Abstract
Family planning is essential for any comprehensive treatment plan for women of reproductive age with multiple sclerosis (MS), including counseling on using effective contraception to optimally time desired and prevent unintended pregnancies. This topical review summarizes the first evidence-based recommendations on contraception safety for women with MS. In 2016, evidence-based recommendations for contraceptive use by women with MS were included in US Medical Eligibility Criteria for Contraceptive Use. They were developed after review of published scientific evidence on contraception safety and consultation with experts. We summarize and expand on the main conclusions of the Centers for Disease Control and Prevention guidance. Most contraceptive methods appear based on current evidence to be safe for women with MS. The only restriction is use of combined hormonal contraceptives among women with MS with prolonged immobility because of concerns about possible venous thromboembolism. Disease-modifying therapies (DMTs) do not appear to decrease the effectiveness of hormonal contraception although formal drug–drug interaction studies are limited. Neurologists can help women with MS make contraceptive choices that factor their level of disability, immobility, and medication use. For women with MS taking potentially teratogenic medications, highly effective methods that are long-acting (e.g. intrauterine devices, implants) might be the best option.
Keywords: Multiple sclerosis, contraception, evidence-based practice, practice guidelines
Introduction
Contraception is an important consideration for women with multiple sclerosis (MS). MS is more prevalent among females, and the peak age of onset for women is during the childbearing years.1,2 MS does not seem to significantly impair fertility although there is emerging data on decreased ovarian reserve and higher prevalence of thyroid autoimmunity in MS patients, possibly affecting fertility.2–5 A chronic neurologic illness may also influence pregnancy intentions. Although some patients report having completed their families prior to MS diagnosis, one study found that among women with MS who did not become pregnant after diagnosis, nearly one-third cited MS-related concerns such as symptoms interfering with parenting, burdening their partner, and children inheriting MS.6 Many women with MS use disease modifying therapies (DMTs). DMTs are generally not recommended for women trying to become pregnant and there are known risks to the fetus associated with some treatments, and none are specifically approved for use in pregnancy.7 If a woman is on certain DMTs, a washout period before conception is recommended.2 Table 1 summarizes the DMTs in wide use today with known or suspected pregnancy risks. Providers are always encouraged to review up-to-date product-specific information for their practice location and scope, prior to giving advice to their patients.
Table 1.
FDA- and EMA-approved disease-modifying therapies in wide use today, with known or suspected pregnancy risksa.8–13
| Name | Teratogenic in animal models | Signal for increased malformations in human pregnancies | Recommended washout period before conception attempt |
|---|---|---|---|
| Fingolimod | Yes | Yes | 2 months |
| Dimethyl fumarate | Yes | No | None |
| Teriflunomide | Yes | Yes in precursor leflunomide | Until plasma levels are below 0.02 μg/mL |
| Alemtuzumab | No (decreased embryonic survival observed in some species) | No | 4 months |
| Natalizumab | No (decreased embryonic survival observed in some species) | Nob (transient hematologic abnormalities in exposed newborns observed) | 1–2 menstrual cycles unless concern for disease reactivation in pregnancy |
| Daclizumab | No | None in clinical development programs | 4 months |
FDA: Food and Drug Administration; EMA: European Medicines Agency.
Extensive body of published literature does not support any increased pregnancy risks with the glatiramer acetate and interferons. Off-label treatments or treatments not in wide clinical use today, such as mitoxantrone, are not included in this table. FDA pregnancy risk categories B through X have been replaced by new risk classification; the older drug pregnancy risk designations are not included in this table.
May not be enough published data to make final conclusions.
The optimal time for a woman with MS to conceive should be considered individually, based on the activity of her disease, her response to treatment, and the availability of resources to manage the challenges of early motherhood. As such, family planning should be an essential part of any comprehensive treatment plan for women of reproductive age with MS, including regular counseling on the use of effective contraception to optimally time desired pregnancies and prevent unintended pregnancies. However, neurologists may not be well equipped to discuss contraception with patients. A survey of female neurologists from the United States and Canada found that most referred their patients to an obstetrician–gynecologist or internist for contraceptive counseling, and many were unsure whether their MS patients used contraception or the type of method used.14
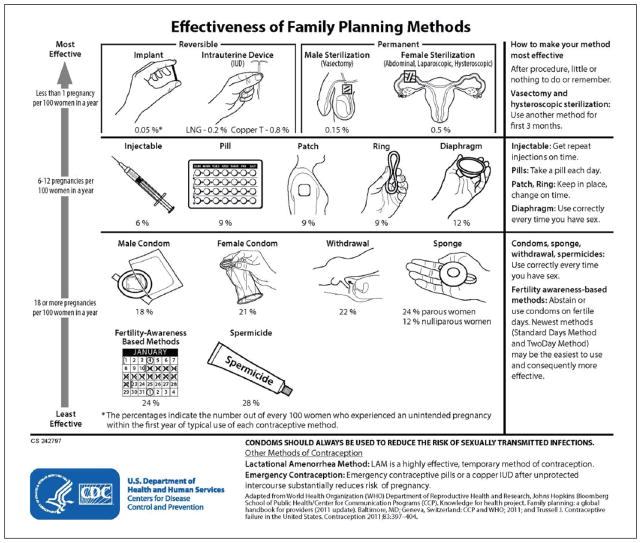
Many methods of contraception are available to women and couples. When choosing an appropriate contraceptive method, factors to consider include safety, availability, acceptability, and effectiveness. For women with MS, additional issues may include difficulty swallowing pills or manual dexterity needed for placing vaginal rings and barrier methods. The effectiveness of a contraceptive method depends on the inherent effectiveness of the method itself and on how consistency and correctly the method is used (Figure 1).15 Whereas pregnancy rates during perfect use show how effective a method is in a hypothetical “perfect use” scenario, pregnancy rates during typical use show how effective a method is during actual use, including inconsistent and/or incorrect use.16 The most effective reversible methods of contraception during typical use are intrauterine devices (IUDs) and implants, collectively known as long-acting, reversible contraception (LARC). LARC methods are highly effective because once in place, they do not require regular user compliance. LARC methods provide pregnancy protection for 3–10 years depending on the device but can be removed at any time if the woman chooses to become pregnant (or for any other reason). Methods that are user-dependent, such as oral contraceptive pills and condoms, rely on consistent and correct use and, as a result, are less effective during typical use. When counseling women about contraceptive options, the full range and effectiveness of methods for which they are medically eligible should be discussed.
Figure 1.
Effectiveness of family planning methods.
Although several clinical reviews are available on the management of and therapeutic considerations for women with MS during the reproductive years,2,7,17,18 the focus of these reviews has largely been on issues around the time pregnancy. Topics have included the effect of pregnancy on the MS disease course; the management of MS during pregnancy, labor, and postpartum; and safety of breastfeeding while on DMTs. Safe and effective contraceptive choices for women with MS have not been included. This topical review will specifically focus on the safety of contraception being used for contraceptive purposes for women with MS; it does not consider the safety of contraception as primary or adjunct MS therapy.
Contraception guidance for healthcare providers
The US Centers for Disease Control and Prevention (CDC) publishes the US Medical Eligibility Criteria for Contraceptive Use15 (US MEC), adapted from the World Health Organization Medical Eligibility Criteria for Contraceptive Use19 (WHO MEC), which provides evidence-based guidance on the safety of contraceptive methods for women with certain characteristics or medical conditions. The recommendations refer to contraceptive methods being used for contraceptive purposes and do not consider the use of contraceptive methods for treatment of medical conditions because the eligibility criteria in these situations might be different. The goal of the recommendations is to remove unnecessary medical barriers to accessing and using contraception, thus reducing unintended pregnancies. They are intended to be a source of clinical guidance assisting healthcare providers when they counsel women, men, and couples about family planning. Although adapted from the WHO MEC to be specific to the US context (e.g. addition of recommendations for new medical conditions), the recommendations in the US MEC are based on rigorous identification and critical appraisal of the scientific evidence and input from experts, and can reasonably be applied in other settings.
For each medical condition and personal characteristic included in the US MEC, eligibility categories have been assigned assessing the safety of the specific contraceptive method for women with the specific medical condition or personal characteristic:15
Category 1 comprises conditions for which no restrictions exist for use of the contraceptive method.
Category 2 comprises conditions for which the contraceptive method generally can be used although careful follow-up might be required.
Category 3 comprises conditions for which use of the contraceptive method usually is not recommended unless other more appropriate contraceptive methods are not available or acceptable. The severity of the condition and the availability, practicality, and acceptability of alternative contraceptive methods should be considered, and careful follow-up is required. Hence, provision of a contraceptive method to a woman with a condition classified as category 3 requires careful clinical judgment and access to clinical services.
Category 4 comprises conditions that represent an unacceptable health risk if the contraceptive method is used.
The US MEC was first published in 2010, but did not include recommendations for contraceptive use by women with MS. The WHO MEC also does not include recommendations for contraceptive use by women with MS.
Updating the contraception guidance to include MS
As part of a process to update the US MEC, the CDC held a meeting with family planning experts and representatives from partner organizations to solicit input on the scope and process for updating the US MEC. Based on CDC receipt of feedback from healthcare providers via email and questions received at conferences about contraception for women with MS, MS was identified as a new medical condition to add.
A systematic review was conducted to evaluate the evidence on the safety of contraceptive use among women with MS.20 The PubMed database was searched for peer-reviewed articles published in any language from database inception through July 2015. The review included studies that examined health outcomes among women diagnosed with MS initiating or continuing a contraceptive method, but excluded studies that evaluated the risk of developing MS among women using contraception. Prior to conducting the review, potential safety concerns were identified in consultation with MS experts. These included possible worsening of disease through relapse or disease progression with hormonal method use; compounded risk for venous thromboembolism (VTE) with hormonal method use among MS patients with increased risk for VTE due to immobility and comprised bone health with use of progestin-only injectables (depot medroxyprogesterone acetate (DMPA)). Potential logistical concerns related to contraceptive use among women with MS were also identified during scoping discussions. These included potential difficulty with placement of IUDs, vaginal rings, and diaphragms in women with vaginal stenosis, and potential difficultly with patient placement of vaginal rings and diaphragms for women with impaired fine motor control in the hands or significant spasticity in the legs.
The systematic review identified 111 articles, of which four studies met our inclusion criteria: one randomized controlled trial (Italy),21 two retrospective cohort studies (Italy and Portugal),22,23 and one cross-sectional study (Germany).24 Although the body of evidence was limited in quantity and quality of studies, the review concluded that the use of combined oral contraceptives or oral contraceptives (type not specified) does not worsen the clinical course of MS, defined as disability level, disease severity or progression, relapse, or number of new brain lesions on magnetic resonance imaging after 96 weeks of follow-up. No studies were identified that examined the safety of other contraceptive methods, and no studies were identified that examined other safety outcomes (e.g. VTE or changes in bone mineral density). Research gaps were identified including the need for additional studies with strong designs that examine a broader range of contraceptive methods and outcomes, report the timing of contraceptive use relative to outcome measurement, and include women with different types of MS. The evidence was presented to a national panel of experts invited to provide their individual perspectives on the scientific evidence presented and potential recommendations. After the meeting, CDC developed the recommendations taking into consideration the perspectives provided during the meeting; further details on the process are described elsewhere.15
Recommendations for women with MS
The US MEC 201615 includes recommendations for contraceptive use by women with MS (Table 2).
Table 2.
Summary of classificationsa for intrauterine devices, hormonal contraceptive methods, and barrier methods for women with multiple sclerosis.
| Cu-IUD | LNG-IUD | Implants | DMPA | POPs | CHCs | Barrier methods | |
|---|---|---|---|---|---|---|---|
| Multiple sclerosis | |||||||
| a. With prolonged immobility | 1 | 1 | 1 | 2 | 1 | 3 | 1 |
| b. Without prolonged immobility | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
CHCs: combined hormonal contraceptives; Cu-IUD: copper-containing intrauterine device; DMPA: depot medroxyprogesterone acetate; LNG-IUD: levonorgestrel-releasing intrauterine device; POPs: progestin-only pills.
1 = A condition for which there is no restriction for the use of the contraceptive method; 2 = A condition for which the advantages of using the method generally outweigh the theoretical or proven risks; 3 = A condition for which the theoretical or proven risk usually outweigh the advantages of using the method; and 4 = A condition that represents an unacceptable health risk if the contraceptive method is used.
IUDs
IUDs include the copper-containing IUD (Cu-IUD) and the levonorgestrel-releasing IUD (LNG-IUD). For both the Cu-IUD and the LNG-IUD, there are no restrictions for use by women with MS (Category 1).
Progestin-only contraceptives
Progestin-only contraceptives include etonogestrel implants, DMPA, and progestin-only pills (POPs). For implants and POPs, there are no restrictions for use by women with MS (Category 1). For DMPA, women with MS can generally use the method although careful follow-up might be required (Category 2) related to concerns about bone health. Women with MS might have compromised bone health from disease-related disability, immobility, or use of corticosteroids,20 and use of DMPA has been associated with small changes in bone mineral density.25
Combined hormonal contraceptives
Combined hormonal contraceptives (CHCs) include low-dose combined oral contraceptives (containing ≤35 μg ethinyl estradiol), the hormonal patch, and the vaginal ring. Classifications for CHCs for women with MS differ based on immobility status. For women with MS without prolonged immobility, there are no restrictions for use of CHCs (Category 1). However, for women with MS with prolonged immobility, CHCs are usually not recommended unless other more appropriate contraceptive methods are not available or acceptable (Category 3). This is because of inferred concerns about VTE risk. Although no evidence was found examining the effect of CHCs on VTE among women with MS, women with MS are at higher risk than unaffected women for VTE,26,27 and CHCs increase VTE risk.28
Barrier methods
Barrier methods include condoms (male and female), spermicides, and diaphragm with spermicide or cervical cap. For these methods, there are no restrictions for use by women with MS (Category 1).
Other methods
Other methods of contraception are included in the US MEC 2016 including fertility awareness-based methods, the lactational amenorrhea method, coitus interruptus (withdrawal), and female and male sterilization. None of these methods are restricted for women with MS.
Although a specific contraceptive method may be classified as a Category 1 (which means that the method can be used with no restrictions related to safety), it does not necessarily mean that the method is the best choice for the patient. When counseling women of reproductive age with MS about contraception, providers, including neurologists, should always consider the individual social, cultural, and clinical circumstances of the patient seeking advice. For example, for a woman with MS taking potentially feto-toxic DMTs, more effective methods such as LARC might be the best option to avoid unintended pregnancy or delay pregnancy until teratogenic medications are no longer needed.
Drug interaction considerations
DMTs that were approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for treatment of relapsing forms of MS prior to July 2015 were considered as related to drug interactions with contraception. Alemtuzumab, approved in the United States in 2014, was the most recently approved DMT considered. DMTs do not appear to decrease the effectiveness of hormonal contraception although formal drug–drug interaction studies are limited.29 However, all medications should be reviewed at every visit, as some therapies taken for MS symptom management may affect contraceptive efficacy. A number of medications used for management of specific MS symptoms and other common illness may have interactions with OCPs. These interactions may result in either decreased efficacy of oral contraceptives or a change in efficacy of the other medications. One notable example is modafinil, commonly used (off label) to treat MS fatigue.2 Oral modafinil has been shown to decrease the level of ethinyl estradiol by altering drug metabolism through the cytochrome P450-mediated oxidative pathways.30,31 Thus, efficacy of oral contraceptives may be reduced in MS patients taking modafinil, potentially leading to unintended pregnancy. Another example is anticonvulsant therapy. Certain anticonvulsants (phenytoin, carbamazepine, barbiturates, primidone, topiramate, and oxcarbazepine) lower the effectiveness of progestin-only contraception and CHCs.15 Women who are long-term users of these drugs should be encouraged to use another contraceptive method. Lamotrigine does not appear to affect the efficacy of progestin-only contraception, but pharmacokinetic studies have shown that levels of lamotrigine decrease significantly during the use of combined oral contraceptives.15 The US MEC includes recommendations for contraceptive use for select drug interactions.
Other contraception guidance and available healthcare provider tools
The CDC also publishes the US Selected Practice Recommendations for Contraceptive Use32 (US SPR), adapted from the WHO Selected Practice Recommendations for Contraceptive Use33 (WHO SPR), a companion document to the US MEC that addresses a select group of common, yet complex, issues regarding initiation and use of specific contraceptive methods. The US SPR includes topics such as how to be reasonably certain that a woman is not pregnant, when to start using specific contraceptive methods, examinations and tests needed before initiation of contraceptive methods, routine follow-up after contraceptive initiation, and management of women with bleeding irregularities while using contraception. The US SPR also includes recommended actions for women in the event of late DMPA reinjections, late or missed doses of CHCs, and missed POPs. While it is less likely that MS neurologists will be routinely handling contraceptive management issues such as side effects, the general awareness of these resources can be very helpful.
Additionally, CDC, in collaboration with the US Office of Population Affairs, publishes Providing Quality Family Planning Services34 (QFP). The QFP provides recommendations on how to provide family planning services so that individuals can achieve their desired number and spacing of children, increase the chance that a baby will be born healthy, and improve their health even if they choose to not have children. The QFP is intended for all current or potential providers of family planning services and includes topics such as providing contraceptive counseling, helping clients achieve pregnancy, basic infertility services, preconception health services, and sexually transmitted disease services.
In an effort to disseminate and promote use of the US MEC, US SPR, and QFP, several provider tools have been developed and are available on CDC’s Division of Reproductive Health website at no cost for download or order (Table 3). Although these guidance documents and provider tools were developed to assist US healthcare providers, the recommendations and tools can reasonably be used in other countries in the absence of locally adapted guidance.
Table 3.
US MEC, US SPR, and QFP provider tools.a
| Provider tool | Description |
|---|---|
| US MEC, US SPR, QFP | Print versions of the guidance documents |
| US MEC/US SPR app | An easy to use reference that combines information from both the US MEC and US SPR that features a streamlined interface so providers can access the guidance quickly and easily (available for iOS and Android operating systems) |
| QFP app | An easy to use reference to help providers make the best use of every family planning visit by streamlining decisions on what services to offer and how to provide them (available for iOS and Android operating systems) |
| US MEC Wheel | An easy to use tool similar to a pregnancy gestation wheel that summarizes US MEC classifications; the medical conditions and personal characteristics are around the outside of the wheel, the contraceptive methods around the inside of the wheel, and the recommendations are revealed as the wheel turns |
| US MEC Summary Chart | A color-coded summary chart of US MEC classifications for IUDs, implants, DMPA, POPs, and CHCs |
| US SPR Charts | Three charts are available that cover two main topics each including:
|
| Effectiveness of Family Planning Methods Chart | A chart that lists family planning methods by level of effectiveness during typical use from most to least effective |
US MEC: US Medical Eligibility Criteria for Contraceptive Use; US SPR: US Selected Practice Recommendations for Contraceptive Use; QFP: Quality Family Planning Services; IUD: intrauterine devices; DMPA: depot medroxyprogesterone acetate; POPs: progestin-only pills; CHCs: combined hormonal contraceptives.
Conclusion
Contraception is important for women of reproductive age with MS to optimally time desired pregnancies and prevent unintended pregnancies. In 2016, the US MEC published evidence-based recommendations for contraceptive use by women with MS to assist health-care providers, including neurologists, when counseling women about contraception. Most methods of contraception appear to be safe for women with MS based on current evidence—the only exception is use of CHCs by women with MS with prolonged immobility due to concerns about possible VTE risk. Although neurologists may frequently refer MS patients to other providers for contraceptive care, neurologists can play a key role in promoting reproductive health by routinely assessing their patients’ pregnancy intentions and helping women make contraceptive choices that factor in their level of disability, immobility, and medication use. When counseling women with MS about contraception, the full range of methods for which they are medically eligible should be discussed in order for women to choose the best method for their personal circumstances. For women with MS taking potentially teratogenic medications, highly effective methods that are long-acting, such as IUDs and implants, might be the best option to avoid unintended pregnancy.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. L.Z., K.C., and M.W. are authors of CDC’s US Medical Eligibility Criteria for Contraceptive Use (US MEC); M.H. served as an expert in multiple sclerosis at the 2015 CDC meeting to develop these recommendations.
Contributor Information
Maria K Houtchens, Department of Neurology, Partners Multiple Sclerosis Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Lauren B Zapata, Division of Reproductive Health, US Centers for Disease Control and Prevention, Chamblee, GA, USA.
Kathryn M Curtis, Division of Reproductive Health, US Centers for Disease Control and Prevention, Chamblee, GA, USA.
Maura K Whiteman, Division of Reproductive Health, US Centers for Disease Control and Prevention, Chamblee, GA, USA.
References
- 1.Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler. 2014;20:520–526. doi: 10.1177/1352458513519181. [DOI] [PubMed] [Google Scholar]
- 2.Bove R, Alwan S, Friedman JM, et al. Management of multiple sclerosis during pregnancy and the reproductive years: A systematic review. Obstet Gynecol. 2014;124:1157–1168. doi: 10.1097/AOG.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 3.Coyle PK. Management of women with multiple sclerosis through pregnancy and after childbirth. Ther Adv Neurol Disord. 2016;9:198–210. doi: 10.1177/1756285616631897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellwig K, Brune N, Haghikia A, et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. 2008;118:24–28. doi: 10.1111/j.1600-0404.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- 5.Thone J, Kollar S, Nousome D, et al. Serum anti-Müllerian hormone levels in reproductive-age women with relapsing-remitting multiple sclerosis. Mult Scler. 2015;21:41–47. doi: 10.1177/1352458514540843. [DOI] [PubMed] [Google Scholar]
- 6.Alwan S, Yee IM, Dybalski M, et al. Reproductive decision making after the diagnosis of multiple sclerosis (MS) Mult Scler. 2013;19:351–358. doi: 10.1177/1352458512452920. [DOI] [PubMed] [Google Scholar]
- 7.Houtchens MK, Kolb CM. Multiple sclerosis and pregnancy: Therapeutic considerations. J Neurol. 2013;260:1202–1214. doi: 10.1007/s00415-012-6653-9. [DOI] [PubMed] [Google Scholar]
- 8.Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-beta exposure in multiple sclerosis. Neurology. 2010;75:1794–1802. doi: 10.1212/WNL.0b013e3181fd62bb. [DOI] [PubMed] [Google Scholar]
- 9.Fragoso YD, Adoni T, Alves-Leon SV, et al. Long-term effects of exposure to disease-modifying drugs in the offspring of mothers with multiple sclerosis: A retrospective chart review. CNS Drugs. 2013;27:955–961. doi: 10.1007/s40263-013-0113-7. [DOI] [PubMed] [Google Scholar]
- 10.Hellwig K. Pregnancy in multiple sclerosis. Eur Neurol. 2014;72(Suppl 1):39–42. doi: 10.1159/000367640. [DOI] [PubMed] [Google Scholar]
- 11.Ramoz LL, Patel-Shori NM. Recent changes in pregnancy and lactation labeling: Retirement of risk categories. Pharmacotherapy. 2014;34:389–395. doi: 10.1002/phar.1385. [DOI] [PubMed] [Google Scholar]
- 12.Sahin L, Nallani SC, Tassinari MS. Medication use in pregnancy and the pregnancy and lactation labeling rule. Clin Pharmacol Ther. 2016;100:23–25. doi: 10.1002/cpt.380. [DOI] [PubMed] [Google Scholar]
- 13.Vukusic S, Marignier R. Multiple sclerosis and pregnancy in the “treatment era. Nat Rev Neurol. 2015;11:280–289. doi: 10.1038/nrneurol.2015.53. [DOI] [PubMed] [Google Scholar]
- 14.Coyle PK, Christie S, Fodor P, et al. Multiple sclerosis gender issues: Clinical practices of women neurologists. Mult Scler. 2004;10:582–588. doi: 10.1191/1352458504ms1083oa. [DOI] [PubMed] [Google Scholar]
- 15.Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use. MMWR Recomm Rep. 2016;2016(65):1–103. doi: 10.15585/mmwr.rr6503a1. [DOI] [PubMed] [Google Scholar]
- 16.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83:397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buraga I, Popovici RE. Multiple sclerosis and pregnancy: Current considerations. ScientificWorldJournal. 2014;2014:513160. doi: 10.1155/2014/513160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCombe PA, Greer JM. Female reproductive issues in multiple sclerosis. Mult Scler. 2013;19:392–402. doi: 10.1177/1352458512452331. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Medical eligibility criteria for contraceptive use. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 20.Zapata LB, Oduyebo T, Whiteman MK, et al. Contraceptive use among women with multiple sclerosis: A systematic review. Contraception. 2016;94:612–620. doi: 10.1016/j.contraception.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozzilli C, De Giglio L, Barletta VT, et al. Oral contraceptives combined with interferon beta in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e120. doi: 10.1212/NXI.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gava G, Bartolomei I, Costantino A, et al. Long-term influence of combined oral contraceptive use on the clinical course of relapsing-remitting multiple sclerosis. Fertil Steril. 2014;102:116–122. doi: 10.1016/j.fertnstert.2014.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Sena A, Couderc R, Vasconcelos JC, et al. Oral contraceptive use and clinical outcomes in patients with multiple sclerosis. J Neurol Sci. 2012;317:47–51. doi: 10.1016/j.jns.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Poser S. Contraception and multiple sclerosis. Der Nervenarzt. 1982;53:323–326. [PubMed] [Google Scholar]
- 25.Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: A systematic review. Contraception. 2006;73:470–487. doi: 10.1016/j.contraception.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Peeters PJ, Bazelier MT, Uitdehaag BM, et al. The risk of venous thromboembolism in patients with multiple sclerosis: The Clinical Practice Research Datalink. J Thromb Haemost. 2014;12:444–451. doi: 10.1111/jth.12523. [DOI] [PubMed] [Google Scholar]
- 27.Roshanisefat H, Bahmanyar S, Hillert J, et al. Multiple sclerosis clinical course and cardiovascular disease risk—Swedish cohort study. Eur J Neurol. 2014;21:1353–e88. doi: 10.1111/ene.12518. [DOI] [PubMed] [Google Scholar]
- 28.Lidegaard O, Milsom I, Geirsson RT, et al. Hormonal contraception and venous thromboembolism. Acta Obstet Gynecol Scand. 2012;91:769–778. doi: 10.1111/j.1600-0412.2012.01444.x. [DOI] [PubMed] [Google Scholar]
- 29.David OJ, Ocwieja M, Meiser K, et al. Pharmacokinetics of fingolimod (FTY720) and a combined oral contraceptive coadministered in healthy women: Drug-drug interaction study results. Int J Clin Pharmacol Ther. 2012;50:540–544. doi: 10.5414/CP201675. [DOI] [PubMed] [Google Scholar]
- 30.Robertson P, Jr, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42:123–137. doi: 10.2165/00003088-200342020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Robertson P, Jr, Hellriegel ET, Arora S, et al. Effect of modafinil on the pharmacokinetics of ethinyl estradiol and triazolam in healthy volunteers. Clinical Pharmacol Ther. 2002;71:46–56. doi: 10.1067/mcp.2002.121217. [DOI] [PubMed] [Google Scholar]
- 32.Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use. MMWR Recomm Rep. 2016;2016(65):1–66. doi: 10.15585/mmwr.rr6504a1. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Selected practice recommendations for contraceptive use. 3. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 34.Gavin L, Moskosky S, Carter M, et al. Providing quality family planning services: Recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1–54. [PubMed] [Google Scholar]