Abstract
Individual variations in animal behaviour can be used to describe relationships between different constructs, as well as the underlying neurobiological mechanisms responsible for such variation. In humans, variation in the expression of certain traits contributes to the onset of psychopathologies, such as drug addiction. Addiction is characterised by persistent drug use despite negative consequences, but it occurs in only a sub-population of drug users. Compulsive drug use is modelled in laboratory animals by punishing a drug-reinforced operant response. It has been reported that there is individual variability in the response to punishment, and in this report we aim to further define the conditions under which this variation can be observed. We have previously used footshock punishment to suppress alcohol seeking in an animal model of context-induced relapse to alcohol seeking after punishment-imposed abstinence. Here we present a re-examination of the training and punishment data from a large cohort of rats (n = 499) collected over several years. We found evidence for a bimodal distribution in the response to punishment in alcohol preferring P rats. We only observed this population split when rats received constant shock intensity for three sessions, but not when increasing shock intensity was used. This observation provides evidence for the existence of two distinct groups of rats, defined by their response to punishment, in an otherwise homogeneous population. The implications of this observation are discussed in reference to prior observations using punishment of other addictive drugs (cocaine and methamphetamine), the potential causes of this phenomenon, and with broader implications for the cause of alcohol and drug addiction in humans.
Keywords: Alcohol, Punishment, Individual differences, Compulsive, Addiction
Introduction
Individual variations in animal behaviour can be a source of frustration in behavioural studies, but if predictable, this variability can be used to describe relationships between different constructs, as well as the underlying neurobiological mechanisms responsible for such variation. The causes of individual variability in animal studies are also of interest because, in humans, variation in the expression of certain traits contributes to the onset of psychopathologies. For example, in the addiction literature, it is often noted that only a low percentage of total drug users become addicted (Anthony et al., 1994; Nutt et al., 2007). The causes of the transition from controlled drug use to addiction in this subpopulation of users have been the focus of an extensive amount of preclinical literature (Piazza and Deroche-Gamonet, 2013; Everitt and Robbins, 2016). Behaviours such as locomotor activity in a novel context (Piazza et al., 1989), impulsive choice (Belin et al., 2008), novelty seeking (Belin et al., 2011), sign tracking (Flagel et al., 2008), and choice for sucrose over cocaine (Lenoir et al., 2007), all show population-level variability in response to identical training parameters that have predictive value towards other measures related to the behavioural effects of cocaine or amphetamine (but see (Badiani et al., 2011) showing a different pattern of results with opioid drugs).
One of the core aspects of drug addiction is compulsive use, defined as continued drug use despite the knowledge of the negative consequences that occur because of drug use, such as loss of job, relationship breakdowns, and drug use in the face of danger (Hasin et al., 2013). Indeed, among the 11 criteria used to diagnose addiction according the DSM 5, different aspects of compulsive behaviour and failures of control represent a significant proportion (Hasin et al., 2013). An important question regarding the cause of this phenomenon is: why do the negative consequences cause a change in behaviour in some individuals but not others?
Pre-clinical studies have used footshock punishment of a drug-reinforced action in laboratory animals to study compulsive use (Grove and Schuster, 1974; Smith and Davis, 1974; Spealman, 1979; Panlilio et al., 2003; Deroche-Gamonet et al., 2004), although other methods are also used, including quinine adulteration in alcohol (Wolffgramm, 1991; Hopf et al., 2010), and intravenous histamine injection (Goldberg, 1980). One relatively consistent finding in more recent studies is that a sub-group of rats persist with drug self-administration in the face of punishment (Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004; Pelloux et al., 2007; Seif et al., 2013; Cadet et al., 2016a; Cadet et al., 2016b). Studies have shown that extended access to cocaine is a necessary factor leading to compulsive cocaine seeking in a sub-population of rats (Vanderschuren and Everitt, 2004; Pelloux et al., 2007). However, individual differences in punishment sensitivity emerge in populations with similar levels of intake of cocaine (Deroche-Gamonet et al., 2004), methamphetamine (Cadet et al., 2016a; Cadet et al., 2016b) or alcohol (Seif et al., 2013) self-administration. Whilst sufficient drug intake is a critical experimental factor, there is still insufficient evidence regarding the cause of punishment-resistant, drug-reinforced operant behaviour. A better understanding of the experimental parameters necessary to observe punishment-resistant drug seeking will help clarify the underlying psychological and neural mechanisms.
In this paper we present a re-examination of data from a series of behavioural studies (total n=499 alcohol preferring P rats) conducted over 3 years where the parameters for alcohol self-administration and footshock punishment were consistent (Marchant et al., 2014; Marchant and Kaganovsky, 2015; Marchant et al., 2016). We found that three sessions of punishment at a constant, mild shock intensity (0.3 mA) reveals a population distribution that is best described as bimodal, a feature that has been observed in prior studies of punishment resistance in rats trained to self-administer cocaine (Belin et al., 2011) or alcohol (Seif et al., 2013). However, a different group of rats that received increasing shock intensity (up to 0.5 mA) exhibited almost total suppression of alcohol self-administration. In the sections below, we first describe the experimental findings and then discuss the methodological and theoretical implications of these findings to preclinical addiction research and human addiction.
Methods
Subjects
We received male alcohol-preferring P rats (~30 days old, total n=499) from Indiana University Medical Centre and housed them singly under reverse 12-h light/dark cycle (lights on 08:00) with food and water available ad libitum. We performed all experiments in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition) and the Animal Care and Use Committee of IRP-NIDA approved all protocols.
We conducted all experiments between September 2012 and November 2015 and the data contributed to several papers (Marchant et al., (2014; 2015; 2016)). Most rats underwent a surgery of some kind after the homecage phase but prior to self-administration. The surgical technique was similar for all rats. We anesthetised rats with 100 mg/kg ketamine + 10 mg/kg xylazine (intraperitoneally) or isoflurane (5% induction; 2–3% maintenance). We injected rats with buprenorphine (0.1 mg/kg, s.c.) or ketoprofen (2.5 mg/kg, s.c., Butler Schein) after surgery and the following day (ketoprofen) to relieve pain and decrease inflammation, and we gave rats 3–5 days recovery after surgery before starting the self-administration sessions. The specific surgery varied between different experiments, and within this data set there are rats with intra-cranial cannula implants (n=183), optogenetic virus and fiber implants (n=71), cholera toxin B (CTb, a retrograde tracer) injection (n=69), chemogenetic virus injection (n=166), and surgically naïve rats (n=10). The brain regions targeted throughout these experiments also varied. However, all data presented in this paper are from behavioural sessions that occurred prior to any experimental manipulations of neuronal activity.
Apparatus
We used standard operant chambers (Med Associates), which were enclosed in ventilated sound-attenuating cubicles. Each chamber was equipped with one retractable lever (designated as “active”) and one non-retractable lever (designated as “inactive”). The grid floors were connected to shockers. We used two contexts for self-administration and punishment, as previously described in Marchant et al., (2013). The contexts had the following different features: grid width (narrow/wide), illumination level (white/red house light), background noise (fan on/off), and background cues (food container present/absent within the operant chamber), cabinet doors (closed/open). Whether a context was used for alcohol self-administration or punishment was counterbalanced.
Experimental procedure
The behavioural procedure consisted of three phases (Figure 1):
Figure 1. Outline of the experimental procedure.
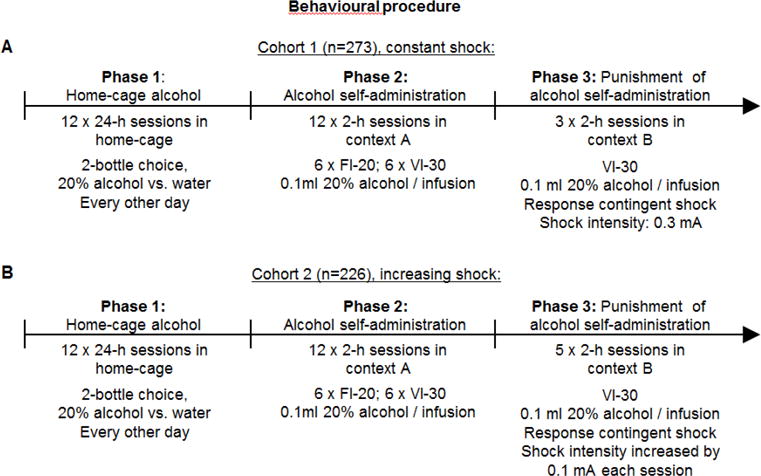
(A) Rats in cohort 1 (n=273) received 3 punishment sessions with constant shock intensity, set at 0.3 mA. (B) Rats in cohort 2 (n=226) received 5 punishment sessions with shock intensity starting at 0.1 mA, and increasing each session by 0.1 mA. FI, fixed-interval; VI, variable-interval.
Phase 1: Home-cage alcohol
We used the intermittent access (3–4 times/week) alcohol procedure (Wise, 1973; Simms et al., 2008), in which rats received 12 × 24-h sessions of access to one bottle of 20% alcohol and one water bottle. Alcohol solutions were prepared in tap water from 100% (v/v) ethanol in standard rat water bottles. Daily sessions began at 09:00. After 24-h, the alcohol bottle was replaced with a second water bottle for a further 24-h. The following day, the second water bottle was replaced with the 20% alcohol bottle and the location of the alcohol bottle was alternated from the previous session. Total alcohol consumption in grams was calculated for each session, using the weight difference between the beginning and end of the session, minus 2 grams for spillage, multiplied by 0.97 (density of 20% ethanol).
Phase 2: Alcohol self-administration
All rats received two 2-h magazine-training sessions where 0.1 ml of alcohol was delivered non-contingently every 5-min and paired with a 2-s tone-light cue. Next, the rats were trained for six 2-h self-administration sessions under a fixed-ratio 1, fixed-interval-20 (FI-20) schedule of reinforcement where an active lever press resulted in the delivery of 0.1 ml of 20% alcohol into the receptacle via a 12-gauge blunt needle connected to a 60-ml syringe controlled by a Razel pump. Each alcohol infusion was paired with a 2-s tone-light cue. This was followed by a 20-s timeout period where lever presses were recorded but not reinforced. The initiation of each session was signaled by the illumination of the house light and the insertion of the active lever into the chamber. Inactive lever presses had no programmed consequences. Following FI-20 training, we trained rats on a fixed-ratio 1, variable-interval 30-s (VI-30) schedule of reinforcement for six 2-h sessions. During the VI-30 sessions, alcohol delivery was available after an active lever press at pseudo-random intervals (from 1 to 59-s) after the preceding alcohol delivery.
Phase 3: Punishment of alcohol self-administration
We then trained the rats, during 2-h sessions, to self-administer alcohol in an alternate context (context B) under the same VI-30 schedule of reinforcement. Active lever presses resulted in the delivery of 0.1 ml of 20% alcohol paired with the 2-s tone-light cue. During punishment, 50% (pseudorandom) of the reinforced active lever presses resulted in a 0.5-s footshock. Punished active lever presses resulted in immediate footshock, 2-s tonelight cue, and alcohol delivery. Unpunished reinforced active lever presses resulted in 2-s tone-light cue and alcohol delivery. Time-out lever presses were never punished. Inactive lever presses had no consequences. We used 2 different punishment conditions. For the first cohort (n=273; Fig. 1A), we gave the rats three 2-h sessions over 3 days with the shock intensity set at 0.3 mA. We gave rats with more than 20 active lever presses additional punishment sessions with higher shock intensity. However these data are not shown because of the variable treatment (typically we did not give the fully suppressed rats more punishment sessions, but this did vary). For the second cohort (n=226; Fig. 1B), we gave the rats five 2-h sessions over 5 days and the shock intensity started at 0.1 mA on the first session, and increased each day by 0.1 mA until session 5 (0.5 mA).
Statistical analysis
We used the suppression ratio to analyze punishment behaviour because this method takes into account baseline alcohol self-administration (Bolles et al., 1980). The suppression ratio is calculated as: (punishment alcohol deliveries)/(punishment alcohol deliveries + mean alcohol deliveries of last 3 non-punished sessions). This suppression ratio has a range of values from 0 to 1. A value of 0.5 represents no change in behaviour from training to punishment. A suppression ratio score of less than 0.5 reflects a reduction in lever pressing during punishment, with 0 representing total suppression. A suppression ratio score greater than 0.5 reflects an increase in lever pressing during punishment. The frequency distributions of the suppression ratio were calculated into bins of 0.05. We used repeated measures ANOVA to compare the suppression ratio between punishment sessions 1, 2, and 3 in the constant shock group (within-subjects). After categorising the rats based on their punishment suppression ratio, we used repeated measures t-tests to compare the suppression ratio between punishment sessions 1 and 3 in cohort 1 (within-subjects). To compare the suppression ratio of cohort 1 punishment session 1 to cohort 2 punishment session 3 (between-subjects), we used an independent-samples t-test. We used repeated-measures ANOVA to compare measures of alcohol self-administration (home-cage alcohol consumption, alcohol deliveries, total active lever presses, time-out lever presses) in rats categorised based on their suppression ratio on punishment day 3 (cohort 1 only). Spearman’s rho correlations were conducted (cohort 1 only), on the following measures: mean home-cage alcohol intake (g/kg/24 h), mean alcohol deliveries (last 3 sessions), mean active lever presses (last 3 sessions), mean timeout lever presses (last 3 sessions), and the suppression ratio for each of the 3 punishment days. We used the non-parametric correlation method (Spearmans) because the suppression ratio on punishment session 3 was not normally distributed. Because of the large sample size (n=499), the significance level was set at 0.01. All statistical analyses were performed using SPSS (IBM SPSS Statistics, version 22).
Results
Population distribution of the suppression ratio in alcohol preferring P rats
Figure 2A–C shows the frequency distribution of suppression ratio for rats in cohort 1, which received constant shock intensity (0.3 mA) over three sessions. In the first session, the population distribution of the suppression ratio approximates a normal distribution. However, a bimodal distribution emerges by the third session. Repeated measures ANOVA revealed a significant effect of punishment session (F(1,272)= 77; p<0.001), and post-hoc t-tests revealed a significant difference in suppression ratio between punishment sessions 1 and 3 in the constant shock group (t(272)= 8.8, p < 0.001). These results show that there is a significant change in the behavioural response to punishment after 3 sessions at the same shock intensity.
Figure 2. Frequency distribution of the suppression ratio.
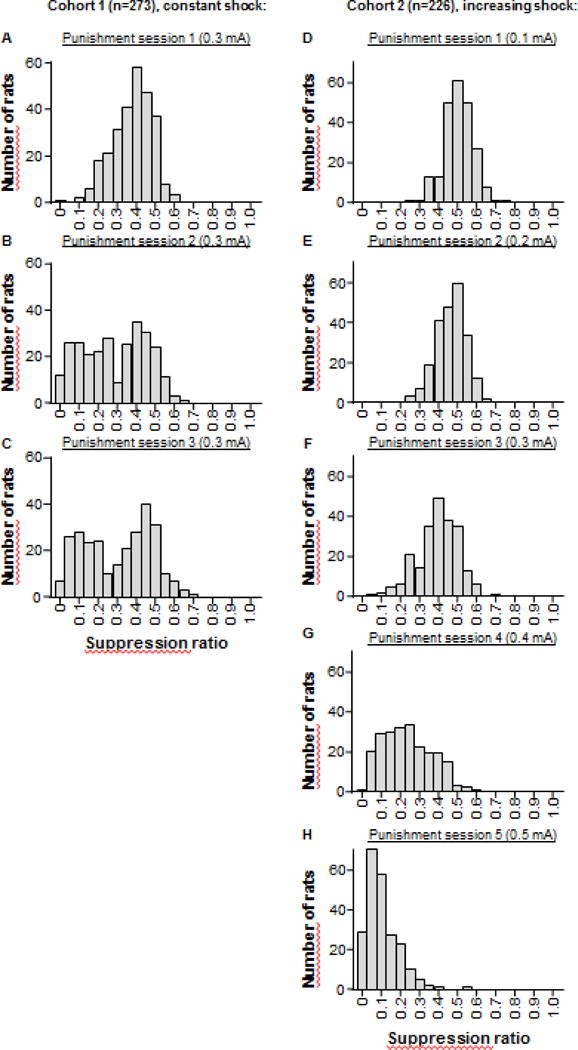
The suppression ratio is calculated using the number of alcohol deliveries on each punishment session divided by (the number of alcohol deliveries on each punishment session + the mean alcohol deliveries of the last 3 sessions of alcohol self-administration). (A–C) Frequency distribution of the suppression ratio for cohort 1, which received constant shock intensity (0.3 mA) over three sessions. (D–H) Frequency distribution of the suppression ratio for cohort 2, which received increasing intensity (+0.1 mA per session) over five sessions.
Figure 2D–H shows the frequency distribution of suppression ratio for rats in cohort 2 on their first (0.1 mA), second (0.2 mA), third (0.3 mA), fourth (0.4 mA), and fifth (0.5 mA) punishment session. The data show that increasing the shock intensity each day to 0.5 mA results in almost total suppression of alcohol self-administration. Because the third punishment session of cohort 2 was the time point that cohort 1 has split into a bimodal population, we were interested in comparing the suppression ratio of the first session with mild shock intensity (0.3 mA) between the two cohorts. Statistical comparison between cohort 1 (Fig. 2A) and cohort 2 (Fig. 2F) revealed no significant difference (t(497)= −2.0; p=0.048). This shows that the first punishment session at 0.3 mA leads to a similar amount of suppression whether it is the first punishment session (Cohort 1; Fig. 2A) or the third punishment session that was preceded by two sessions with lower shock intensity (Cohort 2; Fig. 2F).
In summary, these suppression ratio data show that, under our training parameters, increasing the shock intensity (to 0.5 mA) results in almost total suppression of alcohol self-administration. In contrast, constant shock intensity (0.3 mA) for at least three sessions reveals a bimodal distribution.
Categorisation of groups based on the suppression ratio (cohort 1)
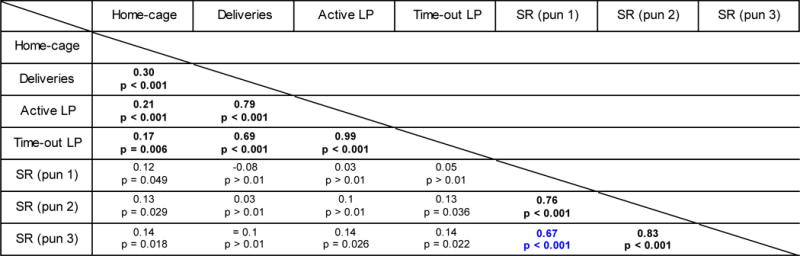
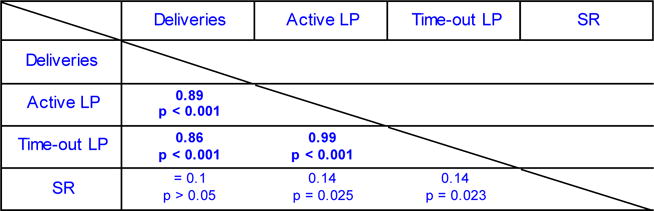
The phenomenon of punishment-resistance has potential utility as a model of aversion-resistant, or compulsive drug seeking. The bimodal distribution in the suppression ratio suggests there are indeed two distinct groups within this population that respond to punishment differently. However, when converting a continuous variable to a categorical variable, it can be difficult to justify where to define the groups. In support of splitting the rats into groups, the correlation analysis (in cohort 1) shows a high correlation between the suppression ratio on punishment session 1 and session 3 (Table 1). This indicates that the rats with the highest suppression ratio on punishment session 1 are generally the same rats with the highest suppression ratio on punishment session 3.
Table 1. Correlations between suppression ratio and measures of alcohol self-administration.
Spearman’s rho correlations from the data of cohort 1 between the suppression ratio of the three punishment sessions and mean home-cage alcohol consumption (g/kg/24 h), the mean of the last three self-administration sessions for alcohol deliveries (Deliveries), total active lever presses (Active LP), total time-out lever presses (Time-out LP). Numbers represent the spearman rho value. Because of the high n (273), alpha was set to 0.01. LP, lever press; SR, suppression ratio.
|
For the categorical analyses, we first divided the rats into two groups, based on a suppression ratio of 0.25. This represents a half-way point between total suppression (0.0) and no suppression (0.5), and it also represents the trough of the bimodal distribution (see Fig. 3C). Rats with a suppression ratio ≤ 0.25 were classified as “punishment-sensitive” (group Low) and rats with suppression ratio > 0.25 were classified as “punishment-resistant”. This resulted in two groups from the original 273 rats, with 116 punishment-sensitive rats, and 157 punishment-resistant rats. We further divided the punishment-resistant rats into groups Medium (suppression ratio: 0.26–0.50, n=128), and High (suppression ratio: 0.51+, n=29). We performed repeated measures t-tests comparing the suppression ratio on punishment session 1 and session 3 for each of the three categories. In group Low we found a significant difference (t(115)= 20; p<0.001), showing that these rats have greater suppression on session 3 compared to session 1. Interestingly, we also found a significant difference in group High (t(28)= −6.9; p<0.001), but in contrast their suppression ratio was higher on session 3 compared to session 1. Finally, in group Medium we found no significant difference (t(127)= 0.7; p>0.01), showing that for this group there was no change in punished alcohol seeking from session 1 to session 3.
Figure 3. Alcohol self-administration from cohort 1.
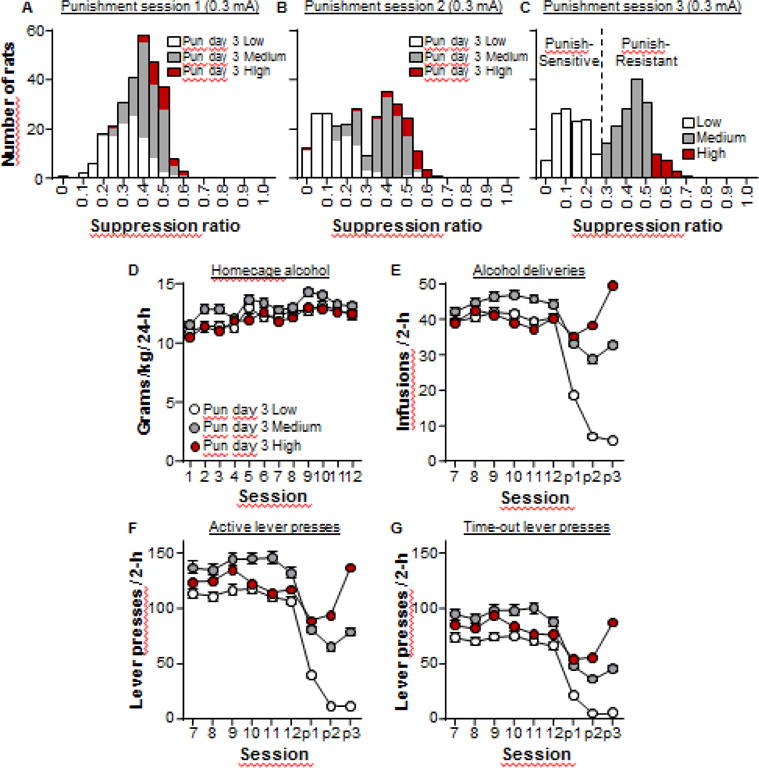
(A–C) The same frequency distribution graphs from Fig. 2A–C. We divided the rats into 3 groups based on the suppression ratio in punishment session 3; the ‘punishment-sensitive’ group (white bars) with suppression ratio ≤ 0.25; the ‘punishment-resistant’ was divided into Medium (grey bars: suppression ratio between 0.25 and 0.50) and High (red bars: suppression ratio ≥ 0.51). (D–F) Alcohol self-administration from cohort 1. (D) Home-cage alcohol consumption (g/kg/24-h) across the 12 home-cages alcohol sessions. (E) Alcohol deliveries across the final 6 self-administration sessions and the 3 punishment sessions. (F) Total active lever presses across the final 6 self-administration sessions and the 3 punishment sessions. (G) Total time-out active lever presses across the final 6 self-administration sessions and the 3 punishment sessions.
In summary, these data show that the shift from a relatively unimodal distribution to a bimodal distribution within the total population primarily comes from the punishment-induced reduction in alcohol seeking in the punishment-sensitive group across the three punishment sessions. In the punishment-resistant group, the majority do not significantly change their responding in response to punishment, but there is a small proportion of this group (~18%) that actually increases alcohol seeking across the three punishment sessions.
Relationship between alcohol self-administration and the punishment suppression ratio
One important question about the cause of the punishment-resistant phenotype is whether rats persist because of higher motivation for alcohol. To answer this question we re-analysed the alcohol self-administration data in two ways based on the categorisation described above. In the first analysis we used two categories (punishment-sensitive, punishment-resistant), and in the second we used three categories, splitting the punishment-resistant rats into Medium and High.
Using two categories, statistical analysis of the 12 home-cage alcohol sessions revealed no effect of the suppression ratio category (F1,271 = 5.0, p=0.03). Statistical analysis of the last 6 sessions of alcohol self-administration (when the reinforcement schedule was the same as during punishment but punishment had not yet commenced) revealed a significant effect of the suppression ratio category on total active lever presses (F1,271 = 20.3, p<0.01), and time-out lever presses (F1,271 = 21.3, p<0.01). Interestingly, for alcohol deliveries there was a trend toward a significant difference (F1,271 = 9.1, p=0.03). There were no significant interactions between these measures and the suppression ratio category (Fs < 1.5). These results show that the rats categorised as punishment-resistant (i.e. suppression ratio > 0.25) had higher alcohol seeking prior to punishment. However, alcohol taking was not significantly different (alcohol deliveries), and the biggest difference between these groups was in the time-out lever presses. Furthermore, the correlation analysis shows no significant correlation between the suppression ratio and measures of alcohol self-administration prior to punishment (see Figure 4 A–C and Table 1), suggesting that responding for alcohol does not reliably predict the response to punishment.
Figure 4. Correlation between the suppression ratio and alcohol self-administration from cohort 1.
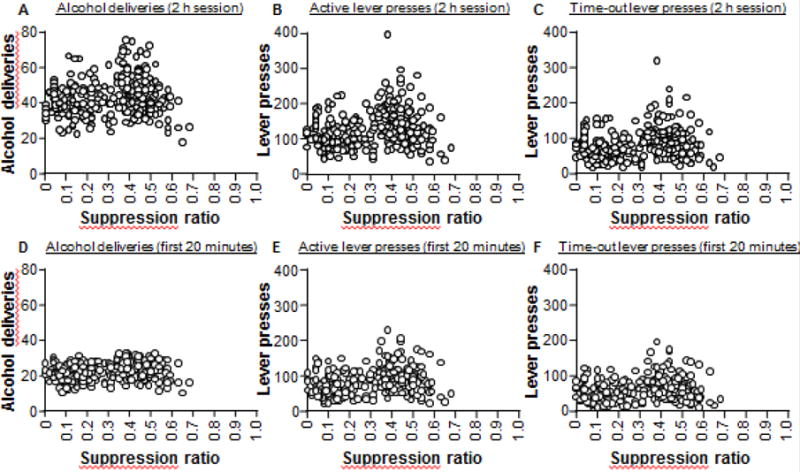
Scatter plots of the correlation between the suppression ratio (punishment day 3) and alcohol deliveries (A, D), total active lever presses (B, E), and time-out active lever presses (C, F). In A–C the measures are the mean counts for the full 2 h session of the last three sessions. In D–F measures are the mean counts for the first 20 minutes of the last three sessions.
We found that alcohol seeking (active lever presses and time-out active lever presses) is higher in the punishment-resistant group, but we failed to observe a significant correlation between the suppression ratio and measures of prior alcohol seeking. In order to explore the source of this variance, we performed a second categorical analysis using three groups. Figure 3D–G shows alcohol self-administration data from rats in cohort 1 based on the three categories (Low (0–0.25, n=116), Medium (0.26–0.50, n=128), and High (0.51+, n=29). It can be seen that the rats in group High are consistently lower than group Medium in these measures and this is supported by statistical analysis. While we found the same main effects of punishment category on measures of alcohol self-administration (F2,270 > 8), the post-hoc analyses revealed that in each measure there was no significant difference between groups Low and High in alcohol deliveries, active lever presses, and time-out lever presses (p>0.05 for each test). Indeed, there was a trend towards significantly lower alcohol deliveries in group High compared to group Medium (p=0.023). Thus, the rats with the highest suppression ratio (i.e. >0.50) had lower alcohol taking and seeking responses than the rats in the middle group (i.e. between 0.26 and 0.50). This shows that that alcohol self-administration is not a reliable predictor of subsequent punishment-imposed suppression.
Finally, the majority of alcohol seeking and taking occurs in the first 20 minutes of a session, so we were interested in whether the rate of alcohol self-administration during this time is related to the suppression ratio. Figure 4 D–F shows scatter plots of the alcohol self-administration data mean of the first 20 minutes for each rat based on their suppression ratio. Statistical analysis revealed no significant relationship between the suppression ratio and the measures of alcohol self-administration in the first 20 minutes (see Table 2).
Table 2. Correlations between suppression ratio and measures of alcohol self-administration in the first 20 minutes.
Spearman’s rho correlations from the data of cohort 1 between the suppression ratio of punishment session 3 and mean of the first 20 minutes of last three self-administration sessions for alcohol deliveries (Deliveries), total active lever presses (Active LP), and total time-out lever presses (Time-out LP). Numbers represent the spearman rho value. Because of the high n (273), alpha was set to 0.01. LP, lever press; SR, suppression ratio.
|
In summary, these results show that, at least under the conditions used here, the amount of suppression induced by punishment of alcohol-reinforced responding is not related to the rate of self-administration prior to punishment.
Discussion
In this report we present a re-examination of alcohol self-administration and punishment data from a large cohort of alcohol preferring P rats. Our results provide evidence for a bimodal distribution in the response to punishment when constant shock intensity (0.3 mA) is used over multiple sessions. In contrast, increasing shock intensity over multiple sessions from 0.1 mA to 0.5 mA resulted in uniform suppression across the population. The underlying cause of individual differences in punishment sensitivity under constant shock intensity is unknown. This data set provides some evidence that the punishment-resistant rats had higher rates of prior alcohol self-administration. However, we observed only weak correlations between several measures of alcohol self-administration and the punishment suppression ratio. Thus, although we have not directly assessed motivation for alcohol, these data highlight the possibility that motivation for alcohol is not necessarily a key factor determining the response to punishment, suggesting that punishment-resistant operant behaviour may be a consequence of factors other than a high motivation for reward.
Limitations and alternative explanations
An important consideration when interpreting these data is whether the underlying cause is a difference in pain threshold or shock sensitivity. Whilst we cannot explicitly exclude this interpretation, we argue that this is not the case because there is a lack of evidence for such a difference in the literature. The few studies that have used footshock in P rats have not reported any within-group differences (McKinzie et al., 2000; Vengeliene et al., 2003). Our own anecdotal observations were that the punishment-resistant rats still displayed a strong reaction when shock was delivered, suggesting they do sense the shock, however, we do not have empirical data on this point.
Another possible alternative explanation is that the analgesic action of alcohol is, in part, responsible for the punishment-resistant phenotype. However, in a study measuring lever pressing for food under risk of punishment, Mitchell et al., (2011) reported that i.p. injections of alcohol up to 1.5 g/kg had no effect on choice for the large reward paired with punishment (0.4 mA). This dose of alcohol is higher than the rats in our studies typically obtained in a 2-h self-administration session (mean ~1 g/Kg/session). Therefore, any potential role for the analgesic effect of alcohol contributing to the data observed in our report can be considered unlikely. However individual differences in the anxiolytic response to alcohol also remain an untested possibility.
Finally, one important consideration when interpreting these data is that different contexts were used for alcohol self-administration and punishment. While the specific contexts that served as the alcohol and punishment context were counterbalanced, it is possible that this manipulation had some impact on the effect of punishment. However, similar types of splits in the population have been reported previously in single context designs (Pelloux et al., 2007; Seif et al., 2013; Krasnova et al., 2014), suggesting that the change in context is not a significant factor causing the individual variability in response to punishment.
Relationship between alcohol seeking and taking prior to punishment and the response to punishment
In this report, we found no evidence that the amount of alcohol intake reliably predicts the response to punishment. This finding is similar to a previous study examining punishment of alcohol self-administration that reported no relationship between baseline alcohol self-administration and subsequent suppression by punishment (Seif et al., 2013). Furthermore, it is worth emphasizing that our report describes a population split in a strain of rats (P rats) that have been selectively bred for high-alcohol consumption (Li et al., 1979). This shows that the genetic mechanism by which the rats were selected for to increase their alcohol intake, of which a loss of the metabotropic glutamate receptor 2 (mGluR2) is one critical factor (Zhou et al., 2013), does not impact the response to punishment. This provides further indirect evidence that alcohol intake is not a critical determinate of the response to punishment of alcohol reinforced responding. These data suggest that motivation for alcohol and the response to punishment are mediated by orthogonal factors. However a recent study by Giuliano et al., (2017) used a progressive ratio (Richardson and Roberts, 1996) test of motivation and found evidence for a relationship between motivation for alcohol and being categorized as compulsive.
In contrast to punishment of alcohol self-administration, prior drug exposure in cocaine-trained rats increases the incidence of compulsive cocaine seeking (Deroche-Gamonet et al., 2004; Vanderschuren and Everitt, 2004; Pelloux et al., 2007). Although we did not explicitly test for this, our data show no evidence that the amount of alcohol taken during self-administration predicts the response to punishment, but this does not rule out the possibility that duration of alcohol exposure contributes to the development of punishment-resistant alcohol seeking. Therefore, it is likely that there are important differences in the factors that lead to punishment-resistant alcohol versus cocaine seeking. However, studies examining the effect of punishment of methamphetamine self-administration have consistently shown no difference in drug intake between groups that are punishment-sensitive or punishment-resistant (Cadet et al., 2016a; Cadet et al., 2016b; Torres et al., 2017). In addition, the conflict model has shown that increasing the shock intensity can fully suppress cocaine seeking (Cooper et al., 2007). This suggests that other experimental factors such as the schedule of punishment, as well as the use of a seeking-taking chain for self-administration and punishment (Pelloux et al., 2007; Chen et al., 2013) contribute to the observation of intake dependent sensitivity to punishment.
Relationship between punishment parameters and compulsive drug seeking
This report shows the emergence of a punishment-resistant phenotype across multiple sessions with the same shock intensity. However, in these experiments we were always able to achieve total suppression by punishing alcohol self-administration with increased shock intensity in the following sessions (data not shown). Thus, even the punishment-resistant rats completely suppress alcohol self-administration given sufficient shock intensity. A similar finding has been observed with methamphetamine self-administration, where daily increments of shock intensity (up to 0.6 mA) lead to total suppression of methamphetamine seeking (Krasnova et al., 2014). However, a similar population split is observed when mild shock intensity (up to 0.36 mA) is used over multiple sessions (Cadet et al., 2016a; Cadet et al., 2016b). These data show that punishment-resistant drug self-administration is only seen when the parameters are set to observe it, and with sufficiently high enough shock intensity all rats are punishment-sensitive.
The fact that sufficiently intense punishment can suppress drug-reinforced operant responding in all rats does not diminish the observation that punishment with mild shock intensity over several sessions reveals two groups of rats. Importantly, the bimodal nature of resistance to punishment has been previously observed in experiments using vastly different self-administration and punishment parameters (Belin et al., 2011; Seif et al., 2013). Furthermore, even punishment of optogenetic self-stimulation of ventral tegmental area dopamine neurons reveals two populations based on the response to punishment (Pascoli et al., 2015). In this study, the proportion of punishment-resistant mice was higher when the response was reinforced with optogenetic stimulation compared to cocaine or sucrose. This finding suggests that the punishment resistant phenotype is dependent upon the nature of the reinforcer. However, individual differences within the population were still evident using a non-physiological reinforcement stimulus. Therefore, the existence of two groups with different responses to punishment is likely a more broadly applicable phenomenon than to just the parameters used in this study. This finding suggests that specific conditions exist to reveal the emergence of a punishment-resistant phenotype. The shock intensity is a primary factor, because if the shock intensity is too high then all rats suppress their intake. However, in this study it is evident that multiple sessions of punishment using mild shock intensity are also necessary to reveal this phenotype. Thus, one important factor contributing to the emergence of the punishment-resistant phenotype might be the type of learning that occurs across the different sessions.
What learning mechanisms are involved in the different responses to punishment?
Relevant to this point is a theoretical account of what learning occurs during punishment. The evidence that instrumental behaviour is controlled by a representation of the outcome has been conclusively shown (Adams and Dickinson, 1981; Balleine and Dickinson, 1998). As such, it is reasonable to assume that punishment learning involves the acquisition of a second instrumental response-outcome (R–O; i.e. lever press-shock) association that is motivationally opposite to the original R–O (i.e. lever press-alcohol) association. However, additional learning of a Pavlovian stimulus-outcome (S–O; i.e. lever-shock) relationship is unavoidable. The extent to which the Pavlovian or instrumental contingencies mediate the punishment-induced suppression of lever pressing is still not fully understood. In a series of elegant studies, Bolles et al. (1980) provided evidence for both S–O learning and R–O learning in punishment. One important observation from this study was that evidence for S–O learning emerged initially, while the evidence for R–O learning emerged towards the end of the first session, and fully by the session on the following day.
The conclusions of Bolles et al., (1980) have implications for interpretation of the data reported in our study. In the constant shock group, the initial punishment session was associated with much less suppression of alcohol self-administration compared to the third session (Fig. 2A vs. Fig. 2C). It may be the case that differences in punishment sensitivity are caused by differential engagement of the learning systems that encode R–O or S–O. However, it is not immediately clear exactly what these differences are for each group. For example, it is possible that the punishment-sensitive rats maintain an S–O association (lever-shock) across the three sessions, and their suppression is more akin to Pavlovian fear conditioning to the lever. In contrast, it is also plausible that the punishment-resistant rats maintain an S–O association and persist with lever pressing because they have not formed an R– O association. Or put more plainly, in the punishment-resistant rats the mild shock is not salient enough to learn that their action is responsible for the negative outcome.
An alternative explanation based on Bolles et al., (1980) is that responding in the third punishment session can be expected to be controlled by R–O associations. Interestingly, when observing our rats, we often noticed an ‘approach-retreat/avoidance’ behavioural pattern, where the rat would place its forepaw on the lever but return to the alcohol receptacle without pressing. Such behaviour has been noted in the literature, where it is referred to as ‘abortive lever pressing responses’, and studies have shown that punishment, but not Pavlovian, contingencies produce this behaviour (Hunt and Brady, 1955). Therefore the presence of this behavioural pattern implies that the punishment-resistant rats have indeed acquired the R–O (shock) association. If we assume that rats in both groups have acquired the R–O (shock) association, then the split in the population may be explained by a difference in the associative strengths of the two motivationally opposite outcomes. In this view, the punishment-resistant rats would have stronger action-alcohol association compared to shock, or the punishment-sensitive rats have a stronger action-shock association.
One important issue that is not addressed in this report is whether the bimodal distribution in punishment sensitivity is independent of punishment history. It is unknown whether mild punishment results in the same population distribution after rats have experienced total punishment-imposed suppression. While we have not directly tested this, a recent study by Giuliano et al., (2017) reported that repeated cycles of punishment and retraining of alcohol self-administration does not alter the phenotype of punishment resistance or sensitivity, and the same pattern of responding to punishment was observed in the same rats over 10 months. Although Giuliano et al. used significantly different training and punishment schedules, such as a seeking-taking chain for reinforcement and punishment of the seeking lever presses, it does offer some insight into the stability of the punishment resistance phenotype. Regardless, it remains an open question whether a rat that has experienced total punishment-imposed suppression would still respond to mild punishment in the same way.
Ultimately, it is unknown if differences in learning mechanisms are responsible for the observed group differences, and future studies directly testing the alternative explanations are needed before any conclusions can be made. Indeed, whether it is different types of learning, increased motivation for alcohol, habituation to the shock, or some other construct (such as impulsivity) is also not known. This is an important question because differences in punishment sensitivity are exploited in animal models of compulsive drug seeking, and the physiological and psychological mechanisms may have parallels with the human condition. As discussed in the next section, human drug abusers have been shown to have dysfunction in fear learning systems, and the extent to which this relates to compulsive drug use is not known.
Relationship between compulsive drug use and fear learning
We propose that the punishment-resistant phenotype in rats is caused by dysfunction in the systems that encode aversive outcomes, and one prediction of this hypothesis is that these rats have predictable differences in other measures of aversive learning. Several studies have shown that both conditioned fear and extinction are affected by acute and chronic exposure to alcohol (Ripley et al., 2003; Bertotto et al., 2006; Lattal, 2007; Holmes et al., 2012), and a similar finding has been reported in human alcoholics (Stephens et al., 2005). However, two studies have directly shown that compulsive drug seeking is not associated with differences in conditioned fear responding (Vanderschuren and Everitt, 2004; Giuliano et al., 2017). Thus, the evidence suggests that while classical fear conditioning is not different between rats that are punishment-resistant or punishment-sensitive, alcohol exposure can cause drug-induced changes in encoding aversive outcomes.
In regards to other drugs of abuse, two recent studies have shown in humans that cocaine addicted individuals have dysfunction in fear and avoidance learning. Kaag et al., (2016) examined the neural response to laboratory based fear conditioning in human cocaine addicts. They found that while there were no differences in physiological measures, the amygdala and insular cortex were hyperactive during fear conditioning in cocaine addicted individuals, compared to controls. Interestingly, the dorsomedial prefrontal cortex was hypoactive at the end of extinction, suggesting enhanced extinction. Ersche et al., (2016) found that cocaine addicted individuals have impairments in an instrumental avoidance learning task. These findings show that cocaine use disorder is associated with dysfunction in the systems that encode aversive learning, and that such dysfunction can lead to significant impairments in behaviour. These findings show that human drug users have dysfunction in the neural systems that encode aversive outcomes. The extent to which this deficit in evaluating negative outcomes contributes to compulsive drug use is not known. Whether the findings in human drug users are because of pre-existing differences, or drug-induced changes, remains to be determined. However, an interesting possibility is that addicted humans, who often display compulsive drug use, might be compulsive because of dysfunction in the systems that encode aversive outcomes in the general sense, and not just in those outcomes that occur because of drug use. If this is the case, such knowledge could be used to predict vulnerable individuals, or may be a target for novel interventions aimed at restoring control over drug use.
Conclusions
Here we describe the emergence of two populations of rats, from an otherwise seemingly homogenous group, defined by their response to punishment. Using two different punishment parameters, we show that multiple punishment sessions with constant shock intensity (0.3 mA) reveal a bimodal distribution, whereas when the shock intensity is incrementally increased to 0.5 mA the bimodal distribution is not observed. Evidence for bimodal distributions in the response to punishment have been demonstrated previously (Belin et al., 2011; Seif et al., 2013), and this report provides a more thorough examination of the parameters that reveal this distribution. We found only weak evidence that the response to punishment is associated with the motivation to consume alcohol. This suggests that the response to punishment and the motivation for alcohol are likely mediated by orthogonal factors. Interestingly, we see this population split in a strain of rats that have been selectively bred for high alcohol intake (alcohol-preferring P rats), providing further evidence in support of this hypothesis. The cause of this individual variability in the response to punishment is not known, but in this paper we have outlined some of the critical parameters that are necessary to observe it.
Highlights.
Individual differences in the sensitivity to punishment are thought to model compulsive drug seeking
We show that alcohol preferring P rats have substantial variability to punishment of alcohol-reinforced behaviour.
Punishment with constant, mild shock intensity over 3 days revealed a bimodal distribution in the population.
Punishment with increasing shock intensity results in total suppression of alcohol seeking.
Acknowledgments
The authors thank Gavan McNally, Phillip Jean-Richard-dit-Bressel, Andrew Lawrence, and Yavin Shaham for their comments on drafts of this manuscript. The authors also thank Jennifer Bossert and Yavin Shaham for their mentorship throughout the duration of these experiments. Research was suppourted by the National Institute on Drug Abuse, Intramural Research Program funds to the Neurobiology of Relapse Section (PI: Yavin Shaham). N.J.M. received suppourt from Early Career Fellowship 1053308; N.J.M and E.J.C received suppourt from Project Grant 1105741 by the National Health and Medical Research Council. The authors declare no conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. The Quarterly Journal of Experimental Psychology Section B. 1981;33:109–121. [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2:244–268. [Google Scholar]
- Badiani A, Belin D, Epstein DH, Calu DJ, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High Impulsivity Predicts the Switch to Compulsive Cocaine-Taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-Novelty-Preference Rats are Predisposed to Compulsive Cocaine Self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertotto ME, Bustos SG, Molina VA, Martijena ID. Influence of ethanol withdrawal on fear memory: Effect of D-cycloserine. Neuroscience. 2006;142:979–990. doi: 10.1016/j.neuroscience.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Holtz R, Dunn T, Hill W. Comparisons of stimulus learning and response learning in a punishment situation. Learning and Motivation. 1980;11:78–96. [Google Scholar]
- Cadet JL, Brannock C, Krasnova IN, Jayanthi S, Ladenheim B, McCoy MT, Walther D, Godino A, Pirooznia M, Lee RS. Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol Psychiatry. 2016a doi: 10.1038/mp.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Walther D, Brannock C, Ladenheim B, McCoy MT, Collector D, Torres OV, Terry N, Jayanthi S. Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats. Sci Rep. 2016b;6:37002. doi: 10.1038/srep37002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for Addiction-like Behavior in the Rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LHE, Luijten M, de Wit S, Sahakian BJ, Bullmore ET, Robbins TW. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352:1468–1471. doi: 10.1126/science.aaf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Pena-Oliver Y, Goodlett CR, Cardinal RN, Robbins TW, Bullmore ET, Belin D, Everitt BJ. Evidence for a long-lasting compulsive alcohol seeking phenotype in rats. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR. Histamine as a punisher in squirrel monkeys: effects of pentobarbital, chlordiazepoxide and H1- and H2-receptor antagonists on behavior and cardiovascular responses. J Pharmacol Exp Ther. 1980;214:726–736. [PubMed] [Google Scholar]
- Grove RN, Schuster CR. Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav. 1974;2:199–208. doi: 10.1016/0091-3057(74)90053-7. [DOI] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt HF, Brady JV. Some effects of punishment and intercurrent anxiety on a simple operant. J Comp Physiol Psychol. 1955;48:305–310. doi: 10.1037/h0042529. [DOI] [PubMed] [Google Scholar]
- Kaag AM, Levar N, Woutersen K, Homberg J, van den Brink W, Reneman L, van Wingen G. Hyperresponsiveness of the Neural Fear Network During Fear Conditioning and Extinction Learning in Male Cocaine Users. Am J Psychiatry. 2016;173:1033–1042. doi: 10.1176/appi.ajp.2016.15040433. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, Shaham Y, Cadet JL. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM. Effects of ethanol on encoding, consolidation, and expression of extinction following contextual fear conditioning. Behavioral neuroscience. 2007;121:1280–1292. doi: 10.1037/0735-7044.121.6.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Waller MB, Hawkins TD. Progress toward a voluntary oral consumption model of alcoholism. Drug and Alcohol Dependence. 1979;4:43–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K. A critical role of nucleus accumbens dopamine D1-family receptors in renewal of alcohol seeking after punishment-imposed abstinence. Behavioral neuroscience. 2015;129:281–291. doi: 10.1037/bne0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y. Context-Induced Relapse to Alcohol Seeking After Punishment in a Rat Model. Biological psychiatry. 2013;73:256–262. doi: 10.1016/j.biopsych.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Rabei R, Kaganovsky K, Caprioli D, Bossert JM, Bonci A, Shaham Y. A Critical Role of Lateral Hypothalamus in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence. The Journal of Neuroscience. 2014;34:7447–7457. doi: 10.1523/JNEUROSCI.0256-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S, Hope BT, Heins RC, Prisinzano TE, Vardy E, Bonci A, Bossert JM, Shaham Y. Role of Ventral Subiculum in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence. J Neurosci. 2016;36:3281–3294. doi: 10.1523/JNEUROSCI.4299-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Sajdyk TJ, McBride WJ, Murphy JM, Lumeng L, Li TK, Shekhar A. Acoustic startle and fear-potentiated startle in alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 2000;65:691–696. doi: 10.1016/s0091-3057(99)00252-x. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology (Berl) 2011;218:703–712. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369:1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- Panlilio L, Thorndike E, Schindler C. Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology. 2003;168:229–235. doi: 10.1007/s00213-002-1193-0. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Luscher C. Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron. 2015;88:1054–1066. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Everitt B, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ripley TL, O’Shea M, Stephens DN. Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. Eur J Neurosci. 2003;18:441–448. doi: 10.1046/j.1460-9568.2003.02759.x. [DOI] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci advance online publication. 2013 doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise RA, Bartlett SE. Intermittent Access to 20% Ethanol Induces High Ethanol Consumption in Long–Evans and Wistar Rats. Alcoholism: Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SG, Davis WM. Punishment of amphetamine and morphine self-administration behavior. The Psychological Record. 1974;24:477–480. [Google Scholar]
- Spealman RD. Comparison of drug effects on responding punished by pressurized air or electric shock delivery in squirrel monkeys: pentobarbital, chlordiazepoxide, d-amphetamine and cocaine. J Pharmacol Exp Ther. 1979;209:309–315. [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, Cadet JL. Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav Brain Res. 2017;326:265–271. doi: 10.1016/j.bbr.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug Seeking Becomes Compulsive After Prolonged Cocaine Self-Administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacology. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. An ethopharmacological approach to the development of drug addiction. Neuroscience & Biobehavioral Reviews. 1991;15:515–519. doi: 10.1016/s0149-7634(05)80142-3. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Karlsson C, Liang T, Xiong W, Kimura M, Tapocik JD, Yuan Q, Barbier E, Feng A, Flanigan M, Augier E, Enoch MA, Hodgkinson CA, Shen PH, Lovinger DM, Edenberg HJ, Heilig M, Goldman D. Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc Natl Acad Sci U S A. 2013;110:16963–16968. doi: 10.1073/pnas.1309839110. [DOI] [PMC free article] [PubMed] [Google Scholar]


