Abstract
With the advent of functional neuroimaging it quickly became apparent that successful episodic memory retrieval was consistently associated with enhanced activity in ventral lateral parietal cortex, especially the left angular gyrus. Here, we selectively review recent neuropsychological and functional neuroimaging evidence relevant to the question of the functional significance of this activity. We argue that the balance of the evidence suggests that the angular gyrus supports the representation of retrieved episodic information, and that this likely reflects a more general role for the region in representing multi-modal and multi-domain information.
It is a privilege to contribute to this special issue honoring Glyn Humphreys. Our aim is to provide a selective review of recent research and theoretical developments relating to the role of lateral parietal cortex in memory retrieval, building on an earlier review by Vilberg and Rugg (2008). In doing so, we will touch on the role of this region in language and attention, topics covered in several papers authored by Glyn (e.g. Humphreys et al., 1994; Shalev et al., 2008; Mavritsaki et al, 2009; Sui et al., 2015).
Our review will focus largely on fMRI studies of retrieval-related BOLD activity in ventral lateral parietal cortex (VLPC), most notably, the angular gyrus. We will also discuss the effects on memory performance of lesions and transcranial magnetic stimulation (TMS) to lateral parietal cortex. Going beyond the scope of the earlier review (Vilberg and Rugg, 2008), we will discuss the mnemonic role of VLPC from the perspective of large-scale brain networks and in light of other cognitive domains in which the region has been implicated. The recent review by Sestieri et al. (2017) provides a complementary perspective to the one presented here.
Neuroanatomy of Lateral Parietal Cortex
Until fairly recently, functional neuroimaging researchers typically divided the VLPC into just two regions, Brodmann Areas (BA) 39 and 40, corresponding with the angular gyrus and supramarginal gyrus respectively. Modern cytoarchitectonic evidence suggests however that the VLPC is a mosaic of at least seven areas (Caspers et al. 2006), including two that fall roughly within the angular gyrus (Figure 1). These anatomical findings correspond well with parcellations of the VLPC based upon functional neuroimaging data derived either from patterns of resting state fMRI connectivity (e.g. Nelson et al., 2010; Wang et al., 2017) or task-related co-activations (Wang et al., 2017). Together, these anatomical and functional data suggest the potential for considerable functional heterogeneity within the VLPC, and caution against attempts to characterize its function in overly general terms. Importantly, there is a high level of inter-individual variability in the sizes and boundaries of the areas illustrated in Figure 1 (Caspers et al., 2006). We discuss a possible implication of this variability toward the end of the present review.
Figure 1.
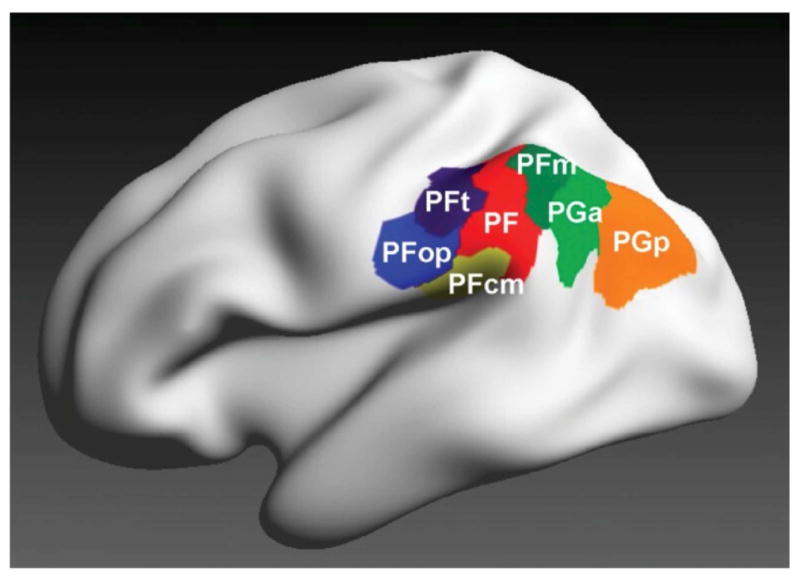
3-D maximum probability parcellation of the VLPC based on the cytoarchitectonic data of Caspers at el. (2006). The angular gyrus comprises regions PGa and PGp. Reproduced from Caspers et al. (2013) with permission of the authors and publisher.
The Dual-Process Framework
It is difficult to discuss possible contributions of lateral parietal cortex to memory retrieval without reference to ‘dual-process’ models of recognition memory. According to these models (e.g. Mandler, 1980; Yonelinas, 2001; Wixted and Mickes, 2010), a recognition memory judgment can be supported by two functionally distinct memory signals, known as ‘recollection’ and ‘familiarity’. Recollection occurs when a recognition test item elicits retrieval of qualitative information about the study episode. This information includes not only the identity of the studied item, but also details about the context in which it was studied. By contrast, familiarity supports an undifferentiated sense of past occurrence that is devoid of contextual information. Whereas both recollection and familiarity can support simple memory judgments such as discriminating between ‘old’ and ‘new’ test items, judgments that require access to the content of an episode – for example, accurate source memory judgments (successful retrieval of a contextual feature of the study episode, as indexed by a discriminative response) or ‘Remember’ judgments (introspective report of contextual retrieval) – are held to depend largely on recollection. Disagreements over important details of the two-process framework remain – for example, whether the memory signal associated with recollection is better modeled as a threshold or a continuous process (Parks and Yonelinas, 2009; Norman, 2010; Wixted and Mickes, 2010), and whether the two memory signals are ever combined to form a unitary signal (Mickes and Wixted, 2010). Nonetheless, the fundamental idea that recognition memory test items can elicit two functionally distinct memory signals is widely, albeit not universally (e.g. Berry et al., 2012), accepted.
A very different dual-process framework - involving the distinction between ‘top-down’ and ‘bottom-up’ attentional control - is also relevant in the present context. According to this framework (e.g. Corbetta et al., 2008), selective attention can be directed to external or internal events through one of two processes: a proactive, goal-directed process, and a reflexive process that operates like a ‘circuit-breaker’ (Astafiev et al., 2006) to orient attention toward a currently unattended but salient event. These two forms of attentional control have been associated with two large-scale brain networks referred to as the ‘dorsal’ (top-down) and ‘ventral’ (bottom-up) attention systems. Of most relevance here, the intra-parietal sulcus (IPS) is held to play a key role in the dorsal system, and a region centered on the temporo-parietal junction (TPJ) (roughly the border between areas PF and PFcm in Figure 1) is considered to be a major component of the ventral system. To accommodate findings from the episodic memory literature (and other cognitive domains, such as semantic memory) some authors have proposed that the role of the VLPC in bottom-up attentional control extends beyond the TPJ to include the angular gyrus (e.g. Cabeza, 2008; Cabeza et al., 2012; G.F Humphreys and Lambon-Ralph, 2015).
Recollection, Familiarity, and Lateral Parietal Cortex
The relevance of dual-process models of recognition memory to retrieval-related lateral parietal activity can be well appreciated from Figures 2 and 3. The figures give clear examples of the striking dissociation in retrieval-related BOLD activity between dorsal and ventral lateral parietal cortex that has been consistently reported over the past 15 or so years (for reviews of early studies see Vilberg and Rugg, 2008; Spaniol et al., 2009; Kim, 2010). As is evident from the figures, BOLD activity within the left intraparietal sulcus (IPS) tracks item familiarity strength, but is insensitive to whether a test item is successfully recollected. By contrast, activity centered on the left angular gyrus is insensitive to differing levels of item familiarity, and is enhanced exclusively for items eliciting successful recollection. Consistent with the previously cited reviews (see also Frithsen and Miller, 2014), Figure 3 demonstrates that this dissociation does not depend on the memory test employed to operationalize recollection and familiarity, and is consistent across a variety of different stimulus materials. In addition, as is clear from Figure 3, retrieval-related activity in both the IPS and VLPC is strongly left-lateralized (see also Guerin and Miller, 2009). In the case of the VLPC, we suspect that this reflects the dependence of episodic memory on semantic and conceptual processing, which is supported by a left-lateralized network that includes the VLPC (see Angular Gyrus and the Core Recollection Network below, and Binder et al., 2009). This is not to say, of course, that lateral parietal cortex in the right hemisphere plays no role in memory retrieval, or that right parietal retrieval-related activity is never detected (see Vilberg and Rugg, 2008, for further discussion).
Figure 2.
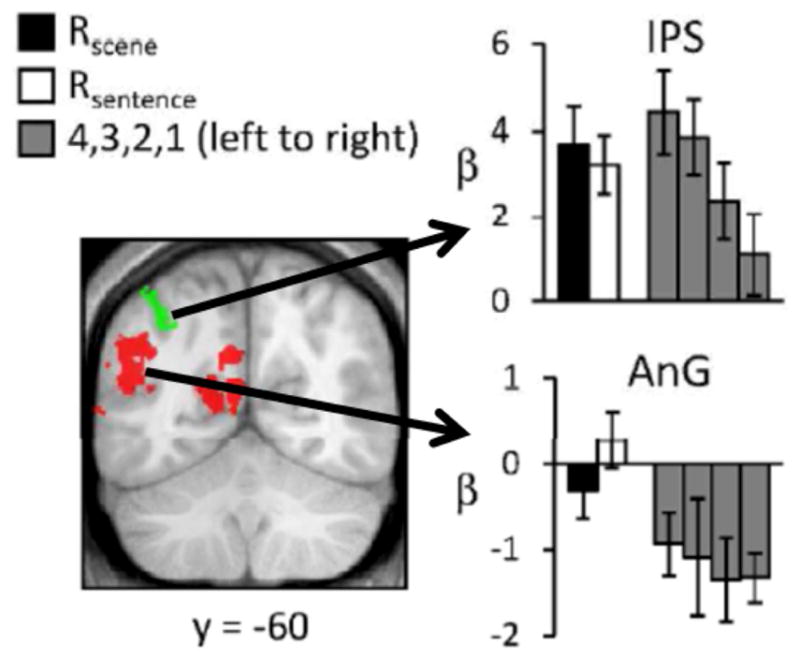
Retrieval-related activity elicited by test items engendering recollection (R scene, R word), and by unrecollected items ranked according to their familiarity strength, as operationalized by confidence ratings (4 = confident old, 1 = confident new). Data from Johnson et al. (2013). Redrawn with permission of the authors.
Figure 3.
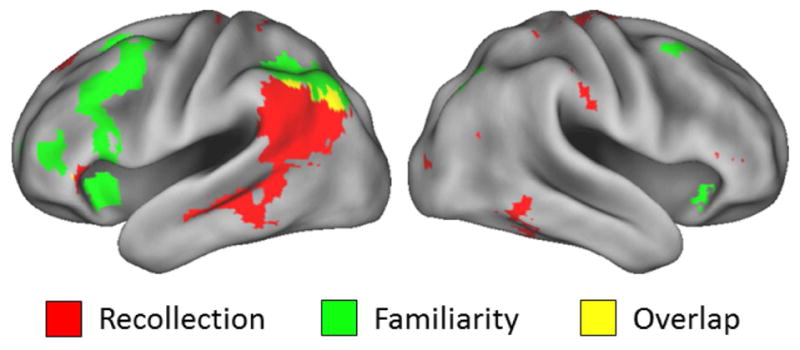
Overlap between recollection and familiarity effects from three independent experiments originally described in King et al. (2015). Experiment 1 (N=36) was a test of associative recognition involving word pairs. At test, ‘intact’ pairs were presented as they were at study, ‘rearranged’ pairs were studied words presented with different words than at study, and ‘new’ pairs were words not presented at study. Recollection was operationalized as the contrast between intact test pairs correctly endorsed as intact vs. intact pairs wrongly endorsed as rearranged. Familiarity was operationalized as the contrast between intact pairs wrongly endorsed as rearranged vs. correctly endorsed novel pairs. Experiment 2 (N=24) employed a Remember/Know/New recognition memory test. Study items were a mixture of words and pictures, and all test items were words. Recollection was operationalized by the contrast between correctly recognized items endorsed as Remembered vs. Known. Familiarity was operationalized by the contrast between items endorsed as Known vs. unstudied items correctly endorsed as New. Experiment 3 (N=28) employed a test of source memory. Test items were studied and unstudied pictures and the requirement was to make a three-way judgment: studied source A, studied source B or unstudied. Recollection was operationalized as the contrast between studied items receiving correct vs. incorrect source judgments, and familiarity was operationalized as the contrast between items receiving incorrect source judgments vs. correctly judged unstudied items. The figure illustrates the main effects of recollection and familiarity (voxel-wise threshold of p<.001), inclusively masked by the simple effect in each experiment (p<.01). Redrawn with permission of the authors.
We have previously argued (e.g. Vilberg and Rugg, 2008; Johnson et al., 2013) that, regardless of the precise functional interpretation of the retrieval effects identified in the IPS and VLPC, these effects constitute strong evidence against ‘single process’ accounts of recognition memory, in which familiarity- and recollection-based recognition reflect differences in the ‘strength’ of a single, continuously-varying memory signal (e.g. Heathcote et al., 2006; Berry et al., 2012). Rather than delve further into the intricacies of models of recognition memory, however, here we use these findings simply to support the claim that retrieval-related activity in the VLPC is selectively associated with retrieval of qualitative information about a prior episode, and not with successful recognition memory more generally. This sets the scene for discussion of the functional significance of this activity and, more broadly, the role of the VLPC in episodic memory retrieval.
Effects of Lateral Parietal Lesions
In light of functional neuroimaging evidence suggesting a mnemonic role for lateral parietal cortex, several studies have examined the effects of lateral parietal lesions on memory performance. Unlike functional neuroimaging, lesion studies can support direct inferences about the causal role of a given brain region in cognition, but three well-known caveats are worth reiterating. First, lesions rarely honor areal boundaries, and typically damage not only cortex, but underlying white matter also. Therefore, the locus of the lesion responsible for an observed impairment can be difficult to determine. Second, patients were typically tested months or years post-lesion. Thus, the functional contribution of the lesioned area in the intact brain runs the risk of underestimation due to the possibility of functional reorganization over the time since the lesion. Third, when memory is tested for information learned post-lesion a mnemonic deficit cannot definitively be ascribed to impaired retrieval, since the possibility of an encoding impairment cannot be ruled out. In the case of the angular gyrus this possibility is not merely theoretical: several fMRI studies have implicated the region in episodic memory encoding (e.g. Wong et al., 2013; de Chastelaine et al., 2016; Lee et al., 2017).
Despite considerable variability in the procedures and materials employed to test memory, early reports of the effects of lateral parietal lesions were fairly consistent: while accuracy on simple recognition and source memory judgments was typically unimpaired, relative to controls, parietal patients expressed lower confidence in these judgments (Ally et al., 2008; Haramati et al, 2008; Simons et al., 2010; Hower et al., 2014), and were less likely to report that recognized items engendered a subjective experience of recollection (as this is indexed by the relative frequency of ‘Remember’ judgments; Davidson et al., 2008). This seeming ‘experiential blunting’ is well illustrated by the findings of Hower et al. (2014). Using a 6-step confidence scale (‘sure new’ to ‘sure old’), the authors contrasted the recognition memory performance of two patients with large bilateral parietal lesions with that of a group of matched controls. Neither recognition accuracy nor the willingness to make highly confident ‘new’ judgments differed between the patients and controls. However, both patients demonstrated a pronounced reluctance to rate their ‘old’ judgments as highly confident, suggestive of impaired conscious access to the quality of the memory signal that served as the basis for the judgments. The findings constitute strong evidence for an association between lateral parietal cortex and memory confidence, but also exemplify the difficulty in ascribing the association to a specific anatomical region (e.g. dorsal vs. ventral). This difficulty is compounded by the employment of a memory test – item recognition memory – that does not permit segregation of familiarity- and recollection-based memory judgments. Thus, it is unclear whether the impairment reported by Hower et al. (2014) is attributable to damage to lateral parietal regions that support recollection specifically, or recognition memory more generally.
Somewhat analogous findings were reported in a recent study by Ciaramelli et al. (in press). Using a multi-featural source memory test, the authors compared the performance of seven patients with unilateral parietal lesions (5 left-sided) with the performance of a large control group. The test required participants to first make a yes/no recognition judgment on a series of studied and unstudied words, rating their confidence in the judgment. For any word judged old, participants went on to make a Remember/Know judgment and then, separately, tried to recall the color and the spatial position in which the word had been studied. As a group, the patients demonstrated no impairment in the accuracy of their recognition or source memory judgments but, echoing prior findings, they showed a weak tendency toward lower confidence in their recognition decisions. More interesting was a striking divergence between patients and controls in the relationship between Remember and source memory judgments. Whereas the likelihood of a Remember judgment did not differ between the groups on trials where one or neither source feature was retrieved, on trials where both features were successfully retrieved, compared to the patients, controls were significantly more likely to make a Remember rather than a Know judgment. Ciaramelli et al. (in press) interpreted these findings as evidence for an impairment of the subjective experience of recollection in their patients. They argued that whereas controls used the richness of the experience accompanying multi-featural recollection as an important basis for endorsing an item as ‘Remembered’, the patients were less able to translate recollection of the two features into a correspondingly rich experience and, instead, endorsed items as Remembered whenever recognition confidence was high. Intriguingly, the authors also reported that while their patients did not demonstrate impairment of either single of multi-featural source memory as a group, loss of tissue specifically in the angular gyrus reduced the likelihood of successfully retrieving both of the source features of a recognized test item. This finding hints at the possibility that the angular gyrus not only contributes to the subjective experience of recollection, but that it also allows access to episodic information that can support performance on ‘objective’ memory tests.
In a striking departure from the findings described thus far, Ben-Zvi et al. (2015) reported that unilateral parietal lesions can be associated with clear impairments in memory accuracy. The authors studied a large sample of patients (up to N=48, 13 left-sided, depending on the experiment). In three different experiments, participants studied pairs of items (word-word, picture-picture, and picture-sound pairs respectively). At test, they were required to recall the second item when cued with the first. Left-lesioned patients demonstrated lower recall than their matched controls in all three experiments, while right-lesioned patients were impaired on the picture-sound test only. Importantly, lesion-behavior correlations identified the angular gyrus as a region critical for the manifestation of these deficits. The findings strongly suggest a necessary role for the VLPC in episodic memory when the test item is an associative rather than a ‘copy’ cue.
Another way to establish the causal role of a brain region in a cognitive process is to temporarily disrupt its function using TMS. This approach has a major advantage over lesion studies for the investigation of memory retrieval since stimulation can be applied after the study phase, eliminating the possibility of disrupted encoding. Despite this advantage, to our knowledge, only four published studies – two employing disruptive stimulation time-locked to the presentation of the experimental items, and two employing ‘off-line’ stimulation - have adopted the approach to examine the role of VLPC in episodic retrieval (we emphasize published studies, wondering, on the basis of our own experience with TMS, whether this might be a research topic that has accumulated a substantial ‘file-drawer’). In an early study, Rossi et al. (2006) reported that TMS targeted at lateral parietal cortex during a recognition memory test had a null effect (although see Vilberg and Rugg, 2008, for further discussion of this study). Three recent studies, each including a condition in which stimulation was targeted specifically at the left angular gyrus, yielded more positive results. In Sestieri et al. (2013), a source memory test was undertaken during sham stimulation, and while stimulation was applied to either left dorsal (IPS) or ventral (posterior angular gyrus – in the vicinity of PGp) parietal cortex. Relative to dorsal parietal stimulation, but not to the sham condition, stimulation of the angular gyrus resulted in a small reduction in recognition accuracy. Additionally, while accuracy of source memory was unaffected, angular gyrus stimulation caused a significant shift in response bias in favor of one of the two sources.
In Yazar et al. (2014) participants encoded word pairs spoken in a male or female voice. In the ensuing test phase one member of each pair was presented and both a source judgment (male or female voice?) and recall of the word’s associate were required. Theta burst TMS (which depresses cortical function for some minutes subsequent to stimulation) was delivered either to the vertex, the IPS, or the angular gyrus (on the border of PGa and PGp) during the study-test interval. The only significant effect was a very modest reduction in the confidence of source judgments following angular gyrus stimulation. In a more recent study (Yazar et al., 2017) participants studied scenes containing a prominent object and concurrently heard the name of the object spoken in one of two accents. In three different test conditions they were presented with the names of studied and unstudied objects and, for objects judged old, were required either to retrieve a spatial feature of the object (e.g. left or right side of the scene), the conjunction of two spatial features (right/top; right/bottom, etc) or the conjunction of a spatial feature and the accent in which the object had been named (e.g. top/scottish; top/english, etc.). Theta burst stimulation was targeted either to the vertex or to the same angular gyrus region as previously. The only significant effect was a small reduction in source accuracy when conjoint retrieval of auditory and visual information was required. The authors interpreted this as evidence that the angular gyrus contributes specifically to the integration of retrieved multi-modal episodic information.
In summary, the balance of the evidence from studies examining memory performance after disruption of lateral parietal cortex suggests that the region likely supports processes responsible for aligning subjective experience with the strength or quality of the memory signal elicited by a retrieval cue, as this is assessed by an ‘objective’ memory test (to our knowledge, it remains to be established whether lateral parietal damage leads to a disconnection between subjective experience and objective performance in other cognitive domains also). Importantly, more recent findings suggest that there are circumstances in which VLPC, and more specifically, the angular gyrus, also contributes to the accuracy of recollection-based memory judgments.
Functional Significance of Recollection-Related activity in the VLPC
The data reviewed so far indicate that the VLPC is not only consistently engaged when a retrieval cue engenders successful recollection, but that it likely has some sort of causal role in recollective processing. The data do not, however, provide a consistent picture of the nature of this role. In this section, we review recent fMRI evidence pertinent to this issue.
One insight into the likely functional significance of retrieval-related activity in the VLPC comes from studies in which the activity was contrasted according to the amount of information recollected. In three early studies (Vilberg and Rugg, 2007, 2009a b) it was reported that angular gyrus recollection effects covaried with amount of recollected information. [Importantly, highly analogous findings have been reported for the modulation of retrieval-related event-related potentials (ERPs) over the left parietal scalp (e.g. Vilberg et al. 2006; Vilberg and Rugg, 2009a). As was discussed by Vilberg and Rugg (2008), the onset latency of these effects (ca. 400 ms post-stimulus) provides some reassurance that recollection-related activity in the VLPC occurs sufficiently early to play a causal role in recollection-based memory judgments.] These findings (see Hutchinson et al., 2014 for similar results) helped motivate an early proposal about the mnemonic role of the VLPC – namely, that it supported, or perhaps served as, an ‘episodic buffer’ (Vilberg and Rugg, 2008; Guerin and Miller, 2011), a putative component of working memory that temporarily stores multi-modal content (Baddeley, 2000). A recent study has revealed an intriguing analog to these earlier findings in the domain of autobiographical memory (Rissman et al. 2016). The authors employed as test items short series of photos that represented either an event that had been personally experienced by a participant 1–3 weeks previously (as recorded by a body camera), or an event experienced by another participant. The test task included the requirement to rate events experienced by the participant as either ‘strongly’ or ‘moderately’ recollected, or as strongly or moderately familiar. Using multi-voxel pattern analysis (MVPA), Rissman et al. (2016) identified populations of voxels where retrieval-related activity reliably discriminated test trials attracting the different ratings. Voxels within the angular gyrus discriminated both between events rated as recollected or familiar, and between events rated as strongly or moderately recollected. The same voxels did not however support above chance classification of trials segregated by familiarity strength. These findings nicely complement the results of the more conventional laboratory-based experiments described previously.
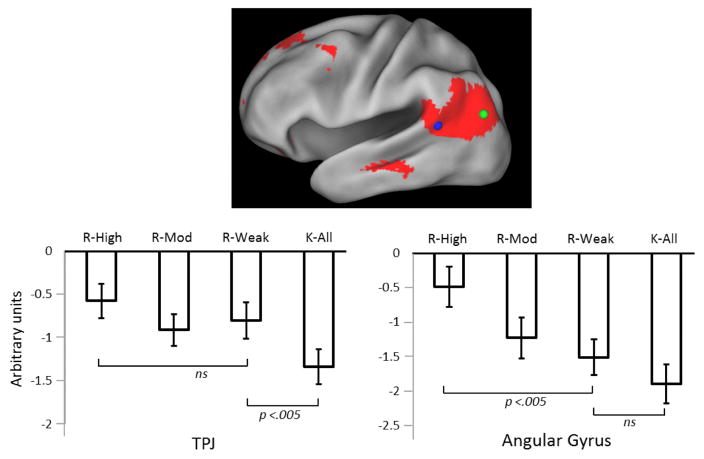
In other recent studies, VLPC retrieval effects were examined as a function of the accuracy and confidence of source memory judgments (Yu et al., 2012; Leiker and Johnson, 2015; Thakral et al., 2015). The rationale for these studies is based on the assumption that confidence covaries with the ‘quality’ or ‘fidelity’ of source-specifying information, and hence serves as a proxy for the amount of task-relevant information retrieved about a prior episode. This assumption is buttressed by the well-attested finding that the confidence of a source judgment is strongly correlated with its accuracy (e.g. Mickes et al., 2009). In each of the above-cited studies, highly confident source judgments elicited greater retrieval-related activity than judgments made with lower confidence. The findings of Yu et al. (2012) are especially instructive. Participants were required to make both a Remember/Know and a source memory judgment for each test item judged to be ‘old’. As is illustrated in Figure 4, the difference in the angular gyrus activity elicited by ‘Remember’ trials associated with inaccurate/low confidence source judgments and ‘Know’ trials was modest (and did not achieve statistical significance). By contrast, Remember trials associated with highly confident source judgments elicited substantially greater activity than that elicited on Remember trials where source judgments were unconfident or inaccurate. The findings suggest that retrieval-related activity in the angular gyrus covaries not with whether a retrieval cue elicits a subjective experience of recollection, but with the amount or the fidelity of the retrieved episodic information. As is also evident from Figure 4, a region in the vicinity of the TPJ demonstrated a qualitatively different pattern of activity. Relative to Know trials, activity was enhanced for Remember trials regardless of the accuracy or confidence of the associated source judgment. This was interpreted as evidence that, as proposed by Cabeza and colleagues (e.g. Cabeza, 2008; Cabeza et al. 2008), items eliciting a subjective sense of recollection can initiate attentional re-orienting, consistent with the proposed role for the TPJ as an attentional ‘circuit-breaker’ (Astafiev et al., 2006). A more striking dissociation between retrieval-related activity in the angular gyrus and the TPJ was reported by Hutchinson et al. (2014). As was already noted, these authors described a graded recollection effect in the angular gyrus. This effect was essentially reversed in the TPJ, such that recollected items elicited lower activity than did unstudied items or items attracting incorrect source judgments. The findings strongly suggest that retrieval-related activity in the TPJ and angular gyrus does not reflect a common process.
Figure 4.
Retrieval-related activity in angular gyrus (green circle) and the TPJ (blue circle) as a function of Remember/Know and source memory judgments. R-High, R-Mod and R-Weak refer to items endorsed as Remembered and attracting highly confident source memory judgments, moderately confident judgments, and a mixture of low confidence and inaccurate judgments respectively. K-All and K-Weak refer to items endorsed as Known collapsed across all levels of source confidence and accuracy, and associated with low confidence/inaccurate judgments respectively. Redrawn from Yu et al. (2012) with permission of the authors.
The idea that the angular gyrus contributes to the fidelity (or, in the authors’ terminology, the precision) of memory judgments receives further support from a recent experiment reported by Richter et al. (2016). The authors employed a memory test that required a rating of the vividness with which each study trial (comprising the pairing of a scene and three objects) was remembered, and in addition, allowed estimation of the precision of the memory for three features (location, color and orientation) of the associated objects. Retrieval-related activity in the angular gyrus did not covary with the vividness of the retrieved memory, but did covary with the precision of memory for the object features. Thus, as with the data of Yu et al. (2012), the findings suggest that the angular gyrus supports processes that contribute not so much to subjective aspects of memory, but to the accuracy of memory-guided judgments.
Angular Gyrus and the Representation of Mnemonic Content
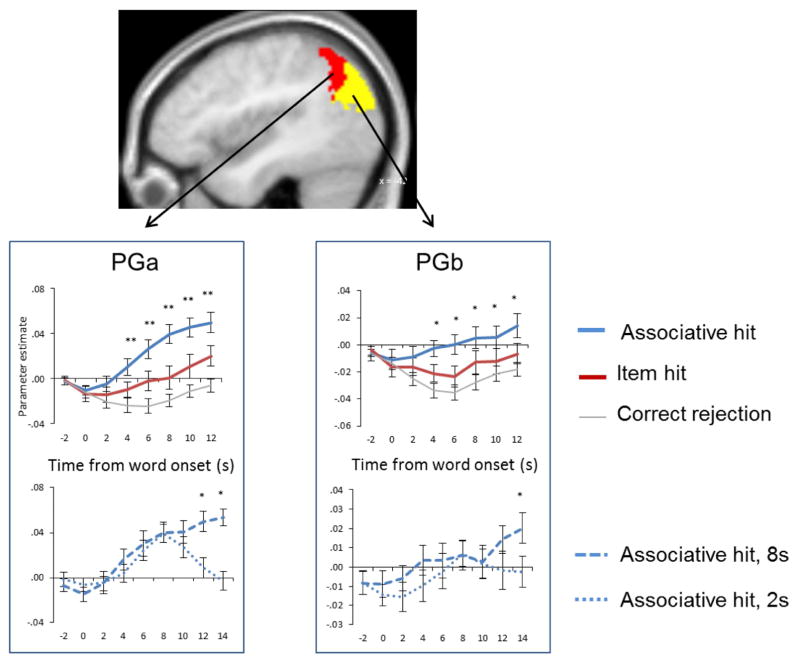
An enduring debate has centered on whether angular gyrus recollection effects reflect a role for the region in the representation of retrieved content (e.g. Vilberg and Rugg, 2008), or in re-directing attention toward the content (e.g. Cabeza, 2008). The finding that the effects covary with the amount and fidelity of recollected information (see above) has been taken as evidence for some kind of representational account, but this has not gone unchallenged (Cabeza et al., 2012). Vilberg and Rugg (2012) reasoned that if the angular gyrus does have a role in representing retrieved content, then activity in the region should persist for as long as the content is held in memory after it has been retrieved. This prediction was examined in two experiments (Vilberg and Rugg, 2012, 2014). In the first experiment, participants studied word-picture pairs. At test, they were presented with studied and unstudied words, followed after an unpredictable delay, ranging between 2s and 8s, by a response cue. For each word judged as studied, the requirement was to retrieve the associated picture and to hold it in mind until the onset of the cue, which signaled which of three possible judgments should be made about the denoted object. If recollection failed, a ‘don’t know’ response was to be made instead. In the second experiment (Vilberg and Rugg, 2014), the identical procedure was employed except that the pictures served as the retrieval cues, and the words as the to-be-retrieved associates. The results of the two studies were very similar: whereas medial temporal and cortical midline regions demonstrated transient, delay-independent responses on trials where the associate was successfully recollected, recollection-related activity in the angular gyrus (PGa and PGb in experiment 1, PGa only in experiment 2) tracked the delay period (see Figure 5), as did activity in middle temporal gyrus. These findings appear incompatible with a role for the region in the triggering of attentional re-orienting to recollected content (a role that would arguably be reflected in a transient, not a sustained response), and point instead to some sort of a role in the representation of the content.
Figure 5.
Sustained angular gyrus recollection effects. Upper graphs show across-subject time-courses for recognized test items for which recollection of the associated picture was successful (associative hit) or unsuccessful (item hit). Lower graphs illustrate time-courses for recollection-related activity across delay periods of 2 and 8s. Redrawn from Vilberg and Rugg (2012) with permission of the authors.
The results offer little insight about the nature of this role, however. Several possibilities seem plausible: for instance, the region might directly support representation of recollected information, perhaps acting as an interface between episodic memory and executive processing, as was suggested by Vilberg and Rugg (2008). Another, related, possibility is that the region acts to bind the different features of an episode into a multi-modal representation (Shimamura, 2011). Alternatively, the region might support attentional or control processes that operate on content that is represented elsewhere (Vilberg and Rugg, 2012; cf. Noonan et al., 2013).
Adjudicating between these alternatives is a formidable task, but some recent fMRI findings hint in favor of some kind of direct representational account. The findings come from studies in which MVPA was employed to examine whether activity within the angular gyrus differs according to mnemonic content. Each of these studies included an approach that has been employed to examine retrieval-related ‘reinstatement’ more generally (e.g. Johnson et al., 2009). The approach involves training a multivariate classifier to discriminate patterns of activity elicited by two or more study conditions, and then using the classifier to ‘decode’ retrieved content from the same voxels during a subsequent test phase. To the extent that decoding is successful, it can be inferred that when the studied information is retrieved from memory it is, in some sense, ‘re-represented’ within the voxel set.
To our knowledge, four studies have examined whether classifier-based reinstatement effects can be identified in voxels restricted to the angular gyrus. In one study (Leiker and Johnson, 2015) classifier performance did not exceed chance level, despite robust evidence for reinstatement in other cortical regions. In contrast, Kuhl et al. (2013) reported that remembered content could be decoded from the angular gyrus. The experiment involved a series of short study-test cycles. Participants studied word-scene and word-face pairs. Some of the studied words were then re-presented and participants attempted to recall either the category of the picture associated with the word (face vs. scene), or the location in which the picture had been presented. Regardless of which memory test was undertaken, a classifier trained on study activity in the angular gyrus to discriminate between the word-scene and word-face pairs was also above chance in classifying the study history of the test words. Kuhl and Chun (2014) employed a similar experimental design. In this case, however, participants attempted to covertly recall the specific picture associated with each test word, rating the vividness of the resulting memory (vivid, weak, or ‘don’t know’). The test phase terminated with an old/new recognition memory test for the studied scenes and faces. A classifier trained to discriminate between the two study categories (i.e. faces and scenes) was able to classify trials in the recall phase on which participants reported vivid or weak memories at above chance level. Classifier accuracy did not vary according to rated vividness (despite robustly greater across-voxel mean BOLD signal on trials rated as vivid rather than weak), but was at chance when recall failed.
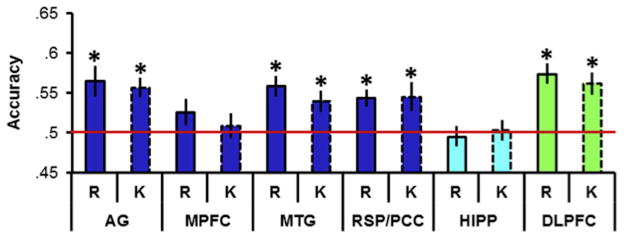
In another experiment examining classifier-based decoding of retrieved content (Thakral et al., 2017), participants studied a series of words and pictures, making different judgments depending on the type of material. Test items comprised words only; some were unstudied, while the remainder corresponded either to studied words or pictures. The task was to make a Remember/Know/New judgment to each item. A classifier trained on voxels within the angular gyrus to discriminate the two study conditions was able to classify the study history of test trials endorsed as Remembered robustly above chance, echoing the findings of Kuhl et al. (2013) and Kuhl and Chun (2014) described above. Classifier performance was however equally accurate for trials attracting a Know judgment (see Figure 6); that is, for trials on which ostensibly there was no conscious recollection of any contextual detail of the study episode. This finding is especially striking given that the mean BOLD signal in the region of the angular gyrus containing the voxel set used to train the classifier demonstrated a robust difference between Remember and Know trials.
Figure 6.
Classification accuracy for retrieved content (words vs. pictures) of a classifier trained on the activity elicited by the two classes of study trial. Accuracy is shown for six regions of interest, angular gyrus (AG), medial prefrontal cortex (MPFC), middle temporal gyrus (MTG), retrosplenial/posterior cingulate cortex (RSC/PCC), hippocampus (HIPP) and left dorsolateral prefrontal cortex (DLPFC). R = trials endorsed as Remember, K = trials endorsed as Know. Red line signifies chance accuracy of 50%. * = classification accuracy significantly (p < .05) greater than chance after Bonferroni correction. Redrawn from Thakral et al. (in press) with permission of the authors.
The MVPA findings described above indicate that, despite the seeming indifference of mean signal changes (recollection effects) in the angular gyrus to the content of recollected information, more fine-grained measurements of neural activity within the region show that it is in fact sensitive to retrieved content. Moreover, the region manifests this sensitivity by recapitulating patterns of activity elicited when the content was initially encoded. These findings suggest that the role of the angular gyrus in memory retrieval extends beyond the support of generic processes that are insensitive to the nature of the retrieved content.
That being said, the findings do not provide unqualified support for the idea that the region plays a role specifically in the representation of recollected content. As already noted, in the two studies where the question was addressed, decoding accuracy did not covary with subjective ratings of the quality of recollection (vividness in Kuhl and Chun, 2014, Remember vs. Know in Thakral et al., 2017), despite robust differences in mean signal across the voxels used to train the MVPA classifiers. There are a number of possible reasons for these findings (discussed in Thakral et al., 2017), but taken at face value they are difficult to reconcile with the notion that angular gyrus recollection effects reflect a role for the region in representing the content specifically of a recollected experience. Furthermore, it is noteworthy that classifier-based decoding of recollected content appears to be near-ubiquitous throughout the cortex. As is illustrated in Figure 6, Thakral et al. (2017) reported similar levels of decoding in some other members of the ‘core recollection network’ (see below) as well as in a region – left dorsolateral prefrontal cortex (PFC) – where recollection effects were undetectable. Similarly, Kuhl and Chun (2014) examined reinstatement in 10 different ROIs, and reported above-chance classification performance in every case (similar findings were also reported by Kuhl et al., 2013). These findings are consistent with other studies in which classifier-based MVPA identified retrieval-related reinstatement in sets of voxels that were widely distributed across the cortex (e.g. Johnson et al., 2009; Wang et al., 2016).
MVPA classifiers are however sensitive only to patterns of activity that are shared across a set of training trials; activity that is idiosyncratic to a specific trial is discounted. Thus, the findings described above may not generalize to MVPA approaches that assess study-test overlap at the single item level, raising the possibility that it is item- rather than category-level measures of reinstatement that track quality or fidelity of recollection. Consistent with this possibility, item-level reinstatement has been reported to covary with ratings of the strength or vividness of retrieval (Ritchey et al., 2013; Wing et al., 2015). In neither of these studies, however, were reinstatement effects identified in the angular gyrus. This is not to say that activity patterns in the region cannot encode information at the single item level. In the study of Kuhl and Chun (2014) described above, activity elicited on successful recall trials (those given strong or weak vividness ratings) was more similar to the activity elicited by the recognition memory test items that corresponded to the recalled item than it was to other test items belonging to the same study category. And Lee and Kuhl (2016) reported that activity patterns in the angular gyrus elicited by a large set of faces could be exploited to ‘reconstruct’ other faces that had been left out of the training set, both while the left-out faces were being viewed and while they were retained in working memory. Equally striking are data reported by Chen et al. (2017). In this study participants were scanned while they viewed a movie, and again while they recalled scenes from it. The authors were able to identify populations of voxels where there was a correspondence between patterns of activity elicited during the viewing of a given scene and when the scene was later recalled. They also identified voxels where patterns of activity during the recall of specific scenes were shared across participants. Both analyses revealed widespread regions where scene-specific pattern similarity was statistically significant, including bilateral angular gyrus. The findings converge with those described earlier to indicate that across-voxel activity patterns in the angular gyrus contain information about specific content, but that this is no less true of several other cortical regions.
In summary, classifier-based MVPA of retrieval-related activity in the VLPC has clearly demonstrated that the activity can discriminate between different categories of retrieved information, but has not convincingly demonstrated that the accuracy of discrimination varies with recollection success or the ‘strength’ of recollection. MVPA has also demonstrated that patterns of activity within the angular gyrus can discriminate between individual items or events. Again, though, it remains to be shown that this capacity covaries with the quality or fidelity of recollection. Thus, present findings are consistent with the idea that the angular gyrus directly supports the representation of recollected content, but they do not compel this conclusion.
Angular Gyrus and the Core Recollection Network
The angular gyrus is only one of a number of regions that are consistently co-activated in association with successful recollection (Rugg and Vilberg, 2013). These regions – which, along with the angular gyrus, include medial PFC, posterior cingulate, hippocampus, parahippocampal cortex and the middle temporal gyrus – have been referred to as the core recollection network (King et al., 2015). Recent evidence indicates that a key property of recollection-related activity in the angular gyrus – its sensitivity to the amount or fidelity of recollected information (see above) – is a characteristic of the network as whole (Hayama et al., 2012; Johnson and Leiker, 2015; Thakral et al., 2015). And, as noted previously and illustrated in Figure 6, the angular gyrus is not the only member of the network from which retrieved content can be decoded. Indeed, perhaps the sole functional characteristic of recollection-related activity in the angular gyrus that consistently distinguishes it from other members of the core network is its time-course (Vilberg and Rugg, 2012, 2014), and even this characteristic is shared with the middle temporal gyrus. [In a seeming contradiction to this claim, Richter et al. (2016; see above) reported that retrieval-related activity in the angular gyrus was sensitive to memory precision, but not to retrieval success, while the opposite pattern prevailed in the hippocampus. Measures of precision and success in this study were correlated, however (r = .53). We think it possible that if the effects of the measures had been examined in separate statistical models, retrieval-related activity in each region would have turned out to be sensitive to both measures].
Of course, we are not suggesting that the functions of the angular gyrus are completely redundant with those of other components of the recollection network (indeed, in the domain of semantic memory, findings from a recent TMS study point to dissociable contributions of the angular gyrus and middle temporal gyrus to the retrieval of semantic information; Davey et al., 2015). Nonetheless, when thinking about possible roles of the angular gyrus in memory retrieval, it is helpful at the same time to think about the role of the network as a whole. This has been conceptualized in a number of ways, but most generally perhaps in terms of the retrieval, representation and manipulation of episodic and self-referential information in service of current and future behavioral goals (e.g. Benoit and Schacter, 2015). Even this very broad conceptualization might not be sufficiently general. Binder and Desai (2011) noted that essentially the same network is activated when semantic rather than episodic information is retrieved, leading these authors to propose that the network supports the representation of conceptual rather than episodic knowledge. From this perspective, the sensitivity of the network to successful recollection is because the content of an episodic memory is to a large extent ‘assembled’ from pre-existing lower-level conceptual representations. Binder and Desai (2009) went on to propose a specific role for the angular gyrus within this framework, namely, the representation of event concepts – concepts that imply an unfolding over time and space, as for example in the concept ‘birthday party’.
Angular Gyrus Engagement in Cognitive Domains other than Episodic and Semantic Memory
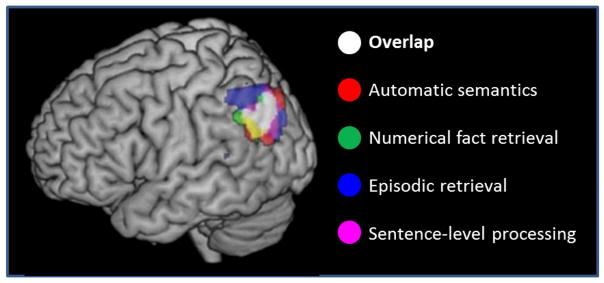
In addition to its putative role in episodic and semantic memory, neuropsychological and functional neuroimaging evidence have implicated the angular gyrus in a wide variety of other cognitive processes, including those supporting functions as disparate as retrieval of arithmetic facts and using ‘theory of mind’ to reason about others’ behavior (see Cabeza et al., 2012, Seghier, 2013, and G.W. Humphreys and Lambon-Ralph, 2015, for recent reviews). Figure 7 illustrates the extent to which experiments manipulating the level of engagement in some of these different domains give rise to anatomically overlapping effects within the angular gyrus. The disparate circumstances in which angular gyrus activation has been reported is a formidable challenge to the attempt to conceive of a single function or computation supported by the region. However, this has not deterred investigators from advancing proposals along these lines. For example, Cabeza et al. (2012) extended their ‘Attention to Memory’ model (Cabeza et al., 2008) to argue that enhanced activity in the angular gyrus (and VLPC more generally) invariably reflects bottom-up attentional capture. By contrast, Seghier (2013) proposed that the region acts as a ‘cross-modal integrative hub’ that can support many different kinds of representation generated by combining bottom-up, multi-modal inputs with top-down biasing signals. In an account that shares some elements of both of these proposals, G.F. Humphreys and Lambon-Ralph (2015) argued that angular gyrus activation reflects the engagement of an ‘automatic buffer’ that reflexively supports the temporary storage of multi-modal information. [On finding that activity in the angular gyrus tracks the difficulty of both semantic and visuospatial decisions (as operationalized by reaction time (RT)), these authors recently revised this proposal to suggest that the region temporarily stores any kind of task-relevant information that automatically captures attention (G.F. Humphreys and Lambon-Ralph, in press). We note that the proposal that angular gyrus activity invariably tracks decision difficulty does not fit well with findings from fMRI studies of episodic retrieval. For instance, as is evident from Figure 2, in the study of Johnson et al. (2013) angular gyrus activity was insensitive to the confidence of familiarity-based memory judgments. This is despite the fact that RTs for ‘unconfident old’ (level 3) judgments were on average some 200–300ms slower than either confident old or confident new judgments. And de Chastelaine et al. (2017) contrasted the activity elicited by familiar but unrecollected test items and new items. Decisions to familiar items were markedly the more difficult (RT was prolonged by approximately 240ms). There was however no evidence of differential activity in left VLPC.]
Figure 7.
Overlap in the left angular gyrus between BOLD signal increases associated with automatic access to semantic information, numerical fact retrieval, successful episodic memory retrieval, and sentence comprehension. Re-drawn from Humphreys and Lambon-Ralph (2015), with the permission of the authors.
We noted earlier that the angular gyrus comprises at least two sub-regions, and cautioned that it would be unwise to assume that the region was functionally homogeneous (see also Seghier, 2013). The reader will not have failed to notice we have largely ignored our own admonition, and have instead discussed possible roles of the angular gyrus in memory retrieval as if the region supported only a single function. While the findings discussed in the preceding paragraph might give anyone holding this view pause for thought, evidence that retrieval-related activity within the angular gyrus is functionally dissociable is sparse (see Vilberg and Rugg (2014) for a hint of such a dissociation, and Seghier (2013) for a review of functional dissociations within the region more generally). One reason for the paucity of this evidence might be the high level of inter-individual variability in the relative sizes and the boundaries of the different areas comprising VLPC, including PGa and PGp (Caspers et al., 2006). In light of this variability, it is possible that functional dissociations in fMRI data that exist at the level of individual subjects are obscured when the data are analyzed using the across-subject approaches that have usually been adopted in the field. Thus, both the homogeneity that has typically been observed within the angular gyrus for recollection effects (e.g. Figure 3), and the extent of across-domain overlap in task-related activity in the region (Figure 7), might be exaggerations or distortions of the true state of affairs. Studies employing methods that allow patterns of functional activity to be rigorously examined at the single subject level (e.g. Nieto-Castañón and Fedorenko, 2012) are needed to address this issue.
Concluding Comments
The neuropsychological and functional neuroimaging evidence that has accrued over the past few years add weight to earlier proposals (e.g. Wagner at al., 2005; Rugg and Vilberg, 2008; Shimamura, 2011) that the VLPC, and the angular gyrus in particular, might contribute directly to the retrieval and representation of episodic information. The evidence strongly suggests that the region makes its contribution as part of a large-scale functional network that is also active during the processing of non-episodic information. While it seems highly likely that the angular gyrus is functionally heterogeneous, little evidence of this has emerged thus far in respect of its putative mnemonic role or roles. This might change when retrieval-related activity in the region is examined at the single subject level (see above). A more nuanced picture might also emerge from studies employing methods – such as EEG, MEG, and electro-corticography (cf. Foster et al., 2015) - that permit retrieval-related VLPC activity to be examined at a markedly higher temporal resolution than that afforded by the fMRI BOLD signal.
What then is the mnemonic role of the angular gyrus? In our view, the balance of the evidence currently favors a role similar to the one that has been suggested in respect of semantic memory, namely, that it serves as a convergence zone (Damasio, 1998), permitting the formation of complex, multi-domain representations assembled out of lower-level representations that are distributed across multiple modality- and domain-specific cortical regions (Binder and Desai, 2009; Segehier, 2013; see also Bonnici et al., 2015, and Price et al. 2016). It has been suggested that the capacity to combine lower level representations in this fashion is quintessentially human (Murray et al., 2017); certainly, it is a capacity that appears necessary for the construction of cohesive episodic memories, which, outside of the laboratory, comprise a plethora of conceptual and perceptual features. It may be no accident that the angular gyrus is one of the cortical regions that has expanded the most disproportionately in humans compared with other primates (Hill et al., 2010).
Acknowledgments
The authors’ research described in this article was supported by National Institutes of Health grants R01MH072966 and R01AG039103.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46:1800–12. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. Eur J Neurosci. 2006;23:591–6. doi: 10.1111/j.1460-9568.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–23. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Schacter DL. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 2015;75:450–7. doi: 10.1016/j.neuropsychologia.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi S, Soroker N, Levy DA. Parietal lesion effects on cued recall following pair associate learning. Neuropsychologia. 2015;73:176–94. doi: 10.1016/j.neuropsychologia.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Berry CJ, Shanks DR, Speekenbrink M, Henson RN. Models of recognition, repetition priming, and fluency: exploring a new framework. Psychol Rev. 2012;119:40–79. doi: 10.1037/a0025464. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–36. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Richter FR, Yazar Y, Simons JS. Multimodal feature integration in the angular gyrus during episodic and semantic retrieval. J Neurosci. 2016;36:5462–71. doi: 10.1523/JNEUROSCI.4310-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–27. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–25. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012;16:338–52. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and inter-individual variability. Neuroimage. 2006;33:430–48. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S, Schleicher A, Bacha-Trams M, Palomero-Gallagher N, Amunts K, Zilles K. Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex. 2013;23:615–28. doi: 10.1093/cercor/bhs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Leong YC, Honey CJ, Yong CH, Norman KA, Hasson U. Shared memories reveal shared structure in neural activity across individuals. Nat Neurosci. 2017;20:115–25. doi: 10.1038/nn.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Faggi G, Scarpazza C, Mattioli F, Spaniol J, Ghetti S, Moscovitch M. Subjective recollection independent from multifeatural context retrieval following damage to the posterior parietal cortex. Cortex. 2017 doi: 10.1016/j.cortex.2017.03.015. in press. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Cornelissen PL, Thompson HE, Sonkusare S, Hallam G, Smallwood J, Jefferies E. Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. J Neurosci. 2015;35:15230–9. doi: 10.1523/JNEUROSCI.4705-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Ciaramelli E, Cohn M, Kim AS, Murphy KJ, Troyer AK, Moscovitch M, Levine B. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–55. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. The relationships between age, associative memory performance, and the neural correlates of successful associative memory encoding. Neurobiol Aging. 2016;42:163–76. doi: 10.1016/j.neurobiolaging.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. Independent contributions of fMRI familiarity and novelty effects to recognition memory and their stability across the adult lifespan. Neuroimage. 2017;156:340–351. doi: 10.1016/j.neuroimage.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Rangarajan V, Shirer WR, Parvizi J. Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex. Neuron. 2015;86:578–90. doi: 10.1016/j.neuron.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frithsen A, Miller MB. The posterior parietal cortex: comparing remember/know and source memory tests of recollection and familiarity. Neuropsychologia. 2014;61:31–44. doi: 10.1016/j.neuropsychologia.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB. Lateralization of the parietal old/new effect: an event-related fMRI study comparing recognition memory for words and faces. Neuroimage. 2009;44:232–42. doi: 10.1016/j.neuroimage.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB. Parietal cortex tracks the amount of information retrieved even when it is not the basis of a memory decision. Neuroimage. 2011;55:801–7. doi: 10.1016/j.neuroimage.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA. The posterior parietal cortex in recognition memory: a neuropsychological study. Neuropsychologia. 2008;46:1756–66. doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Hayama HR, Vilberg KL, Rugg MD. Overlap between the neural correlates of cued recall and source memory: Evidence for a generic recollection network? J Cognit Neurosci. 2012;24:1127–37. doi: 10.1162/jocn_a_00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote A, Raymond F, Dunn J. Recollection and familiarity in recognition memory: evidence from ROC curves. J Mem Lang. 2006;55:495–514. [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107:13135–40. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hower KH, Wixted J, Berryhill ME, Olson IR. Impaired perception of mnemonic oldness, but not mnemonic newness, after parietal lobe damage. Neuropsychologia. 2014;56:409–17. doi: 10.1016/j.neuropsychologia.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Fusion and fission of cognitive functions in the human parietal cortex. Cereb Cortex. 2015;25:3547–60. doi: 10.1093/cercor/bhu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Mapping domain-selective and counterpointed domain-general higher cognitive functions in the lateral parietal cortex: evidence from fMRI comparisons of difficulty-varying semantic versus visuo-spatial tasks, and functional connectivity analyses. Cereb Cortex. doi: 10.1093/cercor/bhx107. in press. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Romani C, Olson A, Riddoch MJ, Duncan J. Non-spatial extinction following lesions of the parietal lobe in humans. Nature. 1994;372:357–9. doi: 10.1038/372357a0. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Weiner KS, Bressler DW, Silver MA, Preston AR, Wagner AD. Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cereb Cortex. 2014;24:49–66. doi: 10.1093/cercor/bhs278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, McDuff SG, Rugg MD, Norman KA. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron. 2009;63:697–708. doi: 10.1016/j.neuron.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Suzuki M, Rugg MD. Recollection, familiarity, and content-sensitivity in lateral parietal cortex: a high-resolution fMRI study. Front Hum Neurosci. 2013;7:219. doi: 10.3389/fnhum.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50:1648–57. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- King DR, de Chastelaine M, Elward RL, Wang TH, Rugg MD. Recollection-related increases in functional connectivity predict individual differences in memory accuracy. J Neurosci. 2015;35:1763–72. doi: 10.1523/JNEUROSCI.3219-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Johnson MK, Chun MM. Dissociable neural mechanisms for goal-directed versus incidental memory reactivation. J Neurosci. 2013;33:16099–109. doi: 10.1523/JNEUROSCI.0207-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Chun MM. Successful remembering elicits event-specific activity patterns in lateral parietal cortex. The Journal of Neuroscience. 2014;34:8051–8060. doi: 10.1523/JNEUROSCI.4328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kuhl BA. Reconstructing perceived and retrieved faces from activity patterns in lateral parietal Cortex. J Neurosci. 2016;36:6069–82. doi: 10.1523/JNEUROSCI.4286-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chun MM, Kuhl BA. Lower parietal encoding activation is associated with sharper information and better memory. Cereb Cortex. 2017;27:2486–2499. doi: 10.1093/cercor/bhw097. [DOI] [PubMed] [Google Scholar]
- Leiker EK, Johnson JD. Pattern reactivation co-varies with activity in the core recollection network during source memory. Neuropsychologia. 2015;75:88–98. doi: 10.1016/j.neuropsychologia.2015.05.021. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: the judgment of previous occurrence. Psychol Rev. 1980;87:252–71. [Google Scholar]
- Mavritsaki E, Heinke D, Deco G, Humphreys GW. Simulating posterior parietal damage in a biologically plausible framework: neuropsychological tests of the search over time and space model. Cogn Neuropsychol. 2009;26:343–90. doi: 10.1080/02643290903424329. [DOI] [PubMed] [Google Scholar]
- Mickes L, Wais PE, Wixted JT. Recollection is a continuous process: implications for dual-process theories of recognition memory. Psychol Sci. 2009;20:509–15. doi: 10.1111/j.1467-9280.2009.02324.x. [DOI] [PubMed] [Google Scholar]
- Murray E, Wise S, Graham K. The Evolution of Memory Systems. Oxford University Press; New York: 2017. [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, Petersen SE. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–70. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Castañón A, Fedorenko E. Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage. 2012;63:1646–69. doi: 10.1016/j.neuroimage.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013;25:1824–50. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 2010;20:1217–27. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CM, Yonelinas AP. Evidence for a memory threshold in second-choice recognition memory responses. Proc Natl Acad Sci U S A. 2009;106:11515–9. doi: 10.1073/pnas.0905505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter FR, Cooper RA, Bays PM, Simons JS. Distinct neural mechanisms underlie the success, precision, and vividness of episodic memory. Elife. 2016;5:e18260. doi: 10.7554/eLife.18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Chow TE, Reggente N, Wagner AD. Decoding fMRI signatures of real-world autobiographical memory retrieval. J Cogn Neurosci. 2016;28:604–20. doi: 10.1162/jocn_a_00920. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Wing EA, LaBar KS, Cabeza R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb Cortex. 2013;23:2818–28. doi: 10.1093/cercor/bhs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Zito G, Vecchio F, Cappa SF, Miniussi C, Babiloni C, Rossini PM. Prefrontal and parietal cortex in human episodic memory: an interference study by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2006;23:793–800. doi: 10.1111/j.1460-9568.2006.04600.x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013;23:255–60. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. The contribution of the human posterior parietal cortex to episodic memory. Nat Rev Neurosci. 2017;18:183–192. doi: 10.1038/nrn.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Capotosto P, Tosoni A, Luca Romani G, Corbetta M. Interference with episodic memory retrieval following transcranial stimulation of the inferior but not the superior parietal lobule. Neuropsychologia. 2013;51:900–6. doi: 10.1016/j.neuropsychologia.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Shalev L, Mevorach C, Humphreys GW. Letter position coding in attentional dyslexia. Neuropsychologia. 2008;46:2145–51. doi: 10.1016/j.neuropsychologia.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11:277–91. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb Cortex. 2010;20:479–85. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–79. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sui J, Liu M, Mevorach C, Humphreys GW. The salient self: the left intraparietal sulcus responds to social as well as perceptual-salience after self-association. Cereb Cortex. 2015;25:1060–8. doi: 10.1093/cercor/bht302. [DOI] [PubMed] [Google Scholar]
- Thakral PP, Wang TH, Rugg MD. Cortical reinstatement and the confidence and accuracy of source memory. Neuroimage. 2015;109:118–29. doi: 10.1016/j.neuroimage.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Wang TH, Rugg MD. Decoding the content of recollection within the core recollection network and beyond. Cortex. 2017;91:101–113. doi: 10.1016/j.cortex.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg K, Moosavi R, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Res. 2006;1122:161–70. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–25. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;34:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Functional significance of retrieval-related activity in lateral parietal cortex: evidence from fMRI and ERPs. Hum Brain Mapp. 2009a;30:1490–501. doi: 10.1002/hbm.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Left parietal cortex is modulated by amount of recollected verbal information. Neuroreport. 2009b;20:1295–9. doi: 10.1097/WNR.0b013e3283306798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. The neural correlates of recollection: transient versus sustained fMRI effects. J Neurosci. 2012;32:15679–87. doi: 10.1523/JNEUROSCI.3065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Temporal dissociations within the core recollection network. Cogn Neurosci. 2014;5:77–84. doi: 10.1080/17588928.2013.860088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Xie S, Guo X, Becker B, Fox PT, Eickhoff SB, Jiang T. Correspondent functional topography of the human left inferior parietal lobule at rest and under task revealed using resting-state fMRI and coactivation based parcellation. Hum Brain Mapp. 2017;38:1659–1675. doi: 10.1002/hbm.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Johnson JD, de Chastelaine M, Donley BE, Rugg MD. The effects of age on the neural correlates of recollection success, recollection-related cortical reinstatement, and post-retrieval monitoring. Cereb Cortex. 2016;26:1698–714. doi: 10.1093/cercor/bhu333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing EA, Ritchey M, Cabeza R. Reinstatement of individual past events revealed by the similarity of distributed activation patterns during encoding and retrieval. J Cogn Neurosci. 2015;27:679–91. doi: 10.1162/jocn_a_00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Mickes L. A continuous dual-process model of remember/know judgments. Psychol Rev. 2010;117:1025–54. doi: 10.1037/a0020874. [DOI] [PubMed] [Google Scholar]
- Wong JX, de Chastelaine M, Rugg MD. Comparison of the neural correlates of encoding item-item and item-context associations. Front Hum Neurosci. 2013;7:436. doi: 10.3389/fnhum.2013.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazar Y, Bergström ZM, Simons JS. Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One. 2014;9:e110414. doi: 10.1371/journal.pone.0110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazar Y, Bergström ZM, Simons JS. Reduced multimodal integration of memory features following continuous theta burst stimulation of angular gyrus. Brain Stimul. 2017;10:624–629. doi: 10.1016/j.brs.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–74. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SS, Johnson JD, Rugg MD. Dissociation of recollection-related neural activity in ventral lateral parietal cortex. Cogn Neurosci. 2012;3:142–49. doi: 10.1080/17588928.2012.669363. [DOI] [PMC free article] [PubMed] [Google Scholar]