Abstract
Epidemiologic studies report improved breast cancer survival in women who receive ketorolac (Toradol) for postoperative pain relief compared with other analgesic agents. Ketorolac is a racemic drug. The S-enantiomer inhibits cyclooxygenases; R-ketorolac is a selective inhibitor of the small GTPases Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell division control protein 42 (Cdc42), which are signaling molecules up-regulated during breast cancer progression and metastasis. The goal of this study was to determine whether R-ketorolac altered breast cancer development in the mouse mammary tumor virus-polyoma middle T-antigen model. Mice were administered ketorolac orally at 1 mg/kg twice daily to approximate the typical human dose. Mammary glands were analyzed for tumor number and immunohistochemical markers of proliferation and differentiation. R-ketorolac treatment significantly reduced mammary epithelial proliferation, based on Ki67 staining, and suppressed tumor development. Proliferative mammary epithelium from R-ketorolac–treated mice displayed greater differentiation, based on significantly higher total E-cadherin and decreased keratin 5 staining than epithelium of placebo-treated mice. No differences were detected in estrogen receptor, progesterone receptor, β-catenin, or vimentin expression between placebo and R-ketorolac treatment groups. These findings indicate that R-ketorolac treatment slows tumor progression in an aggressive model of breast cancer. R-ketorolac may thus represent a novel therapeutic approach for breast cancer prevention or treatment based on its pharmacologic activity as a Rac1 and Cdc42 inhibitor.
Epidemiologic evidence indicates that women who receive ketorolac (Toradol) for pain relief after breast cancer surgery have improved survival, although the basis for this effect has not been determined.1, 2, 3, 4, 5 Ketorolac is produced and administered as a 1:1 racemic mix of the R- and S-enantiomers. The S-form is a nonsteroidal anti-inflammatory drug that inhibits cyclooxygenase (COX) enzymes, whereas the R-form has little or no COX inhibitory activity.6, 7, 8, 9 However, evidence is mounting that R-enantiomers of certain nonsteroidal anti-inflammatory drugs are distinct chemical entities with pharmacologic activities against novel non-COX targets.10, 11, 12, 13 We previously reported that the R-enantiomer of ketorolac is an allosteric inhibitor of the Rac1 [Ras homolog (Rho)-family small GTPases Ras-related C3 botulinum toxin substrate 1] and Cdc42 (cell division control protein 42) GTPases.13 R-ketorolac is a robust inhibitor of growth factor or serum activation of Cdc42 and Rac1, with a potency and efficacy similar to the small-molecule Cdc42 and Rac1 inhibitors, CID2950007/ML141 and NSC23766, respectively.13, 14 Furthermore, R-ketorolac, but not the S-enantiomer, inhibited Rac1- and Cdc42-dependent downstream signaling, growth factor-stimulated actin cytoskeleton rearrangements, cell adhesion, migration, and invasion in tumor cells.13, 14
In many human cancers, aberrant Rho-family signaling because of changes in GTPase expression or activity is a critical contributor to tumor development and progression.15, 16, 17, 18, 19, 20, 21, 22 Rac and Cdc42 GTPases regulate cytoskeletal dynamics important in adhesion, migration, and invasion and other cancer-relevant functions such as gene transcription, cell cycle progression, cell survival, and transformation. Several studies have demonstrated increased Rac1 and Cdc42 activity in cancer by mechanisms that include GTPase overexpression, activating mutations, changes in expression or activity of proteins that control Rho-family GTPase activation, or changes in activity of GTPase effector molecules.15, 23, 24, 25, 26, 27 Furthermore, Rac1 and Cdc42 knockdown or inhibition have been reported to decrease breast cancer cell growth, migration, and invasion; enhance sensitivity to ionizing radiation; and restore sensitivity to therapeutic agents such as trastuzumab and tamoxifen.28, 29, 30, 31, 32, 33, 34, 35, 36 These findings suggest that Rac1 and Cdc42 may be promising targets for clinical intervention in breast cancer.37
Given the evidence that activated Rac1 and Cdc42 are negative prognostic indicators in breast cancer and that ketorolac treatment may prolong survival after surgery for breast cancer, therapeutic targeting of these proteins by R-ketorolac may lead to a novel approach for breast cancer treatment. To test this hypothesis, we examined the effects of the dual Rac1 and Cdc42 inhibitor R-ketorolac on tumor progression with the use of the mouse mammary tumor virus-polyoma middle T-antigen (MMTV-PyMT) model of breast cancer.38 This genetically engineered model was selected because of its reported similarities to human disease.38 With the use of this model, we demonstrated that chronic treatment with R-ketorolac reduced proliferation of mammary epithelium, delayed mammary tumor development, and slowed mammary lesion progression.
Materials and Methods
Oral Dosage Preparation
Racemic ketorolac-tris salt was purchased from Sigma-Aldrich (St. Louis, MO), and individual R-enantiomer was purchased from Toronto Research Chemicals (Toronto, ON, Canada). As an alternative to gavage for oral delivery, the drug was administered in pills formed from ketorolac powder mixed into transgenic dough (BioServ, Flemington, NJ).39 Ketorolac, as racemic compound or individual enantiomer, was stable for at least 3 months as determined by high-performance liquid chromatography.
Animal Model
FVB/N-Tg(MMTV-PyVT)634Mul/J mice, hereafter referred to as MMTV-PyMT mice, were originally obtained from The Jackson Laboratory (Bar Harbor, ME). The colony was maintained by the University of New Mexico Comprehensive Cancer Center Small Animal Models and Imaging Shared Resource and housed at the Animal Research Facility at the University of New Mexico Health Sciences Center. Mice were maintained at a controlled temperature of 22°C to 23°C, with a 12-hour light/12-hour dark cycle. Water and standard mouse chow were available ad libitum. All procedures were approved by the University of New Mexico Institutional Animal Care and Use Committee and performed in accordance with the NIH's Guide for the Care and Use of Laboratory Animals.40
MMTV-PyMT female transgenic mice 5 to 6 weeks of age were placed into groups of two to three mice per cage and acclimated to pills by offering placebo pills twice a day for 3 days. Consumption was confirmed by direct observation. Once treatment began, mice received one pill that contained 1.0 mg/kg body weight of drug or placebo twice daily for the 3-week study or twice daily 5 days per week for the 7-week study.
Tissue Analysis
Mammary tissue whole mounts were prepared from fourth abdominal mammary glands in the 3-week study. Resected mammary glands were fixed in 4% paraformaldehyde, followed by two changes of acetone over 8 to 24 hours, then placed in water for 1 hour. After overnight staining in carmine alum (0.2% carmine dye, 0.5% aluminum potassium sulfate), tissues were destained.
For histologic examination and immunohistochemistry of mammary tissue from the 7-week study, the fourth abdominal mammary glands were resected, fixed in neutral buffered formalin, embedded in paraffin, and sectioned longitudinally at 5 to 6 μm for routine hematoxylin and eosin (H&E) staining and immunohistochemistry. For all immunohistochemical staining, sections were rehydrated, endogenous peroxidase activity was blocked with 3% H2O2 in water, and antigen retrieval was performed with 10 mmol/L citrate buffer, pH 6.0, in a microwave oven. Nonspecific antibody binding was blocked with Biocare Medical Blocking Reagent (Concord, PA). Immunohistochemistry was performed with the following primary antibodies: anti-keratin 5 (PRB-160P; Covance, Princeton, NJ) rabbit polyclonal diluted 1:500 for 1 hour; anti-vimentin rabbit monoclonal antibody (ab92547; Abcam, Cambridge, MA) diluted 1:500 for 1 hour; anti–E-cadherin rabbit polyclonal antibody (7870; Santa Cruz Biotechnology, Dallas, TX) diluted 1:50 for 1 hour; anti–β-catenin mouse monoclonal antibody (610153; BD Biosciences, San Jose, CA) diluted 1:500 for 30 minutes; and mouse monoclonal anti-cyclin D1 (sc-8396; Santa Cruz Biotechnology) diluted 1:500 for 2 hours. All incubations were performed at room temperature. Secondary reagents included Envision+ labeled polymer, anti–rabbit-horseradish peroxidase (HRP; Dako, Santa Clara, CA) applied for 30 minutes at room temperature for rabbit antibodies and streptavidin-HRP (Biocare Medical) applied for 30 minutes at room temperature for mouse antibodies. Reactivity was detected using diaminobenzidine (Dako).
Tumor Scoring and Morphometry
Carmine-stained mammary gland whole mounts were imaged with MoticCam 2300 running Motic Images Plus software version 2.0 (Hong Kong, China) on an Olympus (Tokyo, Japan) SZH dissection microscope. Pixel intensity of tumor and nontumor areas was determined with ImageJ software version 1.47 (NIH, Bethesda, MD; https://imagej.nih.gov/ij). H&E and immunohistochemistry slides were scanned by an Aperio CS2 scanner (Leica Biosystems, Buffalo Grove, IL), and morphometry was performed with the HALO image analysis platform (Indica Labs, Albuquerque, NM). Tumors were identified on H&E-stained slides as discrete masses >3 mm2 that had uniform structure and immunohistochemical staining characteristics and that compressed or infiltrated surrounding parenchyma. Tumor areas were determined using the manual selection tool to outline the tumors. In addition, total epithelial area in each mammary gland was determined on H&E-stained slides by training the HALO Classifier algorithm to distinguish mammary epithelium, both hyperplastic and neoplastic, from stroma. This classifier was then applied to the entire mammary gland section. For quantification of each immunohistochemical stain, the Classifier algorithm was trained to distinguish epithelium from nonepithelial tissue; the epithelium was then analyzed with the appropriate Analysis algorithm modified as necessary for each stain.
RNA Isolation and qPCR
Samples of tumor tissue (30 mg) were frozen in liquid nitrogen and disrupted in buffer provided in an RNeasy Mini Kit (Qiagen, Valencia, CA), using an electric hand drill fitted with nuclease-free 1.5-mL pestles (Kimble-Chase, Vineland, NJ). The tissue lysate was homogenized with the QIAshredder (Qiagen), and RNA was isolated with the RNeasy Mini Kit according to the manufacturer's protocols. RNA was converted into cDNA with the use of a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and a TC-3000X Thermocycler (Techne Inc., Burlington, NJ). cDNA was generated from 1000 ng of RNA of each sample. The resulting cDNA samples were diluted 1:3 with nuclease-free water.
Real-time quantitative PCR (qPCR) was conducted with the use of six mouse primers: Rac1, Rac1b, RhoA, Cdc42, PyMT, and β-80 actin (Qiagen; excluding PyMT; catalog numbers QT01070146, QT00127673, QT00197568, QT00091560, QT00095242, respectively). PyMT primers used were PyMT forward (5′-CGGCGGAGCGAGGAACTGAGGAGAG-3′) and reverse (5′-TCAGAAGACTCGGCAGTCTTA-3′).41 Fast SYBR Green Master Mix (Applied Biosystems) was used to make a 1:5 master mix for each primer. Samples were loaded in triplicate in 384-well plates with 6 μL of master mix and 4 μL of sample per well. A nuclease-free water sample was used as a negative control, and β-actin was included as a positive control. Genes were amplified on a 7900 HT Fast Real-Time PCR System (Applied Biosystems). Relative expression was calculated with the ΔΔct method, using β-actin for normalizing and analyzing the treated samples in reference to placebo samples.
Protein Isolation and Western Blot Procedures
Proteins were extracted with frozen tumor tissues with the use of protocols modified from Zakharchenko et al.42 Briefly, 10 to 20 mg of frozen tissue was homogenized in 250 μL of RIPA buffer (50 mmol/L Tris-HCl pH 7.5, 105 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, and 2 mmol/L EDTA) with protease inhibitors (catalog number 78439; Thermo Fisher Scientific, Waltham, MA) and phosphatase inhibitors (catalog number 78445; Thermo Fisher Scientific) with the use of an electric drill with a plastic pellet pestle (catalog number 7495150000; Kimble-Chase) attached. Homogenized lysates were incubated on ice for 30 minutes, mixed with a vortex mixer, and briefly sonicated. Lysates were incubated for 30 additional minutes and spun at 16,500 × g for 20 minutes at 4°C to remove debris, and the supernatant was divided into aliquots into a new tube. Protein concentrations were measured with a Pierce BCA Protein Assay Kit (catalog number 23227; Thermo Fisher Scientific).
Proteins (20 μg) were resolved by polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Membranes were blocked in 5% milk for 1 hour at 4°C, then blotted with the following antibodies and conditions. GTPase Western blot analyses used anti-Rac1 mouse monoclonal antibody ARC03, anti-Cdc42 mouse monoclonal antibody ACD03, anti-RhoA mouse monoclonal antibody ARH04 (Cytoskeleton, Inc., Denver, CO) at 1:500 dilution in 0.1% milk in TBST (13 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.05% Tween-20 at pH 7.4), RhoA antibody was diluted in TBST only. The following rabbit polyclonal antibodies from Cell Signaling (Danvers, MA) were used at 1:1000 dilution: phospho (p)-P12 (RAC1) activated kinase (PAK)1 (Ser144)/PAK2 (Ser141) antibody number 2606, PAK1 antibody number 2602, PAK1/2/3 antibody number 2604, p-p44/42 mitogen-activated protein kinase [MAPK; extracellular signal-related kinase (Erk)1/2] (Thr202/Tyr204) antibody number 9101, p44/42 MAPK (Erk1/2) antibody number 9102, Akt antibody number 9272, and mouse monoclonal p-Akt (Ser473) (587F11) number 4051. All p-PAK, PAK, p-Erk, Erk, and Akt antibodies were diluted in 5% bovine serum albumin in TBST, whereas p-Akt was diluted in 5% milk in TBST. Secondary antibody Anti-Rabbit IgG (H+L) HRP Conjugate W401B and Anti-Mouse IgG (H+L) HRP Conjugate W402B (Promega, Madison, WI) were used at 1:5000 dilution in 1% milk in TBST. Blots were stripped at 50°C in stripping buffer (62.5 mmol/L Tris-HCl pH 6.7, 100 mmol/L β-mercaptoethanol, 2% SDS) for 30 minutes, followed by thorough rinsing with MilliQ water and three 10-minute washes in TBST. Blots were reprobed with Vinculin XP rabbit monoclonal antibody (HRP conjugate) number 18799 from Cell Signaling at a 1:1000 dilution in 5% bovine serum albumin in TBST. Vinculin was selected because it did not change as a consequence of Rac1 overexpression in SKOV3ip cells. Western blot images were acquired on ProteinSimple FluorChem R system and analyzed with AlphaView SA software version 3.4.0.0 (ProteinSimple, San Jose, CA). Values for specific proteins were normalized to the vinculin loading control or total protein based on image analysis of Ponceau-stained membrane.43 Analysis was completed in Prism software version 6 (GraphPad Software, Inc., La Jolla, CA) with the use of unpaired t-tests that assumed Gaussians distributions with two-tailed P values at 95% confidence intervals. To confirm no difference in variances between treatments the F test was used.
GTPase Activity Measurement
Rac1-GTP levels in mouse breast tumors were quantified with the use of a G-LISA kit (BK128) according to the manufacturer's instructions (Cytoskeleton, Inc.). Briefly, mouse tumors (5–6 mm3 in size) isolated from the left second and third mammary glands of placebo and R-ketorolac–treated mice were lysed in 700 to 900 μL of the provided lysis buffer (GL36-composed of a proprietary formulation of Tris pH 7.5, MgCl2, NaCl, IGEPAL, and SDS), aliquots were removed for protein assay with the use of the Precision Red Advanced Protein Assay, and the remaining lysates were snap-frozen to minimize GTP hydrolysis. Lysates were thawed, and 50-μL samples were immediately transferred to individual Rac1-GTP binding wells for a 30-minute incubation period at 4°C. Wash steps were performed as described, and bound Rac1-GTP was detected with the provided anti-Rac1 primary and secondary HRP-labeled antibodies. After addition of the stop buffer, absorbances of each well were read immediately at 490 nm on a BioTek (Winooski, VT) Synergy Neo2 plate reader. Positive Rac1-GTP controls (100 ng constitutively active Rac1) were diluted and assayed at 3 ng/well, and absorbances were used to calculate the nanogram of Rac1-GTP as a function of total milligram of protein for each sample as follows: [(ODsample − ODblank) × 3/(ODRac1–GTPstandard − ODblank)]/(0.05 mL/mg/mL protein). Samples for which subtraction of the blank resulted in a negative value or there was insufficient material to measure protein were not considered.
Statistical Analysis
The number of tumors per mouse, average tumor size, and total tumor area per mouse in placebo-treated versus ketorolac-treated mice were compared with the t-test. The number of mice with tumors in each group were compared by Fischer's exact test. HALO morphometry provided quantitative measures of total epithelial area and immunohistochemical staining for keratin 5, vimentin, E-cadherin, β-catenin, Ki-67, and cyclin D1, allowing these results to be compared with the t-test. All statistical analyses were performed with Prism software (GraphPad Software, Inc.).
Results
R-Ketorolac Reduces Epithelial Content of Mammary Glands
To test the impact of R-ketorolac on mammary epithelial proliferation, 5- to 6-week–old MMTV-PyMT female mice were treated with twice daily oral doses of 1.0 mg/kg R-ketorolac or placebo for 3 weeks. This dosing regimen achieved serum concentrations comparable with those in humans.8, 9 No significant difference was found between the two treatment groups in palpable mammary nodules or body weight at sacrifice (Supplemental Figure S1). To evaluate epithelial content of the mammary glands, left and right fourth abdominal mammary glands were removed, stained with carmine alum, imaged as whole mounts, and analyzed separately. The results of the analysis were averaged for the histogram. Representative whole-mount mammary gland images for placebo versus R-ketorolac treatment groups are shown in Figure 1, A and B. Quantification of pixel intensity demonstrated a significant reduction in Carmine-stained epithelium in the R-ketorolac treatment group compared with the placebo group (Figure 1C). This indicated reduced epithelial volume in R-ketorolac–treated mice.
Figure 1.
Three-week treatment with R-ketorolac reduces epithelial area. A and B: Carmine staining of mammary gland whole mounts obtained from mice after 3-week dosing with placebo (A) or R-ketorolac (B). Image analysis was performed on left and right fourth mammary glands; the values were averaged for each mouse with nine mice per treatment group. Darker pixels represent epithelial tissue, whereas lighter pixels represent more fatty tissue. C: Pixel intensity throughout the glands was measured with ImageJ version 1.47 software (NIH, Bethesda, MD; https://imagej.nih.gov/ij). Placebo-treated mice had significantly greater density of staining in the mammary glands than mice treated with R-ketorolac. ∗∗∗∗P < 0.0001. Significance was determined with the t-test.
From evidence presented in Figure 1, the effects of R-ketorolac treatment were then examined on epithelial proliferation in mice with somewhat more advanced lesions. Mice received 1.0 mg/kg R-ketorolac or placebo twice daily 5 days per week for 7 weeks. No significant difference was found between placebo and R-ketorolac treatment groups in weight gain over the course of the study or in total mammary gland weight at sacrifice (Supplemental Figure S2). As determined by morphometric analysis of mammary gland sections, total epithelial area (hyperplastic and neoplastic) was significantly greater in placebo-treated mice (57.96 ± 19.13 mm2) than in R-ketorolac–treated mice (43.37 ± 8.58 mm2) (Figure 2, A and B). Moreover, the percentage of the mammary gland area occupied by epithelium was significantly higher in placebo-treated mice (26.8% ± 5.6% versus 21.6% ± 6.5%) (Figure 2B). These findings were in agreement with the first experiment in which reduced mammary epithelium was found in mice treated with R-ketorolac for 3 weeks (Figure 1). A significant difference was found between placebo mice (30.8% ± 8.8% positive nuclei) and R-ketorolac–treated mice (21.9% ± 11.1% positive nuclei) in Ki67 staining (Figure 2, C and D), but no significant change in nuclear cyclin D1 staining between the groups (Supplemental Figure S3). These findings indicated that R-ketorolac treatment reduced epithelial proliferation.
Figure 2.
Seven-week treatment with R-ketorolac reduces epithelial proliferation. A: Comparison of mammary glands in mice receiving placebo or R-ketorolac for 7 weeks. Upper panels show examples of mammary glands stained with hematoxylin and eosin (H&E), and lower panels show morphometric analyses of the same images performed to quantify total epithelial area. B: Quantification of epithelial area of the fourth mammary gland from each mouse treated with placebo or R-ketorolac shown as total epithelial area. Ketorolac-treated mice had smaller amounts of mammary epithelial tissue than placebo-treated mice. C: Representative images of Ki67 immunostaining (upper panels) and corresponding morphometric analyses (lower panels) used to quantify Ki67-positive nuclei. D: Quantification of Ki67-positive nuclei from the fourth mammary gland from each mouse treated with placebo or R-ketorolac. Only the nuclei with the strongest staining (shown in red) were included. Fewer Ki67-positive nuclei in ketorolac-treated versus placebo-treated mice indicate reduced epithelial proliferation in ketorolac-treated mice. ∗P < 0.05. Significance was determined with the one-tailed t-test. Scale bars: 2 mm (A); 100 μm (C). IHC, immunohistochemistry.
R-Ketorolac Slows Tumor Development
The mammary glands of mice in the 7-week study contained a complex admixture of epithelial lesions, including hyperplasia, adenomas, and carcinomas, as well as intermediate lesions; there were few advanced carcinomas. This made identification and classification of individual tumors difficult. Thus, stringent criteria were applied to reproducibly distinguish tumors from surrounding lesions (see Materials and Methods). Results showed that significantly fewer R-ketorolac–treated mice had tumors than placebo-treated mice (Figure 3A). Although 100% of placebo-treated mice had one or more discrete mammary tumors, only 50% of R-ketorolac–treated mice had similar tumors. The mean number of tumors per mouse, when all mice were considered, was significantly lower in R-ketorolac–treated mice (0.90 ± 0.99 tumors per mouse) than in placebo-treated control mice (1.73 ± 0.79 tumors per mouse) (Figure 3B). However, when only mice with tumors were considered, no significant difference was found between R-ketorolac–treated and placebo-treated mice in number of tumors per mouse, average tumor size, or total tumor area per mouse (Supplemental Figure S4). These findings suggested that R-ketorolac decreased mammary tumor development in MMTV-PyMT mice based on number of mice developing tumors but did not reduce tumor size or area once tumors were present.
Figure 3.
R-ketorolac slows mammary tumor development. A: Fourth mammary glands from mice treated with placebo or R-ketorolac were analyzed to distinguish tumors from other epithelial lesions as described in Materials and Methods. Fewer mice receiving R-ketorolac for 7 weeks develop tumors compared with placebo-treated mice. B: The number of tumors per mouse was determined as in A. All mice, including those with and without tumors, were represented in this analysis. Treatment with R-ketorolac reduces the number of tumors per mouse. ∗P < 0.05. Significance was determined by Fischer's exact test.
R-Ketorolac Does Not Alter GTPase or PyMT Transcript Expression but Decreases GTPase Protein Levels
Expression of the PyMT transcript, as measured by qPCR, was not altered in the mammary glands of R-ketorolac–treated mice (Supplemental Figure S5). This indicated that reduced expression of PyMT in the mammary glands of R-ketorolac–treated mice was not responsible for decreased tumor development. Similarly, R-ketorolac did not alter transcripts of the Rho-family GTPases, Rac1, a constitutively active variant Rac1b, Cdc42, or RhoA in the mammary glands of ketorolac-treated mice (Supplemental Figure S5). In contrast to the mRNA findings, a significant decrease in Rac1 protein levels and trend toward decrease in Cdc42 protein levels in tumors from R-ketorolac–treated mice compared with placebo control mice was detected (Supplemental Figure S6A). A trend was found toward a corresponding decrease in Rac1 activity (Supplemental Figure S6B). The decrease in the R-ketorolac target GTPases Rac1 and Cdc42 was selective with neither a decrease in the related family member RhoA (Supplemental Figure S6A) nor in total ERK or AKT proteins (Supplemental Figure S7). R-ketorolac decreased AKT signaling as detected by decreased phosphorylated AKT that may account, in part, for the observed reduction in epithelial proliferation in R-ketorolac–treated mice. Rac1 degradation has been reported43, 44, 45 and highlighted the complexity of mechanisms that regulate Rac1.
R-Ketorolac Treatment Delays Lesion Progression
The MMTV-PyMT mouse model of breast cancer displays many hallmarks of human breast cancer development and progression,38 including that progression associated with epithelial-to-mesenchymal transition (EMT). To determine the effect of R-ketorolac on lesion progression, immunohistochemistry and morphometry were used to measure expression of select EMT markers in proliferative mammary epithelium from MMTV-PyMT mice on the 7-week study. Staining for the epithelial marker E-cadherin was almost entirely restricted to cell margins. This staining was quantified as the number of positive pixels per squared millimeter of mammary epithelium (Figure 4, A and B). Significant maintenance of staining for E-cadherin was found in the mammary glands of mice treated with R-ketorolac (2.83 ± 0.67 ×105 positive pixels/mm2) compared with placebo (2.33 ± 0.35 ×105 positive pixels/mm2). However, no difference was found between treatment groups in staining for the progression markers β-catenin and cytoplasmic vimentin (Supplemental Figure S8).
Figure 4.
R-ketorolac delays mammary lesion progression. A: Representative images for E-cadherin immunostaining (upper panels) and corresponding morphometric analysis (lower panels) used to quantify E-cadherin staining. B: Quantification of E-cadherin staining from the fourth mammary gland from each mouse treated with placebo or R-ketorolac. Significantly higher E-cadherin staining, indicating reduced epithelial-to-mesenchymal transition, was found in R-ketorolac–treated mice. C: Representative images for keratin 5 immunostaining (upper panels) and corresponding morphometric analysis (lower panels) used to quantify cytokeratin 5. D: Quantification of cytokeratin 5 staining from the fourth mammary gland from each mouse treated with placebo or R-ketorolac. Significantly less keratin 5 staining, suggesting slowed lesion progression, was found in ketorolac-treated mice. Results are shown as the total positive pixel area (μm2) per total epithelial area (mm2). ∗P < 0.05, ∗∗P < 0.01. Significance was determined with the one-tailed t-test. Scale bars = 100 μm. IHC, immunohistochemistry.
The MMTV-PyMT model is generally considered to represent a luminal type of mammary cancer, and mammary epithelial cells in this model predominantly express keratin 8. However, when polyoma middle T antigen or ErbB2 signaling is activated in these cells, more keratin 5-positive cells appear, indicating a more basal phenotype.46 Increased expression of keratin 5 in human breast tumors is associated with poorer prognosis and enhanced resistance to therapy.47, 48 Because of the large size and irregular contour of keratin 5-positive cells in MMTV-PyMT mice, the number of keratin 5-positive cells was estimated by measuring keratin 5 staining per squared millimeter of mammary epithelium. In R-ketorolac–treated mice significantly less keratin 5 staining was found than in placebo-treated mice (3.13 ± 029 ×104 versus 3.60 ± 0.42 × 104 positive pixels/mm2 epithelium) (Figure 4, C and D), indicative of a less basal phenotype.
Advanced tumors in MMTV-PyMT mice contain fewer estrogen receptor-α–positive (ER+) and progesterone receptor positive (PR+) cells than less advanced lesions.38 Because of the relatively early stage of the lesions in our study, the number of epithelial cells with ER+ and PR+ nuclei was determined in the entire mammary gland, rather than in individual tumors. R-ketorolac treatment did not alter the total number of ER+ or PR+ cells in the mammary epithelium compared with placebo treatment (Supplemental Figure S9). Although there were more tumors in placebo-treated mice than in R-ketorolac–treated mice, this difference was not reflected in an overall loss of ER or PR expression. This was consistent with the observation that few advanced carcinomas were present either in placebo-treated or R-ketorolac–treated mice.
Taken together, these findings indicated that R-ketorolac decreased selected aspects of lesion progression in the MMTV-PyMT model of human breast cancer. Although R-ketorolac treatment resulted in E-cadherin retention, presumably representing decreased EMT, it did not alter expression of the other EMT markers β-catenin and vimentin. Furthermore, reduced numbers of keratin 5-positive cells in the mammary glands of R-ketorolac–treated mice suggested reduced metastatic potential and decreased likelihood of treatment resistance.
Discussion
The Rho-family GTPases Rac1 and Cdc42 are key regulators of the actin cytoskeleton reorganization necessary for normal cell adhesion and migration, and are signaling molecules that modulate broad aspects of cancer cell function.15, 16, 17, 18, 19, 20, 21, 22 Our previous studies identified R-ketorolac as a dual Rac1 and Cdc42 inhibitor with the use of a combination of high-throughput screening and computational simulation.13 R-ketorolac is one enantiomer of a racemic drug (Toradol) approved by the Food and Drug Administration for pain relief. Because epidemiologic studies indicate survival advantage for breast cancer patients who received racemic ketorolac postoperatively,1, 2, 3, 4, 5 the potential benefits of the R-enantiomer alone were assessed on the development and progression of early mammary gland lesions in the MMTV-PyMT mouse model of breast cancer that recapitulates many key features of human disease.38
The impact of Rac1 and Cdc42 activity on breast cancer development has not been previously studied in the MMTV-PyMT model, but there are similarities between our findings and results reported in other in vivo studies that used genetic or xenograft models. Inhibition of Rac1 by the inhibitor EHop-016 in a xenograft model that used MDA-MB-435 mammary tumor cells decreased tumor growth and angiogenesis.49 Knockdown of Cdc42 modestly decreased growth of MDA-MB-231 cells in vivo, and pretreatment of tumor cells with the Cdc42 selective inhibitor ML141 decreased the number of mice that developed tumors.35 Genetic ablation of T-cell lymphoma invasion and metastasis 1, a Rac1-activating guanine nucleotide exchange factor, delayed tumor development, decreased the number of tumors per mouse, and led to an increase in tumor-free mice in an MMTV-c-neu breast cancer model.50 Similarly, knockout of dedicator of cytokinesis 1, another Rac1 guanine nucleotide exchange factor, in a breast cancer model that depended on human epidermal growth factor receptor 2 decreased mammary intraepithelial lesions and decreased Ki67+ tumor cells by approximately 30%.51 These two studies of genetic disruption of the Rac1 signaling pathway in breast cancer models driven by human epidermal growth factor receptor 2 are consistent with the outcomes of pharmacologic inhibition by R-ketorolac that were observed in MMTV-PYMT mice shown here.
The present study provides additional insights into the potential value of Rac1 and Cdc42 inhibition by R-ketorolac in breast cancer prevention or treatment. Decreased tumor development in R-ketorolac–treated mice was accompanied by retention of epithelial characteristics as determined by the persistence of E-cadherin expression, and reduced basal cell characteristics, as indicated by decreased keratin 5-expressing cells. In studies of MMTV-PyMT tumor organoids, cells with a basal phenotype were shown to be invasive.52 In the present study, R-ketorolac treatment is likely to contribute to reduced invasiveness by reducing the number of keratin 5-positive cells with a basal phenotype. In the MMTV-PyMT model, reduced E-cadherin and β-catenin expression and de novo expression of vimentin in mammary tumor cells are associated with EMT and increased tumor invasiveness and metastasis.53, 54, 55, 56 Of interest, loss of E-cadherin in MMTV-PyMT mammary epithelium during mammary carcinogenesis appears to be Rac1 dependent.56 Loss of E-cadherin is generally the earliest sign of EMT57; thus, it is possible that similar staining for β-catenin and vimentin in R-ketorolac– and placebo-treated mice reflected the relatively early stage of mammary lesion development in these samples. Taken together, our observations indicate that ketorolac treatment reduced the progression and invasive potential of mammary gland lesions and motivates future studies in other models to further delineate the effects and benefits of Rac1 and Cdc42 inhibition on breast cancer development and progression.
The present and earlier studies on the antitumor effects of Rac1 and/or Cdc42 inhibition support further investigations of R-ketorolac as a novel therapeutic approach in breast cancer. Indeed, there are active efforts to identify Rac1 and Cdc42 inhibitors, but the agents identified thus far have not been translated to human use.18, 19, 20, 23, 32, 36, 37, 47, 58 Retrospective studies demonstrated breast and ovarian cancer survival benefit in patients who received racemic ketorolac for indication of pain management as approved by the Food and Drug Administration,1, 2, 3, 4, 5, 59 but toxicity risks associated with COX inhibition by the S-enantiomer limit racemic ketorolac use to 5 days. The limits are due to the gastrointestinal and cardiovascular side effects and to renal toxicity that are generally attributed to the potent COX inhibitory activities of S-ketorolac (Toradol Oral ketorolac tromethamine tablets; F. Hoffmann-LaRoche, Basel, Switzerland60). The lack of COX inhibition by R-ketorolac9, 10, 15 and the demonstrated inhibition of Rac1 and Cdc42 by racemic ketorolac in humans59 suggest that R-ketorolac may offer a tractable option for targeting Rac1 and Cdc42 in human disease through rapid repurposing and testing in clinical prevention or treatment trials.
Acknowledgments
Frederick Schultz (Human Tissue Repository and Tissue Analysis Shared Resource, Department of Pathology and Comprehensive Cancer Center, University of New Mexico) and Nancy Otto (Histology & Tissue Processing Facility Core, The Virginia Harris Cockrell Cancer Research Center, MD Anderson Cancer Center, University of Texas) made important contributions to this study.
Footnotes
Supported by NIH grants R21 CA170375 and 1R21CA170375-01S (L.G.H. and A.W.-N.) and Administrative Diversity Supplement to P50 GM085273 (M.R.R.). Additional support was provided by the University of New Mexico Comprehensive Cancer Center Animal Models and Human Tissue Repository Shared Resources funded by National Cancer Institute 2P30 CA118100 (principal investigator: C. Willman).
D.F.K. and L.G.H. contributed equally as senior authors.
Disclosures: A.W.-N. and L.G.H. are inventors on US patent 9,125,899 for therapeutic uses of nonsteroidal anti-inflammatory drug R-enantiomers.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2017.10.018.
Supplemental Data
Supplemental Figure S1.
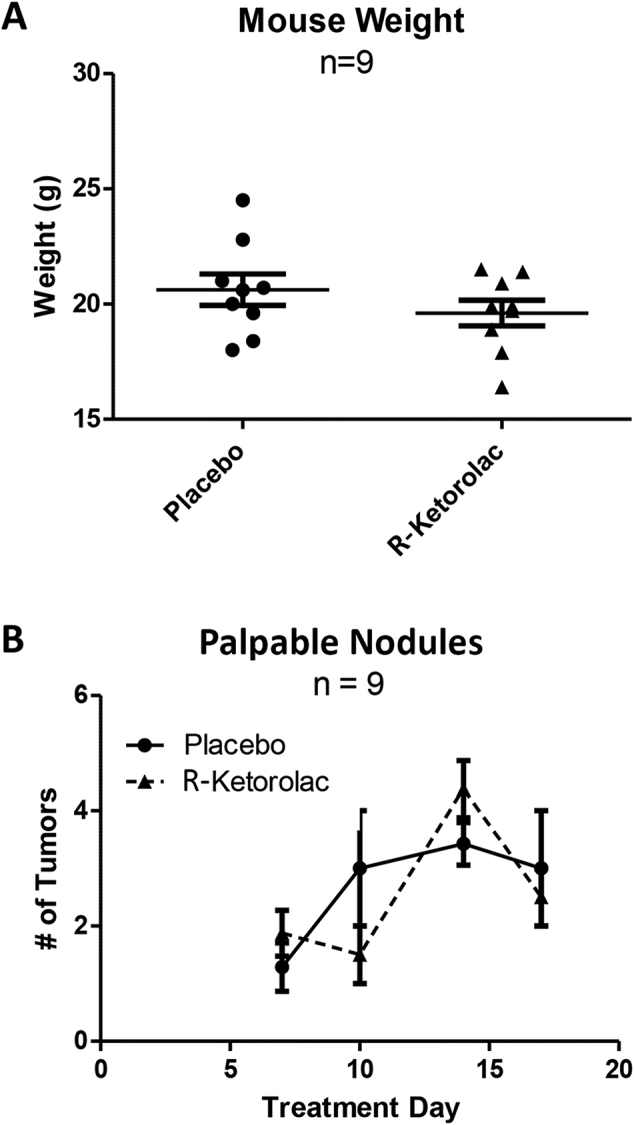
Mouse weight and palpable mammary nodules after 3-week treatment with R-ketorolac. A: Mouse weights were recorded routinely, and final mouse weights were recorded at the end of the study from mice receiving placebo or R-ketorolac. No significant difference was detected between the two groups. B: Over the course of the study, mouse mammary glands were palpated for presence of tumor growth. The number of nodules increased over time, but the difference between groups was not significant. Statistical analysis was conducted with a two-way analysis of variance with a Bonferroni posttest.
Supplemental Figure S2.

Mouse weight and mammary gland weight after 7-week treatment with R-ketorolac. A: Mouse weights were recorded routinely, and final mouse weights were recorded at the end of the study from mice receiving placebo or R-ketorolac. No significant difference was detected between the two groups. B: At the termination of the study, the mammary glands were excised and weighed. The tumor–mass to mouse–mass ratio was calculated. No significant differences were found between the two treatment groups as determined with the unpaired t-test.
Supplemental Figure S3.

R-ketorolac does not change cyclin D staining in nuclei. Cyclin D nuclear staining was quantified from the fourth mammary gland from each mouse treated with placebo or R-ketorolac with the use of morphometric analysis as described in Materials and Methods. No significant difference was found between the groups as determined with the one-tailed t-test.
Supplemental Figure S4.

R-ketorolac does not modify tumor parameters in mice with tumors. Fourth mammary glands from mice treated with placebo or R-ketorolac were analyzed to distinguish tumor versus other epithelial lesions as described in Materials and Methods. A–C: Tumor number per mouse in mice with tumors (A), average tumor size in mice with tumors (B), and total tumor area per mouse in mice with tumors (C) did not differ between the two groups. No significant differences were found between groups for any of these tumor parameters. Significance was determined with the one-tailed t-test.
Supplemental Figure S5.

Gene expression levels of polyoma middle T-antigen (PyMT) or Rho-GTPases does not change with R-ketorolac treatment. RNA was isolated and real-time quantitative PCR was performed on tumor tissue isolated from mice treated with placebo or R-ketorolac for 7 weeks as described in Materials and Methods. Gene expression levels in the tumors of (R)-ketorolac–treated mice are not different from the control placebo-treated mice. No significant differences were determined with the one-tailed t-test. Cdc42, cell division control protein 42; Rac1, Ras homolog (Rho)-family small GTPases Ras-related C3 botulinum toxin substrate 1.
R-ketorolac decreases Ras homolog (Rho)-family small GTPases Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell division control protein 42 (Cdc42) protein levels. A and B: GTPase protein (A) and Rac1 (B) activity of tumors isolated from placebo versus R-ketorolac–treated mice was measured as described in Materials and Methods. GTPase protein (A) and activity of tumors isolated from placebo versus R-ketorolac–treated mice (B) was measured as described in Materials and Methods. A: A significant decrease in Rac1 protein levels and a trend toward decrease in Cdc42 protein level that did not achieve significance without decrease in the related Rho family member RhoA was observed. Significance was determined with unpaired t tests as described further in Materials and Methods. B: Breast glands (R1 and L2/3) from animals treated for 7 weeks with placebo or R-ketorolac were individually excised at time of sacrifice, snap-frozen, and stored at −80°C. Breast glands were thawed in ice-cold lysis buffer and Rac1-GTP levels were measured using GLISA kit from Cytoskeleton. Although there was a trend for reduced Rac1-GTP in R-ketorolac–treated samples, unpaired, two-tailed t-test (P = 0.39) did not reach statistical significance. ∗P < 0.05.
R-ketorolac decreases phospho (p)-AKT in tumors. Mammary tumor protein isolated from placebo- or R-ketorolac–treated mice was analyzed by Western blot analysis as described in Materials and Methods. A: A significant decrease in phosphorylated AKT was detected in protein isolated from R-ketorolac–treated mice, and no significant difference was found in total AKT between the two treatment groups. B: No significant decreases were detected in phosphorylated extracellular signal-related kinase (ERK) or total ERK protein isolated from R-ketorolac–treated mice. Statistical P values were obtained with unpaired t-tests, further described in Materials and Methods. ∗∗P < 0.01.
Supplemental Figure S8.

R-ketorolac does not change β-catenin or vimentin staining. A and B: β-Catenin (A) and vimentin (B) staining were quantified from the fourth mammary gland from each mouse treated with placebo or R-ketorolac with the use of morphometric analysis as described in Materials and Methods. The one-tailed t-test did not reveal significant differences between groups for either β-catenin or vimentin staining.
Supplemental Figure S9.

R-ketorolac did not change nuclear estrogen receptor or total progesterone receptor staining. A and B: Estrogen receptor-positive (ER+) nuclei (A) and total progesterone receptor (B) staining were quantified from the fourth mammary gland from each mouse treated with placebo or R-ketorolac with the use of morphometric analysis as described in the Materials and Methods. No significant differences were determined with the one-tailed t-test.
References
- 1.Forget P., Vandenhende J., Berliere M., Machiels J.P., Nussbaum B., Legrand C., De Kock M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110:1630–1635. doi: 10.1213/ANE.0b013e3181d2ad07. [DOI] [PubMed] [Google Scholar]
- 2.Retsky M., Rogers R., Demicheli R., Hrushesky W.J., Gukas I., Vaidya J.S., Baum M., Forget P., Dekock M., Pachmann K. NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Res Treat. 2012;134:881–888. doi: 10.1007/s10549-012-2094-5. [DOI] [PubMed] [Google Scholar]
- 3.Retsky M., Demicheli R., Hrushesky W.J., Forget P., De Kock M., Gukas I., Rogers R.A., Baum M., Sukhatme V., Vaidya J.S. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem. 2013;20:4163–4176. doi: 10.2174/09298673113209990250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forget P., De Kock M. Perspectives in anaesthesia for cancer surgery. J Cancer Res Clin Oncol. 2014;140:353–359. doi: 10.1007/s00432-013-1522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forget P., Bentin C., Machiels J.P., Berliere M., Coulie P.G., De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth. 2014;113:i82–i87. doi: 10.1093/bja/aet464. [DOI] [PubMed] [Google Scholar]
- 6.Kean W.F., Lock C.J., Howard-Lock H.E. Chirality in antirheumatic drugs. Lancet. 1991;338:1565–1568. doi: 10.1016/0140-6736(91)92382-c. [DOI] [PubMed] [Google Scholar]
- 7.Carabaza A., Cabré F., Rotllan E., Gómez M., Gutiérrez M., García M.L., Mauleón D. Stereoselective inhibition of inducible cyclooxygenase by chiral nonsteroidal antiinflammatory drugs. J Clin Pharmacol. 1996;36:505–512. doi: 10.1002/j.1552-4604.1996.tb05040.x. [DOI] [PubMed] [Google Scholar]
- 8.Mroszczak E., Combs D., Chaplin M., Tsina I., Tarnowski T., Rocha C., Tam Y., Boyd A., Young J., Depass L. Chiral kinetics and dynamics of ketorolac. J Clin Pharmacol. 1996;36:521–539. doi: 10.1002/j.1552-4604.1996.tb05042.x. [DOI] [PubMed] [Google Scholar]
- 9.Jett M.F., Ramesha C.S., Brown C.D., Chiu S., Emmett C., Voronin T., Sun T., O'Yang C., Hunter J.C., Eglen R.M., Johnson R.M. Characterization of the analgesic and anti-inflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther. 1999;288:1288–1297. [PubMed] [Google Scholar]
- 10.Yasui H., Hideshima T., Hamasaki M., Roccaro A.M., Shiraishi N., Kumar S., Tassone P., Ishitsuka K., Raje N., Tai Y.T., Podar K., Chauhan D., Leoni L.M., Kanekal S., Elliott G., Munshi N.C., Anderson K.C. SDX-101, the R-enantiomer of etodolac, induces cytotoxicity, overcomes drug resistance, and enhances the activity of dexamethasone in multiple myeloma. Blood. 2005;106:706–712. doi: 10.1182/blood-2005-02-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolluri S.K., Corr M., James S.Y., Bernasconi M., Lu D., Liu W., Cottam H.B., Leoni L.M., Carson D.A., Zhang X. The R-enantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. Proc Natl Acad Sci U S A. 2005;102:2525–2530. doi: 10.1073/pnas.0409721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue T., Murano M., Yoda Y., Kuramoto T., Kakimoto K., Ishida K., Kawakami K., Abe Y., Morita E., Murano N., Tokioka S. R-etodolac induces E-cadherin and suppresses colitis-related mouse colon tumorigenesis. Oncol Rep. 2010;24:1487–1492. doi: 10.3892/or_00001009. [DOI] [PubMed] [Google Scholar]
- 13.Oprea T.I., Sklar L.A., Agola J.O., Guo Y., Silberberg M., Roxby J., Vestling A., Romero E., Surviladze Z., Murray-Krezan C., Waller A., Ursu O., Hudson L.G., Wandinger-Ness A. Novel activities of select NSAID R-enantiomers against Rac1 and Cdc42 GTPases. PLoS One. 2015;10:e142182. doi: 10.1371/journal.pone.0142182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y., Kenney S.R., Muller C.Y., Adams S., Rutledge T., Romero E., Murray-Krezan C., Prekeris R., Sklar L.A., Hudson L.G., Wandinger-Ness A. R-ketorolac targets Cdc42 and Rac1 and alters ovarian cancer cell behaviors critical for invasion and metastasis. Mol Cancer Ther. 2015;14:2215–2227. doi: 10.1158/1535-7163.MCT-15-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alan J.K., Lundquist E.A. Mutationally activated Rho GTPases in cancer. Small GTPases. 2013;4:159–163. doi: 10.4161/sgtp.26530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orgaz J.L., Herraiz C., Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019. doi: 10.4161/sgtp.29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridley A.J. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–112. doi: 10.1016/j.ceb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pajic M., Herrmann D., Vennin C., Conway J.R., Chin V.T., Johnsson A.K., Welch H.C., Timpson P. The dynamics of Rho GTPase signaling and implications for targeting cancer and the tumor microenvironment. Small GTPases. 2015;6:123–133. doi: 10.4161/21541248.2014.973749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zandvakili I., Lin Y., Morris J.C., Zheng Y. Rho GTPases: anti- or pro-neoplastic targets? Oncogene. 2017;36:3213–3222. doi: 10.1038/onc.2016.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smithers C., Overduin M. Structural mechanisms and drug discovery prospects of Rho GTPases. Cells. 2016;5:E26. doi: 10.3390/cells5020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riching K.M., Keely P.J. Rho family GTPases: making it to the third dimension. Int J Biochem Cell Biol. 2015;59:111–115. doi: 10.1016/j.biocel.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H., Peyrollier K., Kilic G., Brakebusch C. Rho GTPases and cancer. Biofactors. 2014;40:226–235. doi: 10.1002/biof.1155. [DOI] [PubMed] [Google Scholar]
- 23.Zuo Y., Oh W., Ulu A., Frost J.A. Minireview: mouse models of Rho GTPase function in mammary gland development, tumorigenesis, and metastasis. Mol Endocrinol. 2016;30:278–289. doi: 10.1210/me.2015-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnelzer A., Prechtel D., Knaus U., Dehne K., Gerhard M., Graeff H., Harbeck N., Schmitt M., Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 25.Fritz G., Brachetti C., Bahlmann F., Schmidt M., Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y., Olufemi L., Wang M.-T., Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008;13:759–776. doi: 10.2741/2718. [DOI] [PubMed] [Google Scholar]
- 27.Wertheimer E., Gutierrez-Uzquiza A., Rosemblit C., Lopez-Haber C., Sosa M.S., Kazanietz M.G. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal. 2012;24:353–362. doi: 10.1016/j.cellsig.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida T., Zhang Y., Rivera Rosado L.A., Chen J., Khan T., Moon S.Y., Zhang B. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657–1668. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 29.Yan Y., Greer P.M., Cao P.T., Kolb R.H., Cowan K.H. RAC1 GTPase plays an important role in gamma-irradiation induced G2/M checkpoint activation. Breast Cancer Res. 2012;14:R60. doi: 10.1186/bcr3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hein A.L., Post C.M., Sheinin Y.M., Lakshmanan I., Natarajan A., Enke C.A., Batra S.K., Ouellette M.M., Yan Y. RAC1 GTPase promotes the survival of breast cancer cells in response to hyper-fractionated radiation treatment. Oncogene. 2016;35:6319–6329. doi: 10.1038/onc.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi K., Li X., Zheng Y., Takano Y. Invasion of breast cancer cells into collagen matrix requires TGF-alpha and Cdc42 signaling. FEBS Lett. 2011;585:286–290. doi: 10.1016/j.febslet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Cardama G.A., Comin M.J., Hornos L., Gonzalez N., Defelipe L., Turjanski A.G., Alonso D.F., Gomez D.E., Menna P.L. Preclinical development of novel Rac1-GEF signaling inhibitors using a rational design approach in highly aggressive breast cancer cell lines. Anticancer Agents Med Chem. 2014;14:840–851. doi: 10.2174/18715206113136660334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dokmanovic M., Hirsch D.S., Shen Y., Wu W.J. Rac1 contributes to trastuzumab resistance of breast cancer cells: rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther. 2009;8:1557–1569. doi: 10.1158/1535-7163.MCT-09-0140. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez N., Cardama G.A., Comin M.J., Segatori V.I., Pifano M., Alonso D.F., Gomez D.E., Menna P.L. Pharmacological inhibition of Rac1-PAK1 axis restores tamoxifen sensitivity in human resistant breast cancer cells. Cell Signal. 2017;30:154–161. doi: 10.1016/j.cellsig.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Chen H.Y., Yang Y.M., Stevens B.M., Noble M. Inhibition of redox/Fyn/c-Cbl pathway function by Cdc42 controls tumour initiation capacity and tamoxifen sensitivity in basal-like breast cancer cells. EMBO Mol Med. 2013;5:723–736. doi: 10.1002/emmm.201202140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montalvo-Ortiz B.L., Castillo-Pichardo L., Hernández E., Humphries-Bickley T., De la Mota-Peynado A., Cubano L.A., Vlaar C.P., Dharmawardhane S. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. 2012;287:13228–13238. doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y., Zheng Y. Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin Drug Discov. 2015;10:991–1010. doi: 10.1517/17460441.2015.1058775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin E.Y., Jones J.G., Li P., Zhu L., Whitney K.D., Muller W.J., Pollard J.W. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker M.K., Boberg J.R., Walsh M.T., Wolf V., Trujillo A., Duke M.S., Palme R., Felton L.A. A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol Appl Pharmacol. 2012;260:65–69. doi: 10.1016/j.taap.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Research Council . National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 41.Marjon N.A., Hu C., Hathaway H.J., Prossnitz E.R. G protein-coupled estrogen receptor regulates mammary tumorigenesis and metastasis. Mol Cancer Res. 2014;12:1644–1654. doi: 10.1158/1541-7786.MCR-14-0128-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zakharchenko O., Greenwood C., Alldridge L., Souchelnytskyi S. Optimized protocol for protein extraction from the breast tissue that is compatible with two-dimensional gel electrophoresis. Breast Cancer (Auckl) 2011;5:37–42. doi: 10.4137/BCBCR.S6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldridge G.M., Podrebarac D.M., Greenough W.T., Weiler I.J. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazanietz M.G., Caloca M.J. The Rac GTPase in cancer: from old concepts to new paradigms. Cancer Res. 2017;77:5445–5451. doi: 10.1158/0008-5472.CAN-17-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam B.D., Hordijk P.L. The Rac1 hypervariable region in targeting and signaling: a tail of many stories. Small GTPases. 2013;4:78–89. doi: 10.4161/sgtp.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hein S.M., Haricharan S., Johnston A.N., Toneff M.J., Reddy J.P., Dong J., Bu W., Li Y. Luminal epithelial cells within the mammary gland can produce basal cells upon oncogenic stress. Oncogene. 2016;35:1461–1467. doi: 10.1038/onc.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kabos P., Haughian J.M., Wang X., Dye W.W., Finlayson C., Elias A., Horwitz K.B., Sartorius C.A. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2011;128:45–55. doi: 10.1007/s10549-010-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alshareeda A.T., Soria D., Garibaldi J.M., Rakha E., Nolan C., Ellis I.O., Green A.R. Characteristics of basal cytokeratin expression in breast cancer. Breast Cancer Res Treat. 2013;139:23–37. doi: 10.1007/s10549-013-2518-x. [DOI] [PubMed] [Google Scholar]
- 49.Castillo-Pichardo L., Humphries-Bickley T., De La Parra C., Forestier-Roman I., Martinez-Ferrer M., Hernandez E., Vlaar C., Ferrer-Acosta Y., Washington A.V., Cubano L.A., Rodriguez-Orengo J., Dharmawardhane S. The Rac inhibitor EHop-016 inhibits mammary tumor growth and metastasis in a nude mouse model. Transl Oncol. 2014;7:546–555. doi: 10.1016/j.tranon.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strumane K., Rygiel T., van der Valk M., Collard J.G. Tiam1-deficiency impairs mammary tumor formation in MMTV-c-neu but not in MMTV-c-myc mice. J Cancer Res Clin Oncol. 2009;135:69–80. doi: 10.1007/s00432-008-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laurin M., Huber J., Pelletier A., Houalla T., Park M., Fukui Y., Haibe-Kains B., Muller W.J., Côté J.F. Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis. Proc Natl Acad Sci U S A. 2013;110:7434–7439. doi: 10.1073/pnas.1213050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheung K.J., Gabrielson E., Werb Z., Ewald A.J. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin L., Liao L., Redmond A., Young L., Yuan Y., Chen H., O'Malley B.W., Xu J. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937–5950. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flowers M., Schroeder J.A., Borowsky A.D., Besselsen D.G., Thomson C.A., Pandey R., Thompson P.A. Pilot study on the effects of dietary conjugated linoleic acid on tumorigenesis and gene expression in PyMT transgenic mice. Carcinogenesis. 2010;31:1642–1649. doi: 10.1093/carcin/bgq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson J., Berg T., Kurzejamska E., Pang M.F., Tabor V., Jansson M., Roswall P., Pietras K., Sund M., Religa P., Fuxe J. MiR-155-mediated loss of C/EBPbeta shifts the TGF-beta response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32:5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shahi P., Slorach E.M., Wang C.Y., Chou J., Lu A., Ruderisch A., Werb Z. The transcriptional repressor ZNF503/Zeppo2 promotes mammary epithelial cell proliferation and enhances cell invasion. J Biol Chem. 2015;290:3803–3813. doi: 10.1074/jbc.M114.611202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin T.A., Ye L., Sanders A.J., Lane J., Jiang W.G. Cancer invasion and metastasis: molecular and cellular perspective. In: Jandial R., editor. Metastatic Cancer: Clinical and Biological Perspectives. Landes Bioscience; Austin, TX: 2013. pp. 135–168. [Google Scholar]
- 58.Arias-Romero L.E., Chernoff J. Targeting Cdc42 in cancer. Expert Opin Ther Targets. 2013;17:1263–1273. doi: 10.1517/14728222.2013.828037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo Y., Kenney S.R., Cook L.S., Adams S.F., Rutledge T., Romero E., Oprea T., Sklar L.A., Bedrick E., Wiggins C.L., Kang H., Lomo L., Muller C.Y., Wandinger-Ness A., Hudson L.G. A novel pharmacologic activity of ketorolac for therapeutic benefit in ovarian cancer patients. Clin Cancer Res. 2015;21:5064–5072. doi: 10.1158/1078-0432.CCR-15-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warner T.D., Giuliano F., Vojnovic I., Bukasa A., Mitchell J.A., Vane J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
R-ketorolac decreases Ras homolog (Rho)-family small GTPases Ras-related C3 botulinum toxin substrate 1 (Rac1) and cell division control protein 42 (Cdc42) protein levels. A and B: GTPase protein (A) and Rac1 (B) activity of tumors isolated from placebo versus R-ketorolac–treated mice was measured as described in Materials and Methods. GTPase protein (A) and activity of tumors isolated from placebo versus R-ketorolac–treated mice (B) was measured as described in Materials and Methods. A: A significant decrease in Rac1 protein levels and a trend toward decrease in Cdc42 protein level that did not achieve significance without decrease in the related Rho family member RhoA was observed. Significance was determined with unpaired t tests as described further in Materials and Methods. B: Breast glands (R1 and L2/3) from animals treated for 7 weeks with placebo or R-ketorolac were individually excised at time of sacrifice, snap-frozen, and stored at −80°C. Breast glands were thawed in ice-cold lysis buffer and Rac1-GTP levels were measured using GLISA kit from Cytoskeleton. Although there was a trend for reduced Rac1-GTP in R-ketorolac–treated samples, unpaired, two-tailed t-test (P = 0.39) did not reach statistical significance. ∗P < 0.05.
R-ketorolac decreases phospho (p)-AKT in tumors. Mammary tumor protein isolated from placebo- or R-ketorolac–treated mice was analyzed by Western blot analysis as described in Materials and Methods. A: A significant decrease in phosphorylated AKT was detected in protein isolated from R-ketorolac–treated mice, and no significant difference was found in total AKT between the two treatment groups. B: No significant decreases were detected in phosphorylated extracellular signal-related kinase (ERK) or total ERK protein isolated from R-ketorolac–treated mice. Statistical P values were obtained with unpaired t-tests, further described in Materials and Methods. ∗∗P < 0.01.






