Summary
Epigenetic modifications have emerged as attractive molecular substrates that integrate extrinsic changes into the determination of cell identity. Since stroke-related brain damage releases micro-environmental cues, we examined the role of a signaling-induced epigenetic pathway, an atypical protein kinase C (aPKC)-mediated phosphorylation of CREB-binding protein (CBP), in post-stroke neurovascular remodeling. Using a knockin mouse strain (CbpS436A) where the aPKC-CBP pathway was defective, we show that disruption of the aPKC-CBP pathway in a murine focal ischemic stroke model increases the reprogramming efficiency of ischemia-activated pericytes (i-pericytes) to neural precursors. As a consequence of enhanced cellular reprogramming, CbpS436A mice show an increased transient population of locally derived neural precursors after stroke, while displaying a reduced number of i-pericytes, impaired vascular remodeling, and perturbed motor recovery during the chronic phase of stroke. Together, this study elucidates the role of the aPKC-CBP pathway in modulating neurovascular remodeling and functional recovery following focal ischemic stroke.
Keywords: aPKC-CBP pathway, ischemic stroke, cellular reprogramming, pericyte, neural precursors, vascular remodeling
Highlights
-
•
CbpS436A increases the reprogramming efficiency of i-pericytes to NPCs in culture
-
•
CbpS436A increases the number of locally derived NPCs from i-pericyte in vivo
-
•
CbpS436A shows impaired vascular remodeling and functional recovery after stroke
Wang and colleagues used a knockin mouse model CbpS436A to show that the disruption of the aPKC-CBP pathway increases the reprogramming efficiency of ischemia-activated pericytes to neural precursors (NPCs). As an outcome, CbpS436A mice show an increase in the transient population of locally derived NPCs shortly after stroke, while displaying a reduced number of pericytes and impaired vascular remodeling and motor recovery during the chronic phase of stroke.
Introduction
Stroke is a major public health burden with 40% of patients suffering from permanent neurological disabilities, and novel post-stroke interventions are urgently needed (Krueger et al., 2015). Increasing evidence shows that multipotent neural precursors (NPCs) in the injury site not only migrate from the lateral ventricle subventricular zone (SVZ) in adult brain (Jin et al., 2003, Dibajnia and Morshead, 2013) but are also reprogrammed from local non-neuronal cells following stroke (Shimada et al., 2012, Nakagomi et al., 2015). Pericytes that wrap endothelial cells in the capillaries have emerged as an attractive cell resource, which can be reprogrammed to tissue-specific stem cells following injury (Dellavalle et al., 2011, Tatebayashi et al., 2017). In stroke-related brain injury, a mesenchymal-epithelial transition process is involved in cellular reprogramming of pericytes to multipotent NPCs (Nakagomi et al., 2015). In addition to its plasticity in cellular reprogramming, pericytes are also important for the vascular remodeling post stroke by stabilizing the newly generated microvessel walls and maintaining the blood brain barrier (Liu et al., 2012, Armulik et al., 2010).
We recently identified a signaling-directed epigenetic pathway, atypical protein kinase C (aPKC)-mediated S436 phosphorylation in CREB-binding protein (CBP), and its roles in regulating neuronal differentiation of NPCs in both the developing and aging brain, where marked changes in cell-extrinsic signals occur (Wang et al., 2010, Gouveia et al., 2016). Since stroke-related brain injury releases a plethora of cytokines and inflammatory and growth factors (Kalluri and Dempsey, 2008, Doll et al., 2014), such a pool of enriched extrinsic signals, resembling what occurs in the developing or aging brain, may act on the aPKC-CBP pathway to modulate cell fate, including cellular reprogramming and differentiation. Since CBP is a key epigenetic switch to regulate the epithelial-mesenchymal transition process (Abell et al., 2011), it is intriguing to examine the role of the aPKC-CBP pathway following stroke in regulating NPC reprogramming from pericytes.
Here, we identify a transient population of locally derived NPCs that are reprogrammed from pericytes in the injury site shortly after stroke. The permanent deletion of the aPKC-CBP pathway enhances the pericyte reprogramming efficiency to multipotent NPCs, consequently exhausting proliferative pericytes and compromising vascular remodeling to impair post-stroke functional recovery. Here, we show that reactivation of the aPKC-CBP pathway is required for stroke functional recovery in the chronic phase through neurovascular remodeling.
Results
Disruption of the aPKC-CBP Pathway Increases NPC Reprogramming from i-Pericytes in Culture
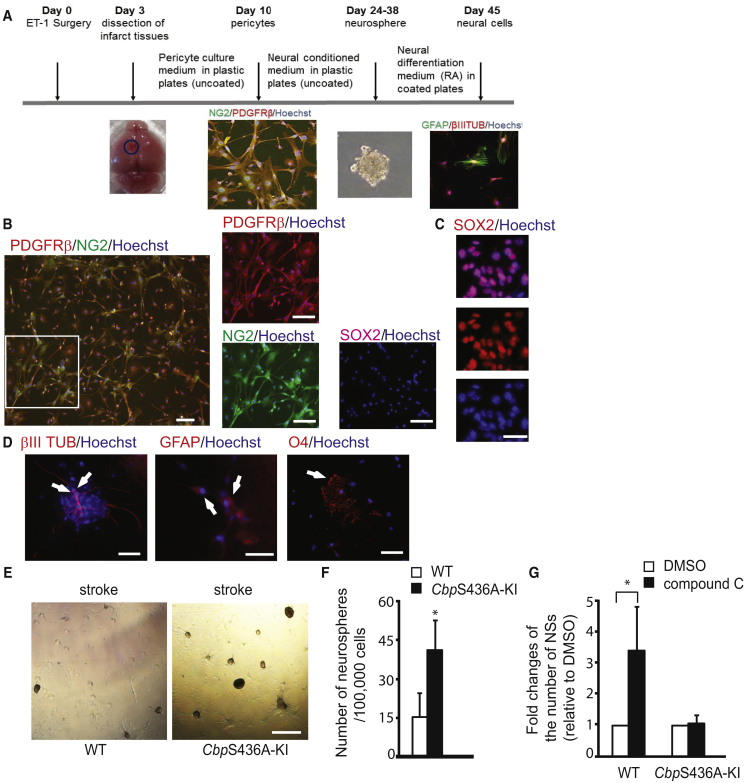
We used endothelin-1 (ET-1)/L-NAME stereotaxic co-injections to induce focal ischemic stroke in the sensorimotor cortex and examined the reprogramming capability of ischemia-activated pericytes (i-pericytes) to SOX2+ NPCs. To test this, we dissected the peri-infarct/infarct cortex tissues 3 days after ET-1/L-NAME injections and selectively isolated pericytes (PDGFRβ+/NG2+/SOX2−) by culturing single dissociated cells on an uncoated plastic culture dish. These i-pericytes were reprogrammed into NPCs in culture by forming neurospheres expressing SOX2 (Figures 1A–1C and S1). These i-neurospheres reprogrammed from i-pericytes were able to further differentiate into neurons, astrocytes, and oligodendrocytes (Figure 1D). Interestingly, i-pericytes from CbpS436A-KI mice produced a greater number of neurospheres (Figures 1E and 1F). In addition, compound C, an AMPK inhibitor, showed the same potency to increase the number of neurospheres that were reprogrammed from wild-type (WT) i-pericytes (Figure 1G), while having no effect on CbpS436A-KI i-pericytes. Together, these data suggest that disruption of the aPKC-CBP pathway improves the reprogramming efficiency of i-pericytes to NPCs.
Figure 1.
CbpS436A Enhances the Reprogramming Efficiency of i-Pericytes to NPCs in Culture
(A) Schematic of experimental flowchart.
(B) Images of i-pericytes cultured for 7 days, showing PDGFRβ+ (red)/NG2+ (green)/SOX2− (purple) cells. Scale bar, 25 μm.
(C) A neurosphere reprogrammed from i-pericytes expressed Sox2 (red) after cytospin. Scale bar, 10 μm.
(D) Differentiation of neurospheres that were reprogrammed from i-pericytes into βIII TUBULIN+ neurons (left panel), GFAP+ astrocytes (middle panel), and O4+ oligodendrocytes (right panel) in the presence of retinoic acid. Arrows denote positive cells for βIII TUBULIN, GFAP, or O4. Scale bar, 20 μm.
(E and F) Images (E) and quantitative analysis (F) of the number of neurospheres reprogrammed from i-pericytes isolated from wild-type (WT) and CbpS436A-KI stroke tissues. Scale bar, 150 μm. ∗p ≤ 0.05, n = 4/group.
(G) Quantitative analysis of the number of neurospheres reprogrammed from i-pericytes in the absence and presence of compound C (1 μM). ∗p ≤ 0.05, n = 4/group.
Error bars in this figure represent the SEM.
Disruption of the aPKC-CBP Pathway Increases the Transient Population of Locally Derived NPCs that Are Reprogrammed from i-Pericytes in the Post-stroke Brain
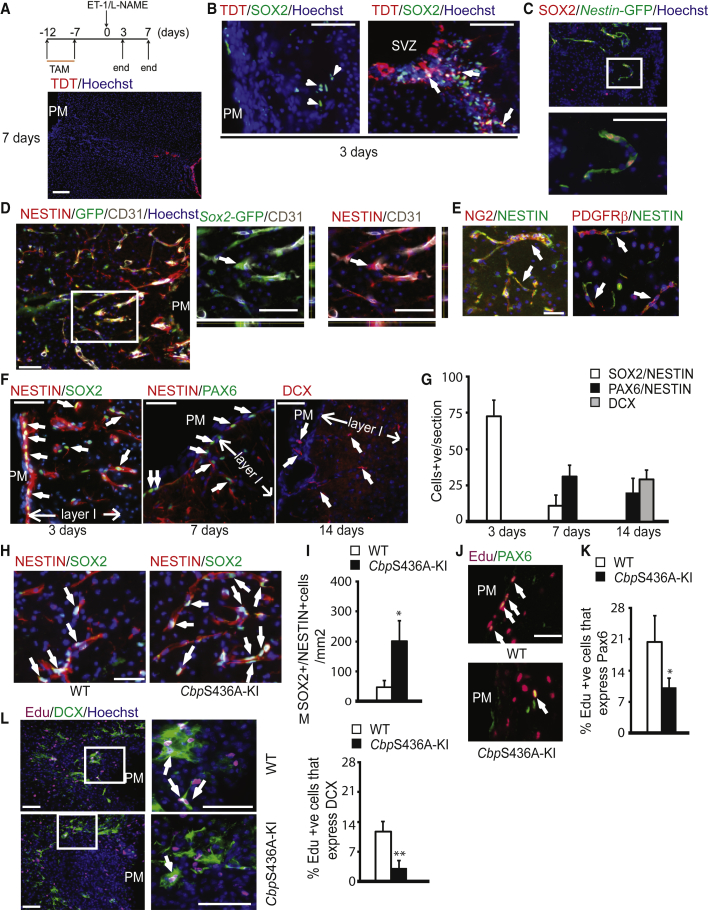
To ask whether i-pericytes can be reprogrammed to NPCs in vivo, we investigated the locally derived NPCs in our focal ischemic stroke model. To first confirm if the SOX2+ NPCs in post-stroke cortex layer I were locally derived and did not migrate from the SVZ, we used a NestinCre-ERT2/tdTomatoflx/Stop/flx reporter line to trace SVZ NPCs by injecting tamoxifen 7 days before stroke surgery (Figure 2A). We found that tdTomato (TDT)+ cells in the SVZ were co-labeled with SOX2, and the TDT+SVZ NPCs did not migrate up to layer I at 7 days after stroke (Figure 2A). In addition, SOX2+NPCs in post-stroke cortex layer I were negative for TDT (Figure 2B). Next, to test whether the SOX2+NPCs in layer I were derived from pericytes following stroke, we performed an in vivo labeling experiment using the NeuroTrace 500/525 technology to label capillary pericytes in the live brain (Damisah et al., 2017). NeuroTrace 500/525 (300 nL) was injected intracortically just below the pia surface before ET-1/L-NAME injections. Three days post stroke, NeuroTrace-labeled pericytes were imaged ex vivo in the stroke-injured live cortical sections using two-photon microscopy. Sequentially, the same injured cortical sections were fixed and immunostained for SOX2 and imaged using confocal microscopy. Using the needle track landmarks to merge the two images, we were able to detect NeuroTrace+/SOX2+ cells, suggesting the pericyte origin of these SOX2+NPCs (Figure S2A). Third, we injected 5-bromo-2′-deoxyuridine (BrdU) 24 hr before surgery (Figure S2B) and sacrificed mice 3 days post-stroke. SOX2+NPCs in the injured cortex were negative for BrdU (Figure S2B), suggesting that they were derived from non-cycling cells pre-stroke. In addition, we used a Sox2-GFP reporter line, together with ethynyl 2′ deoxyuridine (EdU) post-stroke injections (Figure S2C), to show that Sox2-GFP+NPCs presented in both the SVZ and injured cortex layer I, co-labeling with EdU that marked proliferating cells. Together, these data suggest that SOX2+NPCs in injured cortex layer I were locally derived following stroke, possibly from capillary pericytes. Since SOX2+ cells from injured cortex were also NESTIN+ (detected either by a Nestin-GFP reporter line or Nestin-antibody) and adjacent to CD31+ microvessels (Figures 2C, 2D, and S2D), we further examined the identity of the specific population of NESTIN+ cells that were adjacent to microvessels and found that these NESTIN+ cells were co-labeled with two pericyte markers, PDGFRβ and NG2 (Figure 2E). These data further confirmed that the locally derived SOX2+NPCs in the injured cortex are reprogrammed from pericytes following stroke. We then assessed dynamic changes of NESTIN+/SOX2+ NPCs that were adjacent to microvessels at 3, 7, and 14 days post stroke in injured cortex layer I and observed that the locally derived NESTIN+/SOX2+ cells peaked 3 days post stroke and gradually diminished 14 days post stroke (Figures 2F, 2G, and S2E). Interestingly, another population of dorsal committed NPCs expressing both PAX6 and NESTIN surged to peak 7 days post stroke, followed by a rise of DCX+ neuroblasts 14 days post stroke in the same infarct region (Figures 2F and 2G). The sequential wave of three populations of cells in cortex layer I infarct implies a developmental process of locally derived NPCs that were reprogrammed from pericytes following stroke.
Figure 2.
CbpS436A Increases the Transient Population of Locally Derived NPCs that Are Reprogrammed from Pericytes in the Post-stroke Brain
(A and B) Expression of SOX2+ (green)/TDT+ (red) NPCs in the SVZ of NestinCre-ERT2/tdTomatoflx/Stop/flx mice, receiving tamoxifen before stroke, at 3 days (B) and 7 days (A) post stroke, while SOX2+NPCs in injured cortex layer I were negative for TDT 3 days post stroke. Arrows denote co-labeled cells, while arrowheads denote single-labeled cells with SOX2+. Scale bar, 100 μm (A); 40 μm (B). PM, pia mater; LV, lateral ventricle; TDT, TdTomato.
(C) Expression of GFP+ (green) and SOX2+ (red) cells in injured cortex of Nestin-GFP mice 3 days post stroke. White boxed image is enlarged in the bottom panel. Scale bar, 25 μm.
(D) Expression of GFP+ (green)/NESTIN+ (red) co-labeled NPCs that were adjacent to CD31+ (white) endothelial cells in injured cortex of Sox2-GFP mice 3 days post stroke. White boxed image is enlarged in the right panels. Arrows denote co-labeled GFP+/NESTIN+ cells. Scale bar, 25 μm.
(E) NESTIN+ cells within the infarct core are co-labeled with other pericyte markers, NG2 (red, left panel) and PDGFRβ (red, right panel). Arrows denote co-labeled cells. Scale bar, 20 μm.
(F and G) Images (F) and quantification (G) of SOX2 (green)/NESTIN+ (red, left panel) NPCs, PAX6 (green)/NESTIN+ (red, middle panel) NPCs and DCX+ (red, right panel) neuroblasts in injured cortex layer I 3, 7, and 14 days post stroke. Arrows represent positive cells that were quantified for (G). Scale bar, 20 μm.
(H and I) Images (H) and quantification (I) of co-labeled SOX2+/NESTIN+ NPCs in WT and CbpS436A ipsilateral cortex 3 days post stroke. Arrows denote co-labeled cells. Scale bar, 20 μm. ∗p < 0.05, n = 3–4/group.
(J–M) Images (J and L) and quantification (K and M) of co-labeled PAX6+/EdU+ NPCs (J and K) and DCX+/EdU+ neuroblasts (L and M) in WT and CbpS436A ipsilateral cortex 14 days post stroke, injected with EdU days 2–5 post stroke. White boxed image is enlarged in the right panels. Arrows denote co-labeled cells. Scale bar, 25 μm. ∗p < 0.05; ∗∗p < 0.01, n = 3–4/group.
Error bars in this figure represent the SEM.
After identifying the transient population of locally derived NPCs, we further examined their developmental process in CbpS436A-KI mice. The quantification analysis revealed that CbpS436A-KI mice displayed an increase in the population of NESTIN+/SOX2+ NPCs 3 days post stroke (Figures 2H and 2I), while showing a reduced number of PAX6+ dorsal committed NPCs (Figure S2F) and DCX+ neuroblasts (Figure S2F) in layer I of the infarct cortex. We further used EdU pulse labeling post stroke (2–5 days post stroke) and showed that the proportion of PAX6+ dorsal committed NPCs (Figures 2J and 2K) and the proportion of DCX+ neuroblasts (Figures 2L and 2M) over total EdU+ cells were decreased in CbpS436A-KI mice 14 days post stroke.
The aPKC-CBP Pathway Regulates Post-stroke Vascular Remodeling
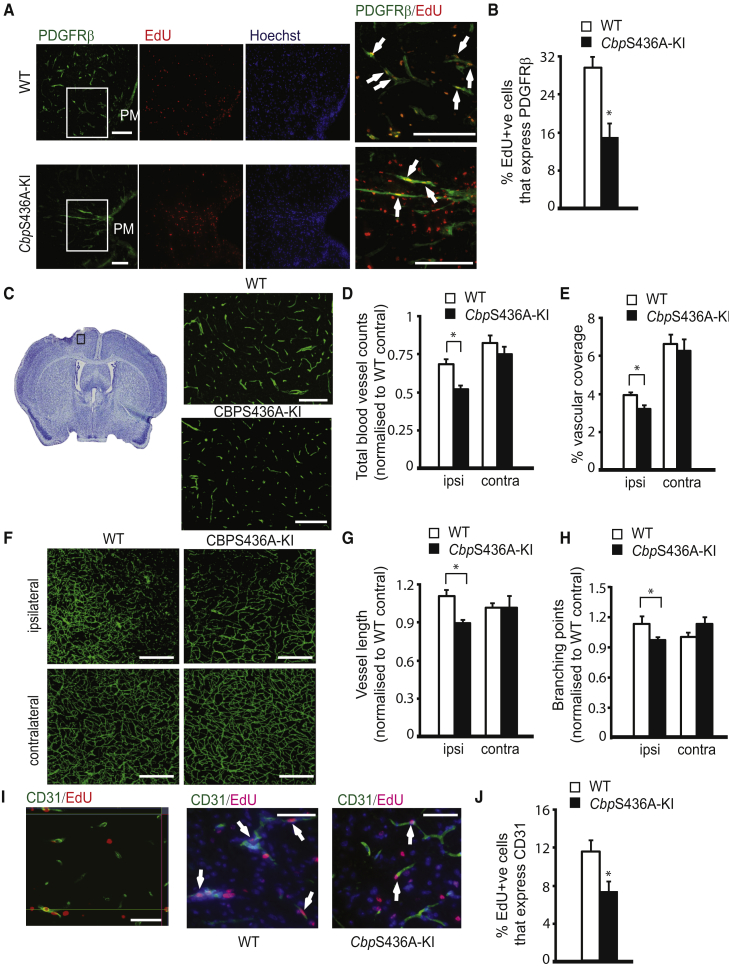
To further confirm that the increased SOX2+/NESTIN+ NPCs in CbpS436A-KI mice indeed originate from increased reprogramming of i-pericytes, we showed that the proportion of PDGFRβ+ pericytes over total EdU+ cells was decreased in CbpS436A-KI mice (Figures 3A and 3B). The effect occurred independently of any difference between WT and CbpS436A-KI in terms of basal number of pericytes under physiological conditions (Figures S3A and S3B) and the number of apoptotic i-pericytes following stroke (Figure S3C). These data suggest that the pool of proliferative i-pericytes exhausted over time due to increased reprogramming. Since pericytes are known to stabilize newly formed blood vessels and neurovascular units (Daneman et al., 2010, Sweeney et al., 2016), we further determined whether post-stroke vascular remodeling and angiogenesis were hampered by the increased reprogramming process. To assess vascular remodeling post stroke, brain sections collected at 14 days post stroke from both genotypes were immunostained for CD31 to label endothelial cells. The density of CD31+ microvessels and the area of vascular coverage were reduced in the ipsilateral cortex from both genotypes (Figures 3C–3E) relative to contralateral. Importantly, CbpS436A-KI mice showed a significant decrease in the number of CD31+ microvessels and vascular coverage when compared with WT mice in the ipsilateral cortex (Figures 3C–3E). In addition, we performed three-dimensional (3D) cortical vasculature analysis using brains collected at 25 days post stroke (Figures 3F–3H). Ipsilateral vessel length and branchpoints from WT mice showed a trend of being higher than those in the contralateral WT cortex, implying a sign of vascular remodeling. However, CbpS436A-KI mice displayed a consistent reduction in the ipsilateral vessel length and branchpoints when compared with their contralateral count and with those from WT mice (Figures 3F–3H). To assess post-stroke angiogenesis, we performed EdU pulse labeling as previously described and examined brain sections 14 days after stroke by immunohistochemistry. The percentage of EdU+/CD31+ newly generated blood vessels was significantly reduced in CbpS436A-KI mice (Figures 3I and 3J). Thus, disruption of the aPKC-CBP pathway increases cellular reprogramming of i-pericytes at the expense of vascular remodeling post stroke.
Figure 3.
CbpS436A regulates Post-stroke Vascular Remodeling
(A and B) Images (A) and quantification (B) of co-labeled PDGFRβ+/EdU+ pericytes in WT and CbpS436A ipsilateral cortex 14 days post stroke. Arrows denote co-labeled cells. Scale bar, 50 μm. ∗p < 0.05, n = 3/group.
(C) Images of CD31 (green) in injured cortex from WT and CbpS436A mice 14 days post stroke (right panel). Scale bar, 100 μm.
(D and E) Quantification of total blood vessel counts (D) and the percentage of vascular coverage (E) from ipsilateral and contralateral cortex of WT and CbpS436A mice as shown in (C), normalized to the average counts of the WT contralateral group. Data were analyzed by two-way ANOVA(Genotype × Hemisphere interaction F(1,24) = 1.116, p = 0.3022, Genotype F(1,24) = 18.52, p = 0.0003, n = 7/per group for total blood vessel counts; Genotype × Hemisphere interaction F(1,12) = 0.225, p = 0.649, Genotype F(1,12) = 0.58 p = 0.0001, n = 4/per group for vascular coverage), with Tukey's post hoc test, ∗p < 0.05, n = 4–7/group.
(F) Confocal images of cortex of WT and CbpS436A mice 25 days post stroke, stained for CD31 (green). Scale bar, 100 μm.
(G and H) Quantitative analysis of vessel length (G) and branchpoints (H), normalized as fold change over contralateral (Genotype × Hemisphere interaction F(1,12) = 4.317, p = 0.3022 for vessel length; Genotype × Hemisphere interaction F(1,12) = 5.372, p = 0.0389 for branchpoints) (n = 5 for WT, n = 3 for KI), with Tukey's post hoc test, ∗p < 0.05, n = 3–5/group.
(I and J) Images (I) and quantification (J) of co-labeled CD31+/EdU+ endothelial cells in WT and CbpS436A ipsilateral cortex 14 days post stroke. Arrows denote co-labeled cells. Scale bar, 20 μm. ∗p < 0.05, n = 3/group.
Error bars in this figure represent the SEM.
The aPKC-CBP Pathway Modulates Post-stroke Functional Recovery without Altering Stroke Volume
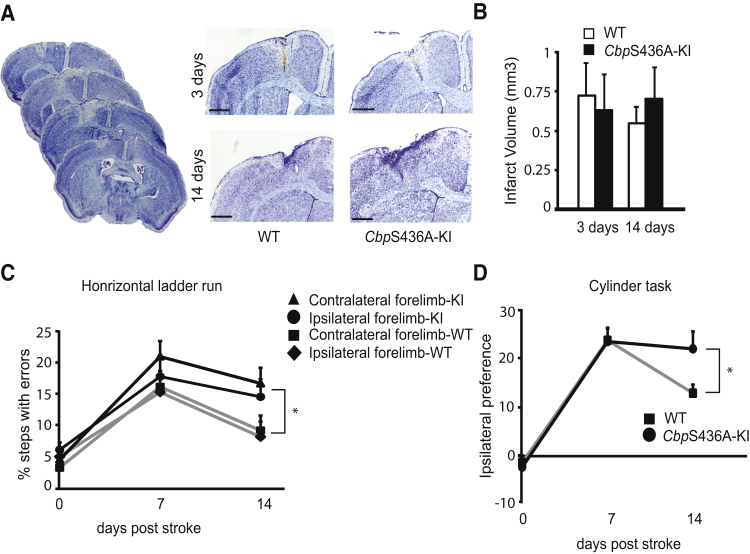
To assess outcomes of the altered neurovascular remodeling in CbpS436A-KI mice, we examined stroke volume and performed behavioral analysis. There was no significant difference in infarct volume between WT and CbpS436A-KI mice at 3 and 14 days post stroke (Figures 4A and 4B). To assess post-stroke functional recovery, both genotypes of mice were tested on two sensitive behavioral tests that measure motor deficits: the horizontal ladder run and cylinder tasks. On the horizontal ladder task, WT and CbpS436A-KI mice showed a comparable baseline pre-stroke and a similar increase in step error 7 days post stroke in both the contralateral and ipsilateral forelimbs (Figure 4C). By 14 days post stroke, CbpS436A-KI mice had significantly more deficits compared with WT mice on both limbs. A very similar pattern was shown in the cylinder test. Both WT and CbpS436A-KI mice displayed no preference in using forepaws to touch the cylinder wall pre-stroke and a similar preference to use the ipsilateral forepaw 7 days post stroke (Figure 4D), while at 14 days post stroke CbpS436A-KI mice had a significantly higher ipsilateral preference, implicating less recovery in CbpS436A-KI mice. Thus, the aPKC-CBP pathway modulates post-stroke functional recovery without altering stroke volume.
Figure 4.
CbpS436A Modulates Post-stroke Functional Recovery without Altering Stroke Volume
(A and B) Images (A) and quantification (B) of infarct volumes at 3 and 14 days post stroke from WT and CbpS436A-KI mice. Scale bars, 50 μm (n = 6/group for 3 days; n = 9/group for 14 days).
(C) The percentage of forelimb error step at 7 and 14 days post stroke in WT and CbpS436A mice, analyzed by two-way ANOVA (Time × Genotype with forelimbs F(6,168) = 0.76, p = 0.7587, n = 17 for WT, n = 13 for KI), with Tukey's post hoc test, ∗p < 0.05 compared with WT, n = 13–17/group.
(D) The ipsilateral preference in the cylinder test at 7 and 14 days post stroke in WT and CbpS436A-KI mice, analyzed by two-way ANOVA (Time × Genotype F(2,42) = 2.395, p = 0.1035, n = 17 for WT, n = 13 for KI) with Tukey's post hoc test, ∗p < 0.05 compared with WT, n = 13–17/group.
Error bars in this figure represent the SEM.
Discussion
The current study identifies a transient population of locally derived NPCs following focal ischemic stroke, reprogrammed from i-pericytes. We further demonstrate that phosphomutant CbpS436A enhances the reprogramming efficiency of i-pericytes to NPCs both in culture and in vivo. As a consequence, permanent deletion of the aPKC-CBP pathway reduces the population of proliferating pericytes following stroke, leading to perturbed vascular remodeling and stroke motor functional recovery deficits.
Increasing evidence suggested that pericytes have the capability to be reprogrammed into multipotent NPCs or mature neurons both in vitro and in vivo (Nakagomi et al., 2015, Tatebayashi et al., 2017, Karow et al., 2012). Here, we demonstrate that i-pericytes following focal ischemic stroke can be reprogrammed to SOX2+NPCs, transiently arising shortly after stroke (3 days) followed by another wave of PAX6+ dorsal committed NPCs peaking 7 days after stroke. More importantly, we found that a single S436 phosphorylation mutation in CBP significantly increases the reprogramming efficiency of i-pericytes to SOX2+ NPCs, while arresting the reprogrammed NPCs at the early stage without progressing into PAX6+ dorsal committed NPCs. This is a very interesting observation. Previously, we showed that CbpS436A led to differentiation deficits in the hippocampal subgranular zone (SGZ) SOX2+NPCs, arrested at an early stage without progressing to mature neurons (Gouveia et al., 2016). This suggests that CbpS436A has two roles in regulating NPC reprogramming and differentiation: facilitating NPC reprogramming from i-pericytes while simultaneously preventing their further differentiation. Another possible explanation for the reduction of PAX6+ dorsal committed NPCs is due to fate changes of SOX2+ multipotent stem cells. SOX2 is not only considered as a marker for neural precursors but also as a marker for pluripotency. A previous study has shown that pluripotent-like stem cells were detected in the injured cortex following transient middle cerebral artery occlusion, labeled with pluripotent markers, SOX2, KL4, and c-MYC (Nakagomi et al., 2015). In addition, IBA1+ microglia have been shown to be reprogrammed from i-pericytes (Sakuma et al., 2016). To assess the possibility that the reduced number of PAX6+ cells in CbpS436A-KI is due to an increased number of Iba1+ microglia, possibly generated from SOX2+ pluripotent/multipotent stem cells, we examined the proportion of IBA1+ microglia in the total EdU+ cells 14 days post stroke. Indeed, the proportion of IBA1+ microglia was increased in CbpS436A-KI compared with their WT littermates (Figures S3D and S3E). These findings suggest that there could be a change in fate between PAX6+ NPCs and IBA1+ microglial cells, which remains to be confirmed in future studies utilizing lineage tracing experiments.
Histone acetyltransferases such as CBP are well known to regulate cell-fate changes (Krishnakumar and Blelloch, 2013, Murao et al., 2016). Increasing studies have targeted either bromodomain or histone deacetylase activity to increase the reprogramming efficiency of nonneruonal cells to NPCs and mature neurons (Li et al., 2015, Pfisterer et al., 2016, Gao et al., 2017). Despite this, a direct signal that modulates histone acetyltransferase (HAT) activity to alter cell fate remains largely unknown. We previously showed that the aPKC-CBP pathway can be activated by metformin to enhance the neuronal differentiation of NPCs (Wang et al., 2012). Our current finding, instead, revealed that inactivation of the aPKC-CBP pathway enhances NPC reprogramming from i-pericytes. Thus, the aPKC-mediated single phosphorylation of CBP serves as a signaling sensor to fine-tune cell-fate decisions through reprogramming or differentiation processes. This phosphorylation in CBP may directly alter either the histone acetyltransferase activities or their chromatin-binding profiles. Identification of downstream targets of the aPKC-CBP pathway at both genomic and epigenomic levels will be further explored.
Experimental Procedures
Animals
All animal use was approved by the Animal Care Committees of the University of Ottawa in accordance with the Canadian Council of Animal Care policies. Transgenic mouse lines, CbpS436A, Sox2-GFP, Nestin-GFP, and NestinCre-ERT2/tdTomatoflx/Stop/flx mice were maintained on a 12 hr light/12 hr dark cycle with access to food and water ad libitum.
Detailed information is described in Supplemental Experimental Procedures.
ET-1/L-NAME Surgery
Mice (2–4 months) were anesthetized using 4%–5% isoflurane and 1.5% oxygen and mounted on a stereotaxic frame for ET-1/L-NAME injections, detailed in Supplemental Experimental Procedures.
Pericyte Culture and Sphere Formation
Three days following ET-1 surgery, mice were killed and their brains were dissected out. Pericyte culture isolated from injured cortex is detailed in Supplemental Experimental Procedures.
Drug Treatment
To label dividing cells or SVZ NPCs, mice received an intraperitoneal injection (i.p.) with EdU, BrdU, or tamoxifen, detailed in Supplemental Experimental Procedures.
Immunohistochemistry, Microscopy, and Quantification
Immunostaining of brain sections was performed as described previously (Gouveia et al., 2016), detailed in Supplemental Experimental Procedures. Detailed image acquisition and quantification is also described in Supplemental Experimental Procedures.
EdU Click-iT Chemistry Labeling
EdU was visualized using Click-iT chemical reaction kits from Cell Signaling (C10338) according to the manufacturer’s instructions.
Three-Dimensional Blood Vessel Staining, Imaging, and Quantification
Mice were killed at 25 days post surgery, and the cortices were dissected, flattened between two layers of glass, and immersed in 4% paraformaldehyde overnight at 4°C. The detailed procedure is described in Supplemental Experimental Procedures.
Two-Photon Ex Vivo Imaging
Coronal slices were generated as described previously. Detailed imaging procedures are described in Supplemental Experimental Procedures.
Horizontal Ladder Test and Cylinder Test
The two tests were performed at the Behavioral Core at the University of Ottawa, detailed in Supplemental Experimental Procedures.
Cresyl Violet Staining and Infarct Volume Measurement
Slides containing serially collected sections were dried at 37°C for 15 min and stained with cresyl violet solution (Sigma-Aldrich, C5402), detailed in Supplemental Experimental Procedures.
Statistical Analysis
Data analysis was performed using GraphPad Prism 6 (Graphpad Software, La Jolla, CA). Behavioral analysis was performed using two-way ANOVA with Tukey's post hoc test. Single comparisons were performed using two-tailed Student's t test. n in the figure legends refers to individual mice.
Author Contributions
A.G. performed stroke surgery, behavioral analysis, immunostaining, and pericyte culture; M.S. maintained the mouse colony; L.H. and F.W. generated the CbpS436A knockin mouse strain. B.L., C.H.C., and L.d.F.C. designed and performed 3D vasculature assessments; D.C.L. designed and provided mice for the NestinCreER2 reporter mice experiments. J.-C.B., D.C.L., and T.S.K. designed and performed two-photon imaging on live brain sections. A.G. and J.W. contributed to experimental design, data interpretation, and writing the paper.
Acknowledgments
This work was supported by J.P. Bickell Foundation, Ottawa Hospital Foundation and the Heart and Stroke Foundation (HSF) through a grant-in-aid (GIA-16-00014650) to J.W.; CNPq (307333/2013-2) and FAPESP (11/50761-2) grants to L.d.F.C.; FAPESP (15/18942-8) grant to C.H.C.; HSF Canadian Partnership for Stroke Recovery (CPSR) Trainee Award to T.S.K.; HSF CPSR and CIHR grants (MOP378718) to D.C.L.; and HSF CPSR, CIHR (MOP142420), and CFI (23915) grants to J.-C.B. We thank Anthony Carter (HSF CPSR) for ET-1 stroke surgery training, Dr. Hsiao-Huei Chen for a gift of anti-IBA1 and Dr. Ruth Slack for a gift of Sox2-GFP reporter mice. We also thank the uOttawa’s Core facility program (CBIA) for infrastructure support.
Published: November 22, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.10.021.
Supplemental Information
References
- Abell A.N., Jordan N.V., Huang W., Prat A., Midland A.A., Johnson N.L., Granger D.A., Mieczkowski P.A., Perou C.M., Gomez S.M. MAP3K4/CBP-regulated H2B acetylation controls epithelial-mesenchymal transition in trophoblast stem cells. Cell Stem Cell. 2011;8:525–537. doi: 10.1016/j.stem.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A., Genove G., Mae M., Nisancigglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Linblom P., Strittmatter K. Pericytes regulate the blood-brain barrier. Nature. 2010;25:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Damisah E.C., Hill R.A., Tong L., Murray K.N., Grutzendler J. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat. Neurosci. 2017;20:1023–1032. doi: 10.1038/nn.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A., Maroli G., Covarello D., Azzoni E., Innocenzi A., Perani L., Antonini S., Sambasivan R., Brunelli S., Tajbakhsh S., Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Dibajnia P., Morshead C. Role of neural precursor cells in promoting repair following stroke. Acta Pharmacol. Sin. 2013;34:78–90. doi: 10.1038/aps.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll D.N., Barr T.L., Simpkins J.W. Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 2014;5:294–306. doi: 10.14336/AD.2014.0500294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Guan W., Wang M., Wang H., Yu J., Liu Q., Qiu B., Yu Y., Ping Y., Bian X. Direct generation of human neuronal cells from adult astrocytes by small molecules. Stem Cell Reports. 2017;8:538–547. doi: 10.1016/j.stemcr.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia A., Hsu K., Niibori Y., Seegobin M., Cancino G.I., He L., Wondisford F.E., Bennett S., Lagace D., Frankland P.W., Wang J. The aPKC-CBP pathway regulates adult hippocampal neurogenesis in an age-dependent manner. Stem Cell Reports. 2016;11:719–734. doi: 10.1016/j.stemcr.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Sun Y., Xie L., Peel A., Mao X.O., Batteur S., Greenberg D.A. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol. Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Kalluri H.S., Dempsey R.J. Growth factors, stem cells, and stroke. Neurosurg. Focus. 2008;24:E14. doi: 10.3171/FOC/2008/24/3-4/E13. [DOI] [PubMed] [Google Scholar]
- Karow M., Sánchez R., Schichor C., Masserdotti G., Ortega F., Heinrich C., Gascón S., Khan M.A., Lie D.C., Dellavalle A. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Krishnakumar R., Blelloch R.H. Epigenetics of cellular reprogramming. Curr. Opin. Genet. Dev. 2013;23:548–555. doi: 10.1016/j.gde.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger H., Koot J., Hall R.E., O’Callaghan C., Bayley M., Corbett D. Prevalence of individuals experiencing the effects of stroke in Canada: trends and projections. Stroke. 2015;46:2226–2231. doi: 10.1161/STROKEAHA.115.009616. [DOI] [PubMed] [Google Scholar]
- Li X., Zuo X., Jing J., Ma Y., Wang J., Liu D., Zhu J., Du X., Xiong L., Du Y. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17:195–203. doi: 10.1016/j.stem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Liu S., Agalliu D., Yu C., Fisher M. .The role of pericytes in blood brain barrier function and stroke. Curr. Pharm. Des. 2012;18:3653–3662. doi: 10.2174/138161212802002706. [DOI] [PubMed] [Google Scholar]
- Murao N., Noguchi H., Nakashima K. Epigenetic regulation of neural stem cell property from embryo to adult. Neuroepigenetics. 2016;5:1–10. [Google Scholar]
- Nakagomi T., Kubo S., Nakano-Doi A., Sakuma R., Lu S., Narita A., Kawahara M., Taguchi A., Matsuyama T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33:1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- Pfisterer U., Ek F., Lang S., Soneji S., Olsson R., Parmar M. Small molecules increase direct neural conversion of human fibroblasts. Sci. Rep. 2016;6:38290. doi: 10.1038/srep38290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma R., Kawahara M., Nakano-Doi A., Takahashi1 A., Tanaka Y., Narita A., Kuwahara-Otani S., Hayakawa T., Yagi H., Matsuyama T., Nakagomi T. Brain pericytes serve as microglia generating multipotent vascular stem cells following ischemic stroke. J. Neuroinflammation. 2016;13:57. doi: 10.1186/s12974-016-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada I.S., LeComte M.D., Granger J.C., Quinlan N.J., Spees J.L. Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J. Neurosci. 2012;32:7926–7940. doi: 10.1523/JNEUROSCI.4303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Ayyadurai S., Zlokovic B.V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi K., Tanaka Y., Nakano-Doi A., Sakuma R., Kamachi S., Shirakawa M., Uchida K., Kageyama H., Takagi T., Yoshimura S. Identification of multipotent stem cells in human brain tissue following stroke. Stem Cells Dev. 2017;26:787–797. doi: 10.1089/scd.2016.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Weaver I.C.G., Gauthier-Fisher A., Wang H., He L., Yeomans J., Wondisford F.E., Kaplan D.R., Miller F.D. CBP histone acetyltransferase activity regulates embryonic neural differentiation in the normal and Rubinstein-Taybi syndrome brain. Dev. Cell. 2010;18:114–125. doi: 10.1016/j.devcel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Wang J., Gallagher D., DeVito L., Cancino I., Tsui D., He L., Keller G., Frankland P.W., Kaplan D.R., Miller F.D. Metformin activates atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.