Abstract
Importance
A recent published study of national data showed increased risk of schizophrenia (SCZ) in offspring associated with both early and delayed parental age, consistent with a U-shaped relationship. However, it remains unclear if the risk to the child is due to psychosocial factors associated with parental age, or if those at higher risk for SCZ tend to have children at an earlier or later age.
Objective
To determine if there is a genetic association between SCZ and age at first birth (AFB) using genetically informative but independently ascertained data sets.
Design, Setting, and Participants
We used genome-wide association study (GWAS) data from 18,957 SCZ cases and 22,673 controls and from 12,247 women from independent community samples not enriched for psychiatric disorders measured for AFB. SCZ genetic risk for each woman in the AFB sample was estimated using genetic effects inferred from the SCZ GWAS.
Main Outcomes and Measures
We tested if SCZ genetic risk was a significant predictor of response variables based on published polynomial functions that described the relationship between maternal age and SCZ risk in offspring in Denmark. We substituted AFB for maternal age in these functions, one of which was corrected for age of the father, and found that the fit was superior in the model without adjustment for father’s age.
Results
We observed a U-shaped relationship between SCZ risk and AFB, consistent with the previously reported relationship between SCZ risk in offspring and maternal age when not adjusted for age of the father. We confirmed that SCZ risk profile score significantly predicted the response variables reflecting the published relationship between maternal age and SCZ risk in offspring from McGrath et al. (2014) (p-value=4E-04).
Conclusions and Relevance
It has been reported that SCZ risk associated with increased maternal age is explained by age of the father, and that de novo mutations that occur more frequently in the germline of older men are the underlying causal mechanism. This explanation may need to be revised if, as suggested here, and if replicated in future studies, there is also an increased genetic risk of SCZ in older mothers.
INTRODUCTION
Parental age is a risk factor for a range of adverse mental health outcomes in children, including common psychiatric disorders such as schizophrenia (SCZ)1-3. Advanced paternal age has received the most attention, with risk to children widely assumed to be explained by de novo mutations that occur more frequently in the germline of older men4-6, although there are studies questioning the de novo mutation hypothesis7,8. There is also emerging evidence to suggest that children of both younger9 and older10 mothers are at increased risk of psychosis.
One recent major study is from McGrath et al. (2014)11 who performed a comprehensive analysis using family data extracted from the Danish Psychiatric Central Register and reported a U-shaped relationship between maternal age and risk of SCZ in offspring. They showed that there was higher risk in children of younger and older mothers compared to those of intermediate age (25-29 years). In their second analyses that were adjusted for age of the father, which tends to be highly correlated with mother’s age, increased risk of SCZ was associated with younger mothers (<25 years), but not older mothers. Conversely, children of older fathers (>29 years) were at increased risk, even after correcting for age of the mother, but children of younger fathers were not. These epidemiological findings for the association of maternal age and risk of SCZ cannot dissect cause from consequence. That is, it is unclear if risk to offspring is due to psychosocial, lifestyle or biological ageing factors associated with maternal age, or if women at higher risk for SCZ tend, on average, to have their (first) child at an earlier or later age. Here our analyses use a novel design to explore the relationship between age at motherhood and schizophrenia.
Recently developed whole genome analysis methods provide an opportunity to investigate this question in a novel way that is independent of potential confounding factors. Genomic relationships and risk profile scores on unrelated individuals derived from genome-wide single nucleotide polymorphism (SNP) data can be used to dissect the (shared) genetic architecture of complex traits12. For instance, independently unrelated individuals can be linked through genomic information, correlating their genotype sharing with similarities in their phenotypes. Since the individuals who are compared are not related in the conventional sense, any covariance between their shared genome and their phenotype is most likely genetic, and likely free of confounding environmental effects. This novel design enables using independent genome wide association studies (GWAS) datasets to quantify the extent of genetic overlap across complex traits.
Here, we investigated the genetic relationship between SCZ and age at first birth in women (AFB) using multiple independent GWAS data sets. Specifically, we used genetic risk alleles and their effect sizes estimated from SCZ GWAS data to create a genetic risk profile score of SCZ for each woman in the AFB data set. The AFB data set comprises community samples of women, (i.e., not ascertained for psychiatric disorders) who had their age at the birth of their first child recorded. While both SCZ and AFB are heritable traits13-15, here the genetic contribution underpinning the trait age at first motherhood is not dependent on the age of her partner. We tested if the relationship between a woman’s AFB and her SCZ risk profile score matched that reported by McGrath et al.(2014)11 between age of mothers and the incidence rate ratio (IRR) for SCZ in their children .
METHODS
Data and statistical analysis
The SCZ sample comprised 18,957 SCZ cases and 22,673 controls in a GWAS from the second phase of the Psychiatric Genomics Consortium (PGC2-SCZ)15 and the AFB sample comprised 12,247 genotyped women measured for AFB from four community cohorts (from Estonia (EGCUT), the Netherlands (Lifelines), Sweden (Swedish Twin Registry) and the UK (TwinsUK) (see eAppendix 1 in the Supplement for full details)). These community cohorts measured for AFB were not enriched for psychiatric disorders. We used genomic best linear unbiased prediction (GBLUP), implemented in the MTG software16, to estimate SNP effects in our QCed PGC2-SCZ dataset (N=41630, eTable 1 provides sample details). Briefly, SNP effects were estimated jointly within a linear mixed model that intrinsically accounts for linkage disequilibrium between SNPs. In estimating SNP effects, we fitted sex, cohort and 20 principal components obtained from the sample to control for potential confounding effects such as population stratification and potential batch effects. The SNP risk predictors were projected into the AFB samples, resulting in a GBLUP risk profile score for each individual. We repeated our analyses using standard genetic risk profile scores17,18, (see eAppendix 2 in the Supplement), in which the SCZ risk scores in the women measured for AFB were estimated by summing the count of the SCZ risk alleles weighted by their effect sizes (log(odds ratio)) estimated from our QCed SCZ GWAS. A regression of phenotype on genetic risk profile score tests for association between the measures. The GBLUP risk profile score is expected to be more accurate than a standard risk profile score as the available data in the SCZ sample is used more optimally in the GBLUP linear mixed model methodology. In particular, arbitrary decisions about p-value and linkage disequilibrium clumping that are inherent in standard profile scoring are avoided.
The distribution of AFB and year of birth for each cohort is described in Table 1 and eFigures 1 and 2. In analyses for AFB, we generated two response variables using polynomial functions derived from McGrath et al. (2014) to describe the relationship between IRR for SCZ in offspring and maternal age (Model1 = 2.7214 + 0.0018x2 – 0.1105x), or maternal age adjusted for age of other parent and urbanization of place of birth (Model2 = 2.5438 + 0.0012x2 + 0.0889x). eFigure 3 and eFigure 4 show how we derived the polynomial functions. In generating the Model1 and Model2 values for each individual, AFB was used as the x variable in the equations. In analyses, we used the residuals of the Model1 or Model2 values after regression on covariates such as age at interview, 20 ancestry principal components and cohort. We did not include year of birth as a covariate because its negative value was highly correlated with age at interview (>0.9). The fitted residuals were regressed against the risk profile scores and we report the proportion of variance (R2) attributable to the polygenic score. The p-value results from the test of the hypothesis R2 = 0. The purpose of these analyses was to test if the SCZ polygenic risk values are a better fit to the phenotypic risk values predicted by Model 1 or Model 2. A smaller p-value for Model1 would imply a better fit to the AFB data compared to Model2 for which the linear and quadratic coefficients were derived from models that adjusted for the age of partner. Analyses were performed for the entire AFB sample and also for subsets of women stratified by mean AFB (26 years) into younger AFB (<26) and older AFB (≥26).
Table 1.
The number of sample size and distribution of AFB across four cohorts.
| Cohort | Sample size | Mean Age (interview) | Range Age | Mean AFB | Range AFB |
|---|---|---|---|---|---|
| EGCUT | 1660 | 67.3 | 45–101 | 24.8 | 15–46 |
| LifeLines | 5344 | 53.1 | 27–93 | 26.7 | 16–43 |
| STR | 3262 | 58.2 | 45–86 | 25.1 | 14–52 |
| TwinsUK | 1981 | 55.4 | 25–86 | 25.7 | 14–44 |
Abbreviation: AFB, maternal age at first birth. EGCUT, the Estonian Genome Center Biobank, University of Tartu. STR, the Swedish Twin Registry.
RESULTS
We sought to provide insight to the relationship between maternal age and SCZ by exploring the genetic overlap between SCZ risk in women measured for AFB. We observed a U-shaped relationship between AFB and SCZ risk profile scores derived using GBLUP (Figure 1), consistent with the relationship reported by McGrath and colleagues (2014) between SCZ risk in offspring and maternal age not adjusted for father’s age. The mean risk profile score from GBLUP analysis was significantly higher in women with early AFB (i.e. < 20) than in women with intermediate AFB (p-value=9E-03 for 25≤AFB<30 and p-value=1E-02 for 30≤AFB<35) (eTable 2). The results from standard profile score were very similar to those from GBLUP but showed weaker signals (eFigure 5).
Figure 1.
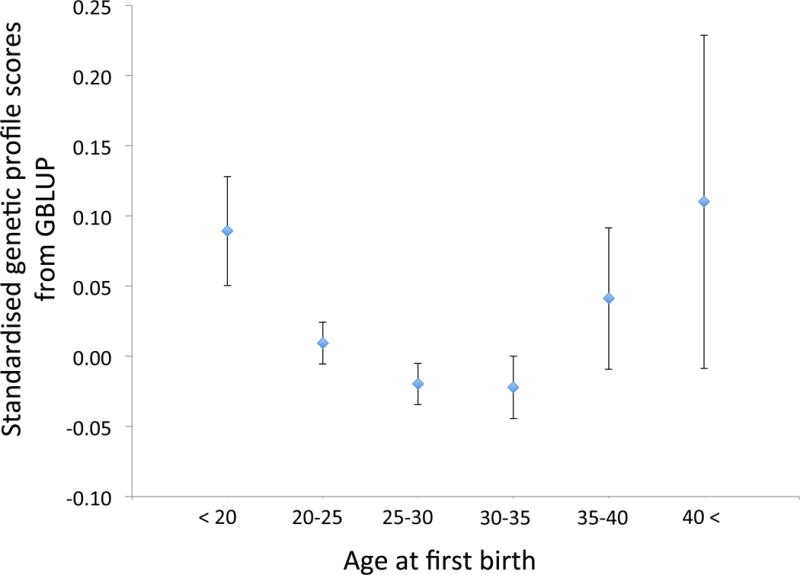
Mean SCZ profile scores from GBLUP in community samples of women (no ascertainment based on psychiatric disorders) grouped by age at birth of their first child. The error bars are SE.
We further investigated if SCZ genetic risk profiles significantly predicted the Model1 and/or Model2 response variables, which are the phenotypic predictors of SCZ risk derived from the results reported by McGrath et al (2014) (Table 2). We initially focused on a subsample of women aged ≥45 years at recruitment (N=10836), to avoid any potential bias owing to the inclusion of women who were childless at recruitment but still of childbearing age. In analyses of the full range of AFB in this sample, the Model1 and Model2 response variables were strongly predicted by GBLUP-derived SCZ risk profile scores (p-value=4E-04 and 5E-03 for Model1 and Model2, respectively) (Table 2). The smaller p-value for Model1 implies a better fit to the AFB data compared to the Model2, which is consistent with the suggestion that adjusting for paternal age removes a true association between maternal age and IRR for SCZ. When restricting the analysis to younger mothers whose AFB was less than the average (AFB<26), the p-value was 6E-04 and 5E-04 for Model1 and Model2, respectively (Table 2). On the other hand, assessing only older mothers (AFB≥26), GBLUP profile score weakly predicted Model1 (p-value=1E-02) but not Model2 (p-value= 2E-01) (Table 2). We repeated the analyses using the total sample of 12247 women and found almost identical results (eTable 3), suggesting very little if any bias arising from the inclusion of an additional 1411 women aged <45 years at recruitment. Again, analyses based on the profile score approach gave similar but weaker significance than GBLUP (eTable 4).
Table 2.
The coefficient of determination (R2) and p-valuea
| Data set | Response variable | R2 | p-valued |
|---|---|---|---|
| Full range of AFB (N=10836) |
Model1b | 1.1E-03 | 4.1E-04 |
| Model2c | 6.4e-04 | 5.0E-03 | |
| AFB < 26 (N=5827) |
Model1 | 1.8E-03 | 6.2E-04 |
| Model2 | 1.9E-03 | 5.1E-04 | |
| AFB >= 26 (N=5009) |
Model1 | 1.0E-03 | 1.4E-02 |
| Model2 | 6.5E-05 | 2.5E-01 |
Abbreviation: GBLUP, genomic best linear unbiased linear prediction. AFB age at birth in women.
In the analyses, either the Model1 or Model2 variables was used as the dependent variables in regression on GBLUP risk profile score using only women aged 45 years or over at recruitment. AFB was used as the x variable in this study. The analysed Model1 and Model2 variables were the residuals from a regression fitting potential confounders as covariates.
Model1: Polynomial function derived from incidence ratio for schizophrenia by maternal age (x) presented in McGrath et al (2014) (Model1 = 2.7214 + 0.0018x2 – 0.1105x).
Model2: polynomial function derived from incidence ratio for schizophrenia by maternal age adjusted for age of other parent and urbanization of place of birth (x) presented in McGrath et al (2014) (Model2 = 2.5438 + 0.0012x2 + 0.0889x).
Including genetic profile score for educational attainment as a covariate had little impact on these results (eTable 8).
P-value for H0: R2 = 0
In our primary analyses we excluded women without children, since the appropriate way to include such women in our analyses is difficult to determine. Delayed AFB and childlessness might have a common genetic predisposition or might be on distinct dimensions of liability. To explore this, we repeated analyses including women having no children, whose age at recruitment was 45 years or over. In this analysis, we assigned women having no children an AFB=45 years, which has been reported as the end of reproductive age19. Both response variables were predicted in analyses across the full range of AFB, but the significance of the prediction was considerably decreased, and neither response variable was predicted in analyses of women with AFB >=26 (eTable 5). These results must be interpreted with caution since there could be many reasons why women have no children, the most common being infertility which is likely to be genetically independent of AFB amongst those able to conceive.
In addition to analyses of the total AFB sample, we examined the relationship between SCZ risk profile scores from GBLUP and the Model1 and Model2 response variable in each cohort (eTable 6), acknowledging that due to smaller sample size, the power to detect a relationship was substantially reduced. For Estonia, risk profile scores were not significantly associated with AFB (see Discussion). However, a significant association remained in the other cohorts for at least one response variable. The prediction for LifeLines was significant for Model1 when using the whole range of AFB or AFB<26 years. For the Swedish Twin Registry (STR), it was significant only for Model1 in analyses of women with AFB≥26 years. For the UK, both response variables were predicted in analyses of the full range of AFB and AFB<26, but not in analyses of women with AFB≥26 (eTable 6). Due to smaller sample size, the U-shaped relationship observed between SCZ risk and AFB was less apparent in each cohort (eFigure 6).
We stratified the AFB sample into birth cohorts born before or after 1945, a demarcation based on the Second Demographic Transition that is linked in the literature to postponement of AFB20. For individuals born after 1945, SCZ risk profile scores from GBLUP significantly predicted AFB for the entire sample and the younger AFB group, but not for the older AFB group (eTable 7). For individuals born before 1945, SCZ risk profile scores from GBLUP did not significantly predict AFB (eTable 7).
DISCUSSION
Maternal age is a risk factor for a number of adverse mental health outcomes, including common psychiatric disorders such as schizophrenia21,22;34. In a recent study, McGrath et al. (2014)11 reported a U-shaped relationship between maternal age and risk of SCZ in children, although in analyses corrected for age of the other parent only children of younger mothers were at increased risk. Their study implied that risk of SCZ in children of older mothers was attributable to the father’s age, consistent with the hypothesis of risk arising from paternal age-related de novo mutations and the presence of a strong correlation between maternal and paternal age. However, since a larger proportion of older men have younger partners than is the case for older women, there is a possibility that correcting for age of spouse may remove a true effect due to advanced maternal age. In other words, the well-known problem of collinearity23-25, which can lead to biased estimates for explanatory variables that are adjusted for a correlated variable (e.g. maternal age adjusted for paternal age) may result in an underestimate of the risk due to older maternal age in analyses such as that reported by McGrath et al (2014). Our study uses a novel experimental design that is free of the effects of partner’s age, and therefore, there is no concern about such a collinearity problem in our analyses.
Maternal age is a proxy for factors other than parental age, including de novo chromosomal abnormalities, and psychosocial factors related to socioeconomic status and educational attainment, both of which are associated with risk of SCZ. Conversely, relatives of individuals affected with SCZ tend to have relatively poor social interactions26, which may increase the time taken to find a mate and thereby delay age at first birth. SCZ risk may also predispose to risk taking and impulsive behaviour, which is associated with early pregnancy and childbirth in females27-29. Consequently, risk of SCZ in offspring due to maternal AFB may be influenced by shared genetic factors between mothers and offspring, as has been suggested for paternal AFB7,8.
In this study, we investigated the genetic overlap between SCZ and AFB using a novel experimental design based on genomic data that enables genetic effects to be disentangled from other confounding factors. This is achieved through independently collected SCZ and AFB data sets that are genetically informative. The AFB data are collected from community samples with no known enrichment for psychiatric disorders. In particular, we sought to determine if response variables based on the relationships reported by McGrath et al. (2014) between maternal age and SCZ risk in children (Model1 and Model2) could be predicted by SCZ risk profile scores in our independent data. We found that SCZ risk profile scores predicted both Model1 and Model2, implying that polygenic variation for SCZ contributes to the relationships reported by McGrath et al. (2014) between maternal age and risk of SCZ.
Our results were stronger for the U-shaped Model1 response variable (based on unadjusted IRR) than Model2 (in which IRR was adjusted for paternal age and urbanization of place of birth), suggesting that polygenic variation contributes to risk of SCZ associated with both early and delayed maternal AFB. In analyses stratified by mean AFB, evidence for genetic overlap between SCZ and AFB was strongest in women with early AFB (AFB<26). In analyses of women with delayed AFB (AFB≥26 years), we observed an association with SCZ risk profile score, but only for the Model1 response based on unadjusted IRR. Given our methodology explores the relationship between AFB genetic risk to SCZ in a way that is independent of the correlation between the ages of the parents, our analyses suggest that risk of SCZ in children of older mothers is not solely attributable to factors associated with advanced paternal age.
A caveat of our findings is that the genetic association between risk of SCZ and delayed AFB were only marginally significant given the number of analyses performed, perhaps reflecting smaller sample size and correspondingly larger standard errors for women with delayed AFB, and so require replication in a larger sample. Provided this finding is confirmed in a future study, it has important implications because it implies that the risk of SCZ associated with increased age in mothers is not entirely due to the father’s age.
Although we demonstrate that SCZ genetic risk can be disentangled from psychosocial, lifestyle and/or biological ageing factors in assessing the relationship between SCZ risk and AFB, we cannot explicitly determine mechanism and it is possible that subtle correlations between genetic and non-genetic factors might influence this relationship. Several mechanisms such as correlation of SCZ risk with socioeconomic status and other environmental and biological factors might also influence the genetic relationship between SCZ and AFB. For instance, SCZ risk could predispose to impulsive behaviour 29 and substance use 27 that are associated with early pregnancy and childbirth 28. On the other hand, poor social competence in SCZ might delay the time to meet a partner and/or have offspring, causing delayed AFB. Other plausible mechanisms such as low IQ and poor educational attainment that are strong risk factors for both SCZ 30 and early pregnancy21 need to be explored although including genetic profile score for educational attainment as a covariate had little impact on our results (eTable 8). Assessing these factors is beyond the scope of this study. Future research including social science measures and information on the non-genetic factors will facilitate a deeper understanding of causal pathways underlying the association between SCZ and AFB.
The analyses of McGrath et al (2014) explored the relationship between parental age and a broad range of psychiatric disorders. Here, we have only considered genetic risk for schizophrenia because samples size, and hence power to estimate risk allele effect sizes, is greatest. For example, for SCZ 108 independent risk loci have been identified as genome-wide significant15, compared to only 3 for bipolar disorder (PGC-BPD, 2011)31. As GWAS sample sizes for other psychiatric disorders increase, then replication of our analyses based on these disorders will be possible.
In summary, this study provides evidence for a significant overlap between genetic factors associated with risk of SCZ and genetic factors associated with AFB. This is the first study to explore a genetic relationship between SCZ and AFB using independent unrelated samples based on genomic data. We conclude that women with high genetic predisposition to SCZ tend to have their first child at an early or later age.
Supplementary Material
Acknowledgments
This research is supported by the Australian National Health and Medical Research Council (grants 613602, 1047956, 1078901, 1080157, 1087889, 1067795) and the Australian Research Council (DE130100614). The research leading to these results received funding from the European Research Council via an ERC Consolidator Grant SOCIOGENOME (615603 awarded to M. Mills, see www.sociogenome.com). Felix C. Tropf additionally received funding from sciencestarter.de for the research visit to the University of Queensland. The TwinsUK study was funded by the Wellcome Trust; European Community’s Seventh Framework Programme (FP7/2007–2013).
EGCUT received targeted financing from Estonian Research Council grant IUT20-60, Center of Excellence in Genomics (EXCEGEN) and University of Tartu (SP1GVARENG). We acknowledge EGCUT technical personnel, especially Mr V. Soo and S. Smit. Data analyses were carried out in part in the High Performance Computing Center of University of Tartu.
TwinsUK. The study was funded by the Wellcome Trust; European Community’s Seventh Framework Programme (FP7/2007-2013). The study also receives support from the National Institute for Health Research (NIHR)- funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. SNP Genotyping was performed by The Wellcome Trust Sanger Institute and National Eye Institute via NIH/CIDR
The SNP&SEQ Technology Platform at the Uppsala University are acknowledged for performing genotyping and for excellent technical assistance. The Swedish Twin Registry is supported by Karolinska Institutet
Primary analyses of the Schizophrenia Working Group of the Psychiatric Genomics Consortium data were conducted on the Genetic Cluster Computer (http://www.geneticcluster.org) hosted by SURFsara and financially supported by the Netherlands Scientific Organization (NWO 480-05-003) along with a supplement from the Dutch Brain Foundation and the VU University Amsterdam.
The acknowledgements for Schizophrenia Working Group of the Psychiatric Genomics Consortium data are listed in reference #15 (and repeated in the Supplementary Material).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Lee and Mehta had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lee, Wray.
Acquisition, analysis, or interpretation of data: Lee, Wray, Mehta, Tropf, Mills, Gratten, McGrath, Kendler, Bakshi, Zhu, Hemani, Magnusson, Esko, Metspalu, Snieder.
Drafting of the manuscript: Lee, Mehta, Wray, Tropf, Gratten, Kendler.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Lee, Mehta.
Obtained funding: Lee, Wray.
Administrative, technical, or material support: All authors.
Study supervision: Lee.
Conflict of Interest Disclosures: None reported.
Data Availability Statement:
This study was conducted through approved data analysis applications made to the consortia involved. For ethical and legal reasons we are not allowed to distribute them. The data of Schizophrenia Working Group of the Psychiatric Genomics Consortium can be acessed through secondary analysis proposals see http://www.med.unc.edu/pgc/documents/documents-for-investigators#access. The TwinsUK data are available on request by contacting the Twin Research Unit at www.twinsuk.ac.uk/data-access/ submission-procedure. The data cannot be released without assessment by a steering committee with transfer agreements as the phenotypic data can be sensitive and may in some cases lead to the identification of the twins involved in the study. These procedures have been put in place by the local ethics committee and the Wellcome Trust. The LifeLines data are available by contacting the LifeLines Research Office (LLscience@umcg.nl)(https://www.lifelines.nl/lifelines-research/access-tolifelines/application-process). The data cannot be released without assessment by the LifeLines Scientific Committee with transfer agreements since the phenotypic data can be sensitive and has the potential in some cases to lead to the identification of individuals involved in the study. These procedures have been put in place by the LifeLines Scientific Board and local ethic committees. For more information see: Scholtens, S., N. Smidt, M.A. Swertz, S.J.J. Bakker, A. Dotinga, J.M. Vonk, F. Van Dijk, S.K.R. van Zon, C. Wijmenga, B.H.R. Wolffenbuttel & R. P. Stolk. (2014). Cohort Profile: LifeLines, a three-generation cohort study and biobank, International Journal of Epidemiology, 1-9.
References
- 1.Bassett AS, Bury A, Hodgkinson KA, Honer WG. Reproductive fitness in familial schizophrenia. Schizophr Res. 1996;21(3):151–160. doi: 10.1016/0920-9964(96)00018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacCabe JH, Koupil I, Leon DA. Lifetime reproductive output over two generations in patients with psychosis and their unaffected siblings: the Uppsala 1915–1929 Birth Cohort Multigenerational Study. Psychological medicine. 2009;39(10):1667–1676. doi: 10.1017/S0033291709005431. [DOI] [PubMed] [Google Scholar]
- 3.McGrath JJ, Hearle J, Jenner L, Plant K, Drummond A, Barkla JM. The fertility and fecundity of patients with psychoses. Acta psychiatrica Scandinavica. 1999;99(6):441–446. doi: 10.1111/j.1600-0447.1999.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 4.D’Onofrio BM, Rickert ME, Frans E, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA psychiatry. 2014;71(4):432–438. doi: 10.1001/jamapsychiatry.2013.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das M, Al-Hathal N, San-Gabriel M, et al. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. Journal of assisted reproduction and genetics. 2013;30(6):843–848. doi: 10.1007/s10815-013-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffe AE, Eaton WW, Straub RE, Marenco S, Weinberger DR. Paternal age, de novo mutations and schizophrenia. Mol Psychiatry. 2014;19(3):274–275. doi: 10.1038/mp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry. 2011;168(1):82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- 9.Eaton WW, Mortensen PB, Frydenberg M. Obstetric factors, urbanization and psychosis. Schizophr Res. 2000;43(2–3):117–123. doi: 10.1016/s0920-9964(99)00152-8. [DOI] [PubMed] [Google Scholar]
- 10.Hultman CM, Ohman A, Cnattingius S, Wieselgren IM, Lindstrom LH. Prenatal and neonatal risk factors for schizophrenia. The British journal of psychiatry : the journal of mental science. 1997;170:128–133. doi: 10.1192/bjp.170.2.128. [DOI] [PubMed] [Google Scholar]
- 11.McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA psychiatry. 2014;71(3):301–309. doi: 10.1001/jamapsychiatry.2013.4081. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Ripke S, Neale BM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tropf FC, Stulp G, Barban N, et al. Human fertility, molecular genetics, and natural selection in modern societies. PLoS One. 2015;10(6):e0126821. doi: 10.1371/journal.pone.0126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills M, Tropf F. The biodemography of fertility: A review and future research frontiers. Kölner Zeitschrift für Soziologie und Sozialpsychologie. 2015 doi: 10.1007/s11577-015-0319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier R, Moser G, Chen GB, et al. Joint analysis of psychiatric disorders increases accuracy of risk prediction for schizophrenia, bipolar disorder, and major depressive disorder. Am J Hum Genet. 2015;96(2):283–294. doi: 10.1016/j.ajhg.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, Middeldorp CM. Research Review: Polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry. 2014;55(10):1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- 19.Leridon H. A new estimate of permanent sterility by age: sterility defined as the inability to conceive. Population studies. 2008;62(1):15–24. doi: 10.1080/00324720701804207. [DOI] [PubMed] [Google Scholar]
- 20.Lesthaeghe R. The unfolding story of the second demographic transition. Population and development review. 2010;36(2):211–251. doi: 10.1111/j.1728-4457.2010.00328.x. [DOI] [PubMed] [Google Scholar]
- 21.Fergusson DM, Woodward LJ. Maternal age and educational and psychosocial outcomes in early adulthood. Journal of child psychology and psychiatry, and allied disciplines. 1999;40(3):479–489. [PubMed] [Google Scholar]
- 22.Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small- and large-for-gestational-age infants. American journal of obstetrics and gynecology. 2012;206(4):314, e311–319. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu YK, Kellett M, Clerehugh V, Gilthorpe MS. Problems of correlations between explanatory variables in multiple regression analyses in the dental literature. British dental journal. 2005;199(7):457–461. doi: 10.1038/sj.bdj.4812743. [DOI] [PubMed] [Google Scholar]
- 24.Belsley D. A Guide to using the collinearity diagnostics. Computer Science in Economics and Management. 1991;4(1):33–50. [Google Scholar]
- 25.Farrar DE, Glauber RR. Multicollinearity in Regression Analysis: The Problem Revisited. The Review of Economics and Statistics. 49:92–107. [Google Scholar]
- 26.Bellack AS, Morrison RL, Wixted JT, Mueser KT. An analysis of social competence in schizophrenia. The British journal of psychiatry : the journal of mental science. 1990;156:809–818. doi: 10.1192/bjp.156.6.809. [DOI] [PubMed] [Google Scholar]
- 27.Giordano GN, Ohlsson H, Sundquist K, Sundquist J, Kendler KS. The association between cannabis abuse and subsequent schizophrenia: a Swedish national co-relative control study. Psychological medicine. 2015;45(2):407–414. doi: 10.1017/S0033291714001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salas-Wright CP, Vaughn MG, Ugalde J, Todic J. Substance use and teen pregnancy in the United States: evidence from the NSDUH 2002-2012. Addictive behaviors. 2015;45:218–225. doi: 10.1016/j.addbeh.2015.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy LF, Lee J, Davis MC, et al. Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(2):456–463. doi: 10.1038/npp.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morra LF, Gold JM, Sullivan SK, Strauss GP. Predictors of neuropsychological effort test performance in schizophrenia. Schizophrenia research. 2015;162(1–3):205–210. doi: 10.1016/j.schres.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sklar P, Ripke S, Scott LJ, et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature Genetics. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


