Abstract
Background
To understand the impact of radiotherapy on the development of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) among elderly prostate cancer patients
Methods
We performed a retrospective cohort study of elderly prostate cancer patients diagnosed during 1999–2011 by using the National Cancer Institute’s Surveillance, Epidemiology and End Results–Medicare linked database. Competing risk analyses adjusting for patient characteristics were conducted to assess the impact of radiotherapy on the development of subsequent MDS/AML, compared with surgery.
Results
Of 32,112 prostate cancer patients, 14,672 underwent radiotherapy, and 17,440 received surgery only. The median follow-up was 4.68 years. A total of 157 (0.47%) prostate cancer patients developed subsequent MDS or AML, and the median time to develop MDS/AML was 3.30 (range: 0.16–9.48) years. Compared with prostate cancer patients who received surgery only, patients who underwent radiotherapy had a significantly increased risk of developing MDS/AML (hazard ratio [HR] =1.51, 95% confidence interval [CI]: 1.07–2.13). When radiotherapy was further categorized by modalities (brachytherapy, conventional conformal radiotherapy, and intensity-modulated radiotherapy [IMRT]), increased risk of second MDS/AML was only observed in the IMRT group (HR=1.66, 95% CI: 1.09–2.54).
Conclusions
Our findings suggest that radiotherapy for prostate cancer increases the risk of MDS/AML, and the impact may differ by modality. Additional studies with longer follow-up are needed to further clarify the role of radiotherapy in the development of subsequent myeloid malignancies. A better understanding may help patients, physicians and other stakeholders make more informed treatment decisions.
Keywords: myelodysplastic syndromes, acute myeloid leukemia, prostate cancer, radiotherapy, second cancer
Introduction
Prostate cancer is common among men in developed countries. In the United States (US), approximately 3 million men live with prostate cancer, and one out of seven men is expected to be diagnosed with prostate cancer during his lifetime.(1,2) About 93% of newly diagnosed prostate cancers are in the local or regional stages, for which the 15-year relative survival is 94.3%.(1) Approximately 40% of patients aged 70 years or older receive radiotherapy as initial treatment.(1) Nearly 1 out of 70 prostate cancer patients who undergo radiotherapy and survive more than 10 years will develop secondary cancer.(3) With a high rate of long-term survivorship, the risk of late treatment effects, such as second cancer, becomes critical for prostate cancer patients.(4)
Radiation is a known risk factor for myeloid malignancies.(5) A retrospective cohort study reported a dose-response effect in the incidence of myelodysplastic syndromes (MDS) among Nagasaki atomic bomb survivors 40 to 60 years after radiation exposure.(6) In Life Span Studies, Japanese atomic bomb survivors showed excess risk of acute myeloid leukemia (AML), which persisted throughout the 55-year follow-up.(7,8) Previous studies also observed an increased risk of MDS and/or AML among patients with lymphoma, breast cancer or testicular cancer who received radiotherapy.(9–13)
Prostate cancer patients may be at risk of second myeloid malignancies following localized radiotherapy. The os coxae, an area that contains the largest proportion of hematopoietically active bone marrow in adults,(14) may receive >50% of the dose from localized prostate irradiation.(15) This exposure may result in a substantial number of hematopoietic stem cells being exposed to the mutagenic effects of radiation. However, the impact of radiotherapy on the incidence of second myeloid malignancies among prostate cancer patients has not been sufficiently elucidated. As patients who received chemotherapy would have an increased risk to develop MDS/AML,(5,16) we conducted a population-based retrospective cohort study of elderly prostate cancer patients who did not undergo any chemotherapy to evaluate the role of radiotherapy in the development of second MDS/AML.
Materials and Methods
Data Sources and Study Population
We used the Surveillance, Epidemiology and End Results (SEER)–Medicare linked database, which links patient-level information on incident cancer diagnoses reported to the SEER registries with a master file of Medicare enrollment and claims for inpatient, outpatient, and physician services(17). The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
We assembled a retrospective cohort of patients who were diagnosed with incident, first primary, and localized prostate adenocarcinoma with clinical stages T1-T3 during 1999–2011. All patients fulfilled the following eligibility criteria: 1) aged 66–99 years at diagnosis, 2) had known month of diagnosis, 3) was not reported from autopsy or death certificate only, and 4) had continuous Medicare fee-for-service coverage (Parts A and B) and was not enrolled in health maintenance organizations from 12 months before diagnosis through death or end of study (12/31/2010 for those diagnosed in 1999–2003, and 12/31/2012 for those diagnosed in 2004–2011), whichever was earlier. We excluded patients who had other cancers or died within one month after initial treatment, who had second malignancies with unknown dates of diagnosis, and who received chemotherapy any time after prostate cancer diagnosis. To ensure that patients did not have radiotherapy for prostate cancer recurrence, patients who underwent radiotherapy 330 days after the last day of initial radiotherapy were also excluded from the analysis.
Identification of Treatment for Prostate Cancer
We used Medicare claims to identify initial treatment for prostate cancer, defined as surgery or radiotherapy started within 9 months after prostate cancer diagnosis. Receipt and type of radiotherapy were ascertained using Healthcare Common Procedure Coding System codes. A patient was considered to have received radiotherapy if there were any treatment delivery codes for brachytherapy or at least four treatment delivery codes for conventional conformal radiotherapy or intensity-modulated radiotherapy (IMRT). We employed the four-treatment restriction to increase the likelihood that patients actually received a full course of radiotherapy.(18) To assess the impact of different types of radiotherapy (brachytherapy, conventional conformal radiotherapy and IMRT), we excluded patients who received more than one type.
Ascertainment of Second MDS, AML and Other Second Malignancies
SEER is usually considered an authoritative source for new cancer diagnoses, but we identified two potential limitations for purposes of this study. First, MDS was not reported to SEER until 2001, therefore, we could not rely on SEER to identify MDS diagnoses in 1999 or 2000. Second, myeloid malignancies have been underreported in SEER.(19) To overcome these limitations, we used both SEER records and Medicare claims to ascertain new MDS and AML diagnoses after the diagnosis of prostate cancer in our cohort. If a prostate cancer patient had a second malignancy reported by SEER and a correspondent histological code for MDS or AML, the patient was considered to have a SEER-reported second MDS/AML. To be ascertained from Medicare claims as having second MDS/AML, a patient needed to have: 1) one inpatient claim with an MDS or AML diagnosis (MDS: International Classification of Disease, 9th revision [ICD-9] code 238.7 prior to October 2006, 239.72–5 beginning October 2006; AML: ICD-9 code 205.0) or two outpatient claims at least 30 days apart but within 12 months; 2) a claim for a bone marrow aspirate or biopsy within 60 days before or after the initial diagnosis, and 3) at least one claim with an MDS/AML diagnosis after the bone marrow claim.(20) Cases were assigned an index diagnosis date based on the date of the first qualifying MDS/AML claim. A washout period of 12 months without any MDS/AML diagnosis was used to ensure that we capture newly diagnosed incident cases, instead of prevalent cases. If a patient was identified by both SEER and Medicare claims, we used the earlier date as the date of MDS/AML diagnosis. Second malignancies other than MDS and AML were identified based on SEER records.
Other Variables of Interest
To assess comorbidity, all inpatient, outpatient and carrier claims within 12 months before the date of diagnosis were identified to calculate a modified Elixhauser comorbidity score.(18,21) As prior history of anemia may suggest underlying MDS/AML and confound a potential association between radiation and second MDS/AML, we recorded whether patients had claims for anemia within 12 months before prostate cancer diagnosis. We also recorded age at prostate cancer diagnosis, cancer stage at diagnosis, and race for each beneficiary.
Statistical Analysis
Frequency distribution of demographic and socioeconomic characteristics across treatment groups were compared using Pearson’s Chi-square tests. Medians, interquartile ranges, and ranges for duration of follow-up and age at diagnosis were calculated for each treatment group. Student t-test tests were used to compare durations of follow-up and age at diagnosis across treatment groups. Patients were followed from the beginning of prostate cancer treatment through the diagnosis of a second malignancy, death or end of study, whichever came first. Competing risks arise in the analysis of time-to-event data, if the event of interest is impossible to observe due to a different type of event occurring before. In this study, death and developing a second malignancy other than MDS/AML were considered competing events. Cumulative incidence of MDS/AML was calculated and comparisons of cumulative incidence across treatment groups were performed using Gray’s test (22),(23). Competing risks regression models were performed using the Fine and Gray method(22) to provide estimates of the crude and adjusted hazard ratios (HRs) for second myeloid malignancies. Age at prostate cancer diagnosis (66–69, 70–74, 75–79, 80–84, 85+ years), year of diagnosis (in calendar year), stage of prostate cancer at diagnosis, race (white, black, and other), Elixhauser score without anemia (0, 1–2, 3+), and previous anemia (yes/no) were included in the model. In addition to analyzing MDS/AML as one group, we also analyzed MDS separately when appropriate. Furthermore, we conducted sensitivity analyses to restrict the analysis to prostate cancer patients who were followed up for ≥ 1 year or diagnosed in 2004–2009 when data on Gleason score were available, or to expand the analysis to prostate cancer patients who received more than one of the three specific radiation modalities or other types of radiotherapy. Proportional hazard assumption was tested by plotting Schoenfeld residuals. All analyses were two-sided and were conducted using SAS Version 9.4 (SAS Inc. Cary, North Carolina), with p < 0.05 indicating statistical significance.
Results
A total of 32,112 prostate cancer patients were included in this study, with a median follow-up of 4.68 (range: 0.09–11.95) years. Compared with those who underwent radiotherapy (n=14,672), patients who received surgery (n=17,440) had shorter follow-up, were younger, were more likely to be white, had fewer comorbidities, and were more likely to be in stages II–III (Table 1).
Table 1.
Characteristics of Prostate Cancer Patients by Treatment Received, 1999–2011
| Surgery
|
Radiotherapy
|
p | |
|---|---|---|---|
| n (%) | n (%) | ||
| Total | 17440 | 14672 | |
| Follow-up time(years) | |||
| Median (IQR) | 4.79(2.60–7.29) | 4.54(2.51–6.98) | <0.01 |
| Range | 0.09–11.91 | 0.09–11.95 | |
| Age at diagnosis(years) | |||
| Median (IQR) | 69 (67–72) | 73 (69–76) | |
| 66–69 | 9559(54.8) | 3774(25.7) | <0.01 |
| 70–74 | 6327(36.3) | 5587(38.1) | |
| 75–79 | 1316(7.5) | 3971(27.1) | |
| 80–84 | 199(1.1) | 1155(7.87) | |
| 85+ | 39(0.2) | 185(1.26) | |
| Race | |||
| White | 15442(88.5) | 12375(84.3) | <0.01 |
| Black | 1119(6.4) | 1427(9.73) | |
| Other | 879(5.0) | 870(5.93) | |
| Year of diagnosis | |||
| 1999–2001 | 2013(11.5) | 1613(11.0) | 0.05 |
| 2002–2004 | 3094(17.7) | 2720(18.5) | |
| 2005–2007 | 4781(27.4) | 4121(28.1) | |
| 2008–2011 | 7552(43.3) | 6218(42.4) | |
| Elixhauser score, excluding anemia | |||
| 0 | 11744(67.3) | 7949(54.2) | <0.01 |
| 1 | 3950(22.6) | 3918(26.7) | |
| 2+ | 1746(10.0) | 2805(19.1) | |
| Anemia | |||
| No | 17085(98.0) | 14250(97.1) | <0.01 |
| Yes | 355(2.0) | 422(2.9) | |
| Stage | |||
| I | 10391(59.6) | 9566(65.2) | <0.01 |
| II–III | 7049 (40.4) | 5106 (34.8) | |
Abbreviation: IQR, interquartile range.
IMRT was the most common type of radiotherapy received (n=8,798, 60.0%; median follow-up: 3.67 years), followed by brachytherapy (n=3,185, 21.7%; median follow-up: 5.46 years) and conventional conformal radiotherapy (n=2,689, 18.3%; median follow-up:7.65 years).
We observed 157 (0.5% of 32,112) incident cases of MDS/AML (122 MDS and 35 AML cases) after the diagnosis of prostate cancer. Among patients who received surgery, 0.3% (n=60) developed MDS/AML, and the median time to develop MDS/AML was 3.43 (range: 0.13–9.01) years. In patients who underwent radiotherapy, 0.7% (n=97) developed MDS/AML, and the median time to develop MDS/AML was 2.95 (range: 0.27–9.48) years for radiotherapy overall. Patients who underwent IMRT (median=2.28 years, range: 0.27–6.38 years) developed MDS/AML earlier than those who received brachytherapy (median=3.78 years, range: 0.58–7.87 years) or conventional conformal radiotherapy (median =4.17 years, range: 0.54–9.48 years) (all p<0.05).
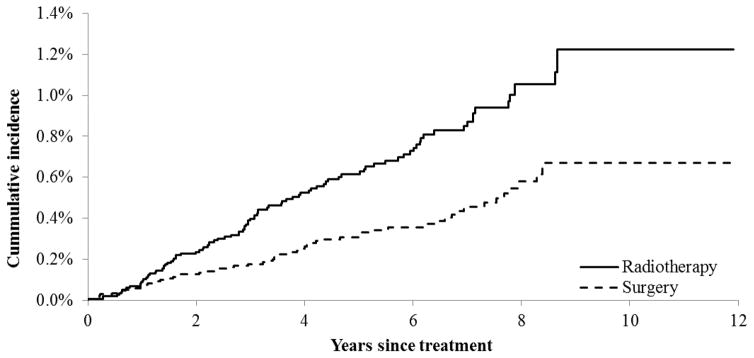
Patients who underwent radiotherapy had a higher cumulative incidence of MDS/AML than those receiving surgery only (Figure 1; Gray’s test, χ2=16.41, p< 0.01). Taking competing risk into consideration, at the end of the study, the cumulative incidence of MDS/AML was 0.07% and 0.12% for surgery and radiotherapy, respectively. The unadjusted cumulative incidence of MDS/AML did not differ across different radiotherapy modalities.
Figure 1.
Cumulative Incidence of MDS/AML among Prostate Cancer Patients
In competing risk analysis using multivariate Cox regression models, compared with prostate cancer patients who received surgery only, patients who received radiotherapy were 51% more likely to develop MDS/AML (HR=1.51, 95% CI: 1.07–2.13). When radiotherapy was further stratified by modalities (brachytherapy, IMRT, and conventional conformal radiotherapy), increased risk of MDS/AML was only observed in the IMRT group (HR=1.66, 95% CI: 1.09–2.54) (Table 2).
Table 2.
Risk of Second MDS/AML after Radiotherapy for Prostate Cancer
| Cancer of interest
|
Unadjusted
|
Adjusted
* |
|||
|---|---|---|---|---|---|
| n (%) | HR (95% CI) | p | HR (95% CI) | p | |
| MDS/AML (n=157) | |||||
| Surgery | 60(0.34) | 1.00 | 1.00 | ||
| Radiotherapy | 97(0.66) | 1.92(1.39–2.65) | <.01 | 1.51(1.07–2.13) | 0.02 |
| Brachytherapy | 19(0.60) | 1.58(0.95–2.65) | 0.08 | 1.35(0.80–2.28) | 0.26 |
| EBRT | 28(1.04) | 2.03(1.29–3.19) | <.01 | 1.41(0.86–2.32) | 0.18 |
| IMRT | 50(0.57) | 2.02(1.39–2.93) | <.01 | 1.66(1.09–2.54) | 0.02 |
| MDS(n=122) | |||||
| Surgery | 49(0.28) | 1.00 | 1.00 | ||
| Radiotherapy | 73(0.50) | 1.77(1.23–2.54) | <.01 | 1.40(0.94–2.07) | 0.09 |
| Brachytherapy | 15(0.47) | 1.54(0.86–2.74) | 0.14 | 1.32(0.74–2.35) | 0.35 |
| Conventional conformal radiotherapy | 23(0.86) | 2.05(1.25–3.37) | <.01 | 1.40(0.80–2.45) | 0.23 |
| IMRT | 35(0.40) | 1.72(1.12–2.66) | 0.01 | 1.44(0.88–2.35) | 0.14 |
Abbreviation: AML, acute myeloid leukemia; CI, confidence interval; HR, hazard ratio; IMRT, intensity-modulated radiotherapy; MDS, myelodysplastic syndromes.
Adjusted for age at prostate diagnosis (65–69, 70–74, 75–79, 80–84, 85+ years), year of diagnosis, stage of prostate cancer at diagnosis (I, II, III), race (white, black, other), Elixhauser score (0, 1–2, 3+), and history of anemia (yes vs. no).
The median time to develop MDS was 2.97 (range: 0.13–9.48) years. In each treatment group, the median time to develop MDS was 3.23 (range: 0.16–9.01) years for surgery, 3.14 (range: 0.58–7.87) years for brachytherapy, 4.63 (range: 0.54–9.48) years for conventional conformal radiotherapy, and 2.33 (range: 0.27–6.06) years for IMRT, respectively. While prostate cancer patients who underwent radiotherapy appeared to have an increased risk of MDS in unadjusted models, the associations diminished in magnitude and were no longer statistically significant in the multivariate models (Table 2).
Among 35 patients who developed AML, 15 men received IMRT. The median time to develop AML was 3.26 years, ranging from 2.12 years in the IMRT group to 4.89 years in the brachytherapy group. In the multivariate models, compared with patients who received surgery only, increased risks of AML were observed among patients who underwent radiotherapy overall and patients who received each specific type of radiotherapy, but the increase only reached statistical significance in the IMRT group (HR=2.57, 95% CI: 1.11–5.95). Per SEER-Medicare’s policy to protect confidentiality, detailed results are not shown due to small numbers of patients.
In sensitivity analyses restricted to prostate cancer patients who were followed up for ≥ 1 year, or expanded to include those who received more than one of the three specific radiation modalities or other types of radiotherapy, we observed very similar results (detailed data not presented). Among a subgroup of prostate cancer patients who had data on Gleason score, we additionally adjusted for the score in multivariate models, and the findings were essentially the same (detailed results not shown).
Discussion
In this population-based study, compared with elderly prostate cancer patients who received surgery only, those who underwent radiotherapy, especially IMRT, had an increased risk of MDS/AML. We initially considered the possibility of including chronic myeloid leukemia as another endpoint. However, only 16 patients developed second chronic myeloid leukemia, and including these patients did not impact our study findings. Thus we focused on second MDS/AML.
To date, only a small number of studies have evaluated the risk of developing myeloid malignancies after radiotherapy for prostate cancer, and the results have been inconsistent.(24–30) While one study observed no difference in the risk of leukemia between prostate cancer patients who received radiotherapy or surgery,(24) two other studies reported increased risks of AML among those who received external beam radiotherapy when compared with patients who did not receive radiation(28) or patients who did not receive definitive therapy(30), respectively. Using SEER data, Ojha et al. observed that, compared with prostate cancer patients without definitive therapy, external beam radiotherapy nearly doubled risk of second AML (HR = 2.05, 95% CI: 1.29–3.26), but neither brachytherapy (HR = 1.22, 95% CI: 0.46–3.22) nor surgery showed any impact.(30) It is important to note that SEER-based studies are unable to account for the impact of chemotherapy, which is an established risk factor for AML(5,16) but not included in SEER records. In order to overcome this limitation, we chose to use the SEER-Medicare database.
A case-control study from Taiwan reported a nearly-doubled risk of MDS among prostate cancer patients who received radiotherapy versus those who did not receive radiotherapy or chemotherapy.(29) However, a recent SEER-Medicare study found that compared with brachytherapy, external beam radiotherapy reduced the risk of second AML (relative risk = 0.53, 95% CI: 0.31–0.92) and had no effect on second MDS among prostate cancer survivors.(31) Although our study also used the SEER-Medicare database, there are several major differences in our approach. First of all, to eliminate the potential confounding effect of chemotherapy, we excluded all prostate cancer patients who received chemotherapy any time after prostate cancer diagnosis, while the other study included patients regardless of chemotherapy status and only adjusted for chemotherapy that was received in the first year(31). Second, as SEER is known to under-report myeloid malignancies,(19) we used an established algorithm(20) to identify MDS and AML patients from both SEER records and Medicare claims, while the other study ascertained MDS and AML diagnoses using SEER records only(31). Third, we included prostate cancer patients diagnosed through 2011 and the development of second MDS/AML through 2012, while the other study only included prostate cancer diagnoses through 2007 and second MDS/AML through 2009(31). As a result, our study reflects more recent treatment regimens for prostate cancer; in fact, the other study did not even include IMRT in the analysis(31), likely due to the small number of patients receiving IMRT in their study. Lastly, we compared different radiotherapy regimens to surgery in terms of risk of second MDS/AML, while the other study removed all patients who received surgery and focused on comparing various radiotherapy regimens(31).
After a median follow-up of 3.05 years, a Cleveland Clinic study found that prostate patients who underwent radiotherapy (brachytherapy, external beam radiotherapy) did not have elevated risk of MDS than those treated by radiation-free radical prostatectomy.(27) Compared with the Cleveland Clinic study,(27) our study included only older prostate cancer patients (aged ≥ 66 years), were larger in sample size, and had a longer follow-up (median=4.68 years). We believe it is more informative to follow prostate cancer patients over a longer period of time and to evaluate the development of both MDS and AML, as MDS and AML share common biological and clinical features, and approximately 30% of MDS patients progress to AML.
We observed an increased risk of MDS/AML among older prostate cancer patients who underwent radiotherapy, especially among those who received IMRT. IMRT treatment of prostate cancer uses either multiple beams or beam arcs to deliver radiation with varying cross-sectional intensities to the target.(32) The radiation dose is expected to conform tightly to the target areas and fall off rapidly in the surrounding healthy tissues.(32,33) On the other hand, IMRT has been criticized for delivering a “low dose bath” to surrounding normal tissues,(34) and it may significantly increase total body dose from leakage(35). A study evaluating radiation dose to normal pelvic tissues of prostate cancer patients found that, compared with the conventional technique, IMRT not only increased the pelvic volume covered by the isodose surfaces below 15 Gy, but also increased the relative dose delivered to the body volume outside the pelvis.(36) As IMRT requires more monitor units to deliver than conventional treatment, it may yield higher out-of-field doses than do conventional treatments.(37,38) It has been estimated that, compared with conventional radiotherapy, IMRT would increase the incidence of second malignancies from about 1% to 1.75% for patients surviving 10 years.(37) Furthermore, prostate cancer patients who received IMRT might have an 30% of increased risk to develop second malignancies than those who received conventional radiotherapy.(39) Findings from a review on planning studies also suggested that IMRT for prostate cancer increased the risk of second primary cancers.(40) Ruben et al. observed a 0.8-time higher out-of-field dose from IMRT than that of three-dimensional conformal radiotherapy, although, the increase in absolute terms was small.(41) As MDS and AML are rare endpoints in the study population, the reason that we only observed increased risk when MDS and AML are combined as one group could be the small number of patients and the limited statistical power for any single endpoint. Given the possibility that IMRT may be linked to a higher risk of secondary cancer compared to other forms of radiotherapy and the widespread adoption of IMRT in recent years, more research is warranted.
Our findings need to be considered in the context that no single treatment option for clinically localized prostate cancer is superior with respect to the risk of complications.(42–44) Previous studies reported that men who underwent surgery were more likely to report urinary incontinence and erectile dysfunction than those who received radiotherapy.(45,46) Compared with prostate cancer patients who received conformal radiotherapy, old men who received IMRT were less likely to develop gastrointestinal morbidities and hip fractures but more likely to report erectile dysfunction.(47) Taken together, it is important for clinicians to balance the benefit and risk of each treatment option and inform patients of the potential short and long term sequellae.
This study has many strengths. First, this was a large, population-based cohort study conducted in prostate cancer patients residing in 17 SEER areas, which account for 28% of the US population(2). Second, the nationwide Medicare claims data cover all health services provided, regardless of where the patients seek their care, thus ensuring comprehensive information on the treatment received by patients. Third, as chemotherapy is a known risk factor for AML/MDS,(5,16) we excluded all prostate cancer patients who received chemotherapy any time since after prostate cancer diagnosis and therefore eliminated the possibility of confounding by chemotherapy. Previous studies using SEER data (25,26,28,30) were unable to account for chemotherapy, and the other study that used SEER-Medicare database(31) only included chemotherapy in the first year as a covariate in the model. Fourth, the SEER-Medicare database also enables us to control for other factors that may influence prostate cancer treatment decisions and possible risk factors for MDS and AML, such as patients’ age, stage of prostate cancer, and comorbid conditions including anemia.
On the other hand, our study population included only elderly patients without private insurance who received surgery or specific types of radiotherapy for their prostate cancer, so the results may not be applicable to all patients. In our study population, older patients were more likely to receive radiotherapy. Recognizing that adjusting for age groups may not be sufficient to control for potential confounding by age, we also repeated all analyses by using age as a continuous variable, and the results were essentially the same. As the majority of the study population was younger than 75 years at the time of prostate cancer diagnosis, we additionally assessed the impact of radiotherapy on risk of second MDS/AML in the subgroup of patients < 75 years at diagnosis and observed nearly identical associations as in the overall study population. Although we used an established algorithm to ascertain incident MDS/AML cases from Medicare claims, it is possible that some patients were misclassified. However, it is unlikely that potential misclassification differed by treatment group. Furthermore, patients who received radiotherapy had more claims for physician office visit than those who underwent surgery only. This might have increased the chance of a prostate cancer patient who underwent radiotherapy being diagnosed with MDS/AML and/or being diagnosed early. In addition, we were unable to obtain detailed information on the dose of radiation or the exact fields treated. There might be other confounding factors that we could not obtain from claims. Lastly, previous studies of radiation-induced malignancies suggest a latency time of 1–5 years to occurrence of myeloid leukemia.(48,49) In our cohort, the median follow-up was 7.65 years for conventional conformal radiotherapy but only 3.67 years for IMRT.
Conclusions
Our findings suggest that radiotherapy for prostate cancer increases the risk of MDS/AML, and the impact may differ by radiation modality. Additional studies with longer follow-up are needed to further clarify the role of radiotherapy in the development of subsequent myeloid malignancies. A better understanding may help patients, physicians and other stakeholders make more informed treatment decisions.
Acknowledgments
Funding: This research was partly funded by R03 CA173810 and R01 CA149045 from the National Cancer Institute.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute (50)’s Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Footnotes
Disclosures: Drs. Yu and Gross receive research support from 21st Century Oncology. Drs. Davidoff and Gore received research funding from Celgene. Drs. Zeidan and Gore serve as a consultant for Celgene. Dr. Gross receives support from Medtronic, Inc. and Johnson & Johnson, Inc. Dr. Ma was a consultant for Celgene. These sources of support were not used for any portion of the current study. None of the other authors have disclosures.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Sountoulides P, Koletsas N, Kikidakis D, Paschalidis K, Sofikitis N. Secondary malignancies following radiotherapy for prostate cancer. Therapeutic advances in urology. 2010;2(3):119–125. doi: 10.1177/1756287210374462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberts DS. Second cancers are killing us! Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(11):2019. doi: 10.1158/1055-9965.EPI-06-0417. [DOI] [PubMed] [Google Scholar]
- 5.Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107(9):2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 6.Iwanaga M, Hsu WL, Soda M, Takasaki Y, Tawara M, Joh T, Amenomori T, Yamamura M, Yoshida Y, Koba T, Miyazaki Y, Matsuo T, Preston DL, Suyama A, Kodama K, Tomonaga M. Risk of myelodysplastic syndromes in people exposed to ionizing radiation: a retrospective cohort study of Nagasaki atomic bomb survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(4):428–434. doi: 10.1200/JCO.2010.31.3080. [DOI] [PubMed] [Google Scholar]
- 7.Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, Kamada N, Dohy H, Matsuo T, Matsui T, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiation research. 1994;137(2 Suppl):S68–97. [PubMed] [Google Scholar]
- 8.Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, Iwanaga M, Miyazaki Y, Cullings HM, Suyama A, Ozasa K, Shore RE, Mabuchi K. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiation research. 2013;179(3):361–382. doi: 10.1667/RR2892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosing C, Munsell M, Yazji S, Andersson B, Couriel D, de Lima M, Donato M, Gajewski J, Giralt S, Korbling M, Martin T, Ueno NT, Champlin RE, Khouri IF. Risk of therapy-related myelodysplastic syndrome/acute leukemia following high-dose therapy and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2002;13(3):450–459. doi: 10.1093/annonc/mdf109. [DOI] [PubMed] [Google Scholar]
- 10.Milligan DW, Ruiz De Elvira MC, Kolb HJ, Goldstone AH, Meloni G, Rohatiner AZ, Colombat P, Schmitz N. Secondary leukaemia and myelodysplasia after autografting for lymphoma: results from the EBMT. EBMT Lymphoma and Late Effects Working Parties. European Group for Blood and Marrow Transplantation British journal of haematology. 1999;106(4):1020–1026. doi: 10.1046/j.1365-2141.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan H, Malmgren J, De Roos AJ. Risk of myelodysplastic syndrome and acute myeloid leukemia post radiation treatment for breast cancer: a population-based study. Breast cancer research and treatment. 2013;137(3):863–867. doi: 10.1007/s10549-012-2386-9. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan HG, Malmgren JA, Atwood MK. Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990–2005. BMC cancer. 2011;11:260. doi: 10.1186/1471-2407-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis LB, Andersson M, Gospodarowicz M, van Leeuwen FE, Bergfeldt K, Lynch CF, Curtis RE, Kohler BA, Wiklund T, Storm H, Holowaty E, Hall P, Pukkala E, Sleijfer DT, Clarke EA, Boice JD, Jr, Stovall M, Gilbert E. Treatment-associated leukemia following testicular cancer. Journal of the National Cancer Institute. 2000;92(14):1165–1171. doi: 10.1093/jnci/92.14.1165. [DOI] [PubMed] [Google Scholar]
- 14.Valentin J. Basic anatomical and physiological data for use in radiological protection: reference values: ICRP Publication 89. Annals of the ICRP. 2002;32(3–4):1–277. [PubMed] [Google Scholar]
- 15.Gershkevitsh E, Rosenberg I, Dearnaley DP, Trott KR. Bone marrow doses and leukaemia risk in radiotherapy of prostate cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1999;53(3):189–197. doi: 10.1016/s0167-8140(99)00145-0. [DOI] [PubMed] [Google Scholar]
- 16.Strom SS, Velez-Bravo V, Estey EH. Epidemiology of myelodysplastic syndromes. Seminars in hematology. 2008;45(1):8–13. doi: 10.1053/j.seminhematol.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Potosky A, Riley G, Lubitz J, Mentnech R, Kessler L. Potential for cancer related health services research using a linked Medicare-tumor related database. Medical Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 18.Roberts KB, Soulos PR, Herrin J, Yu JB, Long JB, Dostaler E, Gross CP. The adoption of new adjuvant radiation therapy modalities among Medicare beneficiaries with breast cancer: clinical correlates and cost implications. International journal of radiation oncology, biology, physics. 2013;85(5):1186–1192. doi: 10.1016/j.ijrobp.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(3):474–481. doi: 10.1158/1055-9965.EPI-11-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrick F, Davidoff AJ, Zeidan AM, Gore SD, Baer MR. Effect of erythropoiesis-stimulating agent policy decisions on off-label use in myelodysplastic syndromes. Medicare & medicaid research review. 2014;4(4) doi: 10.5600/mmrr.004.04.a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kohl M, Heinze G. %PSHREG: A SAS(r) Macro for Proportional and Nonproportional Substribution Hazards Regression for Survival Analyses with Competing risks. 2014 http://cemsiis.meduniwien.ac.at/kb/wf/software/statistische-software/pshreg/
- 23.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 24.Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88(2):398–406. doi: 10.1002/(sici)1097-0142(20000115)88:2<398::aid-cncr22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.McMaster ML, Feuer EJ, Tucker MA. New Malignancies Following Cancer of the Male Genital Tract. In: Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JFJ, editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. Bethesda, MD: National Cancer Institute; 2006. NIH Publ. No. 05-5302. [Google Scholar]
- 26.Neugut AI, Ahsan H, Robinson E, Ennis RD. Bladder carcinoma and other second malignancies after radiotherapy for prostate carcinoma. Cancer. 1997;79(8):1600–1604. doi: 10.1002/(sici)1097-0142(19970415)79:8<1600::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee S, Reddy CA, Ciezki JP, Abdel-Wahab M, Tiu RV, Copelan E, Advani AA, Saunthararajah Y, Paulic K, Hobson S, Maciejewski JP, Bolwell BJ, Kalaycio M, Dreicer R, Klein EA, Sekeres MA. Risk for developing myelodysplastic syndromes in prostate cancer patients definitively treated with radiation. Journal of the National Cancer Institute. 2014;106(3):djt462. doi: 10.1093/jnci/djt462. [DOI] [PubMed] [Google Scholar]
- 28.Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate cancer. Cancer. 2006;107(5):991–998. doi: 10.1002/cncr.22083. [DOI] [PubMed] [Google Scholar]
- 29.Sun LM, Lin CL, Lin MC, Liang JA, Kao CH. Radiotherapy- and chemotherapy-induced myelodysplasia syndrome: a nationwide population-based nested case-control study. Medicine. 2015;94(17):e737. doi: 10.1097/MD.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ojha RP, Fischbach LA, Zhou Y, Felini MJ, Singh KP, Thertulien R. Acute myeloid leukemia incidence following radiation therapy for localized or locally advanced prostate adenocarcinoma. Cancer epidemiology. 2010;34(3):274–278. doi: 10.1016/j.canep.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Berrington de Gonzalez A, Wong J, Kleinerman R, Kim C, Morton L, Bekelman JE. Risk of second cancers according to radiation therapy technique and modality in prostate cancer survivors. International journal of radiation oncology, biology, physics. 2015;91(2):295–302. doi: 10.1016/j.ijrobp.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bortfeld T. IMRT: a review and preview. Physics in medicine and biology. 2006;51(13):R363–379. doi: 10.1088/0031-9155/51/13/R21. [DOI] [PubMed] [Google Scholar]
- 33.Leibel SA, Fuks Z, Zelefsky MJ, Wolden SL, Rosenzweig KE, Alektiar KM, Hunt MA, Yorke ED, Hong LX, Amols HI, Burman CM, Jackson A, Mageras GS, LoSasso T, Happersett L, Spirou SV, Chui CS, Ling CC. Intensity-modulated radiotherapy. Cancer journal. 2002;8(2):164–176. doi: 10.1097/00130404-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins A, Parker C. Treating prostate cancer with radiotherapy. Nature reviews Clinical oncology. 2010;7(10):583–589. doi: 10.1038/nrclinonc.2010.135. [DOI] [PubMed] [Google Scholar]
- 35.Glatstein E. Intensity-modulated radiation therapy: the inverse, the converse, and the perverse. Seminars in radiation oncology. 2002;12(3):272–281. doi: 10.1053/srao.2002.32433. [DOI] [PubMed] [Google Scholar]
- 36.Tao Y, Lefkopoulos D, Ibrahima D, Bridier A, del Polizzi MP, Wibault P, De Crevoisier R, Arriagada R, Bourhis J. Comparison of dose contribution to normal pelvic tissues among conventional, conformal and intensity-modulated radiotherapy techniques in prostate cancer. Acta Oncol. 2008;47(3):442–450. doi: 10.1080/02841860701666055. [DOI] [PubMed] [Google Scholar]
- 37.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. International journal of radiation oncology, biology, physics. 2003;56(1):83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 38.Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, Rosen II. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. International journal of radiation oncology, biology, physics. 2005;62(4):1195–1203. doi: 10.1016/j.ijrobp.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 39.Stathakis S, Roland T, Papanikolaou N, Li J, Ma C. A prediction study on radiation-induced second malignancies for IMRT treatment delivery. Technology in cancer research & treatment. 2009;8(2):141–148. doi: 10.1177/153303460900800207. [DOI] [PubMed] [Google Scholar]
- 40.Murray L, Henry A, Hoskin P, Siebert FA, Venselaar J ESTRO BPgotG. Second primary cancers after radiation for prostate cancer: a review of data from planning studies. Radiation oncology. 2013;8:172. doi: 10.1186/1748-717X-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruben JD, Lancaster CM, Jones P, Smith RL. A comparison of out-of-field dose and its constituent components for intensity-modulated radiation therapy versus conformal radiation therapy: implications for carcinogenesis. International journal of radiation oncology, biology, physics. 2011;81(5):1458–1464. doi: 10.1016/j.ijrobp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Litwin MS, Gore JL, Kwan L, Brandeis JM, Lee SP, Withers HR, Reiter RE. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239–2247. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 43.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin X, Greenfield TK, Litwin MS, Saigal CS, Mahadevan A, Klein E, Kibel A, Pisters LL, Kuban D, Kaplan I, Wood D, Ciezki J, Shah N, Wei JT. Quality of life and satisfaction with outcome among prostate-cancer survivors. The New England journal of medicine. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 44.Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, Nyquist L, Sanda MG. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(2):557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 45.Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Potosky AL, Stanford JL, Stroup AM, Van Horn RL, Penson DF. Long-term functional outcomes after treatment for localized prostate cancer. The New England journal of medicine. 2013;368(5):436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardo Y, Guedea F, Aguilo F, Fernandez P, Macias V, Marino A, Hervas A, Herruzo I, Ortiz MJ, Ponce de Leon J, Craven-Bratle J, Suarez JF, Boladeras A, Pont A, Ayala A, Sancho G, Martinez E, Alonso J, Ferrer M. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(31):4687–4696. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 47.Sheets NC, Goldin GH, Meyer AM, Wu Y, Chang Y, Sturmer T, Holmes JA, Reeve BB, Godley PA, Carpenter WR, Chen RC. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA : the journal of the American Medical Association. 2012;307(15):1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neugut AI, Weinberg MD, Ahsan H, Rescigno J. Carcinogenic effects of radiotherapy for breast cancer. Oncology. 1999;13(9):1245–1256. discussion 1257, 1261–1245. [PubMed] [Google Scholar]
- 49.Roychoudhuri R, Evans H, Robinson D, Moller H. Radiation-induced malignancies following radiotherapy for breast cancer. British journal of cancer. 2004;91(5):868–872. doi: 10.1038/sj.bjc.6602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong FL, Bhatia S, Landier W, Francisco L, Leisenring W, Hudson MM, Armstrong GT, Mertens A, Stovall M, Robison LL, Lyman GH, Lipshultz SE, Armenian SH. Cost-effectiveness of the children’s oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 160(10):672–683. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]