Abstract
Harnessing the power of the human immune system to treat cancer is the essence of immunotherapy. Monoclonal antibodies engage the innate immune system to destroy targeted cells. For the last 30 years, antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity have been the main mechanisms of anti-tumor action of unconjugated antibody drugs. Efforts to exploit the potentials of other immune cells, in particular T cells, culminated in the recent approval of two T cell engaging bispecific antibody (T-BsAb) drugs, thereby stimulating new efforts to accelerate similar platforms through preclinical and clinical trials. In this review, we have compiled the worldwide effort in exploring T cell engaging bispecific antibodies. Our special emphasis is on the lessons learned, with the hope to derive insights in this fast evolving field with tremendous clinical potential.
Keywords: cancer immunotherapy, bispecific antibodies, T cell engaging bispecific antibodies, potency of bispecific antibodies, TDCC, bivalent CD3 binding
1. Introduction
Cancer remains one of the leading causes of death, with the accompanying social and economic burden worldwide. While surgery is effective for locoregional control, chemotherapy and radiation have been mostly ineffective for metastatic cancers, even when pushed to dose and intensity limits, which alone can be harmful because of their inability to discriminate cancer cells from normal bystanders. To minimize toxicity, much efforts have been devoted to identify therapeutic agents that can selectively inhibit the growth of or eradicate cancer cells, while leaving normal cells unscathed – a concept dubbed the “magic bullet” by Paul Ehrlich more than 100 years ago. Before the advent of pathway-specific small molecule inhibitors, antibody-based drugs had been the centerpiece of these efforts and they will likely remain a major player in the coming decades in cancer therapy.
Antibodies are extraordinary molecules vetted through millions of years of evolution. Each antibody molecule has two identical antigen binding sites at the N-terminal variable region that are responsible for the exquisite antigen binding specificity and the binding affinity of these molecules, and a constant fragment crystallizable (Fc) region at the C-terminus that triggers multiple effector mechanisms (Vidarsson, Dekkers, & Rispens, 2014). Depending on the specific antigen/antibody pair, binding alone can physically block the antigen (receptor) or initiate/inhibit signaling through the antigen (receptor) leading to apoptosis of target cells. For the majority of cancer therapeutic IgG antibodies, they execute their immune functions through recruitment of natural killer cells or myeloid cells/macrophages via the Fc region. Furthermore, the Fc region can initiate the classical complement cascade to deposit membrane attack complex on the surface membrane of target cells. These Fc-dependent tumor lysis mechanisms have been extensively studied and exploited in human medicine.
Soon after the discovery of the hybridoma technique by Hans Kohler and Caesar Milstein (Kohler & Milstein, 1975) to immortalize B-cells, the first monoclonal antibody muromonab-CD3 (OKT3) specific for human CD3 was developed and approved in 1985 for treating organ transplant rejection. It took the next decade before the first cancer therapeutic antibody rituximab was approved in 1997 to treat CD20(+) non-Hodgkin lymphoma. Since then, at least 27 therapeutic antibodies for a broad spectrum of human cancers have been approved. The success of these antibody therapeutics firmly established cancer immunotherapy as the fourth modality (after surgery, chemotherapy and radiation) whereby existing defense mechanisms of the human immune system can be mobilized to specifically kill cancer cells. However, naturally occurring IgG antibodies do not have the functionality to directly engage the most efficient “killer” in the immune system, namely, the cytotoxic T lymphocyte (CTL). In order to do that, antibodies have to be engineered to include a second specificity, hence bispecific antibodies (BsAb).
The concept of bispecific antibodies dates back to the 1960s, when Alfred Nisonoff envisioned the potential of replacing one of the two identical antigen binding arms with a different antigen binding specificity (NISONOFF A, 1961; Nisonoff, Wissler, & Lipman, 1960). This concept was developed further in the 1980s to include a second specificity against T cell determinants. CTLs, like all T cells, express variable T-cell receptors (TCRs) associated with invariable CD3 subunits. Binding of TCR by cognate peptide-major histocompatibility complex (pMHC) initiates the signaling through the CD3 complex, which in turn relays the signal internally to activate T cells. By binding to the CD3 complex, CD3-binding monoclonal antibody can bypass the pMHC restriction, thereby activating polyclonal CTLs. When such CD3 binding specificity was engineered into antibodies that bind to tumor specific antigens, CTL response can be redirected to cancer cells (Perez, Hoffman, Shaw, Bluestone, & Segal, 1985; Staerz, Kanagawa, & Bevan, 1985). This strategy gave rise to a completely new class of therapeutic antibodies for cancer immunotherapy. Although it was later found that this class of antibodies could also activate through CD3 on non-T cells, for the purpose of this review, we refer to them as T cell engaging bispecific antibody, or T-BsAb for short.
Over the past three decades a myriad of T-BsAbs have been developed (discussed below). Although the molecular details differ considerably, they are all grounded on the basic design of combining tumor antigen binding specificity and T cell binding specificity into one molecule, with or without an Fc region. To date, only two T-BsAbs, catumaxomab and blinatumomab, have been approved for clinical use in humans, as compared to the other 25 IgG based antibody drugs. The lag is largely attributed to the difficulties in protein engineering during the manufacture of these antibodies and the uncertain clinical toxicities with these novel constructs. Nevertheless, over the past 30 years, multiple molecular designs have been invented, some of which have entered clinical stages of development and many more are in preclinical testing. In this review, we have compiled all the molecular designs that have been developed so far and discussed different aspects of T-BsAbs, including molecular details of their mechanisms of action, factors that may determine their potency, as well as different challenges lying ahead. We hope to provide a timely summary of all the lessons learned that may provide insights to help T-BsAb development in the coming decades.
2. T-BsAbs developed to date
A few recent comprehensive reviews (Brinkmann & Kontermann, 2017; Kontermann & Brinkmann, 2015; Spiess, Zhai, & Carter, 2015) have summarized the various bispecific antibody designs currently under development or approved. To be consistent, this review will follow the same nomenclature they adopted whenever possible. Multiple technologies have been developed to generate human IgG-like molecules; in this review we refer to them as hIgG. Figure 1 summarizes the major formats discussed in this review.
Figure 1. Different formats of T-BsAbs.
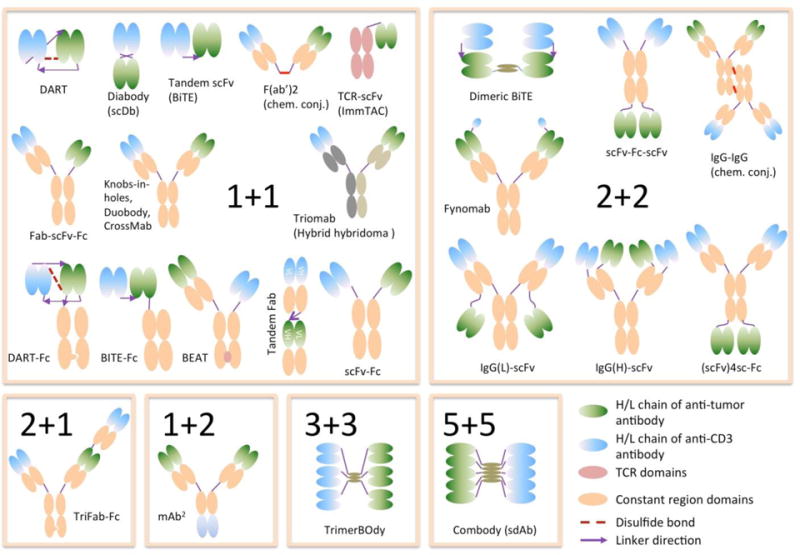
The different molecular designs are grouped by the valency of binding to tumor antigen (first number) and the valency of binding to CD3 (second number). For example, 2+1 denotes bivalent tumor antigen binding and monovalent CD3 binding.
2.1. T-BsAbs in clinical development
Table 1 summarizes all T-BsAbs that have reached clinical stages so far. Out of these 23 antibodies, blinatumomab was approved for treatment of refractory/relapse Ph(−) B-ALL and catumaxomab was approved for malignant ascites derived from EpCAM(+) carcinomas. The rest are mostly ongoing or completed phase I clinical trials, except two trifunctional antibodies, FBTA05 and ertumaxomab, which have entered phase II trials for intravenous infusion. However, both studies have since been terminated.
Table 1.
T-BsAb in clinical development*
| Name | Clinical Phase1 | Tumor Antigen | αCD3 clone used2 | Formats | References |
|---|---|---|---|---|---|
| AMG 420 (a.k.a.duvortuxizumab, BI 836909) | I (2015/NCT02514239) | BCMA | n.a. | BiTE | (Hipp, et al., 2017) |
| JNJ-63709178 | I (2016/NCT02715011) | CD123 | n.a. | hIgG | (Gaudet, et al., 2016) |
| MGD006 | I (2014/NCT02152956) | CD123 | proprietary | DART | (Chichili, et al., 2015; L. Huang & Johnson, 2014) |
| XmAb14045 | I (2016/NCT02730312) | CD123 | n.a. | Fab-scFv-Fc | (Chu, Pong, et al., 2014) |
| AFM11 | I (2014/NCT02106091) | CD19 | UCHT1 (h) | TandAb | (Uwe Reusch, et al., 2015) |
| MGD011 (a.k.a. JNJ-64052781) | I (2016/NCT02743546) | CD19 | XR32 (h) | DART-Fc | (L. Liu, et al., 2016) |
| MT103 (Blinatumomab) | Approved | CD19 | L2K | BiTE | (Dreier, et al., 2003; Dreier, et al., 2002; Löffler, et al., 2000; Mølhøj, et al., 2007) |
| Bi20 (FBTA05) | I/II (2010/NCT01138579) | CD20 | 26II6 (r) | m/rIgG | (Stanglmaier, et al., 2008) |
| CD20-TDB (a.k.a. BTCT4465A, RG7828) | I (2015/NCT02500407) | CD20 | UCHT1 (h) | hIgG | (Liping L. Sun, et al., 2015) |
| REGN1979 | I (2014/NCT02290951) | CD20 | n.a. | hIgG | (E. J. Smith, et al., 2015) From VelocImmune mice |
| AMG-330 | I (2015/NCT02520427) | CD33 | n.a. | BiTE | (Friedrich, et al., 2014; Harrington, et al., 2015; Laszlo, Gudgeon, Harrington, & Walter, 2015) |
| CEA TCB (RG7802, RO6958688) | I (NCT02324257 and NCT02650713) | CEA | proprietary | TriFab-Fc | (Bacac, et al., 2016) |
| MEDI-565 (a.k.a. AMG-211) | I (2011/NCT01284231) | CEA | L2K (de) | BiTE | (Oberst, et al., 2014) |
| MCLA-117 | I (2017/NCT03038230) | CLEC12A, a.k.a. CLL-1 | proprietary | hIgG | (Bakker, VAN LOO, & Logtenberg, 2014; Van Loo, Doornbos, Dolstra, Shamsili, & Bakker, 2015) |
| AMG110 (a.k.a. MT110, Solitomab) | I (2008/NCT00635596) | EpCAM | L2K (de) | BiTE | (Brischwein, et al., 2006; Herrmann, et al., 2010) |
| Catumaxomab | Approved | EpCAM | 26II6 (r) | m/rIgG | (Chelius, et al., 2010; Ruf, et al., 2004; Zeidler, et al., 1999) |
| MGD007 | I (2014/NCT02248805) | GPA33 | n.a. | DART-Fc | (P. A. Moore, et al., 2014) |
| ERY 974 | I (2016/NCT02748837) | GPC3 | n.a. | hIgG | (Ishiguro, et al., 2016) |
| Ertumaxomab | II (2007/NCT00522457) | Her2 | 26II6 (r) | m/rIgG | |
| GBR1302 | I (2016/NCT02829372) | Her2 | n.a. | BEAT | (Croset, et al., 2014) |
| IMCgp100 | Ib/II (2015/NCT02535078) | HLA-A2/gp100 | n.a. | TCR-αCD3 | (Liddy, et al., 2012) |
| PF-06671008 | I (2016/NCT02659631) | p-cadherin | XR32 (h) | DART-Fc | (Root, et al., 2016) |
| BAY2010112 (AMG212, Pasotuxizumab) | I (2012/NCT01723475) | PSMA | proprietary | BiTE | (Friedrich, et al., 2012; WHO, 2014) |
| MOR209/ES414 | I (2014/NCT02262910) | PSMA | n.a. | scFv-Fc-scFv | (Hernandez-Hoyos, et al., 2016) |
This table excludes trials using pre-arm ATC.
Clinical trial stage shows the most advanced clinical phases for the molecule to date. The year of the trial is based on the date published on clinicaltrials.gov.
n.a. denotes clones whose information is not disclosed in the literature; proprietary denotes clones whose information is available in the patent issued or patent pending, as cited in the references; (h):humanized; (r):rat; (de):deimmunized.
Besides T-BsAbs against antigens expressed by hematopoietic cells, namely, B cells (CD19, CD20, BCMA) and myeloid cells (CD33, CD123andCLEC12A), it is encouraging to note that T-BsAbs against antigens expressed by solid tumors (CEA, EpCAM, HER2, PSMA, p-Cadherin, pMHC, GPC3, GPA33) are also being tested. Results from these trials will inform future strategies to optimize T-BsAbs. CD19, CD20, EpCAM, CD33 and HER2 are clinically proven targets, as they are also targets of approved IgG drugs; whereas other targets like p-Cadherin, pMHC and GPC3 are important novel targets that have not been drugged with FDA-approved or EMA-approved antibodies.
The most common format is tandem single chain variable fragment (scFv) based on blinatumomab. However, newer formats like tandem diabody (TandAb), DART and DART-Fc, hIgG, Fab-scFv-Fc, TriFab-Fc, scFv-Fc-scFv, BEAT and TCR-αCD3 are also being investigated. The m/rIgG trifunctional format was used by the first T-BsAb approved. However, apart from immunogenicity, it was severely limited by toxicity when delivered systematically (Sebastian, et al., 2007). This is likely due to their wildtype Fc with full effector functions; and as a result it has not been widely adopted. All the molecular designs incorporate monovalent CD3 binding except for TandAb and scFv-Fc-scFv, which, at least structurally, could mediate bivalent CD3 binding. The prevalence of monovalent anti-CD3 design probably stemmed from the observation that bivalent anti-CD3 antibodies could result in activation induced T cell death (AICD) (Kuhn & Weiner, 2016) and the concerns that it might cause target independent T cell activation. However, AICD in T-BsAb will likely be platform-specific, since for at least 3 formats using bivalent anti-CD3 design, T cells seemed to be fully functional in vitro and in animal models (discussed below). Therefore, the clinical outcomes of these bivalent formats (two are currently in trial) would be informative in the future design of the optimal T-BsAb.
2.2. Preclinical T-BsAbs
The concept of T-BsAb was explored initially in 1985 in murine system using anti-mouse CD3 antibody; but within a few months the first T-BsAb using anti-human CD3 was developed (Perez, et al., 1985; Staerz, et al., 1985). The following decades saw an “explosion” of bispecific antibody development (Riethmüller, 2012). T-BsAbs engineered for human use were dominated initially by chemical conjugation of either full-length IgG or F(ab’), or by hybrid hybridoma technology. Since then, a plethora of T-BsAb formats have been described (Table 2). These include most of the formats used by non-T bispecific antibodies (Brinkmann & Kontermann, 2017; Kontermann & Brinkmann, 2015; Spiess, et al., 2015). The most frequently used format is tandem scFv (BiTE), partly because it avoids issues of cognate chain pairing in multichain constructs, and partly because of its clinical success epitomized by blinatumomab. With the advent of full length bispecific Ig formats that overcome these pairing issues (Figure 1), T-BsAbs with more native conformations can now be more easily manufactured while achieving more desirable PK-profiles than BiTEs (discussed below) and are becoming more widely adopted.
Table 2.
Past and existing T-BsAbs under preclinical development
| Name1 | Target Antigen | Format/Year2 | αCD3 clone used3 | Reference |
|---|---|---|---|---|
| A300E-BiTE | ADAM17 | BiTE/2012 | L2K | (Yamamoto, et al., 2012) |
| BiFab-BCMA | BCMA | Chem. Conj via unnatural aa/2015 | UCHT1 | (Ramadoss, et al., 2015) |
| EM801 | BCMA | TriFab-Fc/2017 | n.a. | (Seckinger, et al., 2017) |
| CD10xCD3 | CD10 | F(ab’)2 by Chem. Conj/1991 | OKT3 | (Oshimi, et al., 1991) |
| CD123xCD3 | CD123 | scFv-Fc-scFv/2012 | UCHT1 | (Kuo, Wong, & Liu, 2012) |
| Xmab14045 | CD123 | Fab-scFv-Fc/2014 | n.a. | (Chu, Pong, et al., 2014) |
| CD133xCD3 | CD133 | Chem. conj+pre-armed ATC/2013 |
OKT3 | (J. Huang, et al., 2013) |
| MS133 | CD133 | Fab-scFv-Fc/2015 | OKT3 (h) | (Zhao, 2015) |
| STL001 | CD138 | BiTE-Fc/2015 | (Kufer, Lutterbuse, Kohleisen, Zeman, & Bauerle, 2009) | (J. Zou, et al., 2015) |
| (19)-3s | CD19 | Dock-and-lock/2014 | OKT3 | (D. L. Rossi, Rossi, Cardillo, Goldenberg, & Chang, 2014) |
| bscCD19xCD3 | CD19 | BiTE/2000 | TR66 | (Löffler, et al., 2000) |
| CD19xCD3 | CD19 | Hybrid hybridoma/1998 | OKT3 | (Daniel, et al., 1998) |
| CD19xCD3 | CD19 | Tandab/1999 | OKT3 | (Cochlovius, Kipriyanov, Stassar, Schuhmacher, et al., 2000; Kipriyanov, et al., 1999) |
| CD19xCD3 | CD19 | Diabody/2000 | OKT3 | (Cochlovius, Kipriyanov, Stassar, Christ, et al., 2000) |
| CD19xCD3 | CD19 | DART/2011 | TR66 | (P. A. Moore, et al., 2011) |
| CD19xTCR | CD19 | DART/2011 | hBMA031 | (P. A. Moore, et al., 2011) |
| HD37xT5.16 | CD19 | Hybrid hybridoma/2007 | Anti-CD5 | (Tita-Nwa, et al., 2007) |
| (20)-3s | CD20 | Dock-and-lock/2014 | n.a. | (D. L. Rossi, et al., 2014) |
| BIS20X3 | CD20 | F(ab’)2 by Chem. Conj/2004 | 37-6673 | (Stel, et al., 2004) |
| CD20xCD3 | CD20 | Diabody/2002 | HIT3a | (Xiong, et al., 2002) |
| CD20xCD3 | CD20 | Chem. conj+pre-armed ATC/2005 | OKT3 | (Gall, Davol, Grabert, Deaver, & Lum, 2005) |
| CD20XCD3 | CD20 | IgG(H)-scFv/2016 | n.a. | (Lu, et al., 2016) |
| (22)-3s | CD22 | Dock-and-lock/2014 | (D. L. Rossi, et al., 2014; E. A. Rossi, Rossi, Cardillo, Chang, & Goldenberg, 2014) | |
| CD22XCD3-RicinA | CD22 | Hybrid hybridoma/1994 | 64.1 (mIgG2a) | (Shen, Li, & Vitetta, 1994) |
| CD30xCD3 | CD30 | Hybrid hybridoma/1993 | OKT3 | (Pohl, et al., 1993; Renner & Pfreundschuh, 1995) |
| AMV564 | CD33 | TandAb/2016 | n.a. | (Uwe Reusch, et al., 2016) |
| CD33xCD3 | CD33 | BiTE/2011 | n.a. | (Stamova, et al., 2011) |
| CD33xCD3 | CD33 | Pre-targeting/2014 | MT-301 | (Arndt, et al., 2014) |
| Xmab13551 | CD38 | Fab-scFv-Fc/2014 | n.a. | (Chu, Miranda, et al., 2014) |
| aCEAxaCD3 | CEA | Diabody/2003 | OKT3 | (Blanco, Holliger, Vile, & Álvarez-Vallina, 2003) |
| CEAxCD3 | CEA | BiTE/2015 | L2K (de) | (Osada, et al., 2015) |
| MF23B/OKT3 | CEA | Diabody/1999 | OKT3 | (Holliger, et al., 1999) |
| Claudin6XCD3 | Claudin6 | BiTE/2016 | TR66 | (Stadler, et al., 2016) |
| CCL1xCD3 | CLL-1 | hIgG/2017 | n.a. | (Leong, et al., 2017) |
| CMVBi | CMV | Chem. conj+pre-armed ATC/2012 |
OKT3 (m) | (Lum, et al., 2012) |
| BiAb(OKT3x cetuximab) | EGFR | Chem. conj+pre-armed ATC/2006 |
OKT3 | (Ursula Reusch, et al., 2006) |
| biMAbM26. 1 | EGFR | Hybrid hybridoma/1993 | 289.1 (mIgG2a) |
(Ferrini, et al., 1993; Negri, et al., 1995) |
| CD3xEGFR | EGFR | Orthogonal Fab | n.a. | (Lewis, et al., 2014) |
| CD3xEGFR | EGFR | Tandem Fab, BiTE, IgG | n.a. | (X. Wu, et al., 2015) |
| hEx3 | EGFR | Diabody, scFv4-Ig and scDb-Fc/2004 | OKT3 | (Asano, et al., 2014; Asano, et al., 2007; Hayashi, et al., 2004; Watanabe, 2011) |
| M2xEGFR | EGFR | F(ab’)2/1999 | Anti-CD2 | (Wild, Strittmatter, Matzku, Schraven, & Meuer, 1999) |
| EGFRvIIIxCD3 | EGFRvIII | BiTE/2013 | OKT3 | (Choi, et al., 2013) |
| 1H8/CD3 | EpCAM | BiTE/2014 | (Dorken, et al., 2006) | (Zhang, et al., 2014) |
| BiTE-KIH | EpCAM | BiTE, BiTE-Fc/2015 | diL2K | (Y. Xu, et al., 2015) |
| E3Bi | EpCAM | BiTE/2004 | n.a. | (Ren-Heidenreich, Davol, Kouttab, Elfenbein, & Lum, 2004) |
| EpCAMxCD3 | EpCAM | BiTE/1997 | TR66 | (Mack, Gruber, Schmidt, Riethmüller, & Kufer, 1997) |
| HEA125XOKT3 | EpCAM | Hybrid hybridoma/2009 | OKT3 | (Salnikov, et al., 2009) |
| EphA10xCD3 | EphA10 | BiTE/2015 | OKT3 | (Taki, et al., 2015) |
| FcRH5xCD3 | FcRH5 | hIgG/2017 | n.a. | (Li, et al., 2017) |
| TR66XMOv18 | Folate Receptor |
F(ab’)2 by Chem. Conj/1991 | TR66 | (Mezzanzanica, et al., 1991) |
| G250XCD3 | G250 | Chimeric IgG/1996 | 4B5 | (Luiten, Coney, Fleuren, Warnaar, & Litvinov, 1996) |
| hu3F8-BsAb | GD2 | IgG(L)-scFv/2015 | OKT3 (h) | (Cheng, Ahmed, Xu, & Cheung, 2015; H. Xu, et al., 2015) |
| hCD4IgGxCD3 | gp120 | Fab-Fc+hCD4-Fc/1994 | UCHT1 | (Chamow, et al., 1994) |
| Janusins | gp120 | scFv-Fc+hCD4-Fc/1991 | n.a. | (Traunecker, Lanzavecchia, & Karjalainen, 1991) |
| VRC07xCD3 | gp120 | Tandem FAB (VRC07)-scFv/2015 | n.a. | (Pegu, et al., 2015; Petrovas, et al., 2017) |
| HIVxCD3 | gp120/gp41 | DART/2015 | XR32 | (Sung, et al., 2015) |
| CD3xAntag2 | GRPR | Chem. Conj/2006 | OKT3 | (Zhou, et al., 2006) |
| COVA420 | HER2 | FynomAb/2014 | OKT3 (h) | (Brack, et al., 2014; Wuellner, et al., 2015) |
| FcabCD3 | HER2 | mAb2/2010 | (Hofmeister, et al., 2005) | (Wang, et al., 2013; Wozniak-Knopp, et al., 2010) |
| HER2-BsAb | HER2 | IgG(L)-scFv/2017 | OKT3 (h) | (Lopez-Albaitero, et al., 2017) |
| HER2xCD3 | HER2 | F(ab’)2 by Chem. Conj/1992 | UCHT1 (h) | (M R Shalaby, 1992) |
| HER2xCD3 | HER2 | F(ab’)2 by Chem. Conj/1993 | OKT3 | (Tsukamoto, et al., 1993) |
| HER2xCD3 | HER2 | Chem. conj+pre-armed ATC/2001 |
OKT3 | (Sen, et al., 2001) |
| HER2xCD3 | HER2 | F(ab’)2 by Chem. Conj/2002 | UCHT1 (h) | (Scheffold, Kornacker, Scheffold, Contag, & Negrin, 2002) |
| HER2xCD3 | HER2 | F(ab’)2 by by unnatural aa/2012 | UCHT1 | (Kim, et al., 2012) |
| HER2xCD3 | HER2 | Chem. conj+pre-armed ATC/2014 | OKT3 | (Han, 2014) |
| Her2xCD3 | HER2 | Universal adaptor/2014 | n.a. | (H. Y. Liu, Zrazhevskiy, & Gao, 2014) |
| HER2xCD3 | HER2 | Tetra-IgG, tri-IgG, Tri-Fab, BiFab by unnatural amino acid/2015 | UCHT1 | (Cao, et al., 2015) |
| mAb-Fv | HER2 | IgG(H)-scFv/2011 | OKT3 (h) | (G. L. Moore, et al., 2011) |
| HER2xCD3/CA-125xCD3 | HER2/CA-125 | Chem. Conj/2006 | OKT3 | (Chan, et al., 2006) |
| pMHCxCD3 | HLA-A2/AFP15 8-166 | BiTE/2017 | n.a. | (H. Liu, et al., 2017) |
| pMHCxCD3 | HLA-A2/NY-ESO-1, LAGE-1, gp100, MAGE-A3, Melan-A | ImmTAC/2012 | n.a. | (Liddy, et al., 2012; McCormack, et al., 2013) |
| pMHCxCD3 | HLA-A2/WT1 | BiTE/2015 | n.a. | (Dao, et al., 2015) |
| pMHCxCD3 | HLA-A2-MART-1 | Combody (sdAb)/2010 | n.a. | (Zhu, et al., 2010) |
| (x)-3s | HLA-DR, CEAC AM5, CEA CAM6, Trop-2 | Dock-and-lock/2014 | n.a. | (D. L. Rossi, et al., 2014; E. A. Rossi, et al., 2014) |
| LamininxCD3 | Laminin | Trimerbody/2013 | OKT3 | (Blanco-Toribio, et al., 2013) |
| MCSPxCD3 | MCSP | BiTE/2011 | n.a. | (Torisu-Itakura, et al., 2011) |
| MCSP (CSPG4) |
BiTE/2010 | L2K | (Bluemel, et al., 2010) | |
| SEA D227A-Mx3 | MUC-1 | SA-fused to diabody/2002 | OKT3 | (Takemura, et al., 2002) |
| 5.2-CD3 | PfMSP-119 | BiTE/2003 | OKT3 (m) | (Yoshida, et al., 2003) |
| PgpxCD3 | pgp | dsDb (disulfide diabody)/2004 | HIT3a | (Gao, et al., 2004; J. Liu, et al., 2009) |
| PSCAxCD3 | PSCA | scDiabody, BiTE/2011 | MT-301 | (Feldmann, et al., 2012; Feldmann, et al., 2011) |
| PSMA | PSMA | Diabody/2008 | n.a. | (Bühler, et al., 2008) |
| Xmab18087 | SSTR2 | Fab-scFv-Fc/2017 | n.a. | (Lee, et al., 2017) |
| TenascinxCD3 | Tenascin | hybrid hybridoma/1995 | CBT3G | (Bonino, et al., 1995) |
| Fab-sec conjugate | α4β7, folate receptor | Fab-sec conjugate/2012 | v9 (h) | (Cui, Thomas, Burke, & Rader, 2012) |
T-BsAbs that did not have a specific name from the reference were named as antigenxCD3.
year after “/” is the year of publication.
n.a. denotes clones whose information is not available.
In addition to the large number of formats, more than 44 antigens have been targeted, with varying degrees of success in preclinical models. The majority of these antigens are oncogenic proteins, except for a few targets in infectious diseases which are not the focus of this review. The most commonly targeted antigens are EGFR, CD19, CD20, CD33, CEA, EpCAM and HER2, all of which have been targeted by more than one format. Peptide-MHC is an interesting class of antigens that has emerged in recent years. Traditional targets for therapeutic antibodies are expressed on cell surface, while most oncoproteins are expressed intracellularly and inaccessible to conventional antibodies. However, peptide fragments of some of these proteins generated via protein turnover can be presented by MHC on the cell surface, which greatly expand the repertoire of “druggable” targets. Immunocore Limited has pioneered the affinity maturation of TCR fused to anti-CD3 scFv. Moreover, TCR-like therapeutic antibodies that target pMHC in a similar fashion as TCR are also emerging in the past few years and are currently actively pursued (Dao, et al., 2015).
Most T-BsAbs developed so far utilize anti-CD3 moiety for T cell recruitment. Excluding those T-BsAbs that did not disclose their anti-CD3 sequences, most of the T-BsAbs developed to date used clones derived from OKT3, UTCH1, L2K or TR66. These mouse-derived antibodies have been humanized, affinity matured or deimmunized, depending on the formats/developers. Based on the available kinetic data, the affinities of these anti-CD3 antibodies span a wide range from 1-200nM by surface plasmon resonance analysis and 8-500nM by flow cytometry analysis (The effect of anti-CD3 affinity will be discussed in the next section). Our compilation also showed that other triggering molecules like TCR, CD5 and CD2 have also been successfully used in the past. In a study using anti-CD19 antibody in a BiTE format, Moore et al. compared the effects of different T cell triggering modules and showed that there was no significant differences between anti-TCR and anti-CD3 in T-cell dependent cellular cytotoxicity (TDCC) assays (P. A. Moore, et al., 2011). Similarly, Tita-Nwa et al. also showed that CD19xCD5 from hybrid hybridoma lysed lymphoma cells with potency comparable to CD19xCD3 when activated T cells was used as effector cells, except that it did not induce resting T cell proliferation and induced less AICD (Tita-Nwa, et al., 2007). Anti-CD2 antibodies alone usually do not activate T cells; by using two anti-CD2 antibodies M1 and M2, Wild et al. demonstrated that M2xEGFR could activate TDCC in an M1-dependent manner (Wild, et al., 1999). All these results suggest that triggering molecules other than CD3 can be viable alternatives for engaging T cells.
3. Lessons learned over three decades of T-BsAb research
3.1. Mechanisms of action
The original intent of developing bispecific antibodies with anti-CD3 specificity was to recruit CTL to kill tumor cells (Staerz, et al., 1985). However, other immune cells, including γδ T cells, natural killer T cells and CD4(+) T cells, also express CD3 and in theory they all can be activated by T-BsAbs. Indeed, it has been shown that γδ T cells are as potent as CD8(+) T cells in TDCC assay by an EGFR T-BsAb (Ferrini, et al., 1993). NKT cells can be activated by anti-CD3 antibodies (Iyoda, et al., 2010) and have been shown using anti-EpCAM BiTE MT110 to have cytotoxic activity (Kischel, Hausmann, Baeuerle, & Kufer, 2009). NKT cells could be a good source of effector cells as they express invariant TCR (thus limiting potential toxicity) and can undergo robust ex vivo expansions (Heczey, et al., 2014). Both CD4(+) and CD8(+) T cells can be activated and contribute to cytotoxicity induced by T-BsAb, although CD4(+)T cells generally do so with delayed kinetics (Haas, et al., 2009). Interestingly, in a subcutaneous model of ovarian cancer, Stadler et al. analyzed the cellular composition of tumor infiltrating lymphocytes (TILs) and found that there were more CD4(+) T cells than CD8(+) T cells, consisting of TH17, TH1 and TH2 subsets based on gene expression profiling (Stadler, et al., 2016). Similar results regarding the presence of both CD4(+) and CD8(+) T cell subsets in tumors have also been observed with the IgG(L)-scFv modular platform (Lopez-Albaitero, et al., 2017; H. Xu, et al., 2015). While CD8(+) CTLs perform anti-tumor effect, CD4(+) T cells also play an important role in tumor eradication, either directly or indirectly, as suggested by other studies (Matsuzaki, et al., 2015; Quezada, et al., 2010). The relative potency of CD8(+) T cells versus CD4(+) T cells in TDCC is inconclusive in the published literature. In terms of maximal killing, some T-BsAbs (e.g., anti-CD20) induced higher maximal killing in the presence of CD8(+) T cells than that of CD4(+) T cells (Liping L. Sun, et al., 2015); whereas others (e.g., anti-BCMA, anti-PSCA and AFM11) induced similar levels of maximal cell killing for both T cell subsets (Feldmann, et al., 2012; Hipp, et al., 2017; Uwe Reusch, et al., 2015). Data regarding EC50 is more limited and equally inconclusive. Within each of the two major αβ T cell subsets, effector memory T cells appeared to be the major mediators of TDCC in the presence of EpCAM BiTE; naïve T cells on the other hand, mediated limited levels of TDCC (Dreier, et al., 2002; Kischel, et al., 2009). Interestingly, expansion of effector memory T cells following blinatumomab treatment was also associated with the anti-tumor response (Bargou, et al., 2008).
To be able to kill tumor cells, T-BsAb must be in contact with both tumor target cells and effector cells. After intravenous infusion of the antibody, it is likely that at least a proportion of the T-BsAb will bind first to the effector cells. However, the majority of T-BsAbs currently under development have a relatively fast koff when binding to CD3, leading to a short residence time (dissociation t1/2) in minutes. Whether and how this initial contact of T-BsAb with T cells can prime them to change their migration behavior likely depends on the structural design of the T-BsAb, a topic that has not been carefully investigated. One can speculate that stronger binding of CD3 through bivalency lengthens the residence time and primes T cells more efficiently. In an extreme example, catumaxomab was shown to be able to activate T cell in an antigen-independent manner (Stanglmaier, et al., 2008); and in a separate study, TNFα released from catumaxomab activated T cells could increase ICAM-1 and CD62E expression on endothelial cells to facilitate T cell adhesion (Dettmar, et al., 2012), an important step in their migration out of the vasculature. Nevertheless, most other T-BsAbs cannot activate T cells independently of target cells, at least not to the extent of catumaxomab; thus the detection of changes in T cell behavior, or lack thereof, may require more sensitive tools. Of note, catumaxomab activates T cells to such an extent that its toxicity has limited further dose escalation in human trials.
Inside solid tumor vasculature, macromolecules like T-BsAbs extravasate through transvascular pores with sizes between 200nm–1.2μm to reach tumor by diffusion (Hobbs, et al., 1998). Multiple solid tumors have been shown to contain TIL, the frequency of which is associated strongly with prognosis and tumor response to immune checkpoint inhibitors (ICI) (Gajewski, et al., 2013; Gooden, de Bock, Leffers, Daemen, & Nijman, 2011). In tumors without TILs (Spranger, 2016), they rely on the ability of T-BsAbs to recruit T cells from the blood. Under normal conditions, naïve T cells are activated by antigens in the lymph nodes and mature into effector T cells. Effector T cells then exit into the blood and migrate into tissues via a coordinated process of rolling, adhesion and transmigration, orchestrated by the sequential interactions of selectin with selectin ligand, chemokine with chemokine receptor, and integrin with adhesion molecules (Nolz, 2015). How T-BsAbs influence these steps and which subsets of T cells are recruited into tumors remain open questions. Much of the clinical experience with T-BsAbs is based on liquid tumors and malignant ascites, which do not possess the same complex architecture of solid tumors, whose vasculature can severely hinder T cell trafficking. Nevertheless, in tumor xenografts in mice, T-BsAb was shown to be able to recruit T cells from the peritoneal cavity into tumors (Bacac, et al., 2016; Stadler, et al., 2016), or from bloodstream into solid tumor masses (Lopez-Albaitero, et al., 2017; H. Xu, et al., 2015). Whether this can be translated into efficacy in human patients will have to await future clinical trials.
Besides bringing together tumor target cells and effector cells, T-BsAbs can exert adhesive forces between the two apposing cells, as measured by atomic force microscopy (S. C. Hoffmann, Wabnitz, Samstag, Moldenhauer, & Ludwig, 2011; Seckinger, et al., 2017). T-BsAbs can induce a more stable conjugate formation between target cells and effector cells, increasing the contact time by as much as 3-fold (Bacac, et al., 2016; Salnikov, et al., 2009) and providing additional time for full activation of T cells to occur. This engagement of target cells and effector cells by T-BsAbs was shown to induce the formation of immunological synapse that is indistinguishable from the synapse formed between TCR and pMHC complex (H. Xu, et al., 2015). The basic geometry of these synapses have TCR-CD3 concentrated in the middle, LFA-1 and F-actin forming ring-like structure at the periphery, and CD45 excluded from the synapse (Griffiths, Tsun, & Stinchcombe, 2010; Li, et al., 2017; Offner, Hofmeister, Romaniuk, Kufer, & Baeuerle, 2006). Formation of immunological synapse is accompanied by the redistribution of signaling and secretory granule proteins in the cell, which eventually leads to the release of perforin and granzymes (Offner, et al., 2006). Release of perforin causes transient pore formation in the juxtaposed target cells and endocytosis of both perforin and granzyme into “gigantosomes”. Inside these enlarged endosomes, perforin again forms pores and releases granzymes into the cytoplasm to cause apoptosis of target cells (Thiery, et al., 2011). This contact-dependent cytotoxicity is likely the main mechanism for T-BsAb induced direct killing of tumor cells, as EGTA chelation of Ca2+, which is required for perforin multimerization and pore formation, led to the complete inhibition of target cell apoptosis by T-BsAb (Haas, et al., 2009; Lyubchenko, Wurth, & Zweifach, 2001). Activation of T cells also results in the secretion of cytokines and T cell proliferation (Nguyen, et al., 2016), which may be required to sustain the immune reactions and their anti-tumor effects.
Although formation of immunological synapse coincides with T-BsAb binding and cytotoxicity, two molecular details have not been fully understood. First, it is unclear how monovalent anti-CD3 binding can lead to clustering of CD3 molecules on T cells. Based on the relative affinities of anti-CD19 and anti-CD3, Hoffmann et al. proposed that T-BsAb binds to target cells and serves as a T cell activation “matrix” that captures and activates mobile T cells (P. Hoffmann, et al., 2005). How this occurs mechanistically is unclear. It is possible that the close approximation of antigens leads to clustering of TCRs on T cells and their subsequent activation. Indeed, both tumor antigen and CD3 have been shown to cluster at the synapse when the target cell and T cell were brought together by T-BsAb (Blank-Voorthuis, et al., 1993; Li, et al., 2017). This is reminiscent of the effect of secondary antibody cross linking primary antibodies attached to antigen on cell surface. However, in a comparison among 3 antibodies that bind to 3 epitopes on FcRH5, it was found that the antibody which bound to the most membrane distal epitope could not induce clustering of antigen or antibody (Li, et al., 2017), arguing that simple approximation of target antigens is insufficient to activate T cells and that other factors also need to be considered, as explained in the next section. It is important to note that the binding behavior of T-BsAbs to membrane-anchored antigens in the interface between target cells and effector cells may be very different from when they are in solution, as the antigens are constrained in two-dimensional planes, with possible boundaries set by immunological synapse (Valitutti, Coombs, & Dupré, 2010). The second unresolved molecular aspect is whether the formation of immunological synapses is absolutely required for cytotoxicity to occur. In the case of pMHC and TCR interaction, it has been shown that cytotoxicity can be uncoupled from TCR clustering and formation of mature immune synapse. This was done by the use of low concentration of pMHC that triggered maximal cytotoxicity but only minimal TCR modulation and IFNγ secretion, and the formation of rudimentary synapse. Ca2+ flux still occurred but displayed a spike-like pattern, in contrast to the smooth and sustained pattern observed by fully activated CTLs (Faroudi, et al., 2003). Indeed, as few as three pMHC molecules was sufficient to trigger cytotoxicity, whereas formation of mature synaspse required about ten. With three pMHC, signs of cell death could occur as early as 5-15min (Faroudi, et al., 2003; Purbhoo, Irvine, Huppa, & Davis, 2004). Interestingly, in a study where T-BsAb bound to target cell through the FcR (not through tumor antigen), T cell killing of target cells did not require TCR clustering (Blank-Voorthuis, et al., 1993). In another study that demonstrated the serial killing ability of anti-CD19 BiTE using cytotoxic T cell line MC-15, the authors did not observe any stable clustering of target cells around T cells and killing occurred in as early as a few minutes, limited seemingly by T cell movement during target cell scanning. However, formation of synapses or lack thereof was not investigated in that study (P. Hoffmann, et al., 2005). Thus, it appears that under optimal conditions (activated T cells, high effector to target ratio, and homogeneous or cloned effector cells), cytotoxicity elicited by T-BsAbs can occur with very fast kinetics, raising the possibility that it may not require the formation of mature synapses. Although further studies are required to clarify this issue, it is tempting to speculate that such mechanism will be beneficial in the diffusion front of T-BsAbs inside tumor, where antibody concentration may be low (Adams, et al., 2001).
3.2. Factors that affect the potency of therapeutic T-BsAb
Multiple factors can affect the potency of a particular T-BsAb, including the antigen itself, binding epitope, antibody affinities, and the specific format used. All these variables interact to generate a specific context that determines the efficiency of T cell activation and ensuing target cell killing. Individual variables have been investigated over the years and in the following paragraphs their importance will be reviewed.
3.2.1. Antigens and epitopes
In an elegant study, Bluemel et al. (Bluemel, et al., 2010) used a set of T-BsAbs that bind to different epitopes along the length of the melanoma antigen MCSP and compared the potency of these antibodies in TDCC assay. They found that antibodies binding to membrane proximal region of MCSP were more potent than those binding to membrane distal region. Consistently, MT110 (anti-EpCAM) gradually lost TDCC activity when the cognate antigen was artificially displaced away from the membrane by increasing number of MCSP spacer domains. In addition, this study also demonstrated that increasing the size of antigens could also block T cell cytotoxicity in the presence of T-BsAb, probably through steric hindrance that prevented T cells from accessing the target cell membrane. Using a different antigen system, Li et al. (Li, et al., 2017) similarly used a set of three antibodies against FcRH5 to compare the effects of distance from target cell membrane on the potency of T-BsAbs. They found that only the membrane proximal antibody 1G7 efficiently caused target antigen clustering and CD45 exclusion from immunological synapse, which translated into higher potency in TDCC. On the other hand, truncating the target antigen to draw it closer to membrane renders the membrane distal clone efficient in TDCC. In another series of T-BsAbs against p-Cadherin developed by Root et al., two antibodies that bound to the distal domains of p-Cadherin with high affinities failed to exert any cytotoxic activity (Root, et al., 2016). Thus, evidences available so far consistently suggest that bringing epitopes closer to the cell membrane can be beneficial in inducing more efficient target cell killing. Intuitively, shorter distance between target cells and effector cells could alter the interaction of activating or inhibitory ligand-receptor pairs, or it could directly influence the transport of cytotoxic molecules into the target cells.
Two other important factors pertaining to T-BsAb targets are the expression levels of antigens and the behavior of antigens on cell membrane, i.e., their mobility and distribution pattern. Evidences regarding the effect of antigen expression levels have been inconsistent in the literature. Some studies have shown positive correlation between half maximal effective concentration (EC50) with antigen expression level (e.g., GB1302, hu3F8-BsAb and HER2-BsAb) (Croset, et al., 2014; Lopez-Albaitero, et al., 2017; H. Xu, et al., 2015), while others demonstrated otherwise (e.g., MEDI-565 and AMG-330) (Friedrich, et al., 2014; Oberst, et al., 2014). These results are difficult to reconcile, but they could be attributed to the different assay systems in different laboratories, the number of cell targets examined, the different culture conditions of cell lines, the potency of the specific T-BsAbs, as well as the physical-biochemical properties of the particular antigens studied. Another interesting aspect of antigen that has not been extensively studied is their mobility and distribution pattern on the cell membrane. In a study with human glioblastoma that express both EGFR and CSPG, it was found that EGFR-specific T-BsAb induced higher cytotoxicity than CSPG-specific T-BsAb, although the cell expressed higher CSPG level. Immunofluorescence staining showed that EGFR formed “patchy” staining pattern, whereas CSPG had uniform pattern (Pfosser, Brandl, Salih, Grosse-Hovest, & Jung, 1999). One plausible explanation, albeit speculative, was that antigens that form microclusters on cell surface may have a higher chance of clustering TCR and activate T cells. This would add an interesting dimension to the properties of antigens. However, more studies are obviously required to substantiate such proposals and to untangle the effects of membrane proximity, expression levels, mobility and microclusters of antigens on the potency of T-BsAb. It is also important to note that for T-BsAbs with bivalent antigen binding, extremely high antigen density may in fact generate steric hindrance for T-BsAb binding, whereas too low a density may exceed the maximal distance between the paratopes, as suggested by Plückthun and Pack (Plückthun & Pack, 1997), leading to less effective effector cell activation.
3.2.2. Anti-tumor antigen and anti-CD3 avidity
Under normal conditions, CTLs make transient contacts with target cells that usually result in futile signaling. Higher avidity of T-BsAb may increase the contact time between target cells and effector cells and therefore increase the likelihood of T cell activation. Indeed, by comparing different affinity matured mutants of an anti-p-Cadherin antibody, Root et al. (Root, et al., 2016) demonstrated that within the same epitope, increasing the affinity from 43.4nM to 3.9nM and 0.2nM also increased the potency by 10 folds and 130 folds, respectively, as measured by EC50. These increases in affinity were mainly the result of decrease in koff by as much as 800 folds, which translated to an increase in interaction t1/2 from 1.2min to 16hrs, a timeframe that will increase the efficiency of tumor cytotoxicity. Similarly, data from Reusch et al. showed that in one set of affinity matured TandAb antibodies (T597, T613, T605) derived from the same anti-CD33 clone, increasing the affinity from 9.7nM to 0.7nM decreased the EC50 of TandAbs to induce PBMC proliferation from 500pM to 7pM; similar trend was observed for another set of antibodies (T479, T481, T480, T478) fused to a different anti-CD3 clone (Uwe Reusch, et al., 2016). In another affinity maturation experiment using TCR ImmTAC, Liddy et al. (Liddy, et al., 2012) demonstrated that increasing affinities of ImmTAC from 30μM to 0.32nM markedly improved the activation of T cells as measured by IFNγ secretion. However, increasing affinity from 0.32nM to 0.03nM did not seem to further increase T cell activation significantly, suggesting that when a threshold was reached, further decrease in koff (hence increase in t1/2) could not increase activation much further, although no detailed kinetic parameters were provided in that study.
To date, 3 reports have compared the effects of changing the avidity of anti-CD3 antibody on cytotoxicity. Bortoletto et al. (Bortoletto, Scotet, Myamoto, D’Oro, & Lanzavecchia, 2002) used mutants of clone TR66 with either increased or decreased affinity to test their ability to activate T cells and exert cytotoxicity. They found that the wildtype version was better than either mutant in T cell activation and cytotoxicity, and that the low affinity mutant was better than the high affinity mutant, suggesting that there exists an optimal kinetics for CD3 binding in order to exert optimal cytotoxicity and that increasing affinity did not always lead to increased function. These interpretations are consistent with observations in classic CTLs, where low affinity interaction of TCR and pMHC is necessary to permit serial TCR triggering (Valitutti, Muller, Cella, Padovan, & Lanzavecchia, 1995). However, it should be noted that the experiments of Bortoletto et al. (Bortoletto, et al., 2002) were done using antibody supernatants from CHO cells and not purified antibodies, potentially weakening their conclusions. Two more recent studies suggested pharmacokinetics and toxicity as important factors in designing high affinity T-BsAbs. In one study, Leong et al. (Leong, et al., 2017) compared the in vitro and in vivo efficacy of 3 highly purified T-BsAbs with CD3 binding affinities of 50nM, 0.5nM and 0.05nM. Variants with lower affinities had EC50 that were 4-100 folds higher (less potent) than those with higher affinities, most likely due to the lower activation of CD8(+) T cells. However, higher affinity variants had 2-4 fold faster clearance when injected into mice and they were associated with severe cytokine storm when injected into cynomolgus monkeys. In another study using Xmab13551, an anti-CD38 T-BsAb developed by Xencor, two variants with lower anti-CD3 affinity were compared with the original antibody for CD38(+) cell depletion and cytokine secretion. It was found that in vitro TDCC potency correlated with anti-CD3 affinity. However, when injected in into cynomolgus monkeys, the variant with intermediate anti-CD3 affinity mediated more sustained CD38(+) cell depletion compared to the other two versions; moreover, the it caused less cytokine release than the parental T-BsAb, hence less toxicity (G. L. Moore, et al., 2015). All these suggest that potency, pharmacokinetics and toxicity need to be balanced while manipulating the affinity of the anti-CD3 arm.
3.2.3. Formats of T-BsAbs
More than 32 bispecific formats have been employed for T cell engaging bispecific antibody generation (Table 1 & 2). Currently only the tandem scFv (BiTE) and Triomab trifunctional format have been approved for clinical use, although there are multiple promising formats under active preclinical and clinical development. Different formats differ in molecular size, stability, flexibility, compactness, ease of production, valency of antigen binding, mode of interaction with target cells and effector cells, as well as pharmacokinetics. There is probably no one format suitable for all applications. Which design to use very likely depends on the specific antigen and the specific application, although a few studies have compared side-by-side the potency of different designs with the same antigen binding components.
In an interesting comparison among Fab-Fab (tandem Fab), tandem scFv and full-length IgG (orthogonal Fab) that all target EGFR, it was shown that in a FACS-based cytotoxicity assay, tandem scFv was roughly 10 fold more potent than Fab-Fab format (EC50<10pM vs EC50<100pM), whereas both were more potent than the full-length IgG format (X. Wu, et al., 2015). Full-length IgG is highly flexible in the hinge region, causing the angular distance between the two Fab arms to vary from 20 to 180 degrees, equivalent to be around 10 nm on average (Bongini, et al., 2004; Oda, et al., 2006). Based on the molecular weight, it is possible that the distance between the two paratopes in tandem scFv is closer than those in Fab-Fab and IgG format, hence it is able to bring the effector cells closer to the target cells. This is indeed supported by another study using HER2 specific FynomAb (Wuellner, et al., 2015). In vitro TDCC assay demonstrated that a FynomAb with N-terminal fusion had ~ 8-fold higher potency than the same antibody with C-terminal fusion. In a study that demonstrated a cell-free expression system, anti-EpCAM BiTE and BiTE-Fc (monovalent) were compared with scFv-Fc (two scFv fused to two heavy chains at N-termini); and it was found that BiTE and BiTE-Fc have similar activity in T cell activation and TDCC assay and both were better than scFv-Fc (Y. Xu, et al., 2015), likely for the same reason of paratope distance. However, it should be noted that the relative sizes of the different molecules could also influence T cell interaction with their targets. Contradicting data in the literature also exist. For example, diabody, which is much smaller than F(ab’)2 and scFv4-Fc format (4 scFv fused at the N-termini of four antibody chains), had similar potency as the former (Hayashi, et al., 2004) and 500-fold less potent than the latter (Asano, et al., 2007), likely because of the differences in the valency of antigen binding.
Two interesting formats show higher potency than tandem scFv in the context of anti-CD19 T-BsAb. The first one is TandAb (tandem diabody). For example, AFM11 (anti-CD19xanti-CD3 TandAb) when produced in CHO cells was 16-34 fold more potent than tandem scFv derived from blinatumomab (Uwe Reusch, et al., 2015). The second one is DART format (MGD011), which exhibited up to 60-fold higher potency than the tandem scFv format with the same antibody components in TDCC, T cell activation and proliferation, as well as IFNγ secretion (P. A. Moore, et al., 2011). Both molecules have entered clinical trial and it would be interesting to see how they perform in comparison to blinatumomab.
In addition to in vitro potency in TDCC assays, the in vivo efficacy of T-BsAbs is also influenced by their pharmacokinetic profile. Half-life of T-BsAbs in vivo is mainly determined by first-pass renal filtration and antibody recycling. The former is determined by the size of the molecule – drugs smaller than ~ 60kDa can be rapidly eliminated by glomerular filtration in the kidney. The latter is mediated by the neonatal FcR (FcRn) that can bind to Fc-bearing molecules in the acidic compartment of endosomes and recycle them back to the extracellular space. T-BsAb formats consisting of antibody fragments that are small in size and lack Fc region are quickly eliminated from the body, resulting in shorter half-life and the requirement for continuous infusion. For example, diabody, tandem scFv, F(ab’)2 and DART all have short half-lives between 2-8 hours (Cochlovius, Kipriyanov, Stassar, Christ, et al., 2000; Friedrich, et al., 2012; Negri, et al., 1995). TandAb has slightly longer half-lives of 7-22 hours (Kipriyanov, et al., 1999; Uwe Reusch, et al., 2015), whereas Fc containing formats like EM801, MGD011, COVA420, PF-06671008 have half-lives between 96-135 hours (Brack, et al., 2014; L. Liu, et al., 2016; Root, et al., 2016; Seckinger, et al., 2017). Incorporation of the Fc region is beneficial for sustaining the effective concentration of T-BsAbs in serum and tumor, and there seems to be a trend to include Fc in T-BsAbs. Inclusion of Fc creates challenges in generating heterodimers required to form T-BsAbs; fortunately, multiple technologies have now been in place to address this issue.
Higher valency of tumor antigen binding is generally desirable since it usually leads to increased avidity of binding and potency of T-BsAbs, as shown in a functional comparison between tandem scFv and dimeric tandem scFv against GD2 (Ahmed, Cheng, Cheung, & Cheung, 2015). Multiple strategies have been employed to achieve this, e.g., IgG(L)-scFV, triFab-Fc, TandAb, scFv-Fc-scFv, Fynomab and Dock-And-Lock. The effect of valency for CD3 binding is less clear, since there are concerns that bivalent CD3 binding will lead to T cell deletion (Kuhn & Weiner, 2016) and antigen-independent activation. Therefore, many designs have purposely avoided bivalent CD3 binding by creating asymmetric molecules with bivalent tumor antigen binding and monovalent CD3 binding, e.g., triFab-Fc, DART-Fc and BiTE-Fc (monomeric). Some technologies were also developed to create symmetric molecules with monovalent tumor antigen and CD3 binding, e.g., knobs-in-holes, crossMab and Fab-arm exchange. It is unclear at present whether bivalent CD3 binding is problematic, since no antigen-independent activation of T cells was observed in a small number of bivalent-designed T-BsAbs (Lopez-Albaitero, et al., 2017; Uwe Reusch, et al., 2015; Wuellner, et al., 2015; H. Xu, et al., 2015). However, given the protection against organ rejection by the bivalent OKT3 antibody, whether T-BsAb with bivalent designs are more tolerogenic than those with monovalent designs need to be carefully examined.
4. Challenges and perspectives
4.1. Quantity and quality of TILs
With their ability to elicit polyclonal anti-tumor T cell responses, T-BsAbs represent an important alternative strategy to adoptive transfer of engineered T cells (e.g., CAR-T cells). Since they rely on the reprogramming of autologous T cell specificity to kill cancerous cells, the quantity and quality of autologous T cells present will very likely determine the effectiveness of T-BsAbs, as demonstrated ex vivo (Harrington, et al., 2015) and in a small clinical study with catumaxomab (Ströhlein, Lefering, Bulian, & Heiss, 2014). In theory, autologous T cells can be manipulated and expanded ex vivo and infused into patients to be used as effector cells. On this front, it is encouraging to see technological advances in the ex vivo expansion of autologous T cells for clinical applications (Restifo, Dudley, & Rosenberg, 2012; C. Smith, et al., 2015). Alternatively, in vivo expansion using engineered cytokines (e.g. IL15Ra-IL15) may also be a good option. Future strategies with emphasis in the stratification of patients based on T cell content and activity (Becht, et al., 2016) may be critical for assuring clinical success of T-BsAbs.
4.2. Cytokine release syndrome (CRS)
Common with T cell-based immunotherapies, the injection of T-BsAbs is associated with CRS, characterized by sharp increases in serum levels of inflammatory cytokines such as IL-6, TNFα and IFNγ, as seen with blinatumomab or catumaxomab (Mau-Sørensen, et al., 2015; Teachey, et al., 2013). CRS is thought to be caused by overactivation of immune cells beyond the point where it can no longer be self-contained. In the clinic, such activation may be necessary for the efficacy of the therapeutic agent; but its current management is still suboptimal, leading to life-threatening complications. Although the effects of CRS on tumor microenvironment have not been fully understood, IL-6, TNFα and IFNγ have been linked to tumor growth and/or immune evasion (Abiko, et al., 2015; Fisher, Appenheimer, & Evans, 2014; Landskron, De la Fuente, Thuwajit, Thuwajit, & Hermoso, 2014).
Since cytokine release is intimately linked to T cell activation, the same factors that affect the potency of T-BsAb will likely also affect cytokine releases. For example, increasing the affinity of ImmTAC molecule to pMHC increases IFN secretion (Liddy, et al., 2012); similarly, increasing the affinity of anti-CD3 in the context of anti-CLL1 T-BsAb resulted in life-threatening cytokine release in cynomolgus monkeys (Leong, et al., 2017). It should be noted that T cell activation is a loose term that entails different T cell behaviors, including but not limited to upregulation of surface activation markers, release of cytokines, and release of cytotoxic molecules. It is not entirely clear at present if these events can be individually manipulated by changing the T-BsAbs. However, in an interesting study using an anti-PSMA T-BsAb, Hernandez-Hoyos et al. demonstrated that it was possible to generate potent TDCC in vitro while reducing cytokine secretion via the use of scFv-Fc-scFv T-BsAb format (Hernandez-Hoyos, et al., 2016), suggesting that tumor cytotoxicity and cytokine storm may be separable events or an optimal balance between potency and toxicity can be achieved by changing T-BsAb design. A better understanding of how the molecular formats and other T-BsAb properties (e.g., affinities) influence these events will undoubtedly be instrumental in the future design of better T-BsAb candidates to alleviate CRS and avoid cytokine-induced enhancement of tumor growth.
4.3. Specific tumor target antigens
The third challenge faced by T-BsAb therapeutics, or rather monoclonal antibody therapeutics in general, is the identification of therapeutic targets that are specific enough to discriminate between cancer cells and normal cells. Most of the therapeutic targets for antibody drugs under development are differentiation markers that are also expressed in normal cells, albeit at a lower level or with a more restricted pattern. This narrows the therapeutic window and poses a great challenge to the design and development of T-BsAbs. Most of the drugs developed to date represent compromises between clinical efficacy and toxicity. An “ideal target” that can clearly demarcate normal versus diseased cells has yet to be discovered. Intracellular oncoproteins, which comprise most of the cancer “drivers” and some of which can be presented by pMHC, seem to be the closest we can ascertain as an “ideal target”. However, targeting these antigens requires isolation of TCR or TCR-like antibodies, with substantial issues of cross-reactivity which could compromise organ or tissue selectivity. With the ability to produce more and more high quality “omics” data and their wide accessibility, future target discovery and testing will require integration, plus sophisticated analysis and interpretation of big data. The caveat of this endeavor is that targets may not be of sufficient density, and will likely require extensive testing before specificity can be proven. To create safe and effective clinical T-BsAb for such targets will take time.
4.4. T cell homing, activation and survival
To date, no data on T cell homing are available in the clinic for targeting solid tumors using intravenous T-BsAbs. The ideal T-BsAb should drive T cells into solid tumors, activate TILs to proliferate, and carry out the anti-tumor function despite the presence of immune checkpoints in the tumor stroma. Successful T-BsAb therapy requires the efficient implementation of each of these steps. However, multiple obstacles exist (Figure 2).
Figure 2. Hurdles for T cell-mediated tumor surveillance.
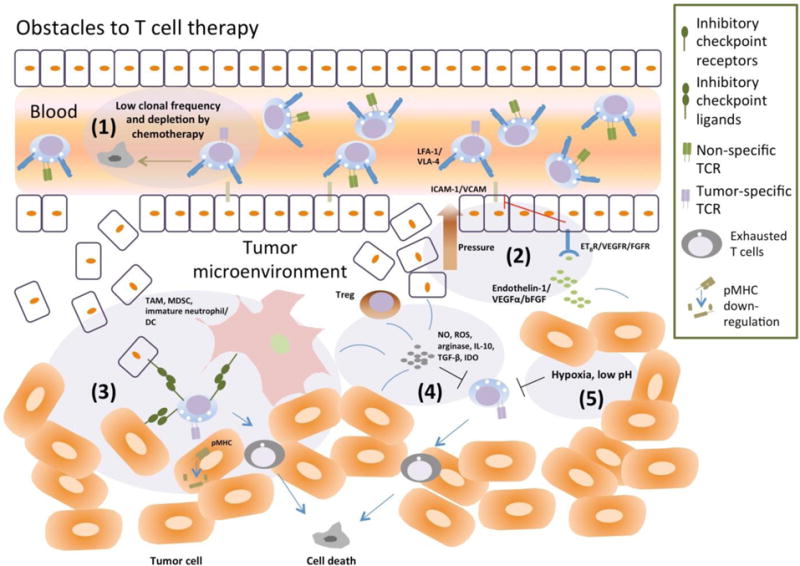
Insufficient tumor infiltrating lymphocytes (TILs) (both antigen-specific and antigen-nonspecific) can be caused by: (1) low clonal frequency of tumor specific T cells and depletion of lymphocytes by chemotherapy; (2) denial of T cell entry due to increase in interstitial pressure (abnormal angiogenesis and irregular endothelium) and down-regulation of adhesion molecules on endothelial cells (“anergic” EC), both controlled by soluble factors (e.g. endothelin-1, VEGFα and bFGF) secreted by tumor cells and other immunosuppressive cells present in the tumor microenvironment (TME). Mechanisms used by tumor cells to evade T cell killing mainly consist of: (3) downregulation of MHC and the cognate T-cell receptor (TCR) target (peptide-MHC) on tumor cells and suppression by inhibitory immune checkpoint receptor-ligand interactions (e.g. PD1 with PD-L1/PD-L2, CTLA4 with CD80/CD86); (4) anergy mediated by secreted immunosuppressive molecules (e.g., NO, ROS, arginase, IL-10, TGFβ, IDO); (5) alteration by tumor cells of the metabolic environment making it hypoxic and acidic, which can be detrimental to T cell function. Processes (2)-(5) can be executed by the different cellular components in the TME, such as tumor cells, endothelial cells, regulatory T cells (Treg), tumor associated macrophage (TAM), myeloid derived suppressor cells (MDSC), immature neutrophil and immature DC
First of all, T cells, whether they are already residing in the tumors (TILs) or are driven by T-BsAb to infiltrate tumors, are the ingredients of “inflamed tumors”, a prerequisite for response to T cell-based therapies. Preclinical studies have consistently shown that the presence of TILs correlates with therapeutic efficacy of immune checkpoint inhibitors (Gajewski, et al., 2013; Gooden, et al., 2011; Spranger, 2016); one might expect it to also predict tumor response to T-BsAbs. However, T cell numbers may be low due to depletion by prior chemotherapy or cachexia. The hostile microenvironment of tumor, which is hypoxic, acidic and immunosuppressive, could also contribute to the suppression and further deletion of TILs (Bellone & Calcinotto, 2013). Furthermore, exhaustion may also be due to the repeated over-stimulation of TILs by T-BsAbs. In an in vitro serial TDCC assay, Osada et al. showed that the cytotoxicity of T cells induced by anti-CEA BiTE was impaired when the same T cells were transferred from the first culture and applied to a second culture of target cells (Osada, et al., 2015). In a syngeneic mouse model, T-BsAb could cause apoptosis of TILs by reactivation induced cell death (Hettich, Lahoti, Prasad, & Niedermann, 2016). Thus, how to maintain and expand a functional population of cytotoxic T cells is a pressing question faced by T-BsAb therapy. It may be unrealistic to expect T-BsAb to provide all the signals for these purposes inside the tumor stroma. In this regard, cytokines such as IL15 are viable candidates to aid the survival and expansion of T cells (Huarte, et al., 2009; Rettinger, et al., 2012).
Second, T cells recruited by T-BsAbs need to overcome high interstitial pressure and a molecular network that “discourages” their entry. Tumor vasculatures are highly disorganized; and tumors secreted factors like VEGFα and FGFs can cause tumor endothelial cells to become “anergic”, i.e., losing adhesion molecules like ICAM-1/2, VCAM-1 and CD34, which are important for T cell transmigration (Bellone & Calcinotto, 2013). Moreover, tumor cells can secrete chemokines that attract immunosuppressive cells and repel CTLs (Oelkrug & Ramage, 2014). How and whether T-BsAbs can help overcome these limitations remain to be seen. However, in xenograft models, some T-BsAb formats (e.g. IgG(L)-scFv) can drive T cells from the blood into solid tumors and effect tumor ablation, despite the upregulation of PD-L1 in the tumor stroma (Lopez-Albaitero, et al., 2017; H. Xu, et al., 2015). It is noteworthy also that inflammatory cytokines secreted by T-BsAb activated T cells, e.g., TNFα, can apparently overcome some of these barriers, as demonstrated by an NGR-TNF molecule that specifically targeted tumor vasculature through NGR peptide and that was able to increase infiltration of CD8(+) T cells (Calcinotto, et al., 2012). This molecule is currently in late clinical development. It would be interesting to test the combination of vasoactive cytokines with T-BsAbs for solid tumor immunotherapy. Apart from cytokines, which usually have pleiotropic effects, CTL-attracting chemokines (e.g., CXCL9, CXCL10, CCL3) may be another viable alternatives, e.g., by incorporating a chemokine component in T-BsAb designs. The challenge with such designs will be the maintenance of a chemokine concentration gradient between blood and tumor, which is essential for chemokines to recruit T cells (Siddiqui, Erreni, van Brakel, Debets, & Allavena, 2016); extensive testing and optimization will be required both in vitro and in vivo.
Another obstacle for T cell based immunotherapy is the prevalence of immunosuppressive molecules in the tumor microenvironment, both cell surface bound and secreted. Tumor endothelial cells, immunosuppressive myeloid cells, and tumor cells can all express immunosuppressive molecules like PD-L1 and PD-L2 (Motz & Coukos, 2011; W. Zou, Wolchok, & Chen, 2016). In this regard, the ability of T cells to survive and not become exhausted, while being driven by T-BsAb into the tumor, should benefit from the explosive developments in the field of ICIs. In preclinical models, Junttila et al. demonstrated in a HER2(+) CT26 subcutaneous model that combining anti-PD-L1 antibody and anti-HER2 T-BsAb induced a stronger and a more durable anti-tumor response, when compared to T-BsAb alone (Junttila, et al., 2014). Similarly, combination of anti-PD-1 or anti-PD-L1 antibodies with CD20-TDB also showed greater anti-tumor effects than single-agents alone (Liping Laura Sun, et al., 2016). In clinical settings, screening of co-signaling molecules on B-ALL blasts from blinatumomab treated patients identified PD-L1 as the marker that was significantly upregulated in non-responders. Moreover, combination of blinatumomab and pembrolizumab induced responses in a previously nonresponding 12-year-old patient (Feucht, et al., 2016). Clinical trials of similar combination therapies for solid tumors are currently underway, e.g., RO6958688 (anti-CEA T-BsAb) is in a phase 1b study in combination with atezolizumab to treat CEA-positive tumors.
One interesting proposal to enhance T cell homing, infiltration and survival is to combine T-BsAb with low-dose chemotherapy. Although high dose of chemotherapy is immunosuppressive, low dose of selected chemotherapy can modify subsets of T cells to the host’s advantage. In a murine mesothelioma model, the addition of cisplatin to anti-CTLA4 enhanced infiltration of TILs accompanied by higher anti-tumor efficacy (L. Wu, Yun, Tagawa, Rey-McIntyre, & de Perrot, 2012). Fan et al. also showed that low-dose cytosine arabinoside increased the expression of CD80 and CD86 on B-ALL patient-derived samples, sensitizing them to anti-CD19 T-BsAb killing (Fan, et al., 2015). In summary, the success of T-BsAbs will likely require a combination of strategies and modalities to enhance T cell homing, activation and proliferation, as well as derepression in the tumor microenvironment. Some of these can be integrated into the design of T-BsAbs to include more specificity, whereas others may be better achieved with a cocktail of drugs/biologics.
5. Conclusions
This is an exciting decade for antibody drug development. Since the discovery of monoclonal antibody technology, over the last 30 years, advances in protein engineering and manufacture, coupled with better understanding of cancer biology and immunology, have enabled better design and faster clinical translation of novel immunotherapeutics to address unmet medical needs. Future progress in the fight against cancer will likely require the integration of multiple ttreatment strategies, and antibodies will play a pivotal role. In addition to normal IgG antibodies, immune checkpoint inhibitors, antibody drug conjugates, radioimmunoconjugates, and CAR-T-cells, T-BsAbs provide another exciting and potent class of antibody-based immunotherapeutics, whose potential has yet to be fully realized. Although currently only two T-BsAb antibodies have been approved for clinical use, with more than 60 bispecific designs and some promising candidates, we are optimistic that more approvals will follow in the near future.
Acknowledgments
Funding
This work was supported in part by Funds from Enid A. Haupt Endowed Chair, Kids Walk for Kids with Cancer NYC, Katie Find a Cure Foundation, Isabella Santos Foundation, the Robert Steel Foundation, NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbrevation
- CRS
cytokine release syndrome
- CTL
cytotoxic T lymphocyte
- EC50
half maximal effective concentration
- Fc
fragment crystallizable
- ICI
immune checkpoint inhibitor
- pMHC
peptide-major histocompatibility complex
- scFv
single chain variable fragment
- TandAb
tandem diabody
- T-BsAb
T cell engaging bispecific antibody
- TCR
T cell receptor
- TDCC
T cell dependent cellular cytotoxicity
- TIL
tumor infiltrating lymphocyte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Memorial Sloan Kettering Cancer Center and NK Cheung have financial interest in Y-mabs Therapeutics, Inc. and Abpro, Inc.
References
- Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. British journal of cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. High Affinity Restricts the Localization and Tumor Penetration of Single-Chain Fv Antibody Molecules. Cancer Research. 2001;61:4750–4755. [PubMed] [Google Scholar]
- Ahmed M, Cheng M, Cheung IY, Cheung NKV. Human derived dimerization tag enhances tumor killing potency of a T-cell engaging bispecific antibody. OncoImmunology. 2015;4:e989776. doi: 10.4161/2162402X.2014.989776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt C, Feldmann A, von Bonin M, Cartellieri M, Ewen EM, Koristka S, Michalk I, Stamova S, Berndt N, Gocht A, Bornhauser M, Ehninger G, Schmitz M, Bachmann M. Costimulation improves the killing capability of T cells redirected to tumor cells expressing low levels of CD33: description of a novel modular targeting system. Leukemia. 2014;28:59–69. doi: 10.1038/leu.2013.243. [DOI] [PubMed] [Google Scholar]
- Asano R, Shimomura I, Konno S, Ito A, Masakari Y, Orimo R, Taki S, Arai K, Ogata H, Okada M, Furumoto S, Onitsuka M, Omasa T, Hayashi H, Katayose Y, Unno M, Kudo T, Umetsu M, Kumagai I. Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics. MAbs. 2014;6:1243–1254. doi: 10.4161/mabs.29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano R, Watanabe Y, Kawaguchi H, Fukazawa H, Nakanishi T, Umetsu M, Hayashi H, Katayose Y, Unno M, Kudo T, Kumagai I. Highly Effective Recombinant Format of a Humanized IgG-like Bispecific Antibody for Cancer Immunotherapy with Retargeting of Lymphocytes to Tumor Cells. Journal of Biological Chemistry. 2007;282:27659–27665. doi: 10.1074/jbc.M704719200. [DOI] [PubMed] [Google Scholar]
- Bacac M, Fauti T, Sam J, Colombetti S, Weinzierl T, Ouaret D, Bodmer W, Lehmann S, Hofer T, Hosse RJ, Moessner E, Ast O, Bruenker P, Grau-Richards S, Schaller T, Seidl A, Gerdes C, Perro M, Nicolini V, Steinhoff N, Dudal S, Neumann S, von Hirschheydt T, Jaeger C, Saro J, Karanikas V, Klein C, Umaña P. A Novel Carcinoembryonic Antigen T-Cell Bispecific Antibody (CEA TCB) for the Treatment of Solid Tumors. Clinical Cancer Research. 2016;22:3286–3297. doi: 10.1158/1078-0432.CCR-15-1696. [DOI] [PubMed] [Google Scholar]
- Bakker ABH, VAN LOO PF, Logtenberg T. Bispecific igg antibodies as t cell engagers. Google Patents 2014 [Google Scholar]
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmüller G, Reinhardt C, Baeuerle PA, Kufer P. Tumor Regression in Cancer Patients by Very Low Doses of a T Cell–Engaging Antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- Becht E, Giraldo NA, Germain C, de Reyniès A, Laurent-Puig P, Zucman-Rossi J, Dieu-Nosjean MC, Sautès-Fridman C, Fridman WH. Immune Contexture, Immunoscore, and Malignant Cell Molecular Subgroups for Prognostic and Theranostic Classifications of Cancers. Advances in Immunology. 2016;130:95–190. doi: 10.1016/bs.ai.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Bellone M, Calcinotto A. Ways to Enhance Lymphocyte Trafficking into Tumors and Fitness of Tumor Infiltrating Lymphocytes. Frontiers in oncology. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Toribio A, Sainz-Pastor N, Álvarez-Cienfuegos A, Merino N, Cuesta ÁM, Sánchez-Martín D, Bonet J, Santos-Valle P, Sanz L, Oliva B, Blanco FJ, Álvarez-Vallina L. Generation and characterization of monospecific and bispecific hexavalent trimerbodies. MAbs. 2013;5:70–79. doi: 10.4161/mabs.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco B, Holliger P, Vile RG, Álvarez-Vallina L. Induction of Human T Lymphocyte Cytotoxicity and Inhibition of Tumor Growth by Tumor-Specific Diabody-Based Molecules Secreted from Gene-Modified Bystander Cells. The Journal of Immunology. 2003;171:1070–1077. doi: 10.4049/jimmunol.171.2.1070. [DOI] [PubMed] [Google Scholar]
- Blank-Voorthuis CJ, Braakman E, Ronteltap CP, Tilly BC, Sturm E, Warnaar SO, Bolhuis RL. Clustered CD3/TCR complexes do not transduce activation signals after bispecific monoclonal antibody-triggered lysis by cytotoxic T lymphocytes via CD3. The Journal of Immunology. 1993;151:2904–2914. [PubMed] [Google Scholar]
- Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, Kufer P. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunology, Immunotherapy. 2010;59:1197–1209. doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongini L, Fanelli D, Piazza F, De Los Rios P, Sandin S, Skoglund U. Freezing immunoglobulins to see them move. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6466–6471. doi: 10.1073/pnas.0400119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonino LD, De Monte LB, Spagnoli GC, Vola R, Mariani M, Barone D, Moro AM, Riva P, Nicotra MR, Natali PG, Malavasi F. Bispecific monoclonal antibody anti-CD3 X anti-tenascin: An immunotherapeutic agent for human glioma. International Journal of cancer. 1995;61:509–515. doi: 10.1002/ijc.2910610414. [DOI] [PubMed] [Google Scholar]
- Bortoletto N, Scotet E, Myamoto Y, D’Oro U, Lanzavecchia A. Optimizing anti-CD3 affinity for effective T cell targeting against tumor cells. European Journal of Immunology. 2002;32:3102–3107. doi: 10.1002/1521-4141(200211)32:11<3102::AID-IMMU3102>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Brack S, Attinger-Toller I, Schade B, Mourlane F, Klupsch K, Woods R, Hachemi H, von der Bey U, Koenig-Friedrich S, Bertschinger J, Grabulovski D. A Bispecific HER2-Targeting FynomAb with Superior Antitumor Activity and Novel Mode of Action. Molecular cancer therapeutics. 2014;13:2030–2039. doi: 10.1158/1535-7163.MCT-14-0046-T. [DOI] [PubMed] [Google Scholar]
- Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, Offner S, Locher M, Urbig T, Raum T, Kleindienst P, Wimberger P, Kimmig R, Fichtner I, Kufer P, Hofmeister R, da Silva AJ, Baeuerle PA. MT110: A novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Molecular Immunology. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Bühler P, Wolf P, Gierschner D, Schaber I, Katzenwadel A, Schultze-Seemann W, Wetterauer U, Tacke M, Swamy M, Schamel WWA, Elsässer-Beile U. A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells. Cancer Immunology, Immunotherapy. 2008;57:43–52. doi: 10.1007/s00262-007-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, Corti A, Bellone M. Targeting TNF-α to Neoangiogenic Vessels Enhances Lymphocyte Infiltration in Tumors and Increases the Therapeutic Potential of Immunotherapy. The Journal of Immunology. 2012;188:2687–2694. doi: 10.4049/jimmunol.1101877. [DOI] [PubMed] [Google Scholar]
- Cao Y, Axup JY, Ma JSY, Wang RE, Choi S, Tardif V, Lim RKV, Pugh HM, Lawson BR, Welzel G, Kazane SA, Sun Y, Tian F, Srinagesh S, Javahishvili T, Schultz PG, Kim CH. Multiformat T-Cell-Engaging Bispecific Antibodies Targeting Human Breast Cancers. Angewandte Chemie. 2015;127:7128–7133. doi: 10.1002/anie.201500799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamow SM, Zhang DZ, Tan XY, Mhatre SM, Marsters SA, Peers DH, Byrn RA, Ashkenazi A, Junghans RP. A humanized, bispecific immunoadhesin-antibody that retargets CD3+ effectors to kill HIV-1-infected cells. The Journal of Immunology. 1994;153:4268–4280. [PubMed] [Google Scholar]
- Chan JK, Hamilton CA, Cheung MK, Karimi M, Baker J, Gall JM, Schulz S, Thorne SH, Teng NN, Contag CH, Lum LG, Negrin RS. Enhanced Killing of Primary Ovarian Cancer by Retargeting Autologous Cytokine-Induced Killer Cells with Bispecific Antibodies: A Preclinical Study. Clinical Cancer Research. 2006;12:1859–1867. doi: 10.1158/1078-0432.CCR-05-2019. [DOI] [PubMed] [Google Scholar]
- Chelius D, Ruf P, Gruber P, Plöscher M, Liedtke R, Gansberger E, Hess J, Wasiliu M, Lindhofer H. Structural and functional characterization of the trifunctional antibody catumaxomab. MAbs. 2010;2:309–319. doi: 10.4161/mabs.2.3.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Ahmed M, Xu H, Cheung NKV. Structural design of disialoganglioside GD2 and CD3-bispecific antibodies to redirect T cells for tumor therapy. International Journal of cancer. 2015;136:476–486. doi: 10.1002/ijc.29007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichili GR, Huang L, Li H, Burke S, He L, Tang Q, Jin L, Gorlatov S, Ciccarone V, Chen F, Koenig S, Shannon M, Alderson R, Moore PA, Johnson S, Bonvini E. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: Preclinical activity and safety in nonhuman primates. Science Translational Medicine. 2015;7:289ra282–289ra282. doi: 10.1126/scitranslmed.aaa5693. [DOI] [PubMed] [Google Scholar]
- Choi BD, Kuan CT, Cai M, Archer GE, Mitchell DA, Gedeon PC, Sanchez-Perez L, Pastan I, Bigner DD, Sampson JH. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Miranda Y, Phung S, Chen H, Rashid R, Endo NA, Chan EW, Pong E, Bonzon C, Muchhal US, Leung IWL, Bernett MJ, Moore GL, Szymkowski DE, Desjarlais JR. Immunotherapy with Long-Lived Anti-CD38 × Anti-CD3 Bispecific Antibodies Stimulates Potent T Cell-Mediated Killing of Human Myeloma Cell Lines and CD38+ Cells in Monkeys: A Potential Therapy for Multiple Myeloma. Blood. 2014;124:4727–4727. [Google Scholar]
- Chu SY, Pong E, Chen H, Phung S, Chan EW, Endo NA, Rashid R, Bonzon C, Leung IWL, Muchhal US, Moore GL, Bernett MJ, Szymkowski DE, Desjarlais JR. Immunotherapy with Long-Lived Anti-CD123 × Anti-CD3 Bispecific Antibodies Stimulates Potent T Cell-Mediated Killing of Human AML Cell Lines and of CD123+ Cells in Monkeys: A Potential Therapy for Acute Myelogenous Leukemia. Blood. 2014;124:2316–2316. [Google Scholar]
- Cochlovius B, Kipriyanov SM, Stassar MJJG, Christ O, Schuhmacher J, Strauß G, Moldenhauer G, Little M. Treatment of Human B Cell Lymphoma Xenografts with a CD3 × CD19 Diabody and T Cells. The Journal of Immunology. 2000;165:888–895. doi: 10.4049/jimmunol.165.2.888. [DOI] [PubMed] [Google Scholar]
- Cochlovius B, Kipriyanov SM, Stassar MJJG, Schuhmacher J, Benner A, Moldenhauer G, Little M. Cure of Burkitt’s Lymphoma in Severe Combined Immunodeficiency Mice by T Cells, Tetravalent CD3 × CD19 Tandem Diabody, and CD28 Costimulation. Cancer Research. 2000;60:4336–4341. [PubMed] [Google Scholar]
- Croset A, Macoin J, Ollier R, Pluess M, Delon C, Skegro D, Blein S, Hou S, Back J. 139 GBR1302: a BEAT® bispecific antibody for the treatment of HER2 positive cancers. European Journal of Cancer. 2014;50:48. [Google Scholar]
- Cui H, Thomas JD, Burke TR, Rader C. Chemically Programmed Bispecific Antibodies That Recruit and Activate T Cells. Journal of Biological Chemistry. 2012;287:28206–28214. doi: 10.1074/jbc.M112.384594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel PT, Kroidl A, Kopp J, Sturm I, Moldenhauer G, Dörken B, Pezzutto A. Immunotherapy of B-Cell Lymphoma With CD3×19 Bispecific Antibodies: Costimulation via CD28 Prevents “Veto” Apoptosis of Antibody-Targeted Cytotoxic T Cells. Blood. 1998;92:4750–4757. [PubMed] [Google Scholar]
- Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y, Xiang J, Yan S, de Morais Guerreiro MD, Veomett N, Dubrovsky L, Curcio M, Doubrovina E, Ponomarev V, Liu C, O’Reilly RJ, Scheinberg DA. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotech. 2015;33:1079–1086. doi: 10.1038/nbt.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmar K, Seitz-Merwald I, Lindemann C, Schroeder P, Seimetz D, Atz J. Transient lymphocyte decrease due to adhesion and migration following catumaxomab (anti-EpCAM × anti-CD3) treatment in vivo. Clinical and Translational Oncology. 2012;14:376–381. doi: 10.1007/s12094-012-0811-5. [DOI] [PubMed] [Google Scholar]
- Dorken B, Riethmuller G, Kufer P, Lutterbuse R, Bargou R, Loffler A. CD 19×CD3 specific polypeptides and uses thereof. Google Patents 2006 [Google Scholar]
- Dreier T, Baeuerle PA, Fichtner I, Grün M, Schlereth B, Lorenczewski G, Kufer P, Lutterbüse R, Riethmüller G, Gjorstrup P, Bargou RC. T Cell Costimulus-Independent and Very Efficacious Inhibition of Tumor Growth in Mice Bearing Subcutaneous or Leukemic Human B Cell Lymphoma Xenografts by a CD19-/CD3-Bispecific Single-Chain Antibody Construct. The Journal of Immunology. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, Kufer P, Riethmuller G, Bargou R, Baeuerle PA. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. International Journal of Cancer. 2002;100:690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- Fan D, Li W, Yang Y, Zhang X, Zhang Q, Yan Y, Yang M, Wang J, Xiong D. Redirection of CD4+ and CD8+ T lymphocytes via an anti-CD3 × anti-CD19 bi-specific antibody combined with cytosine arabinoside and the efficient lysis of patient-derived B-ALL cells. Journal of Hematology & Oncology. 2015;8:108. doi: 10.1186/s13045-015-0205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroudi M, Utzny C, Salio M, Cerundolo V, Guiraud M, Müller S, Valitutti S. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: Manifestation of a dual activation threshold. Proceedings of the National Academy of Sciences. 2003;100:14145–14150. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann A, Arndt C, Töpfer K, Stamova S, Krone F, Cartellieri M, Koristka S, Michalk I, Lindemann D, Schmitz M, Temme A, Bornhäuser M, Ehninger G, Bachmann M. Novel Humanized and Highly Efficient Bispecific Antibodies Mediate Killing of Prostate Stem Cell Antigen-Expressing Tumor Cells by CD8<sup>+</sup> and CD4<sup>+</sup> T Cells. The Journal of Immunology. 2012;189:3249–3259. doi: 10.4049/jimmunol.1200341. [DOI] [PubMed] [Google Scholar]
- Feldmann A, Stamova S, Bippes CC, Bartsch H, Wehner R, Schmitz M, Temme A, Cartellieri M, Bachmann M. Retargeting of T cells to prostate stem cell antigen expressing tumor cells: Comparison of different antibody formats. The Prostate. 2011;71:998–1011. doi: 10.1002/pros.21315. [DOI] [PubMed] [Google Scholar]
- Ferrini S, Cambiaggi A, Sforzini S, Marciano S, Canevari S, Mezzanzanica D, Colnaghi MI, Grossi CE, Moretta L. Targeting of T lymphocytes against egf-receptor+ tumor cells by bispecific monoclonal antibodies: Requirement of CD3 molecule cross-linking for t-cell activation. International Journal of cancer. 1993;55:931–937. doi: 10.1002/ijc.2910550610. [DOI] [PubMed] [Google Scholar]
- Feucht J, Kayser S, Gorodezki D, Hamieh M, Döring M, Blaeschke F, Schlegel P, Bösmüller H, Quintanilla-Fend L, Ebinger M, Lang P, Handgretinger R, Feuchtinger T. T-cell responses against CD19(+) pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7:76902–76919. doi: 10.18632/oncotarget.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DT, Appenheimer MM, Evans SS. The Two Faces of IL-6 in the Tumor Microenvironment. Seminars in immunology. 2014;26:38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M, Henn A, Raum T, Bajtus M, Matthes K, Hendrich L, Wahl J, Hoffmann P, Kischel R, Kvesic M, Slootstra JW, Baeuerle PA, Kufer P, Rattel B. Preclinical Characterization of AMG 330, a CD3/CD33-Bispecific T-Cell–Engaging Antibody with Potential for Treatment of Acute Myelogenous Leukemia. Molecular cancer therapeutics. 2014;13:1549–1557. doi: 10.1158/1535-7163.MCT-13-0956. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Raum T, Lutterbuese R, Voelkel M, Deegen P, Rau D, Kischel R, Hoffmann P, Brandl C, Schuhmacher J, Mueller P, Finnern R, Fuergut M, Zopf D, Slootstra JW, Baeuerle PA, Rattel B, Kufer P. Regression of Human Prostate Cancer Xenografts in Mice by AMG 212/BAY2010112, a Novel PSMA/CD3-Bispecific BiTE Antibody Cross-Reactive with Non-Human Primate Antigens. Molecular Cancer Therapeutics. 2012;11:2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Gall JM, Davol PA, Grabert RC, Deaver M, Lum LG. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B cells and bypass complement-mediated rituximab resistance in vitro. Experimental Hematology. 2005;33:452–459. doi: 10.1016/j.exphem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Gao Y, Xiong D, Yang M, Liu H, Peng H, Shao X, Xu Y, Xu C, Fan D, Qin L, Yang C, Zhu Z. Efficient inhibition of multidrug-resistant human tumors with a recombinant bispecific anti-P-glycoprotein [times] anti-CD3 diabody. Leukemia. 2004;18:513–520. doi: 10.1038/sj.leu.2403267. [DOI] [PubMed] [Google Scholar]
- Gaudet F, Nemeth JF, McDaid R, Li Y, Harman B, Millar H, Teplyakov A, Wheeler J, Luo J, Tam S, Wu SJ, Chen E, Rudnick S, Chu G, Hughes A, Luistro L, Chin D, Babich A, Kalota A, Singh I, Salvati M, Elsayed Y, Attar RM. Development of a CD123xCD3 Bispecific Antibody (JNJ-63709178) for the Treatment of Acute Myeloid Leukemia (AML) Blood. 2016;128:2824–2824. [Google Scholar]
- Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. British journal of cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GM, Tsun A, Stinchcombe JC. The immunological synapse: a focal point for endocytosis and exocytosis. The Journal of Cell Biology. 2010;189:399–406. doi: 10.1083/jcb.201002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbüse R, Schlereth B, Kufer P, Baeuerle PA. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214:441–453. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Han H, Ma J, Zhang K, Li W, Liu C, Zhang Y, Zhang G, Ma P, Wang L, Zhang G, Tao H, Gao B. Bispecific anti‑ CD3 × anti-HER2 antibody mediates T cell cytolytic activity to HER2‑ positive colorectal cancer in vitro and in vivo. International journal of oncology. 2014;45:2446–2454. doi: 10.3892/ijo.2014.2663. [DOI] [PubMed] [Google Scholar]
- Harrington KH, Gudgeon CJ, Laszlo GS, Newhall KJ, Sinclair AM, Frankel SR, Kischel R, Chen G, Walter RB. The Broad Anti-AML Activity of the CD33/CD3 BiTE Antibody Construct, AMG 330, Is Impacted by Disease Stage and Risk. PLOS ONE. 2015;10:e0135945. doi: 10.1371/journal.pone.0135945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Asano R, Tsumoto K, Katayose Y, Suzuki M, Unno M, Kodama H, Takemura S-I, Yoshida H, Makabe K, Imai K, Matsuno S, Kumagai I, Kudo T. A highly effective and stable bispecific diabody for cancer immunotherapy: cure of xenografted tumors by bispecific diabody and T-LAK cells. Cancer Immunology, Immunotherapy. 2004;53:497–509. doi: 10.1007/s00262-003-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, Gao X, Guo L, Yvon E, Hicks J, Liu H, Dotti G, Metelitsa LS. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124:2824–2833. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Hoyos G, Sewell T, Bader R, Bannink J, Chenault RA, Daugherty M, Dasovich M, Fang H, Gottschalk R, Kumer J, Miller RE, Ravikumar P, Wiens J, Algate PA, Bienvenue D, McMahan CJ, Natarajan SK, Gross JA, Blankenship JW. MOR209/ES414, a Novel Bispecific Antibody Targeting PSMA for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Molecular Cancer Therapeutics. 2016;15:2155–2165. doi: 10.1158/1535-7163.MCT-15-0242. [DOI] [PubMed] [Google Scholar]
- Herrmann I, Baeuerle PA, Friedrich M, Murr A, Filusch S, Rüttinger D, Majdoub MW, Sharma S, Kufer P, Raum T, Münz M. Highly Efficient Elimination of Colorectal Tumor-Initiating Cells by an EpCAM/CD3-Bispecific Antibody Engaging Human T Cells. PLoS One. 2010;5:e13474. doi: 10.1371/journal.pone.0013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettich M, Lahoti J, Prasad S, Niedermann G. Checkpoint antibodies but not T cell-recruiting diabodies effectively synergize with TIL-inducing γ-irradiation. Cancer Research. 2016 doi: 10.1158/0008-5472.CAN-15-3451. [DOI] [PubMed] [Google Scholar]
- Hipp S, Tai YT, Blanset D, Deegen P, Wahl J, Thomas O, Rattel B, Adam PJ, Anderson KC, Friedrich M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017 doi: 10.1038/leu.2016.388. [DOI] [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proceedings of the National Academy of Sciences. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, Itin C, Prang N, Baeuerle PA. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. International Journal of Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- Hoffmann SC, Wabnitz GH, Samstag Y, Moldenhauer G, Ludwig T. Functional analysis of bispecific antibody (EpCAMxCD3)-mediated T-lymphocyte and cancer cell interaction by single-cell force spectroscopy. International Journal of cancer. 2011;128:2096–2104. doi: 10.1002/ijc.25556. [DOI] [PubMed] [Google Scholar]
- Hofmeister R, Kohleisen B, LENKKERI-SCHÜTZ U, Itin C, BÄUERLE P, Carr FJ, Hamilton AA, Williams S. Multispecific deimmunized cd3-binders. Google Patents 2005 [Google Scholar]
- Holliger P, Manzke O, Span M, Hawkins R, Fleischmann B, Qinghua L, Wolf J, Diehl V, Cochet O, Winter G, Bohlen H. Carcinoembryonic Antigen (CEA)-specific T-Cell Activation in Colon Carcinoma Induced by Anti-CD3×Anti-CEA Bispecific Diabodies and B7×Anti-CEA Bispecific Fusion Proteins. Cancer Research. 1999;59:2909–2916. [PubMed] [Google Scholar]
- Huang J, Li C, Wang Y, Lv H, Guo Y, Dai H, Wicha MS, Chang AE, Li Q. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133high cancer stem cells in vitro and in vivo. Clinical Immunology. 2013;149:156–168. doi: 10.1016/j.clim.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Huang L, Johnson LS. CD3-Binding Molecules Capable of Binding to Human and Non-Human CD3. Google Patents 2014 [Google Scholar]
- Huarte E, Fisher J, Turk MJ, Mellinger D, Foster C, Wolf B, Meehan KR, Fadul CE, Ernstoff MS. Ex vivo expansion of tumor specific lymphocytes with. Cancer Letters. 2009;285:80–88. doi: 10.1016/j.canlet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro T, Kinoshita Y, Sano Y, Azuma Y, Tsunenari T, Ono N, Kayukawa Y, Kamata-Sakurai M, Shiraiwa H, Kaneko A, Frings W, Komatsu S, Nezu J, Endo M. Abstract DDT01-05: First-in-class T cell-redirecting bispecific antibody targeting glypican-3: a highly tumor-selective antigen. Cancer Research. 2016;76 DDT01-05-DDT01-05. [Google Scholar]
- Iyoda T, Ushida M, Kimura Y, Minamino K, Hayuka A, Yokohata S, Ehara H, Inaba K. Invariant NKT cell anergy is induced by a strong TCR-mediated signal plus co-stimulation. Int Immunol. 2010;22:905–913. doi: 10.1093/intimm/dxq444. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Li J, Johnston J, Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li G, Young J, Luis E, Lewis Phillips G, Stefanich E, Spiess C, Polson A, Irving B, Scheer JM, Junttila MR, Dennis MS, Kelley R, Totpal K, Ebens A. Antitumor Efficacy of a Bispecific Antibody That Targets HER2 and Activates T Cells. Cancer Research. 2014;74:5561–5571. doi: 10.1158/0008-5472.CAN-13-3622-T. [DOI] [PubMed] [Google Scholar]
- Kim CH, Axup JY, Dubrovska A, Kazane SA, Hutchins BA, Wold ED, Smider VV, Schultz PG. Synthesis of Bispecific Antibodies using Genetically Encoded Unnatural Amino Acids. Journal of the American Chemical Society. 2012;134:9918–9921. doi: 10.1021/ja303904e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Von der Lieth CW, Matys ER, Little M. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics1. Journal of molecular biology. 1999;293:41–56. doi: 10.1006/jmbi.1999.3156. [DOI] [PubMed] [Google Scholar]
- Kischel R, Hausmann S, Baeuerle P, Kufer P. Abstract #3252: Effector memory T cells make a major contribution to redirected target cell lysis by T cell-engaging BiTE antibody MT110. Cancer Research. 2009;69:3252–3252. [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discovery Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Kufer P, Lutterbuse R, Kohleisen B, Zeman S, Bauerle P. Pharmaceutical compositions comprising bispecific anti-cd3, anti-cd19 antibody constructs for the treatment of b-cell related disorders. Google Patents 2009 [Google Scholar]
- Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016;8:889–906. doi: 10.2217/imt-2016-0049. [DOI] [PubMed] [Google Scholar]
- Kuo SR, Wong L, Liu JS. Engineering a CD123xCD3 bispecific scFv immunofusion for the treatment of leukemia and elimination of leukemia stem cells. Protein Engineering, Design and Selection. 2012;25:561–570. doi: 10.1093/protein/gzs040. [DOI] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic Inflammation and Cytokines in the Tumor Microenvironment. Journal of Immunology Research. 2014;2014:19. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG 330. Blood Cancer Journal. 2015;5:e340. doi: 10.1038/bcj.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Chu SY, Rashid R, Phung S, Leung IW, Muchhal US, Moore GL, Bernett MJ, Schubbert S, Ardila C, Bonzon C, Foster P, Szymkowski DE, Desjarlais JR. Abstract 3633: Anti-SSTR2 × anti-CD3 bispecific antibody induces potent killing of human tumor cells <em>in vitro</em> and in mice, and stimulates target-dependent T cell activation in monkeys: A potential immunotherapy for neuroendocrine tumors. Cancer Research. 2017;77:3633–3633. [Google Scholar]
- Leong SR, Sukumaran S, Hristopoulos M, Totpal K, Stainton S, Lu E, Wong A, Tam L, Newman R, Vuillemenot BR, Ellerman D, Gu C, Mathieu M, Dennis MS, Nguyen A, Zheng B, Zhang C, Lee G, Chu YW, Prell RA, Lin K, Laing ST, Polson AG. An anti-CD3/anti–CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood. 2017;129:609–618. doi: 10.1182/blood-2016-08-735365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, Hansen-Estruch C, Chamberlain AK, Truhlar SM, Conner EM, Atwell S, Kuhlman B, Demarest SJ. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotech. 2014;32:191–198. doi: 10.1038/nbt.2797. [DOI] [PubMed] [Google Scholar]
- Li J, Stagg NJ, Johnston J, Harris MJ, Menzies SA, DiCara D, Clark V, Hristopoulos M, Cook R, Slaga D, Nakamura R, McCarty L, Sukumaran S, Luis E, Ye Z, Wu TD, Sumiyoshi T, Danilenko D, Lee GY, Totpal K, Ellerman D, Hötzel I, James JR, Junttila TT. Membrane-Proximal Epitope Facilitates Efficient T Cell Synapse Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell. 2017;31:383–395. doi: 10.1016/j.ccell.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, Gavarret J, Bianchi FC, Pumphrey NJ, Ladell K, Gostick E, Sewell AK, Lissin NM, Harwood NE, Molloy PE, Li Y, Cameron BJ, Sami M, Baston EE, Todorov PT, Paston SJ, Dennis RE, Harper JV, Dunn SM, Ashfield R, Johnson A, McGrath Y, Plesa G, June CH, Kalos M, Price DA, Vuidepot A, Williams DD, Sutton DH, Jakobsen BK. Monoclonal TCR-redirected tumor cell killing. Nat Med. 2012;18:980–987. doi: 10.1038/nm.2764. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu Y, Xiang J, Long L, Green S, Yang Z, Zimdahl B, Lu J, Cheng N, Horan LH, Liu B, Yan S, Wang P, Diaz J, Jin L, Nakano Y, Morales JF, Zhang P, Liu L-X, Staley BK, Priceman SJ, Brown CE, Forman SJ, Chan VW, Liu C. Targeting Alpha-Fetoprotein (AFP)–MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clinical Cancer Research. 2017;23:478–488. doi: 10.1158/1078-0432.CCR-16-1203. [DOI] [PubMed] [Google Scholar]
- Liu HY, Zrazhevskiy P, Gao X. Solid-Phase Bioconjugation of Heterobifunctional Adaptors for Versatile Assembly of Bispecific Targeting Ligands. Bioconjugate Chemistry. 2014;25:1511–1516. doi: 10.1021/bc5002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang M, Wang J, Xu Y, Wang Y, Shao X, Yang C, Gao Y, Xiong D. Improvement of tumor targeting and antitumor activity by a disulphide bond stabilized diabody expressed in Escherichia coli. Cancer Immunology, Immunotherapy. 2009;58:1761–1769. doi: 10.1007/s00262-009-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Lam CYK, Long V, Widjaja L, Yang Y, Li H, Jin L, Burke S, Gorlatov S, Brown J, Alderson R, Lewis MD, Nordstrom JL, Koenig S, Moore PA, Johnson S, Bonvini E. MGD011, A CD19 × CD3 Dual-Affinity Retargeting Bi-specific Molecule Incorporating Extended Circulating Half-life for the Treatment of B-Cell Malignancies. Clinical Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-0666. [DOI] [PubMed] [Google Scholar]
- Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmüller G, Dörken B, Bargou RC. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- Lopez-Albaitero A, Xu H, Guo H, Wang L, Wu Z, Tran H, Chandarlapaty S, Scaltriti M, Janjigian Y, de Stanchina E, Cheung NKV. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. OncoImmunology. 2017;6:e1267891. doi: 10.1080/2162402X.2016.1267891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Chen GJ, Tai PH, Yang YC, Hsu YS, Chang M, Hsu CL. Tetravalent anti-CD20/CD3 bispecific antibody for the treatment of B cell lymphoma. Biochemical and biophysical research communications. 2016;473:808–813. doi: 10.1016/j.bbrc.2016.03.124. [DOI] [PubMed] [Google Scholar]
- Luiten RM, Coney LR, Fleuren GJ, Warnaar SO, Litvinov SV. Generation of chimeric bispecific G250/anti-CD3 monoclonal antibody, a tool to combat renal cell carcinoma. British journal of cancer. 1996;74:735–744. doi: 10.1038/bjc.1996.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum LG, Ramesh M, Thakur A, Mitra S, Deol A, Uberti JP, Pellett PE. Targeting Cytomegalovirus-Infected Cells Using T Cells Armed with Anti-CD3 × Anti-CMV Bispecific Antibody. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18:1012–1022. doi: 10.1016/j.bbmt.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubchenko TA, Wurth GA, Zweifach A. Role of Calcium Influx in Cytotoxic T Lymphocyte Lytic Granule Exocytosis during Target Cell Killing. Immunity. 2001;15:847–859. doi: 10.1016/s1074-7613(01)00233-3. [DOI] [PubMed] [Google Scholar]
- Shalaby MR, S HM, Presta L, Rodrigues ML, Beverley PC, Feldmann M, Carter P. Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. The Journal of Experimental Medicine. 1992;175:217–225. doi: 10.1084/jem.175.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M, Gruber R, Schmidt S, Riethmüller G, Kufer P. Biologic properties of a bispecific single-chain antibody directed against 17-1A (EpCAM) and CD3: tumor cell-dependent T cell stimulation and cytotoxic activity. The Journal of Immunology. 1997;158:3965–3970. [PubMed] [Google Scholar]
- Matsuzaki J, Tsuji T, Luescher IF, Shiku H, Mineno J, Okamoto S, Old LJ, Shrikant P, Gnjatic S, Odunsi K. Direct tumor recognition by a human CD4+ T-cell subset potently mediates tumor growth inhibition and orchestrates anti-tumor immune responses. Sci Rep. 2015;5:14896. doi: 10.1038/srep14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau-Sørensen M, Dittrich C, Dienstmann R, Lassen U, Büchler W, Martinius H, Tabernero J. A phase I trial of intravenous catumaxomab: a bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Cancer Chemotherapy and Pharmacology. 2015;75:1065–1073. doi: 10.1007/s00280-015-2728-5. [DOI] [PubMed] [Google Scholar]
- McCormack E, Adams KJ, Hassan NJ, Kotian A, Lissin NM, Sami M, Mujić M, Osdal T, Gjertsen BT, Baker D, Powlesland AS, Aleksic M, Vuidepot A, Morteau O, Sutton DH, June CH, Kalos M, Ashfield R, Jakobsen BK. Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunology, Immunotherapy. 2013;62:773–785. doi: 10.1007/s00262-012-1384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanzanica D, Garrido MA, Neblock DS, Daddona PE, Andrew SM, Zurawski VR, Segal DM, Wunderlich JR. Human T-Lymphocytes Targeted against an Established Human Ovarian Carcinoma with a Bispecific F(ab′)<sub>2</sub> Antibody Prolong Host Survival in a Murine Xenograft Model. Cancer Research. 1991;51:5716–5721. [PubMed] [Google Scholar]
- Mølhøj M, Crommer S, Brischwein K, Rau D, Sriskandarajah M, Hoffmann P, Kufer P, Hofmeister R, Baeuerle PA. CD19−/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Molecular Immunology. 2007;44:1935–1943. doi: 10.1016/j.molimm.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Moore GL, Bautista C, Pong E, Nguyen DHT, Jacinto J, Eivazi A, Muchhal US, Karki S, Chu SY, Lazar GA. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs. 2011;3:546–557. doi: 10.4161/mabs.3.6.18123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GL, Lee SH, Schubbert S, Miranda Y, Rashid R, Pong E, Phung S, Chan EW, Chen H, Endo N, Ardila MC, Bernett MJ, Chu S, Leung IWL, Muchhal U, Bonzon C, Szymkowski DE, Desjarlais J. Tuning T Cell Affinity Improves Efficacy and Safety of Anti-CD38 × Anti-CD3 Bispecific Antibodies in Monkeys – a Potential Therapy for Multiple Myeloma. Blood. 2015;126:1798–1798. [Google Scholar]
- Moore PA, Alderson R, Shah K, Yang Y, Burke S, Li H, Ciccarone V, Bonvini E, Johnson S. Abstract 669: Development of MGD007, a gpA33 × CD3 bi-specific DART for T-cell immunotherapy of metastatic colorectal cancer. Cancer Research. 2014;74:669–669. [Google Scholar]
- Moore PA, Zhang W, Rainey GJ, Burke S, Li H, Huang L, Gorlatov S, Veri MC, Aggarwal S, Yang Y, Shah K, Jin L, Zhang S, He L, Zhang T, Ciccarone V, Koenig S, Bonvini E, Johnson S. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117:4542–4551. doi: 10.1182/blood-2010-09-306449. [DOI] [PubMed] [Google Scholar]
- Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- Negri DR, Tosi E, Valota O, Ferrini S, Cambiaggi A, Sforzini S, Silvani A, Ruffini PA, Colnaghi MI, Canevari S. In vitro and in vivo stability and anti-tumour efficacy of an anti-EGFR/anti-CD3 F(ab’)2 bispecific monoclonal antibody. British journal of cancer. 1995;72:928–933. doi: 10.1038/bjc.1995.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HH, Kim T, Song SY, Park S, Cho HH, Jung SH, Ahn JS, Kim HJ, Lee JJ, Kim HO, Cho JH, Yang DH. Naïve CD8+ T cell derived tumor-specific cytotoxic effectors as a potential remedy for overcoming TGF-β immunosuppression in the tumor microenvironment. Sci Rep. 2016;6:28208. doi: 10.1038/srep28208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A, R M. Recombination of a mixture of univalent antibody fragments of different specificity. Archives of Biochemistry and biophysics. 1961:460–462. doi: 10.1016/0003-9861(61)90296-x. [DOI] [PubMed] [Google Scholar]
- Nisonoff A, Wissler FC, Lipman LN. Properties of the Major Component of a Peptic Digest of Rabbit Antibody. Science. 1960;132:1770–1771. doi: 10.1126/science.132.3441.1770. [DOI] [PubMed] [Google Scholar]
- Nolz JC. Molecular mechanisms of CD8+ T cell trafficking and localization. Cellular and Molecular Life Sciences. 2015;72:2461–2473. doi: 10.1007/s00018-015-1835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst MD, Fuhrmann S, Mulgrew K, Amann M, Cheng L, Lutterbuese P, Richman L, Coats S, Baeuerle PA, Hammond SA. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs. 2014;6:1571–1584. doi: 10.4161/19420862.2014.975660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M, Uchiyama S, Robinson CV, Fukui K, Kobayashi Y, Azuma T. Regional and segmental flexibility of antibodies in interaction with antigens of different size. FEBS Journal. 2006;273:1476–1487. doi: 10.1111/j.1742-4658.2006.05168.x. [DOI] [PubMed] [Google Scholar]
- Oelkrug C, Ramage JM. Enhancement of T cell recruitment and infiltration into tumours. Clinical & Experimental Immunology. 2014;178:1–8. doi: 10.1111/cei.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Molecular Immunology. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Osada T, Patel SP, Hammond SA, Osada K, Morse MA, Lyerly HK. CEA/CD3-bispecific T cell-engaging (BiTE) antibody-mediated T lymphocyte cytotoxicity maximized by inhibition of both PD1 and PD-L1. Cancer Immunology, Immunotherapy. 2015;64:677–688. doi: 10.1007/s00262-015-1671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshimi K, Seto T, Oshimi Y, Masuda M, Okumura K, Mizoguchi H. Increased lysis of patient CD10-positive leukemic cells by T cells coated with anti-CD3 Fab’ antibody cross-linked to anti-CD10 Fab’ antibody. Blood. 1991;77:1044–1049. [PubMed] [Google Scholar]
- Pegu A, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, Guo X, Shi W, Georgiev I, Zhou T, Chen X, O’Dell S, Todd JP, Kwong PD, Rao SS, Yang Z-Y, Koup RA, Mascola JR, Nabel GJ. Activation and lysis of human CD4 cells latently infected with HIV-1. Nature Communications. 2015;6:8447. doi: 10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985;316:354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, Cooper A, Hataye J, Andrews S, Ambrozak D, Del Río Estrada PM, Boritz E, Paris R, Moysi E, Boswell KL, Ruiz-Mateos E, Vagios I, Leal M, Ablanedo-Terrazas Y, Rivero A, Gonzalez-Hernandez LA, McDermott AB, Moir S, Reyes-Terán G, Docobo F, Pantaleo G, Douek DC, Betts MR, Estes JD, Germain RN, Mascola JR, Koup RA. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Science translational medicine. 2017;9 doi: 10.1126/scitranslmed.aag2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfosser A, Brandl M, Salih H, Grosse-Hovest L, Jung G. Role of target antigen in bispecific-antibody-mediated killing of human glioblastoma cells: A pre-clinical study. International Journal of cancer. 1999;80:612–616. doi: 10.1002/(sici)1097-0215(19990209)80:4<612::aid-ijc21>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Plückthun A, Pack P. New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnology. 1997;3:83–105. doi: 10.1016/s1380-2933(97)00067-5. [DOI] [PubMed] [Google Scholar]
- Pohl C, Denfeld R, Renner C, Jung W, Bohlen H, Sahin U, Hombach A, van Lier R, Schwonzen M, Diehl V, Pfreundschuh M. CD30-antigen-specific targeting and activation of T cells via murine bispecific monoclonal antibodies against CD3 and CD28: Potential use for the treatment of hodgkin’s lymphoma. International Journal of cancer. 1993;54:820–827. doi: 10.1002/ijc.2910540517. [DOI] [PubMed] [Google Scholar]
- Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4<sup>+</sup> T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. The Journal of Experimental Medicine. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss NS, Schulman AD, Choi S-H, Rodgers DT, Kazane SA, Kim CH, Lawson BR, Young TS. An Anti-B Cell Maturation Antigen Bispecific Antibody for Multiple Myeloma. Journal of the American Chemical Society. 2015;137:5288–5291. doi: 10.1021/jacs.5b01876. [DOI] [PubMed] [Google Scholar]
- Ren-Heidenreich L, Davol PA, Kouttab NM, Elfenbein GJ, Lum LG. Redirected T-cell cytotoxicity to epithelial cell adhesion molecule-overexpressing adenocarcinomas by a novel recombinant antibody, E3Bi, in vitro and in an animal model. Cancer. 2004;100:1095–1103. doi: 10.1002/cncr.20060. [DOI] [PubMed] [Google Scholar]
- Renner C, Pfreundschuh M. Treatment of Heterotransplanted Hodgkin’s Tumors in SCID Mice by a Combination of Human NK or T Cells and Bispecific Antibodies. Journal of Hematotherapy. 1995;4:447–451. doi: 10.1089/scd.1.1995.4.447. [DOI] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger E, Meyer V, Kreyenberg H, Volk A, Kuçi S, Willasch A, Koscielniak E, Fulda S, Wels WS, Boenig H, Klingebiel T, Bader P. Cytotoxic Capacity of IL-15-Stimulated Cytokine-Induced Killer Cells Against Human Acute Myeloid Leukemia and Rhabdomyosarcoma in Humanized Preclinical Mouse Models. Frontiers in oncology. 2012;2:32. doi: 10.3389/fonc.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss SHJ, Fucek I, Eser M, McAleese F, Molkenthin V, Le Gall F, Topp M, Little M, Zhukovsky EA. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. MAbs. 2015;7:584–604. doi: 10.1080/19420862.2015.1029216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch U, Harrington K, Gudgeon C, Fucek I, Ellwanger K, Weichel M, Knackmuss S, Zhukovsky E, Fox JA, Kunkel LA, Guenot J, Walter RB. Characterization of CD33/CD3 Tetravalent Bispecific Tandem Diabodies (TandAbs) for the Treatment of Acute Myeloid Leukemia. Clinical Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-0350. [DOI] [PubMed] [Google Scholar]
- Reusch U, Sundaram M, Davol PA, Olson SD, Davis JB, Demel K, Nissim J, Rathore R, Liu PY, Lum LG. Anti-CD3 × Anti-Epidermal Growth Factor Receptor (EGFR) Bispecific Antibody Redirects T-Cell Cytolytic Activity to EGFR-Positive Cancers <em>In vitro</em> and in an Animal Model. Clinical Cancer Research. 2006;12:183–190. doi: 10.1158/1078-0432.CCR-05-1855. [DOI] [PubMed] [Google Scholar]
- Riethmüller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immunity. 2012;12:12. [PMC free article] [PubMed] [Google Scholar]
- Root A, Cao W, Li B, LaPan P, Meade C, Sanford J, Jin M, O’Sullivan C, Cummins E, Lambert M, Sheehan A, Ma W, Gatto S, Kerns K, Lam K, D’Antona A, Zhu L, Brady W, Benard S, King A, He T, Racie L, Arai M, Barrett D, Stochaj W, LaVallie E, Apgar J, Svenson K, Mosyak L, Yang Y, Chichili G, Liu L, Li H, Burke S, Johnson S, Alderson R, Finlay W, Lin L, Olland S, Somers W, Bonvini E, Gerber HP, May C, Moore P, Tchistiakova L, Bloom L. Development of PF-06671008, a Highly Potent Anti-P-cadherin/Anti-CD3 Bispecific DART Molecule with Extended Half-Life for the Treatment of Cancer. Antibodies. 2016;5:6. doi: 10.3390/antib5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DL, Rossi EA, Cardillo TM, Goldenberg DM, Chang CH. A new class of bispecific antibodies to redirect T cells for cancer immunotherapy. MAbs. 2014;6:381–391. doi: 10.4161/mabs.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA, Rossi DL, Cardillo TM, Chang CH, Goldenberg DM. Redirected T-Cell Killing of Solid Cancers Targeted with an Anti-CD3/Trop-2–Bispecific Antibody Is Enhanced in Combination with Interferon-α. Molecular cancer therapeutics. 2014;13:2341–2351. doi: 10.1158/1535-7163.MCT-14-0345. [DOI] [PubMed] [Google Scholar]
- Ruf P, Jäger M, Ellwart J, Wosch S, Kusterer E, Lindhofer H. Two new trifunctional antibodies for the therapy of human malignant melanoma. International Journal of cancer. 2004;108:725–732. doi: 10.1002/ijc.11630. [DOI] [PubMed] [Google Scholar]
- Salnikov AV, Groth A, Apel A, Kallifatidis G, Beckermann BM, Khamidjanov A, Ryschich E, Büchler MW, Herr I, Moldenhauer G. Targeting of cancer stem cell marker EpCAM by bispecific antibody EpCAMxCD3 inhibits pancreatic carcinoma. Journal of Cellular and Molecular Medicine. 2009;13:4023–4033. doi: 10.1111/j.1582-4934.2009.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffold C, Kornacker M, Scheffold YC, Contag CH, Negrin RS. Visualization of Effective Tumor Targeting by CD8+ Natural Killer T Cells Redirected with Bispecific Antibody F(ab′)<sub>2</sub>HER2xCD3. Cancer Research. 2002;62:5785–5791. [PubMed] [Google Scholar]
- Sebastian M, Passlick B, Friccius-Quecke H, Jäger M, Lindhofer H, Kanniess F, Wiewrodt R, Thiel E, Buhl R, Schmittel A. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM × anti-CD3): a phase I study. Cancer Immunology, Immunotherapy. 2007;56:1637–1644. doi: 10.1007/s00262-007-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, Lipp S, Merino J, Prosper F, Emde M, Delon C, Latzko M, Gianotti R, Lüoend R, Murr R, Hosse RJ, Harnisch LJ, Bacac M, Fauti T, Klein C, Zabaleta A, Hillengass J, Cavalcanti-Adam EA, Ho AD, Hundemer M, San Miguel JF, Strein K, Umaña P, Hose D, Paiva B, Vu MD. Target Expression, Generation, Preclinical Activity, and Pharmacokinetics of the BCMA-T Cell Bispecific Antibody EM801 for Multiple Myeloma Treatment. Cancer Cell. 2017;31:396–410. doi: 10.1016/j.ccell.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Sen M, Wankowski DM, Garlie NK, Siebenlist RE, Van Epps D, LeFever AV, Lum LG. Use of Anti-CD3 × Anti-HER2/neu Bispecific Antibody for Redirecting Cytotoxicity of Activated T Cells Toward HER2/neu+ Tumors. Journal of Hematotherapy & Stem Cell Research. 2001;10:247–260. doi: 10.1089/15258160151134944. [DOI] [PubMed] [Google Scholar]
- Shen GL, Li JL, Vitetta ES. Bispecific anti-CD22/anti-CD3-ricin A chain immunotoxin is cytotoxic to Daudi lymphoma cells but not T cells in vitro and shows both A-chain-mediated and LAK-T-mediated killing. The Journal of Immunology. 1994;152:2368–2376. [PubMed] [Google Scholar]
- Siddiqui I, Erreni M, van Brakel M, Debets R, Allavena P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into Fractalkine/CX3CL1 expressing tumors: importance of the chemokine gradient. J Immunother Cancer. 2016;4:21. doi: 10.1186/s40425-016-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Okern G, Rehan S, Beagley L, Lee SK, Aarvak T, Schjetne KW, Khanna R. Ex vivo expansion of human T cells for adoptive immunotherapy using the novel Xeno-free CTS Immune Cell Serum Replacement. Clin Trans Immunol. 2015;4:e31. doi: 10.1038/cti.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD, Oyejide A, Kirshner JR, Canova L, Menon J, Principio J, MacDonald D, Kantrowitz J, Papadopoulos N, Stahl N, Yancopoulos GD, Thurston G, Davis S. A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep. 2015;5:17943. doi: 10.1038/srep17943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Molecular Immunology. 2015;67:95–106. doi: 10.1016/j.molimm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383–391. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler CR, Bähr-Mahmud H, Plum LM, Schmoldt K, Kölsch AC, Türeci Ö, Sahin U. Characterization of the first-in-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6. OncoImmunology. 2016;5:e1091555. doi: 10.1080/2162402X.2015.1091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]
- Stamova S, Cartellieri M, Feldmann A, Arndt C, Koristka S, Bartsch H, Bippes CC, Wehner R, Schmitz M, von Bonin M, Bornhäuser M, Ehninger G, Bachmann M. Unexpected recombinations in single chain bispecific anti-CD3–anti-CD33 antibodies can be avoided by a novel linker module. Molecular Immunology. 2011;49:474–482. doi: 10.1016/j.molimm.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Stanglmaier M, Faltin M, Ruf P, Bodenhausen A, Schröder P, Lindhofer H. Bi20 (fBTA05), a novel trifunctional bispecific antibody (anti-CD20 × anti-CD3), mediates efficient killing of B-cell lymphoma cells even with very low CD20 expression levels. International Journal of cancer. 2008;123:1181–1189. doi: 10.1002/ijc.23626. [DOI] [PubMed] [Google Scholar]
- Stel AJ, Kroesen BJ, Jacobs S, Groen H, de Leij LFMH, Kluin-Nelemans HC, Withoff S. The Role of B Cell-Mediated T Cell Costimulation in the Efficacy of the T Cell Retargeting Bispecific Antibody BIS20×3. The Journal of Immunology. 2004;173:6009–6016. doi: 10.4049/jimmunol.173.10.6009. [DOI] [PubMed] [Google Scholar]
- Ströhlein MA, Lefering R, Bulian DR, Heiss MM. Relative lymphocyte count is a prognostic parameter in cancer patients with catumaxomab immunotherapy. Med Hypotheses. 2014;82:295–299. doi: 10.1016/j.mehy.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E, Mai E, Young J, Johnson C, Huseni M, Wang X, Chen Y, Wang P, Wang H, Dybdal N, Chu YW, Chiorazzi N, Scheer JM, Junttila T, Totpal K, Dennis MS, Ebens AJ. Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Science translational medicine. 2015;7:287ra270–287ra270. doi: 10.1126/scitranslmed.aaa4802. [DOI] [PubMed] [Google Scholar]
- Sun LL, Wang P, Clark R, Hristopoulos M, Ellerman D, Mathieu M, Chu YW, Wang H, Totpal K, Ebens AJ, Polson AG, Gould S. Preclinical Characterization of Combinability and Potential Synergy of Anti-CD20/CD3 T-Cell Dependent Bispecific Antibody with Chemotherapy and PD-1/PD-L1 Blockade. Blood. 2016;128:4168–4168. [Google Scholar]
- Sung JAM, Pickeral J, Liu L, Stanfield-Oakley SA, Lam CYK, Garrido C, Pollara J, LaBranche C, Bonsignori M, Moody MA, Yang Y, Parks R, Archin N, Allard B, Kirchherr J, Kuruc JD, Gay CL, Cohen MS, Ochsenbauer C, Soderberg K, Liao HX, Montefiori D, Moore P, Johnson S, Koenig S, Haynes BF, Nordstrom JL, Margolis DM, Ferrari G. Dual-Affinity Re-Targeting proteins direct T cell–mediated cytolysis of latently HIV-infected cells. The Journal of clinical investigation. 2015;125:4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S-I, Kudo T, Asano R, Suzuki M, Tsumoto K, Sakurai N, Katayose Y, Kodama H, Yoshida H, Ebara S, Saeki H, Imai K, Matsuno S, Kumagai I. A mutated superantigen SEA D227A fusion diabody specific to MUC1 and CD3 in targeted cancer immunotherapy for bile duct carcinoma. Cancer Immunology, Immunotherapy. 2002;51:33–44. doi: 10.1007/s00262-001-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki S, Kamada H, Inoue M, Nagano K, Mukai Y, Higashisaka K, Yoshioka Y, Tsutsumi Y, Tsunoda S-I. A Novel Bispecific Antibody against Human CD3 and Ephrin Receptor A10 for Breast Cancer Therapy. PLoS One. 2015;10:e0144712. doi: 10.1371/journal.pone.0144712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M, Berg RA, Fitzgerald JC, Aplenc R, Gore L, Grupp SA. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, Goping IS, Bleackley RC, Kirchhausen T, Lieberman J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12:770–777. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tita-Nwa F, Moldenhauer G, Herbst M, Kleist C, Ho AD, Kornacker M. Cytokine-induced killer cells targeted by the novel bispecific antibody CD19xCD5 (HD37xT5.16) efficiently lyse B-lymphoma cells. Cancer Immunology, Immunotherapy. 2007;56:1911–1920. doi: 10.1007/s00262-007-0333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisu-Itakura H, Schoellhammer HF, Sim MS, Irie RF, Hausmann S, Raum T, Baeuerle PA, Morton DL. Redirected Lysis of Human Melanoma Cells by a MCSP/CD3-bispecific BiTE Antibody that Engages Patient-derived T Cells. Journal of immunotherapy (Hagerstown, Md: 1997) 2011;34:597–605. doi: 10.1097/CJI.0b013e3182307fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunecker A, Lanzavecchia A, Karjalainen K. Bispecific single chain molecules (Janusins) target cytotoxic lymphocytes on HIV infected cells. The EMBO Journal. 1991;10:3655–3659. doi: 10.1002/j.1460-2075.1991.tb04932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Nakamura Y, Masuko T, Hashimoto Y, Habu S, Nishimura T. Specific targeting of in vitro-activated human antitumour effector cells using anti-CD3 [times] anti-c-erbB-2 bispecific antibody. Immunol Cell Biol. 1993;71:109–115. doi: 10.1038/icb.1993.11. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Coombs D, Dupré L. The space and time frames of T cell activation at the immunological synapse. FEBS Letters. 2010;584:4851–4857. doi: 10.1016/j.febslet.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Van Loo PF, Doornbos R, Dolstra H, Shamsili S, Bakker L. Preclinical Evaluation of MCLA117, a CLEC12AxCD3 Bispecific Antibody Efficiently Targeting a Novel Leukemic Stem Cell Associated Antigen in AML. Blood. 2015;126:325–325. [Google Scholar]
- Vidarsson G, Dekkers G, Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, He Y, Zhang G, Ma J, Liu C, He W, Wang W, Han H, Boruah BM, Gao B. Retargeting T Cells for HER2-Positive Tumor Killing by a Bispecific Fv-Fc Antibody. PLoS One. 2013;8:e75589. doi: 10.1371/journal.pone.0075589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Asano R, Arai K, Shimomura I, Ogata H, Kawaguchi H, Hayashi H, Ohtsuka H, Yoshida H, Katayose Y, Egawa S, Nakanishi T, Umetsu M, Yasui H, Ishida T, Imai K, Kudo T, Unno M, Kumagai I. In vitro and in vivo antitumor effects of recombinant bispecific antibodies based on humanized anti-EGFR antibody. Oncology Reports. 2011;26:949–955. doi: 10.3892/or.2011.1382. [DOI] [PubMed] [Google Scholar]
- WHO. List 111. World Health Organization; 2014. Proposed International Nonproprietary Names; pp. 251–252. (Vol. WHO Drug Information). [Google Scholar]
- Wild MK, Strittmatter W, Matzku S, Schraven B, Meuer SC. Tumor Therapy with Bispecific Antibody: The Targeting and Triggering Steps Can Be Separated Employing a CD2-Based Strategy. The Journal of Immunology. 1999;163:2064–2072. [PubMed] [Google Scholar]
- Wozniak-Knopp G, Bartl S, Bauer A, Mostageer M, Woisetschläger M, Antes B, Ettl K, Kainer M, Weberhofer G, Wiederkum S, Himmler G, Mudde GC, Rüker F. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Engineering, Design and Selection. 2010;23:289–297. doi: 10.1093/protein/gzq005. [DOI] [PubMed] [Google Scholar]
- Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M. CTLA-4 Blockade Expands Infiltrating T Cells and Inhibits Cancer Cell Repopulation during the Intervals of Chemotherapy in Murine Mesothelioma. Molecular cancer therapeutics. 2012;11:1809–1819. doi: 10.1158/1535-7163.MCT-11-1014. [DOI] [PubMed] [Google Scholar]
- Wu X, Sereno AJ, Huang F, Lewis SM, Lieu RL, Weldon C, Torres C, Fine C, Batt MA, Fitchett JR, Glasebrook AL, Kuhlman B, Demarest SJ. Fab-based bispecific antibody formats with robust biophysical properties and biological activity. MAbs. 2015;7:470–482. doi: 10.1080/19420862.2015.1022694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuellner U, Klupsch K, Buller F, Attinger-Toller I, Santimaria R, Zbinden I, Henne P, Grabulovski D, Bertschinger J, Brack S. Bispecific CD3/HER2 Targeting FynomAb Induces Redirected T Cell-Mediated Cytolysis with High Potency and Enhanced Tumor Selectivity. Antibodies. 2015;4:426. [Google Scholar]
- Xiong D, Xu Y, Liu H, Peng H, Shao X, Lai Z, Fan D, Yang M, Han J, Xie Y, Yang C, Zhu Z. Efficient inhibition of human B-cell lymphoma xenografts with an anti-CD20×anti-CD3 bispecific diabody. Cancer Letters. 2002;177:29–39. doi: 10.1016/s0304-3835(01)00758-3. [DOI] [PubMed] [Google Scholar]
- Xu H, Cheng M, Guo H, Chen Y, Huse M, Cheung NKV. Retargeting T cells to GD2 pentasaccharide on human tumors using bispecific humanized antibody. Cancer Immunol Res. 2015;3:266–277. doi: 10.1158/2326-6066.CIR-14-0230-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lee J, Tran C, Heibeck TH, Wang WD, Yang J, Stafford RL, Steiner AR, Sato AK, Hallam TJ, Yin G. Production of bispecific antibodies in “knobs-into-holes” using a cell-free expression system. MAbs. 2015;7:231–242. doi: 10.4161/19420862.2015.989013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Trad A, Baumgart A, Hüske L, Lorenzen I, Chalaris A, Grötzinger J, Dechow T, Scheller J, Rose-John S. A novel bispecific single-chain antibody for ADAM17 and CD3 induces T-cell-mediated lysis of prostate cancer cells. Biochemical Journal. 2012;445:135–144. doi: 10.1042/BJ20120433. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kobayashi T, Matsuoka H, Seki C, Gosnell WL, Chang SP, Ishii A. T-cell activation and cytokine production via a bispecific single-chain antibody fragment targeted to blood-stage malaria parasites. Blood. 2003;101:2300–2306. doi: 10.1182/blood-2002-03-0831. [DOI] [PubMed] [Google Scholar]
- Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, Lindhofer H. Simultaneous Activation of T Cells and Accessory Cells by a New Class of Intact Bispecific Antibody Results in Efficient Tumor Cell Killing. The Journal of Immunology. 1999;163:1246–1252. [PubMed] [Google Scholar]
- Zhang P, Shi B, Gao H, Jiang H, Kong J, Yan J, Pan X, Li K, Zhang P, Yao M, Yang S, Gu J, Wang H, Li Z. An EpCAM/CD3 bispecific antibody efficiently eliminates hepatocellular carcinoma cells with limited galectin-1 expression. Cancer Immunology, Immunotherapy. 2014;63:121–132. doi: 10.1007/s00262-013-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yudan Y, Zhou Pengfei, Ma Hong, Zhao Xiaolai, He Xin, Wang Tao, Zhang Jing, Liu Yang, Zhang Tao. Targeting CD133high Colorectal Cancer Cells In Vitro and In Vivo With an Asymmetric Bispecific Antibody. Journal of Immunotherapy. 2015;38:217–228. doi: 10.1097/CJI.0000000000000086. [DOI] [PubMed] [Google Scholar]
- Zhou J, Chen J, Zhong R, Mokotoff M, Shultz LD, Ball ED. Targeting Gastrin-Releasing Peptide Receptors on Small Cell Lung Cancer Cells with a Bispecific Molecule that Activates Polyclonal T Lymphocytes. Clinical Cancer Research. 2006;12:2224–2231. doi: 10.1158/1078-0432.CCR-05-1524. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang L, Liu R, Flutter B, Li S, Ding J, Tao H, Liu C, Sun M, Gao B. COMBODY: one-domain antibody multimer with improved avidity. Immunol Cell Biol. 2010;88:667–675. doi: 10.1038/icb.2010.21. [DOI] [PubMed] [Google Scholar]
- Zou J, Chen D, Zong Y, Ye S, Tang J, Meng H, An G, Zhang X, Yang L. Immunotherapy based on bispecific T-cell engager with hIgG1 Fc sequence as a new therapeutic strategy in multiple myeloma. Cancer Science. 2015;106:512–521. doi: 10.1111/cas.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8:328rv324–328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]


