Abstract
Disseminated intravascular coagulation (DIC) is an acquired condition that develops as a complication of systemic and sustained cell injury in conditions such as sepsis and trauma. It represents major dysregulation and increased thrombin generation in vivo. A poor understanding and recognition of the complex interactions in the coagulation, fibrinolytic, inflammatory, and innate immune pathways have resulted in continued poor management and high mortality rates in DIC. This review focuses attention on significant recent advances in our understanding of DIC pathophysiology. In particular, circulating histones and neutrophil extracellular traps fulfil established criteria in DIC pathogenesis. Both are damaging to the vasculature and highly relevant to the cross talk between coagulation and inflammation processes, which can culminate in adverse clinical outcomes. These molecules have a strong potential to be novel biomarkers and therapeutic targets in DIC, which is still considered synonymous with ‘death is coming’.
Keywords: DIC, neutrophil extracellular, Thrombin
Introduction
Disseminated intravascular coagulation (DIC) represents a major dysfunction in the hemostatic system, which is a physiological response to vascular injury. Upon injury, immediate interactions between components of the vessel wall and circulating blood lead to activation of the extrinsic and intrinsic pathways of coagulation to generate a burst of thrombin. Thrombin is immediately pro-coagulant in converting fibrinogen to fibrin but also mediates the anti-coagulant pathway by interacting with thrombomodulin (TM) to activate the protein C (PC) pathway. This controls the extent of localized clot formation, but when injury is of a systemic or sustained nature, regulation of thrombin generation is lost with adverse functional consequences. Owing to the ubiquitous nature of thrombin in affecting coagulation, fibrinolysis, and inflammation 1– 3, this can result in DIC and cause organ failure from microvascular thrombosis and endothelial barrier disruption.
This dynamic complexity in DIC pathogenesis is clinically important because its presence is well validated as an independent predictor of mortality 4. Development of DIC significantly increases risk of death beyond that of the underlying pathology. For example, DIC development in patients with sepsis and trauma doubles the risk of mortality 5. Despite this awareness, and the fact that its acronym could stand for ‘death is coming’, it remains poorly recognized by critical care clinicians and poorly managed because of the lack of high-quality evidence. DIC diagnosis is based on scoring a number of hemostatic parameters 6. Although measurements of the prothrombin time, fibrinogen, platelets, and fibrin-related products are generally available, changes in all of these parameters may not occur at the same time and can delay recognition and diagnosis. In some critical care settings, not all of these tests might be requested. Together with the lack of understanding that the manifestation of DIC can vary depending on primary disease-specific drivers of thrombin generation in causing multi-organ failure, this has resulted in poor management and mortality rates of 50% 7.
With the overall aim of improving our understanding of how DIC contributes to adverse clinical outcomes, this review will build upon key criteria in DIC. These were set out by the International Society on Thrombosis and Hemostasis (ISTH) Scientific and Standardization Sub-Committee (SSC) in describing how DIC can arise from the vasculature but also cause damage to the vasculature 4. Furthermore, the direct coupling of inflammation to coagulation processes facilitates the development of organ dysfunction. Specifically in this review, we will examine how extracellular histones and neutrophil extracellular traps (NETs) fit the DIC criteria and key principles in our understanding of DIC pathogenesis. Insight into this rapidly growing area of research could pave the way for improved clinician understanding and better approaches to manage the patient with DIC.
Extracellular histones and neutrophil extracellular traps
While the intra-nuclear function of histones as proteins that package DNA into nucleosomes has been well understood 7, 8, it is their role when released extracellularly upon cellular damage or death that is of interest and relevance to DIC. The cytotoxicity of extracellular histones, whereby their neutralization in sepsis models with anti-histone antibodies or activated PC (APC) conveyed survival benefit were first described in 2009 9. This discovery, followed by further in vitro and in vivo work, has translated into studies in patients with sepsis, trauma, and pancreatitis to illustrate the clinical relevance of extracellular histones and histone-DNA complexes (nucleosomes) to systemic inflammation, microvascular thrombosis 10– 14, organ injury 10, 11, 15– 18, and death. Extracellular histones may be found in the circulation (either free or complexed with DNA ‘nucleosomes’) or localized (and modified) as part of the extracellular traps released upon damage or activation of nucleated cells, primarily neutrophils. The various roles of neutrophils as key modulators of the complex interaction between innate immunity, inflammation, and coagulation (also known as ‘immunothrombosis’) are increasingly recognized and were recently well reviewed by Stiel et al. 19. The focus of this review is on one aspect of neutrophil contribution; which is mediation by NETs.
NETs were first described in 2004 as a neutrophil-derived amalgam of elastases, histones, and DNA that collectively trap and kill bacteria 20. Like histones, NETs have important physiological but potential pathological manifestations. Their uncontrolled or inappropriate release by neutrophils can contribute to the pathogenesis of sepsis, micro- and macro-vascular thrombosis, and multiple organ injury 21– 26. Consequences of NETs are typically site-specific, but breakdown products, such as cell-free DNA (cfDNA) or DNA-myeloperoxidase (DNA-MPO) complexes, can be found in the circulation 27– 31. However, cfDNA can arise from damaged cells and not only from NETs 32. As such, high circulating cfDNA levels should not be assumed to correlate directly with in vivo NET formation. Furthermore, intact NETs are structurally different and functionally dependent on its associated contents both locally and when cleaved and present in the circulation 33, 34.
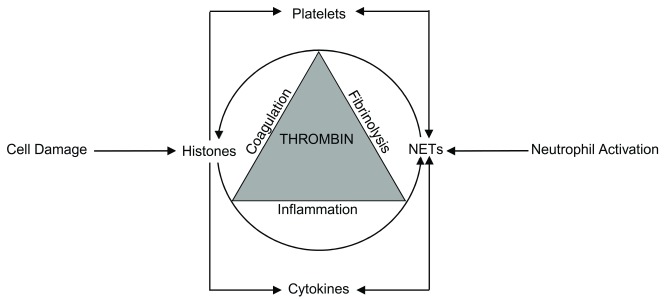
Importantly, there is a bi-directional relationship between NETs and histones ( Figure 1). First, NETs bear exposed histones (and numerous potent enzymes such as elastase) on their meshwork and therefore facilitate local histone-mediated cytotoxicity, pro-coagulant and pro-inflammatory effects. Histones can also be released from NETs into the circulation to disseminate its adverse effects 35, 36. Second, histones can directly stimulate neutrophils to form NETs 10, 37, 38 and therefore there is a vicious circle triggered by cellular injury that is then propagated by this bi-directional relationship between histones and NETs to promote further thrombin generation and contribute to DIC pathogenesis. These will be specifically detailed below in addressing how the various pathophysiological aspects of DIC can be contributed to by circulating histones and NETs.
Figure 1. Bi-directional relationship between histones and neutrophil extracellular traps (NETs).
Cell damage releases histones which trigger NET formation and the formed NETs are a source for both localized and systemic histone release. The increased thrombin generation, which is the hallmark of disseminated intravascular coagulation, simultaneously affects coagulation, fibrinolysis, and inflammation processes to amplify the reciprocal relationship between histones and NETs.
Relevance of histones and neutrophil extracellular traps to disseminated intravascular coagulation
The discussion will be divided into how histones and NETs contribute to the initiation, amplification, and propagation of coagulation activation ( Table 1).
Table 1. Mechanisms by which histones and neutrophil extracellular traps cause disseminated intravascular coagulation.
|
Triggering coagulation
• Tissue factor expression on endothelial cells and macrophages (mediated by Toll-like receptors and pro-inflammatory pathways: nuclear factor-kappa B and activator protein 1) 12, • Neutrophil extracellular traps bearing tissue factor 40– 42 • Pro-inflammatory cytokine release, production, and activation 10, 43– 48 • Cellular activation and injury, including platelets 9, 10, 13, 26, 49– 54 |
|
Amplifying coagulation
• Reduced endogenous anti-coagulant activity by consumption, liver damage, and extravascular extravasation 12, 16, 55– 59 • Intrinsic pathway activation 60– 63 • Impaired fibrinolysis 64– 66 |
|
Propagating coagulation
• Cytotoxic effects in circulation 9, 10, 15, 49, 59, 67– 69 • Circulating microparticle effects 70– 73 • Distant organ injury 9, 10, 15, 74 |
Factors that trigger coagulation in disseminated intravascular coagulation
Tissue factor expression. It is widely acceptable that the most important trigger of coagulation in sepsis and trauma-associated DIC is excessive tissue factor (TF) expression by circulating monocytes and its exposure from the vascular sub-endothelium following injury 75. This is supported by the observation that patients with DIC have significantly higher levels of circulating TF compared with controls 76. Exaggerated TF expression in septic and trauma DIC was historically attributed to systemic inflammation triggered by the invading microorganisms or their toxins (such as lipopolysaccharides) 39– 41. However, two recent studies have shown that extracellular histones can directly induce TF expression in a dose- and time-dependent manner on the surface of endothelial cells and macrophages via Toll-like receptor-4 (TLR-4) and TLR-2 and activation of the nuclear factor-kappa B (NF-κB) and activator protein 1 (AP-1) pathways 12, 42. With regard to NETs, recent studies have reported that NETs bear TF and contribute to thrombosis in myocardial infarction 77 and anti-neutrophil cytoplasmic antibody-associated vasculitis 78, 79. One study in the cancer setting reported significant interactions and correlations between NET components (primarily circulating DNA and nucleosomes) and TF-bearing microparticles culminating in overt DIC 43. Interestingly, both TF-bearing NETs and TF-bearing microparticles were found to be triggered by complement C5a 79, highlighting a significant interaction between the coagulant and innate immune systems that include complement in contributing to pathology.
Pro-inflammatory cytokine involvement. In sepsis and trauma, the DIC process is directly linked into, and triggered by, the systemic inflammatory host response, of which pro-inflammatory cytokines play critical roles beyond induction of TF expression 44. Excessive cytokine activities disrupt the fine balance and cross talk between coagulant, anti-coagulant, and inflammatory pathways to augment the pro-coagulant phenotype 44, 45. Histones can directly induce the release of several pro-inflammatory cytokines, including interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-alpha (TNF-α) 10, 46– 48. NETs act as scaffolds to potentiate IL production and activation 80– 82. Conversely, cytokines can induce NETosis 83 in a bi-directional relationship akin to that between histones and NETs ( Figure 1).
Platelet activation. Platelets are important for both the initial burst and further sustenance of thrombin generation by acting as scaffolds on which further coagulation activation takes place 84 and through platelet-derived polyphosphate activation of factor XI 85. Platelets also promote a pro-coagulant phenotype via P-selectin expression which enables adherence to the vascular endothelium and leukocytes while also augmenting TF expression 49 and phosphatidylserine exposure on monocytes 50, 51 which collectively enhances thrombin generation 51. Histones can directly induce platelet activation via calcium influx with subsequent platelet aggregation and consumption in vitro and in vivo 13, 52, 86. Histones can also promote thrombin generation in a platelet-dependent manner via P-selectin expression, phosphatidylserine exposure, and FV/Va availability on platelet surfaces 87. Histone-induced TF expression and subsequent thrombin generation can further activate platelets. In terms of clinical relevance, a case control study in intensive care patients recently illustrated a strong association between high histone levels and subsequent platelet consumption and thrombocytopenia to translate the findings of histone-induced thrombocytopenia in animal models 88. In parallel, NETs can interact with platelets to induce platelet aggregation, polyphosphate release, and subsequent thrombin generation to cause intravascular coagulation in septic mice 26. Conversely, platelets can directly induce NETosis 33, 89, 90 ( Figure 1) and contribute to pathology, including that of transfusion-associated acute lung injury 33, 90.
Vascular endothelial injury. The vascular endothelium, one of the biggest organs in the body, has a natural anti-coagulant surface mediated by the generation of APC 91, tissue factor pathway inhibitor (TFPI) 92, and expression of heparan sulfate and glycosaminoglycans that convey anti-thrombin (AT) activity 93. Endothelial cells also participate in fibrinolysis through release of tissue plasminogen activator (tPA) upon activation 94, 95. Therefore, damage or dysfunction of the vascular endothelium is another vital aspect of DIC pathogenesis. To this end, histone-induced toxicity on the vascular endothelium is well documented 9 and further extended in clinical studies with strong correlations observed between levels of circulating histones and soluble TM, a marker of endothelial cell injury, in critically ill patients 10. In addition, histones have been reported to induce the release of ultra-large von Willebrand factor (vWF) multimers 96, which are involved in platelet adhesion, platelet consumption, and microvascular thrombosis. Reports in patients with sepsis have indeed documented elevated levels of ultra-large multimers of vWF together with deficiency of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13), which is responsible for degradation of these multimers 53, 54, but the direct effect of histones on ADAMTS-13 is not currently known. As to NETs, a recent study showed that vascular endothelial cells have limited phagocytic capacity for NETs, which could result in poor NET clearance and subsequent damage to endothelial tight junctions to increase vascular permeability 97. Another study illustrated that matrix metalloproteinases within NETs contribute to endothelial dysfunction 67. Indeed, activated endothelial cells can also induce NETosis 98. As such, these histone- and NET-induced changes are also likely to play relevant roles in DIC pathogenesis.
A physical consequence of cellular injury induced by histones and NETs is the exposure of negatively charged phospholipid surfaces from membrane disruption 99. Availability of such phospholipid surfaces is highly pro-coagulant and can accelerate the prothrombinase reaction by 250,000-fold 100. In addition, histones can directly bind prothrombin and cause its auto-activation into thrombin. The implications would be that histones can directly generate thrombin without requiring coagulation activation 101. Although this reaction takes about eight hours and therefore may not be physiologically relevant, the available evidence points to the diversity in how histones directly contribute to thrombin generation and disseminate coagulation activation.
Factors that amplify coagulation in disseminated intravascular coagulation
Reduced endogenous anti-coagulant activity. The three endogenous anti-coagulant pathways—AT, PC, and TFPI—play a critical role in regulating the extent of clot generation. All of these pathways are significantly compromised in DIC. Declining trends in AT and PC levels can be used to identify patients in a non-overt stage of DIC before full decompensation 6. Low AT and PC levels have been validated as strong predictors of mortality in patients with sepsis and DIC 55, 102.
Although low levels of endogenous anti-coagulants can be due to pathological consumption by the excessive coagulopathy in DIC, other mechanisms also contribute to reduced activity and levels 103. Related to histones and NETs are two studies showing that histones can disrupt the PC pathway by downregulating TM 12 or dampening TM-dependent PC activation 56. This would impair APC anti-coagulant, anti-inflammatory, and cyto-protective functions 57, including its ability to proteolytically cleave histones 9. NET-associated elastases can potently degrade AT and TFPI 58– 60. In addition, two other potential mechanisms for endogenous anti-coagulant loss are reduced synthesis by the liver and loss to the extravascular space from enhanced vascular permeability 60. Histones can potentially contribute to both of these mechanisms by inducing liver injury and inflammation 16, 61 and significantly increasing vascular permeability through endothelial damage 9, 10.
Intrinsic pathway activation. In physiological terms, the intrinsic pathway of coagulation plays an important role in increasing thrombin generation and accelerating hemostatic clot formation. This is well exemplified by the significant bleeding issues in patients without factor VIII or IX. cfDNA- and NET-bound DNA can exert pro-coagulant effects through activating the intrinsic pathway of coagulation via FXI and FXII 62, 63. Likewise, histones can activate the intrinsic pathway through an FXII-dependent mechanism, and histone-DNA complexes significantly contribute to elevated FXII in patients with overt DIC 104. Indirectly, histone-induced release of platelet polyphosphate can stimulate factor XI auto-activation as well as accelerate its thrombin-mediated activation 105.
Impaired fibrinolysis. Impaired or excessive fibrinolysis is an important aspect of sepsis- and trauma-induced DIC, respectively 64,65, 106. All studies investigating the effects of histones, cfDNA, and NETs on the fibrinolytic system have consistently shown an overwhelmingly anti-fibrinolytic effect 66, 68, 107. This effect is mediated by enhanced clot resistance to fibrinolysis by plasmin and downregulation of plasminogen activation by tPA 66, 68, 107. As such, it appears that the effects of histones and NETs on the fibrinolytic system are relevant for sepsis-induced DIC but may not directly account for the hyper-fibrinolytic phenotype in trauma-associated DIC, although high histone levels in such patients may increase tPA release through significant endothelial stimulation and damage 69.
Factors that propagate coagulation in disseminated intravascular coagulation
The development of multiple organ injury further augments thrombin generation and dysfunction in DIC. Microvascular thrombosis can be triggered by the factors discussed above in leading to organ ischemia and failure. There is increasing understanding of the role mediated by circulating histones in particular. In addition to causing direct injury to endothelial and other hematopoietic cells 9, 10, 52, 99, histones have been shown to mediate distant organ injury and dysfunction. In mice models of trauma 10 and sepsis 15, histones are major mediators of lung and cardiac injury and dysfunction, respectively. The clinical relevance of these findings has also been demonstrated in cohorts of critically ill patients with trauma 10 and sepsis 15. The evidence for the distant organ-damaging properties of histones in trauma comes from the consequent development of acute lung injury after significant non-thoracic trauma. Translational relevance is supported by the increased development of acute lung injury in patients with severe non-thoracic trauma with high histone levels. Similarly, sepsis patients without pre-existing cardiac disease were significantly more likely to develop new-onset cardiac arrhythmia (nine-fold increase) and left ventricular dysfunction (two-fold increase) if they had high histone levels 15. Equally, circulating histones can mediate renal 108, liver 61, 109, and brain 109 injury. Notably, the incubation of plasma or serum from critically ill patients (with sepsis, trauma, or pancreatitis) with cultured endothelial cells or cardiomyocytes induces cell death, which can be prevented in the presence of an antibody to histones 10, 15.
Cellular damage is associated with microparticle formation and release 70, 71. Their pro-coagulant properties include TF expression 43, 78, 79, as discussed above. Circulating microparticles from damaged or activated hematopoietic cells also have exposed phosphatidylserine and these surfaces provide attachment sites for coagulation factors, which contribute to thrombotic complications in inflammatory disorders 72. Significantly high levels of microparticles from activated endothelial cells and neutrophils were recently demonstrated in septic shock-induced DIC patients in whom elevated levels of NET surrogate markers (for example, nucleosomes and circulating DNA-MPO complexes) were evident 73. These microparticles may have synergistic pro-coagulant effects with NETs 72 and prime neutrophils to undergo NETosis by facilitating a pro-inflammatory environment, including the release of pro-inflammatory cytokines 74, 110.
These histone-induced cytotoxic effects not only are a manifestation of micro- and macro-vascular thrombosis due to the pro-coagulant effects described above but also are from direct cytotoxicity mediated by histone binding to cellular membranes with consequent pore formation, calcium influx, and overload 10, 11, 52, 99, 111. Fattahi et al. have demonstrated that after histone infusion into mice, histones localize (in order of concentration) in the lungs, spleen, kidneys, plasma, liver, heart, and brain 112. As histones unravel from DNA binding as part of nucleosomes, their cytotoxicity becomes apparent because of their ability to bind cell membrane phospholipids. However, in intact nucleosomes, where histone binding sites are covered by DNA, no cytotoxicity could be elicited 86. Collectively, these data suggest that circulating histones in patients with sepsis- or trauma-associated DIC are major mediators of distant organ injury and adverse clinical outcome.
Summary and insights for the future
In this review, we have highlighted how extracellular histones and NETs fulfil the most important principles of DIC pathophysiology, as established by the ISTH SSC 4. First, histones can arise from endothelial cells following damage or from an exaggerated inflammatory response and in turn can mediate further significant damage to vascular endothelial cells. Directly and indirectly, histones can cause pro-inflammatory cytokine release and contribute to ‘inflammation gone amok’, as described in the ISTH communication 4. With the bi-directional relationship between histones and NETs, along with their functional consequences ( Figure 2), histones can be considered mediators of distant organ injury with NETs being the effectors of multi-organ failure.
Figure 2. Functional consequences of circulating histones and neutrophil extracellular traps (NETs).
Summary of pro-coagulant, anti-fibrinolytic, pro-inflammatory, and cytotoxic effects of histones and NETs. IL, interleukin; NF-κB, nuclear factor-kappa B; TF, tissue factor; TNF-α, tumor necrosis factor-alpha.
As to how these findings can translate into better recognition of DIC, there are a number of studies showing histone-DNA complexes as important prognosticators in patients with DIC 113, and their levels correlate with increasing DIC scores 114. As such, there appears to be potential in using these molecules as biomarkers in early DIC before full decompensation occurs. This could be a major advancement in the diagnosis and management of critically ill patients at risk of DIC. One reason for this is that the current recommendations rely on a panel of coagulation tests collectively forming the ‘DIC score’, which signifies that the phenomenon is already under way or indeed advanced (overt DIC) rather than presents a target for early therapeutic intervention. Furthermore, the scoring system can vary between different societies and countries (for example, ISTH score, the Japanese Association for Acute Medicine Criteria, and the Japanese Ministry of Health and Welfare score), and this impacts considerably on specificities/sensitivities of detection, stage of DIC (for example, overt or non-overt) identification, and prognostic values 90. Therefore, there is an area of unmet need for biomarkers that can better improve standardization in diagnosing DIC.
One difficulty facing the implementation of such biomarkers is that there is no simple, rapid test for quantifying histones that could be suitable for the acute hospital setting. Furthermore, there is controversy regarding the level of circulating histones; some papers quote levels in the microgram-per-milliliter range using Western blotting quantification 10, 15, 88, whereas others suggest that levels are in the nanogram-per-milliliter or picogram-per-milliliter range using enzyme-linked immunosorbent assay (ELISA) 13, 115, 116. From our experience, current ELISAs are not sufficiently specific for histone measurement in clinical samples and this is due to interference from other plasma proteins. The same issue applies to NET measurement in patient samples. Currently, most studies rely on measuring cfDNA, histone-DNA, and DNA-MPO complexes as surrogate markers of NET formation 27– 31. Although these assays are a good development, they are also associated with problems relating to specificity, especially when the cfDNA may be released from other dying cells and not necessarily from NETs. Recent studies have illustrated promising potential for the use of neutrophil side fluorescence as a marker of neutrophil chromatin decondensation (hence NETosis) in predicting DIC development in patients with septic shock 117. This new marker correlated significantly (yet with a weak correlation coefficient) with circulating nucleosomes and DNA-MPO complexes in patients with DIC 73. Standardization for histone and NET measurements using accurate high output techniques is therefore a pressing need.
Nonetheless, these are exciting challenges to overcome. There is the potential for novel therapeutic approaches using modalities that neutralize histones (APC 9, anti-histone antibodies 10, 11, 15, recombinant TM 13, and heparin 118) or NETs (DNase 20, ADAMTS-13 119, and PAD4-targeted therapy 34) or both. Many of these interventions are clearly anti-coagulant and could convey bleeding risk in patients with DIC if not used with caution. These would require well-designed randomized control trials using appropriate DIC patient populations (for example, with high circulating histones or NETs or both) as well as the potential for modified non-anti-coagulant versions as is the case with non-anti-coagulant heparins, which can neutralize circulating histones 118.
Abbreviations
ADAMTS-13, disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; APC, activated protein C; AT, anti-thrombin; cfDNA, cell-free DNA; DIC, disseminated intravascular coagulation; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; ISTH, International Society on Thrombosis and Hemostasis; MPO, myeloperoxidase; NET, neutrophil extracellular trap; PC, protein C; SSC, Scientific and Standardization Sub-Committee; TF, tissue factor; TFPI, tissue factor pathway inhibitor; TLR, Toll-like receptor; TM, thrombomodulin; tPA, tissue plasminogen activator; vWF, von Willebrand factor.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Julie Helms, Strasbourg University Hospital, Strasbourg, France
Craig N Jenne, Department of Microbiology, Immunology and Infectious Diseases, University of Calgary , Calgary, Canada
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Wang W, Boffa MB, Bajzar L, et al. : A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1998;273(42):27176–81. 10.1074/jbc.273.42.27176 [DOI] [PubMed] [Google Scholar]
- 2. Chen D, Dorling A: Critical roles for thrombin in acute and chronic inflammation. J Thromb Haemost. 2009;7 Suppl 1:122–6. 10.1111/j.1538-7836.2009.03413.x [DOI] [PubMed] [Google Scholar]
- 3. Narayanan S: Multifunctional roles of thrombin. Ann Clin Lab Sci. 1999;29(4):275–80. [PubMed] [Google Scholar]
- 4. Taylor FB, Jr, Toh CH, Hoots WK, et al. : Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30. [PubMed] [Google Scholar]
- 5. Fourrier F, Chopin C, Goudemand J, et al. : Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest. 1992;101(3):816–23. 10.1378/chest.101.3.816 [DOI] [PubMed] [Google Scholar]
- 6. Toh CH, Hoots WK, SSC on Disseminated Intravascular Coagulation of the ISTH: The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007;5(3):604–6. 10.1111/j.1538-7836.2007.02313.x [DOI] [PubMed] [Google Scholar]
- 7. Smith MM: Histone structure and function. Curr Opin Cell Biol. 1991;3(3):429–37. 10.1016/0955-0674(91)90070-F [DOI] [PubMed] [Google Scholar]
- 8. Kornberg RD, Lorch Y: Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98(3):285–94. 10.1016/S0092-8674(00)81958-3 [DOI] [PubMed] [Google Scholar]
- 9. Xu J, Zhang X, Pelayo R, et al. : Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–21. 10.1038/nm.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Abrams ST, Zhang N, Manson J, et al. : Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187(2):160–9. 10.1164/rccm.201206-1037OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alhamdi Y, Zi M, Abrams ST, et al. : Circulating Histone Concentrations Differentially Affect the Predominance of Left or Right Ventricular Dysfunction in Critical Illness. Crit Care Med. 2016;44(5):e278–88. 10.1097/CCM.0000000000001413 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Kim JE, Yoo HJ, Gu JY, et al. : Histones Induce the Procoagulant Phenotype of Endothelial Cells through Tissue Factor Up-Regulation and Thrombomodulin Down-Regulation. PLoS One. 2016;11(6):e0156763. 10.1371/journal.pone.0156763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakahara M, Ito T, Kawahara K, et al. : Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One. 2013;8(9):e75961. 10.1371/journal.pone.0075961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alhamdi Y, Toh CH: The role of extracellular histones in haematological disorders. Br J Haematol. 2016;173(5):805–11. 10.1111/bjh.14077 [DOI] [PubMed] [Google Scholar]
- 15. Alhamdi Y, Abrams ST, Cheng Z, et al. : Circulating Histones Are Major Mediators of Cardiac Injury in Patients With Sepsis. Crit Care Med. 2015;43(10):2094–103. 10.1097/CCM.0000000000001162 [DOI] [PubMed] [Google Scholar]
- 16. Wen Z, Lei Z, Yao L, et al. : Circulating histones are major mediators of systemic inflammation and cellular injury in patients with acute liver failure. Cell Death Dis. 2016;7(9):e2391. 10.1038/cddis.2016.303 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Liu T, Huang W, Szatmary P, et al. : Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br J Surg. 2017;104(9):1215–25. 10.1002/bjs.10538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allam R, Kumar SV, Darisipudi MN, et al. : Extracellular histones in tissue injury and inflammation. J Mol Med. 2014;92(5):465–72. 10.1007/s00109-014-1148-z [DOI] [PubMed] [Google Scholar]
- 19. Stiel L, Meziani F, Helms J: Neutrophil activation during septic shock. Shock. 2017. 10.1097/SHK.0000000000000980 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Brinkmann V, Reichard U, Goosmann C, et al. : Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Kaplan MJ, Radic M: Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189(6):2689–95. 10.4049/jimmunol.1201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korkmaz HI, Ulrich MMW, Vogels S, et al. : Neutrophil extracellular traps coincide with a pro-coagulant status of microcirculatory endothelium in burn wounds. Wound Repair Regen. 2017;25(4):609–17. 10.1111/wrr.12560 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Gloude NJ, Khandelwal P, Luebbering N, et al. : Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood. 2017;130(10):1259–66. 10.1182/blood-2017-05-782870 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Laridan E, Denorme F, Desender L, et al. : Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82(2):223–32. 10.1002/ana.24993 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Dicker AJ, Crichton ML, Pumphrey EG, et al. : Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2017; pii: S0091-6749(17)30746-7. 10.1016/j.jaci.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. McDonald B, Davis RP, Kim SJ, et al. : Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129(10):1357–67. 10.1182/blood-2016-09-741298 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Sil P, Yoo D, Floyd M, et al. : High Throughput Measurement of Extracellular DNA Release and Quantitative NET Formation in Human Neutrophils In Vitro. J Vis Exp. 2016; (112): e52779. 10.3791/52779 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Hashiba M, Huq A, Tomino A, et al. : Neutrophil extracellular traps in patients with sepsis. J Surg Res. 2015;194(1):248–54. 10.1016/j.jss.2014.09.033 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Kraaij T, Tengström FC, Kamerling SW, et al. : A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun Rev. 2016;15(6):577–84. 10.1016/j.autrev.2016.02.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Marin Oyarzún CP, Carestia A, Lev PR, et al. : Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci Rep. 2016;6:38738. 10.1038/srep38738 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Arai Y, Yamashita K, Mizugishi K, et al. : Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(12):1683–9. 10.1016/j.bbmt.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 32. Choi JJ, Reich CF, 3rd, Pisetsky DS: Release of DNA from dead and dying lymphocyte and monocyte cell lines in vitro. Scand J Immunol. 2004;60(1–2):159–66. 10.1111/j.0300-9475.2004.01470.x [DOI] [PubMed] [Google Scholar]
- 33. Caudrillier A, Kessenbrock K, Gilliss BM, et al. : Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–71. 10.1172/JCI61303 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Kolaczkowska E, Jenne CN, Surewaard BG, et al. : Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. 10.1038/ncomms7673 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Kumar SV, Kulkarni OP, Mulay SR, et al. : Neutrophil Extracellular Trap-Related Extracellular Histones Cause Vascular Necrosis in Severe GN. J Am Soc Nephrol. 2015;26(10):2399–413. 10.1681/ASN.2014070673 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Saffarzadeh M, Juenemann C, Queisser MA, et al. : Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2):e32366. 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakazawa D, Kumar SV, Marschner J, et al. : Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI. J Am Soc Nephrol. 2017;28(6):1753–68. 10.1681/ASN.2016080925 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Huang H, Tohme S, Al-Khafaji AB, et al. : Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62(2):600–14. 10.1002/hep.27841 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Aras O, Shet A, Bach RR, et al. : Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103(12):4545–53. 10.1182/blood-2003-03-0713 [DOI] [PubMed] [Google Scholar]
- 40. Franco RF, de Jonge E, Dekkers PE, et al. : The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96(2):554–9. [PubMed] [Google Scholar]
- 41. Osterud B, Flaegstad T: Increased tissue thromboplastin activity in monocytes of patients with meningococcal infection: related to an unfavourable prognosis. Thromb Haemost. 1983;49(1):5–7. [PubMed] [Google Scholar]
- 42. Yang X, Li L, Liu J, et al. : Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-κB and AP-1. Thromb Res. 2016;137:211–8. 10.1016/j.thromres.2015.10.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Hell L, Thaler J, Martinod K, et al. : OC-16 - Neutrophil extracellular traps and tissue factor-bearing microvesicles: a liaison dangereuse causing overt DIC in cancer patients? Thromb Res. 2016;140 Suppl 1:S174–5. 10.1016/S0049-3848(16)30133-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Levi M, van der Poll T, ten Cate H, et al. : The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27(1):3–9. 10.1046/j.1365-2362.1997.570614.x [DOI] [PubMed] [Google Scholar]
- 45. van der Poll T, de Jonge E, Levi M: Regulatory role of cytokines in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27(6):639–51. 10.1055/s-2001-18868 [DOI] [PubMed] [Google Scholar]
- 46. Xu J, Zhang X, Monestier M, et al. : Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187(5):2626–31. 10.4049/jimmunol.1003930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Allam R, Darisipudi MN, Tschopp J, et al. : Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol. 2013;43(12):3336–42. 10.1002/eji.201243224 [DOI] [PubMed] [Google Scholar]
- 48. Bosmann M, Grailer JJ, Ruemmler R, et al. : Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27(12):5010–21. 10.1096/fj.13-236380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Celi A, Pellegrini G, Lorenzet R, et al. : P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci U S A. 1994;91(19):8767–71. 10.1073/pnas.91.19.8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shebuski RJ, Kilgore KS: Role of inflammatory mediators in thrombogenesis. J Pharmacol Exp Ther. 2002;300(3):729–35. 10.1124/jpet.300.3.729 [DOI] [PubMed] [Google Scholar]
- 51. del Conde I, Nabi F, Tonda R, et al. : Effect of P-selectin on phosphatidylserine exposure and surface-dependent thrombin generation on monocytes. Arterioscler Thromb Vasc Biol. 2005;25(5):1065–70. 10.1161/01.ATV.0000159094.17235.9b [DOI] [PubMed] [Google Scholar]
- 52. Fuchs TA, Bhandari AA, Wagner DD: Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118(13):3708–14. 10.1182/blood-2011-01-332676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bockmeyer CL, Claus RA, Budde U, et al. : Inflammation-associated ADAMTS13 deficiency promotes formation of ultra-large von Willebrand factor. Haematologica. 2008;93(1):137–40. 10.3324/haematol.11677 [DOI] [PubMed] [Google Scholar]
- 54. Kremer Hovinga JA, Zeerleder S, Kessler P, et al. : ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007;5(11):2284–90. 10.1111/j.1538-7836.2007.02743.x [DOI] [PubMed] [Google Scholar]
- 55. Fijnvandraat K, Derkx B, Peters M, et al. : Coagulation activation and tissue necrosis in meningococcal septic shock: severely reduced protein C levels predict a high mortality. Thromb Haemost. 1995;73(1):15–20. [PubMed] [Google Scholar]
- 56. Ammollo CT, Semeraro F, Xu J, et al. : Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9(9):1795–803. 10.1111/j.1538-7836.2011.04422.x [DOI] [PubMed] [Google Scholar]
- 57. Bouwens EA, Stavenuiter F, Mosnier LO: Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost. 2013;11 Suppl 1:242–53. 10.1111/jth.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Levi M, van der Poll T: Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–34. 10.1097/CCM.0b013e3181c98d21 [DOI] [PubMed] [Google Scholar]
- 59. Massberg S, Grahl L, von Bruehl ML, et al. : Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887–96. 10.1038/nm.2184 [DOI] [PubMed] [Google Scholar]
- 60. Gando S, Levi M, Toh CH: Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2: 16037. 10.1038/nrdp.2016.37 [DOI] [PubMed] [Google Scholar]
- 61. Wen Z, Liu Y, Li F, et al. : Circulating histones exacerbate inflammation in mice with acute liver failure. J Cell Biochem. 2013;114(10):2384–91. 10.1002/jcb.24588 [DOI] [PubMed] [Google Scholar]
- 62. Swystun LL, Mukherjee S, Liaw PC: Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9(11):2313–21. 10.1111/j.1538-7836.2011.04465.x [DOI] [PubMed] [Google Scholar]
- 63. Kannemeier C, Shibamiya A, Nakazawa F, et al. : Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104(15):6388–93. 10.1073/pnas.0608647104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gando S, Sawamura A, Hayakawa M: Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann Surg. 2011;254(1):10–9. 10.1097/SLA.0b013e31821221b1 [DOI] [PubMed] [Google Scholar]
- 65. Hayakawa M: Pathophysiology of trauma-induced coagulopathy: disseminated intravascular coagulation with the fibrinolytic phenotype. J Intensive Care. 2017;5:14. 10.1186/s40560-016-0200-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Varjú I, Longstaff C, Szabó L, et al. : DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb Haemost. 2015;113(6):1289–98. 10.1160/TH14-08-0669 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Carmona-Rivera C, Zhao W, Yalavarthi S, et al. : Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74(7):1417–24. 10.1136/annrheumdis-2013-204837 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Gould TJ, Vu TT, Stafford AR, et al. : Cell-Free DNA Modulates Clot Structure and Impairs Fibrinolysis in Sepsis. Arterioscler Thromb Vasc Biol. 2015;35(12):2544–53. 10.1161/ATVBAHA.115.306035 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Levin EG, Marzec U, Anderson J, et al. : Thrombin stimulates tissue plasminogen activator release from cultured human endothelial cells. J Clin Invest. 1984;74(6):1988–95. 10.1172/JCI111620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoyer FF, Nickenig G, Werner N: Microparticles--messengers of biological information. J Cell Mol Med. 2010;14(9):2250–6. 10.1111/j.1582-4934.2010.01114.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Deng F, Wang S, Zhang L: Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: a literature review. J Cell Mol Med. 2017;21(9):1698–710. 10.1111/jcmm.13125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. He Z, Si Y, Jiang T, et al. : Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb Haemost. 2016;115(4):738–51. 10.1160/TH15-09-0710 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Delabranche X, Stiel L, Severac F, et al. : Evidence of Netosis in Septic Shock-Induced Disseminated Intravascular Coagulation. Shock. 2017;47(3):313–7. 10.1097/SHK.0000000000000719 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Gupta AK, Hasler P, Holzgreve W, et al. : Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66(11):1146–54. 10.1016/j.humimm.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 75. Østerud B, Bjørklid E: The tissue factor pathway in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27(6):605–17. 10.1055/s-2001-18866 [DOI] [PubMed] [Google Scholar]
- 76. Shimura M, Wada H, Wakita Y, et al. : Plasma tissue factor and tissue factor pathway inhibitor levels in patients with disseminated intravascular coagulation. Am J Hematol. 1997;55(4):169–74. [DOI] [PubMed] [Google Scholar]
- 77. Stakos DA, Kambas K, Konstantinidis T, et al. : Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36(22):1405–14. 10.1093/eurheartj/ehv007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. : Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73(10):1854–63. 10.1136/annrheumdis-2013-203430 [DOI] [PubMed] [Google Scholar]
- 79. Huang YM, Wang H, Wang C, et al. : Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 2015;67(10):2780–90. 10.1002/art.39239 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Clancy DM, Henry CM, Sullivan GP, et al. : Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines. FEBS J. 2017;284(11):1712–25. 10.1111/febs.14075 [DOI] [PubMed] [Google Scholar]
- 81. Hu Z, Murakami T, Tamura H, et al. : Neutrophil extracellular traps induce IL-1β production by macrophages in combination with lipopolysaccharide. Int J Mol Med. 2017. 10.3892/ijmm.2017.2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Keshari RS, Jyoti A, Kumar S, et al. : Neutrophil extracellular traps contain mitochondrial as well as nuclear DNA and exhibit inflammatory potential. Cytometry A. 2012;81(3):238–47. 10.1002/cyto.a.21178 [DOI] [PubMed] [Google Scholar]
- 83. Keshari RS, Jyoti A, Dubey M, et al. : Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 2012;7(10):e48111. 10.1371/journal.pone.0048111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Versteeg HH, Heemskerk JW, Levi M, et al. : New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–58. 10.1152/physrev.00016.2011 [DOI] [PubMed] [Google Scholar]
- 85. Müller F, Mutch NJ, Schenk WA, et al. : Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143–56. 10.1016/j.cell.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Abrams ST, Zhang N, Dart C, et al. : Human CRP defends against the toxicity of circulating histones. J Immunol. 2013;191(5):2495–502. 10.4049/jimmunol.1203181 [DOI] [PubMed] [Google Scholar]
- 87. Semeraro F, Ammollo CT, Morrissey JH, et al. : Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–61. 10.1182/blood-2011-03-343061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alhamdi Y, Abrams ST, Lane S, et al. : Histone-Associated Thrombocytopenia in Patients Who Are Critically Ill. JAMA. 2016;315(8):817–9. 10.1001/jama.2016.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Clark SR, Ma AC, Tavener SA, et al. : Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463–9. 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- 90. Carestia A, Kaufman T, Schattner M: Platelets: New Bricks in the Building of Neutrophil Extracellular Traps. Front Immunol. 2016;7:271. 10.3389/fimmu.2016.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Esmon CT: The endothelial cell protein C receptor. Thromb Haemost. 2000;83(5):639–43. [PubMed] [Google Scholar]
- 92. Broze GJ, Jr: Tissue factor pathway inhibitor. Thromb Haemost. 1995;74(1):90–3. [PubMed] [Google Scholar]
- 93. Bombeli T, Mueller M, Haeberli A: Anticoagulant properties of the vascular endothelium. Thromb Haemost. 1997;77(3):408–23. [PubMed] [Google Scholar]
- 94. van den Eijnden-Schrauwen Y, Kooistra T, de Vries RE, et al. : Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995;85(12):3510–7. [PubMed] [Google Scholar]
- 95. Suzuki Y, Mogami H, Ihara H, et al. : Unique secretory dynamics of tissue plasminogen activator and its modulation by plasminogen activator inhibitor-1 in vascular endothelial cells. Blood. 2009;113(2):470–8. 10.1182/blood-2008-03-144279 [DOI] [PubMed] [Google Scholar]
- 96. Lam FW, Cruz MA, Parikh K, et al. : Histones stimulate von Willebrand factor release in vitro and in vivo. Haematologica. 2016;101(7):e277–9. 10.3324/haematol.2015.140632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pieterse E, Rother N, Garsen M, et al. : Neutrophil Extracellular Traps Drive Endothelial-to-Mesenchymal Transition. Arterioscler Thromb Vasc Biol. 2017;37(7):1371–9. 10.1161/ATVBAHA.117.309002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Gupta AK, Joshi MB, Philippova M, et al. : Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584(14):3193–7. 10.1016/j.febslet.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 99. Semeraro F, Ammollo CT, Esmon NL, et al. : Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J Thromb Haemost. 2014;12(10):1697–702. 10.1111/jth.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nesheim ME, Tracy RP, Mann KG: “Clotspeed,” a mathematical simulation of the functional properties of prothrombinase. J Biol Chem. 1984;259(3):1447–53. [PubMed] [Google Scholar]
- 101. Barranco-Medina S, Pozzi N, Vogt AD, et al. : Histone H4 promotes prothrombin autoactivation. J Biol Chem. 2013;288(50):35749–57. 10.1074/jbc.M113.509786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Choi Q, Hong KH, Kim JE, et al. : Changes in plasma levels of natural anticoagulants in disseminated intravascular coagulation: high prognostic value of antithrombin and protein C in patients with underlying sepsis or severe infection. Ann Lab Med. 2014;34(2):85–91. 10.3343/alm.2014.34.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Asakura H, Ontachi Y, Mizutani T, et al. : Decreased plasma activity of antithrombin or protein C is not due to consumption coagulopathy in septic patients with disseminated intravascular coagulation. Eur J Haematol. 2001;67(3):170–5. 10.1034/j.1600-0609.2001.5790508.x [DOI] [PubMed] [Google Scholar]
- 104. Park HS, Gu J, You HJ, et al. : Factor XII-mediated contact activation related to poor prognosis in disseminated intravascular coagulation. Thromb Res. 2016;138:103–7. 10.1016/j.thromres.2015.12.011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Morrissey JH, Smith SA: Polyphosphate as modulator of hemostasis, thrombosis, and inflammation. J Thromb Haemost. 2015;13 Suppl 1:S92–7. 10.1111/jth.12896 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Asakura H: Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2(1):20. 10.1186/2052-0492-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Longstaff C, Varjú I, Sótonyi P, et al. : Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288(10):6946–56. 10.1074/jbc.M112.404301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Allam R, Scherbaum CR, Darisipudi MN, et al. : Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23(8):1375–88. 10.1681/ASN.2011111077 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. Yang R, Zou X, Tenhunen J, et al. : HMGB1 and Extracellular Histones Significantly Contribute to Systemic Inflammation and Multiple Organ Failure in Acute Liver Failure. Mediators Inflamm. 2017;2017:5928078. 10.1155/2017/5928078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dieker J, Tel J, Pieterse E, et al. : Circulating Apoptotic Microparticles in Systemic Lupus Erythematosus Patients Drive the Activation of Dendritic Cell Subsets and Prime Neutrophils for NETosis. Arthritis Rheumatol. 2016;68(2):462–72. 10.1002/art.39417 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 111. Gamberucci A, Fulceri R, Marcolongo P, et al. : Histones and basic polypeptides activate Ca2+/cation influx in various cell types. Biochem J. 1998;331(Pt 2):623–30. 10.1042/bj3310623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fattahi F, Grailer JJ, Jajou L, et al. : Organ distribution of histones after intravenous infusion of FITC histones or after sepsis. Immunol Res. 2015;61(3):177–86. 10.1007/s12026-015-8628-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 113. Kim JE, Lee N, Gu JY, et al. : Circulating levels of DNA-histone complex and dsDNA are independent prognostic factors of disseminated intravascular coagulation. Thromb Res. 2015;135(6):1064–9. 10.1016/j.thromres.2015.03.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 114. Walborn A, Patel P, Hoppensteadt D, et al. : Extracellular Nucleosome Levels in the Etiopathogenesis of Sepsis Associated Coagulopathy. Am Soc Hematology. Blood. 2016;128:564 Reference Source [Google Scholar]
- 115. Ekaney ML, Otto GP, Sossdorf M, et al. : Impact of plasma histones in human sepsis and their contribution to cellular injury and inflammation. Crit Care. 2014;18(5):543. 10.1186/s13054-014-0543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gao H, Zhang N, Lu F, et al. : Circulating histones for predicting prognosis after cardiac surgery: a prospective study. Interact Cardiovasc Thorac Surg. 2016;23(5):681–7. 10.1093/icvts/ivw198 [DOI] [PubMed] [Google Scholar]
- 117. Stiel L, Delabranche X, Galoisy AC, et al. : Neutrophil Fluorescence: A New Indicator of Cell Activation During Septic Shock-Induced Disseminated Intravascular Coagulation. Crit Care Med. 2016;44(11):e1132–e1136. 10.1097/CCM.0000000000001851 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 118. Wildhagen KC, García de Frutos P, Reutelingsperger CP, et al. : Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood. 2014;123(7):1098–101. 10.1182/blood-2013-07-514984 [DOI] [PubMed] [Google Scholar]
- 119. Savchenko AS, Borissoff JI, Martinod K, et al. : VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood. 2014;123(1):141–8. 10.1182/blood-2013-07-514992 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation