Summary
RHOA, a founding member of the Rho GTPase family, is critical for actomyosin dynamics, polarity, and morphogenesis in response to developmental cues, mechanical stress, and inflammation. In murine small intestinal epithelium, inducible RHOA deletion causes a loss of epithelial polarity, with disrupted villi and crypt organization. In the intestinal crypts, RHOA deficiency results in reduced cell proliferation, increased apoptosis, and a loss of intestinal stem cells (ISCs) that mimic effects of radiation damage. Mechanistically, RHOA loss reduces YAP signaling of the Hippo pathway and affects YAP effector epiregulin (EREG) expression in the crypts. Expression of an active YAP (S112A) mutant rescues ISC marker expression, ISC regeneration, and ISC-associated Wnt signaling, but not defective epithelial polarity, in RhoA knockout mice, implicating YAP in RHOA-regulated ISC function. EREG treatment or active β-catenin Catnblox(ex3) mutant expression rescues the RhoA KO ISC phenotypes. Thus, RHOA controls YAP-EREG signaling to regulate intestinal homeostasis and ISC regeneration.
Keywords: mouse model, intestinal stem cell, regeneration, Rho GTPase, RhoA, Hippo signaling, YAP, Wnt signaling
Highlights
-
•
Inducible RHOA deletion in mice causes defects in intestinal structure and polarity
-
•
RHOA controls intestinal stem cell proliferation and Hippo signaling
-
•
Active YAP/EREG, as well as β-catenin, rescues ISC loss caused by RHOA deficiency
-
•
RHOA-YAP-EREG signaling mediates intestinal maintenance and ISC regeneration
In this article, Zheng and colleagues show that inducible RHOA deletion in mice causes defects in intestine epithelial polarity and deficiencies in intestinal stem cell proliferation, survival, and regeneration. They further demonstrate by genetic rescues that RHOA controls a YAP-EREG axis to mediate canonical Wnt signaling, intestinal stem cell function, and intestinal homeostasis.
Introduction
Intestinal epithelium, one of the most vigorously self-renewing tissues in an adult mammal, is composed of four major cell types: absorptive enterocytes, mucus-secreting goblet cells, antimicrobial Paneth cells, and hormone-secreting enteroendocrine cells (Barker and Clevers, 2010, Barker et al., 2007). More differentiated cell types reside within the villus, while the base of each villus is surrounded by multiple epithelial crypt invaginations (Clevers, 2013). At the bottom of each crypt, crypt base columnar cells (CBCs) (Cheng and Leblond, 1974) occupy cell positions +1 through +5 from the base and are intercalated between Paneth cells, which together constitutes a stem cell niche (Sato et al., 2011, Snippert et al., 2010).
The CBCs, containing intestinal stem cell (ISC) activity, proliferate constantly and give rise to rapidly proliferating transient amplifying cells just above Paneth cells. Transient amplifying cells then migrate upward and differentiate at the crypt-villus boundary into one of the four mature cell types (Tian et al., 2015). Several molecular markers have been used to identify ISCs, including Lgr5, Ascl2, Olfm4, and Bmi1 (Barker and Clevers, 2010, Barker et al., 2007, van der Flier et al., 2009). The Lgr5-positive cells are actively cycling, long lived, and give rise to all four epithelial lineages (Sato et al., 2011, Snippert et al., 2010). Single Lgr5-expressing ISCs can be cultured in vitro to form long-lived, self-organizing crypt-villus organoids in the absence of non-epithelial niche cells (Sato and Clevers, 2013, Sato et al., 2009, Sato et al., 2011).
The regeneration and differentiation process of the small intestine is stringently regulated to ensure a balanced homeostasis. Primarily known as an important regulator in organ size control and cell proliferation (Zhao et al., 2010), the Hippo/Yes-Associated Protein (YAP) pathway has emerged recently as an important regulator of ISC regeneration and intestinal tumorigenesis (Barry and Camargo, 2013, Hong et al., 2016). YAP1 and TAZ have been shown to promote ISC proliferation (Imajo et al., 2015), but surprisingly, mice with depleted YAP1 and/or TAZ protein in the intestine have normal homeostasis (Azzolin et al., 2014, Barry et al., 2013, Cai et al., 2015), suggesting that YAP1 and TAZ are dispensable under normal conditions. Further studies show that YAP1 is involved in intestinal regeneration after drug- or irradiation-induced injury (Gregorieff et al., 2015, Taniguchi et al., 2015), and the YAP1 target, epidermal growth factor receptor ligand EREG, modulates ISC proliferation and regeneration (Gregorieff et al., 2015). Multiple studies suggest that the Hippo-YAP pathway interacts with the Wnt/β-catenin pathway (Pinto et al., 2003, van der Flier and Clevers, 2009) to regulate the function of ISCs (Azzolin et al., 2014, Barry and Camargo, 2013, Cai et al., 2015, Imajo et al., 2012). However, conflicting interpretations of these studies led to the proposal that YAP may both enhance and inhibit Wnt signaling in ISCs (Azzolin et al., 2014, Barry and Camargo, 2013, Imajo et al., 2012, Rosenbluh et al., 2012), possibly reflecting the complexity of the signaling network in ISC regulation.
While many of the current studies focus on the intracellular signaling mechanisms of ISCs, the apical-basal polarity and cell adhesion junctions play key roles in intestinal epithelium morphogenesis. As a key member of the Rho guanosine triphosphatase (GTPase) family, RHOA has been found to be involved in regulating tissue-specific cytoskeleton dynamics, cell adhesion, survival, cell-cycle progression, and transcription (Etienne-Manneville and Hall, 2002, Wang and Zheng, 2007). RHOA acts as a molecular switch to control signal transduction by shifting between a guanosine diphosphate (GDP)-bound, inactive form and a GTP-bound, active form (Etienne-Manneville and Hall, 2002, Karlsson et al., 2009, Liu et al., 2012, Wang and Zheng, 2007). Dysfunction of RHOA and related GTPases can cause cancer, neurological abnormalities, immunological disorders, and several other diseases (Wang and Zheng, 2007, Zhou and Zheng, 2013). Interestingly, in the Hippo pathway, YAP1 and TAZ transcriptional activities are regulated by mechanical actomyosin signal and G-protein-coupled receptor (GPCR)-mediated extracellular signals through Rho GTPases (Dupont et al., 2011, Rauskolb et al., 2014, Yu et al., 2012, Zhao et al., 2012), suggesting a connection between RHOA and Hippo-mediated transcription.
In the current work, we use an intestinal epithelium-specific inducible gene deletion in a mouse model of Villin-CreERT2;RhoAflox/flox to define the physiological function and molecular mechanisms of RHOA in ISC regulation. Our study shows that RHOA-mediated YAP-EREG signaling plays a critical role during intestine homeostasis and ISC regeneration, implicating a relationship between RHOA-controlled epithelial integrity/polarity and intracellular YAP/Wnt signaling in ISC regulation.
Results
Inducible Deletion of RhoA Causes Defects in Small Intestinal Epithelium
RHOA is unanimously expressed throughout the small intestine in adult mice, including crypt and villi (Figures S1A and S1B). Inside the crypt, RHOA protein is detected in both Lgr5−GFP+ ISCs and Lgr5−GFP− Paneth cells in the small intestine of Lgr5-EGFP-IRES-CreERT2 knockin mice (Figures S1A and S1B).
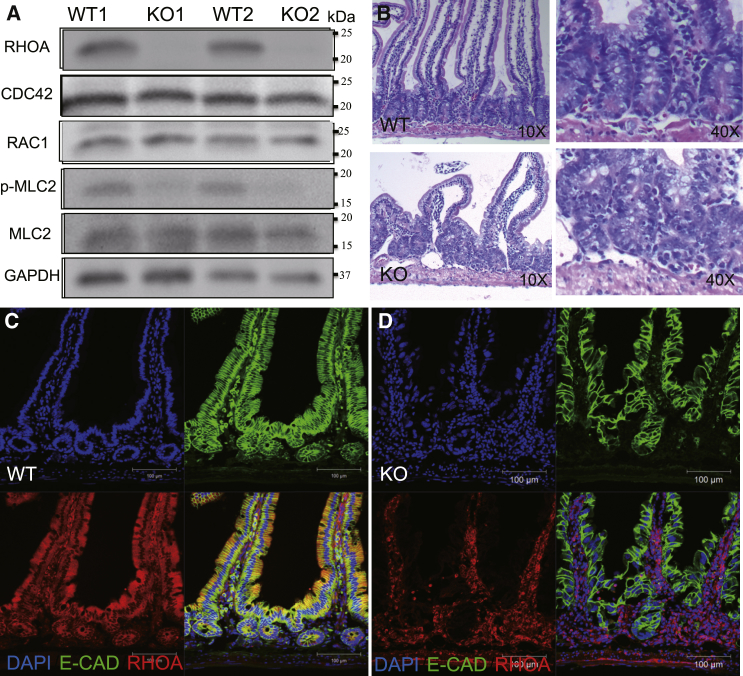
To investigate the function of RHOA in mammalian intestinal epithelium, we generated an inducible intestine-specific RhoA-ablation mouse line, which uses the intestinal epithelium-specific Villin promoter to deliver tamoxifen-inducible Cre activity in the intestine. Villin-CreERT2; RhoAfl/fl mice (hereafter called RhoA knockout [KO]) and respective control wild-type mice RhoAfl/fl (hereafter called WT) were injected with tamoxifen intraperitoneally, twice a day for 3 straight days at 4–8 weeks of age. One day post tamoxifen administration, the RhoA KO mice were depleted of the RhoA gene and protein in the small intestine but not in the surrounding stroma (Figures S1C, S1D, and 1A). Compensatory expression of other Rho GTPase family members such as Rac1 and CDC42 was not detected, and the downstream effector of RHOA, phospho-MLC2, was downregulated upon RHOA deletion (Figure 1A). H&E staining of duodenum in RhoA KO mice revealed a severe disruption of crypt-villus architecture compared with WT mice (Figure 1B). The villi in the RhoA KO mice were relatively intact but noticeably shorter than that of WT mice (Figure 1B). Transmission electron microscopy analysis confirmed that the intestinal epithelial architecture was disorganized in RhoA KO mice (Figure S2A). Similar but more moderate structural defects in villi and crypts were observed in jejunum and ileum (Figures S2B and S2C). We chose duodenum as the focus of this study.
Figure 1.
Conditional Deletion of RhoA in the Small Intestine Causes Defects in Epithelial Architecture
(A) Western blotting of RHOA, Cdc42, Rac1, and effectors of RHOA (MLC2 and p-MLC2) in isolated small intestinal crypts from 1- to 2-month-old control WT or RhoA KO mice.
(B) Representative H&E staining of control WT or RhoA KO duodenum sections.
(C and D) Representative immunofluorescence by anti-RHOA and anti-E-cadherin in control WT (C) or RhoA KO (D) duodenum sections.
Further analysis comparing RHOA and E-cadherin protein expression in WT and KO duodenum confirms a complete deletion of RHOA in both villi and crypts of KO mice, while E-cadherin levels were reduced with a disrupted expression pattern in the KO (Figures 1C and 1D). Noticeably, there is no detectable difference between heterozygous RhoA KO (Villin-CreERT2+; RhoAfl/wt) mice and control WT (RhoAfl/fl) litters (data not shown), indicating that there is no haplodeficient effect with RhoA gene in the small intestine.
Loss of RhoA Disrupts Epithelial Polarity, Adhesion, and Organization in the Small Intestine
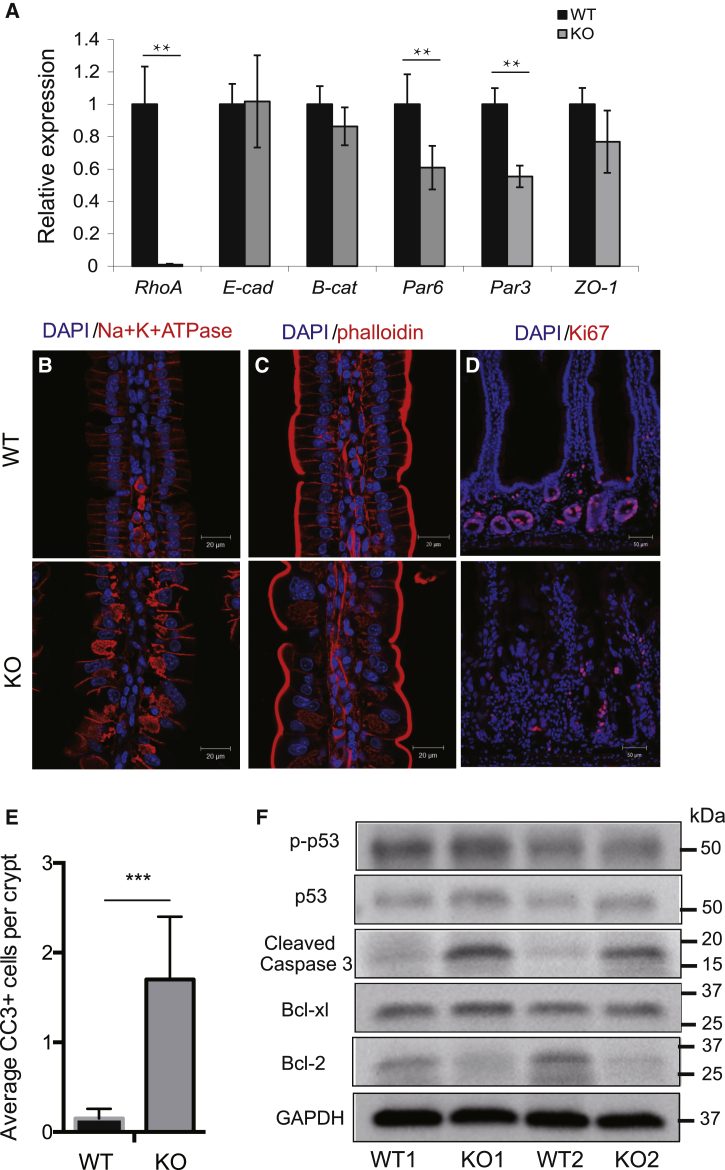
RHOA is involved in actin stress fiber formation, actomyosin contractility, focal adhesion, and adherens junction (AJ) complex (Kaibuchi et al., 1999, Melendez et al., 2011, Rajasekaran et al., 2001). To further characterize the intestinal defects in RhoA KO mice, we analyzed the transcription pattern of markers for AJ, polarity, and basolateral compartments. The transcription expression of polarity-regulating molecules Par3 and Par6 was significantly reduced in RhoA KO intestine (Figure 2A) while the mRNA level of adhesion molecules E-cadherin and β-Catenin were not significantly altered, suggesting disrupted epithelial polarity. Immunofluorescence staining found that in RhoA KO mice, ion transporter Na+,K+-ATPase, a basolateral marker, was more diffusely localized, confirming the polarity defect (Figure 2B). Analysis of actin cytoskeleton using phalloidin staining showed a disrupted junctional actin network in RhoA KO intestinal epithelial cells (Figure 2C), consistent with the disruption of the E-cadherin complex (Figure 1D).
Figure 2.
Loss of RhoA Disrupts Epithelial Polarity and Adhesion, while Reducing Proliferation and Inducing Apoptosis in Small Intestine
(A) qRT-PCR analysis showing gene expression of cell junction and polarity markers in control WT and RhoA KO small intestine. Data are normalized to GAPDH expression; n = 3 mice per genotype. Error bars represent SD from three independent experiments.
(B–D) Representative immunofluorescence staining of Na+,K+-ATPase α (B), phalloidin (C), and Ki67 (D) in control WT and RhoA KO duodenum.
(E) Quantification of cleaved caspase-3-positive cells per crypt in control WT and RhoA KO duodenum. n = 4 mice for each genotype. Error bars represent SD from three independent experiments.
(F) Western blotting of apoptotic related proteins Bcl-2, Bcl-xL, p53, p-P53, and cleaved caspase-3 in duodenal crypts isolated from control and RhoA KO mice.
∗∗p < 0.01, ∗∗∗p < 0.001.
We next examined whether loss of RHOA affects different cell types in small intestine. Paneth cells are exclusively located at the bottom of the crypts in WT intestine, as shown by lysozyme immunostaining; in the KO mice, most Paneth cells remained in the crypts but the villi/crypt structures were disrupted (Figures S3A and S3B). In addition, the number and localization of goblet cells and enteroendocrine cells marked by mucin-2 and chromogranin A staining, respectively, were unaffected by RhoA KO (Figures S3C and S3D). We conclude that RHOA is critically involved in basolateral polarity, cell adhesion, and epithelium organization in the small intestine.
Loss of RhoA Reduces Proliferation and Induces Apoptosis in Small Intestinal Crypts
RHOA has been implicated in regulating epithelial cell proliferation in the small intestine (Benoit et al., 2009), and RHOA-regulated cell adhesion/polarity is closely related to cell-cycle regulation. We next examined whether the crypt cell proliferation and cell cycle are affected by RHOA loss of function. The number of proliferating crypt cells marked by Ki67 (non-G0 cycling cells) (Figure 2D) and phosphor-histone H3 (M-phase cells) (Figure S3E) are both dramatically decreased in RhoA KO intestinal tissue, which may account for the disorganized epithelial structure. Reduced proliferation occurs in all sections of the intestine, including duodenum, jejunum, and ileum (data not shown), suggesting that RHOA is critical for cell proliferation in the whole small intestine. Recent genetic studies have revealed a critical function of RHOA during mitosis in different biological contexts including blood progenitor cells, keratinocytes, and mouse embryonic fibroblasts (Jackson et al., 2011, Melendez et al., 2011, Zhou et al., 2013). Interestingly, RHOA deficiency in small intestine also induced multinucleated cells (Figure S3F), suggesting a mitotic arrest.
Aside from the effect on proliferation, the RhoA KO increased the cleaved caspase-3-positive cells significantly throughout the crypts and villi (Figure S3G, quantified in Figure 2E). Consistently, total cleaved caspase-3 protein level was dramatically increased in RhoA KO epithelium as found by western blotting (Figure 2F), which could, at least in part, account for the partial loss of the crypt upon RHOA deletion. Molecularly, RHOA deletion caused a reduced pro-survival signal marked by Bcl-2, with no obvious change of pro-apoptotic proteins such as p53 (Figure 2F), indicating an altered apoptotic mechanism.
Inducible RhoA Deletion Leads to a Loss of Intestinal Stem Cells and Reduced Canonical Wnt Signaling In Vivo and In Vitro
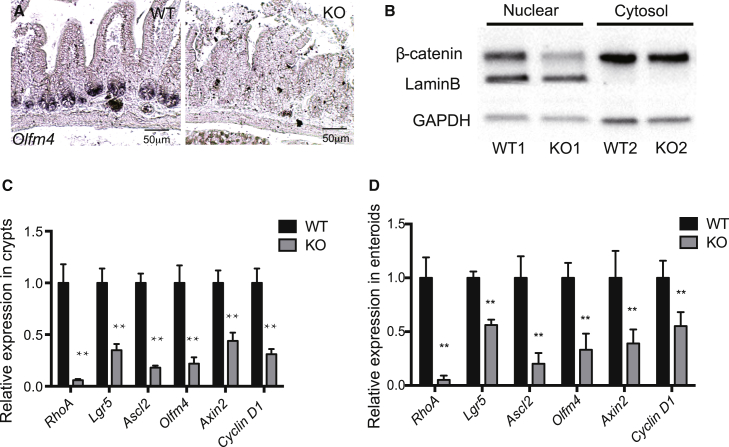
Disrupted crypts and reduced proliferation in RhoA KO small intestine is indicative of ISC malfunction. To determine the requirement of RHOA in ISCs, we crossed Lgr5-EGFP-IRES-CreERT2 mouse (Barker et al., 2007), which has the eGFP marking Lgr5+ ISCs in the crypts, with RhoA KO and WT mice (the CreERT2 in this model could not effectively delete RhoA gene upon tamoxifen induction; data not shown). In this model, Lgr5−eGFP+ ISCs were drastically reduced in small intestine crypts 2 days after tamoxifen induction (Figure S4A). An in situ hybridization analysis showed that the ISC marker Olfm4 was also dramatically decreased in RHOA-depleted small intestine (Figure 3A). A qRT-PCR test confirmed that three ISC markers, i.e., Lgr5, Olfm4, and Ascl2, were significantly reduced in RhoA KO crypts (Figure 3C).
Figure 3.
RhoA Deletion Leads to a Loss of Intestinal Stem Cells and Reduced Canonical Wnt Signaling In Vivo and In Vitro
(A) Representative images of in situ hybridization of Olfm4 RNA in control WT and RhoA KO duodenum sections.
(B) Western blotting of cytosolic and nuclear fractions of β-catenin protein from control WT and RhoA KO duodenal crypts. GAPDH and laminin B protein expressions were used as controls for the cytosolic and nuclear fractions, respectively.
(C and D) qRT-PCR analysis of RNA expressions of ISC markers and Wnt target genes in isolated control WT and RhoA KO duodenal crypts (C) and enteroids (D). Data are normalized to GAPDH; n = 3 mice for each genotype. ∗∗p < 0.01. Error bars represent SD from three independent experiments.
Since canonical Wnt signaling is associated with and is required for self-renewal of ISCs and ISC-niche interaction (Pinto et al., 2003, van der Flier and Clevers, 2009), we investigated the canonical Wnt signaling in RhoA KO crypts. A qRT-PCR analysis showed that the canonical Wnt pathway targets Axin2 and Cyclin D1 were both significantly reduced in the RHOA loss-of-function crypts (Figure 3C), suggesting impaired canonical Wnt signaling upon RhoA KO. Consistently, the protein level of nuclear β-catenin, as a readout of canonical Wnt signaling, was correspondingly reduced in RhoA KO crypts (Figure 3B).
To further examine whether RHOA regulates the function of ISCs, we performed an in vitro intestinal organoid (enteroid) culture assay using enteroids derived from Lgr5-EGFP-IRES-CreERT2; Villin-CreERT2;RhoAfl/fl (KO) and Lgr5-EGFP-IRES-CreERT2; Villin-CreERT2; RhoAfl/+ mice (WT). Four days after culture, Lgr5−eGFP+ stem cells were localized at the tip of crypt-like intrusions surrounding the central lumen (green due to autofluorescence) lined by villus-like epithelium (Figure S4C). Consistent with the loss of ISC phenotype upon RHOA deletion in vivo, 4-OH tamoxifen-induced RHOA deletion in vitro inhibited the formation of crypt-like domains, leading to the loss of Lgr5−eGFP+ ISCs (Figure S4C). Addition of Y27632, an inhibitor of the major RHOA signaling effector ROCK, into the medium (for 4 days) phenocopied RHOA deletion, causing a loss of Lgr5−eGFP+ ISCs (Figure S4C). qRT-PCR performed 1 day after tamoxifen-induced RHOA deletion revealed a similar transcriptional reduction of ISC markers, i.e., Lgr5, Ascl2, and Olfm4, which is associated with a reduction of canonical Wnt markers Axin2 and Cyclin D1 in RhoA KO enteroids (Figure 3D). An examination of non-canonical Wnt antagonists DKK1 and Wnt5A in WT and KO enteroids by qRT-PCR found their transcription levels very low and remained unchanged upon RhoA KO (data not shown). Together, these results suggest that RHOA signaling regulates ISC cell fate.
YAP Signaling Mediates the RhoA KO Phenotype of ISC Loss
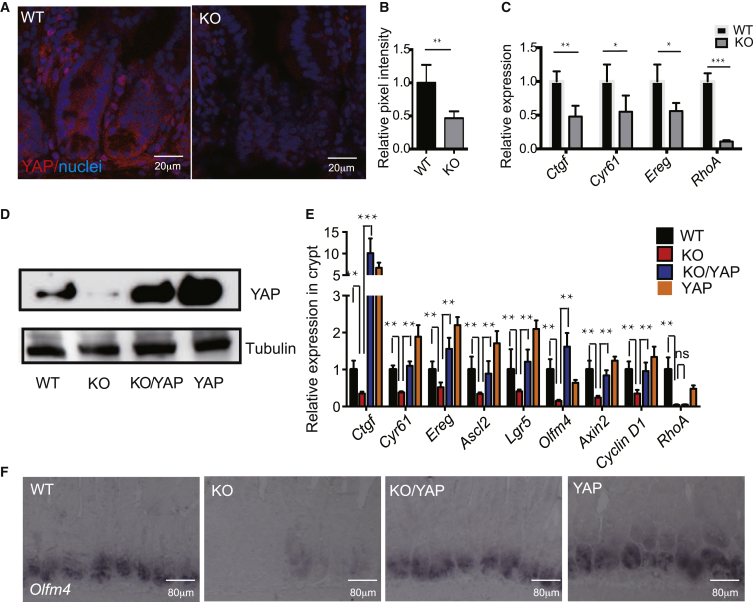
The Hippo/YAP pathway has been reported as an important regulator during ISC regeneration (Barry and Camargo, 2013), and RHOA GTPases regulate YAP1 and TAZ transcriptional activities (Yu et al., 2012). To examine whether Hippo signaling is involved in the RhoA KO phenotypes, we first determined the expression of total YAP protein in the crypts by immunostaining. RhoA KO crypts exhibited significantly lower total YAP and nuclear YAP expressions, indicative of reduced YAP signaling activity (Figures 4A and 4B). Consistently, the transcripts of the YAP signaling targets, Ctgf, Cyr61, and Ereg, were repressed in RhoA KO crypts as well (Figure 4C), and western blotting of total YAP showed a significant reduction of YAP protein in RhoA KO crypts (Figure 4D).
Figure 4.
YAP Signaling Mediates the RhoA KO Phenotype of ISC Loss
(A) Representative immunofluorescence staining of total YAP in control and RhoA KO small intestine.
(B) Quantification of fluorescence intensity of nuclear YAP staining in control and RhoA KO crypts. Normalized to DAPI staining, n = 4 mice for each genotype. Error bars represent SD from three independent experiments.
(C) qRT-PCR showing expression of YAP signaling targets in control and RhoA KO duodenal crypts. Data are normalized to GAPDH expression; n = 3 mice for each genotype. Error bars represent SD from three independent experiments.
(D) Western blotting of total YAP protein using duodenal crypts isolated from control WT, RhoA KO, KO/YAP (S112A) rescue, and YAP (S112A) expressing enteroids.
(E) qRT-PCR analysis showing the expression of YAP transcriptional targets, ISC markers, and canonical Wnt signaling target genes in control WT, RhoA KO, KO/YAP(S112A) rescue, and YAP(S112A) expressing duodenal crypts. Data are normalized to GAPDH; n = 3 mice for each genotype. Error bars represent SD from three independent experiments.
(F) In situ hybridization analysis of Olfm4 RNA in control WT, RhoA KO, KO/YAP(S112A) rescue, and YAP(S112A) expressing duodenum.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant.
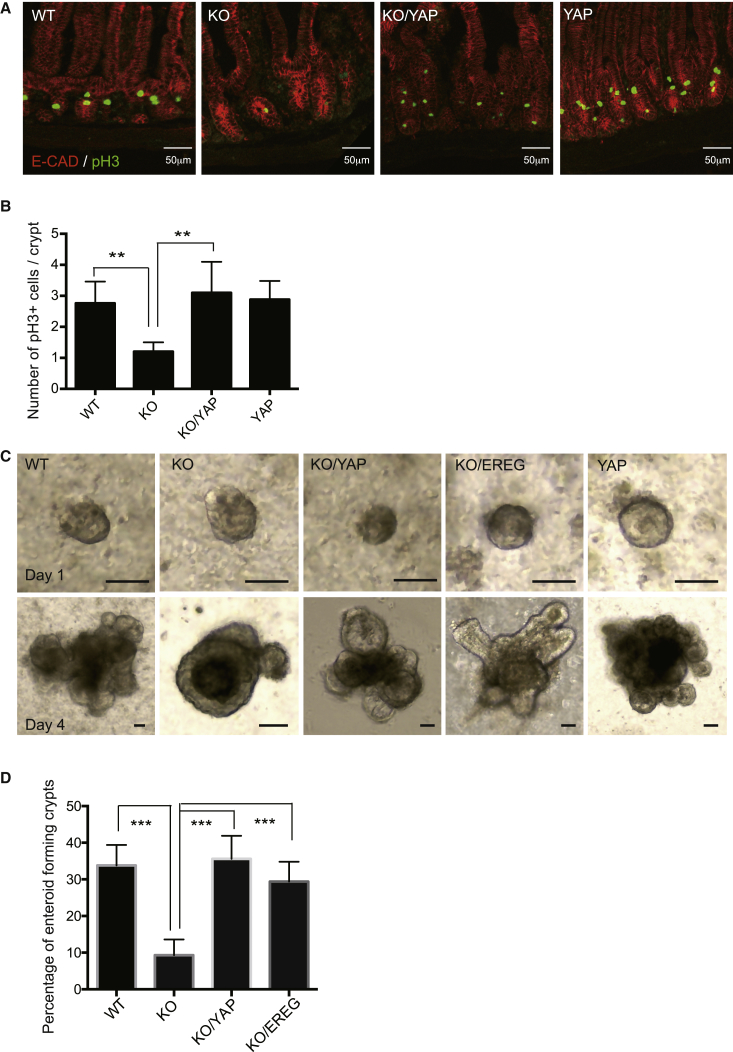
To further determine whether YAP signaling plays a role in the RHOA-mediated ISC regulation, we took a genetic rescue approach to cross Villin-CreERT2; RhoAfl/fl (KO) mice or Villin-CreERT2; RhoAfl/+ (WT) with mice expressing an inducible active YAP (S112A) mouse model, which turns on YAP nuclear activity upon Cre induction with tamoxifen. The active YAP mutant expression readily rescued the transcription of the YAP signaling targets, Ctgf, Cyr61, and Ereg, as well as the ISC markers, Ascl2, Lgr5, and Olfm4. Interestingly, canonical Wnt targets Axin2 and Cyclin D1 were also rescued by the active YAP mutant in RhoA KO (Figure 4E), consistent with a rescue of ISCs by the active YAP mutant signaling. An in situ hybridization with a probe against Olfm4 confirmed that the active YAP mutant rescued the ISC marker expression in the RhoA KO (Figure 4F), while H&E staining showed that the active YAP mutant could not restore the disrupted villi structure (Figure S5A).
As RhoA KO has significantly reduced proliferation and disrupted structure in small intestinal epithelium, we tested whether active YAP is able to rescue the defects by immunostaining of pH3, E-cadherin, and β-catenin. The active YAP mutant rescued cell proliferation in the crypts, but not the disrupted epithelial organization (Figures 5A, 5B, and S5B). Surprisingly, we saw increased cytoplasmic YAP staining besides nuclear YAP in our YAP mutant rescue mice, which may be due to a compensatory reaction of the endogenous YAP (Figure S5B). We also used enteroid culture assays to determine the function of YAP signaling in ISC regeneration. In a 1-day culture, the isolated crypts of all genotypes formed similar small sphere-like enteroids; however, after 4 days in culture there was little budding or growth of the RhoA KO enteroids, and significantly fewer buds developed from RhoA KO enteroids compared with WT control (Figures 5C and 5D). Inducible expression of the active YAP(S112A) mutant, or an addition of the YAP target EREG protein to the culture medium, rescued enteroid-forming efficiency and enteroid growth (Figures 5C and 5D). These results indicate that YAP signaling mediates ISC homeostasis and regeneration under the RHOA null genetic conditions.
Figure 5.
YAP Signaling Rescues Proliferation and Enteroid Growth in RhoA KO
(A) Representative immunofluorescence staining of phosphor-histone H3 in control WT, RhoA KO, KO/YAP (S112A) rescue, and YAP (S112A) mice small intestine.
(B) Quantification of number of phosphor-histone H3-positive cells per crypt in control WT, RhoA KO, KO/YAP (S112A) rescue, and YAP (S112A) small intestine. n = 3 mice for each genotype. Error bars represent SD from three independent experiments.
(C) Representative images of growth of control WT, RhoA KO, KO/YAP(S112A) rescue, KO/EREG rescue (0.5 μg/mL), and YAP(S112A) expressing enteroids after 1 day and 4 days of culture.
(D) Percentage of enteroids developed from same number of crypts planted. n = 3 mice for each genotype. Error bars represent SD from three independent experiments.
∗∗p < 0.01, ∗∗∗p < 0.001.
Constitutively Active β-Catenin Overcomes the Loss of ISC Phenotype in RhoA KO Crypts Independent of YAP Signaling
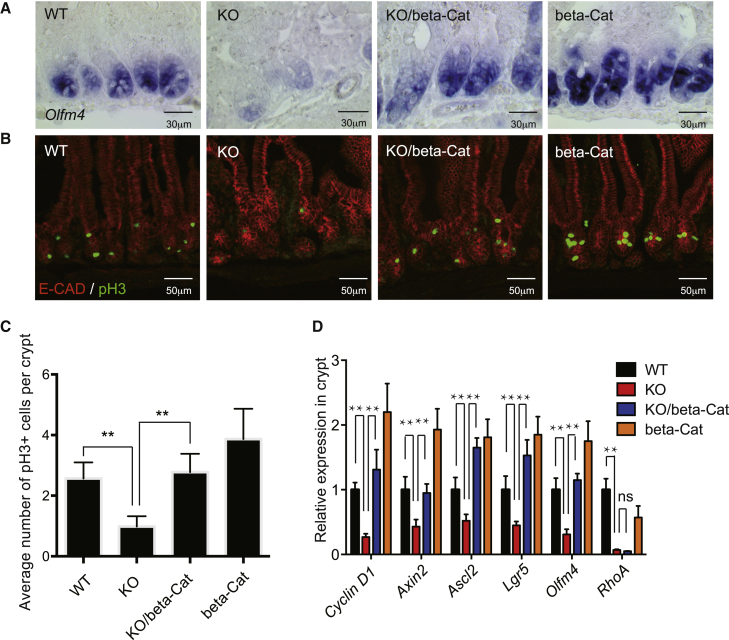
Since canonical Wnt signaling, known to be critical for ISC homeostasis and regeneration (Clevers, 2013, Reya and Clevers, 2005), is reduced in RhoA KO small intestinal crypts but can be rescued by active YAP mutant, we next tested the hypothesis that active canonical Wnt signaling may rescue ISC defects in RhoA KO mice. To this end, we crossed Villin-CreERT2; RhoAfl/fl (KO) mice or Villin-CreERT2; RhoAfl/+ (WT) with Catnblox(ex3) mice, which express an active β-catenin mutant upon Cre induction (Harada et al., 1999). H&E staining found that upon tamoxifen injection, Villin-CreERT2; RhoAfl/fl; Catnblox(ex3) mice (KO/β-Cat) showed a more organized crypt structure compared with the RhoA KO (Figure S6A), whereas Villin-CreERT2; RhoAfl/+; Catnblox(ex3) (β-Cat) mice had expanded crypts with large Paneth cells, consistent with canonical Wnt overexpression phenotype (Farin et al., 2012). In situ hybridization analysis of the ISC marker Olfm4 showed that the Wnt activation by Catnblox(ex3) expression rescued the loss of ISC phenotype of RhoA KO mice (Figure 6A), while β-Cat Catnblox(ex3) mice displayed expanded Olfm4 expressing crypts. Immunostaining of pH3 and E-cadherin suggested that active canonical Wnt signaling could rescue the proliferation defects in RHOA-depleted crypts, and the crypt/villi structures also seemed to be partially restored (Figures 6B and 6C). Finally, qRT-PCR analysis of ISC markers Lgr5, Ascl2, and Olfm4 in isolated crypts further verified that expression of these markers in KO ISCs were restored by the active β-catenin expression (Figure 6D).
Figure 6.
Constitutively Active β-Catenin Overcomes the Loss of ISC Phenotype in RhoA KO Crypts
(A) In situ hybridization of Olfm4 RNA in control, RhoA KO, KO/β-catenin Catnblox(ex3) rescue, and β-catenin Catnblox(ex3) expressing duodenum.
(B) Representative immunofluorescence staining of phosphor-histone H3 in control WT, RhoA KO, KO/β-catenin Catnblox(ex3) rescue, and β-catenin Catnblox(ex3) expressing small intestine.
(C) Quantification of number of phosphor-histone H3-positive cells per crypt in control WT, RhoA KO, KO/β-catenin Catnblox(ex3) rescue, and β-catenin Catnblox(ex3) expressing small intestine. n = 3 mice for each genotype. Error bars represent SD from three independent experiments.
(D) qRT-PCR showing expression of ISCs markers and Wnt target genes in WT, RhoA KO, KO/β-catenin Catnblox(ex3) rescue, and β-catenin Catnblox(ex3) expressing crypts. Data are normalized to GAPDH expression; n = 3 mice for each genotype. Error bars represent SD from three independent experiments.
∗∗p < 0.01; ns, not significant.
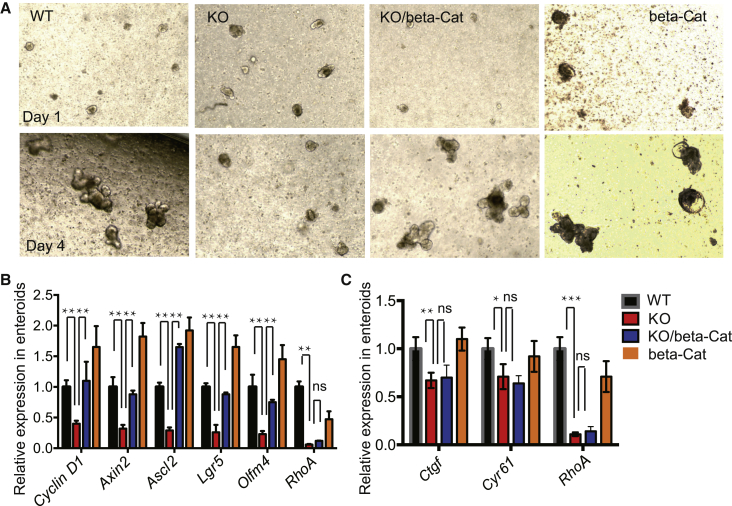
We further examined whether active canonical Wnt signaling is sufficient to rescue the RhoA KO ISC defects in vitro. Crypts from KO/β-Cat Catnblox(ex3) mice formed enteroids that mimicked WT enteroids after a 4-day culture, with many buds forming in the enteroids, whereas the enteroids grown from β-Cat Catnblox(ex3) mice formed a large balloon-like structure that is indicative of excessive Wnt activity (Figure 7A). qRT-PCR analysis of the enteroids showed similar results to that of in vivo analysis, i.e., the ISC markers Lgr5, Ascl2, and Olfm4, as well as the Wnt target Axin2, were readily rescued in KO/β-Cat Catnblox(ex3) enteroids (Figure 7B). Interestingly, we found that active canonical Wnt signaling did not affect YAP signaling activity as assayed by a YAP transcription target gene analysis (Figure 7C) and YAP protein immunostaining (Figure S6B), suggesting that canonical Wnt-activity involved in ISC regeneration may function downstream of YAP signaling in the RhoA KO background.
Figure 7.
Constitutively Active β-Catenin Rescues RhoA KO Enteroid Growth Independent of YAP Signaling
(A) Representative images of WT, RhoA KO, KO/β-catenin Catnblox(ex3) rescue, and β-catenin Catnblox(ex3) expressing enteroids after 1 day and 4 days of culture.
(B and C) qRT-PCR showing expression of ISCs markers, Wnt target genes, YAP target genes in WT, RhoA KO, KO/β-catenin Catnblox(ex3) rescue, and β-catenin Catnblox(ex3) expressing enteroids. Data are normalized to GAPDH expression; n = 3 mice for each genotype. Error bars represent SD from three independent experiments.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; ns, not significant.
Discussion
As a founding member of the Rho GTPase family, RHOA regulates multiple important cellular functions including cytoskeleton dynamics, cell adhesion, survival, and cell cycle in various tissues (Zhou and Zheng, 2013), but little is known about its physiological function in small intestine in mammals. In this study, we utilize a genetic approach to inducibly and specifically delete RHOA in mouse small intestinal epithelium, and find that RHOA is essential for the maintenance of gut epithelial architecture and homeostasis of ISCs. Loss of RHOA causes a disrupted villi and crypt structure in the small intestine, with defects in cell adhesion and junction. In addition, RHOA-depleted epithelium loses polarity organization and epithelial integrity.
Five days after the absence of RHOA, crypt cell proliferation is significantly reduced while apoptosis is elevated, along with a drastically decreased number of ISCs. This is consistent with a multitude of studies showing that RHOA positively regulates cell proliferation in several tissues (Melendez et al., 2011). These effects upon RHOA loss are reminiscent of certain stress conditions, such as irradiation. For example, mice treated with 10 Gy of radiation share a similar phenotype with RHOA loss-of-function mice, including disrupted villi, reduced proliferation, ectopic apoptosis, and repressed ISC marker expressions (Figure S7). Interestingly, most of our RhoA KO mice were able to recover from the induced changes in the small intestine and eventually survive, possibly due to adaptive compensations from other Rho family members such as RhoB and RhoC (data not shown). EREG from the surrounding stroma cells may also play a compensatory role, as previously reported in YAP-depleted intestines (Gregorieff et al., 2015). A recent report using a non-inducible RHOA loss-of-function model showed chronic intestinal inflammation (Lopez-Posadas et al., 2016), which is not observed in our transiently inducible RhoA KO mice, suggesting that RHOA may have different roles during embryonic and early postnatal stages versus adult stage, or the compensatory effects from RhoA KO differ in short-term versus long-term reactions.
In the intestine, YAP1 protein is enriched at the bottom of the crypt where the ISCs congregate (Cai et al., 2010, Camargo et al., 2007), and multiple loss-of-function studies have shown that YAP/TAZ promotes proliferation and regeneration of ISCs (Hong et al., 2016). However, there is conflicting evidence that intestine-specific induction of YAP1 would reduce ISC number, suggesting a growth-suppressive function of overexpressed YAP1 (Barry et al., 2013). We find that YAP and its targets such as EREG are significantly reduced in RhoA KO crypts, and our genetic rescue experiment with a constitutively active form of YAP mutant can rescue ISC number and function of the KO mice, suggesting that YAP acts downstream of RHOA to positively regulate ISC regeneration. YAP and TAZ transcriptional activities can be regulated by multiple signals including mechanical stress, polarity, and Rho GTPases (Dupont et al., 2011, Rauskolb et al., 2014, Yu et al., 2012, Zhao et al., 2012); thus, possibly, RHOA-mediated epithelial polarity can regulate YAP/TAZ through Lats1/2 as previously reported (Yu et al., 2012).
Previous loss-of-function studies have found that YAP1 is dispensable during normal intestinal homeostasis, whereas it is essential for regeneration after irradiation injury (Gregorieff et al., 2015). Since RhoA KO shows multiple defects similar to those of irradiation-induced injuries, reduced YAP signaling in the RhoA KO could contribute to the defects in ISC regeneration as in the YAP-deleted intestine (Gregorieff et al., 2015). However, no reduced ISC number or ISC maker expression is observed in our YAP-overexpressing mice, differing from a previous report (Barry et al., 2013).
Wnt signaling is well established as being essential for the maintenance of the proliferative crypt compartment, and loss-of-function studies support the idea that the Wnt pathway constitutes the master regulator for intestinal homeostasis and maintenance of ISCs (Fevr et al., 2007, Korinek et al., 1998, Kuhnert et al., 2004, Pinto et al., 2003). However, the interaction between canonical Wnt and YAP signaling remains controversial, as some reports propose that YAP1 maintains the proliferation and function of ISCs by activating canonical Wnt signaling activity (Camargo et al., 2007) while others show that cytoplasmic YAP1 inhibits canonical Wnt signaling (Barry et al., 2013). Our study indicates that in the RHOA-depleted background, ectopic YAP activity promotes ISC regeneration and canonical Wnt signaling, while canonical Wnt signaling has no obvious impact on YAP signaling activity. How active YAP restores canonical Wnt signaling in RhoA KO small intestine needs further investigation, but it is not through a crosstalk with non-canonical Wnt signaling as previously reported in other model systems (Park et al., 2015), and may be via an indirect effect such that YAP, through EREG, restores the ISC proliferation and homeostasis that affects canonical Wnt activity. Our genetic rescue experiment with a stabilized β-catenin mutant suggests that canonical Wnt signaling is sufficient for ISC regeneration in the absence of RHOA and/or intact YAP signaling. This discovery implicates canonical Wnt signaling for small intestine regeneration after injury. However, exaggerated Wnt signaling can promote ISC hyperproliferation, and an optimal Wnt signaling dose needs to be precisely modulated for proper intestinal homeostasis (Clevers, 2013).
RHOA is a molecular switch in transducing diverse extracellular signals and is activated from a GDP-bound inactive form to a GTP-bound active form in response to stimuli (Etienne-Manneville and Hall, 2002, Karlsson et al., 2009, Liu et al., 2012, Wang and Zheng, 2007). The positive stimuli of RHOA include mechanical stress post injury (Aikawa et al., 1999, Smith et al., 2003), cytokines released due to inflammation (Campos et al., 2009, Iwata et al., 2012), and GPCRs hijacked in colon cancer conditions (Dorsam and Gutkind, 2007), which are involved in multiple disease states. Such altered pathophysiological status may regulate RHOA activity, which in turn affects ISCs. Further examination of how altered pathologic signals may be transduced through the identified RHOA-YAP-EREG pathway in intestinal diseases that result in altered ISC homeostasis will be of future interest.
Experimental Procedures
Animal Studies
We used a RhoAlox/flox mouse line described previously (Katayama et al., 2011). The RhoAflox/flox mouse was crossed with Villin-CreERT2 mice provided by Dr. Helen Piwnica-Worms at the University of Texas MD Anderson Center. Littermate controls were generated by standard pairings. Animal protocols were approved by the Cincinnati Children's Hospital Medical Center Committee on the Ethics of Animal Experiments. All mice were housed in a specific pathogen-free breeding barrier. Euthanasia was performed by CO2 followed by cervical dislocation. To induce Cre recombinase, we intraperitoneally injected 6- to 8-week-old adult mice with 1 mg of tamoxifen (Sigma-Aldrich, Switzerland) dissolved in corn oil for 4 consecutive days. Mice were euthanized and analyzed 1 day after the last injection. For the genetic rescue experiment, we crossed RhoAflox/flox; Villin-CreERT2 mice (Melendez et al., 2011) to knockin mice containing either an inducible active YAP mutant allele (S112A mutation [Xin et al., 2011], under the control of CMV-LSL) or an inducible active β-catenin mutant allele catnblox(ex3) (Harada et al., 1999).
H&E Staining and Tissue Preparation
Intestinal tissues were flushed with PBS and fixed in 10% formalin or 4% paraformaldehyde overnight at 4°C. Tissues were then embedded in paraffin or cold optimum cutting temperature (OCT) embedding medium. Processing of tissues for paraffin embedding and H&E staining was performed by the CCHMC Digestive Health Center Morphology Core.
Immunofluorescence and Confocal Microscopy
Immunofluorescence was performed on frozen sections. Intestinal tissues were flushed with PBS and fixed in 4% paraformaldehyde overnight at 4°C, cryoprotected in 30% sucrose, embedded in OCT compound, and sectioned at 12 μm. Antibodies for immunofluorescence are: RHOA (Cell Signaling Technology, #2117, 1:250), cleaved caspase-3 (Cell Signaling, #9661, 1:250), p-histone H3 (Cell Signaling, #9701, 1:250), laminin B (Cell Signaling, #12586, 1:250), E-cadherin (BD Biosciences, #610181, 1:250), β-catenin (BD Biosciences, #610153, 1:250), Na+,K+-ATPase α (MBL, #D154-3, 1:250), Ki67 (Abcam, #ab15580, 1:200), chromogranin A (Abcam, # ab15160, 1:200), lysozyme (Dako, #A0099, 1:2000), and mucin-2 (Thermo Scientific, #MS-1037, 1:200).
Images were acquired on a Zeiss 710 and Nikon A1R GaAsP Inverted Confocal Microscope. Signal intensity was optimized for control samples and the same settings (laser power/gain/offset) were used for RhoA KO tissue and cells. Phase-contrast images of enteroids were captured on an Olympus IX51 microscope equipped with a Q Color 5 camera and acquired with Q Capture Pro 6.0 software.
In Situ Hybridization
Intestinal tissues were flushed with PBS and fixed in 10% formalin or 4% paraformaldehyde overnight at 4°C. Tissues were then embedded in paraffin or cold OCT embedding medium. Processing of tissues for paraffin embedding was performed by the CCHMC Digestive Health Center Morphology Core, and in situ hybridization was performed following a published protocol (Gregorieff and Clevers, 2010).
Western Blotting
Intestinal epithelium from villi and crypts were isolated as previously described (Melendez et al., 2013). Tissues were homogenized in lysis buffer containing protease and phosphatase inhibitors, sonicated at 4°C, mixed with 4× SDS loading buffer, and heated at 100°C for 5 min. A Bradford assay was used to determine protein concentration (Bio-Rad). Antibodies for western blotting are: anti-RHOA (#2117), Cdc42 (#2462), MLC2 (#3671), p-MLC2 (#3675), Bcl-2 (#15071), BCL-xL (#2764), p53 (#2527), p-p53 (#2521), cleaved caspase-3 (#9661), YAP (#14074), and GAPDH (#5174) (all from Cell Signaling), and anti-Rac1 antibody (#610650, BD Biosciences). The FOCUS SubCell kit (G Biosciences) was used to separate crypt cell cytosolic and nuclear fractions.
Transmission Electron Microscopy
For transmission electron microscopy, small intestinal sections were dissected, flushed with cold PBS supplemented with protease inhibitors, and fixed in 4% glutaraldehyde/0.175 M cacodylate buffer followed by 1% osmium tetroxide/0.175 M cacodylate. Mouse tissues were then dehydrated, embedded in LX-112 resin, and imaged by the CCHMC Digestive Health Center Morphology Core.
RNA Extraction and Real-Time qPCR
mRNA was isolated from intestinal crypts using an RNA Mini Kit (Qiagen). RNA concentration was measured by Nanodrop and cDNA was produced with a cDNA synthesis kit (ABI). qPCR was performed using SYBR qPCR master mix (ABI) or TaqMan qPCR master mix (ABI) on an ABI700 qRT-PCR instrument. Relative expression levels were determined by the ΔΔCt method standardized to gapdh. Primer sequences for SYBR reaction were obtained from web source http://medgen.ugent.be/rtprimerdb or http://pga.mgh.harvard.edu/primerbank (see below for sequences).
SYBR Reaction qRT-PCR Primer Sequences
Bmi1: 5′-CAC CCA CAG TTC CCT CAC ATT-3′ and 5′-TCG AGG TCT ACT GGC AAA GGA-3′
Axin2: 5′-GTG GAT ACG CTG GAC TTC TGG-3′ and 5′-GAG CCG ATC TGT TGC TTC TTG-3′
Cyclin D 1: 5′-GTG AGG GAA GAG GTG AAG GTG-3′ and 5′-GAT CCT GGG AGT CAT CGG TAG-3′
E-cadherin: 5′-CAC CTG GAG AGA GGC CAT GT-3′ and 5′-TGG GAA ACA TGA GCA GCT CT-3′
Β-catenin: 5′-ATG GAG CCG GAC AGA AAA GC-3′ and 5′-CTT GCC ACT CAG GGA AGG A-3′
Par3: 5′-CAC CAT GCG GAA GAT GAT CTG-3′ and 5′-GAG CCG TTT TTA TTG CAC ACC-3′
Par6: 5′-TCT GTA CGG GTG CAT GTT CAG-3′ and 5′-TGC CAG GTT AAT CAT GTA CGT TG-3′
Zo-1: 5′-GAA CCA ATT CGC CGA GAA GG-3′ and 5′-CTG TTG CCC GCA TGT GAT G-3′
Gapdh: H: 5′-CGT ATT GGG CGC CTG GTC AC-3′ and 5′-ATG ATG ACC CTT TTG GCT CC-3′
TaqMan reaction qRT-PCR primer item number (Thermo Fisher)
Ctgf: Mm01192933_g1; Cyr61: Mm00487499_g1;
Ereg: Mm00514794_m1; RhoA: Mm00834507_g1;
Lgr5: Mm00438890_m1; Olfm4: Mm01320260_m1;
Ascl2: Mm01268891_g1.
RhoA-Inducible Deletion in Enteroid Culture and Staining
Duodenal crypts from 6- to 8-week old control and RhoA KO mice were isolated and cultured in vitro as previously described (Mahe et al., 2013, Sato et al., 2011, Snippert et al., 2010). At 24 hr post passage, RHOA deletion was induced with 1 μM 4-OHT and complete deletion of RhoA allele was observed after 48 hr and detected by genomic PCR. At 5–7 days post induction, enteroids were fixed in 4% paraformaldehyde for 1.5 hr at room temperature and then washed in TBS/0.3% Tween 20. Fixed enteroids were blocked for 3 hr at room temperature in 2% normal goat serum/4% BSA/TBS/0.1% Tween 20, followed by an antibody.
Detailed protocols of quantification of Paneth cells, Goblet cells, and enteroendocrine cells are described elsewhere (Melendez et al., 2013).
Quantification
Paneth cell number was quantified by counting the average number of lysosome-positive cells in crypts and villi (50 crypts and villi per mouse). Nuclear YAP signaling was quantified by measuring the YAP immunostaining intensity using ImageJ, which is normalized to DAPI nuclei staining (50 crypts per mouse).
Statistics
Results are expressed as the mean ± SD. Significance was calculated by Student's t test. p < 0.05 was considered significant.
Author Contributions
M.L., Z.Z., L.S., X.Z., and Y.Z. designed the study and developed the methodologies. M.L., Z.Z., L.S., X.Z., Y.F., S.A., J.M., A.K.D, F.B., and M.X. collected data, performed the analysis, and contributed critical reagents. K.N. and H.G. contributed critical reagents and technical expertise. M.L., Z.Z., and Y.Z. wrote the manuscript.
Acknowledgment
This work was in part supported by NIH grants R01 DK104814, R01 HL134617, R01 CA193350, and P30 DK078392 (Pathology Core).
Published: November 9, 2017
Footnotes
Supplemental Information includes seven figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.10.004.
Supplemental Information
References
- Aikawa R., Komuro I., Yamazaki T., Zou Y., Kudoh S., Zhu W., Kadowaki T., Yazaki Y. Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circ. Res. 1999;84:458–466. doi: 10.1161/01.res.84.4.458. [DOI] [PubMed] [Google Scholar]
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Barker N., Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barry E.R., Camargo F.D. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr. Opin. Cell Biol. 2013;25:247–253. doi: 10.1016/j.ceb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Barry E.R., Morikawa T., Butler B.L., Shrestha K., de la Rosa R., Yan K.S., Fuchs C.S., Magness S.T., Smits R., Ogino S. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit Y.D., Lussier C., Ducharme P.A., Sivret S., Schnapp L.M., Basora N., Beaulieu J.F. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol. Cell. 2009;101:695–708. doi: 10.1042/BC20090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Maitra A., Anders R.A., Taketo M.M., Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Zhang N., Zheng Y., de Wilde R.F., Maitra A., Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo F.D., Gokhale S., Johnnidis J.B., Fu D., Bell G.W., Jaenisch R., Brummelkamp T.R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Campos S.B., Ashworth S.L., Wean S., Hosford M., Sandoval R.M., Hallett M.A., Atkinson S.J., Molitoris B.A. Cytokine-induced F-actin reorganization in endothelial cells involves RhoA activation. Am. J. Physiol. Ren. Physiol. 2009;296:F487–F495. doi: 10.1152/ajprenal.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Leblond C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Dorsam R.T., Gutkind J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Farin H.F., Van Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Fevr T., Robine S., Louvard D., Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A., Clevers H. In situ hybridization to identify gut stem cells. Curr. Protoc. Stem Cell Biol. 2010;Chapter 2 doi: 10.1002/9780470151808.sc02f01s12. Unit 2F.1. [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Liu Y., Inanlou M.R., Khomchuk Y., Wrana J.L. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M.M. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong A.W., Meng Z., Guan K.L. The Hippo pathway in intestinal regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2016;13:324–337. doi: 10.1038/nrgastro.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo M., Ebisuya M., Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat. Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- Imajo M., Miyatake K., Iimura A., Miyamoto A., Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata A., Shirai R., Ishii H., Kushima H., Otani S., Hashinaga K., Umeki K., Kishi K., Tokimatsu I., Hiramatsu K. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin. Exp. Immunol. 2012;168:234–240. doi: 10.1111/j.1365-2249.2012.04564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B., Peyrollier K., Pedersen E., Basse A., Karlsson R., Wang Z., Lefever T., Ochsenbein A.M., Schmidt G., Aktories K. RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol. Biol. Cell. 2011;22:593–605. doi: 10.1091/mbc.E09-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Kuroda S., Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Pedersen E.D., Wang Z., Brakebusch C. Rho GTPase function in tumorigenesis. Biochim. Biophys. Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Katayama K., Melendez J., Baumann J.M., Leslie J.R., Chauhan B.K., Nemkul N., Lang R.A., Kuan C.Y., Zheng Y., Yoshida Y. Loss of RhoA in neural progenitor cells causes the disruption of adherens junctions and hyperproliferation. Proc. Natl. Acad. Sci. USA. 2011;108:7607–7612. doi: 10.1073/pnas.1101347108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V., Barker N., Moerer P., van Donselaar E., Huls G., Peters P.J., Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kuhnert F., Davis C.R., Wang H.T., Chu P., Lee M., Yuan J., Nusse R., Kuo C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Bi F., Zhou X., Zheng Y. Rho GTPase regulation by miRNAs and covalent modifications. Trends Cell Biol. 2012;22:365–373. doi: 10.1016/j.tcb.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Posadas R., Becker C., Gunther C., Tenzer S., Amann K., Billmeier U., Atreya R., Fiorino G., Vetrano S., Danese S. Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation. J. Clin. Invest. 2016;126:611–626. doi: 10.1172/JCI80997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe M.M., Aihara E., Schumacher M.A., Zavros Y., Montrose M.H., Helmrath M.A., Sato T., Shroyer N.F. Establishment of gastrointestinal epithelial organoids. Curr. Protoc. Mouse Biol. 2013;3:217–240. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez J., Liu M., Sampson L., Akunuru S., Han X., Vallance J., Witte D., Shroyer N., Zheng Y. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology. 2013;145:808–819. doi: 10.1053/j.gastro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez J., Stengel K., Zhou X., Chauhan B.K., Debidda M., Andreassen P., Lang R.A., Zheng Y. RhoA GTPase is dispensable for actomyosin regulation but is essential for mitosis in primary mouse embryonic fibroblasts. J. Biol. Chem. 2011;286:15132–15137. doi: 10.1074/jbc.C111.229336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.W., Kim Y.C., Yu B., Moroishi T., Mo J.S., Plouffe S.W., Meng Z., Lin K.C., Yu F.X., Alexander C.M. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran S.A., Palmer L.G., Moon S.Y., Peralta Soler A., Apodaca G.L., Harper J.F., Zheng Y., Rajasekaran A.K. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol. Biol. Cell. 2001;12:3717–3732. doi: 10.1091/mbc.12.12.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C., Sun S., Sun G., Pan Y., Irvine K.D. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Rosenbluh J., Nijhawan D., Cox A.G., Li X., Neal J.T., Schafer E.J., Zack T.I., Wang X., Tsherniak A., Schinzel A.C. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Smith P.G., Roy C., Zhang Y.N., Chauduri S. Mechanical stress increases RhoA activation in airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2003;28:436–442. doi: 10.1165/rcmb.4754. [DOI] [PubMed] [Google Scholar]
- Snippert H.J., van der Flier L.G., Sato T., van Es J.H., van den Born M., Kroon-Veenboer C., Barker N., Klein A.M., van Rheenen J., Simons B.D. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Wu L.W., Grivennikov S.I., de Jong P.R., Lian I., Yu F.X., Wang K., Ho S.B., Boland B.S., Chang J.T. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Biehs B., Chiu C., Siebel C.W., Wu Y., Costa M., de Sauvage F.J., Klein O.D. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep. 2015;11:33–42. doi: 10.1016/j.celrep.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van der Flier L.G., Haegebarth A., Stange D.E., van de Wetering M., Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Wang L., Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. doi: 10.1016/j.tcb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Xin M., Kim Y., Sutherland L.B., Qi X., McAnally J., Schwartz R.J., Richardson J.A., Bassel-Duby R., Olson E.N. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H., Zhao J., Yuan H., Tumaneng K., Li H. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Lei Q., Guan K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Wang L., Wang C.Y., Yu J., Guan K.L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zheng Y. Cell type-specific signaling function of RhoA GTPase: lessons from mouse gene targeting. J. Biol. Chem. 2013;288:36179–36188. doi: 10.1074/jbc.R113.515486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Florian M.C., Arumugam P., Chen X., Cancelas J.A., Lang R., Malik P., Geiger H., Zheng Y. RhoA GTPase controls mitosis and programmed necrosis of hematopoietic progenitors. J. Exp. Med. 2013;210:2371–2385. doi: 10.1084/jem.20122348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.