Abstract
The demand for synthetic biomaterials in medical devices, pharmaceutical products and, tissue replacement applications are growing steadily due to ageing population worldwide. The use for patient matched devices is also increasing due to availability and integration of new technologies. Applications of additive manufacturing (AM) or 3D printing (3DP) in biomaterials have also increased significantly over the past decade towards traditional as well as innovative next generation Class I, II and III devices. In this review, we have focused our attention towards the use of AM in surface modified biomaterials to enhance their in vitro and in vivo performances. Specifically, we have discussed the use of AM to deliberately modify the surfaces of different classes of biomaterials with spatial specificity in a single manufacturing process as well as commented on the future outlook towards surface modification using AM.
Keywords: Additive manufacturing, 3D Printing, Biomaterials, Surface modification
Graphical abstract
The demand for synthetic biomaterials in medical devices, pharmaceutical products and, tissue replacement applications are growing steadily due to ageing population worldwide. The use for patient matched devices are also increasing due to availability and integration of new technologies. Applications of additive manufacturing (AM) or 3D printing (3DP) in biomaterials have also increased significantly over the past decade towards traditional as well as innovative next generation Class I, II and III devices. In this review, we have focused our attention towards the use of AM in surface modified biomaterials to enhance their in vitro and in vivo performances. Specifically, we have discussed the use of AM to deliberately modify the surfaces of different classes of biomaterials with spatial specificity in a single manufacturing process as well as commented on the future outlook towards surface modification using AM.
1. Introduction
Since its advent in the mid 80’s, rapid prototyping (RP) or solid freeform fabrication (SFF) has gained significant interest in both research and industrial applications for biomedical and pharmaceutical products. Researchers and product designers use these technologies to quickly test and iterate prototypes designs; to model with a high degree of customization. While prototyping is almost always a low-throughput phase of development, there is a push to bring RP to its full manufacturing potential. The idea that full production can be done with RP is defined as additive manufacturing (AM). The distinction is that an AM part not only mimic the shape and size, but also has the mechanical and chemical properties to be functional for end use.
In early industry applications, AM techniques were employed as a way for manufacturers to quickly produce prototypes prior to expensive retooling or slow tool room design [1]. More recently, AM has gained interest as a way to create novel or patient-specific biomedical devices in fields such as biotechnology, healthcare, and pharmaceuticals. New designs and modifications of implants, prostheses, drug delivery systems (DDS) that were previously impossible to manufacture under traditional methods are quickly becoming available with the advancement of AM [2].
1.1. Aim of Surface Modification
While bulk properties may initially determine material suitability for an application, the physical aspects of the material surface as well as the chemistry are paramount to the function of many biomedical devices. Surface modification falls under two categories: physical and chemical. Physical modification results in a change in the topography or morphology of the surface with little to no change in the chemistry, such as etching, grit-blasting, and machining. Well-established chemical techniques include plasma and chemical vapor deposition, atomic layer depostion, and electro-chemical deposition [3], [4], [5], [6]. Chemical treatment can result in oxideing/nitriding/carbiding a surface, surface functionalization, ion infusion, single layer coatings, or coatings comprising many layers of different compositions [7], [8], [9]. The goal of modifying the surface of a biomaterial is to create a specific chemical and physical environment that offers a favorable cellular response in hard or soft tissue. In cases where tissue integration is desired the physical environment includes macro, micro, and even nanoscale features that allow for cells to adhere, proliferate, and migrate. However, it is important to note that in some instances, textured surfaces are detrimental to the function of the device such as articulating surfaces or cardiovascular devices.
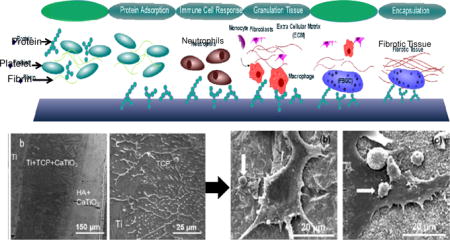
At the root, tissue integration can be achieved by initiating specific protein adsorption that leads to ECM formation or by providing a surface that acts as a simulated ECM. The specific proteins and glycosaminoglycans (GAGs) within the ECM play an important role in creating an environment for the targeted cells to adhere, proliferate and differentiate [10], [11], [12]. In some cases, the implant of interest is cultured with cells in vitro to develop a natural ECM and then decellularized prior to implantation, leaving behind a scaffolding for host cells to grow into. It should be noted that the body is also capable of creating an ECM around the implant surface, but without the proper chemistry or physical cues such as surface texture to facilitate cell recruitment and proper host tissue integration, the result is the haphazard adsorption of proteins that mark the biomaterial as a foreign body [13], [14], [15]. Implantation generally initiates a very specific inflammatory response, called the foreign body response (FBR). In the last stages of the FBR, macrophages can develop into foreign body giant cells and granulation tissue comprised of fibroblasts, macrophages, and new vascular tissue develops into a fibrous encapsulation, or scar tissue, surrounding the implant [14]. Figure 1 illustrates how microscopic topography and porosity can promote cell adhesion, and cause tissue to mechanically interlock with the device. It has been shown that topographical cues may bear more influence on cellular alignment and activity than varying chemistry. Smooth muscle cells were shown to have similarly increased alignment and decreased proliferation on micro/nano patterned poly(methyl methacrylate) (PMMA) and poly(dimethylsiloxane) (PDMS) substrates, even after the addition of a collagen coating [16]. Additionally, the use of both chemical and these topographical cues can work synergistically to direct cell growth [17], [18].
Figure 1.
The Foreign Body Response (Top) shows how an implant surface is tagged by proteins marking it as a foreign body. Further response by immune cells results in inflammation and finally encapsulation of the implant by fibrotic tissue. Surface texturing on the nano/micro scale (Bottom) can give the body physical cues that allow for normal cell adhesion that forms a base for normal tissue growth [19].
Augmenting and utilizing initial protein adsorption is a common approach to decrease inflammation and promote favorable biological responses. In the early 90’s, a process called layer-by-layer (LbL) was developed that used polyelectrolyte solutions to create complex surface layering [20], [21]. Briefly, the principle behind LbL is to alternatively dip the substrate of interest into solutions of oppositely charged particles to create bilayer coatings. LbL offers coating control on the nanoscale [21], and its exploitation of natural electrostatic attraction allows for facile, non-line-of-sight coatings of polymers and proteins. A physical surface modification method that has gained popularity, especially in groups examining the micro-environment of cells in vivo, is a process called soft lithography (SL). Soft lithography works by creating a master die, and using that to create elastomer molds. The master die can be used to create dozens of molds, and in turn the molds are utilized to create micro and nano-sized features on the substrates of interest [22], [23]. Controlling the physical features of the microenvironment influences cells’ preferences to grow on a surface or along certain directions. In some cases, AM techniques is used to create the master die [23], but the issue with all of these techniques is that there are post-processing steps that occur after the completion of a manufactured part. Post-processing has its own issues such as difficulty in localizing surface modification, restrictions in techniques due to composition, line-of-sight restrictions, and the extra time required to finish the part.
2. Current Additive Manufacturing Technology
2.1. Printing Processes
The often-overlooked application of AM is surface modification. The layer-by-layer processing allows tight control over compositional gradients as well as physical features down to the micro-scale. Metal, polymer, and ceramic parts can all be fabricated. And using different techniques, composite materials can be created with low spatial tolerances that allow researchers to modify surface properties. The precise control over a surface’s physical and chemical features that AM offers opens the door to create a single-step manufacturing process for highly customizable products, as well as a powerful post-processing tool for site-specific surface modification. Other inherent advantages of AM such as on demand manufacturing, smaller foot-print for manufacturing facility, and agile manufacturing ability even in remote areas further strengthens the argument for use of AM in such devices to enhance surface quality during manufacturing.
Additive manufacturing processes can be broken down into six broad methods as defined by ASTM, and further differentiated by technique as described in Table 1. Not all techniques listed have been used for surface modification as of today, but that does not preclude their use as a surface modification tool in such a rapidly evolving field as AM.
Table 1.
| Method | Manufacturing Technique | Description |
|---|---|---|
| Materials Extrusion | Fused Deposition Modeling (FDM) | Heated thermopolymer is extruded from print nozzle |
| Fused deposition of Ceramics (FDC) | Thermopolymer is loaded with ceramic material and extruded. Polymer burnout leaves ceramic part behind | |
| Binder Jetting | Drop on powder (DOP) | Binding agent is dispensed as droplets onto powder particles causing them to adhere and form support for subsequent layers |
| Powder Bed Fusion | Selective Laser Sintering (SLS) | A focused laser beam is used to sinter powder particles |
| Selective Laser Melting (SLM) | A focused laser beam creates a melt pool from powder particles to create fully dense scan lines | |
| Selective Heat Sintering (SHS) | A heated print head passes over powder particles to create sintered pattern | |
| Electron Beam Melting (EBM) [27], [28] | An electron beam within a vacuum chamber is used to either melt powder particles similar to SLM | |
| Directed Energy Deposition | Laser metal deposition (LMD) [27] | Metal powder is carried by gas into the focal point of a laser creating a melt pool. |
| Electron Beam Free-Form Fabrication (EBF3) | An electron beam within a vacuum chamber is used to either melt either directly fed powder particles or wire-fed substrate. | |
| Vat Polymerization | Stereolithography (SLA) | A laser initiates free radical polymerization within a bath of photopolymer, solidifying resin wherever the light touches. |
| Digital Light Processing (DPL) [29] | Similar to SLA, but a projected mask is used to cure each layer | |
| Continuous Liquid Interface Production (CLIP) [29] | A mask from the bottom of the vat polymerizes the photopolymer beyond a barrier region where no polymerization occurs. The build plate raises from the barrier upwards, continuously. | |
| Material Jetting | Drop on Demand (DOD) | Material is deposited onto substrate or build plate as droplets from piezoelectric or thermal actuators within a print head. |
| Multi-Jet Modeling (MJM) | Similar to DOD, multiple materials can be dispensed from the same print head. | |
| Laser Induced Forward Transfer (LIFT) | Material on a quartz ribbon is ejected by selective laser heating on the ribbon backside. |
3. Modifying Material Surfaces
3.1. Metallic biomaterials
3.1.1. Applications
Metals are commonly found in biomedical devices; used as the bulk material in heart pumps, stents, hip/knee/shoulder implants, pins and plates, dental posts, and many other applications. Standard metals used are stainless steels, cobalt-chrome and titanium, and their alloys such as Ti6Al4V, Ti6Al7Nb, NiTi (Nitinol), and CoCrMo. Alloyed metals are most commonly used in load-bearing implant applications in an attempt to modify characteristics such as fatigue and wear resistance, modulus, and density to more closely match the biological application [30]. When compared to other materials, metals provide the necessary mechanical and chemical attributes desired for orthopedic and dental applications. Additionally, the ease of manufacturing allows for high-throughput and complex shape construction of devices at low expense, making metals the long-term standard for implant materials.
3.1.2. Current challenges with metallic biomaterials
While metals and their alloys are sought for their bulk material properties, their surface characteristics can pose challenges. One such challenge that plagues metallic implants is corrosion. Even in alloyed metals pitting, stress, and fretting corrosion are still sources of implant failure and revision surgery [31]. Another key challenge is the inability to interact with the surrounding tissue, known as being bioinert. Without proper implant-host tissue interaction, a thin fibrotic lining, or scar tissue, forms at the interface [32]. In bone replacement applications this scar tissue encapsulation prevents proper fixation, and implants are prone to loosen with time. In addition, a modulus mismatch can lead to stress shielding, where the greater modulus of the metal results in force transfer through the implant rather than the surrounding bone. Lack of use and force-induced osteocyte signaling results in diminished bone remodeling, reducing density and strength of the surrounding bone that leads to loosening and fracture.
3.1.3. Surface modifications of metallic biomaterials using AM
Addressing intrinsic material issues is critical for the success and longevity of all metallic devices. Modifying the surfaces of the implants can prevent corrosion, enhance biocompatibility, and improve osseointegration without compromising the bulk properties. Incorporating passivating elements (Al, Cr, Ti), and augmenting surface energy [33], [34], topography [33], [34], and crystalline structure [35] are the best routes for improved corrosion resistance and implant-host interaction.
Metal implants generally have a high surface energy (low wettability) and have smooth surfaces, creating a poor environment for cell adhesion. If the surface has been modified to introduce roughness, the mechanical properties of the implant may suffer. Samples prepared via SLM processes showed that the inherent surface defects left from building reduce the fatigue stress of the material drastically. Fatigue samples of 316L stainless steel with as-prepared surfaces exhibited fatigue limits of 108 MPa compared to 267 MPa for SLM samples that have been turned [36]. Similarly, SLM Ti-6Al-4V samples with as-prepared surfaces showed fatigue limits of 210 MPa compared to ~500 MPa for the same samples with a polished surface [37].
To combat poor tissue integration, metal implant surfaces have been designed with macro and micro-sized pores, and designed surface topography to promote cell growth on the implant surface. While there is a great amount of research showing porosity enhances cell and nutrient infiltration into an implant, ultimately the structure of the surface dictates the degree of cellular adhesion and differentiation as well as gene and protein expressions [38]. Because of its extensive use in orthopedic and dental applications, titanium is most often used in studying the effects of surface roughness. As depicted in figure 1, discrepancies in cell behavior have been seen between unmodified, micro-roughened surfaces, and even nano-roughened surfaces [5], [39], [40]. The effect of surface topography is not limited to only roughness, but in fact intentional structures have been shown to induce adhesion and favorable differentiation [41], [42], [43]. To this end, AM has exceptional capabilities to modify surface topography and can be implemented into the design of the build. The intrinsic roughness of these implants can increase tissue integration and implant fixation without sacrificing the near-net shape manufacturing ability of the implants [44], [45]. Additionally, inherent roughness of sintered parts increases mechanical adherence of coatings that can be added at later stages to better suit the implant application [46], [47], [48]. This may not however be considered true design modification as the roughness is a natural byproduct of processes rather than intentional placement. A more deliberate approach for implementing surface porosity is to create a functionally graded implant with a porous structure on the outmost layer and a partially or fully dense body inside [49]. The effects of different lattice structures on cells has been observed in vitro where greater mineralization was seen when compared to flat or even sand-blasted titanium [35]. Control of lattice parameters, specifically pore size, is critical to cell motility and adhesion [50]. Similarly, AM is uniquely utilized for creating biomimetic surface structures based on computer tomography (CT) images [18], [51], [52]. This leads to nearly identical surface features experienced by the cells in vivo, a powerful implication for cells that are highly influenced by physical cues. EBM and SLM have less inherent surface roughness compared to laser sintering or powder bed printing due to the full melting of powder particles. These processes have been utilized to print microscale porous structures while achieving stiffness and strength values in-between that of trabecular and cortical bone [53], [54]. Without changing the underlying chemical structure of a material AM can influence the efficacy of an implantable device. Intrinsic roughness and graded porosity of designed, biomimetic, or random nature gives manufacturers precise control over mechanical properties as well as biological responses; coupled with any desired post-treatment such as acid-etching, nanotube formation, drug loading, or coating only furthers the impact of AM metal structures in biomedical applications.
While physical challenges are being met with AM, a separate issue experienced with metal alloys is happening on chemical level. Corrosion and wear are inevitable when metals are placed in such an environment as the human body, essentially a warm salt bath with repetitive loading and articulation. Both of these forms of degradation of metals and their alloys cause the constituent metals leach from the implant into the surrounding tissue. With time, the accumulation of heavy metals in the cells results in poisoning and death known as metallosis. Incorporating elements that self-passivate (Ti, Cr, AL) is the most common method to prevent corrosion. Titanium-based implants are particularly stable as they form a protective, dense oxide film in situ, which prevents corrosion and enhances biocompatibility. Certain alloys like CoCrMo have favorable wear properties compared to softer metals like titanium, increasing the longevity of the implant by preserving the condition of articulating surfaces. Yet, even these prostheses are susceptible to wear over time, releasing cobalt and chromium ions, which are known to be particularly poisonous, causing metallosis and osteolysis. Due to issues with fibrosis and metallosis, there is limited interest in metal-on-metal implants for joint replacement as opposed to conventional metal-on-polymer joints consisting of CoCrMo and ultra-high molecular weight polyethylene (UHMWPE). However, the mismatch of hardness between the metal and polymer can results in UHMWPE wear that in turn results in micro-particulate formation [55]. In this form, PE is ingested by cells and causes osteolysis in the implants’ surrounding [55], [56], [57]. The idea behind metal-on-metal implants is that lower coefficients of friction and similar hardness values will reduce fretting and wear; however Titanium, Cobalt, and Chromium ions have shown to up-regulate the production of bone resorbing cytokines and down-regulate the production of bone forming cytokines such as TGF-β1 [58]. Additional studies have suggested that improper mechanical forces experienced by the bone as in slippage of an implant [59], or fluid pressure in bone-implant cavities [60], [61], are also causes of osteolysis. Further studies have shown that particulates and cyclic pressure loading increased possibilities of osteolysis through synergistic mechanisms [17].
One method of AM that has garnered much attention for its capability in producing compositionally complex materials, as well its ability to modify existing surface microstructure is Laser Engineered Net Shaping (LENS™). In LENS™, the substrate of interest is fed as a powder into the path of a focused laser beam to create a molten pool of substrate material at specific x, y, and z coordinates. This method has the advantage to feed multiple powders into the focal point of the laser, allowing for the build to be a full or gradient composite.
Laser surface modification (LSM) using LENS™ of Ti6Al4V to modify granular structure thereby changing the hardness while creating a passive oxide layer that significantly reduces the in vivo wear when compared to as-received Ti6Al4V samples [62]. LSM of 316 L SS with HA powder fed in to LENS™ resulted in the formation of an iron/HA composite layer that showed a diffuse interface suggesting good interfacial strength. Additionally, the modified substrate showed better apatite precipitation in SBF when compared to the unmodified SS, suggesting better osseointegration properties [63]. In studies by Roy et al., LENS™ processing technique was again used to create compositionally gradient titanium/HA and tantalum/MgO implants [64] [65]. The studies showed that differences in microstructure can be elicited by varying the processing parameters such as laser scan speed and thickness of the gradient layer as seen in figure 2. The effect that the resultant TCP and MgO layers have on in vitro results is further discussed in figure 3.
Figure 2.
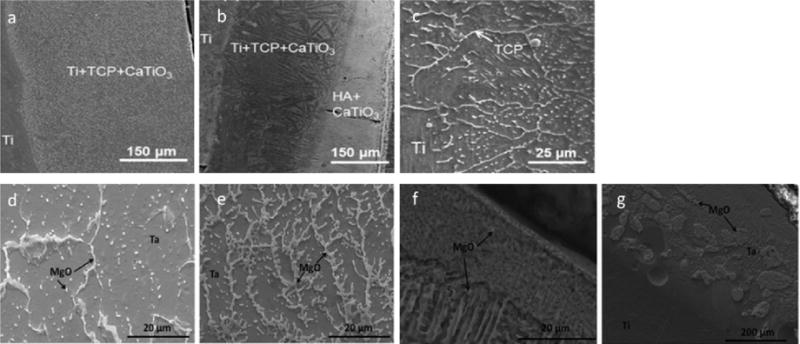
Cross-sectional images of LENS™ deposited metal-ceramic composites showing the microstructural differences in Ti/TCP coatings (a) single layer, (b) multi-layer, and (c) single layer samples at higher magnification [64], and Ta/MgO coatings with scan speeds of (d) 10 mm/s and (e–g) 15 mm/s [65].
Figure 3.
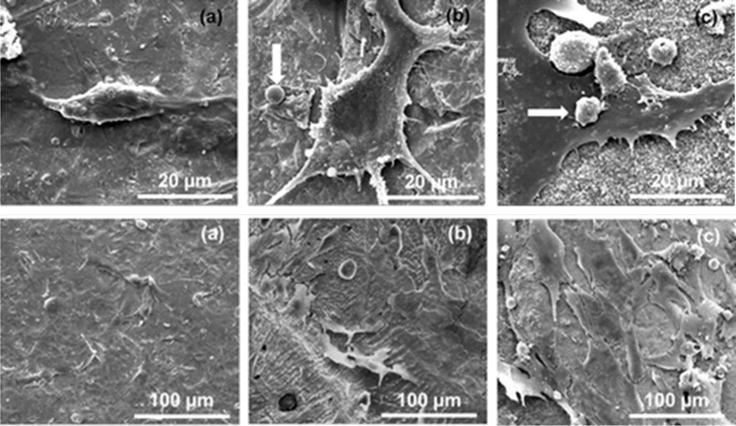
SEM images of human osteoblasts at 3 day (top) and 7 day (bottom) time points for (a) Ti, (b) Ta, and (c) Ta+MgO coatings [71].
The significance of using titanium and HA is that while powder-fed methods seem to have the upper hand in metal and composite printing, an innate challenge with composite builds in laser beam melting (LBM) methods is the differences in laser absorption and melting points. A ceramic in LBM may absorb energy, but cools so slowly that a local hot spot can form and overly heat the metals causing liquidation of more than the layer of interest and may introduce thermal stresses that cause cracking. Another significant challenge met by the use of a ceramic is the powder feed rate, an important parameter for LBM. For lightweight materials such as polymeric or ceramic stock, controlling the feed rate is hindered by frictional and van der Waals interactions within the relatively low density material [66]. Further, the use of bioceramics in metal composites alters the physical surface properties of the metal such as wear resistance and the coefficient of friction as well as the chemical properties such as biocompatibility. Figure 3 shows that by coating Ti with tantalum (Ta) and MgO-doped Ta the biocompatibility of Ti implants was further enhanced. Increasing proliferation and adhesion of human fetal osteoblasts (hFOBs) was seen on Ti, Ta, and Ta+MgO coatings, respectively [65]. Similarly, increased flattening and adhesion of hFOBs were also found for Ti substrates with composite coatings consisting of Ti and TCP [64].
Metals have also seen extensive use in soft tissue applications, mainly within the circulatory system. In these applications where the implant is in direct contact with the circulatory system, cell damage and clotting caused by the device requires patients to be on anticoagulants for the duration of the implantation [15]. Endothelial cells (EC) are the normal interior lining to blood vessels, and are therefore considered to be the best possible coating for any implant in these applications [67], [68]. EC produce antithrombogenic factors that prevent platelet aggregation and promote fibrinolysis, and otherwise naturally prevent and degrade thrombus formations. Endothelial cells are very sensitive to the environmental conditions such as substrate composition, flow shear forces, ECM composition, etc. [69]. For in vivo work micro-patterned surfaces on long-term cardiovascular devices have been shown to promote endothelial cell growth over typical smooth features on implant surfaces [70]. Additional research has focused on adsorbing proteins to the substrate surface to attract EC or endothelial progenitor cells (EPC) in vivo rather than ex vivo implant seeding [71].
The adhesion of platelets is initiated by the adhesion of proteins to the surface of the biomaterial which in turn activate fibrinogen [15]. Fibrinogen was shown to be the main component in platelet adhesion during a clotting event [72]. Other important factors are von Willebrand factor (vWF), vitronectin and fibronectin that activate transmembrane G-protein receptors on the surface of platelets propagating signaling and clotting. The adsorption of proteins to metal surfaces is caused by charge attraction between the surface and the protein. Proteins consist of amino acid chains containing different functional groups that can carry charges and adhere to substrate surfaces. Metals can also experience in situ oxidation resulting in positively charged surface environments. The result of the new surface charge is adsorption of proteins, which can result in cell signaling to cause inflammatory responses or in the blood stream, coagulation cascades [73], [74]. PEG/PEO coatings have been studied extensively as anti-fouling coating for implants to reduce protein adsorption to surfaces in contact with blood [3], [4], [73], and that silver nanoparticles (SNP) coated with PEG can be potential cardiovascular implant coatings due to reduced thrombotic effect and infections [75]. It is obvious that the ability to print an antifouling coating can be beneficial for stents and other applications such as membranes. To this end, an SLA method has been recently used to coat membranes or porous materials with PEG to prevent bacterial adhesion [76].
Not all proteins adsorbed cause a negative effect. If the device is not directly in contact with the cardiovascular system specific protein adsorption to the metal’s surface can actually facilitate host tissue growth and integration [77], [78]. Further, in order to overcome some of the limitations presented by natural protein adsorption such as enzymatic degradation and nonspecific cell receptor attraction, synthetic peptides are used to specifically target receptors of desired cell populations [79].
3.2. Ceramic biomaterials
3.2.1. Applications
Ceramics are commonly used in orthopedics and dentistry, however they are also used in drug delivery applications [80], [81]. This class of materials is ideally suited for bone scaffolding applications towards critical fracture/injury repair, where bone’s natural ability to heal into the wound is suppressed [80]. Additionally, ceramics are seen in hip/knee/shoulder implants as coatings, ceramic-on-ceramic joints’ articulating surfaces, bone cement for non-critical injury repair or increasing implant fixation, dental work for enamel and root replacement, and in different drug release applications. Commonly used ceramics are calcium phosphates (CaPs) in the form of α or β-tricalcium phosphate or hydroxyapatite, silicates like 45S5 bioglass, alumina, and porcelain and zirconia [81].
In the human body the fifth most occurring element is calcium, 99 percent of which is stored in bones and teeth. It is not a surprise that many biomedical ceramics are calcium based. Bone and teeth are both largely ceramic-based structures (bone is a composite of collagen fibers and hydroxyl apatite), thus ceramic materials provide chemical composition as well as crystallography appropriate for those applications. Nearly identical chemical and crystal structure along with similar micro/nano topography creates what is called a biomimetic device that mirrors the form and function of the biological tissue. The greater familiarity reduces inflammation or rejection due to immunological responses to foreign bodies. This form of biomimicry has the additional benefits of degrading into ions and CaPs that the body can readily excrete or resorb. As a resorbable material, the effects of degradation are next to nil, and the rate at which the material is resorbed can be tailored to match the tissue healing rate by changing the crystal structure, CaP phase, or surface modification. In applications that come into contact with bone CaP scaffolds share a majority of elemental similarity to natural bone, yet they lack the trace elements such as Si4+, Mg2+, Sr2+, Zn2+, Cu2+ and Fe2+/Fe3+ among others. Recent research has been done on the addition of ionic elements found naturally in bone to bone scaffolds. These ions have been shown to play a role in angiogenesis, osteogenesis, and osteoinduction [82].
3.2.2. Current challenges with ceramic biomaterials
To date, additively manufactured ceramics are most common in dental applications, where they have a natural advantage over metals and polymers in their high compressive strength and high corrosion resistance, but surface modification of ceramics using AM occurs mostly in the field of resorbable drug delivery devices. One of the biggest challenges is the inability to sinter an AM ceramic after it has been loaded with drugs or biomolecules because of the low degradation temperature of the additional material. Despite the presence of binder holding the ceramic powder particles together, these parts are susceptible to bulk degradation and burst release of loaded molecules.
3.2.3. Surface modified ceramics using AM
In order to combat burst drug release and maintain steady release profiles, as well as implant integrity in vivo surface modification of resorbable bioceramics is focused towards applying layers of polymeric coatings. In drug delivery applications such as drug-loaded bone scaffolds, CaP materials must be heated to extreme temperatures to reach a crystalline phase, which prevents polymers and drugs from being applied to the “green” scaffolds. Post-calcination, the ceramic can be dosed with any drug desired and coated with polymer to control drug elution. Tarafder et al. used 3D printed β-TCP bone tissue scaffolds with designed porosity as carriers of an osteoporosis treatment drug, Alendronate. These scaffolds were subsequently coated in PCL to control the release kinetics and showed that TCP+Alendronate+PCL scaffolds were able to significantly increase bone formation over TCP+Alendronate scaffolds at 6 weeks post-op seen in figure 4 [83]. This surface modification of the ceramic+drug implantable drug delivery system (DDS) with PCL illustrated the ability to tailor release kinetics by tweaking the hydrophilic/hydrophobic surface interactions between the polymer and solution, and the polymer and drug.
Figure 4.
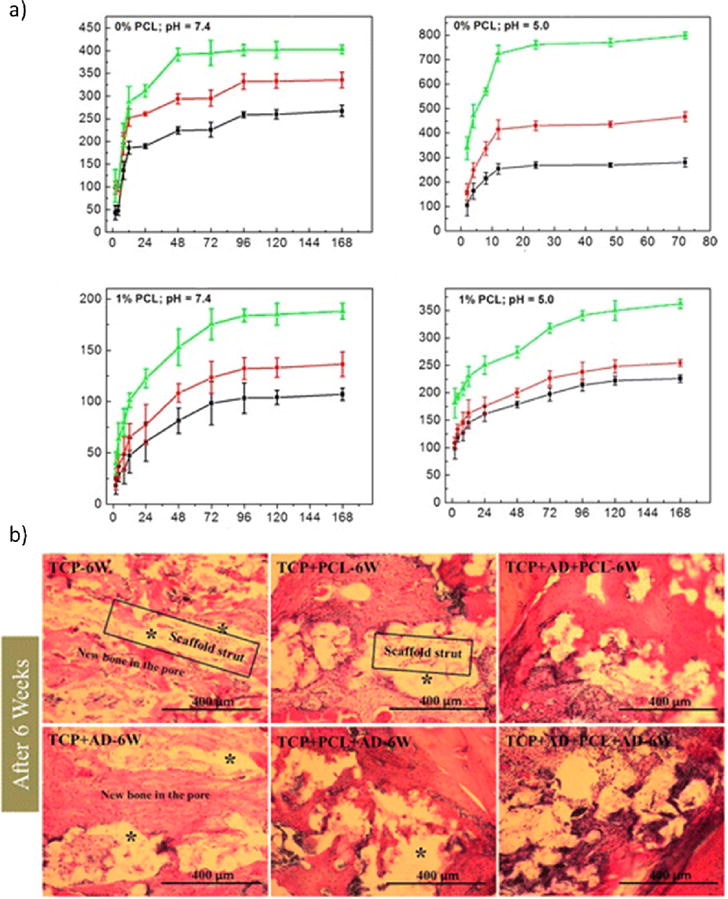
a) Release kinetics of drug-loaded 3D printed scaffolds loaded with 1000 (green), 800 (red), and 500 (black) μg of Alendronate. Scaffolds without PCL (top) and with PCL coating (bottom) at a simulated post-op pH of 5.0 (right) and physiological pH of 7.4 (left). b) New bone formation (red) into implantation site and around the scaffolds (white) at 6 weeks post-op. [83].
Alternatively, additives or dopants like trace elements (Mg, Sr, Si, Zn) found in normal tissue have been used as methods to enhance osteogenesis and angiogenesis (bone growth and vascularization, respectively) in similar ways as drugs. These dopants can be included in the manufacturing process, however, as sintering does not destroy the molecules or hamper their effects. Figure 5 shows how the inclusion of dopants in the Drop-on-demand printing (DoD) process can affect the microscale morphology while maintaining macroscale features [84, 85]
Figure 5.
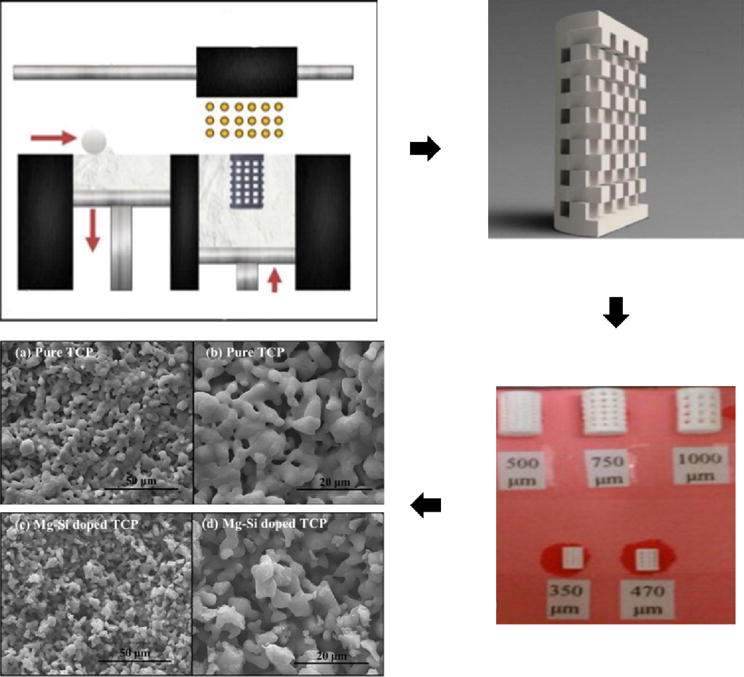
(Clockwise from top left) Drop-on-demand printing (DoD) set up, CAD file of actual scaffold design, scaffolds printed and sintered with various pore diameters, effect of Mg-Si dopants on sintered surface microstructure. Adapted from [84] and [85].
In applications where the DDS is subcutaneous, gastrointestinal, or otherwise non-weight-bearing different binder formulations have been used to create a part with a bulk internal chemistry—usually containing the drug—and a less soluble, polymer supplemented outer surface chemistry. In a study by Song et al., the release profile of adsorbed ibuprofen and bovine serum albumin on mesoporous silica/bioglass scaffolds could be regulated by surface modification in post-processing as well as surface modification through a one-pot process [86]. In a later study by Zhu et al., surface modified and 3D printed composite scaffolds were studied for use in bone replacement therapy for patients undergoing surgical bone removal due to osteoarticular tuberculosis. In their study, mesoporous silica nanoparticles and mesoporous bioactive glasses were modified with silanes that grafted carboxyl (−COOH) or methyl (−CH3) functional groups to the surfaces to eliminate the burst release of anti-tuberculosis (TB) drugs. These modified powders were mixed with the Anti-TB drugs and further mixed into a polymer solution containing poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx). The composite was printed into porous scaffolds (full process is shown in figure 6) and studied in vitro and in vivo using rabbit femur models for drug release profiles, with bone regeneration observed in the latter. Findings showed ~80% release of both drugs at 84 days in the composite scaffolds compared to ~100% release at five days in pure CaP scaffolds with drugs loaded in a traditional drop-by-drop method. No significant difference was seen in the amount of new bone formation over 12 weeks between the two scaffolds, but the ability of the printed scaffold to rival CaP scaffolds while retaining a consistent drug release profile shows promise for future bone therapy studies [87].
Figure 6.
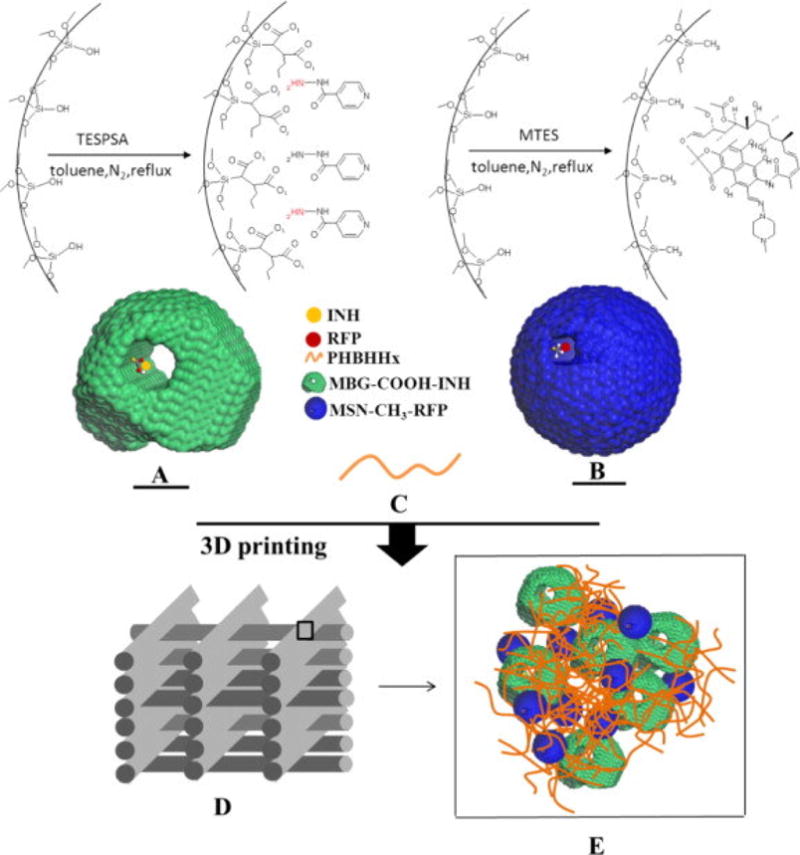
(Top left) The processing of mesoporous bioglasses to graft carboxyl groups, (Top Right) processing mesoporous silica nanoparticles to graft methyl groups. (Middle) the anti-TB drugs INH and RFP shown adsorbed to the respective particles. (Bottom) the 3D-printed scaffold and molecular representation of the drug-loaded particles adhered together with the polymer [87].
3.3. Polymeric biomaterials
3.3.1. Applications
Polymers are also commonly used as biomaterials e.g. pins and plates for bone and cartilage repair, hip/knee/shoulder articulating surfaces, DDS, skin coatings for burn repair, and in optical applications. One of the unique features of polymers is the ability to biodegrade into constituents that can be processed by the body. Frequently used biodegradable polymers include polyethylene glycol/oxide (PEG/PEO), polylactic acid (PLA), polyglycolic acid (PGA), Tri-methyl carbonate (TMC), and polydioxanone (PDS) [3], [88], [89]. More recently, other synthetic polymers like polycaprolactone (PCL), Polyether ether ketone (PEEK), Polypropylene fumarate (PPF) and functionalized polyurethanes have received much attention for use in soft and hard tissue repair [89], [90], [91], [92]. Natural polymers like collagen and fibrin, polysaccharides like alginate, silk, hyaluronic acid and chitosan have been explored for use in biomedical applications for their degradation properties or appeal as naturally occurring biopolymers [89], [93]. Collagen is the most abundant protein and natural polymer in the ECM that provides support and adhesion for cell movement, hence its used widespread in surface modification [94], [95]. Other polymers like poly(methyl methacrylate) (PMMA) and polytetraflouroethylene (PTFE) are less biodegradable, to the point of being permanent, and in the case of PMMA excellent optical properties make it superior for use in contacts and intraocular lenses. Yet, despite the seemingly removed nature of these lenses, foreign body reactions can foul the lenses and the need to modify the surface has been explored [93], [96]. PMMA is also widely used in bone cement for implant anchoring and fixation.
3.3.2. Current challenges with polymeric biomaterials
Polymers mechanical properties such as toughness and stiffness, and chemical properties like biodegradability can be further tailored by co-polymerization. Different compositions are used to match the application needs. For example, devices like sutures or bone screws need greater toughness or higher modulus, and must also degrade at a rate similar to the tissue’s ability to heal. Poly (lactic-co-glycolic acid) (PLGA) is one of the most widely used polymers due to its good biocompatibility and ability to easily modify its degradability, where higher lactide content causes the material to degrade at a much slower rate than compositions with higher glycolide content.
Despite a high level of adaptability and promising biodegradable traits, polymer systems still have pitfalls. While used extensively and being comprised of components found in metabolic pathways, PLA/PGA polymers and co-polymers break down into acid compounds causing local acidosis. Extensive release of these and other polymers’ constituents can cause inflammatory responses in the body that can hinder or undo the healing process. Additionally, polymers are often associated with a high surface energy that reduces the wettability of the implant and lack charges that assist in protein and cell adhesion. This limited bioactivity can impair cell adhesion thus delaying the healing process, and can even induce fibrotic tissue formation [14].
3.3.3. Surface modification of polymeric biomaterials using AM
Bulk material properties are easily achieved and are desired for the success of the devices, however the surface characteristics have limited their biomedical applications. The first methods used to increase bioactivity involve bulk material changes such as porous scaffolding, allowing for greater cell migration and blood perfusion deeper into the implant. While AM techniques like 3DP, multi-jet printing, SLA, and FDM have given researchers almost unlimited control over hatch (pore) and strut dimensions, these changes in design do little to modify surface energy. A significant challenge for biopolymers researchers is to increase surface energy, thereby affecting cell adhesion. This is where AM can be used to its fullest potential. In all polymer construction, often without major changes to the design file of the device, the starting material composition can be modified to induce nano and micro topography, variation in chemical composition and even crystallography. Studies have been conducted that introduce ceramic materials like calcium phosphates (CaPs) such as hydroxyapatite (HA) for bone scaffolding applications to the polymer constituents prior to SLA [97]. The resulting scaffold is polymer-based with nano-sized and fully crystallized granules of ceramic that protrude from the surface, giving the implant a nano/mirco roughness, and a chemistry and crystallography similar to that of bone.
In a study by Dawson et al. [95], the effect that collagen has in inducing osteogenic differentiation in human bone marrow mesenchymal stem cells as well as chondrogenesis was observed. Composite scaffolds of HA and type I collagen were able to successfully induce osteoblastogenesis and showed increased mineralization at 28 days in vitro and in vivo when compared to pure calcium phosphate scaffolds. Similarly, the collagen scaffolds were able to successfully induce chondrogenesis. Perhaps more interesting is the discovery that better chondrogenesis was seen at the center of channeled collagen scaffolds rather than the outer surface, further elucidating the importance and synergistic effects of surface chemistry and 3D complexity. While AM was used in this study, the purpose was for crating molds to prepare the scaffolds rather than the scaffolds themselves. Taking the use of collagen and AM techniques one step further, Inzana et al. [98] incorporated type I collagen into the binder of a ceramic 3DP process. Interestingly, the results of the study concluded that 3D scaffold promoted better bone replacement of the scaffold or “engraftment” than CaP or CaP/5% wt % collagen scaffolds after 9 weeks. As the scaffold degrades, allowing for appropriate replacement by natural bone, the effective “surface” of the scaffold is changing. As evidenced by the study, a surface coated in collagen by dip coating was less effective, which illustrates the need for composite surface chemistry, and further advocates the importance of AM in producing effective scaffolds versus traditional coating methods. Traditional methods will only coat the outer surfaces, whereas AM offers a way to control the chemistry of the exposed material throughout the degradation process i.e., the life of the device.
In exploring polymers as a base material for drug delivery systems, work has been put towards attaching functional groups through plasma [99], [100] or other chemical methods [101], [102], then adsorb proteins and growth factors to these functional groups. These methods prove effective at mitigating blood-related adverse events and increase cell adhesion and proliferation of stem cells, osteoblasts, endothelial and epithelial cells [103]; however, they are lengthy methods that require multi-stage post-processing. Without AM, post-processing is necessary as two of the most important factors in controlling the release kinetics of DDS are the porosity of the device as well as the surface chemistry [104], [105]. Figure 7 shows two different examples of surface modified DDS using polymer and ceramic based AM methods. Yu et al. demonstrated the ability of using the 3DP method to create a DDS with multiple surface chemistries towards specific drug release profiles. Both changes in powder and binder composition were utilized to create a hollow, cylinder-shaped DDS with insoluble top and bottom layers, a less soluble outer ring layer, and an inner bulk composition laden with acetaminophen [104]. Similarly, dual quick and slow release DDS systems have been fabricated by modifying the top of slow-release ceramic pills [106], [107], and a cellulose modified tablet was fabricated using material extrusion of multiple materials [108]. In a study done by Water et al., multiple morphologies were created during the fabrication of a drug eluting polymer/ceramic composite. Using PLA as the bulk material, feed stock material was created by melting and extruding different compositions of hydroxyapatite (HA, 0 and 5%) and Nitrofurantoin (NF, 0-30%). The feedstock was used in a MakerBot Replicator 2 (New York City, New York) to extrude the composite into disks for their drug-elution study [105]. It can be seen that the surface topography of the extrudate and printed material is increasingly rougher with the addition of HA particles or NF doses, which is amplified when both are present. This increase in surface roughness correlates to an increase in surface area, which affects the performance of the implantable or ingestible device by increasing the effective area in contact with the body. Increased exposure of the surface to the body would increase the rate of dissolution of the drug into the surrounding tissue as well as the increased rate of device resorption. There is a dual function of the increased roughness, which can be an increase in the fixation of the implant. This demonstration of the ability to tailor surface characteristics using HA also has other implications such as increased surface crystallinity [97], [105], reduced surface energy, increased hydrophilicity, and increased protein adsorption [11], [97], reduced pre-osteoblast cell apoptosis [11] and increased proliferation from pre-osteoblast cells [97].
Figure 7.
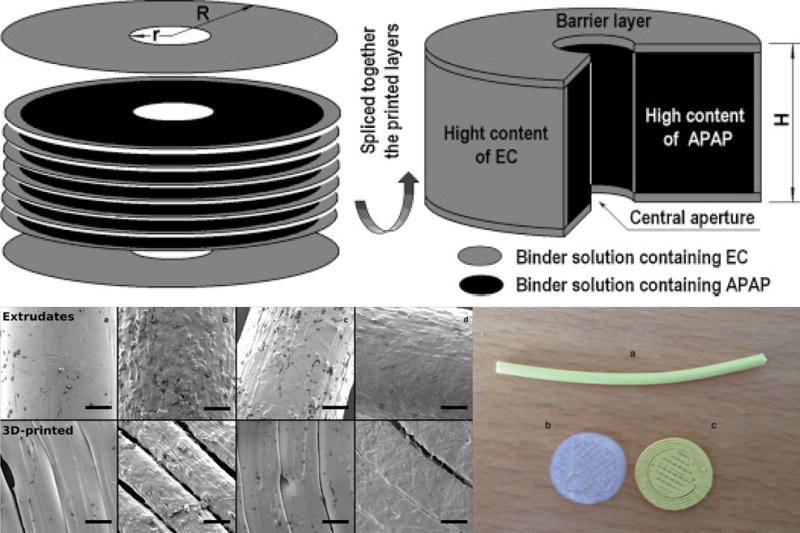
(Top left) Exploded and (top right) slice view of 3D printed DDS with hydrophobic ethyl cellulose (EC) outer layers protecting the acetaminophen (APAP)-laden bulk, allowing for linear drug release [104]. (Bottom left) an extruded PLA + 20% NF strand (a) used as feedstock for printing the disks below (b) PLA and (c) PLA-20% NF. (Bottom right) SEM images of feedstock strands and the 3D printed strands with varying compositions. (a) PLA+5% HA, (b) PLA+5% HA+30% NF, (c) PLA+5% HA+10% NF, and (d) PLA+30% NF [105].
Further study on drug eluting stents has shown that FDM is a viable process to create an end-use resorbable, implantable stent that has the necessary mechanical stability for over four weeks [109]. Despite the lack of surface modification using FDM itself, it has been demonstrated that an inner core of the implant can be drug loaded as is the case above, while an outer surface can be printed to control drug release [110].
Bioprinting, another form of additive manufacturing, is also becoming very popular lately to fabricate biological scaffolds. The idea behind bioprinting is to recreate scaffolds that are composed of degradable materials laced with cells for tissue growth. The purpose of creating these scaffolds is two-fold: one goal is to produce replacement organs or a cellular construct that the body can use to make a fully functioning organ, and the second is to produce standardized tissue models for in vitro studies [111].
A relatively newer 3DP machine, laser induced forward transfer (LIFT) utilizes a quartz ribbon that has a biological coating on the side of the build platform. The ribbon can move in the X and Y direction while the build platform below can move along all three axes. From above, a stationary laser pulses and strikes the quartz side of the printing ribbon, causing localized heating that results in vaporization of a small fraction of the printing material. The formation of a vapor bubble at the interface ejects a small droplet of printing material to the desired location on the build platform. This is a very powerful tool for 3D printing biological materials. The quartz ribbon can be coated with a wide variety of compositions, or alternate ribbons can be replaced during mid build [112] A modified version of LIFT exists as biological laser printing (BioLP™), which takes a three-layer approach in the ribbon in which a sacrificial energy-absorbing layer between the quartz and the printing material is added [111], [113].
Bioprinting methods by design utilize extrusion, inkjet and laser-assisted methods. These free-forming techniques have a major drawback in that the substrate material is generally non-rigid and there is no base to support overhangs as there is in powder-bed methods. Especially in biological scaffolds, overhangs such as horizontal orifices or vessel tunnels are common as illustrated in figure 8. To accommodate this, the most common method in use is solid support material, which can be easily removed by increasing temperature (for acellular constructs) or rinsing/incubation [114], [115].
Figure 8.
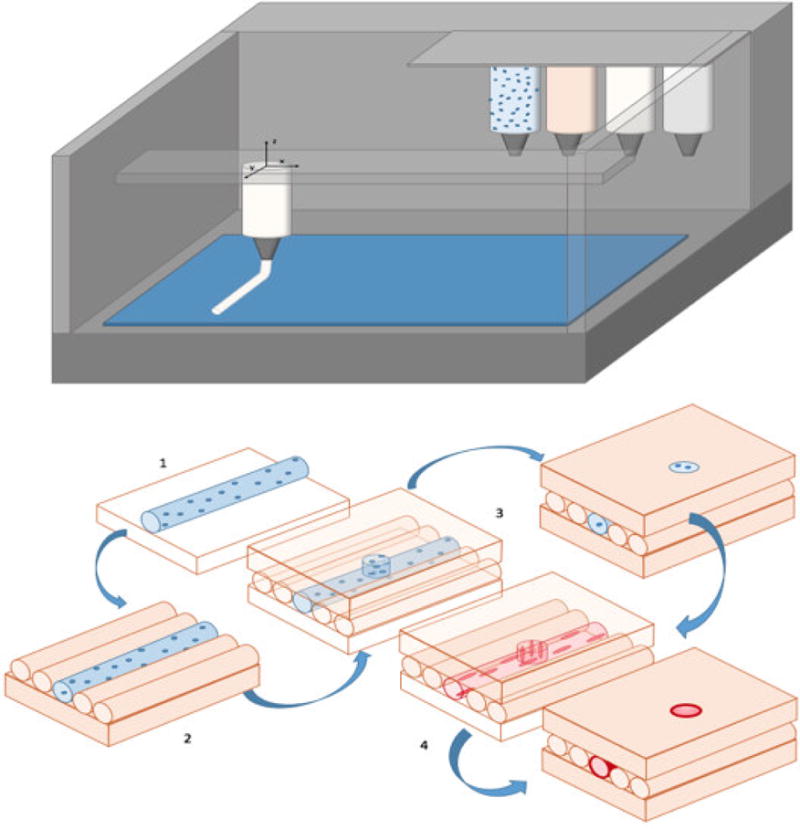
Top) Schematic of a multi-material bioprinter with extrusion nozzles for material deposition - the canisters represent ceramics (white), cells (blue), and polymers (orange). Bottom) Progression of vascularization of a bioprinted 3D scaffold. Base (orange) can be any appropriate biopolymer (alginate, fibrin, or collagen) matrix material. 1) Cell-laden material (blue) is printed onto the base in any direction 2) matrix material fills surrounding area of build layer 3) additional matrix or vascularization material added 4) cell-laden tubule rinsed to wash away excess material and incubated, leaving pre-endothelial cells lining the support matrix allowing them to culture under biological conditions.
Another less common method is the use of a liquid bath that supports the build by a buoyant force that can act also as a cross-linking agent [116]. In the case of Christensen et al., a CaCl2 solution was used to cross-link sodium alginate polymers, forming a calcium alginate gel. Similarly, other divalent cations can be used in place of Ca2+ to create a similar cross-linked structure. Trivalent cations (Al3+, Fe3+) will result in greater cross-linking than chemistries [117]. Additionally, alginate can be covalently bonded with materials like PEG that increases the mechanical strength of the hydrogel, shown in figure 9 [118].
Figure 9.
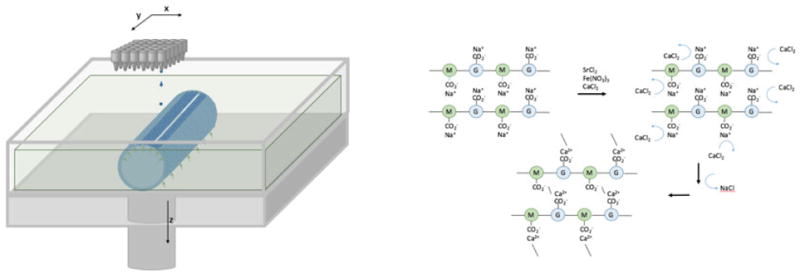
Left) simple schematic of drop-by-demand printing. In this case buoyant forces (green arrows) of the vat solution are used to provide support for an alginate substrate in the form of an open tubule. Right) Sodium alginate polymers of (1-4)-linked β-D-mannuronate and α-L-guluronate (M and G, respectively) react with calcium chloride to produce the cross-linked hydrogel, calcium alginate [118].
Bioprinting using DoD can produce surfaces patterned with cells or proteins for use in directed cells growth [112], [119], [120] and is utilized extensively in the areas of biosensors [121], [122]. As shown in Figure 10, droplet-based bioprinting is used for site-specific modification of biosensors that can be additional biomaterials, enzymes, proteins, or even cells [123].
Figure 10.
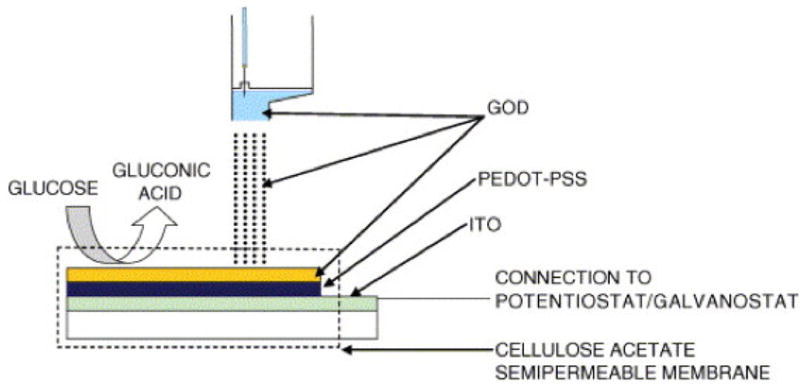
An example of printed biosensors where bioprinting is used to adhere an enzyme (Glucose Oxidase, GOD) to a conductive polymer surface (poly(3, 4-ethylenedioxythiophene/polystyrene sulfonic acid) (PEDOT/PSS) for use as glucose monitor [122].
Nanomaterial inclusion in AM has been specifically utilized by DoD as a way to print conductive materials onto substrates. Although most of the progress has been geared towards printing on organic photovoltaics, such techniques could be used to create conductive surfaces. Inks containing silver nanoparticles have been used to create interconnected, conductive patterns on surfaces with wire a width around 100 microns and height of 300 nm [124]. Additionally, Ni/NiO surface features on the order of a few hundred nanometers can be printed using colloidal inks of nickel using DoD [125]. Biosensors using this method were created in a scalable design as inexpensive and sufficiently accurate microfluidic diagnostic devices [126]. While many biosensors are external devices, these sensors must be able to interact with bodily fluids and tissues without causing adverse reactions in order to provide and accurate diagnosis. Looking forward, these types of devices can be utilized for in situ diagnostic analysis for or actuating as was done for neural prostheses [127].
Future trends in AM of biomaterials surfaces
Current challenges
It is clear that use of AM in biomaterials and biomedical devices will continue to grow. However, despite significant growth in the field during the past decade, there are many challenges that needs attention for the continued growth. Low throughput of manufactured pieces, material selection, and the resolution with which the parts can be made are some of the global challenges in AM. Moreover, in many applications, existing surface modification approaches are well established, which offers a wider selection of materials, and are easy to perform in comparison to various AM techniques. In LbL technique, the depth of surface layers and compositions can be controlled down to nanoscale resolution [21]. Researchers have shown the diversity of different polymeric compositions and varying application times. These precisely controlled bilayers exhibit linear or exponential growth of layer thickness with subsequent layer application [20]. Another method that has very fine control of surface coating thickness is atomic/molecular layer deposition (ALD/MLD). This post-processing method submits the device to different gases in a reaction chamber, allowing for the attachment of molecules to exposed surfaces for a non-line-of-sight process. Pulses of different gases allows for a LbL approach to create atomic and molecular-scale bilayers [128]. For drug and protein surface adhesion, common methods such as dip-coating, incubation in solution, and drop-wise addition can also be used with any of the implant materials. Solvent removal after coating leaves behind surfaces that are only as thick as the adsorbed molecules. In contrast, AM is limited in surface modification by its relatively low resolution. Extrusion techniques like FDM are limited by the size of the extrusion nozzle, which in turn is constrained by flow rate and viscosity of the build material. Achieving designed nanoscale features or layers on a surface is all but impossible simply due to the shear forces necessary to extrude a viscous thermoplastic, let alone a solids loaded composite. The other manufacturing methods that use a powder bed (3DP), or are powder fed (SLS, DMLS, EBM, etc.) are limited by the particle size being used as well as binder dispersion volume and melt pool size [129], respectively. In bioprinting, the amount of liquid material that can be administered is controlled to the pico-liter level allowing for excellent resolution; however, in order to print on such a fine scale requires the dispensed liquid of low viscosity i.e., around 30 mPa [121]. The trade-off for resolution is the viscosity of the liquid and therefore the availability of materials that can be used in this process. Any process that utilizes a laser to sinter, melt, or cure the stock material requires that that material can absorb the laser’s energy and thus be heated. In the case of UV laser-curing in SLA, the material selection is limited to thermoset polymers that polymerize via UV light. Material selection can be broadened in part by the use of added photoinitiators which absorb the UV rays and start free-radical polymerization.
Finally, AM parts most often still need post-processing modifications like polishing or sintering. Methods like inkjet 3DP, or FDC require a post-manufacture heat treatment to burn off the binder and sinter the green parts after the build is finished. Similarly, metal powder-sintering methods also require some form of final sintering to achieve the desired mechanical properties. While post-processing heat treatment is inherent in some systems to densify the metal or ceramic powder, others such as EBM or LENS will still require cutting and polishing to get a final part. In short, while it increases autonomy and can reduce multiple manufacturing stages, end-use-ready parts directly from the build stage are still unavailable using AM alone. To this end, the implementation of multiple techniques such as DMLS and CNC in a single system has been shown to produce ready to use parts right after the build [130].
Regulatory issues
The drive towards finer features and interacting with the body at a nanoscale level unlocks a plethora of possibilities for AM, but nevertheless poses new problems for AM: regulation. Regulation of AM materials that are in contact with human body fluids and membranes is absolutely necessary, especially nanomaterials where the safety risks when in contact with the human body are still poorly understood. Nanoparticles pose a particular danger where the surface area of a material is so large that it can have significant chemical reactions with the surrounding environment, but particles are small enough to pass through membranes like the blood-brain barrier [131]. The FDA has planned since 2013 to create a set of regulatory guidelines that manufacturers of devices made form AM methods will follow [132]. It was noted that the FDA is well aware of the importance of personalized medicine and the possibilities AM can offer in advancing healthcare, and in May 2016 released a draft guidance for 3D printed medical devices [133]. The draft guidance is a general outline for manufacturers that details the FDA’s current thoughts on device design and testing. The biggest hurdle the industry faces will be getting materials FDA certified. On one hand the variability in mechanical properties from build to build has to be reduced meaning standards for testing have to be created. As of today, there are only three ASTM standards in place for testing materials made by additive manufacturing. On the other hand, how does the FDA assure that the device meets regulatory standards when considering devices that are patient specific? Patient specific devices, also known as point-of-care products, are as to date not listed in the draft guidance along with drug and cell therapy devices made via AM.
Future trends
Significant development is taking place in different areas of additive manufacturing over the past two decades. The first issue was reproducibility and reliability. Parts produced by the same machine at different time points or by different machines of the same model at the same time should yield the same part quality. Almost after 30 years since the first AM system was conceptualized, part quality has reached at a level when General Electric is comfortable to print parts for aerospace engines or US Food and Drug Administration (FDA) is fine with approving AM processed parts for human use. Such accomplishments are not trivial. In the future, it can be envisioned that AM technology will be utilized not only for complex geometries, but also on implementing surface modification during part fabrication to reduce multiple processing steps and tailor properties to enhance tissue-materials interactions. Specifically, surface technology can influence drug release and targeting, antimicrobial, and cell recruitment properties. To accomplish such advancements, AM systems should be able to deposit multiple materials in one operation with high degree of build resolution. Single material builds, i.e., non-composite materials, are limited by their homogeneous properties. Limited by a printer’s ability to process only a select few materials, the AM devices are usually made of a single type of material e.g., only polymers or ceramics or metals with trace amounts of other material phases. The current route for AM surface modification is largely done by modifying the substrates prior to printing by adding small amounts of filler made from other material types to create a composite surface. Similarly, the substrate materials prior to printing can be functionalized, resulting in homogeneous bulk and surface chemistry. Multi-material printing methods, including LENS, FDC, and bioprinters with multiple dispensing heads allow for a good degree of control over composition during the actual printing phase. The advantage to in situ control is the ability to choose to create chemically homogeneous or gradient areas within the build. This is not only limited to the chemistry being heterogeneous if desired, but physical properties such as surface texture or crystal structure near the surface can be differentiated from the bulk structure. However, challenges of multi-material printing are also significant particularly towards understanding compatibility among different materials during building due to thermal stresses or chemical interactions.
Next generation AM capabilities for biomaterials surfaces
As it was also pointed out that AM techniques are limited by the resolution that the machines are capable of printing. Some of the current efforts are focused on refining methods to increase the range of printable features. One of the more notable advances in both resolution and speed came from the development of continuous liquid interface production (CLIP). A better understanding of the effects of oxygen as a barrier to prevent the photoinitiation process led to the creation of CLIP, where the build plate is drawn upwards from the barrier as a mask causes polymerization at the build plate surface. As a continuous process, the slice resolution is limited only by the refresh frequency of the laser mask, and the in-plane spatial resolution can range 10-100 μm [29]. Binder-jet printing already disperses droplets in the tens of picoliters, but is limited by powder size and. Current developments in metallic materials printing have led to liquid-metal droplets using 3D inkjet printing. This process, called gallium-iridium direct writing, reduces metal to the nanoparticle range using a solvent such as ethanol, which allows the new NP-laced ink to still be deposited onto surfaces through piezoelectric dispensing heads [134], [135]. Gallium-Iridium metals cannot be exposed to the surface in contact with the body however as they would be in liquid form, and other metals processed through binder-jet methods as a dispersion in ink, makes larger scale builds impractical. In addressing scale-up of printing liquid metals there have been advances in laser-induced forward transfer (LIFT) that has recently led to stacking drops of copper one micrometer in diameter to nearly one millimeter in height [136]. While the focus is currently on wearable technology, methods similar to the gallium-iridium ink-jetting may be developed for use in internal wearable devices such as electrodes for monitoring and shock delivery as would be seen in a pacemaker, or signal transmission for intraocular and intracochlear devices.
Breakthroughs such as this show that AM is entering an arena where metal or ceramic can be printed with no restrictions set by powder or melt pool size or binder jetting and opens the door for multi-scale printing. The ability to print in the macro, micro and nanoscale in a single build platform could not only allow for fine compositional control of the device, but also introduces spatially specific topography, geometry and porosity [5] [137]. In this way the rapid production of bulk materials could be done with a low resolution method, and near-net shape can be achieved using a micro or nano resolution method. It is also well-known that cells are sensitive to nano and micro-topography, but even the chemical interactions vary from macro to micro to nano-scale particles changing the way a nano-featured device interacts with the body compared to a micro-featured device of the same composition.
4. Summary
Today’s implantable devices are the pinnacle of biomaterial science and the culmination of decades of research, yet they are fraught with the same issues that have limited implants as a secondary option to non-surgical or grafting procedures. Additive manufacturing, though still in its infancy, has already attracted the interest of biomaterial’s researchers as a new and powerful tool to develop novel devices, and the way we think about design in general.
Material’s whose bulk properties are desired, but surface properties eliminate them from use in certain applications are no longer restricted. A metal implant susceptible to corrosion and wear can be reinforced with hard, corrosion resistant ceramic layers. Ceramic or polymer components that lack bioactivity can be incorporated with bioactive molecules such as drugs, proteins, and ions that interact with the surrounding tissue. All of this can be done in a single build as a monolithic part, and can be prepared on a case by case basis using AM. And that is the true power of AM: its capacity to give manufacturers direct control over the bulk properties, and more importantly over the surface properties in three-dimensional space. While composite surfaces engineered to mitigate the deleterious effects of traditional implants are actively being researched, AM offers designed control of said surfaces. Implants are like the transplants, drugs, and therapies used for patients today in that there is no one-size-fits-all approach. In the near future, we hope see implants designed with AM that meet each patients’ needs, perform with reliability, and become a go-to therapy for the millions suffering from disease and debilitation world-wide.
Acknowledgments
The authors would like to acknowledge financial support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers NIAMS-R01-AR-066361 and R01 AR067306-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bandyopadhyay A, Bose S, editors. Additive Manufacturing. CRC Press; 2015. [Google Scholar]
- 2.Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A. Additive Manufacturing of Biomaterials. Progress in Materials Science. 2017 doi: 10.1016/j.pmatsci.2017.08.003. https://doi.org/10.1016/j.pmatsci.2017.08.003. [DOI] [PMC free article] [PubMed]
- 3.Stallard CP, Solar P, Biederman H, Dowling DP. Deposition of Non-Fouling PEO-Like Coatings Using a Low Temperature Atmospheric Pressure Plasma Jet. Plasma Process Polym. 2015 May;:n/a–n/a. [Google Scholar]
- 4.Roy M, Bandyopadhyay A, Bose S. Induction Plasma Sprayed Nano Hydroxyapatite Coatings on Titanium for Orthopaedic and Dental Implants. Surface & Coatings Technology. 2011;205(8-9):2785–2792. doi: 10.1016/j.surfcoat.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, Bose S. In vivo response of laser processed porous titanium implants for load-bearing implants. Annals of biomedical engineering. 2017;45(1):249–260. doi: 10.1007/s10439-016-1673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Shaughnessy WS, Gao M, Gleason KK. Initiated Chemical Vapor Deposition of Trivinyltrimethylcyclotrisiloxane for Biomaterial Coatings. Langmuir. 2006 Aug;22(16):7021–7026. doi: 10.1021/la0607858. [DOI] [PubMed] [Google Scholar]
- 7.Roy M, Fielding GA, Beyenal H, Bandyopadhyay A, Bose S. Mechanical, In Vitro Antimicrobial and Biological Properties of Plasma Sprayed Silver-Doped Hydroxyapatite Coating. ACS Applied Materials and Interfaces. 2012;4:1341–1349. doi: 10.1021/am201610q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das K, Bose S, Bandyopadhyay A, Karandikar B, Gibbins BL. Influence of silver on antibacterial activities of surface modified Ti. Journal of Biomedical Materials Research Part B. 2008;87B(2):455–460. doi: 10.1002/jbm.b.31125. [DOI] [PubMed] [Google Scholar]
- 9.Vahabzadeh S, Roy M, Bandyopadhyay A, Bose S. Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater. 2015 Apr;17:47–55. doi: 10.1016/j.actbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pieper JS, van Wachem PB, van Luyn MJA, Brouwer LA, Hafmans T, Veerkamp JH, van Kuppevelt TH. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials. 2000 Aug;21(16):1689–1699. doi: 10.1016/s0142-9612(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 11.Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007 Jun;28(16):2622–2630. doi: 10.1016/j.biomaterials.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma PX. Scaffolds for tissue fabrication. Mater Today. 2004 May;7(5):30–40. [Google Scholar]
- 13.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005 Jan;11(1–2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 14.Bose S, Bandyopadhyay A, editors. Materials and Devices for Bone disorders. Elsevier; 2016. [Google Scholar]
- 15.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015 Jun;13:S72–S81. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 16.Yim EKF, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005 Sep;26(26):5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEvoy A, Jeyam M, Ferrier G, Evans CE, Andrew JG. Synergistic effect of particles and cyclic pressure on cytokine production in human monocyte/macrophages: proposed role in periprosthetic osteolysis. Bone. 2002 Jan;30(1):171–177. doi: 10.1016/s8756-3282(01)00658-5. [DOI] [PubMed] [Google Scholar]
- 18.Lim JY, Donahue HJ. Cell Sensing and Response to Micro- and Nanostructured Surfaces Produced by Chemical and Topographic Patterning. Tissue Eng. 2007 Jun;13(8):1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 19.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999 Mar;20(6):573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 20.Boudou T, Crouzier T, Ren K, Blin G, Picart C. Multiple Functionalities of Polyelectrolyte Multilayer Films: New Biomedical Applications. Adv Mater. 2010;22(4):441–467. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- 21.Hammond PT. Engineering materials layer-by-layer: Challenges and opportunities in multilayer assembly. AIChE J. 2011;57(11):2928–2940. [Google Scholar]
- 22.Xia Y, Whitesides GM. Soft Lithography. Annu Rev Mater Sci. 1998;28(1):153–184. [Google Scholar]
- 23.Carvalho A, Pelaez-Vargas A, Gallego-Perez D, Grenho L, Fernandes MH, Ferraz MP, Hansford DJ, Monteiro FJ. Micropatterned silica thin films with nanohydroxyapatite micro-aggregates for guided tissue regeneration. Dent Mater Off Publ Acad Dent Mater. 2012 Dec;28(12):1250–1260. doi: 10.1016/j.dental.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.ASTM F2792-12a. Standard Terminology for Additive Manufacturing Technologies’ (Withdrawn 2015) ASTM International; West Conshohocken, PA: 2012. www.astm.org. [Google Scholar]
- 25.Wendel B, Rietzel D, Kühnlein F, Feulner R, Hülder G, Schmachtenberg E. Additive Processing of Polymers. Macromol Mater Eng. 2008 Oct;293(10):799–809. [Google Scholar]
- 26.Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010 Aug;31(24):6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 27.Yan M, Yu P. An Overview of Densification, Microstructure and Mechanical Property of Additively Manufactured Ti-6Al-4V — Comparison among Selective Laser Melting, Electron Beam Melting, Laser Metal Deposition and Selective Laser Sintering, and with Conventional Powder. 2015 [Google Scholar]
- 28.Gong X, Anderson T, Chou K. Review on powder-based electron beam additive manufacturing technology. Manuf Rev. 2014;1:2. [Google Scholar]
- 29.Tumbleston JR, Shirvanyants D, Ermoshkin N, Janusziewicz R, Johnson AR, Kelly D, Chen K, Pinschmidt R, Rolland JP, Ermoshkin A, Samulski ET, Desimone JM. Continuous liquid interface production of 3D objects. Science. 2015 Mar;347(6228):1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 30.Bandyopadhyay A, Bose S, Das S. 3D printing of biomaterials. MRS Bulletin. 2015;40(02):108–115. [Google Scholar]
- 31.Aksakal B, Yildirim ÖS, Gul H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J Fail Anal Prev. 2004 Jun;4(3):17–23. [Google Scholar]
- 32.Puleo DA, Nanci A. Understanding and controlling the bone–implant interface. Biomaterials. 1999 Dec;20(23):2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 33.Stevens MM, George JH. Exploring and Engineering the Cell Surface Interface. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 34.Sousa SR, Moradas-Ferreira P, Saramago B, Viseu Melo L, Barbosa MA. Human Serum Albumin Adsorption on TiO2 from Single Protein Solutions and from Plasma. Langmuir. 2004 Oct;20(22):9745–9754. doi: 10.1021/la049158d. [DOI] [PubMed] [Google Scholar]
- 35.Michiardi A, Aparicio C, Ratner BD, Planell JA, Gil J. The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces. Biomaterials. 2007 Feb;28(4):586–594. doi: 10.1016/j.biomaterials.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 36.Riemer A, Leuders S, Thöne M, Richard HA, Tröster T, Niendorf T. On the fatigue crack growth behavior in 316L stainless steel manufactured by selective laser melting. Eng Fract Mech. 2014 Apr;120:15–25. [Google Scholar]
- 37.Wycisk E, Solbach A, Siddique S, Herzog D, Walther F, Emmelmann C. Effects of Defects in Laser Additive Manufactured Ti-6Al-4V on Fatigue Properties. Phys Procedia. 2014 Jan;56:371–378. [Google Scholar]
- 38.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 39.Biggs MJP, Richards RG, Gadegaard N, Wilkinson CDW, Dalby MJ. Regulation of implant surface cell adhesion: characterization and quantification of S-phase primary osteoblast adhesions on biomimetic nanoscale substrates. J Orthop Res. 2007 Feb;25(2):273–282. doi: 10.1002/jor.20319. [DOI] [PubMed] [Google Scholar]
- 40.Wang XJ, Li YC, Lin JG, Yamada Y, Hodgson PD, Wen CE. In vitro bioactivity evaluation of titanium and niobium metals with different surface morphologies. Acta Biomater. 2008 Sep;4(5):1530–1535. doi: 10.1016/j.actbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Mcnamara LE, Sjöström T, Burgess KEV, Kim JJW, Liu E, Gordonov S, Moghe PV, Meek RMD, Oreffo ROC, Su B, Dalby MJ. Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials. 2011;32(30):7403–7410. doi: 10.1016/j.biomaterials.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 42.de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A. Enhancement ofin vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res A. 2007 Mar;80A(3):554–564. doi: 10.1002/jbm.a.30955. [DOI] [PubMed] [Google Scholar]
- 43.Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007 Jul;28(21):3188–3197. doi: 10.1016/j.biomaterials.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Stübinger S, Mosch I, Robotti P, Sidler M, Klein K, Ferguson SJ, Rechenberg B. Histological and biomechanical analysis of porous additive manufactured implants made by direct metal laser sintering: A pilot study in sheep: Analysis of Porous Additive Manufactured Implants. J Biomed Mater Res B Appl Biomater. 2013 Oct;101(7):1154–1163. doi: 10.1002/jbm.b.32925. [DOI] [PubMed] [Google Scholar]
- 45.Balla VK, Xue W, Bose S, Bandyopadhyay A. Engineered Porous Metals for Implants. JOM. 2008;60(5):45–49. [Google Scholar]
- 46.Akova T, Ucar Y, Tukay A, Balkaya MC, Brantley WA. Comparison of the bond strength of laser-sintered and cast base metal dental alloys to porcelain. Dent Mater. 2008 Oct;24(10):1400–1404. doi: 10.1016/j.dental.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Fu Q, Hong Y, Liu X, Fan H, Zhang X. A hierarchically graded bioactive scaffold bonded to titanium substrates for attachment to bone. Biomaterials. 2011 Oct;32(30):7333–7346. doi: 10.1016/j.biomaterials.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Yang W, Li X, Zhang X, Wang C, Meng X, Pei Y, Fan X, Lan P, Wang C, Li X, Guo Z. Improving Osteointegration and Osteogenesis of Three-Dimensional Porous Ti6Al4V Scaffolds by Polydopamine-Assisted Biomimetic Hydroxyapatite Coating. ACS Appl Mater Interfaces. 2015 Mar;7(10):5715–5724. doi: 10.1021/acsami.5b00331. [DOI] [PubMed] [Google Scholar]
- 49.Shah FA, Omar O, Suska F, Snis A, Matic A, Emanuelsson L, Norlindh B, Lausmaa J, Thomsen P, Palmquist A. Long-term osseointegration of 3D printed CoCr constructs with an interconnected open-pore architecture prepared by electron beam melting. Acta Biomater. 2016 May;36:296–309. doi: 10.1016/j.actbio.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 50.Hollander DA, Von Walter M, Wirtz T, Sellei R, Schmidt-Rohlfing B, Paar O, Erli H-J. Structural, mechanical and in vitro characterization of individually structured Ti–6Al–4V produced by direct laser forming. Biomaterials. 2006 Mar;27(7):955–963. doi: 10.1016/j.biomaterials.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 51.Sutradhar A, Park J, Carrau D, Miller MJ. Experimental validation of 3D printed patient-specific implants using digital image correlation and finite element analysis. Comput Biol Med. 2014 Sep;52:8–17. doi: 10.1016/j.compbiomed.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Cohen J, Reyes SA. Creation of a 3D printed temporal bone model from clinical CT data. Am J Otolaryngol. 2015 Sep;36(5):619–624. doi: 10.1016/j.amjoto.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Heinl P, Müller L, Körner C, Singer RF, Müller FA. Cellular Ti–6Al–4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008 Sep;4(5):1536–1544. doi: 10.1016/j.actbio.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Sallica-Leva E, Jardini AL, Fogagnolo JB. Microstructure and mechanical behavior of porous Ti–6Al–4V parts obtained by selective laser melting. J Mech Behav Biomed Mater. 2013 Oct;26:98–108. doi: 10.1016/j.jmbbm.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Elfick APD, Green SM, Krikler S, Unsworth A. The nature and dissemination of UHMWPE wear debris retrieved from periprosthetic tissue of THR. J Biomed Mater Res A. 2003 Apr;65A(1):95–108. doi: 10.1002/jbm.a.10455. [DOI] [PubMed] [Google Scholar]
- 56.Utzschneider S, Lorber V, Dedic M, Paulus A, Schröder C, Gottschalk O, Schmitt-Sody M, Jansson V. Biological activity and migration of wear particles in the knee joint: an in vivo comparison of six different polyethylene materials. J Mater Sci-Mater Med. 2014;25(6):1599–1612. doi: 10.1007/s10856-014-5176-6. [DOI] [PubMed] [Google Scholar]
- 57.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005 Apr;26(11):1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 58.Wang JY, Wicklund BH, Gustilo RB, Tsukayama DT. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomaterials. 1996;17(23):2233–2240. doi: 10.1016/0142-9612(96)00072-5. [DOI] [PubMed] [Google Scholar]
- 59.Pijls BG, Valstar ER, Nouta K-A, Plevier JWM, Fiocco M, Middeldorp S, Nelissen RGHH. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop. 2012 Dec;83(6):614–624. doi: 10.3109/17453674.2012.747052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van der Vis HM, Aspenberg P, Marti RK, Tigchelaar W, Van Noorden CJ. Fluid pressure causes bone resorption in a rabbit model of prosthetic loosening. Clin Orthop. 1998 May;(350):201–208. [PubMed] [Google Scholar]
- 61.Aspenberg P, Van der Vis H. Migration, particles, and fluid pressure. A discussion of causes of prosthetic loosening. Clin Orthop. 1998 Jul;(352):75–80. [PubMed] [Google Scholar]
- 62.Balla VK, Soderlind J, Bose S, Bandyopadhyay A. Microstructure, mechanical and wear properties of laser surface melted Ti6Al4V alloy. J Mech Behav Biomed Mater. 2014 Apr;32:335–344. doi: 10.1016/j.jmbbm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Balla VK, Das M, Bose S, Ram GDJ, Manna I. Laser surface modification of 316 L stainless steel with bioactive hydroxyapatite. Mater Sci Eng C. 2013 Dec;33(8):4594–4598. doi: 10.1016/j.msec.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 64.Roy M, Balla VK, Bandyopadhyay A, Bose S. Compositionally graded hydroxyapatite/tricalcium phosphate coating on Ti by laser and induction plasma. Acta Biomater. 2011 Feb;7(2):866–873. doi: 10.1016/j.actbio.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 65.Roy M, Balla VK, Bandyopadhyay A, Bose S. MgO-Doped Tantalum Coating on Ti: Microstructural Study and Biocompatibility Evaluation. ACS Appl Mater Interfaces. 2012;4(2):577–580. doi: 10.1021/am201365e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt J, Sachs M, Blümel C, Winzer B, Toni F, Wirth K-E, Peukert W. A novel process route for the production of spherical LBM polymer powders with small size and good flowability. Powder Technol. 2014 Jul;261:78–86. [Google Scholar]
- 67.Aoki J, Serruys PW, van Beusekom H, Ong ATL, Mcfadden EP, Sianos G, van Der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJB. Endothelial Progenitor Cell Capture by Stents Coated With Antibody Against CD34: The HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J Am Coll Cardiol. 2005 May;45(10):1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 68.Shirota T, He H, Yasui H, Matsuda T. Human Endothelial Progenitor Cell-Seeded Hybrid Graft: Proliferative and Antithrombogenic Potentials in Vitro and Fabrication Processing. Tissue Eng. 2003 Feb;9(1):127–136. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 69.McGuigan AP, Sefton MV. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials. 2007 Jun;28(16):2547–2571. doi: 10.1016/j.biomaterials.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nothhaft M, Lutter C, Rzany A, Cicha I, Garlichs C. TCT-803 Influence of stent surface microstructuring on endothelial cell migration and substrate thrombogenicity. J Am Coll Cardiol. 2013 Oct;62(18, Supplement 1):B244. [Google Scholar]
- 71.Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, Seitz B, Kapurniotu A, Kwok C, Dewor M, Bosserhoff A-K, Bernhagen J, Hanrath P, Hoffmann R, Weber C. A Novel Drug-Eluting Stent Coated With an Integrin-Binding Cyclic Arg-Gly-Asp Peptide Inhibits Neointimal Hyperplasia by Recruiting Endothelial Progenitor Cells. J Am Coll Cardiol. 2006 May;47(9):1786–1795. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 72.Tsai W-B, Grunkemeier JM, McFarland CD, Horbett TA. Platelet adhesion to polystyrene-based surfaces preadsorbed with plasmas selectively depleted in fibrinogen, fibronectin, vitronectin, or von Willebrand’s factor. J Biomed Mater Res. 2002 Jun;60(3):348–359. doi: 10.1002/jbm.10048. [DOI] [PubMed] [Google Scholar]
- 73.Feng W, Zhu S, Ishihara K, Brash JL. Protein resistant surfaces: Comparison of acrylate graft polymers bearing oligo-ethylene oxide and phosphorylcholine side chains. Biointerphases. 2006 Mar;1(1):50–60. doi: 10.1116/1.2187495. [DOI] [PubMed] [Google Scholar]
- 74.Werner C, Maitz MF, Sperling C. Current strategies towards hemocompatible coatings. J Mater Chem. 2007;17(32):3376–3384. [Google Scholar]
- 75.Ragaseema VM, Unnikrishnan S, Kalliyana Krishnan V, Krishnan LK. The antithrombotic and antimicrobial properties of PEG-protected silver nanoparticle coated surfaces. Biomaterials. 2012 Apr;33(11):3083–3092. doi: 10.1016/j.biomaterials.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Wardrip NC, Dsouza M, Urgun-Demirtas M, Snyder SW, Gilbert JA, Arnusch CJ. Printing-Assisted Surface Modifications of Patterned Ultrafiltration Membranes. ACS Appl Mater Interfaces. 2016 Nov;8(44):30271–30280. doi: 10.1021/acsami.6b11331. [DOI] [PubMed] [Google Scholar]
- 77.Collier JH, Segura T. Evolving the use of peptides as components of biomaterials. Biomaterials. 2011 Jun;32(18):4198–4204. doi: 10.1016/j.biomaterials.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirano Y, Mooney DJ. Peptide and Protein Presenting Materials for Tissue Engineering. Adv Mater. 2004 Jan;16(1):17–25. [Google Scholar]
- 79.Fraioli R, Rechenmacher F, Neubauer S, Manero JM, Gil J, Kessler H, Mas-Moruno C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf B Biointerfaces. 2015 Apr;128:191–200. doi: 10.1016/j.colsurfb.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 80.Bose S, Sugiura S, Bandyopadhyay A. Processing of Controlled Porosity Ceramic Structures via Fused Deposition Process. Scripta Materialia. 1999;41(9):1009–14. [Google Scholar]
- 81.Darsell J, Bose S, Hosick H, Bandyopadhyay A. From CT Scans to Ceramic Bone Grafts. Journal of the American Ceramic Society. 2003;86(7):1076–80. [Google Scholar]
- 82.Bose S, Fielding G, Tarafder S, Bandyopadhyay A. Understanding of dopant – induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends in Biotechnology. 2013 Oct;31(10):594–605. doi: 10.1016/j.tibtech.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tarafder S, Bose S. Polycaprolactone-Coated 3D Printed Tricalcium Phosphate Scaffolds for Bone Tissue Engineering: In Vitro Alendronate Release Behavior and Local Delivery Effect on In Vivo Osteogenesis. ACS Appl Mater Interfaces. 2014;6(13):9955–9965. doi: 10.1021/am501048n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fielding G, Bose S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013;9(11):9137–9148. doi: 10.1016/j.actbio.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bose S, Tarafder S, Bandyopadhyay A. Effect of Chemistry on Osteogenesis and Angiogenesis Towards Bone Tissue Engineering Using 3D Printed Scaffolds. Ann Biomed Eng. 2017 Jan;45(1):261–272. doi: 10.1007/s10439-016-1646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song S-W, Hidajat K, Kawi S. Functionalized SBA-15 Materials as Carriers for Controlled Drug Delivery: Influence of Surface Properties on Matrix–Drug Interactions. Langmuir. 2005 Oct;21(21):9568–9575. doi: 10.1021/la051167e. [DOI] [PubMed] [Google Scholar]
- 87.Zhu M, Li K, Zhu Y, Zhang J, Ye X. 3D-printed hierarchical scaffold for localized isoniazid/rifampin drug delivery and osteoarticular tuberculosis therapy. Acta Biomater. 2015 Apr;16:145–155. doi: 10.1016/j.actbio.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 88.Gloria A, Causa F, Russo T, Battista E, Della Moglie R, Zeppetelli S, De Santis R, Netti PA, Ambrosio L. Three-Dimensional Poly(ε-caprolactone) Bioactive Scaffolds with Controlled Structural and Surface Properties. Biomacromolecules. 2012 Nov;13(11):3510–3521. doi: 10.1021/bm300818y. [DOI] [PubMed] [Google Scholar]
- 89.Puppi D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010 Apr;35(4):403–440. [Google Scholar]
- 90.Chiono V, Mozetic P, Boffito M, Sartori S, Gioffredi E, Silvestri A, Rainer A, Giannitelli SM, Trombetta M, Nurzynska D, Di Meglio F, Castaldo C, Miraglia R, Montagnani S, Ciardelli G. Polyurethane-based scaffolds for myocardial tissue engineering. Interface Focus. 2014 Feb;4(1):20130045. doi: 10.1098/rsfs.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007 Nov;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan KH, Chua CK, Leong KF, Cheah CM, Cheang P, Abu Bakar MS, Cha SW. Scaffold development using selective laser sintering of polyetheretherketone–hydroxyapatite biocomposite blends. Biomaterials. 2003 Aug;24(18):3115–3123. doi: 10.1016/s0142-9612(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 93.Lin Q, Xu X, Wang B, Shen C, Tang J, Han Y, Chen H. Hydrated polysaccharide multilayer as an intraocular lens surface coating for biocompatibility improvements. J Mater Chem B. 2015 Apr;3(18):3695–3703. doi: 10.1039/c5tb00111k. [DOI] [PubMed] [Google Scholar]
- 94.Wang H, Leeuwenburgh SCG, Li Y, Jansen JA. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng Part B Rev. 2012 Feb;18(1):24–39. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dawson JI, Wahl DA, Lanham SA, Kanczler JM, Czernuszka JT, Oreffo ROC. Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2008 Jul;29(21):3105–3116. doi: 10.1016/j.biomaterials.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 96.Li DJ, Cui FZ, Gu HQ. F+ ion implantation induced cell attachment on intraocular lens. Biomaterials. 1999 Oct;20(20):1889–1896. doi: 10.1016/s0142-9612(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 97.Lee K-W, Wang S, Yaszemski MJ, Lu L. Physical properties and cellular responses to crosslinkable poly(propylene fumarate)/hydroxyapatite nanocomposites. Biomaterials. 2008 Jul;29(19):2839–2848. doi: 10.1016/j.biomaterials.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014 Apr;35(13):4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siow KS, Britcher L, Kumar S, Griesser HJ. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization - A Review. Plasma Process Polym. 2006 Aug;3(6–7):392–418. [Google Scholar]
- 100.Tsougeni K, Petrou PS, Awsiuk K, Marzec MM, Ioannidis N, Petrouleas V, Tserepi A, Kakabakos SE, Gogolides E. Direct Covalent Biomolecule Immobilization on Plasma-Nanotextured Chemically Stable Substrates. ACS Appl Mater Interfaces. 2015 Jul;7(27):14670–14681. doi: 10.1021/acsami.5b01754. [DOI] [PubMed] [Google Scholar]
- 101.Truica-Marasescu F, Pham S, Wertheimer MR. VUV processing of polymers: Surface modification and deposition of organic thin films. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At. 2007 Dec;265(1):31–36. [Google Scholar]
- 102.Ino K, Ito A, Wu Y, Saito N, Hibino E, Takai O, Honda H. Application of Ultra-Water-Repellent Surface to Cell Culture. J Biosci Bioeng. 2007;104(5):420–423. doi: 10.1263/jbb.104.420. [DOI] [PubMed] [Google Scholar]
- 103.Wei J, Igarashi T, Okumori N, Igarashi T, Maetani T, Liu B, Yoshinari M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed Mater. 2009;4(4):45002. doi: 10.1088/1748-6041/4/4/045002. [DOI] [PubMed] [Google Scholar]
- 104.Yu D-G, Branford-White C, Ma Z-H, Zhu L-M, Li X-Y, Yang X-L. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int J Pharm. 2009 Mar;370(1–2):160–166. doi: 10.1016/j.ijpharm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Water JJ, Bohr A, Boetker J, Aho J, Sandler N, Nielsen HM, Rantanen J. Three-Dimensional Printing of Drug-Eluting Implants: Preparation of an Antimicrobial Polylactide Feedstock Material. J Pharm Sci. 2015 Mar;104(3):1099–1107. doi: 10.1002/jps.24305. [DOI] [PubMed] [Google Scholar]
- 106.Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015 Oct;494(2):643–650. doi: 10.1016/j.ijpharm.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 107.Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Controlled Release. 2015 Nov;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 108.Khaled SA, Burley JC, Alexander MR, Roberts CJ. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharm. 2014 Jan;461(1–2):105–111. doi: 10.1016/j.ijpharm.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 109.Park SA, Lee SJ, Lim KS, Bae IH, Lee JH, Kim WD, Jeong MH, Park J-K. In vivo evaluation and characterization of a bio-absorbable drug-coated stent fabricated using a 3D-printing system. Mater Lett. 2015 Feb;141:355–358. [Google Scholar]
- 110.Moulton SE, Wallace GG. 3-dimensional (3D) fabricated polymer based drug delivery systems. J Controlled Release. 2014 Nov;193:27–34. doi: 10.1016/j.jconrel.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Ringeisen BR, Othon CM, Barron JA, Young D, Spargo BJ. Jet-based methods to print living cells. Biotechnol J. 2006 Sep;1(9):930–948. doi: 10.1002/biot.200600058. [DOI] [PubMed] [Google Scholar]
- 112.Xiong R, Zhang Z, Chai W, Huang Y, Chrisey DB. Freeform drop-on-demand laser printing of 3D alginate and cellular constructs. Biofabrication. 2015;7(4):45011. doi: 10.1088/1758-5090/7/4/045011. [DOI] [PubMed] [Google Scholar]
- 113.Chen CY, Barron JA, Ringeisen BR. Cell patterning without chemical surface modification: Cell–cell interactions between printed bovine aortic endothelial cells (BAEC) on a homogeneous cell-adherent hydrogel. Appl Surf Sci. 2006 Oct;252(24):8641–8645. [Google Scholar]
- 114.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009 Oct;30(31):6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 115.Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo S-S, Vincent PA, Dai G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014 Sep;35(28):8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christensen K, Xu C, Chai W, Zhang Z, Fu J, Huang Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng. 2015 May;112(5):1047–1055. doi: 10.1002/bit.25501. [DOI] [PubMed] [Google Scholar]
- 117.Yang CH, Wang MX, Haider H, Yang JH, Sun J-Y, Chen YM, Zhou J, Suo Z. Strengthening Alginate/Polyacrylamide Hydrogels Using Various Multivalent Cations. ACS Appl Mater Interfaces. 2013 Nov;5(21):10418–10422. doi: 10.1021/am403966x. [DOI] [PubMed] [Google Scholar]
- 118.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012 Jan;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials. 2007 Sep;28(27):3936–3943. doi: 10.1016/j.biomaterials.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 120.Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P, Wang F, Chichkov B, Li W, Steinhoff G. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011 Dec;32(35):9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 121.Derby B. Bioprinting: inkjet printing proteins and hybrid cell-containing materials and structures. J Mater Chem. 2008 Nov;18(47):5717–5721. [Google Scholar]
- 122.Setti L, Fraleoni-Morgera A, Ballarin B, Filippini A, Frascaro D, Piana C. An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens Bioelectron. 2005 Apr;20(10):2019–2026. doi: 10.1016/j.bios.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 123.Barron JA, Rosen R, Jones-Meehan J, Spargo BJ, Belkin S, Ringeisen BR. Biological laser printing of genetically modified Escherichia coli for biosensor applications. Biosens Bioelectron. 2004 Sep;20(2):246–252. doi: 10.1016/j.bios.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 124.Yu J-S, Kim I, Kim J-S, Jo J, Larsen-olsen TT, Sndergaard RR, Hsel M, Angmo D, Jrgensen M, Krebs FC. Silver front electrode grids for ITO-free all printed polymer solar cells with embedded and raised topographies, prepared by thermal imprint, flexographic and inkjet roll-to-roll processes. Nanoscale. 2012;4(19):6032–6040. doi: 10.1039/c2nr31508d. [DOI] [PubMed] [Google Scholar]
- 125.Rho Y, Kang K-T, Lee D. Highly crystalline Ni/NiO hybrid electrodes processed by inkjet printing and laser-induced reductive sintering under ambient conditions. Nanoscale. 2016;8(16):8976–8985. doi: 10.1039/c6nr00708b. [DOI] [PubMed] [Google Scholar]
- 126.Dixon C, Ng AHC, Fobel R, Miltenburg MB, Wheeler AR. An inkjet printed, roll-coated digital microfluidic device for inexpensive, miniaturized diagnostic assays. Lab Chip. 2016;16(23):4560–4568. doi: 10.1039/c6lc01064d. [DOI] [PubMed] [Google Scholar]
- 127.Noolandi J, Peterman MC, Huie P, Lee C, Blumenkranz MS, Fishman HA. Towards a Neurotransmitter-Based Retinal Prosthesis Using an Inkjet Print-head. Biomed Microdevices. 2003 Sep;5(3):195–199. [Google Scholar]
- 128.Vähä-Nissi M, Sievänen J, Salo E, Heikkilä P, Kenttä E, Johansson L-S, Koskinen JT, Harlin A. Atomic and molecular layer deposition for surface modification. J Solid State Chem. 2014 Jun;214:7–11. [Google Scholar]
- 129.Goodridge RD, Tuck CJ, Hague RJM. Laser sintering of polyamides and other polymers. Prog Mater Sci. 2012 Feb;57(2):229–267. [Google Scholar]
- 130.Yamazaki T. Development of A Hybrid Multi-tasking Machine Tool: Integration of Additive Manufacturing Technology with CNC Machining. Procedia CIRP. 2016;42:81–86. [Google Scholar]
- 131.Feng X, Chen A, Zhang Y, Wang J, Shao L, Wei L. Central nervous system toxicity of metallic nanoparticles. Int J Nanomedicine. 2015;10:4321–4340. doi: 10.2147/IJN.S78308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.FDA Plans Meeting to Explore Regulation, Medical Uses of 3D Printing Technology | RAPS. [Online]. Available: http://www.raps.org/regulatory-focus/news/2014/05/19000/FDA-3D-Printing-Guidance-and-Meeting/. [Accessed: 17-Aug-2016].
- 133.C. for D. and R. Health. 3D Printing of Medical Devices - FDA’s Role in 3D Printing. [Online] Available: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/3DPrintingofMedicalDevices/ucm500548.htm [Accessed: 01-Dec-2016]
- 134.Boley JW, White EL, Chiu GT-C, Kramer RK. Stretchable Electronics: Direct Writing of Gallium-Indium Alloy for Stretchable Electronics (Adv. Funct. Mater. 23/2014) Adv Funct Mater. 2014 Jun;24(23):3474–3474. [Google Scholar]
- 135.Materials science: Liquid metal printed in 3D. Nature. 2013 Jul;499(7458):256–257. [Google Scholar]
- 136.Visser CW, Pohl R, Sun C, Römer G-W, in ‘t Veld B Huis, Lohse D. 3D Printing: Toward 3D Printing of Pure Metals by Laser-Induced Forward Transfer (Adv. Mater. 27/2015) Adv Mater. 2015 Jul;27(27):4103–4103. doi: 10.1002/adma.201501058. [DOI] [PubMed] [Google Scholar]
- 137.Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. Microwave Sintered 3D Printed Tricalcium Phosphate Scaffolds for Bone Tissue Engineering. Journal of Tissue Engineering and Regenerative Medicine. 2013;7(8):631–641. doi: 10.1002/term.555. [DOI] [PMC free article] [PubMed] [Google Scholar]


