Abstract
The advent of magnetic resonance imaging (MRI) enables noninvasive measurement of cerebrospinal fluid (CSF) motion, and new information about CSF motion has now been acquired. The driving force of the CSF has long been thought to be choroid plexus (CP) pulsation, but to investigate whether this phenomenon actually occurs, CSF motion was observed in the ventricular system and subarachnoid space using MRI. Eleven healthy volunteers, ranging in age from 23 to 58 years, participated in this study. The MRI sequences used were four-dimensional phase-contrast (4D-PC) and time-spatial labeling inversion pulse (t-SLIP). The 4D-PC images included sagittal images in the cranial midline, coronal images focusing on the foramen of Monro (FOM), and oblique coronal images of the trigone to quantify CSF velocity and acceleration. These values were compared and analyzed as non-parametric data using the Kolmogorov-Smirnov test and the Mann-Whitney U test. 4D-PC showed that the median CSF velocity was significantly lower in the posterior part of the lateral ventricle than in other regions. The quantitative analysis of velocity and acceleration showed that they were decreased around the CP in the trigone. Image analysis of both velocity mapping and t-SLIP showed suppressed CSF motion around the CP in the trigone. These findings cast doubt on CP pulsation being the driving force for CSF motion.
Keywords: choroid plexus, cerebrospinal fluid, magnetic resonance imaging, image analysis, hydromechanics
Introduction
There is an exchange of substances between the cerebrospinal fluid (CSF) and the interstitial fluid of the brain and nerves, and CSF motion through the brain ventricles and subarachnoid space helps maintain homeostasis of the central nervous system (CNS).1,2) Therefore, normal CSF motion is important in the CNS, and disruption of CSF motion can lead to conditions such as hydrocephalus. CSF motion involves flow and circulation of fluid along a pressure gradient and requires fluid pulsation. CSF pulsation has been widely demonstrated in animal studies and in human clinical studies, and imaging studies have helped researchers and clinicians understand the complicated CSF motion. Therefore, CSF pulsation and motion require some type of driving force. However, controversy still exists regarding the origin of this CSF driving force.
Current understanding of the origin of CSF pulsation dates back to the beginning of the 20th century. Namely, CSF is mainly produced by the choroid plexus (CP) in the lateral ventricle, flows from the third and fourth ventricles into the subarachnoid space of the cranium and spinal cord, and is absorbed by venous blood from arachnoid granules in the convexity. This can be thought of as bulk flow like a river in a one-way direction from the site of production to the site of absorption. However, recent studies of CSF motion are gradually challenging these basic concepts about CSF motion.3,4)
Two major theories about the origin of CSF motion are the arterial and respiratory driving force theories. Moreover, whether pulsations are directly transmitted from blood vessels to the CSF, or whether pulsations are indirectly transmitted from blood vessels through other tissues to the CSF, remains unclear. Bering reported that the CP plays an important role in transmitting arterial pulsations to the CSF.5) Later, in regard to any association between the mechanism of ventricular enlargement and CP pulsation, research results were published showing that CP pulsation was indeed an important factor in ventricular enlargement.6,7)
However, there have been many arguments against this theory that CSF pulsation originates in the CP.8–10) These arguments refuting the theory that the CP is the driving force of CSF pulsation include proposed theories such as “arterial pulsation in the subarachnoid space is directly propagated to the CSF,”11,12) and that “capillary pulsation in the brain parenchyma causes expansion of the brain parenchyma, resulting in transmission of brain pulsations to the CSF.”13) Another theory has been that “changes in intrathoracic pressure with respiration are transmitted to the venous plexus in the spinal epidural space and directly to the intracranial venous system, and this in turn is propagated to the CSF.”14,15)
The increased popularity of neuroendoscopic surgery has now made it possible to directly observe the CP. However, Picard commented that, based on his experience, he had never observed volumetric change due to active CP arterial pulsation.16) Furthermore, no imaging studies to observe the CP and surrounding CSF motion have previously been reported. Consequently, there has been increasing skepticism about whether the CP is really the driving force of CSF pulsatile motion.
Therefore, quantitative analysis of both velocity and acceleration of the CSF in the CSF cavity was performed by four-dimensional phase contrast (4D-PC) magnetic resonance imaging (MRI), along with imaging analysis of CSF motion in the CSF cavity using time-spatial labeling inversion pulse (t-SLIP), to investigate whether the CP is the driving force of CSF pulsation. This enables indirect observation of whether surrounding CSF motion occurs due to volumetric change of the CP in the trigone of the lateral ventricle. The CP is widely distributed from the lateral ventricles through the foramen of Monro (FOM) to the third ventricle, as well as partially from the fourth ventricle to the subarachnoid space in the base of the brain. The CP in the ventricles projects from the ventricular wall, MRI can easily observe the posterior part of lateral ventricle, and the CP has a large volume. Thus, CSF motion in the trigone can be observed. Our conclusions about whether the CP in the posterior part of lateral ventricle is actually involved in CSF pulsation are reported.
Materials and Methods
Our institution’s internal review board approved this research. All volunteers were examined after providing appropriate informed consent, consistent with the terms of approval from the internal review board of Tokai University Hospital, Isehara, Kanagawa, Japan.
This study included 11 healthy volunteer participants, ranging in age from 23 to 58 years. To obtain the widest possible view of the CP in the lateral ventricle, oblique coronal views of the posterior part of the lateral ventricle were obtained to observe CSF motion around the CP. MRI imaging was performed as described below.
A 1.5-T scanner (Gyroscan, Philips; Best, The Netherlands) equipped with an 8-channel phased array head/neck coil was used with the following conditions: flow encode directions, foot-head (FH), right-left (RL) and anterior-posterior (AP); number of cardiac phases, 32; repetition time (TR), 9.8–16.4 ms; echo time (TE), 6.6–6.7 ms; flip angle (FA), 20°; field of view (FOV), 22 × 22 or 32 × 32 cm2; velocity encoding (VENC), 5 cm/sec; and spatial resolution, 1.96 mm (isotropic). The four-dimensional velocity field of the CSF was extracted by the Pulsatility-Based Segmentation (PUBS) technique,17) in which a flow segment with a temporal pattern correlating with that at a reference point in an artery or vein was regarded as a blood flow and removed from a velocity map. Vectors then delineated the in-plane velocities, while the through-plane velocities were visualized by colors. The vector-color-coded CSF velocity field was then superimposed on the T2-weighted images with the stationary tissues. The principle of this analysis represents the velocity obtained in the three directions (AP, RL, FH) by the PC method. To evaluate the effect of cardiac pulsation on CSF motion around the CP in the lateral ventricle, acceleration was calculated as the first-order time derivative of the mean velocity in the ROIs. The maximum acceleration was compared among region of interests (ROIs). The details of the ROI setting are shown in a previous report.18)
After color and vector images were obtained, the researchers, who included neurosurgeons, used an originally developed, mouse-operated, graphical user interface to specify the ROI such that the posterior part of the lateral ventricle, in particular most of the CP, was included on oblique coronal views. A partial volume effect due to the relatively large voxel size (about 2 mm) used in this study tends to produce errors with segmentation based on T2-weighted images. Therefore, a segmentation method known as spatial-based fuzzy clustering19) was used to reduce this partial volume effect. This can distinguish CSF spaces from the brain parenchyma.
The quantitative data for both velocity and acceleration of CSF motion around the CP in the trigone were compared to the results on the same volunteers in which a 4D-PC method was used to quantitatively analyze CSF motion of the ventral surface of the brainstem, third ventricle, fourth ventricle, anterior horn of the lateral ventricle, and subarachnoid space of the convexity. Statistical analysis was performed to examine whether CP pulsation in the trigone was indeed the origin of CSF motion. The CSF velocity and acceleration data calculated in the ROIs are shown as box plots.
CSF velocity around the CP of the trigone was compared with data for other intracranial regions. This was evaluated as non-parametric data using the Kolmogorov-Smirnov test and the Mann-Whitney U test. All statistical analyses were performed using SPSS ver. 13 (SPSS Japan Inc., Tokyo, Japan). To correct for the multiple comparisons, the Bonferroni’s method was used to set the threshold of significance to a P value < 0.0033 (0.0033 = 0.05/15).
For imaging analysis of each CSF cavity region, t-SLIP images using the following sequences were used: 2D single-shot T2-weighted imaging, repetition time = 6000 ms, echo time = 83 ms, flip angle = 90°, field of view = 250 × 250 mm2, matrix = 256 × 256, slice thickness = 5 mm, turbo spin echo factor = 97, half scan factor = 0.6, sensitivity encoding factor = 1.6, TI = 2500 ms, k-space trajectory = sequential order, and selective pulse width = 30 mm. The TI value was set close to the null point of the CSF. The t-SLIP images were evaluated in the ventral surface of the brainstem, fourth ventricle, third ventricle, anterior horn, and trigone. The image analysis was performed by three board-certified neurosurgeons.
Results
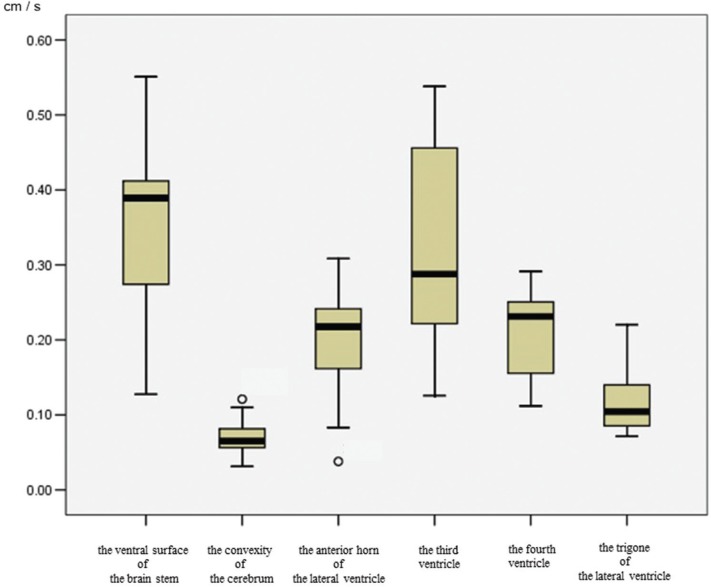
Figure 1 shows a box plot of the CSF velocity at each site. The quantitative velocity values differed among the intracranial sites. The median velocities in each ROI were: ventral surface of the brainstem, 0.40 cm/s; fourth ventricle, 0.23 cm/s; third ventricle, 0.28 cm/s; anterior horn of the lateral ventricle, 0.21 cm/s; trigone, 0.10 cm/s; and convexity, 0.07 cm/s. The CSF velocity was lower in the trigone than in the ventral surface of the brainstem, third ventricle, and fourth ventricle (P < 0.0033). Therefore, CSF motion in the trigone, which includes much of the CP, tended to be slower.
Fig. 1.
Box plots of CSF velocity using 4D-PC in each region. There is markedly increased CSF velocity in the ventral surface of the brainstem. In the ventricular system, the CSF velocity in the third ventricle is increased, followed by the fourth ventricle and the anterior horn of the lateral ventricle. The CSF velocity in the trigone of the lateral ventricle is markedly lower than in other regions. The CSF velocity in the convexity of the cerebrum is also lower than that of the ventral surface of the brainstem. Outside values are indicated by a small “0”.
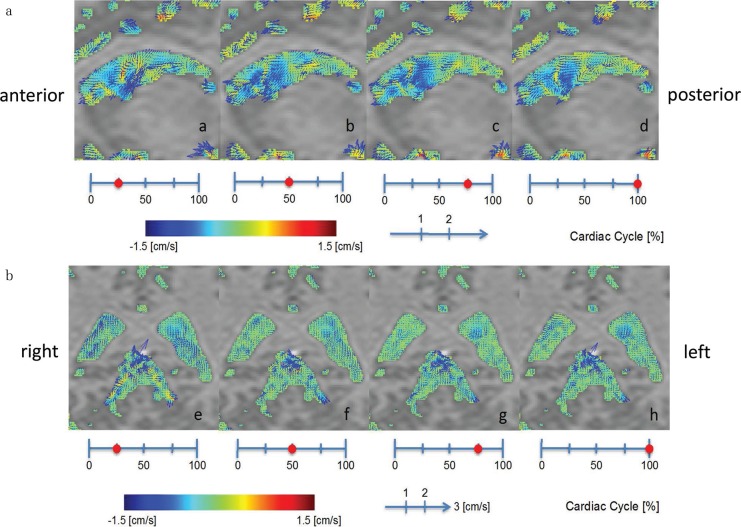
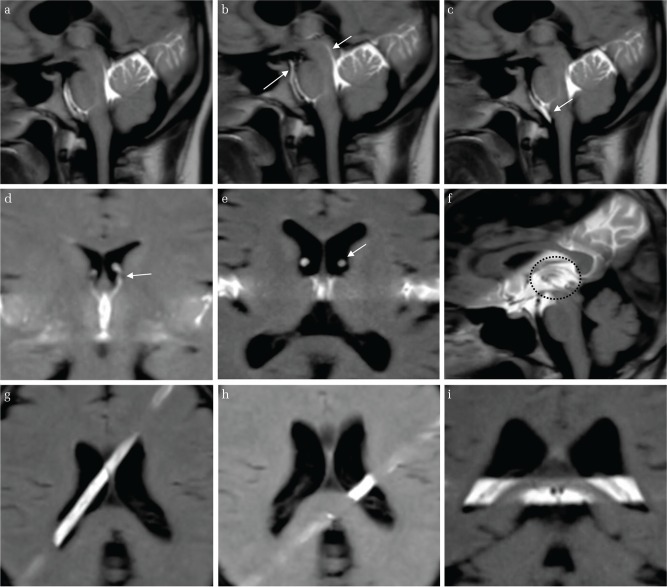
Figure 2 shows color imaging of CSF velocity in a representative volunteer. The sagittal image in Fig. 2a shows an increase in CSF velocity around the FOM. This increased velocity was transmitted from the third ventricle to the anterior horn. However, CSF velocity at the trigone was slower (Fig. 2b), and, moreover, transmission of CSF motion from the trigone anteriorly to the body of the lateral ventricle was not observed (Fig. 2a).
Fig. 2.
Illustrative case of a 29-year-old man. (a) CSF velocity sagittal mapping of the lateral ventricle using the 4D-PC method. Increased CSF velocity is noted at the foramen of Monro. This increased CSF velocity is transmitted from the 3rd ventricle to the anterior horn, but it does not reach the trigone of the lateral ventricle. (b) Oblique coronal CSF velocity imaging. The images show slow CSF velocity in the trigone of the lateral ventricle compared with the quadrigeminal cistern.
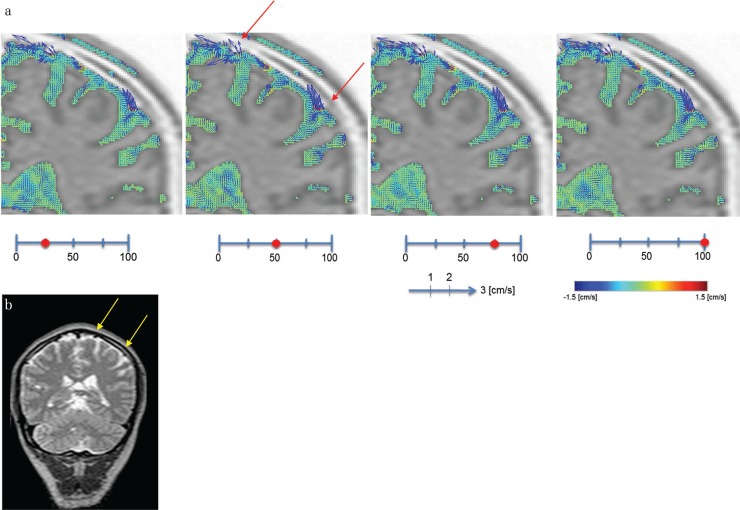
Figure 3 compares CSF velocity changes in the trigone and subarachnoid space of the cerebral convexity. CSF velocity increased around structures appearing to be blood vessels in the subarachnoid space of the cerebral convexity (Fig. 3b), but these changes were localized, and overall CSF velocity was still slow. Overall CSF velocity in the trigone was slow, and even in sites where the CP was thought to be located, no increases in CSF velocity due to pulsatile changes of the CP were observed.
Fig. 3.
Illustrative case of a 54-year-old man. (a) Increased magnification of CSF velocity mapping by the 4D-PC method. A limited increase of CSF velocity around the vessels of the convexity of the subarachnoid space is shown (arrows), while slow CSF velocity in the trigone of the lateral ventricle is also shown. (b) The T2-weighted image of the same volunteer as (a) shows a vascular structure in the convexity of the cerebrum (arrows).
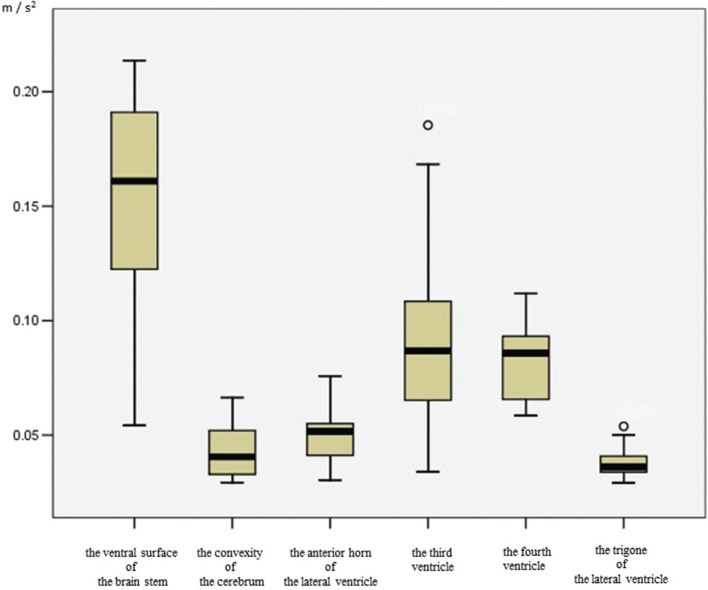
Figure 4 shows a box plot of CSF acceleration at each site. The quantitative acceleration values differed among the intracranial sites. The median acceleration values in each ROI were: ventral surface of the brainstem, 0.17 (m/s2); fourth ventricle, 0.08 (m/s2); third ventricle, 0.08 (m/s2); anterior horn of the lateral ventricle, 0.05 (m/s2); trigone, 0.03 (m/s2); and convexity, 0.03 (m/s2). CSF acceleration tended to be lower in the trigone than in the ventral surface of the brainstem, third ventricle, and fourth ventricle (P < 0.0033). Therefore, CSF motion in the trigone, which includes much of the CP, seemed to show slower motion.
Fig. 4.
Box plots of CSF acceleration using 4D-PC in each region. The CSF acceleration in the trigone of the lateral ventricle tends to be compressed compared with the ventral surface of the brain stem, and both third and fourth ventricle. Outside values are indicated by a small “0”.
Figure 5 shows representative t-SLIP images in each region. In the ventral surface of the brainstem, third ventricle, fourth ventricle, and anterior horn of the lateral ventricle, all volunteers’ images showed marked labeled CSF motion around the tagged area or unlabeled CSF motion came into the tagged area, which means that there was irregular CSF motion in the tagged area. On the other hand, all image interpreters concluded that there was no remarkable CSF movement around the CP in the trigone, and there was also irregular CSF motion inside the CP in the trigone, even in younger volunteers with a small-volume trigone (Fig. 5).
Fig. 5.
The t-SLIP images in various parts of the intracranial CSF cavity (a, b, c) 28-year-old volunteer. The tag was applied at the perpendicular axis of the brainstem. The labelled CSF moves in both upper and lower directions at the ventral surface of the brainstem and Sylvian aqueduct (arrow). (d) 28-year-old volunteer. The tag was applied at the third ventricle in the coronal image. The CSF from the third ventricle surges up into the anterior horn through the FOM (arrow). (e) 56-year-old volunteer. The tag was applied at the third ventricle in the axial image. The labeled CSF from the third ventricle surges up into the anterior horn through the FOM (arrow). (f) 56-year-old volunteer. The tag was applied at the third ventricle in the sagittal image. Marked turbulent CSF motion is observed in the third ventricle (circle). (g) 28-year-old volunteer. The tag was applied above the CP. No obvious CSF movement around the CP is seen. (h) 28-year-old volunteer. The tag was applied perpendicular to the axis of the CP. No CSF upward or downward movement is noted in the trigone. (i) 28-year-old volunteer. The tag was applied at the trigone. No turbulent CSF motion is noted in the trigone.
Discussion
The present study using 4D-PC MRI and t-SLIP found no obvious CSF motion around the CP in the trigone of healthy volunteers. This lack of CSF motion suggests that the CP is not the origin of the driving force for CSF motion from the lateral ventricle to the third ventricle. CSF motion was slow around the CP in the lateral ventricle, except around the FOM. CSF velocity was higher in the third ventricle than in the other ventricles, while CSF acceleration was predominant in both the third and fourth ventricles, which was derived from the quantitative analysis of the CSF velocity and acceleration and image analysis of the t-SLIP images.
Many clinicians, based on their experience observing the CSF during surgery, external ventricular drainage, and lumbar puncture, know that CSF pulsation is synchronous with the heartbeat and respiration. Bering’s research is famous for suggesting that the CP acts as a pump for synchrony between CSF pulsation and the heartbeat.5)
In 1943, O’Connell proposed that arterial blood flow into the brain parenchyma during systole expands the brain parenchyma, thus compressing the third ventricle and acting as a CSF pump.11) These findings were later supported by Du Boulay using videos of oil contrast myelography of many patients showing that rhythmical squeezing of the thalamus bilaterally was transmitted to the CSF in the third ventricle, causing CSF motion.13) In that article, Du Boulay concluded that there was little CSF motion in the lateral ventricles, and that compared to CSF motion in the third ventricle, CSF motion in the lateral ventricles was not significant.13) The present results are in total agreement with O’Connell, who proposed that the third ventricle acts as a CSF pump, because the present result shows that the CSF motion of the third ventricle is predominant in the ventricular system, and CSF motion of the trigone is very weak in the ventricular system. Presumably, squeezing of the thalamus on both sides at the third ventricle causes the capillary pulsation in the thalamus to be the driving force of CSF pulsation; thus, this is the origin of the CSF driving force of the CSF motion in the ventricular system.
Today, the rebuttal of Bering’s proposal is supported by the findings of recent advanced endoscopic views. During endoscopy within the cerebral ventricles (by insertion of an endoscopic sheath to observe under the open cavity), clinicians have never observed pulsatile changes of the CP that are sufficient to pump CSF to other ventricles.16) Therefore, there is little evidence to support the theory that expansion of the CP induces CSF pulsation and is the driving force of CSF motion.
MRI is now being used to analyze CSF motion.3,4,18,20,21) MRI techniques have been widely developed to noninvasively observe and quantify CSF motion in humans. Some researchers have impressed slower CSF motion in the lateral ventricle3,4,22) by MRI, but no quantitative analysis was performed before the present report. Several studies using noninvasive MRI have quantified CSF motion and reported CSF pulsations.23–27) These MRI studies were designed to investigate temporal changes in CSF flow waves and analyze CSF pulsation. However, direct observation of pulsatile CP motion in the posterior part of the lateral ventricle has not been previously reported.
If pulsatile changes occur in response to pressure changes in blood vessels supplying the CP, increased CSF velocity and/or turbulence in that region would be observed. It is important that a high volume compartment such as the trigone showed low CSF velocity, while a low volume compartment such as the third ventricle showed high velocity. Thus, we determined the acceleration (defined as the time rate of change of velocity) of the CSF. Acceleration directly reflects pulsation, such as of the CP, and is not affected by the volume of a cavity.28) Additionally, image analysis using the t-SLIP method, which shows direct CSF movement in the CSF space, was also performed. However, CSF velocity and acceleration around the CP in the trigone were low in the present study, and there was no obvious motion (rapid and/or turbulent) of excited CSF in the observed region (around the CP in the posterior part of the lateral ventricle). There was also no obvious CSF motion around the CP using the t-SLIP method. This low CSF acceleration and velocity and the absence of turbulence on these two MRI imaging techniques can be interpreted as meaning that pulsatile changes of the CP sufficient to drive CSF motion do not occur.
What then is the origin of rhythmical CSF pulsation? According to the results of CSF velocity, the third ventricle plays a central role in the driving force of CSF motion in the ventricular system. We have previously reported the details of velocity and pressure gradient images in the third ventricle (Figs. 2 and 3 in our article).4) Thus, the origin of the driving force of CSF motion is the squeezing of the pump action of the thalamus on the ventricular system, but the origin of CSF motion in the subarachnoid space is still debated; blood pulsation of the vascular system may be directly propagated to the CSF or be the result of transmission of intrathoracic or parenchymal pressure changes. We need to further investigate the origin of CSF pulsation in the subarachnoid space.
The present study has some limitations. The first may be measurement error. Tang et al. reported an error margin of up to 10% using phase-contrast methods.29) The second limitation is the age of the volunteers. The present data were obtained from adult volunteers aged ≥23 years, so caution is necessary in extrapolating these results to the developmental stage of the CP during infancy and childhood. The third limitation is that observation of CSF movement by the present method is limited to one cardiac cycle, so that CSF motion related to respiratory thoracic pressure changes could not be observed.
The present study focused on the CP in the trigone, so the possibility remains that pulsatile changes of the CP in the third and fourth ventricles may still be actively involved in CSF pulsation. It was technically difficult to separately evaluate pulsatile changes of the CP in the other lateral ventricle, 3rd ventricle, and 4th ventricle.
In this study, because the participants were healthy adults, no comment on the relationship between CSF motion and the CP in healthy infants and children, or in patients with hydrocephalus, is possible.
Conclusion
This study investigated whether the CP in the trigone plays an important role in CSF pulsation. 4D-PC was used to measure velocity and acceleration around the CP of the trigone and compare the results with those of other CSF spaces. Observation of the ventricular system showed that CSF acceleration was weak in the trigone compared to other ventricular sites. Additionally, image analysis of the CSF motion showed reduced CSF motion around the CP of the trigone compared to other ventricular sites. These observations seem to suggest that the CP in the trigone is not likely a major source of CSF pulsation.
Acknowledgement
The authors would like to thank Tomohiko Horie, RT, and Nao Kajihara, RT, for their assistance with the MR imaging, and Dr. Takatoshi Sorimach for assistance with the statistical analysis of the data.
Footnotes
Conflicts of Interest Disclosure
This study was supported in part by the Research and Study Project of Tokai University Educational System General Research Organization, Japan Society for the Promotion of Science, and Health and Labor Sciences Research grants from the Japanese government for research on rare and intractable disease. Kagayaki Kuroda is an employee of the Bioview Inc. Other authors have no financial conflicts or any interest with any commercial product used in this study or any substantial relationship with an entity that may impact or benefit from the conclusions of this research. This research was approved by our institution’s Institutional Review Board for Clinical Research, Tokai University Hospital (http://irb.med.u-tokai.ac.jp/) IRB No. 13R-066 (Flow dynamic study of cerebrospinal fluid using MRI).
Contributors
All authors were involved in the conception and design of these experiments. Hirayama, Takizawa, Hayashi, and Sano collected the data. Takizawa and Yatsushiro participated in the analysis and interpretation of the data. Takizawa, Matsumae, and Kuroda wrote the manuscript, and all authors critically revised the article and reviewed the final version prior to submission of the manuscript. Matsumae supervised the study.
References
- 1).Matsumae M, Sato O, Hirayama A, et al. : Research into the physiology of cerebrospinal fluid reaches a new horizon: intimate exchange between cerebrospinal fluid and interstitial fluid may contribute to maintenance of homeostasis in the central nervous system. Neurol Med Chir (Tokyo) 56: 416–441, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Johanson C: Choroid plexus–cerebrospinal fluid circulatory dynamics: impact on brain growth, metabolism, and repair. In Conn PM. (eds): Neuroscience in medicine. Totowa, Humana Press, 2008, pp. 173–200 [Google Scholar]
- 3).Horie T, Kajihara N, Matsumae M, et al. : Magnetic resonance imaging technique for visualization of irregular cerebrospinal fluid motion in the ventricular system and subarachnoid space. World Neurosurg 97: 523–531, 2017 [DOI] [PubMed] [Google Scholar]
- 4).Matsumae M, Hirayama A, Atsumi H, Yatsushiro S, Kuroda K: Velocity and pressure gradients of cerebrospinal fluid assessed with magnetic resonance imaging. J Neurosurg 120: 218–227, 2014 [DOI] [PubMed] [Google Scholar]
- 5).Bering EA: Choroid plexus and arterial pulsation of cerebrospinal fluid; demonstration of the choroid plexuses as a cerebrospinal fluid pump. AMA Arch Neurol Psychiatry 73: 165–172, 1955 [DOI] [PubMed] [Google Scholar]
- 6).Wilson CB, Bertan V: Interruption of the anterior choroidal artery in experimental hydrocephalus. Arch Neurol 17: 614–619, 1967 [DOI] [PubMed] [Google Scholar]
- 7).Bering EA: Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. J Neurosurg 19: 405–413, 1962 [DOI] [PubMed] [Google Scholar]
- 8).Du Boulay G, O’Connell J, Currie J, Bostick T, Verity P: Further investigations on pulsatile movements in the cerebrospinal fluid pathways. Acta Radiol Diagn (Stockh) 13: 496–523, 1972 [DOI] [PubMed] [Google Scholar]
- 9).Feinberg DA, Mark AS: Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology 163: 793–799, 1987 [DOI] [PubMed] [Google Scholar]
- 10).Milhorat TH: The third circulation revisited. J Neurosurg 42: 628–645, 1975 [DOI] [PubMed] [Google Scholar]
- 11).O’Connell JE: The vascular factor in intracranial pressure and the maintenace of the cerebrospinal fluid circulation. Brain 66: 204–228, 1943 [Google Scholar]
- 12).Laitinen L: Origin of arterial pulsation of cerebrospinal fluid. Acta Neurol Scand 44: 168–176, 1968 [DOI] [PubMed] [Google Scholar]
- 13).Du Boulay GH: Pulsatile movements in the CSF pathways. Br J Radiol 39: 255–262, 1966 [DOI] [PubMed] [Google Scholar]
- 14).Friese S, Hamhaber U, Erb M, Kueker W, Klose U: The influence of pulse and respiration on spinal cerebrospinal fluid pulsation. Invest Radiol 39: 120–130, 2004 [DOI] [PubMed] [Google Scholar]
- 15).Klose U, Strik C, Kiefer C, Grodd W: Detection of a relation between respiration and CSF pulsation with an echoplanar technique. J Magn Reson Imaging 11: 438–444, 2000 [DOI] [PubMed] [Google Scholar]
- 16).Picard N: Regarding Bering’s hypothesis on choroid plexus pulsatility. J Neurosurg 122: 479–480, 2015 [DOI] [PubMed] [Google Scholar]
- 17).Alperin N, Lee SH: PUBS: pulsatility-based segmentation of lumens conducting non-steady flow. Magn Reson Med 49: 934–944, 2003 [DOI] [PubMed] [Google Scholar]
- 18).Hayashi N, Matsumae M, Yatsushiro S, Hirayama A, Abdullah A, Kuroda K: Quantitative analysis of cerebrospinal fluid pressure gradients in healthy volunteers and patients with normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 55: 657–662, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Abdullah A, Hirayama A, Yatsushiro S, Matsumae M, Kuroda K: Cerebrospinal fluid image segmentation using spatial fuzzy clustering method with improved evolutionary expectation maximization. Conf Proc IEEE Eng Med Biol Soc 2013: 3359–3362, 2013 [DOI] [PubMed] [Google Scholar]
- 20).Atsumi H, Matsumae M, Hirayama A, Kuroda K: Measurements of intracranial pressure and compliance index using 1.5-T clinical MRI machine. Tokai J Exp Clin Med 39: 34–43, 2014 [PubMed] [Google Scholar]
- 21).Hirayama A, Matsumae M, Yatsushiro S, Abdulla A, Atsumi H, Kuroda K: Visualization of pulsatile CSF motion around membrane-like structures with both 4D velocity mapping and time-slip technique. Magn Reson Med Sci 14: 263–273, 2015 [DOI] [PubMed] [Google Scholar]
- 22).Yamada S, Miyazaki M, Kanazawa H, et al. : Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 249: 644–652, 2008 [DOI] [PubMed] [Google Scholar]
- 23).Bhadelia RA, Bogdan AR, Kaplan RF, Wolpert SM: Cerebrospinal fluid pulsation amplitude and its quantitative relationship to cerebral blood flow pulsations: a phase-contrast MR flow imaging study. Neuroradiology 39: 258–264, 1997 [DOI] [PubMed] [Google Scholar]
- 24).Bhadelia RA, Bogdan AR, Wolpert SM: Analysis of cerebrospinal fluid flow waveforms with gated phase-contrast MR velocity measurements. AJNR Am J Neuroradiol 16: 389–400, 1995 [PMC free article] [PubMed] [Google Scholar]
- 25).Bhadelia RA, Frederick E, Patz S, et al. : Cough-associated headache in patients with Chiari I malformation: CSF flow analysis by means of cine phase-contrast MR imaging. AJNR Am J Neuroradiol 32: 739–742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Henry-Feugeas MC, Idy-Peretti I, Baledent O, et al. : Origin of subarachnoid cerebrospinal fluid pulsations: a phase-contrast MR analysis. Magn Reson Imaging 18: 387–395, 2000 [DOI] [PubMed] [Google Scholar]
- 27).Henry-Feugeas MC, Idy-Peretti I, Blanchet B, Hassine D, Zannoli G, Schouman-Claeys E: Temporal and spatial assessment of normal cerebrospinal fluid dynamics with MR imaging. Magn Reson Imaging 11: 1107–1118, 1993 [DOI] [PubMed] [Google Scholar]
- 28).Feynman RP, Leighton R, Sands M: The Feynman lectures on physics. Addison Wesley Longman; 2, 1970 [Google Scholar]
- 29).Tang C, Blatter DD, Parker DL: Accuracy of phase-contrast flow measurements in the presence of partial-volume effects. J Magn Reson Imaging 3: 377–385, 1993 [DOI] [PubMed] [Google Scholar]