Abstract
Objective
Hypertension is associated with unfavorable changes in adrenergic receptor responsiveness, but the relationship of race and sex to adrenergic receptor responsiveness in the development of cardiovascular disease is unclear. This study examined α-adrenergic and β-adrenergic receptor responsiveness in African-American and white men and women with untreated high blood pressure (BP) (HBP) and with normal BP.
Methods and results
The study sample comprised 161 African-American and white men and women in the age range 25–45 years. Isoproterenol, a nonselective β-adrenergic receptor agonist, was administered intravenously to determine the bolus dose required to increase heart rate by 25 bpm, an index of β-adrenergic receptor responsiveness. Similarly, phenylephrine, an α1-adrenergic receptor agonist, was administered to determine the bolus dose required to increase BP by 25 mmHg, an index of vascular α1-adrenergic receptor responsiveness. HBP (P <0.01), male sex (P =0.04), and higher BMI (P <0.01) were all associated with reduced β-adrenergic receptor responsiveness, with a similar trend observed for African-American race (P =0.07). Conversely, α1-adrenergic receptor responsiveness was increased in association with HBP (P <0.01), female sex (P <0.01), and African-American race (P <0.01).
Conclusion
In the early stages of hypertension, cardiovascular β-adrenergic receptors demonstrate blunted responsiveness, whereas conversely α1-adrenergic receptors exhibit increased responsiveness. This pattern of receptor changes is especially evident in men and African-Americans, is exacerbated by obesity, and may contribute to the development of cardiovascular disease.
Keywords: adrenergic receptors, hypertension, race, sex
INTRODUCTION
Downregulation of peripheral β-adrenergic receptor has been documented in animal models of hypertension [1], and both cardiac and vascular β-adrenergic receptors appear downregulated in human hypertension [2–6]. Evidence for altered responsiveness of peripheral vascular α-adrenergic receptor in hypertension is less clear. Some studies have reported greater pressor responses to α-adrenergic receptor agonists in hypertensive patients compared with normotensive patients [4,7], but others have observed no differences [5,8]. Human studies of adrenergic receptor responsiveness have frequently been carried out in relatively small [4–7,9], sometimes exclusively normotensive, [10–14] samples. Nonetheless, sex and race have emerged as important factors to be taken into consideration [5,12–15].
Research is mixed with respect to whether there are sex differences in adrenergic receptor function, with some studies reporting decreased α-adrenergic receptor and β-adrenergic receptor responsiveness in normotensive women compared with men [10], whereas others have reported increased α-adrenergic receptor responsiveness [11], greater β-adrenergic receptor responsiveness [12], or no sex differences in β-adrenergic receptor responsiveness [9,11]. Although African-Americans appear to have increased α1-adrenergic receptor responsiveness compared with whites [5,14], some, but not all [5], studies have reported reduced β-adrenergic receptor responsiveness in African-Americans compared with white Americans [9,13–15].
Altered adrenergic receptor function plays an important role in the pathophysiology of hypertension and may inform different strategies to treat and prevent high blood pressure (BP) (HBP). Given the sex and racial differences in the prevalence of hypertension [16–18], the hemodynamics of hypertension, the rates of uncontrolled hypertension [19,20], and the target organ damage associated with hypertension, a more comprehensive understanding of individual differences in adrenergic receptor responsiveness is needed. Although prior studies suggest that sex and race may play a role in adrenergic receptor responsiveness, to our knowledge, no studies have examined the combined effects of BP, race, and sex on the responsiveness of α-adrenergic receptor and β-adrenergic receptors in a sample of men and women with unmedicated HBP and with normal BP (NBP).
METHODS
Participants
The study population included 161 men and women, aged 25–45 years, participating in the BIOH study at Duke University Medical Center [21,22]. The study protocol was approved by Duke University Medical Center’s Institutional Review Board, and all participants provided verbal and written consent prior to participation. Individuals with BP exceeding 160 mmHg SBP or 100 mmHg DBP, or a history of antihypertensive medication use, were excluded. Other than HBP, inclusion criteria were designed to establish a study sample in good overall health volunteers with a diagnosis of any other cardiovascular disease, diabetes, or current use of tobacco products were excluded.
Blood pressure screening and classification
BP readings were obtained on three separate visits, approximately 1 week apart, to determine BP status. During each visit, participants were asked to relax in a private room for 5 min prior to BP readings. Three seated BPs were taken, 2 min apart, using an appropriate sized occlusion cuff, mercury column sphygmomanometer, and stethoscope. The nine resulting BP readings were averaged to define BP status. Participants whose mean SBP was within the range of 130–160 mmHg and/or whose mean DBP was within the range of 85–100 mmHg over the three screening sessions were classified as having HBP. All other participants, whose BPs were below these ranges, were classified as having NBP.
Cardiovascular measurements during adrenergic receptor responsiveness testing
All adrenergic receptor responsiveness testing was conducted while participants were completely reclined and at least 6 h after the most recent caffeine consumption. BP was measured continuously using the Finapres Model 2300 (Ohmeda, Madison, Wisconsin, USA) noninvasive BP monitor. This instrument utilizes the vascular unloading technique to measure SBP, DBP, and mean arterial pressure (MAP) on a beat-by-beat basis and has been validated against intra-arterial measures under various conditions including pressor responses to phenylephrine [23]. Heart rate (HR) was derived from the ECG as the interval between successive R-waves.
β-Adrenergic receptor responsiveness
The standardized isoproterenol sensitivity test [dose of isoproterenol to increase HR by 25 bpm (CD25)] was used to evaluate β-adrenergic receptor responsiveness in terms of the chronotropic dose of isoproterenol required to increase HR by 25 bpm [24]. Progressively, increasing bolus doses of isoproterenol (0.125, 0.25, 0.5, 1.0, 2.0, and 4.0 μg) were injected into a vein until an increase in HR of at least 25 bpm was observed. HR responses following each dose were computed as the shortest three successive ECG R–R intervals following drug injection, compared with the shortest three R–R intervals at rest (preinjection). Following each dose, the next higher dose was not injected for at least 5 min, or until cardiovascular activity had returned to resting levels, usually within 5–l0 min. The linear regression model of log-dose/HR response for each participant was used to determine CD25 exactly by interpolation. The CD25 measure provides an index of β-adrenergic receptor responsiveness that is inversely related to adrenergic receptor responsiveness (i.e. higher CD25 values are indicative of reduced or blunted β-adrenergic receptor responsiveness).
α1-Adrenergic receptor responsiveness
A procedure analogous to the β-adrenergic receptor responsiveness test described above was used for assessing α1-adrenergic receptor responsiveness, using the α1-adrenergic receptor pharmacological agonist phenylephrine, to stimulate vascular α1-adrenergic receptor [4]. In this test, the criterion response is defined as the dose required to increase MAP by 25 mmHg (PD25). An initial dose of 25 μg phenylephrine was used, with successive doses doubled until the 25 mmHg response was exceeded, or until a maximum dose of 800 μg. Again, at least 5 min, or longer if required for recovery of MAP to resting levels, preceded administration of successive doses. The linear log-dose/MAP response curve was used to determine the exact PD25 dose. The PD25 index is inversely related to vascular α1-adrenergic receptor responsiveness (i.e. higher PD25 indicates reduced α1-adrenergic receptor responsiveness, and conversely, lower PD25 indicates heightened α1-adrenergic receptor responsiveness).
Statistical analysis
Data are presented in terms of sample composition (n, %) and as means ± SD (M ± SD). Sample characteristics were compared by BP status, sex, and race. Hierarchical regression analyses were used to evaluate the effect of BP status, sex, and race on β-adrenergic receptor responsiveness to isoproterenol (CD25) and α-adrenergic receptor responsiveness to phenylephrine (PD25). Model 1 included the aforementioned independent variables only, whereas Model 2 also controlled for age and BMI. Exploratory analyses assessed the presence of interactions among BP status, sex, and race. All statistical analyses were conducted using the SAS 9.3 system (SAS Institute, Cary, North Caro-lina, USA) with significance set at P =0.05.
RESULTS
Sample characteristics are summarized in Table 1. The sample was 43% women, 48% African-American, and 45% had HBP. Mean BP in those classified as having HBP was 131/89 ± 8/4 mmHg, compared with 110/71 ± 8/ 5 mmHg for those with NBP.
TABLE 1.
Demographic and anthropomorphic characteristics of study sample (mean ± SD)
| Variable | Mean ± SD |
|---|---|
| N | 161 |
| Age (year) | 33 ± 6 |
| Sex (n, % female) | 70 (43%) |
| Race (% AA) | 78 (48%) |
| HBP (n, %) | 73 (45%) |
| BSA (m2) | 1.92 ± 0.19 |
| BMI (kg/m2) | 26.0 ± 3.4 |
| Clinic SBP (mmHg) | 129 ± 15 |
| Clinic DBP (mmHg) | 80 ± 11 |
| Heart rate (bpm) | 66 ± 11 |
AA, African-American; HBP, high blood pressure.
β-Adrenergic receptor responsiveness
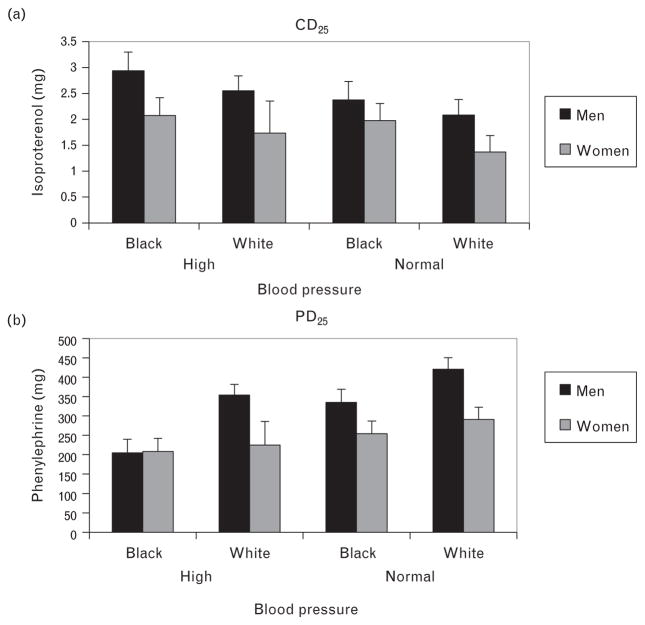
Our initial regression analysis (Model 1) showed that for the CD25 index, there were significant main effects for BP status (b =0.26, P =0.001), sex (b =0.16, P =0.04), and a trend for race (b =0.14, P =0.07) (Table 2). Participants with HBP had higher CD25 (reduced β-adrenergic receptor responsiveness) compared with those with NBP, men had significantly higher CD25 than women, and African-Americans tended to have higher CD25 compared with whites (Table 3). When age and BMI were added in Model 2, an additional 6% of the variance in CD25 was accounted for, that was predominantly due to the effects of BMI (b =0.23, P =0.003), with the effects for BP status (b =0.15, P =0.054) and race (b =0.11, P =0.16) becoming less pronounced (Table 3). Although there were no significant interaction effects between race, sex, and BP status on β-adrenergic receptor responsiveness, corresponding CD25 values adjusted for age and BMI are illustrated in Fig. 1a.
TABLE 2.
Hierarchical regression results predicting dose of isoproterenol to increase heart rate by 25 bpm and dose of phenylephrine to increase mean arterial pressure by 25 mmHg
| Model 1 | CD25 | PD25 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Estimate | P | R2 | Estimate | P | R2 | |
| BP status | 0.26 | 0.001 | −0.20 | 0.007 | ||
|
| ||||||
| Sex | 0.16 | 0.044 | 0.27 | 0.001 | ||
|
| ||||||
| Race | 0.14 | 0.065 | 0.10 | −0.24 | 0.001 | 0.17 |
|
| ||||||
| Model 2 | ||||||
| BP status | 0.15 | 0.054 | −0.21 | 0.009 | ||
| Sex | 0.20 | 0.010 | 0.27 | 0.001 | ||
| Race | 0.11 | 0.159 | −0.24 | 0.001 | ||
| Age | 0.14 | 0.071 | −0.04 | 0.574 | ||
| BMI | 0.23 | 0.003 | 0.16 | 0.06 | 0.436 | 0.16 |
Note: Body size (BSA) was unrelated to both CD25 and PD25 in these models. For BP status 1 = high, 2 =normal; sex 1 =male, 2 =female; race 1 =African-American, 2 =white. CD25, dose of isoproterenol to increase heart rate by 25 bpm; PD25, dose of phenylephrine to increase mean arterial pressure by 25 mmHg.
TABLE 3.
Dose of isoproterenol to increase heart rate by 25 bpm and dose of phenylephrine to increase mean arterial pressure by 25 mmHg by race, sex, and blood pressure status (mean ± SD)
| CD25 (μg isoproterenol) | PD25 (μg phenylephrine) | |
|---|---|---|
| Race | ||
| African-American | 2.36 ± 1.75 | 249.5 ± 149.1 |
| White American | 1.93 ± 1.51 | 347.9 ± 164.2 |
|
| ||
| Sex | ||
| Male | 2.38 ± 1.90 | 340.6 ± 176.2 |
| Female | 1.82 ± 1.17 | 247.8 ± 130.6 |
|
| ||
| Blood pressure | ||
| HBP | 2.65 ± 1.89 | 268.2 ± 156.4 |
| NBP | 1.71 ± 1.26 | 326.8 ± 166.6 |
CD25, dose of isoproterenol to increase heart rate by 25 bpm; HBP, high blood pressure; NBP, normal blood pressure; PD25, dose of phenylephrine to increase mean arterial pressure by 25 mmHg.
FIGURE 1.
Mean dose of isoproterenol to increase heart rate by 25 bpm (μg isoproterenol) and dose of phenylephrine to increase mean arterial pressure by 25 mmHg (μg phenylephrine) by race, sex, and blood pressure status.
α1-Adrenergic receptor responsiveness
For the PD25 index, our initial regression analysis (Model 1) showed significant effects for BP status (b =−0.20, P =0.007), sex (b =0.27, P =0.001), and race (b =−0.24, P =0.001) (Table 2). Participants with HBP had lower PD25 (increased α1-adrenergic receptor responsiveness) compared with those with NBP, women had lower PD25 than men, and African-Americans had significantly lower PD25 compared with whites (Table 3). Neither age (b =−0.04, P =0.57) nor BMI (b =0.06, P =0.44) explained additional variance in PD25 when included in Model 2, and effects for BP status, sex, and race were virtually unchanged (Table 2). Although there were no significant interaction effects between race, sex, and BP status on α1-adrenergic receptor responsiveness, corresponding PD25 values adjusted for age and BMI are illustrated in Fig. 1b.
DISCUSSION
In our biracial sample of men and women, we found that BP status, sex, and race were all associated with differences in cardiovascular α1-adrenergic receptor and β-adrenergic receptor responsiveness. Our results confirm those of earlier studies documenting that cardiovascular β-adrenergic receptor responsiveness is reduced in men and women with HBP or hypertension [5,25,1]. We also observed increased α1-adrenergic receptor responsiveness, evidenced by lower PD25, in people with HBP compared with those with NBP. Previous studies have shown that post-synaptic β-adrenergic receptor function is attenuated, whereas α1-adrenergic receptor functions are potentiated in cardiovascular tissues of hypertensive humans and animals [3,26]. Reduced β-adrenergic receptor responsiveness and heightened α-adrenergic receptor responsiveness is a likely mechanism contributing to elevated systemic vascular resistance and potential amplification of sympathetic stimulation of the vasculature in hypertension [27]. This pathophysiologic milieu also may promote the development of vascular hypertrophy, leading to a sustained elevation in systemic vascular resistance that is characteristic of established hypertension [27,28].
Women, compared with men in our study, demonstrated greater β-adrenergic receptor responsiveness, or lower CD25, as well as greater α1-adrenergic receptor responsiveness, or lower PD25. Although not all studies have found greater α1-adrenergic receptor-mediated responses in women [10], our findings are in keeping with Johansson et al. [29] and Luzier et al. [11] who also reported greater sensitivity to phenylephrine and isoproterenol in women with normal BP. Our observations also are consistent with some [12,30], but not all [9,10], previous studies of sex-related differences in β-adrenergic receptor responsiveness. As noted previously, sympathetic nervous system (SNS) hyperactivity results in downregulation of β-adrenergic receptor responsiveness. There is evidence that men exhibit greater resting SNS activity than women, who may more typically show heightened resting parasympathetic activity [31]. It is possible that over time this increased SNS activity may contribute to a greater reduction in β-adrenergic receptor responsiveness at an earlier age in men than in women. Therefore, our observed sex difference in β-adrenergic receptor responsiveness may reflect the smaller age-related decline in β-adrenergic receptor responsiveness among female compared with male study participants [32].
A significant racial difference was observed for adrenergic receptor responsiveness. African-Americans had reduced β-adrenergic receptor responsiveness coupled with higher α1-adrenergic receptor responsiveness compared with white Americans. These findings are supported by the results of previous studies [5,13–15,33]. However, it is of note that controlling for BMI attenuated the race effect for β-adrenergic receptor responsiveness, suggesting that relative body weight was a contributing factor in our study sample. Growing evidence indicates that adrenergic receptor density, distribution and function, and consequent vasomotor activity are determined in part by genetics, accounting for some of the individual differences both between and within racial groups [34,35]. A recent pharmacogenomics genome-wide meta-analysis of BP responses to β-blockers in African-Americans showed that two genotype variants were related to the antihypertensive efficacy of atenolol and metoprolol [36]. A study examining the effects of β1-adrenergic receptor polymorphisms on the response to β-blockade, however, found that racial differences could not be fully explained by different distributions of functional β1-adrenergic receptor variants [37]. These results suggest that socioeconomic status and lifestyle factors, such as diet, may interact with genetic influences to contribute to racial differences in adrenergic receptor responsiveness.
In contrast to β-adrenergic receptors, α-adrenergic receptors are mainly responsible for sympathetically mediated vasoconstrictive responses [26]. Stein et al. [14] reported that the reduced β-adrenergic receptor responsiveness and increased α-adrenergic receptor responsiveness observed among African-Americans were independent of one another. The additive effects of enhanced vasoconstriction and attenuated vasodilation may amplify vascular tone and vasoconstrictive responses during stressful encounters [14] and may ultimately contribute to the development of the low renin, relatively low cardiac output, and high systemic vascular resistance hemodynamic profile characteristic of African-American hypertensive patients [38]. These physiologic findings and the data from genetic studies are consistent with the clinical observation that men and women of African origin, including African-Americans, derive less benefit than whites and other minorities from the use of beta-blockers [39–42].
We also found that increased BMI was related to β-adrenergic receptor downregulation, contributing to the effects shown in relation to HBP. In addition, controlling for BMI attenuated the race effect for β-adrenergic receptor responsiveness. Previous studies have demonstrated that sympathetic overactivity is a hallmark of obesity and elevated SNS activity is an important pathophysiologic link between obesity and hypertension. However, as others have noted, the precise mechanisms contributing to ‘adrenergic overdrive’ in obese individuals are complex and not fully understood [43,44]. Although there were no differences in BMI between African-Americans and whites in our sample, BMI for both groups did meet criteria for classification as overweight. Collectively, these findings suggest that irrespective of race, relative body weight also may be an important contributing factor to diminished β-adrenergic receptor responsiveness.
The current study has several limitations. We employed a cross-sectional design, precluding inferences as to whether race, sex, and BMI differences in adrenergic receptor responsiveness are causally related to the development and progression of hypertensive disease. BP status, in terms of high versus normal, was based upon clinic BP readings only, whereas inclusion of 24-h ambulatory BP monitoring would have allowed for more precise categorization. Other potential factors related to hypertension such as hormonal, metabolic, and psychological status were not evaluated. Similarly, other potential factors contributing to individual differences in adrenergic receptor responsiveness, such as socioeconomic status; alcohol consumption; and dietary salt, sugar, and fat intake were not assessed. Regarding β-adrenergic receptor responsiveness, in our study, criterion response to isoproterenol was the conventional 25 bpm HR response (CD25), reflecting cardiac β-adrenergic receptor responses and not BP or vascular resistance responses, which would be more likely to capture vascular β-adrenergic receptor responsiveness. However, isoproterenol is a nonselective β-adrenergic receptor agonist, and in a previous study we found that the degree of the vasodilatory response of the systemic vasculature was closely related to the magnitude of HR response [5], suggesting that we may cautiously interpret increased cardiac β-adrenergic receptor responsiveness as also indicative of greater vascular β-adrenergic receptor-mediated vasodilatory responsiveness. We also note that sympathetic and parasympathetic nervous system activity during isoproterenol infusion may also contribute to the measured HR response that is attributed to isoproterenol alone.
In conclusion, the observations from the present study confirm that hypertension is associated with downregulation of cardiovascular β-adrenergic receptor responsiveness. Our findings also indicate that being overweight or obese is likely to contribute to this association. Sex and race were found to be related to the relative responsiveness of α1 and β-adrenergic receptors and thus potentially influencing the pathophysiology of hypertension. Although heightened α1-adrenergic receptor responsiveness occurred in association with HBP, it also was observed to be greater in African-Americans compared with white Americans and in women compared with men. African-Americans are at relatively higher risk for developing hypertension, but younger women are at reduced risk, suggesting that heightened α1-adrenergic receptor responsiveness per se is not a likely mechanism or marker of hypertension [18]. In the context of heightened α-adrenergic receptor responsiveness, the accompanying responsiveness of β-adrenergic receptors may be of particular importance. The characteristically higher β-adrenergic receptor responsiveness seen in women may counteract α-adrenergic receptor-mediated vasoconstriction and facilitate vasodilation. However, downregulated β-adrenergic receptor responsiveness may predispose African-Americans to a predominance of α-adrenergic receptor-mediated vasoconstriction. This racial difference in relative α1 and β-adrenergic receptor responsiveness is exacerbated in the context of HBP and likely contributes to the elevated systemic vascular resistance that is often characteristic of hypertension among African-Americans [45,46]. This pattern of racial differences in α1 and β-adrenergic receptor responsiveness may help to inform clinical decision-making in the optimal management of HBP.
Acknowledgments
We thank Dr Silvina V. Waldman, currently in the Department of Cardiology, FLENI, Buenos Aires, Argentina, and Dr Faye S. Routledge, currently in the School of Nursing, Emory University, Atlanta, Georgia, USA for reading earlier drafts of this manuscript and for providing critical feedback.
The work was supported by grants HL 49427 from the National Heart, Lung and Blood Institute, Bethesda, Maryland, USA and MO1-RR-30 (GCRC) from the National Institutes of Health, Bethesda, Maryland, USA.
Subject codes: Autonomic Nervous System, Clinical Studies, Mechanisms.
Abbreviations
- AR
adrenergic receptor
- BP
blood pressure
- CD25
dose of isoproterenol to increase heart rate by 25 bpm
- HBP
high blood pressure
- NBP
normal blood pressure
- PD25
dose of phenylephrine to increase mean arterial pressure by 25 mmHg
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Michel MC, Brodde OE, Insel PA. Peripheral adrenergic receptors in hypertension. Hypertension. 1990;16:107–120. doi: 10.1161/01.hyp.16.2.107. [DOI] [PubMed] [Google Scholar]
- 2.Bertel O, Buhler FR, Kiowski W, Lutold BE. Decreased beta-adrenoreceptor responsiveness as related to age, blood pressure, and plasma catecholamines in patients with essential hypertension. Hypertension. 1980;2:130–138. doi: 10.1161/01.hyp.2.2.130. [DOI] [PubMed] [Google Scholar]
- 3.Feldman RD. Beta-adrenergic receptor alterations in hypertension –physiological and molecular correlates. Can J Physiol Pharmacol. 1987;65:1666–1672. doi: 10.1139/y87-261. [DOI] [PubMed] [Google Scholar]
- 4.Kotchen TA, Guthrie GP, Jr, McKean H, Kotchen JM. Adrenergic responsiveness in prehypertensive subjects. Circulation. 1982;65:285–290. doi: 10.1161/01.cir.65.2.285. [DOI] [PubMed] [Google Scholar]
- 5.Sherwood A, Hinderliter AL. Responsiveness to alpha- and beta-adrenergic receptor agonists. Effects of race in borderline hypertensive compared to normotensive men. Am J Hypertens. 1993;6:630–635. doi: 10.1093/ajh/6.7.630. [DOI] [PubMed] [Google Scholar]
- 6.Stein CM, Nelson R, Deegan R, He H, Wood M, Wood AJ. Forearm beta adrenergic receptor-mediated vasodilation is impaired, without alteration of forearm norepinephrine spillover, in borderline hypertension. J Clin Invest. 1995;96:579–585. doi: 10.1172/JCI118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jie K, van Brummelen P, Vermey P, Timmermans PB, van Zwieten PA. Alpha 1-and alpha 2-adrenoceptor mediated vasoconstriction in the forearm of normotensive and hypertensive subjects. J Cardiovasc Pharmacol. 1986;8:190–196. doi: 10.1097/00005344-198601000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Egan B, Panis R, Hinderliter A, Schork N, Julius S. Mechanism of increased alpha adrenergic vasoconstriction in human essential hypertension. J Clin Invest. 1987;80:812–817. doi: 10.1172/JCI113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins LL, Dimsdale JE, Ziegler MG. Reduced beta 2-receptor mediated vasodilation in African Americans. Life Sci. 1995;57:1411–1416. doi: 10.1016/0024-3205(95)02103-p. [DOI] [PubMed] [Google Scholar]
- 10.Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res. 1987;61:581–585. doi: 10.1161/01.res.61.4.581. [DOI] [PubMed] [Google Scholar]
- 11.Luzier AB, Nawarskas JJ, Anonuevo J, Wilson MF, Kazierad DJ. The effects of gender on adrenergic receptor responsiveness. J Clin Pharmacol. 1998;38:618–624. doi: 10.1002/j.1552-4604.1998.tb04468.x. [DOI] [PubMed] [Google Scholar]
- 12.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JA, Burlew BS, Stiles RN. Racial differences in beta-adrenoceptor-mediated responsiveness. J Cardiovasc Pharmacol. 1995;25:90–96. doi: 10.1097/00005344-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 15.Jain S, Dimsdale JE, Roesch SC, Mills PJ. Ethnicity, social class and hostility: effects on in vivo beta-adrenergic receptor responsiveness. Biol Psychol. 2004;65:89–100. doi: 10.1016/s0301-0511(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville, MD: US Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Health Statistics; 2016. DHHS Publication Number 2016–1232. [PubMed] [Google Scholar]
- 17.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. 2005;165:2098–2104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 19.Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension. 2008;51:1149–1155. doi: 10.1161/HYPERTENSIONAHA.107.107342. [DOI] [PubMed] [Google Scholar]
- 20.Batson B, Belletti D, Wogen J. Effect of African American race on hypertension management: a real-world observational study among 28 US physician practices. Ethn Dis. 2010;20:409–415. [PubMed] [Google Scholar]
- 21.Hinderliter AL, Blumenthal JA, Waugh R, Chilukuri M, Sherwood A. Ethnic differences in left ventricular structure: relations to hemodynamics and diurnal blood pressure variation. Am J Hypertens. 2004;17:43–49. doi: 10.1016/j.amjhyper.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15:111–118. doi: 10.1016/s0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 23.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension. 1989;13:647–655. doi: 10.1161/01.hyp.13.6.647. [DOI] [PubMed] [Google Scholar]
- 24.Cleaveland CR, Rangno RE, Shand DG. A standardized isoproterenol sensitivity test. The effects of sinus arrhythmia, atropine, and propranolol. Arch Intern Med. 1972;130:47–52. doi: 10.1001/archinte.130.1.47. [DOI] [PubMed] [Google Scholar]
- 25.Valentini M, Julius S, Palatini P, Brook RD, Bard RL, Bisognano JD, Kaciroti N. Attenuation of haemodynamic, metabolic and energy expenditure responses to isoproterenol in patients with hypertension. J Hypertens. 2004;22:1999–2006. doi: 10.1097/00004872-200410000-00024. [DOI] [PubMed] [Google Scholar]
- 26.de Champlain J. Pre and postsynaptic adrenergic dysfunctions in hypertension. J Hypertens Suppl. 1990;8:S77–S85. [PubMed] [Google Scholar]
- 27.Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep. 2009;11:199–205. doi: 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 28.Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. Am J Hypertens. 1996;9:113S–120S. doi: 10.1016/0895-7061(96)00287-7. [DOI] [PubMed] [Google Scholar]
- 29.Johansson SR, Hjalmarson A. Age and sex differences in cardiovascular reactivity to adrenergic agonists, mental stress and isometric exercise in normal subjects. Scand J Clin Lab Invest. 1988;48:183–191. doi: 10.3109/00365518809085411. [DOI] [PubMed] [Google Scholar]
- 30.Mills PJ, Ziegler MG, Nelesen RA, Kennedy BP. The effects of the menstrual cycle, race, and gender on adrenergic receptors and agonists. Clin Pharmacol Ther. 1996;60:99–104. doi: 10.1016/S0009-9236(96)90172-1. [DOI] [PubMed] [Google Scholar]
- 31.Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 32.Turner MJ, Mier CM, Spina RJ, Ehsani AA. Effects of age and gender on cardiovascular responses to phenylephrine. J Gerontol A Biol Sci Med Sci. 1999;54:M17–24. doi: 10.1093/gerona/54.1.m17. [DOI] [PubMed] [Google Scholar]
- 33.Thomas KS, Nelesen RA, Malcarne VL, Ziegler MG, Dimsdale JE. Ethnicity, perceived discrimination, and vascular reactivity to phenylephrine. Psychosom Med. 2006;68:692–697. doi: 10.1097/01.psy.0000238214.80871.e6. [DOI] [PubMed] [Google Scholar]
- 34.Kurnik D, Muszkat M, Sofowora GG, Friedman EA, Dupont WD, Scheinin M, et al. Ethnic and genetic determinants of cardiovascular response to the selective alpha 2-adrenoceptor agonist dexmedetomidine. Hypertension. 2008;51:406–411. doi: 10.1161/HYPERTENSIONAHA.107.098939. [DOI] [PubMed] [Google Scholar]
- 35.Adefurin A, Ghimire LV, Kohli U, Muszkat M, Sofowora GG, Li C, et al. Genetic variation in the alpha1B-adrenergic receptor and vascular response. Pharmacogenomics J. 2016 doi: 10.1038/tpj.2016.29. [Epub ahead of print] doi:10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong Y, Wang Z, Beitelshees AL, McDonough CW, Langaee TY, Hall K, et al. Pharmacogenomic genome-wide meta-analysis of blood pressure response to beta-blockers in hypertensive African Americans. Hypertension. 2016;67:556–563. doi: 10.1161/HYPERTENSIONAHA.115.06345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurnik D, Li C, Sofowora GG, Friedman EA, Muszkat M, Xie HG, et al. Beta-1-adrenoceptor genetic variants and ethnicity independently affect response to beta-blockade. Pharmacogenet Genom. 2008;18:895–902. doi: 10.1097/FPC.0b013e328309733f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosman AR, Goldberg B, Mckechnie JK, Offermeier J, Oosthuizen OJ. South-African multicenter study of metoprolol and propranolol in essential hypertension. S Afr Med J. 1977;51:57–61. [PubMed] [Google Scholar]
- 39.Domanski MJ Investigators B. Beta-blocker Evaluation of Survival Trial (BEST) J Am Coll Cardiol. 2000;35:202a–203a. [Google Scholar]
- 40.Douglas JG, Bakris GL, Epstein M, Ferdinand KC, Ferrario C, Flack JM, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163:525–541. doi: 10.1001/archinte.163.5.525. [DOI] [PubMed] [Google Scholar]
- 41.Seedat YK. Varying responses to hypotensive agents in different racial groups – black versus white differences. J Hypertens. 1989;7:515–518. doi: 10.1097/00004872-198907000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Gupta AK, Poulter NR, Dobson J, Eldridge S, Cappuccio FP, Caulfield M, et al. Ethnic differences in blood pressure response to first and second-line antihypertensive therapies in patients randomized in the ASCOT Trial. Am J Hypertens. 2010;23:1023–1030. doi: 10.1038/ajh.2010.105. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens. 2010;23:1052–1060. doi: 10.1038/ajh.2010.154. [DOI] [PubMed] [Google Scholar]
- 44.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huisman HW, Schutte AE, Schutte R, van Rooyen JM, Fourie CMT, Mels CMC, et al. Exploring the link between cardiovascular reactivity and end-organ damage in African and Caucasian men: the SABPA study. Am J Hypertens. 2013;26:68–75. doi: 10.1093/ajh/hps007. [DOI] [PubMed] [Google Scholar]
- 46.Taherzadeh Z, Brewster LM, van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J Clin Hypertens (Greenwich) 2010;12:431–438. doi: 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]