Abstract
Background
Moraxella catarrhalis (Mcat) is a frequent pathogen of acute otitis media (AOM) in young children. Here we prospectively assessed naturally-induced serum antibodies to four Mcat vaccine candidate proteins in stringently defined otitis prone (sOP) and non-otitis prone (NOP) children age 6 to 36 months old following nasopharyngeal (NP) colonization, at onset of AOM and convalescence from AOM.
Methods
Serum IgG and IgM antibody against recombinant Mcat proteins, oligopeptide permease A (OppA), outer membrane protein (OMP) CD, hemagglutinin (Hag), and PilA clade 2 (PilA2), were quantitated by ELISA.
Results
During NP colonization by Mcat all four antigens were immunogenic in both sOP and NOP children. However, sOP children had lower antibody responses than NOP children across age 6-36 months, similar to our findings for protein vaccine candidates of Streptococcus pneumoniae (Spn) and Nontypeable Haemophilus influenzae (NTHi). sOP children displayed a later and lower peak of antibody rise than NOP children for all four antigens during NP colonization of Mcat. The age-dependent increase of antibody ranked as OppA > Hag5-9 > OMP CD > PilA2 in both sOP and NOP children. Lower serum antibody levels to the Mcat antigens were measured in sOP compared to NOP children at the onset of AOM. We did not find a consistent significant increase of antibody at the convalescence phase after an AOM event.
Conclusions
sOP children is a highly vulnerable population that mount lower serum antibody responses to Mcat candidate vaccine proteins compared to NOP children during asymptomatic NP carriage and at onset of AOM.
Keywords: Otitis prone, Nasopharyngeal colonization, Acute otitis media, Immunogenicity, Recombinant proteins, Carriage
1. Introduction
Acute otitis media (AOM) is the most common infectious disease to cause parents to seek medical care for their child and receive antibiotics. The primary otopathogens causing AOM are Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat).[1] Mcat is also a common cause of acute sinusitis in children and adults and acute exacerbations of chronic bronchitis in adults.[2] Our group has been studying the immune response to protein vaccine candidates of the three major otopathogens in two populations of children: (1) a group that has poor antibody and cellular immune responses to otopathogens and very frequent AOM episodes (termed stringently defined otitis prone (sOP) children) and (2) a group who show strong immune responses and experience few or no AOM episodes (termed non-otitis prone (NOP) children).[3-14] However, among the Spn and NTHi candidate vaccines we have studied thus far some antigens showed significantly greater immunogenicity than others.[3,4,15,16] Moreover, since sOP children would benefit the greatest from a vaccine to prevent AOM and their frequent illnesses cause greater morbidity and consume a disproportionately high amount of health care costs for otitis media care, study of this highly vulnerable population has merit.
Currently, there is no licensed vaccine available for Mcat. A number of vaccine targets have been identified.[17] Here, we studied four highly conserved, surface exposed Mcat proteins: OMP CD, OppA, Hag, and PilA2 in sOP compared to NOP children. OMP CD is a porin and an adhesin.[18] Oligopeptide permease protein A (OppA) is an oligopeptide binding protein of the oligopeptide permease ABC transport system, which mediates uptake of peptides and fitness of Mcat in the respiratory tract.[19,20] Hemagglutinin (Hag), also named Moraxella IgD binding protein (MID), is an adhesin and mediates adherence to primary cultures of human middle ear epithelial cells.[21] PilA clade 2 (PilA2) is a pilin, which is the major protein subunit of type IV pili and is essential for Mcat natural genetic transformation and can also enhance biofilm formation.[22]
Naturally-induced antibodies comprise a fundamental component of humoral immunity against infectious agents. We have previously shown that healthy children develop serum antibodies to OMP CD, OppA, Hag, and PilA2 following Mcat nasopharyngeal (NP) colonization and AOM.[23] In this study, we analyzed serum antibody responses to the same four Mcat proteins in sOP children and compared them to NOP children. We sought to identify Mcat proteins capable of eliciting robust antibody immune responses in sOP children equivalent to those in NOP children, since natural priming in infancy and post vaccination boosting in the second year of life to those antigens among sOP children would be a desirable feature.
2. Materials and Methods
2.1. Subjects and sampling
The samples collected and analyzed were obtained during a prospective study supported by the National Institute of Deafness and Communication Disorders, as previously described.[15,16] Healthy children without previous episodes of AOM were enrolled at 6 mos. of age from a middle class, suburban socio-demographic pediatric practice in Rochester, NY during June, 2008 to March, 2014. Serum samples and NP and oropharyngeal cultures (hereafter termed NP samples) were obtained 7 times during the study period at 6, 9, 12, 15, 18, 24, and 30-36 mos. of age. During the study period whenever children experienced an AOM episode a confirmatory tympanocentesis was performed and middle ear fluid (MEF) samples were microbiologically assessed (an essential component to the definition of “stringently-defined” AOM) since virtually all prior studies have relied on only a clinical diagnosis that is known to be variably accurate). Serum and NP cultures were collected at time of the clinical diagnosis of AOM and, 3 weeks following an AOM (convalescent stage). The study was approved by the Rochester General Hospital Research Subjects Review Boards and written informed consent was obtained for participation and all procedures.
2.2. Enzyme-linked immunosorbent assay
Recombinant Mcat proteins, OppA, OMP CD, Hag5-9 (truncated Hag protein), and PilA2 were expressed and purified as previously described.[18,20-22] All the proteins are conserved among Mcat strains [17] and have been demonstrated to display native epitopes on the surface of Mcat by a flow cytometry assay which showed binding of mouse immune sera against all four recombinant proteins displayed on the surface of multiple Mcat strains (data not shown). Protein-specific antibody concentrations were determined by enzyme-linked immunosorbent assay (ELISA) using purified recombinant proteins. Ninety six-well Nunc MaxiSorp plates were coated with 1 μg/mL of individual proteins (100 μL/well) in phosphate-buffered saline (PBS, pH 7.4) and incubated at 37°C for 1 h. After five washes, the plates were blocked with 10% fetal bovine serum (FBS) in PBS (pH 7.4) at room temperature for 1 h (200 μl per well). After washing, 100 μl of serum 2-fold serially diluted in PBS/0.5%BSA/0.005% tween at a starting dilution of 1:50 was added to each well. Human serum IgGs, Carimune (CSL Behring AG, Bern, Switzerland) and Gammagard (Baxter, Deerfield, IL) were used as references and in-house control sera with high and low titers were run on each plate. The plates were incubated at room temperature for 30 min followed by the addition of affinity purified goat anti-human IgG/IgM antibody conjugated to horseradish peroxidase (Bethyl Laboratories, Montgomery, TX) as a secondary antibody. The reaction products were developed with TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD), stopped by addition of 1.0 M phosphoric acid and read by a Spectramax 340PC plate reader (Molecular Devices, Sunnyvale, CA) using a 450-nm filter.
To provide quantitative results on antibody concentrations, the level of specific antibody in the samples was determined by comparison to an internal reference serum (Carimune for OMP CD and Gammagard for OppA, Hag, and PilA2). The levels of IgG and IgM in the reference serum were quantitatively measured by using a human IgG or IgM ELISA quantitation kit (Bethyl laboratories). A four-parameter logistic-log function was used to form the reference and sample curves. Precision, Calibration of Standard curve, Robustness/Stability and Linearity were done with an in-house reference serum according to International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guidance.[24] The assay lower limit of detection was 171 ng/mL for OppA, 69 ng/mL for OMP CD, 191 ng/mL for Hag5-9, and 47 ng/mL for PilA2 for serum IgG, and 44 ng/mL for OppA, 55 ng/mL for OMP CD, 63 ng/mL for Hag5-9, and 30 ng/mL for PilA2 for serum IgM. Less than 3% of the serum samples had IgG and IgM titers that were below the limit of detection. The inter-assay coefficient of variation was ≤30% for all antigens and secondary antibody combinations.[16,23]
2.3 Microbiology
All NP samples and MEF were tested for the presence of Mcat, Spn and NTHi using standard methods as previously described.[15]
2.4. Statistical analysis
To compare the antibody response to Mcat NP colonization between sOP and NOP children at healthy visits, a linear mixed effects model was used to model response log2 Antibody concentration vs. predictors log10 Age and colonization status (per visit) vs. otitis prone (OP) status (per individual).[25] A time varying OP effect was estimated separately for each visit type, using suitable likelihood ratio tests (see Appendix for details). The model was applied to each antigen-specific IgG separately, and P-values adjusted using the Benjamini-Hochberg procedure.[26] Dunn's multiple comparisons test was employed to compare the difference of antibody concentration among different age time points for sOP and NOP children when NP colonization status was assessed. An unpaired t test was used to compare the difference of antibody concentrations between sOP and NOP children for the data in Gaussian distribution and Mann-Whitney test for data not in Gaussian distribution. A paired t test was used to compare the difference of antibody concentrations between AOM acute and convalescence phase. P values of < 0.05 were considered significant.
3. Results
3.1. Serum IgG antibody levels in sOP and NOP children after NP colonization
Fifty-one sOP children and 131 NOP children who had current and/or prior documented culture positive Mcat NP colonization were studied and their serum antibody levels are displayed in Figure 1 and Figure 2. For OppA, sOP children had significantly lower levels of antibody compared to NOP children at age 6-36 mos. (P = 0.015, Fig 1A and Table 3). At age 18 mos. sOP children had antibody levels higher than those measured at age 6 mos. (P < 0.05; Fig 2A) vs. age 15 mos. for NOP children (P < 0.05; Fig 2B), consistent with a later age for measureable increases in total IgG antibody to OppA. The maximum level of antibody (ng/ml) was 3906 ± 629, measured at age 24 mos. for sOP and 5623 ± 1811 measured at age 24 mos. for NOP children (Fig 2A and 2B). For OMP CD, sOP children had lower levels of antibody compared to NOP children at age 6-36 mos. (P = 0.0055, Fig 1B and Table 3). sOP children did not have an increase of IgG across 6-36 mos. age (P > 0.05, Fig 2C). Differently, NOP children showed an earlier increase of IgG to OMP CD at 12 mos. of age (P < 0.05 compared to 6 mos. age, Fig 2D) and further increases at 18-36 mos. of age (P < 0.001 compared to 6 mos. age, Fig 2D). The maximum level of antibody (ng/ml) was 2487 ± 684, measured at age 18 mos. for sOP and 3537 ± 918 measured at age 18 mos. for NOP children (Fig 2C and 2D). For Hag5-9, sOP children had lower levels of antibody compared to NOP children at age 6-36 mos. old (P = 0.02, Fig 1C and Table 3). At age 18 mos. sOP children had antibody levels higher than those measured at age 6 mos. (P < 0.05; Fig 2E) vs. age 15 mos. for NOP children (P < 0.05; Fig 2F), consistent with a later age for measureable increases in total IgG antibody to Hag5-9. The maximum level of antibody (ng/ml) was 2407 ± 392 measured at age 24 mos. for sOP and 4174 ± 1422 measured at age 24 mos. for NOP children (Fig 2E and 2F). For PilA2, although no difference of antibody levels between sOP and NOP children was observed across age 6-36 mos. (P = 0.32, Fig 1D and Table 3), sOP children displayed a later rise and lower peak of antibody than NOP children (Fig 2G and 2H). sOP children did not have a significant increase of IgG across 6-36 mos. of age (P > 0.05, Fig 2G). In contrast, NOP children showed an increase of IgG at 30-36 mos. of age (P < 0.05 compared to 6 mos. age, Fig 2H). The maximum level of antibody (ng/ml) was 1929 ± 358, measured at age 30-36 mos. for sOP and 3563 ± 1362 measured at age 24 mos. for NOP children (Fig 2G and 2H).
Figure 1.
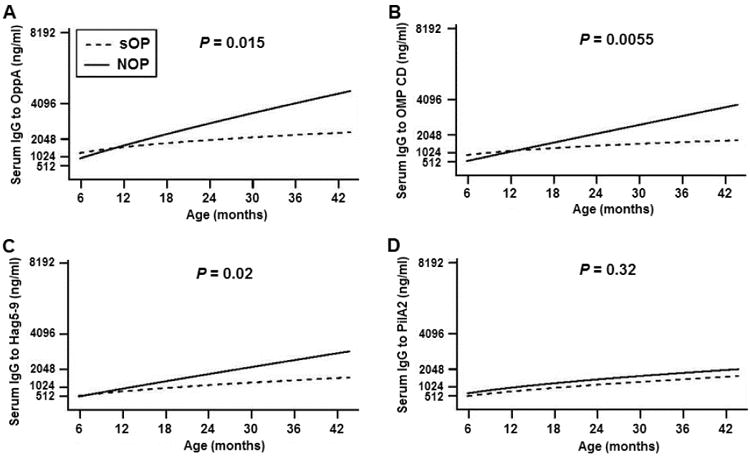
Modeled comparison of serum IgG antibody level against Mcat proteins of OppA (A), OMP CD (B), Hag5-9 (C), and PilA2 (D) between the sOP (dash line) and NOP (solid line) children at their healthy visits. Serum was obtained from the children who had current and/or prior NP colonization of Mcat at each visit confirmed by culture. Serum anti-Mcat protein specific IgG antibody concentrations (ng/ml) were determined with a quantitative ELISA for the sOP children (n = 51) and NOP children (n = 131) at age of 6-36 mos., modeled to 42 mos. Fitted curves are plotted for all antibody values against age of children. P-value is for OP effect among colonization positive visits.
Figure 2.
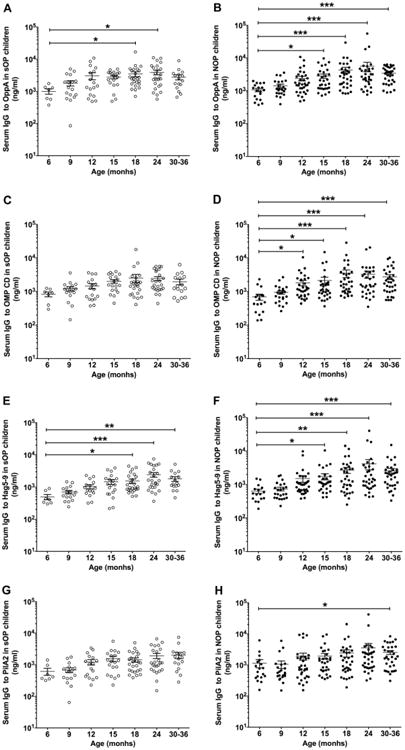
Comparison of age-dependent increase of serum IgG antibody against Mcat proteins of OppA (A and B), OMP CD (C and D), Hag5-9 (E and F), and PilA2 (G and H) between the sOP and NOP children at their healthy visits. Serum was obtained from the children who had NP Mcat colonization prior to and/or at each visit confirmed by culture. Serum anti-Mcat protein specific IgG antibody concentrations (ng/ml) were determined with a quantitative ELISA for the sOP children (n = 51) and NOP children (n = 131) at age of 6-36 mos. Data are represented by mean ± standard error of the mean (SEM). *P < 0.05, **P < 0.01, and ***P < 0.001.
Table 3. Tests for OP effect on antibody response to each antigen for colonization +ve and -ve visits.
| Antibody response | Colonization +vea | Colonization -vea | ||
|---|---|---|---|---|
|
| ||||
| X2 | P-value | X2 | P-value | |
| OppA | 8.40 | 0.0150 | 0.53 | 0.7670 |
| OMP CD | 10.42 | 0.0055 | 1.49 | 0.4754 |
| Hag5-9 | 7.82 | 0.0201 | 3.13 | 0.2095 |
| PilA2 | 2.31 | 0.3154 | 1.65 | 0.4380 |
The likelihood-ratio test statistic X2 for each hypothesis are listed, along with the P-value for rejection of the null hypothesis of no group-specific OP effect. X2 has an approximate χ2 distribution with 2 degrees of freedom under the null hypothesis.
In sum, the increase of antibody to the four Mcat antigens can be ranked as OppA > Hag5-9 > OMP CD > PilA2 in regard to earlier rises and higher peak levels of natural antibody in both sOP and NOP children based on overall.
3.2. Serum IgG and IgM antibody levels in sOP and NOP children at onset of AOM
Serum IgG and IgM were examined in sOP and NOP children at the time of clinical diagnosis of AOM confirmed to be caused by Mcat using tympanocentesis to obtain MEF for microbiologic culture. Because we found that age was a significant variable for both sOP and NOP children with regard to mounting an antibody response (Fig 1 and Fig 2), we confined this analysis of serum antibody response to AOM infections occurring in children at age 11 to 22 months old to minimize an age effect as a confounder for comparisons. For OppA, IgG levels at onset of AOM were significantly higher for NOP compared to sOP children (P = 0.039, Fig 3A), and IgM and total IgM + IgG levels trended higher for NOP children (P = 0.1515, Fig 3A). For Hag5-9, IgG levels at onset of AOM were significantly higher for NOP compared to sOP children (P = 0.025, Fig 3C). The immune response to Hag5-9 was remarkable for the significantly higher IgM compared to IgG levels in both sOP and NOP children (P = 0.0035 and 0.0012, respectively, Fig 3C). For OMP CD (Fig 3B) and PilA2 (Fig 3D), the quantity of IgM and IgG in serum at onset of AOM was not significantly different between sOP and NOP children, suggesting that there was approximately equal early primary responses and late primary or secondary responses to these antigens. There were 8.3% of Mcat-caused AOM episodes in which Mcat was isolated with a second otopathogen (Spn or NTHi) at the same time from the middle ears of these children. We did not find a difference of the serum antibody responses to the Mcat proteins between colonization or infection of Mcat only from co-colonization or co-infection of Mcat with Spn and/or NTHi in the children.
Figure 3.
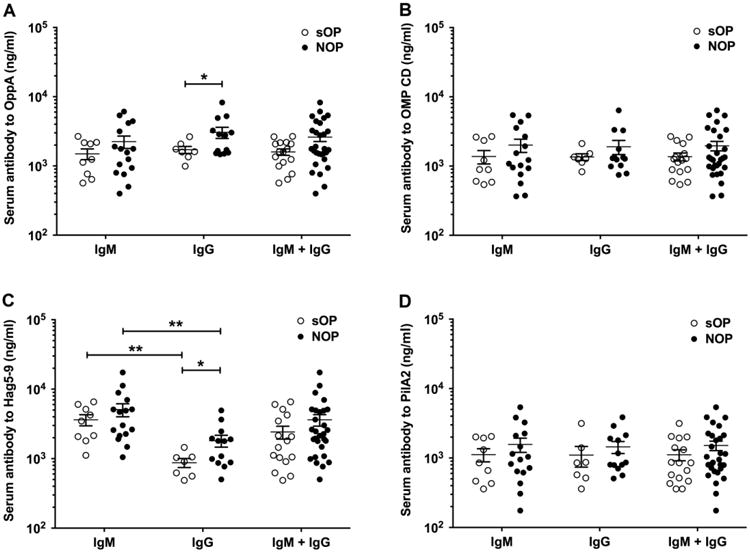
Comparison of serum IgG and IgM antibody against Mcat proteins of OppA (A), OMP CD (B), Hag5-9 (C), and PilA2 (D) between the sOP and NOP children at their acute visit of AOM. Serum anti-Mcat protein specific IgG and IgM antibody concentrations (ng/ml) were determined with a quantitative ELISA for the sOP children (n = 7-9) and NOP children (n = 13-16) at age of 11-22 mos. Data are represented by mean ± SEM. *P < 0.05 and **P < 0.01.
3.3 Serum IgG and IgM antibody levels in sOP and NOP children at onset of AOM vs. convalescence
Fifteen to nineteen pairs of sera from sOP children and 20-22 pairs of sera from NOP children were examined to assess the difference of IgM and IgG levels at onset of AOM compared to after recovery from AOM caused by Mcat for the same child (Table 1). sOP children and NOP children showed 5%-16% and 0-5%, respectively, of > 2-fold increase of serum IgM to Mcat proteins at convalescence stage after an AOM episode (Table 1). There were 0-20% and 0-15% of > 2-fold increases of serum IgG to Mcat proteins in sOP children and NOP children, respectively, at convalescence stage vs. the acute AOM phase (Table 1). Antibody change of each individual child revealed that serum IgM and IgG exhibited a difference between acute and convalescence levels in all three directions: rising, dropping and unchanged among both sOP and NOP children (Table 2).
Table 1.
Comparison of serum IgG and IgM antibody concentration against Mcat proteins between the sOP and NOP children at their AOM vs. convalescence stagea
| Protein | Group of childrenb | N | IgM (ng/ml, mean±SEM) | IgG (ng/ml, mean±SEM) | Percentage of children with >2-fold increase in antibody at convalescence stage vs. AOM | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Acute | Convalescence | Acute | Convalescence | IgM | IgG | |||
| OppA | sOP | 15-19 | 1581±497 | 1352±368 | 2339±564 | 1688±214 | 11% | 0% |
| NOP | 20-22 | 2019±335 | 1536±206 | 2405±420 | 2539±481 | 5% | 0% | |
| OMP CD | sOP | 15-19 | 1471±413 | 1356±314 | 2593±730 | 2567±454 | 16% | 20% |
| NOP | 20-22 | 1799±328* | 1331±199 | 2324±455 | 2663±662 | 5% | 10% | |
| Hag5-9 | sOP | 15-19 | 3196±466 | 2831±337 | 1908±646 | 1109±180 | 11% | 0% |
| NOP | 20-22 | 5205±903* | 3943±603 | 1273±252 | 1697±559 | 0% | 15% | |
| PilA2 | sOP | 15-19 | 1237±387* | 937±286 | 1888±509 | 1315±268 | 5% | 7% |
| NOP | 20-22 | 1437±271* | 1057±171 | 1073±200 | 1545±526 | 5% | 10% | |
children at age 6-36 mos. old were observed.
sOP children have 3-8 AOM episodes each and NOP children have 1-2 AOM episodes each.
P < 0.05 for acute versus convalescence serum.
Table 2.
Change of serum IgG and IgM antibody level against Mcat proteins at convalescence stage vs. AOM in sOP and NOP childrena
| Protein | Group of childrenb | N | Percentage of children with nincrease in antibody at convalescence stage vs. AOM (%) | Percentage of children with no change in antibody at convalescence stage vs. AOM (%) | Percentage of children with decrease in antibody at convalescence stage vs. AOM (%) | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IgM | IgG | IgM | IgG | IgM | IgG | |||
| OppA | sOP | 15-19 | 16 | 33 | 58 | 40 | 26 | 27 |
| NOP | 20-22 | 13 | 29 | 65 | 57 | 22 | 14 | |
| OMP CD | sOP | 15-19 | 21 | 31 | 47 | 63 | 32 | 6 |
| NOP | 20-22 | 9 | 24 | 52 | 71 | 39 | 5 | |
| Hag5-9 | sOP | 15-19 | 21 | 14 | 58 | 64 | 21 | 21 |
| NOP | 20-22 | 9 | 20 | 57 | 65 | 35 | 15 | |
| PilA2 | sOP | 15-19 | 5 | 13 | 53 | 38 | 42 | 50 |
| NOP | 20-22 | 9 | 50 | 43 | 11 | 48 | 39 | |
children at age 6-36 mos. old were observed.
sOP children have 3-8 AOM episodes each and NOP children have 1-2 AOM episodes each.
4. Discussion
Stringently defined otitis prone (sOP) children should be a primary target for novel vaccines against AOM infections caused by Mcat because these children would particularly benefit from vaccination due to their exceptional susceptibility to recurrent middle ear infections. Our previous publication (Ren et al, Vaccine 2015) analyzed the serum antibody responses of the combined sOP and NOP child populations [23], while the current study compared the difference of serum antibody responses between sOP and NOP children. This is the first report of antibody responses to Mcat antigens in sOP children. We have previously shown that sOP children have significantly lower serum antibody responses to Spn and NTHi NP colonization and AOM infections caused by those respiratory pathogens.[3,4] In the current work we similarly found that sOP children displayed impaired serum antibody responses to Mcat candidate protein vaccine antigens compared to age-matched NOP children after asymptomatic Mcat NP colonization and Mcat-caused AOM. The immune deficits among sOP children have been characterized by our group and shown to include immature innate and adaptive immune responses to otopathogens.[5,7,11]
The current results are relevant to consideration of vaccine trials involving young children in hopes of preventing AOM caused by Mcat, as indicated by our previous observations that 23% of sOP children often exhibit absent or significantly reduced antibody responses to many routine pediatric vaccines given in the first year of life.[8] Among the four Mcat candidate protein vaccines we found that OppA was most immunogenic followed by Hag5-9, OMP CD, and then PilA2. The immunogenicity of a protein antigen is mainly determined by the binding strength of epitopes of the protein to B- or T-cell human major histocompatibility complex (MHC) or human leukocyte antigen (HLA) molecules and the density of MHC/HLA binding motifs on each epitope.[27] Differed immunogenicity of these Mcat proteins may be attributed to differing strength of binding to B- and T-cells. However, we note that all four antigens were immunogenic in both sOP and NOP children in the age range of 6 to 36 months old. There are two clades of pilin proteins, PilA1 comprising subclades of PilA1a (32%) and PilA1b (10%), and PilA2 (58%) among Mcat strains.[22] Although there is high conservation among the pilins [22], each protein may elicit serum antibodies without cross reaction with the other pilins upon Mcat NP colonization and middle ear infection because each Mcat strain expresses only one subclade of pilin. In addition, PilA2 is a smaller 16-kD protein [22] and may present fewer reactive epitopes than other larger protein antigens on the surface of Mcat, such as OMP CD, OppA, and Hag. All these factors may be responsible for the lower serum antibody levels detected in young children in this study.
The current results of a more gradual rise in serum antibodies to Mcat antigens in sOP compared to NOP children in response to NP colonization and AOM infections are consistent with our prior observations on the antibody responses to Spn and NTHi.[3,4] In the current work we quantitated antibody by weight whereas in our prior work results were reported in ELISA units [3,4] so direct comparisons in antibody quantity from the current study with prior work by our group is not possible. However, the age of sOP and NOP children when measurable rises to specific candidate protein antigens were identified can be compared. In that regard we observed that natural exposure to Mcat resulted in serum antibody rises to all 4 of the proteins we studied at a later age than we observed for either Spn or NTHi. Specifically for Spn proteins PhtD, LytB, PcpA, PhtE and Ply and NTHi proteins Protein D, P6 and OMP26, significant rises in serum antibody were measured at age 9-12 months.[3,4,15,16] In comparison for Mcat proteins OppA, OMP CD, Hag, and PilA2, significant rises in serum antibody were measured at age 12-36 months. The relevance of these findings relates to potential of natural priming by natural exposure to Mcat prior to and concurrent with the likely age of vaccination of young infants in the first year of life. That is, our data suggest that a natural priming immune response from Mcat exposures may be more delayed than what might occur for Spn and NTHi, suggesting that immune responses to Mcat requires a more mature immune system in infants. We previously found that Spn predominates over Mcat to cause AOM when both organisms co-colonized the NP of young children.[28] Pettigrew et al. showed that Spn colonization is negatively associated with colonization by Haemophilus influenzae on 968 swabs collected from 212 American children.[29] We hypothesize that colonization with Spn and/or NTHi may suppress the colonization and proliferation of Mcat in the NP of children, which may account for the delayed natural priming from Mcat and development of antibodies to Mcat antigens. Future studies on the microbial interactions in the NP among these otopathogens may shed light on the mechanisms of this observation. We did not observe a significant difference of Mcat protein-specific antibodies in the sera of sOP and NOP children when samples were taken during a current vs. a previous Mcat NP colonization. The level of serum Mcat-specific antibody was more associated with the age of the children than the time of the colonization and the gradual effect of repeated exposures to the organism.
Measurement of IgM and IgG antibody levels at the time of clinical diagnosis of AOM allows additional comparisons among vaccine candidate proteins. However, the results do not inform regarding protective levels of antibody. We pursued these studies primarily to understand the relative frequency of early primary serum responses (predominant IgM) to the studied vaccine candidates versus late primary (equivalent IgM and IgG) or secondary (predominant IgG) responses. In our past study of natural antibody levels to NTHi protein antigens D, P6 and OMP26, IgG levels to the individual proteins were higher than the corresponding IgM levels in both sOP and NOP children.[3] In our current Mcat study, we observed similar levels of IgG and IgM to OppA, OMP CD and PilA2 in both sOP and NOP children. However, IgM levels to Hag5-9 were much higher than IgG levels in both sOP and NOP children. We interpret the findings to suggest that earlier exposures to Hag5-9 following NP colonization and AOM infections resulted in less frequent primary immune responses than the other 3 proteins studied because the responses were IgM predominant (Fig 3). The implication might be that natural priming for Hag5-9 responses occurs less frequently in young infants compared to the other vaccine candidates at a younger age.
We found that the Mcat antigens elicited varying IgM and IgG antibody responses in both sOP and NOP children when acute to convalescent measurements were made surrounding an AOM infection. These results are consistent with our earlier studies of Spn and NTHi IgG responses.[3,4] It supports our observation that acute to convalescent changes in antibody to various candidate protein antigens of all three otopathogens surrounding an AOM event reflect an ongoing immune response initiated by NP colonization and not a specific response to the AOM infection. Similar observations of this variability in acute to convalescent antibody levels surrounding an AOM event have been made by another group regarding Mcat protein responses [30].
More than 90% of AOM are preceded by a viral upper respiratory infection (URI).[31] For some children NP colonization may have occurred weeks before an intercurrent viral URI triggered pathogenesis of AOM.[32-34] But for other children acquisition of the potential otopathogen may have occurred only a few days before an intercurrent viral URI, thus accounting for our observing IgM and IgG antibody levels rising, remaining unchanged or falling during the 3 week time span between collection of acute and convalescent sera. We do not consider it likely that the timing of the collection of the acute serum sample in relation to the onset of AOM clinical infection varied widely among the study children because prior work has shown that nearly all AOM infections occur 3 to 5 days after onset of viral URI.[34-36]
Overall this study adds further evidence that sOP children are immunologically different from NOP children. We have previously reported that sOP children suffer from delayed immune maturation and we have proposed the term “prolonged neonatal-like immune profile (PNIP) as applicable to these children because our studies of their B cells and T cells are consistent with a neonatal-like profile.[5,7,8,11,37] The underlying immune immaturity likely accounts for the impaired antibody responses to Mcat infection during asymptomatic colonization and AOM. Although they have not studied Mcat proteins, we note that studies by the group led by Thornton and Richmond involving Australian children who experience recurrent AOM have not observed lower antibody responses to Spn proteins or capsular polysaccharides or to NTHi proteins.[38-40] However there are important differences in the two study populations. We study stringently-defined otitis prone (sOP) children who have every clinical diagnosis confirmed by tympanocentesis and every otopathogen identified by culture, while the Australian study population is diagnosed clinically by health care providers not directly involved in the research and in some cases middle ear fluid obtained from ruptured eardrums.[38-40] Also only 26% of our sOP children require ventilation tubes (unpublished data) while all of the Australian OP children studied for antibody responses to protein antigens were recruited at time of ventilation tube surgery.[38-40] Moreover, they found that Australian aboriginal (possibly similar to OP) children with otitis media displayed lower serum IgG to NTHi but not Spn proteins than non-aboriginal (possibly similar to NOP) or healthy children.[41] Consistent with our findings, these observations also suggest that OP children in different geographic areas may have similar defects in producing serum antibodies to certain otopathogens.
In conclusion, vaccine candidate proteins OppA, OMP CD, Hag5-9 and PilA2 of Mcat are naturally immunogenic in young children who experience Mcat NP colonization and AOM. sOP children produce lower naturally-induced serum antibody against these Mcat protein antigens compared to NOP children, which supports previous work in identifying the immune defects in this population. Purified proteins, particularly when adjuvanted, and administered by inoculation may elicit a different pattern and robustness of antibody response than we measured following natural exposure to Mcat by NP colonization and AOM infection. Further studies of functionality of the serum antibodies and analysis of mucosal antibodies would provide insights to their potential as vaccines and such studies are currently underway in our laboratory.
Acknowledgments
We thank Jill Mangiafesto and Konnor Shares for their ELISA work. We thank Dr. Janet Casey, the nurses and staff of Legacy Pediatrics and the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics and the parents who consented and the children who participated in this challenging study. We thank Dr. Robert Zagursky at Rochester General Hospital Research Institute for his help in reviewing and editing the manuscript. We thank the support of materials and methods from R01 DC012200 to Dr. Timothy F. Murphy.
Funding: This work was supported by the NIH/NIAID under Grant R03 AI113649 to Dabin Ren and NIDCDR01 08671 to Michael E. Pichichero. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- Mcat
Moraxella catarrhalis
- AOM
acute otitis media
- sOP
stringently defined otitis prone
- NOP
non-otitis prone
- OP
otitis prone
- NP
nasopharyngeal
- OMP
outer membrane protein
- Spn
Streptococcus pneumoniae
- NTHi
nontypeable Haemophilus influenzae
- OppA
oligopeptide permease A
- MID
Moraxella IgD-binding protein
- Hag
hemagglutinin
- PilA2
PilA clade 2
- mos
months
- MEF
middle ear fluid
- Escherichia coli
E. coli
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- ICH
International Conference on Harmonisation
- ANOVA
analysis of variance
- SEM
standard error of the mean
- URI
upper respiratory infection
- PNIP
prolonged neonatal-like immune profile
Appendix
Statistical models for OP (otitis prone) effects on antibody response to NP colonization of Mcat
There are n subjects, from which data from multiple visits are observed. Index pair (i, j) refers to the jth of ni visits from subject i. The variables used are:
Aij = Age of subject i at jth visit (in months),
COLij = 1 if subject i is colonized at visit j; = 0 if subject otherwise has colonization history,
OPi = 1 if subject i is sOP,
Yij = Antibody titer of subject i at jth visit. Four antibodies were modeled (OppA, OMP CD, Hag5-9, and PilA2).
The full linear model is
| (1) |
where log10(Aij) × COLij × OPi includes all main effects and interactions, ui is a subject level random effect and eij is the remaining error term. It is assumed that the random effects and error terms are entirely independent, with , eij ∼ N(0, σ2).
Model (1) can be interpreted as 2 × 2 factorial design with age covariate, with design points = (COLij, OPi) ∈ [(0,0), (0,1), (1,0), (1,1)], alternatively denoted t ∈ [ćó, ćo, cć, co] respectively. In the full model a separate simple linear regression fit is associated with each design point t:
| (1) |
with pooled estimates of and σ2 . In this way an OP effect is assessed separately for the colonization +ve (colonization positive at current visit) and -ve (colonization negative at current visit but positive at prior visit (s)) visits, by testing hypotheses:
Hypotheses H1:c and H1:ć are equivalent in the sense that they both represent the full model (1), with 8 model degrees of freedom. Hypotheses H0:c and H0:ć each introduce two constraints to the full model, and are reduced submodels of (1) with 6 degrees of freedom. These represent no OP effect for colonization +ve and -ve visits. The hypotheses can therefore be tested using the likelihood-ratio test statistic X2 = 2log(lFULL/lRED), where lFULL, lRED are the maximum likelihood values of the appropriate full and reduced model. Under the null hypothesis X2 possesses an approximate χ2 distribution with d degrees of freedom, equaling the difference in model degrees of freedom.
The models were fit using function lme from the R package nlme, using the maximum likelihood method (the restricted log-likelihood (REML) method is also available).[25] OP effect on antibody response to each antigen for colonization +ve and -ve visits is shown in Table 3.
Distributional assumptions were assessed by examining subject and population level residuals, and the fitted values for random effect ui. No apparent deviation from normality was observed for the random effect. A moderate amount of right-skewness was observed for some of the residuals. To assess the effect of this on estimates of significant levels, the log transform log2(Y) for antibodies was replaced by the Box-Cox transform Yλ = (Yλ − 1)/λ for values of λ ∈ [−0.5,0.5].[42] This transformation converges to the log transformation as λ approaches 0, and the parameter λ otherwise controls skewness. By adjusting λ, skewness in the residual distributions was reduced without significantly changing the observed significance levels. We therefore conclude that skewness does not affect the inference.
Footnotes
Authors' contribution: DR and MEP conceived and designed the study. DR, MEP, TFM, ERL, AAC and NLM provided materials. DR performed experiments. DR and ALA conducted statistics. DR and MEP wrote the manuscript. All authors have approved the final article.
Disclosure of potential conflicts of interest: Timothy F. Murphy has patents for vaccines for Moraxella catarrhalis. No other potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32:805–809. doi: 10.1097/INF.0b013e31828d9acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruckdeschel EA, Kirkham C, Lesse AJ, Hu Z, Murphy TF. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect Immun. 2008;76:1599–1607. doi: 10.1128/IAI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine. 2011;29:1023–1028. doi: 10.1016/j.vaccine.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J. 2011;30:645–650. doi: 10.1097/INF.0b013e31821c2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204:645–653. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS Immunol Med Microbiol. 2012;65:439–447. doi: 10.1111/j.1574-695X.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. J Infect Dis. 2012;205:1225–1229. doi: 10.1093/infdis/jis179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. Pediatr Infect Dis J. 2013;32:1163–1168. doi: 10.1097/INF.0b013e31829e887e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhoeven D, Nesselbush M, Pichichero ME. Lower nasopharyngeal epithelial cell repair and diminished innate inflammation responses contribute to the onset of acute otitis media in otitis-prone children. Med Microbiol Immunol. 2013;202:295–302. doi: 10.1007/s00430-013-0293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhoeven D, Pichichero ME. Divergent mucosal and systemic responses in children in response to acute otitis media. Clin Exp Immunol. 2014;178:94–101. doi: 10.1111/cei.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basha S, Pichichero ME. Poor memory B cell generation contributes to non-protective responses to DTaP vaccine antigens in otitis-prone children. Clin Exp Immunol. 2015;182:314–322. doi: 10.1111/cei.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Casey JR, Newman E, Pichichero ME. Otitis-prone Children Have Immunologic Deficiencies in Naturally Acquired Nasopharyngeal Mucosal Antibody Response after Streptococcus pneumoniae Colonization. Pediatr Infect Dis J. 2016;35:54–60. doi: 10.1097/INF.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 13.Pichichero ME. Ten-Year Study of the Stringently Defined Otitis-prone Child in Rochester, NY. Pediatr Infect Dis J. 2016;35:1033–1039. doi: 10.1097/INF.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren D, Almudevar AL, Pichichero ME. Synchrony in serum antibody response to conserved proteins of Streptococcus pneumoniae in young children. Hum Vaccin Immunother. 2015;11:489–497. doi: 10.4161/21645515.2014.990861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan MN, Almudevar A. Antibody response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2010;28:7184–7192. doi: 10.1016/j.vaccine.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum Vaccin Immunother. 2012;8:799–805. doi: 10.4161/hv.19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren D, Pichichero ME. Vaccine targets against Moraxella catarrhalis. Expert Opin Ther Targets. 2016;20:19–33. doi: 10.1517/14728222.2015.1081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy TF, Kirkham C, Liu DF, Sethi S. Human immune response to outer membrane protein CD of Moraxella catarrhalis in adults with chronic obstructive pulmonary disease. Infect Immun. 2003;71:1288–1294. doi: 10.1128/IAI.71.3.1288-1294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones MM, Johnson A, Koszelak-Rosenblum M, Kirkham C, Brauer AL, Malkowski MG, et al. Role of the Oligopeptide Permease ABC Transporter of Moraxella catarrhalis in Nutrient Acquisition and Persistence in the Respiratory Tract. Infect Immun. 2014;82:4758–4766. doi: 10.1128/IAI.02185-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang M, Johnson A, Murphy TF. Characterization and evaluation of the Moraxella catarrhalis oligopeptide permease A as a mucosal vaccine antigen. Infect Immun. 2011;79:846–857. doi: 10.1128/IAI.00314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaFontaine ER, Snipes LE, Bullard B, Brauer AL, Sethi S, Murphy TF. Identification of domains of the Hag/MID surface protein recognized by systemic and mucosal antibodies in adults with chronic obstructive pulmonary disease following clearance of Moraxella catarrhalis. Clin Vaccine Immunol. 2009;16:653–659. doi: 10.1128/CVI.00460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luke-Marshall NR, Sauberan SL, Campagnari AA. Comparative analyses of the Moraxella catarrhalis type-IV pilus structural subunit PilA. Gene. 2011;477:19–23. doi: 10.1016/j.gene.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Ren D, Almudevar AL, Murphy TF, Lafontaine ER, Campagnari AA, Luke-Marshall N, et al. Serum antibody response to Moraxella catarrhalis proteins OMP CD, OppA, Msp22, Hag, and PilA2 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2015;33:5809–5814. doi: 10.1016/j.vaccine.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ICH. Switzerland: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; 1994. [Accessed June 15, 2017]. Validation of Analytical Procedures: Text and Methodology. Online. Available: http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. [Google Scholar]
- 25.Pinheiro J, Bates D, DebRoy S, Sarkar D team, RC. Linear and nonlinear mixed effects models. R package version 3. 2017 [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Statistical Methodology) 1995:289–230. [Google Scholar]
- 27.Weber CA, Mehta PJ, Ardito M, Moise L, Martin B, De Groot AS. T cell epitope: friend or foe? Immunogenicity of biologics in context. Adv Drug Deliv Rev. 2009;61:965–976. doi: 10.1016/j.addr.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, Casey JR, Chang A, Pichichero ME. When co-colonizing the nasopharynx haemophilus influenzae predominates over Streptococcus pneumoniae except serotype 19A strains to cause acute otitis media. Pediatr Infect Dis J. 2012;31:638–640. doi: 10.1097/INF.0b013e31824ba6f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathers K, Leinonen M, Goldblatt D. Antibody response to outer membrane proteins of Moraxella catarrhalis in children with otitis media. Pediatr Infect Dis J. 1999;18:982–988. doi: 10.1097/00006454-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Pichichero ME. Otitis media. Pediatr Clin North Am. 2013;60:391–407. doi: 10.1016/j.pcl.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Heikkinen T, Ruuskanen O. Temporal development of acute otitis media during upper respiratory tract infection. Pediatr Infect Dis J. 1994;13:659–661. doi: 10.1097/00006454-199407000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Koivunen P, Kontiokari T, Niemela M, Pokka T, Uhari M. Time to development of acute otitis media during an upper respiratory tract infection in children. Pediatr Infect Dis J. 1999;18:303–305. doi: 10.1097/00006454-199903000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev. 2003;16:230–241. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chonmaitree T, Alvarez-Fernandez P, Jennings K, Trujillo R, Marom T, Loeffelholz MJ, et al. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis. 2015;60:1–9. doi: 10.1093/cid/ciu714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49:3750–3755. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. 2014;10:1171–1184. doi: 10.1586/1744666X.2014.942288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkham LS, Wiertsema SP, Corscadden KJ, Mateus T, Mullaney GL, Zhang G, et al. Otitis-prone children produce functional antibodies to pneumolysin and pneumococcal polysaccharides. Clin Vaccine Immunol. 2017;24:e00497–00416. doi: 10.1128/CVI.00497-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiertsema SP, Corscadden KJ, Mowe EN, Zhang G, Vijayasekaran S, Coates HL, et al. IgG responses to Pneumococcal and Haemophilus influenzae protein antigens are not impaired in children with a history of recurrent acute otitis media. PLoS One. 2012;7:e49061. doi: 10.1371/journal.pone.0049061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon VJ, Corscadden KJ, Fuery A, Thornton RB, Kirkham LA, Richmond PC, et al. Children with otitis media mount a pneumococcal serotype specific serum IgG and IgA response comparable to healthy controls after pneumococcal conjugate vaccination. Vaccine. 2012;30:3136–3144. doi: 10.1016/j.vaccine.2012.01.086. [DOI] [PubMed] [Google Scholar]
- 41.Thornton RB, Kirkham LS, Corscadden KJ, Wiertsema SP, Fuery A, Jones BJ, et al. Australian Aboriginal Children with Otitis Media Have Reduced Antibody Titers to Specific Nontypeable Haemophilus influenzae Vaccine Antigens. Clin Vaccine Immunol. 2017;24 doi: 10.1128/CVI.00556-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Box GE, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society Series B (Statistical Methodology) 1964:211–252. [Google Scholar]


