pang D., Delgado B., Kocherginsky M., Tretiakova M., Krausz T., Pan D., He J., McClintock M.K., Conzen S.D. A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev Res (Phila) 2009;2:850–861. doi: 10.1158/1940-6207.CAPR-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Volden P.A., Wonder E.L., Skor M.N., Carmean C.M., Patel F.N., Ye H., Kocherginsky M., McClintock M.K., Brady M.J., Conzen S.D. Chronic social isolation is associated with metabolic gene expression changes specific to mammary adipose tissue. Cancer Prev Res (Phila) 2013;6:634–645. doi: 10.1158/1940-6207.CAPR-12-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antoni M.H., Lutgendorf S.K., Cole S.W., Dhabhar F.S., Sephton S.E., McDonald P.G., Stefanek M., Sood A.K. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sloan E.K., Priceman S.J., Cox B.F., Yu S., Pimentel M.A., Tangkanangnukul V., Arevalo J.M., Morizono K., Karanikolas B.D., Wu L., Sood A.K., Cole S.W. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Campbell J.P., Karolak M.R., Ma Y., Perrien D.S., Masood-Campbell S.K., Penner N.L., Munoz S.A., Zijlstra A., Yang X., Sterling J.A., Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lutgendorf S.K., DeGeest K., Sung C.Y., Arevalo J.M., Penedo F., Lucci J., III, Goodheart M., Lubaroff D., Farley D.M., Sood A.K., Cole S.W. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Powe D.G., Voss M.J., Zanker K.S., Habashy H.O., Green A.R., Ellis I.O., Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barron T.I., Connolly R.M., Sharp L., Bennett K., Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 110.Melhem-Bertrandt A., Chavez-Macgregor M., Lei X., Brown E.N., Lee R.T., Meric-Bernstam F., Sood A.K., Conzen S.D., Hortobagyi G.N., Gonzalez-Angulo A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jin W., Xu S., Wang H., Yu Y., Shen Y., Wu B., Jin L. Genome-wide detection of natural selection in African Americans pre- and post-admixture. Genome Res. 2012;22:519–527. doi: 10.1101/gr.124784.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Storey J.D., Madeoy J., Strout J.L., Wurfel M., Ronald J., Akey J.M. Gene-expression variation within and among human populations. Am J Hum Genet. 2007;80:502–509. doi: 10.1086/512017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang W., Duan S., Kistner E.O., Bleibel W.K., Huang R.S., Clark T.A., Chen T.X., Schweitzer A.C., Blume J.E., Cox N.J., Dolan M.E. Evaluation of genetic variation contributing to differences in gene expression between populations. Am J Hum Genet. 2008;82:631–640. doi: 10.1016/j.ajhg.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giri V.N., Beebe-Dimmer J.L. Familial prostate cancer. Semin Oncol. 2016;43:560–565. doi: 10.1053/j.seminoncol.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haiman C.A., Chen G.K., Blot W.J., Strom S.S., Berndt S.I., Kittles R.A., Rybicki B.A., Isaacs W.B., Ingles S.A., Stanford J.L., Diver W.R., Witte J.S., Chanock S.J., Kolb S., Signorello L.B., Yamamura Y., Neslund-Dudas C., Thun M.J., Murphy A., Casey G., Sheng X., Wan P., Pooler L.C., Monroe K.R., Waters K.M., Le M.L., Kolonel L.N., Stram D.O., Henderson B.E. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011;7:e1001387. doi: 10.1371/journal.pgen.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmadiyeh N., Pomerantz M.M., Grisanzio C., Herman P., Jia L., Almendro V., He H.H., Brown M., Liu X.S., Davis M., Caswell J.L., Beckwith C.A., Hills A., Macconaill L., Coetzee G.A., Regan M.M., Freedman M.L. 8q24 Prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proc Natl Acad Sci U S A. 2010;107:9742–9746. doi: 10.1073/pnas.0910668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amundadottir L.T., Sulem P., Gudmundsson J., Helgason A., Baker A., Agnarsson B.A. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38:652–658. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 118.Haiman C.A., Patterson N., Freedman M.L., Myers S.R., Pike M.C., Waliszewska A., Neubauer J., Tandon A., Schirmer C., McDonald G.J., Greenway S.C., Stram D.O., Le Marchand L., Kolonel L.N., Frasco M., Wong D., Pooler L.C., Ardlie K., Oakley-Girvan I., Whittemore A.S., Cooney K.A., John E.M., Ingles S.A., Altshuler D., Henderson B.E., Reich D. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat Genet. 2007;39:638–644. doi: 10.1038/ng2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robbins C., Torres J.B., Hooker S., Bonilla C., Hernandez W., Candreva A., Ahaghotu C., Kittles R., Carpten J. Confirmation study of prostate cancer risk variants at 8q24 in African Americans identifies a novel risk locus. Genome Res. 2007;17:1717–1722. doi: 10.1101/gr.6782707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freedman M.L., Haiman C.A., Patterson N., McDonald G.J., Tandon A., Waliszewska A., Penney K., Steen R.G., Ardlie K., John E.M., Oakley-Girvan I., Whittemore A.S., Cooney K.A., Ingles S.A., Altshuler D., Henderson B.E., Reich D. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006;103:14068–14073. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haiman C.A., Chen G.K., Blot W.J., Strom S.S., Berndt S.I., Kittles R.A. Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet. 2011;43:570–573. doi: 10.1038/ng.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berndt S.I., Wang Z., Yeager M., Alavanja M.C., Albanes D., Amundadottir L. Two susceptibility loci identified for prostate cancer aggressiveness. Nat Commun. 2015;6:6889. doi: 10.1038/ncomms7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fanale D., Amodeo V., Corsini L.R., Rizzo S., Bazan V., Russo A. Breast cancer genome-wide association studies: there is strength in numbers. Oncogene. 2012;31:2121–2128. doi: 10.1038/onc.2011.408. [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Closas M., Couch F.J., Lindstrom S., Michailidou K., Schmidt M.K., Brook M.N. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet. 2013;45:392–398.e2. doi: 10.1038/ng.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Purrington K.S., Slager S., Eccles D., Yannoukakos D., Fasching P.A., Miron P. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2014;35:1012–1019. doi: 10.1093/carcin/bgt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pirie A., Guo Q., Kraft P., Canisius S., Eccles D.M., Rahman N. Common germline polymorphisms associated with breast cancer-specific survival. Breast Cancer Res. 2015;17:58. doi: 10.1186/s13058-015-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen F., Stram D.O., Le M.L., Monroe K.R., Kolonel L.N., Henderson B.E., Haiman C.A. Caution in generalizing known genetic risk markers for breast cancer across all ethnic/racial populations. Eur J Hum Genet. 2011;19:243–245. doi: 10.1038/ejhg.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huo D., Zheng Y., Ogundiran T.O., Adebamowo C., Nathanson K.L., Domchek S.M., Rebbeck T.R., Simon M.S., John E.M., Hennis A., Nemesure B., Wu S.Y., Leske M.C., Ambs S., Niu Q., Zhang J., Cox N.J., Olopade O.I. Evaluation of 19 susceptibility loci of breast cancer in women of African ancestry. Carcinogenesis. 2012;33:835–840. doi: 10.1093/carcin/bgs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen F., Chen G.K., Stram D.O., Millikan R.C., Ambrosone C.B., John E.M. A genome-wide association study of breast cancer in women of African ancestry. Hum Genet. 2013;132:39–48. doi: 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feng Y., Stram D.O., Rhie S.K., Millikan R.C., Ambrosone C.B., John E.M., Bernstein L., Zheng W., Olshan A.F., Hu J.J., Ziegler R.G., Nyante S., Bandera E.V., Ingles S.A., Press M.F., Deming S.L., Rodriguez-Gil J.L., Palmer J.R., Olopade O.I., Huo D., Adebamowo C.A., Ogundiran T., Chen G.K., Stram A., Park K., Rand K.A., Chanock S.J., Le Marchand L., Kolonel L.N., Conti D.V., Easton D., Henderson B.E., Haiman C.A. A comprehensive examination of breast cancer risk loci in African American women. Hum Mol Genet. 2014;23:5518–5526. doi: 10.1093/hmg/ddu252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huo D., Feng Y., Haddad S., Zheng Y., Yao S., Han Y.J. Genome-wide association studies in women of African ancestry identified 3q26.21 as a novel susceptibility locus for oestrogen receptor negative breast cancer. Hum Mol Genet. 2016;25:4835–4846. doi: 10.1093/hmg/ddw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haiman C.A., Chen G.K., Vachon C.M., Canzian F., Dunning A., Millikan R.C. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011;43:1210–1214. doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fejerman L., John E.M., Huntsman S., Beckman K., Choudhry S., Perez-Stable E., Burchard E.G., Ziv E. Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res. 2008;68:9723–9728. doi: 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ruiz-Narvaez E.A., Sucheston-Campbell L., Bensen J.T., Yao S., Haddad S., Haiman C.A., Bandera E.V., John E.M., Bernstein L., Hu J.J., Ziegler R.G., Deming S.L., Olshan A.F., Ambrosone C.B., Palmer J.R., Lunetta K.L. Admixture mapping of African-American women in the AMBER Consortium identifies new loci for breast cancer and estrogen-receptor subtypes. Front Genet. 2016;7:170. doi: 10.3389/fgene.2016.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prueitt R.L., Wallace T.A., Glynn S.A., Yi M., Tang W., Luo J., Dorsey T.H., Stagliano K.E., Gillespie J.W., Hudson R.S., Terunuma A., Shoe J.L., Haines D.C., Yfantis H.G., Han M., Martin D.N., Jordan S.V., Borin J.F., Naslund M.J., Alexander R.B., Stephens R.M., Loffredo C.A., Lee D.H., Putluri N., Sreekumar A., Hurwitz A.A., Ambs S. An immune-inflammation gene expression signature in prostate tumors of smokers. Cancer Res. 2016;76:1055–1065. doi: 10.1158/0008-5472.CAN-14-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]