Abstract
Once considered a problem of Western nations, obesity (body mass index ≥30 kg/m2) has rapidly increased since the 1970s to become a major threat to world health. Since 1970, the face of obesity has changed from a disease of affluence and abundance to a disease of poverty. During the last 10 years, studies have mechanistically linked obesity and an obese tumor microenvironment with signaling pathways that predict aggressive breast cancer biology. For example, in the United States, African American women are more likely than non-Hispanic European American women to be obese and to be diagnosed with triple-negative breast cancer (TNBC). In 2008, the Carolina Breast Study found that obesity (increased waist/hip ratio) was linked to an increased incidence of TNBC in premenopausal and postmenopausal African American women. Subsequently, several groups have investigated the potential link between obesity and TNBC in African American women. To date, the data are complex and sometimes contradictory. We review epidemiologic studies that investigated the potential association among obesity, metabolic syndrome, and TNBC in African American women and mechanistic studies that link insulin signaling to the obese breast microenvironment, tissue inflammation, and aggressive TNBC biology.
During the past 20 years, the United States, as well as much of the world, has experienced an increasing obesity epidemic. The obesity epidemic has disproportionately affected the poor. Once a disease of the wealthy, obesity is now a disease of poverty. Possible contributors include disparities in income that promote consumption of inexpensive high-calorie foods with low nutritional value,1 disparities in access to healthy food sources (food deserts),2 and lack of exercise. The obesity epidemic has also disproportionately affected women of African descent. For example, in the United States, >58.6% of African American women are obese (versus 34.5% non-Hispanic European Americans).3, 4 For reasons discussed in this review, these women are also more likely to develop triple-negative breast cancers (TNBCs).
TNBCs lack expression of estrogen receptor (ER) and progesterone receptor (PR) and do not overexpress tyrosine kinase human epidermal growth factor receptor 2.5, 6 The basal subset of TNBC is characterized by expression of basal-type cytokeratin 5 and cytokeratin 6 and high expression of epidermal growth factor receptor, and frequently exhibits aggressive clinical behavior.6 TNBCs most frequently occur in women with germline BRCA1 mutations and in premenopausal women of African descent.7
The Carolina Breast Study found an association between obesity, as measured by increased waist/hip ratio (WHR), and an increased incidence of TNBC in premenopausal and postmenopausal African American women. Subsequently, a number of groups have investigated the potential link between obesity and TNBC in African American women. However, to date, studies have relied on body shape rather than biology; the data are complex and sometimes contradictory.
Nevertheless, the past 10 years has witnessed a substantial increase in our understanding of both the biology of aggressive TNBC and the importance of the breast microenvironment on aggressive cancer biology. Key drivers of TNBC include i) genomic instability, including loss of p53 function8, 9, 10; ii) activation of key signaling networks11, 12, 13, 14, 15, 16; iii) the role of progenitor-like cells in promoting epithelial-to-mesenchymal transition and phenotypic plasticity17; and iv) the obese microenvironment.18, 19 We review epidemiologic studies that investigated the potential association among obesity, metabolic syndrome, and TNBC in African American women and mechanistic studies that link insulin signaling, the obese breast microenvironment, and inflammation with aggressive TNBC biology.
The Changing Face of Obesity in the United States
Definition of Obesity
The Centers for Disease Control and Prevention (CDC) defines a body mass index (BMI) ≥30 kg/m2 as obesity or metabolically unhealthy (CDC, https://www.cdc.gov/obesity/adult/defining.html, last accessed April 3, 2017). Abdominal obesity, as measured by WHR or by waist circumference, is another commonly used measure of obesity or poor metabolic health (metabolically unhealthy). Women with a WHR of ≥0.85 are considered to be at substantially increased risk for metabolic complications by the World Health Organization.20 The CDC considers a waist circumference of ≥88 cm metabolically unhealthy. Although overall obesity is associated with many adverse health outcomes, abdominal obesity may have an even greater adverse effect on metabolic health [heart disease, insulin resistance (prediabetes), type 2 diabetes] and cancer.
Obesity Epidemic in the United States
In 2014, the National Health and Nutrition Examination Surveys reported that in the United States, 35.0% of adult men and 40.4% of adult women were obese as measured by BMI.21, 22 Between 2005 and 2014, there was an increased prevalence of obesity (from 35.3% to 40.4%) and severe obesity (BMI >40 kg/m2) (from 7.4% to 9.9%) in women21, 22; no statistically significant increase was observed in men.21, 22 Similarly, obesity has steadily increased in adolescents 12 to 19 years of age. In particular, severe obesity increased in adolescents from 2.6% in 1988 to 1994 to 9.1% in 2013 to 2014.21, 22, 23 These studies highlight that in the United States obesity is increasing in women and adolescents and remains a pressing public health challenge.
Obesity Is a Disease of the Poor and Malnourished
Before the 1970s, obesity was associated with wealth and nutrition.21, 22, 24, 25 Today, it is common to find obesity to be associated with poverty and malnutrition.25 The reason for this shift is not well understood and is likely multifactorial.25, 26 Possible contributors include socioeconomic factors that promote consumption of inexpensive high-calorie foods with low nutritional value1 and disparities in access to healthy food sources (ie, food deserts).2, 27
Throughout the world, obesity is more prevalent in urban areas and in individuals with less education and income.24, 25 Compared with the poor, affluent individuals have additional income to purchase healthy foods, adequate time for leisure and physical activities, and access to quality health care.28 In contrast, poor households may lack access to healthy foods, which has the potential to exacerbate poverty by trapping the poor in a cycle of obesity and obesity-associated health problems, further increasing economic and health disparities.29
Disparities That Contribute to Obesity
Income Disparities
There is a strong correlation between poverty and obesity. Until approximately 40 years ago, calories were associated with nutrition. With the advent of junk food and fast food, the opposite has become true. Less nutritious, calorie-dense foods are often less expensive than healthier foods; as a result, poor families often eat high-calorie food with low nutritional content (Trust for America's Health, http://healthyamericans.org/report/108, last accessed April 3, 2017). Disparities in income have a particular effect on African Americans. For the past 30 years, African American families have earned $1 for every $2 earned by non-Hispanic European American families (Urban Institute, http://www.urban.org/research/publication/less-equal-racial-disparities-wealth-accumulation, last accessed April 3, 2017). In addition, >30% of African American families with children live below the poverty line (US Census Bureau, https://www.census.gov/content/dam/Census/library/publications/2017/demo/P60-259.pdf, last accessed April 3, 2017), and 12% of African American families live in deep poverty (<50% of the US federal poverty threshold) (US Census Bureau, https://www.census.gov/library/publications/2013/demo/p60-245.html, last accessed April 3, 2017). In African American families, 22.5% do not have consistent access to adequate food because of lack of money or other resources compared with <10% of European American households (US Department of Agriculture, https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/key-statistics-graphics/#insecure, last accessed April 3, 2017). These disparities in income substantially contribute to the effect of poverty on obesity and metabolic health.
Food Deserts
Families in predominantly minority and low-income neighborhoods often have limited access to supermarkets and affordable healthy food (food deserts). The US Department of Agriculture defines a food desert as a geographic region whose inhabitants live >1 mile (urban) or >10 miles (rural) from a grocery store that provides a full range of meats, dairy products, grains, and vegetables (US Department of Agriculture, http://www.ers.usda.gov/data-products/food-access-research-atlas/go-to-the-atlas.aspx, last accessed April 3, 2017). Individuals living in food deserts often rely on convenience stores and fast-food chains that offer few, if any, healthy food choices, such as fruits and vegetables. The failure of supermarket chains to locate stores that offer fresh fruits and vegetables in inner-city communities—a form of food redlining—has had a profound effect on health. African American and Hispanic European American women are more likely to live in food deserts than non-Hispanic women of European or Asian descent.2, 29, 30 Lack of healthy food choices puts African-American and Hispanic-American women at increased risk for insulin-resistance, type 2 diabetes (Figure 1), and obesity.2, 29, 30
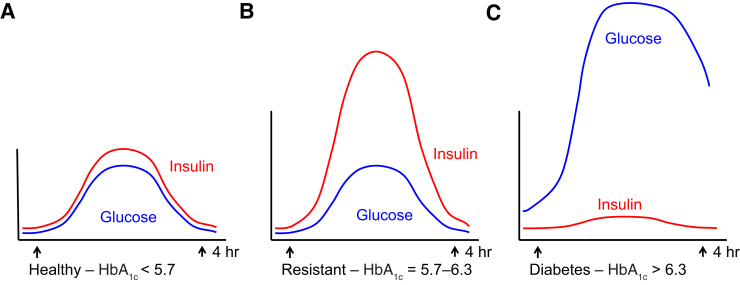
Figure 1.
Relative circulating insulin and glucose levels in a healthy individual (A), an insulin-resistant individual (B), and an individual with diabetes (C). Insulin resistance (prediabetes) occurs when the body demands increasingly greater amounts of insulin to deliver glucose to the muscle and other organs. When individuals with insulin resistance eat, insulin may increase to 5- to 10-fold higher than in individuals without insulin resistance. HbA1c, hemoglobin A1c.
Neighborhood Disparities Are Associated With a Lack of Exercise and Obesity
The lack of safe neighborhoods has a significant effect on obesity and metabolic health in African Americans. In a recent study, African Americans were 80% less likely to engage in physical activity than non-Hispanic European Americans (US Department of Health and Human Services, https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=25, last accessed April 3, 2017). According to the 2013 Youth Risk Behavior Surveillance study, 21.5% of African American youth did not participate in at least 1 hour of daily physical activity during the prior week compared with 12.7% of non-Hispanic European American youth (CDC, https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6304a1.htm, last accessed April 3, 2017).
National studies have found that access to public parks, public pools, and green space is much lower in African American neighborhoods (National Recreation and Park Association, http://www.nrpa.org/uploadedFiles/nrpa.org/Publications_and_Research/Research/Papers/Parks-Rec-Underserved-Areas.pdf, last accessed April 3, 2017). Tellingly, African American children in neighborhoods that lack access to parks, playgrounds, and recreation centers have a 20% to 45% greater risk of becoming overweight (The State of Obesity, http://stateofobesity.org/disparities/blacks, last accessed April 3, 2017). Sidewalks in African American neighborhoods are 38 times more likely to be in poor condition, which is compounded by the threat of violence that strongly influences the amount of daily outdoor play that mothers permit their children.31 Taken together, it is clear that disparities in exercise, access to parks and sidewalks, and a lack of safe neighborhoods disproportionately promote obesity and poor metabolic health in African Americans.
Challenges in Defining, Measuring, and Comparing Obesity in Individuals of Different Racial and Ethnic Groups
BMI, Race, and Risk: An Inexact Measure
BMI ≥30 kg/m2 is the most frequently used measure of obesity (CDC, https://www.cdc.gov/obesity/adult/defining.html, last accessed April 3, 2017).32 Although BMI is a commonly used measure, the appropriateness of BMI as a phenotypic marker of adiposity across populations differing in race and ethnicity is highly controversial,32, 33 and there are several issues with using BMI as a measure of obesity and metabolic health.
First, the association between BMI and obesity varies significantly among individuals of different races and ethnic groups.34, 35 Furthermore, the association between body shape or composition and disease is an inexact and complex science that is only now beginning to be understood (Figure 2).32 For example, African Americans have higher muscle mass than non-Hispanic European Americans and Asian Americans.36, 37 As a result African Americans, with high muscle mass, have high BMI and are disproportionately categorized as obese.
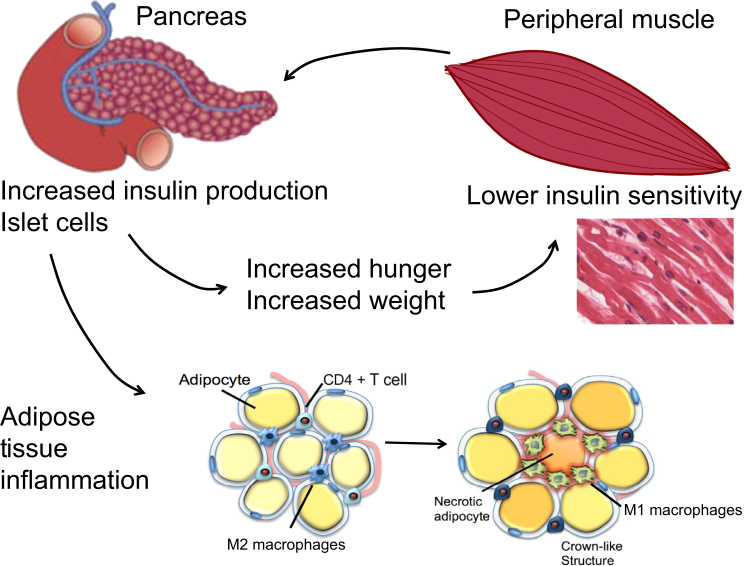
Figure 2.
Insulin resistance promotes weight gain and tissue inflammation. After eating, serum glucose levels increase and trigger the release of the hormone insulin. Normally, this increase in serum insulin promotes glucose uptake into the peripheral tissue. Muscle tissue is the primary user of glucose and a major regulator of insulin sensitivity. In an individual who is insulin resistant, the peripheral tissue progressively loses its ability to transport glucose, leading to an increase in serum glucose. In response to this increased level of serum glucose, β-cells in the pancreas increase production of insulin. Insulin promotes hunger and, as a result, insulin-resistant individuals become hungrier (not full) after eating, leading to a positive feedback cycle of progressively increasing weight gain, insulin resistance, and adipose tissue inflammation. Ultimately, the β-cells of the pancreas are unable to keep up with demand and diabetes (pancreas failure) ensues.
Second, BMI appears to affect individuals of different races and ethnicities disproportionately in their risk for type 2 diabetes and cancer.38, 39 In a large multiethnic cohort study, for an equivalent incidence rate of type 2 diabetes conferred by a BMI of >30 kg/m2 in non-Hispanic European Americans, the BMI cutoff value was 24 kg/m2 in South Asian Americans, 25 kg/m2 in Chinese Americans, and 26 kg/m2 in African Americans.40 These studies provide evidence that other factors beyond BMI, such as body composition and/or the insulin sensitivity of peripheral muscle tissue, may play a role in determining the effect of obesity on disease risk.
Racial Differences in Visceral Adipose Tissue
Abdominal obesity (WHR of ≥0.85 or waist circumference of ≥88 cm) is the second most frequently used measure of obesity (CDC, https://www.cdc.gov/obesity/adult/defining.html, last accessed April 3, 2017). WHR is thought to be a better measure than BMI of type 2 diabetes and metabolic risk in non-Hispanic European Americans (CDC, https://www.cdc.gov/obesity/adult/defining.html, last accessed April 3, 2017). However, the use of abdominal obesity in studies that include individuals of different races and ethnicities may be as problematic as the use of BMI because of the known racial and ethnic differences in body fat composition and distribution.
For example, African American and non-Hispanic European American women have differences in muscle mass, fat distribution, bone mineral density, and bone mass.37 These differences lead to changes in visceral adipose tissue, which positively associates with hyperinsulinemia, insulin resistance, and metabolic syndrome (CDC, https://www.cdc.gov/obesity/adult/defining.html, last accessed April 3, 2017). In contrast, leg fat has the opposite correlation.41 Because African American women have an increased incidence of type 2 diabetes relative to non-Hispanic European American women,42 it would be anticipated that African American women would have a higher incidence of central obesity or increased visceral adipose tissue. However, the reverse is true. For a given BMI, waist circumference, or WHR, African American women have lower amounts of normalized visceral adipose tissue than non-Hispanic European American women.43, 44, 45
It is unclear why African Americans have lower amounts of normalized visceral adipose tissue but a higher incidence of type 2 diabetes and metabolic complications. Although visceral adipose tissue is an important predictor of insulin resistance in non-Hispanic women, the peripheral muscle is the major user of glucose and the major determinant of insulin sensitivity or resistance. Given that African American women have significantly more muscle mass than non-Hispanic European Americans,37 this may account for some of the lowered insulin sensitivity in African American women compared with non-Hispanic European American women.
Challenges in Using Body Shape as a Measure of Obesity and Metabolic Health
Reframing the Question
The biology of ER+ breast cancer and TNBC is clearly distinct. ER+ breast cancer is characterized by estrogen dependency; TNBCs are by definition ER−. In the past, with the exception of aromatization of androgen, adipose tissue was considered a biochemically inert organ. As a result, many investigators believed that excess adipose tissue and obesity may not be drivers of TNBC. However, it is now known that adipose tissue is a metabolically active endocrine organ that provides a rich source of inflammatory cytokines, hormones, and tumor growth factors as well as adipose stem cells.
Epidemiologic studies testing for the association between body shape and TNBC have provided confusing and many times contradictory results. Given our mechanistic understanding of the breast microenvironment, signaling pathways that promote the aggressive biology, and, as described below, an improved ability to differentiate individuals who are metabolically unhealthy, perhaps it is time to go beyond body shape and take mechanism and biology into account.
BMI Does Not Consistently Predict Metabolic Health
During the past 20 years, there is increasing recognition of individuals who, despite having a BMI >30 kg/m2, do not have abnormal metabolic profiles. These individuals are described as metabolically healthy obese46, 47, 48, 49 and do not have insulin resistance, type 2 diabetes, dyslipidemia, or hypertension.50 Specific classification schemes have been developed by individual research groups with variable cutoffs for blood pressure and cholesterol ratios.51, 52, 53 To further complicate matters, each group has used variable definitions for insulin resistance, including fasting plasma glucose, hemoglobin A1c, and/or homeostasis model assessment.46 Each of these measures has its strengths and weaknesses and is variably accurate in identifying insulin-resistant individuals. For instance, one study found that >30% of patients were not correctly diagnosed with insulin resistance when fasting plasma glucose level was used as the primary measure.54 Thus, reproducible, consistent criteria are needed to determine whether an individual is obese but metabolically healthy.46
Racial and Ethnic Differences in Metabolic Health
As described above, the ability of BMI to predict metabolic health is markedly different among individuals of differing race and ethnicity. Peripheral muscle tissue is the major determinant of insulin sensitivity, which is why exercise is hypothesized to greatly improve insulin sensitivity in insulin-resistant individuals. Lower peripheral insulin sensitivity in African American women compared with non-Hispanic European American women may account for these differences and thus make it difficult to determine metabolically healthy BMI cutoff points for studying the association between obesity and TNBC.
TNBC Occurs More Frequently in African American Women
TNBCs lack expression of ER and PR and do not overexpress the tyrosine kinase human epidermal growth factor receptor 2.5, 6, 55 TNBCs most frequently occur in women with germline BRCA1 mutations and in premenopausal women of African descent.7 However, only 20.4% of African American women with TNBC have a germline mutation of BRCA1.56 These data suggest that the molecular events that surround the initiation of TNBC in African American women may be distinct from women of non-African descent.
The Carolina Breast Cancer Study found that the highest prevalence of the basal-like subtype of TNBC occurred in premenopausal African American women [38 of 97 invasive cancers (39%)] and was substantially higher than the prevalence of TNBC in postmenopausal African American women [14 of 99 invasive cancers (14%)] or postmenopausal non-Hispanic European American women [48 of 300 invasive cancers (16%)] (P < 0.001 for both comparisons).55 The high frequency of TNBC in premenopausal African American women has been also observed in several subsequent population-based studies.57, 58, 59, 60
There is evidence that African American women with TNBC have worse survival than non-Hispanic European American women. However, published reports of survival outcomes are conflicting.59, 61, 62, 63, 64, 65, 66 African American women often lack access to breast cancer screening and oncology care and experience treatment delays.67 There is strong evidence that disparities in income and heath care provision and comorbid disease affect the stage of presentation and survival of African American women with TNBC.67, 68 It is unclear whether the observed differences in survival persist after adjusting for these disparities in access, income, and comorbid disease.7
Association between Obesity and Breast Cancer
Obesity and Breast Cancer in African American Women
In 2008, the Carolina Breast Cancer Study found that obesity, as measured by increased WHR, was linked to an increased incidence of TNBC in both premenopausal and postmenopausal African American women.55 Subsequently, a number of groups have investigated the potential link between obesity and TNBC in African American women. To date, the data are complex and sometimes contradictory. One difficulty in performing studies that investigate the potential association between obesity and TNBC is that measurements of obesity are not consistent and are driven by anthropomorphic measurements (BMI and WHR) rather than biology. As outlined above, these measures are not consistent among individuals of different race and ethnicity. With these caveats in mind, we review the existing studies that tested for the potential association between TNBC and obesity, with a focus on African American women.
Carolina Breast Cancer Study
The Carolina Breast Cancer Study is a North Carolina population-based, case-controlled study of breast cancer conducted in three stages.69 The current study stage, Stage 3 (years 2008 to 2014), includes female residents in 44 North Carolina counties.69 The investigators used randomized recruitment to oversample African American women and women <50 years old.69 The WHR was compared between the highest (≥0.84) and lowest (<0.77) groups in relation to the basal-type subset of TNBC. Across all women, there was an increased risk [odds ratio (OR) = 2.3; 95% CI, 1.4–3.6] for developing basal-type TNBC in women with higher WHR.70 Premenopausal women (OR = 1.8; 95% CI, 1.0–3.4) and postmenopausal women (OR = 2.7; 95% CI, 1.3–5.4) with high WHR had a significantly increased risk of developing basal-type TNBC compared with the lowest WHR group.70 The prevalence of basal-type breast cancer was highest among premenopausal African American women, who also had the highest prevalence of basal-type risk factors.70 There was no significant association between increased BMI (defined as BMI ≥30 kg/m2) and the risk of basal-type TNBC.70
Women's CARE Study
The potential association between increased BMI (BMI ≥30 kg/m2) and TNBC in African American women is inconsistent and age dependent across studies.71, 72, 73, 74 The Women's Contraceptive and Reproductive Experiences (CARE) study is a case-controlled study of BMI and breast cancer risk in non-Hispanic European American women and African American women. The Women's CARE study reported an inverse association between a woman's BMI at 18 years of age and premenopausal ER−/PR− breast cancer and positive association between current BMI and postmenopausal ER+/PR+ breast cancer.75
Black Women's Health Study
The Black Women's Health Study (BWHS) is a prospective study among African American women across the United States.76 The study was established in 1995, with 59,000 African American women responding to a 14-page health questionnaire. The BWHS tested for the potential association between body size and breast cancer. In the BWHS, high BMI at 18 years of age was associated with reduced risk of both premenopausal and postmenopausal breast cancer, and current BMI was inversely associated with premenopausal cancer.77 There was also a trend toward a positive association between high BMI and ER+/PR+ breast cancer.
Women's Circle of Health Study
The Women's Circle of Health Study is a multisite case-control study in New York City and New Jersey that aims to identify risk factors for early aggressive breast cancers in African American and non-Hispanic European American women.78, 79 Recently, the Women's Circle of Health Study observed significant inverse associations of high BMI with ER−/PR− breast cancer among postmenopausal women. Similar to the Carolina Breast Cancer Study, increased WHR was associated with an increased risk of premenopausal breast cancer after adjustment for BMI.70, 78, 79
Results from the African American Breast Cancer Epidemiology and Risk Consortium
The African American Breast Cancer Epidemiology and Risk (AMBER) Consortium80 is a collaboration of four studies: the Carolina Breast Cancer Study,70 the Women's Circle of Health Study,75 the BWHS,76 and the Multiethnic Cohort Study.81 The first three studies are described above. The Multiethnic Cohort Study is a prospective study that includes 16,594 African American women from Los Angeles County and the state of Hawaii. The AMBER Consortium was formed, in part, to investigate the inconsistent and confusing results generated in individual studies that tested for the potential association between obesity (measured by BMI and/or WHR) and TNBC.80, 82
The AMBER Consortium investigated the potential association between general and central obesity and breast cancer subtypes in premenopausal and postmenopausal African American women.80, 82 The AMBER Consortium found that the effect of general and central obesity varied by menopausal status and hormone receptor subtype in African American women.80 In postmenopausal women, higher recent BMI was associated with increased risk of ER+ cancer (OR = 1.31; 95% CI, 1.02–1.67 for BMI ≥35 kg/m2 versus <25 kg/m2) and with a decreased risk of TNBC (OR = 0.60; 95% CI, 0.39–0.93 for BMI ≥35 kg/m2 versus <25 kg/m2). In premenopausal women, increased BMI was associated with a decreased incidence of premenopausal ER+ cancer and all subtypes of postmenopausal cancer.80 Furthermore, in premenopausal women, high recent WHR was associated with an increased risk of premenopausal ER+ tumors (OR = 1.35; 95% CI, 1.01–1.80) and all breast cancer subtypes in postmenopausal women (OR = 1.26; 95% CI, 1.02–1.56).80 The investigators concluded that their findings imply that in African American women, there are different mechanisms for the associations between adiposity and TNBC and ER+ breast cancers.80
Mechanisms by Which Obesity May Promote TNBC Initiation, Progression, and Aggressive Biology
Recent studies have identified several potential mechanistic links between obesity and TNBC initiation, progression, and metastasis. Important areas of investigation include the effect of i) insulin on Akt/mammalian target of rapamycin (mTOR) signaling and glycolysis; ii) obesity-mediated tissue inflammatory cytokines, such as leptin, and activation of signaling pathways that promote invasion and metastasis; and iii) the obese tissue microenvironment on aggressive TNBC biology.
Normally, on eating, insulin is secreted in response to increased blood glucose. The increased insulin levels stimulate leptin synthesis and secretion. Circulating leptin then sends a satiety signal through the hypothalamus and acts on the pancreas to inhibit insulin release. In obesity, circulating levels of both insulin and leptin are elevated. Leptin levels are increased even in the absence of hyperinsulinemia in the obese. The feedback loops that limit food consumption and decrease circulating insulin are not functional. High levels of leptin act to directly stimulate mitogenesis and decrease apoptosis in breast cancer cells.83 Furthermore, insulin stimulates the overexpression of leptin and its receptor in breast cancer cells, which sets up an autocrine loop that stimulates breast cancer cell growth.83 Leptin also stimulates the secretion of proinflammatory cytokines from macrophages [IL-6 and tumor necrosis factor (TNF)-α], as well as T cells and mononuclear cells (IL-2 and interferon-γ).84
Effect of Insulin on Akt/mTOR and Glycolysis
The AKT/mTOR-signaling network plays a pivotal role in cell growth, proliferation, and survival.15, 17 Activation of Akt/mTOR signaling predicts poor prognosis in women with TNBC.15, 85, 86 Insulin is a key activator of Akt/mTOR signaling.15, 87 Women with insulin resistance produce chronically elevated levels of insulin (Figure 1).88, 89 Because insulin activates Akt/mTOR and activation of Akt/mTOR predicts aggressive TNBC biology, it is hypothesized that the higher levels of serum insulin observed in insulin-resistant women may promote aggressive biology in women with TNBC.
Another mechanism by which insulin/Akt/mTOR may promote aggressive TNBC biology is via the effect of Akt/mTOR on glucose uptake and glycolysis. Aggressive TNBCs typically are glucose dependent and generate a larger proportion of their energy via aerobic glycolysis as opposed to mitochondrial oxidative phosphorylation.90 Akt/mTOR increases glucose uptake and promotes a switch from mitochondrial respiration (tricarboxylic acid cycle) to aerobic glycolysis (Warburg effect).91, 92 The Warburg effect directly contributes to the aggressive biology of TNBC by increasing glycolysis and glucose uptake, which supplies anabolic precursors for rapid growth and promotes mitochondrial dysfunction that leads to apoptosis resistance.
Tissue Inflammation
Obesity promotes a state of inflammation that is characterized by increased serum and tissue inflammatory cytokines (Figure 2). Inflammatory cytokines increased in obese individuals include IL-6, IL-8, TNF-α, and leptin (Figure 3). Together these inflammatory cytokines promote tissue inflammation and activate signaling pathways that promote aggressive TNBC biology. IL-6, IL-8, and leptin are increased in obese individuals and activate STAT3, NF-κB, and Wnt/EZH2 signaling. Activation of STAT3, NF-κB, and Wnt/EZH2 promotes invasion and metastasis and predict poor prognosis in women with TNBC.93, 94
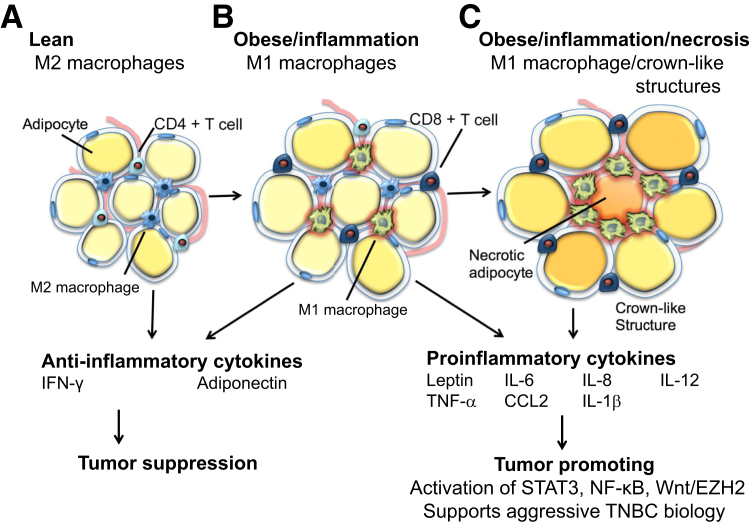
Figure 3.
A: Metabolically normal, lean adipose tissue produces anti-inflammatory cytokines and is associated with CD4+ T cells and M2 macrophages. B: As obesity increases, adipocytes undergo hypertrophy, begin to produce proinflammatory cytokines, and are associated with CD8+ T cells and M1 macrophages. C: Finally, as metabolic dysfunction worsens, adipose tissue produces only proinflammatory cytokines, and crown-like structures of necrotic adipocytes surrounded by M1 macrophages appear. CCL2, chemokine (C-C motif) ligand 2; IFN-γ, interferon-γ; IL, interleukin; TNBC, triple-negative breast cancer.
Obesity, Immune Cell Switching, and a Protumorigenic Tissue Microenvironment
There is increasing evidence that T-cell subsets and macrophages can promote the aggressive biology of TNBC. T cells and macrophages can inhibit or promote tumorigenesis. Classically activated macrophages (M1 type) are regulated by Th1 cytokines (eg, interferon-γ or TNF-α); M1 macrophages possess enhanced cytotoxic activity and are antitumorigenic.95 However, when tissue is exposed to inflammatory cytokines [eg, leptin, IL-6, IL-8, IL-12, chemokine (C-C motif) ligand 2, and IL-1β], there is a switch from M1 macrophages to alternatively activated macrophage (M2 type).96 Rather than aiding in healing, M2 macrophages promote cancer and are frequently observed in aggressive TNBCs.97
In the lean state, M2 macrophages and regulatory T cells help restrain inflammation in adipose tissue and maintain metabolic homeostasis. Eosinophil-derived IL-4 and IL-13 promote the maintenance of M2 macrophages in the fat, and IL-10 secreted by regulatory T cells and M2 macrophages limits local inflammation. Obesity-related factors, including saturated free fatty acids (ceramide and palmitate), trigger secretion of IL-1β. IL-1β, in turn, promotes recruitment of M1 macrophages into adipose tissue. M1 macrophages produce inflammatory cytokines, such as IL-6, IL-12, chemokine (C-C motif) ligand, and IL-1β. The production of inflammatory cytokines by M1 macrophages recruits effector and memory T cells and promotes inflammation and malignancy (Figure 3).
Alternatively activated M2 macrophages are found in high numbers in stroma of TNBCs.97 In addition, M2 macrophages produce epithelial growth factor (EGF) and tumor growth factor (TGF)-β. Poor prognosis TNBC is characterized by activation of EGF and TGF-β signaling. EGF and TGF-β both promote invasion, metastasis, and progenitor cell turnover.
Emerging evidence indicates that EGF and TGFβ- signaling contributes to a protumorigenic microenvironment that contributes to initiation and progression of TNBC.98, 99 Taken together, these observations underscore a potential mechanistic link among obesity, M2 macrophage production of EGF and TGF-β, and aggressive TNBC biology. We hypothesize that this state of adipose tissue inflammation and differentiation may play a role in driving the initiation of TNBC and provide a potential mechanistic link between the obese microenvironment and TNBC.
Conclusions
The association between body shape and risk of TNBC is complex. The AMBER Consortium study is one of the most comprehensive studies conducted to date to test this association. The AMBER Consortium reported that in premenopausal women increased BMI was associated with a decreased incidence of all subtypes (including TNBC) of postmenopausal cancer.80 Furthermore, in premenopausal women, high recent WHR was associated with an increased risk of premenopausal ER+ tumors and all breast cancer subtypes in postmenopausal women.80 The investigators concluded that, in African American women, there were likely different mechanisms for the associations between adiposity and TNBC and ER+ breast cancers.80
Body shape does not always predict metabolic health. Women can be obese and not have metabolic abnormalities (eg, insulin resistance and hyperlipidemia). Conversely, women can be lean and metabolically unhealthy. Furthermore, the ability of BMI to predict metabolic health differs between individuals of differing race and ethnicity.
African American women have a high incidence of obesity, insulin resistance, and premenopausal TNBC. There is increasing evidence to support the mechanistic link between obesity, insulin signaling, and aggressive subtypes of TNBC. Obesity promotes tissue inflammation and elevated inflammatory cytokines (IL-6, IL-8, TNF-α, and leptin). IL-6 and IL-8 signaling in turn activates STAT3, NF-κB, and EZH2 signaling and predicts poor prognosis in women with TNBC.93, 94
Given the complexity of epidemiologic studies, it is important to include biological measures of metabolic disease rather than just measure body shape. Insulin, tissue inflammation, and cytokine signaling all appear to be drivers of aggressive TNBC. To this end, biomarkers that reflect insulin resistance (eg, hemoglobin A1c or homeostasis model assessment), tissue inflammation (eg, leptin/adiponectin ratio, IL-6/IL-8 ratio), or an obese tissue microenvironment (ie, tissue macrophages, collagen composition) may provide increased mechanistic insight into TNBC biology.
Acknowledgment
We thank Chris Gandhi for his editorial contribution.
Footnotes
Race in Cancer Health Disparities Theme Issue
Supported by NIH/National Cancer Institute grants R01CA155664, R01CA158668, R01CA170851, R01CA192914, U01CA189283 (V.L.S.), and P30CA033572.
Disclosures: None declared.
This article is part of a review series on understanding the complex role of race in cancer health disparities.
References
- 1.Hawkes C. Uneven dietary development: linking the policies and processes of globalization with the nutrition transition, obesity and diet-related chronic diseases. Global Health. 2006;2:4. doi: 10.1186/1744-8603-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummins S., Macintyre S. “Food deserts” - evidence and assumption in health policy making. BMJ. 2002;325:436–438. doi: 10.1136/bmj.325.7361.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein L., Teal C.R., Joslyn S., Wilson J. Ethnicity-related variation in breast cancer risk factors. Cancer. 2003;97:222–229. doi: 10.1002/cncr.11014. [DOI] [PubMed] [Google Scholar]
- 4.Kim S.Y., Dietz P.M., England L., Morrow B., Callaghan W.M. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity. 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 5.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 6.Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Thorsen T., Quist H., Matese J.C., Brown P.O., Botstein D., Lonning P.E., Borresen-Dale A.L. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietze E.C., Sistrunk C., Miranda-Carboni G., O'Regan R., Seewaldt V.L. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15:248–254. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann B.D., Pietenpol J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232:142–150. doi: 10.1002/path.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Eirew P., Mullaly S.C., Aparicio S. The omics of triple-negative breast cancers. Clin Chem. 2014;60:122–133. doi: 10.1373/clinchem.2013.207167. [DOI] [PubMed] [Google Scholar]
- 10.Shaver T.M., Lehmann B.D., Beeler J.S., Li C.I., Li Z., Jin H., Stricker T.P., Shyr Y., Pietenpol J.A. Diverse, biologically relevant, and targetable gene rearrangements in triple-negative breast cancer and other malignancies. Cancer Res. 2016;76:4850–4860. doi: 10.1158/0008-5472.CAN-16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wend P., Runke S., Wend K., Anchondo B., Yesayan M., Jardon M., Hardie N., Loddenkemper C., Ulasov I., Lesniak M.S., Wolsky R., Bentolila L.A., Grant S.G., Elashoff D., Lehr S., Latimer J.J., Bose S., Sattar H., Krum S.A., Miranda-Carboni G.A. WNT10B/beta-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol Med. 2013;5:264–279. doi: 10.1002/emmm.201201320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King T.D., Suto M.J., Li Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem. 2012;113:13–18. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kriegsmann M., Endris V., Wolf T., Pfarr N., Stenzinger A., Loibl S., Denkert C., Schneeweiss A., Budczies J., Sinn P., Weichert W. Mutational profiles in triple-negative breast cancer defined by ultradeep multigene sequencing show high rates of PI3K pathway alterations and clinically relevant entity subgroup specific differences. Oncotarget. 2014;5:9952–9965. doi: 10.18632/oncotarget.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon V., Banerji S. Molecular pathways: PI3K pathway targets in triple-negative breast cancers. Clin Cancer Res. 2013;19:3738–3744. doi: 10.1158/1078-0432.CCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 15.Massihnia D., Galvano A., Fanale D., Perez A., Castiglia M., Incorvaia L., Listi A., Rizzo S., Cicero G., Bazan V., Castorina S., Russo A. Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget. 2016;7:60712–60722. doi: 10.18632/oncotarget.10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDaniel J.M., Varley K.E., Gertz J., Savic D.S., Roberts B.S., Bailey S.K., Shevde L.A., Ramaker R.C., Lasseigne B.N., Kirby M.K., Newberry K.M., Partridge E.C., Jones A.L., Boone B., Levy S.E., Oliver P.G., Sexton K.C., Grizzle W.E., Forero A., Buchsbaum D.J., Cooper S.J., Myers R.M. Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget. 2017;8:8226–8238. doi: 10.18632/oncotarget.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangel M.C., Bertolette D., Castro N.P., Klauzinska M., Cuttitta F., Salomon D.S. Developmental signaling pathways regulating mammary stem cells and contributing to the etiology of triple-negative breast cancer. Breast Cancer Res Treat. 2016;156:211–226. doi: 10.1007/s10549-016-3746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundaram S., Johnson A.R., Makowski L. Obesity, metabolism and the microenvironment: links to cancer. J Carcinog. 2013;12:19. doi: 10.4103/1477-3163.119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Esposito V., Liguoro D., Ambrosio M.R., Collina F., Cantile M., Spinelli R., Raciti G.A., Miele C., Valentino R., Campiglia P., De Laurentiis M., Di Bonito M., Botti G., Franco R., Beguinot F., Formisano P. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. 2016;7:24495–24509. doi: 10.18632/oncotarget.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . World Health Organization; Geneva, Switzerland: 2011. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. [Google Scholar]
- 21.Flegal K.M., Kruszon-Moran D., Carroll M.D., Fryar C.D., Ogden C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidell J.C., Halberstadt J. Obesity: the obesity epidemic in the USA - no end in sight? Nat Rev Endocrinol. 2016;12:499–500. doi: 10.1038/nrendo.2016.121. [DOI] [PubMed] [Google Scholar]
- 23.Ogden C.L., Carroll M.D., Lawman H.G., Fryar C.D., Kruszon-Moran D., Kit B.K., Flegal K.M. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malik V.S., Willett W.C., Hu F.B. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 26.Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J., Singh G.M., Gutierrez H.R., Lu Y.A., Bahalim A.N., Farzadfar F., Riley L.M., Ezzati M., Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitacre P.T., Tsai P., Mulligan J. The National Academies Press; Washington, DC: 2009. The Public Health Effects of Food Deserts: Workshop Summary. [PubMed] [Google Scholar]
- 28.Caballero B. A nutrition paradox - underweight and obesity in developing countries. N Engl J Med. 2005;352:1514–1516. doi: 10.1056/NEJMp048310. [DOI] [PubMed] [Google Scholar]
- 29.Cummins S., Macintyre S. Food environments and obesity - neighbourhood or nation? Int J Epidemiol. 2006;35:100–104. doi: 10.1093/ije/dyi276. [DOI] [PubMed] [Google Scholar]
- 30.Beaulac J., Kristjansson E., Cummins S. A Systematic review of food deserts, 1966-2007. Prev Chronic Dis. 2009;6:A105. [PMC free article] [PubMed] [Google Scholar]
- 31.Dias J.J., Whitaker R.C. Black mothers' perceptions about urban neighborhood safety and outdoor play for their preadolescent daughters. J Health Care Poor Underserved. 2013;24:206–219. doi: 10.1353/hpu.2013.0018. [DOI] [PubMed] [Google Scholar]
- 32.Heymsfield S.B., Peterson C.M., Thomas D.M., Heo M., Schuna J.M., Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? a quantitative critical review. Obes Rev. 2016;17:262–275. doi: 10.1111/obr.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson D.A., Feingold M., Bolin F., Parfitt A.M. Principal components analysis of regional bone density in black and white women: relationship to body size and composition. Am J Phys Anthropol. 1991;86:507–514. doi: 10.1002/ajpa.1330860406. [DOI] [PubMed] [Google Scholar]
- 34.Kelly T.L., Wilson K.E., Heymsfield S.B. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heo M., Kabat G.C., Gallagher D., Heymsfield S.B., Rohan T.E. Optimal scaling of weight and waist circumference to height for maximal association with DXA-measured total body fat mass by sex, age and race/ethnicity. Int J Obes. 2013;37:1154–1160. doi: 10.1038/ijo.2012.201. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z., Heo M., Lee R.C., Kotler D.P., Withers R.T., Heymsfield S.B. Muscularity in adult humans: proportion of adipose tissue-free body mass as skeletal muscle. Am J Hum Biol. 2001;13:612–619. doi: 10.1002/ajhb.1099. [DOI] [PubMed] [Google Scholar]
- 37.Silva A.M., Shen W., Heo M., Gallagher D., Wang Z., Sardinha L.B., Heymsfield S.B. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22:76–82. doi: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 39.Kwan M.L., John E.M., Caan B.J., Lee V.S., Bernstein L., Cheng I., Gomez S.L., Henderson B.E., Keegan T.H., Kurian A.W., Lu Y., Monroe K.R., Roh J.M., Shariff-Marco S., Sposto R., Vigen C., Wu A.H. Obesity and mortality after breast cancer by race/ethnicity: the California Breast Cancer Survivorship Consortium. Am J Epidemiol. 2014;179:95–111. doi: 10.1093/aje/kwt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu M., Austin P.C., Manuel D.G., Shah B.R., Tu J.V. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34:1741–1748. doi: 10.2337/dc10-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyer D., Boyko E.J., McNeely M.J., Leonetti D.L., Kahn S.E., Fujimoto W.Y. Subcutaneous thigh fat area is unrelated to risk of type 2 diabetes in a prospective study of Japanese Americans. Diabetologia. 2011;54:2795–2800. doi: 10.1007/s00125-011-2275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis A.A., Kaklamani V.G. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012:809291. doi: 10.1155/2012/809291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman M., Temple J.R., Breitkopf C.R., Berenson A.B. Racial differences in body fat distribution among reproductive-aged women. Metabolism. 2009;58:1329–1337. doi: 10.1016/j.metabol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll J.F., Chiapa A.L., Rodriquez M., Phelps D.R., Cardarelli K.M., Vishwanatha J.K., Bae S., Cardarelli R. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 45.Goedecke J.H., Levitt N.S., Evans J., Ellman N., Hume D.J., Kotze L., Tootla M., Victor H., Keswell D. The role of adipose tissue in insulin resistance in women of African ancestry. J Obes. 2013;2013:952916. doi: 10.1155/2013/952916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathew H., Farr O.M., Mantzoros C.S. Metabolic health and weight: understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016;65:73–80. doi: 10.1016/j.metabol.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrmann W., Reuter W., Schutz C., Lindhofer H.G., Wurzberger G., Schneider P. The behavior of specific parameters of lipid and lipoprotein metabolism in metabolically healthy and obese subjectsZ Gesamte Inn Med. 1982;37:43–50. German. [PubMed] [Google Scholar]
- 48.Bluher S., Schwarz P. Metabolically healthy obesity from childhood to adulthood - does weight status alone matter? Metabolism. 2014;63:1084–1092. doi: 10.1016/j.metabol.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Sims E.A. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 50.Bluher M. Are metabolically healthy obese individuals really healthy? Eur J Endocrinol. 2014;171:R209–R219. doi: 10.1530/EJE-14-0540. [DOI] [PubMed] [Google Scholar]
- 51.Achilike I., Hazuda H.P., Fowler S.P., Aung K., Lorenzo C. Predicting the development of the metabolically healthy obese phenotype. Int J Obes. 2015;39:228–234. doi: 10.1038/ijo.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips C.M. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219–227. doi: 10.1007/s11154-013-9252-x. [DOI] [PubMed] [Google Scholar]
- 53.Velho S., Paccaud F., Waeber G., Vollenweider P., Marques-Vidal P. Metabolically healthy obesity: different prevalences using different criteria. Eur J Clin Nutr. 2010;64:1043–1051. doi: 10.1038/ejcn.2010.114. [DOI] [PubMed] [Google Scholar]
- 54.Gomez-Ambrosi J., Catalan V., Rodriguez A., Andrada P., Ramirez B., Ibanez P., Vila N., Romero S., Margall M.A., Gil M.J., Moncada R., Valenti V., Silva C., Salvador J., Fruhbeck G. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37:2813–2821. doi: 10.2337/dc14-0937. [DOI] [PubMed] [Google Scholar]
- 55.Carey L.A., Perou C.M., Livasy C.A., Dressler L.G., Cowan D., Conway K., Karaca G., Troester M.A., Tse C.K., Edmiston S., Deming S.L., Geradts J., Cheang M.C., Nielsen T.O., Moorman P.G., Earp H.S., Millikan R.C. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 56.Greenup R., Buchanan A., Lorizio W., Rhoads K., Chan S., Leedom T., King R., McLennan J., Crawford B., Kelly Marcom P., Shelley Hwang E. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol. 2013;20:3254–3258. doi: 10.1245/s10434-013-3205-1. [DOI] [PubMed] [Google Scholar]
- 57.Morris G.J., Naidu S., Topham A.K., Guiles F., Xu Y., McCue P., Schwartz G.F., Park P.K., Rosenberg A.L., Brill K., Mitchell E.P. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's surveillance, epidemiology, and end results database. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 58.Stead L.A., Lash T.L., Sobieraj J.E., Chi D.D., Westrup J.L., Charlot M., Blanchard R.A., Lee J.C., King T.C., Rosenberg C.L. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res. 2009;11:R18. doi: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lund M.J., Trivers K.F., Porter P.L., Coates R.J., Leyland-Jones B., Brawley O.W., Flagg E.W., O'Regan R.M., Gabram S.G., Eley J.W. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 60.Stark A., Kleer C.G., Martin I., Awuah B., Nsiah-Asare A., Takyi V., Braman M., Quayson S.E., Zarbo R., Wicha M., Newman L. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. 2010;116:4926–4932. doi: 10.1002/cncr.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bauer K.R., Brown M., Cress R.D., Parise C.A., Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 62.Albain K.S., Unger J.M., Crowley J.J., Coltman C.A., Jr., Hershman D.L. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodward W.A., Huang E.H., McNeese M.D., Perkins G.H., Tucker S.L., Strom E.A., Middleton L., Hahn K., Hortobagyi G.N., Buchholz T.A. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. 2006;107:2662–2668. doi: 10.1002/cncr.22281. [DOI] [PubMed] [Google Scholar]
- 64.Shen Y., Dong W., Esteva F.J., Kau S.W., Theriault R.L., Bevers T.B. Are there racial differences in breast cancer treatments and clinical outcomes for women treated at M.D. Anderson Cancer Center? Breast Cancer Res Treat. 2007;102:347–356. doi: 10.1007/s10549-006-9337-2. [DOI] [PubMed] [Google Scholar]
- 65.Dawood S., Broglio K., Kau S.W., Green M.C., Giordano S.H., Meric-Bernstam F., Buchholz T.A., Albarracin C., Yang W.T., Hennessy B.T., Hortobagyi G.N., Gonzalez-Angulo A.M. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27:220–226. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dean-Colomb W., Yan K., Liedtke C., Symmans W.F., Holmes F.A., O'Shaughnessy J., Asmar L., Hortobagyi G.N., Pusztai L., Gonzalez-Angulo M., American Society of Clinical Oncology Transcriptional profiles of triple receptor-negative breast cancer: are Caucasian, Hispanic, and African-American women different? J Clin Oncol. 2008;26:22014. [Google Scholar]
- 67.Vona-Davis L., Rose D.P. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health. 2009;18:883–893. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 68.Danforth D.N., Jr. Disparities in breast cancer outcomes between Caucasian and African American women: a model for describing the relationship of biological and nonbiological factors. Breast Cancer Res. 2013;15:208. doi: 10.1186/bcr3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newman B., Moorman P.G., Millikan R., Qaqish B.F., Geradts J., Aldrich T.E., Liu E.T. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35:51–60. doi: 10.1007/BF00694745. [DOI] [PubMed] [Google Scholar]
- 70.Millikan R.C., Newman B., Tse C.K., Moorman P.G., Conway K., Dressler L.G., Smith L.V., Labbok M.H., Geradts J., Bensen J.T., Jackson S., Nyante S., Livasy C., Carey L., Earp H.S., Perou C.M. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schatzkin A., Palmer J.R., Rosenberg L., Helmrich S.P., Miller D.R., Kaufman D.W., Lesko S.M., Shapiro S. Risk factors for breast cancer in black women. J Natl Cancer Inst. 1987;78:213–217. [PubMed] [Google Scholar]
- 72.Adams-Campbell L.L., Kim K.S., Dunston G., Laing A.E., Bonney G., Demenais F. The relationship of body mass index to reproductive factors in pre- and postmenopausal African-American women with and without breast cancer. Obes Res. 1996;4:451–456. doi: 10.1002/j.1550-8528.1996.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 73.Austin H., Cole P., Wynder E. Breast cancer in black American women. Int J Cancer. 1979;24:541–544. doi: 10.1002/ijc.2910240504. [DOI] [PubMed] [Google Scholar]
- 74.Zhu K., Caulfield J., Hunter S., Roland C.L., Payne-Wilks K., Texter L. Body mass index and breast cancer risk in African American women. Ann Epidemiol. 2005;15:123–128. doi: 10.1016/j.annepidem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Berstad P., Coates R.J., Bernstein L., Folger S.G., Malone K.E., Marchbanks P.A., Weiss L.K., Liff J.M., McDonald J.A., Strom B.L., Simon M.S., Deapen D., Press M.F., Burkman R.T., Spirtas R., Ursin G. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev. 2010;19:1532–1544. doi: 10.1158/1055-9965.EPI-10-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg L., Adams-Campbell L., Palmer J.R. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50:56–58. [PubMed] [Google Scholar]
- 77.Palmer J.R., Adams-Campbell L.L., Boggs D.A., Wise L.A., Rosenberg L. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev. 2007;16:1795–1802. doi: 10.1158/1055-9965.EPI-07-0336. [DOI] [PubMed] [Google Scholar]
- 78.McGee S.A., Durham D.D., Tse C.K., Millikan R.C. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev. 2013;22:1227–1238. doi: 10.1158/1055-9965.EPI-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ambrosone C.B., Ciupak G.L., Bandera E.V., Jandorf L., Bovbjerg D.H., Zirpoli G., Pawlish K., Godbold J., Furberg H., Fatone A., Valdimarsdottir H., Yao S., Li Y., Hwang H., Davis W., Roberts M., Sucheston L., Demissie K., Amend K.L., Tartter P., Reilly J., Pace B.W., Rohan T., Sparano J., Raptis G., Castaldi M., Estabrook A., Feldman S., Weltz C., Kemeny M. Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol. 2009;2009:871250. doi: 10.1155/2009/871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bandera E.V., Chandran U., Hong C.C., Troester M.A., Bethea T.N., Adams-Campbell L.L., Haiman C.A., Park S.Y., Olshan A.F., Ambrosone C.B., Palmer J.R., Rosenberg L. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150:655–666. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kolonel L.N., Henderson B.E., Hankin J.H., Nomura A.M., Wilkens L.R., Pike M.C., Stram D.O., Monroe K.R., Earle M.E., Nagamine F.S. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palmer J.R., Ambrosone C.B., Olshan A.F. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309–319. doi: 10.1007/s10552-013-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rose D.P., Vona-Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer. 2012;19:R225–R241. doi: 10.1530/ERC-12-0203. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Perez A., Vilarino-Garcia T., Fernandez-Riejos P., Martin-Gonzalez J., Segura-Egea J.J., Sanchez-Margalet V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017;35:71–84. doi: 10.1016/j.cytogfr.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Ueng S.H., Chen S.C., Chang Y.S., Hsueh S., Lin Y.C., Chien H.P., Lo Y.F., Shen S.C., Hsueh C. Phosphorylated mTOR expression correlates with poor outcome in early-stage triple negative breast carcinomas. Int J Clin Exp Pathol. 2012;5:806–813. [PMC free article] [PubMed] [Google Scholar]
- 86.Iqbal J., Thike A.A., Cheok P.Y., Tse G.M., Tan P.H. Insulin growth factor receptor-1 expression and loss of PTEN protein predict early recurrence in triple-negative breast cancer. Histopathology. 2012;61:652–659. doi: 10.1111/j.1365-2559.2012.04255.x. [DOI] [PubMed] [Google Scholar]
- 87.Whiteman E.L., Cho H., Birnbaum M.J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 88.Laakso M. How good a marker is insulin level for insulin resistance? Am J Epidemiol. 1993;137:959–965. doi: 10.1093/oxfordjournals.aje.a116768. [DOI] [PubMed] [Google Scholar]
- 89.Johnson J.L., Duick D.S., Chui M.A., Aldasouqi S.A. Identifying prediabetes using fasting insulin levels. Endocr Pract. 2010;16:47–52. doi: 10.4158/EP09031.OR. [DOI] [PubMed] [Google Scholar]
- 90.Kim J.W., Dang C.V. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 91.Elstrom R.L., Bauer D.E., Buzzai M., Karnauskas R., Harris M.H., Plas D.R., Zhuang H., Cinalli R.M., Alavi A., Rudin C.M., Thompson C.B. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 92.Robey R.B., Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Creighton C.J., Li X., Landis M., Dixon J.M., Neumeister V.M., Sjolund A., Rimm D.L., Wong H., Rodriguez A., Herschkowitz J.I., Fan C., Zhang X., He X., Pavlick A., Gutierrez M.C., Renshaw L., Larionov A.A., Faratian D., Hilsenbeck S.G., Perou C.M., Lewis M.T., Rosen J.M., Chang J.C. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hartman Z.C., Poage G.M., den Hollander P., Tsimelzon A., Hill J., Panupinthu N., Zhang Y., Mazumdar A., Hilsenbeck S.G., Mills G.B., Brown P.H. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73:3470–3480. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Medrek C., Ponten F., Jirstrom K., Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DeNardo D.G., Brennan D.J., Rexhepaj E., Ruffell B., Shiao S.L., Madden S.F., Gallagher W.M., Wadhwani N., Keil S.D., Junaid S.A., Rugo H.S., Hwang E.S., Jirstrom K., West B.L., Coussens L.M. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shree T., Olson O.C., Elie B.T., Kester J.C., Garfall A.L., Simpson K., Bell-McGuinn K.M., Zabor E.C., Brogi E., Joyce J.A. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]