Abstract
The hypomethylating agents (HMA) azacitidine and decitabine are both approved by the FDA for the treatment of myelodysplastic syndromes (MDS). Although heralded as a significant advancement, HMA lead to responses in less than half of patients and for those that respond most will relapse. As such, there is a crucial need to improve frontline therapy approaches. One promising strategy involves combining azacitidine or decitabine with investigational or existing therapies with the goal of achieving synergistic activity and better patient outcomes. The purpose of this paper is to critically review the efficacy and safety of reported HMA-based combination regimens in patients with higher-risk MDS.
Keywords: AML, MDS, hypomethylating agent, DNA methyltransferase inhibitor, epigenetic, combination therapy
Introduction
Myelodysplastic Syndromes (MDS) are heterogeneous clonal hematopoietic neoplasms characterized by ineffective hematopoiesis, peripheral blood cytopenias, and a risk of progression to acute myeloid leukemia (AML).[1] MDS is the most common myeloid malignancy in the United States with an estimated incidence rate of 75 per 100,000 among individuals older than 65 in 2005. [2] The disorder predominantly affects the elderly with 88% of patients older than 60 years at time of diagnosis. [3]
Risk of disease progression and death in MDS correlates with the number and severity of cytopenias; bone marrow blast percentage; and cytogenetic profile, according to the International Prognostic Scoring System (IPSS) and the more recent revised International Prognostic scoring system (IPSS-R) with updated cytogenetic categories.[4, 5] In the World Health Organization Prognostic Scoring System (WPSS), WHO-based morphologic pathology diagnosis contributes significantly to risk assessment. [6] In both current systems, patients with high risk MDS have a poor prognosis with median overall survival of fewer than 21 months for the WPSS and 20 months for the IPSS-R. [7]
There are currently only two treatment modalities shown to improve overall survival in high risk MDS: Allogeneic stem cell transplant and the hypomethylating agent, azacitidine. Allogeneic stem cell transplantation is the only potentially curative therapy for high risk MDS. [8] However, due to advanced age, medical comorbidities, and limited availability of stem cell donors, only a minority of patients actually undergo stem cell transplant. [9, 10] For patients with higher risk MDS, who are not fit for intensive approaches, treatment with hypomethylating agents has become the standard of care.
The hypomethylating agents, azacitidine and decitabine are cytidine nucleoside analogs with similar mechanism of action but with several notable exceptions. Azacitidine is a ribonucleoside that is incorporated into both RNA and DNA at a ratio of ~3/2. [11] Preferential RNA incorporation allows azacitidine to inhibit protein synthesis. Decitabine is incorporated into DNA only and is 10 times more potent than azacitidine on a molar basis. [11] Additionally, azacitidine and decitabine have differential effects on the cell cycle, gene regulation, and apoptosis. [11, 12] Once integrated into the DNA strand, both azacitidine and decitabine inhibit cytosine methylation in newly dividing cells by binding and depleting the DNMT1 enzyme. As a result, hypomethylating agents cause global hypomethylation through ongoing DNA replication cycles in absence of the necessary DNMT, followed by subsequent induction of DNA damage and cell death. [11, 12]
Azacitidine and decitabine received FDA approval in 2004 and 2006 respectively for use in all subtypes of MDS. [13, 14] The main supporting evidence for azacitidine was the Cancer and Leukemia Group B trial (CALGB) 9221. [15] Retrospective analysis of the CALGB combined trials 8421, 8921, and 9221 incorporating updated IPSS criteria showed overall response rates of 33 to 53% when compared to conventional care. [16] In the AZA-001 study azacitidine significantly prolonged overall survival compared to conventional care (24.5 months vs. 15.0 months, respectively; p=0.0001). [17] Evidence supporting use of decitabine in high risk MDS comes from a randomized phase III trial, which showed an overall response rate of 30% and a trend toward a longer median time to AML or death (12.1 months vs. 7.8 months P =0.16 by the log-rank test) when compared to conventional care. [18]
Despite the success of hypomethylating agents, treatment failure is common and most patients either do not respond to treatment or eventually relapse. [15, 16] Another major concern is the inability of HMA to eradicate the malignant clone. This has been exemplified in clinical settings by the dissociation between marrow response, peripheral blood response and survival as well as by the very high relapse rate for patients stopping HMA while in complete remission (CR). Lymphoid-primed multipotential progenitors (LMPP)-like and granulocyte macrophage progenitors (GMP)-like leukemic stem/progenitor population have also been shown to persist in patients treated with azacitidine, who achieved morphologic CR and normalization of blast counts. [19]
Thus, to improve results, over the last decade a large variety of agents have been combined with HMA, including investigational treatments targeting epigenetic regulation, immunomodulation, dysregulated hematopoiesis, the tumor microenvironment, apoptosis, immune evasion, or other markers of leukemogenesis.
This review evaluates the efficacy and safety of current combination therapies with hypomethylating agents in patients with intermediate and high-risk MDS.
I. Combinations with Histone Deacetylase Inhibitors (HDACi)
Histone acetylation is a critical regulator of gene expression and is mediated by histone acetyltransferases (HATs) and histone deacetylases (HDACs). HDACs remove acetyl molecules at lysine residues of H3/H4 to condense chromatin and make DNA inaccessible to transcriptional complexes. [20, 21]. HDACs are classified into 4 classes (I, II, III, and IV) according to their homology to yeast proteins, subcellular location, and enzymatic activities. [22] The class 1 HDACs include HDAC 1, -2, -3, and -8 are nuclear in location with HDAC 1, 2, and 3 being primarily responsible for histone acetylation. [22] Class IIa (HDAC 4, -5, -7, and 9), class IIb (HDAC 6 and 10), and class IV (HDAC 11) de-acetylate non-histone substrates to regulate multiple physiologic processes. [22, 23]
HDAC inhibitors target the zinc-dependent deacetylases of Class I, II, IV and not the Class III HDACs or the sirtuins, which are NAD+ dependent deacetylases. [22, 24] HDAC inhibitors have varying specificity (table 1) and are classified according to their chemical structure: carboxylic acids (Valproate, phenylbutyrate), hydroxamic acids (trichostatin A, vorinostat), aminobenzamides (Entinostat, mocetinostat), and cyclic peptides (apicidin, romidepsin), epoxyketones (trapoxins), and hybrid molecules. [22] Inhibition by HDAC inhibitorsincreases histone acetylation, which opens chromatin leading to transcription of genes mediating cell cycle arrest, differentiation, and apoptosis of cancer cells. [24]
Table 1.
Selected Clinical Trials of HDAC inhibitors in Combination with Hypomethylating Agents in MDS
| HDAC inhibitor | Targets | Selected Study |
Study Intervention |
N % MDS |
MDS Response ORR % CR/ PR/ HI % |
Study Outcome Median OS (mos) |
|---|---|---|---|---|---|---|
| Phenylbutyrate | Class I and IIa |
Gore et al 2006 |
PB+ AZA Phase I |
n=29 36% |
38 14/ 3/ 20 |
- |
| Valproic Acid | Class I and IIa | Issa et al 2015 |
VPA + DAC Phase II, RCT |
n=149 58% DAC, n=70 VPA + DAC, n= 79 |
DAC 51 31/ - /- VPA + DAC 58 [p vs. DAC= 0.5] 37/ -/ - |
DAC 11.2 VPA + DAC 11.2 [ p vs DAC= 0.92) |
| Vorinostat | Class I, II, IV | Sekeres et al 2015 |
VOR + AZA Phase II, RCT |
N=276 AZA, n= 92 VOR + AZA, n= 91 |
AZA 36 23/- / 0/ 13 VOR + AZA 22 [p vs AZA =0.07] 14/ 1/ 7 |
AZA - AZA + VOR - |
| Panobinostat | Class I, II, IV | Garcia- Manero et al 2015 |
PAN + AZA Phase IIb, RCT |
N=82 57% AZA, n= 42 PAN + AZA, n= 40 |
AZA 38 10/ - /- PAN+ AZA 37.5% 15/ - /- |
AZA - PAN + AZA - |
| Pracinostat | Class I, II, IV | Garcia- Manero et al 2015 |
PRA + AZA Phase II, RCT |
N= 1–2 100% AZA, n= PRA+ AZA, n= |
AZA 31/ - /55 PRA+ AZA 18/- /35 |
AZA 18.8 PRA+ AZA 15.7 [HR=1.21, p=NS] |
| Entinostat | Class I | Prebet et al 2014 |
ENT + AZA Phase II, RCT |
n= 149 AZA, n= 74 AZA + ENT, n= 75 |
AZA 32 12/ 8/ 12 AZA + ENT 27 8/ 7/ 12 |
AZA 18 ENT + AZA 13 |
| Mocetinostat | HDAC 1, 2, 3, 11 |
Garcia- Manero et al 2013 |
MOC + AZA | N= 22 100% |
MOC + AZA CR + CRi rate 59% |
- |
Abbreviations: AZA, azacitidine; DAC, decitabine; PB, phenylbutyrate; VPA, valproic acid; VOR, vorinostat; PRA, pracinostat; PAN, panobinostat; ENT, entinostat; MOC, mocetinostat; ms, months, ORR, overall response rate; CR, complete remission; Med, median; N, number of study participants;
Carboxyllic Acids: Phenylbutyrate and Valproic Acid
The carboxylic acid HDACi, phenylbutyrate and valproic acid areclass I and IIa HDACi and the first used in combination with AZA for treatment of MDS and AML. Initial phase I studies with phenylbutyrate and valproic acid showed evidence of synergism with increased clinical response rate with combination therapy compared to historical controls. [25–27] A recent phase II trialrandomized patients to either low dose decitabine monotherapy or combination with valproic acid. The study showed no significant difference in overall response (51 vs. 58%, p=0.407), CR (31 vs. 37%, p =0.497), or median survival (11.2 vs. 11.9, p= 0.92). The combination arm also had more side effects, in particular memory impairment and nausea. [28]
Hydroxamic Acids: Vorinostat, Panobinostat, Pracinostat
Vorinostat, panobinostat and pracinostat are oral, pan- HDAC inhibitor (Class I, II, and IV) with histone and non-histone deacetylase activity. [29, 30] Each of these hydroxamic acid containing HDACi demonstrated early promise when used in combination with hypothmethylating agents in single arm phase I trials. [31, 32] However, more recent multi-arm randomized controlled studies have shown mixed or in some cases negative outcomes when combination therapy was compared with hypomethylating agent monotherapy. In the North American Leukemia Intergroup study, there was no significant difference between combination therapy (azacitidine and vorinostat) and azacitidine monotherapy for overall response (27% vs. 37%, p =0.16), CR (17% vs. 24%, p= 0.5), or median overall survival (17 vs. 15 months, p= 0.17). [33] However, there was a trend toward improved relapse-free survival for combination therapy compared to azacitidine (13 months vs. 7 months, p=0.11). The combination arm had greater grade 3/4 adverse events, in particular gastrointestinal disorders (23 vs. 4 patients). [34] The median number weeks of therapy was also lower for combination treatment than azacitidine monotherapy at 20 vs. 25 weeks suggestive of a higher rate of early discontinuation.[34]
Garcia-Manero et al presented at the ASH 2015 annual meeting a combination of panobinostat and azacitidine with a 2-fold increase in CR (15% vs 9.5 %) but similar ORR (37.5% vs 38.1%) and 1-year survival (70% vs 60%) when compared to azacitidine monotherapy. [35] Combination therapy was associated with higher incidence of grade >3 adverse events including thrombocytopenia, neutropenia, anemia, and pneumonia. Treatment related deaths (13.2% vs 4.8%) and treatment discontinuation due to adverse events (36.8% vs 23.8%) were also more common with combination therapy. [35] The randomized phase II trial of pracinostat and azacitidine in patients with previously untreated intermediate risk-2 or high –risk MDS showed that the combination had 2-fold lower CR (18 vs 31%), nearly 1/3 lower HI (35 vs 55%), lower OS (15.7 vs. 18.8 months, HR 1.21, p= NS). [36] Combination therapy was associated with a higher incidence of grade ≥3 events including thrombocytopenia, febrile neutropenia, and fatigue. The pracinostat and azacitidine arm also had a higher rate of discontinuation for adverse events (26% vs 10%, mostly within the first 2 cycles). [36]
Aminobenzamides: Entinostat and Mocetinostat
Two orally bioavailable aminobenzamides have been studied in combination with hypomethylating agents: entinostat and mocetinostat. Entinostat selectively targets nuclear histone deactylases. In the US Leukemia Intergroup Trial E1905, 149 patients were randomized to either azacitidine 50mg/m2/day over 10 days alone or with concurrent entinostat 4mg/m2/day given on day 3 and day 10. Combination therapy was associated with lower hematologic normalization rate, defined as CR, PR and trilineage normalization, (26.7% vs. 32.5%, respectively) and lower median overall survival (13 months vs. 18 months). The combination arm also had a significantly lower magnitude of methylation difference from start of therapy on day 0 to day 15 on genome wide methylation analysis. The inferior clinical response rate, median overall survival and magnitude of global methylation of combination therapy were attributed to the cell cycle inhibitory effects of entinostat. By causing a G1/S cell cycle arrest, concomitant administration of entinostat in theory would prevent incorporation of hypomethylating agents into DNA and RNA. [37] The trial also investigated patients with therapy related myeloid neoplasms as a separate cohort. In this sub-study, the combination therapy also had lower hematologic normalization rate and median overall survival. The combination arm was also associated with a significant excess of treatment discontinuation related to treatment toxicity or death relative to monotherapy. [38] Mocetinostat inhibits the nuclear histone deacetylases (HDAC 1, 2, 3) as well as non-histone deacetylase HDAC11. A recent phase II study in patients with MDS receiving mocetinostat 35mg 3x/week after day 5 and azacitidine 75 mg/m2 SQ daily for 7 days, showed promising results with an ORR 61% (17/28 patients), CR or CRi in 50% (14/28 patients), median overall survival of 12.9 months. The most common grade III/IV toxicities included fatigue, nausea, diarrhea, vomiting, and thrombocytopenia. [39]
Although combinations of hypomethylating agents and HDACi have shown promise in single arm trials, results from more recent multi-arm randomized controlled studies have been disappointing. To date, combination therapy has shown no significant improvement in clinical response or overall survival when compared to azacitidine monotherapy. This is in part due to a worse side effect profile and early treatment discontinuation, which was seen in combination therapy with vorinostat, pracinostat, and panobinostat. Pharmacodynamic antagonism may also be an issue. The development of more selective HDAC inhibitors, able to targetparticular HDAC isoforms offers potential for improved side effect profile and enhanced efficacy with combination therapy. Lastly, the optimization of dosing schedules with HDACi given sequentially after hypomethylating agents may preserve the efficacy of hypomethylating agents and lead to greater synergy.
II. Combinations with Lenalidomide
Lenalidomide is an oral, thalidomide derived, immunomodulatory drug (IMiD) that has received FDA approval for transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes associated with a deletion of the chromosome 5 long arm (del(5q)). [40, 41] In MDS with the 5q-deletion, lenalidomide activates CRBN-CRL4 E3 ubiquitin ligase leading to increased ubiquitination and degradation of casein kinase 1A1 (CSNK1A1) and p-53 mediated tumor cell depletion. [42, 43] Lenalidomide has also demonstrated induction of transfusion independence in low risk MDS patients without 5q-.[44] Combination therapy with azacitidine and lenalidomide has shown efficacy in trials with or without selection of patients with deletion 5q.
In higher risk MDS, the 5q- deletion in conjunction with increased blast count and greater chromosomal rearrangement carries a poor prognosis. [45] Early clinical trials with sequential dosing of azacitidine 75mg/m2/day followed by lenalidomide in patients with AML, CMML, or MDS carrying 5q- showed variable ORR ranging from 24- 78% and CR 8.2-44%.[46–48] In the largest of these studies, the ORR was merely 24.5% (12/49) and early treatment discontinuation (<4 cycles) in 30/49 patients. Grade >3 adverse events occurred in 38/49 patients with infectious events being most common. [48]
In patients with higher risk MDS not selected for the 5q- deletion, early phase I/II trials with the combination of sequentially dosed azacitidine 75mg/m2/day followed by lenalidomide 10–50mg/day, showed response rates as high as 72%. [49–51]. Based on these promising results, combination with lenalidomide was integrated into the randomized phase II North American Intergroup Study SWOG S1117. The preliminary results presented at ASH 2015 showed no significant difference between combination therapy and azacitidine monotherapy in overall response (45% vs. 37%, p =0.45), CR (21% vs. 24%, p= 0.73), and median overall survival (18 vs. 15 months, p= 0.38). [33] Combination therapy had greater toxicity requiring dose modification and early treatment discontinuation. Grade 3/4 adverse events more common in the azacitidine and lenalidomide combination included rash, febrile neutropenia, and gastrointestinal disorders. [34] There have also been concerns related to the cumulative hematological toxicity of the combination.[51]
III. Growth Factors
Cytopenias are key elements of the diagnosis and prognosis of MDS. Erythropoiesis stimulating agents (ESA) improve quality of life and transfusion dependency in as many as half of lower risk MDS patients. [52, 53] TPO mimetics may decrease platelet transfusion dependency as well as the risk of bleeding. [54] HMAs typically induce cytopenias, particularly in early cycles of treatment, resulting in dose restrictions, treatment delays or discontinuation. [16, 17] The addition of growth factors to HMA treatment could potentially mitigate hematologic toxicity.
Erythropoiesis-stimulating agents
In a retrospective analysis of 282 patients with higher-risk MDS (IPSS Int-2 or higher) treated with azacitidine, patients who also received ESAs concomitantly or before starting azacitidine showed significant increase in transfusion independence (48 and 20%, p=0.01) and median OS 19.6 months and 11.9 months (p=0.04) when compared to azacitidine treatment alone. The relatively higher rate of response to ESAs in this high risk MDS population was thought to be due to a reversal of ESA resistance with azacitidine treatment. [55, 56] Several studies have since investigated the ability of azacitidine to reverse transfusion dependence in ESA non-responders in intermediate to low risk MDS. [56–58] The Nordic NMDSG08A phase II trial, showed that in patients with intermediate and low risk MDS who were transfusion dependent and non-responders to EPO/G-CSF, treatment with ≥1 cycle of azacitidine resulted in transfusion independence in 17% and an ORR of 23%. Combination treatment with ESA and azacitidine in patients still transfusion dependent after 6 cycles of azacitidine monotherapy, resulted in only an additional 6% achieving transfusion independence and only 7% ORR. The duration of response was short-lived, lasting < 6 months in 4 of 6 responders.[58]
Thrombopoiesis-stimulating agents
The two second generation thrombopoietin receptor agonists, romiplostim and eltrombopag are (FDA) approved for use in patients with ITP refractory to corticosteroids or immunoglobulins but both have been used off label for thrombocytopenia in lower risk MDS. [54] In two phase II randomized studies in patients with low and intermediate risk MDS treated with decitabine [59] or azacitidine[60] in combination with romiplostim, combination therapy was associated with a trend towards higher median platelet counts, lower platelet transfusion rates, and smaller percentage of patients with bleeding events when compared to treatment with placebo and hypomethylating agent. The decitabine and romiplastim combination also had a trend towards a higher ORR and CR when compared to decitabine and placebo (table 2). [59] Significant adverse events related to romiplastim treatment included one patient with pulmonary artery thrombosis and DVT. [59] Romiplostim has recently been shown to cause a significant transient elevation in peripheral blast counts relative to placebo, which led to early discontinuation of the randomized controlled phase II study. The transient blast count elevation did not correlate with clinical outcomes; and the overall survival rates and progression to AML-free survival were similar between groups. [61] A phase I dose-finding trial of eltrombopag in patients with higher risk MDS and platelet count <75 ×109 /L, who were also receiving azacitidine showed that platelet count improved or remained stable in 9/12 patients and CR was achieved in 4/12 patients. The study also demonstrated that eltrombopag had no effect on blast count or bone marrow fibrosis. [62]
Table 2.
Selected Clinical Trials of Non- HDAC inhibitor Combination Therapies with Hypomethylating Agents in MDS
| Study | Intervention | Design | N | Med Age |
% MDS |
ORR%* | CR/PR/HI* | Med OS (ms) |
|---|---|---|---|---|---|---|---|---|
| Sekeres et al 2012 |
AZA + LEN | Phase II |
36 | 68 | 100% | 72% | 44% | 13.6 |
| Narayan et al 2015 |
AZA + LEN | Phase II |
32 | 73.5 | 19% | 25% | 12.5% | 5 |
| DiNardo et al 2015 |
AZA + LEN | Phase I/II |
88 | 67 | 51% | 35% | 17% | 32.8 |
| Sekeres et al 2015 |
AZA AZA + LEN |
Phase II, RCT |
Total, n=277 AZA, n=92 AZA + LEN, n= 93 |
- | 100% |
AZA 37% AZA + LEN 45% [p vs AZA= 0.45] |
AZA 24% AZA + LEN 21% [p vs AZA = 0.73] |
AZA 15 AZA + LEN 18 [p vs AZA = 0.38] |
| Mittelman et al 2013 |
AZA + LEN | Phase 2 | 18 | - | 100% HR and LR MDS Selected for 5q- |
78% | 44% | - |
| Platzbecker et al 2013 |
AZA + LEN | Phase I | 19 | 69 | 65% Selected for 5q- |
42% | 11% | |
| Ades et al 2015 |
AZA + LEN | Phase I-II |
49 | 69 | 63% IPSS-2 or high risk MDS Selected for 5q- |
24% | 8.2% | - |
| Itzykson et al 2012 |
AZA + ESA | Retro. | Total, n= 282 AZA, n= 239 AZA + ESA, n= 32 |
72 | Total 77% AZA 84% AZA + ESA 76% |
AZA 43% AZA + ESA 53 % [p vs AZA= 0.34] |
AZA 13% AZA +ESA 19% |
AZA 11.9 AZA + ESA 19.6 [p vs AZA = 0.04] |
| Tobiasson et al 2014 |
AZA + ESA | Phase II |
Total, n=30 AZA, n= 30 AZA + ESA, n=16 |
69 | 100% IPSS low and Int-1 refractory to ESA |
AZA 23% AZA + ESA 7% |
- | - |
| Kantarjian et al 2010 |
AZA + ROM | Phase II, RCT |
Total, n= 40 AZA, n= 13 AZA + ROM 500µg , n= 13 AZA + ROM 750µg, n= 14 |
71 | 100% IPSS Low, Int-1, Int-2 |
AZA 15% AZA + ROM 500µg 8% AZA + ROM 750µg 14% |
-- | |
| Greenberg et al 2013 |
ROM + DAC |
Phase II, RCT |
Total, n=29 DAC, n= 14 DAC + ROM, n=15 |
68 | 100% IPSS low, Int, high risk MDS |
DAC 21% DAC + ROM 33% ** Non- significant |
DAC 7% DAC + ROM 13%** Non- significant |
- |
| Svensson et al 2014 |
ELT + AZA | Phase I | 12 | 74 | 100% | 67% | 33% | - |
| Strati et al 2015 |
MID + AZA | Phase I/II |
54 | 65 | 5% | 26% | 2% | 5.5 |
| Daver et al 2015 |
GEM + AZA | Phase II | 110 | 70 | 22% | - | 35% | 5.7 |
| Fathi et al 2015 |
SGN-33A + HMA |
Phase 1 | 23 | 77 | 0% MDS 100% AML |
65% | 22% | - |
| Ravandi et al 2012 |
SAP + DAC | Phase I/II/III |
33 | 77 | 0% MDS 100% AML |
37% | 30% | 7.8 |
| Nevada et al 2014 |
RIG + AZA | Phase I/II |
18 12 Evaluable |
70.5 | 61% | 50% | 8.3% | - |
| Tibes et al 2015 |
SON + AZA | Phase I/ Ib |
29 | 72 | 31% | - | 40% | - |
| Ritchie et al 2015 |
BIR + AZA | Phase II |
6 | ≥ 60 | 100% | 83% | 50% | - |
Abbreviations: AZA, azacitidine; DAC, decitabine; HMA, hypomethylating agent; LEN, lenalidomide; GEM, gemtuzumab; ESA, erythrocyte stimulating agent; ELT, eltrombopag; MID, midostaurin; SGN-33A, SGN-CD33A; SAP, sapacitabine; RIG, rigosertib; SON, sonidegib; BIR, birinapant; Retro, retrospective study; ms, months, ORR, overall response rate; CR, complete remission; Med, median; N, number of study participants;
IV. Monoclonal Antibodies and Antibody Drug Conjugates
The cell surface molecule CD33 is primarily a marker of myeloid differentiation and commonly expressed on myeloblasts of MDS and AML. [65, 66] Therefore, monoclonal antibodies (mAbs) and antibody drug conjugates directed against CD33 are important treatment approaches for high risk MDS and AML. Gemtuzumab ozogamicin is a humanized anti-CD33 monoclonal antibody covalently linked to calicheamicin a potent antitumor antibiotic that cleaves double-stranded DNA at specific sequences. [67] A phase II study of gemtuzumab given sequentially after decitabine, demonstrated an overall CR/CRi of 33% (5/15 patients), but with inferior median overall survival (5.7 months) in patients with untreated MDS when compared to historical outcomes of hypomethylating agents. [68] The inferior overall median overall survival was attributed to under treatment with decitabine. However, the inferior median overall survival may also be related to excessive toxicity, in particular grade >3 neutropenic fevers, gastrointestinal and mucosal bleeding occurring in 45% and 7% of all patients respectively. [68] Vadastuximab or SGN- CD33A is another humanized anti-CD33 mAb conjugated to a synthetic pyrrolobenzodiazepine dimer, which upon cleavage interrupts cell division by DNA crosslinking. [69] A preclinical study showed that combination therapy with hypomethylating agents increased tumor cell killing in MDR- positive AML cells and significantly reduced tumor growth in mouse xenograft models of AML. The antileukemic activity of combination therapy relied on upregulation of CD33 after treatment with hypomethylating therapy. [69] A phase I study in patients with previously untreated CD33+ AML treated with SGN-CD33A 10mcg/kg IV every 4 weeks in combination with hypomethylating agent therapy (azactidine and decitabine), interim analysis showed that 65% achieved CR (5/24) and CRi (10/24). Grade>3 adverse events included fatigue (54%), febrile neutropenia (46%) but the 30-day mortality and 60-day mortality was low at 0 and 4% respectively. [70] Additional clinical trials evaluating combination therapy withSGN-CD33A and hypomethylating agents are currently under investigation.
V. Nucleoside Analogs
Nucleoside analogs (i.e., cytarabine) are a major class of anti-tumor therapies in leukemia. Sapacitabine is anoral nucleoside analogue that is converted to its major active metabolite2′-C-Cyano-2′-deoxy-b-D-arabino-pentofuranosylcytosine (CNDAC). After triphosphorylation and DNA incorporation, CNDAC results in single-strand breaks and G2/M cell cycle arrest. The fatty-acid side chain on the N4 group of the cytosine moiety prevents degradation by deamination and subsequently improves bioavailability. [71] A phase I/II study in elderly patients (median age 77) with newly diagnosed AML treated with alternating 4 week cycles of decitabine (odd cycles) and sapacitabine 300mg PO BID × 3 days/week for 2 weeks (even cycles), resulted in an overall response rate of 37% (17/46 patients; 10 CR, 2 PR, and 5patient with major hematologic improvement) and median overall survival of 238 days. There were two drug limiting toxicities (pneumonia/sepsis and typhlitis) and six patients died within 60-days with one death from typhlitis possibly related to treatment. Common adverse side effects included asthenia, fatigue, decreased appetite, nausea, vomiting, constipation, diarrhea, peripheral edema, cellulitis, febrile neutropenia, back pain, and cytopenias, mostly moderate in intensity. [72] The SEAMLESS study, a phase III study comparing combination therapy with sapacitabine and decitabine to decitabine monotherapy in elderly patients with newly diagnosed AML is currently under investigation.
VI. Kinase and Multi-kinase Inhibitors
FLT3 inhibitors
Midostaurin is an oral, broad-spectrum tyrosine kinase inhibitor active on both wild type and mutated FLT3. The multi-target kinase inhibitor was recently shown to improve OS of FLT3 mutated AML patients in combination with standard induction regimen. [63] A phase I/II trial of midostaurin sequentially dosed after azacitidine in patients with AML, secondary AML or MDS, demonstrated modest clinical benefit (ORR of 26%, CR rate 2%, median overall survival of 5.5 months) and reduction of bone marrow blast counts in 79% of patients and >50% decrease in bone marrow blast counts in 53% of patients. Major non-hematologic toxicities included infection, reduced ejection fraction, nausea, vomiting, and diarrhea.[64]
Rigosertib
Rigosertib is a benzyl styryl sulfone that binds to RAS-binding domains of multiple RAS effectors including RAF, phosphoinositide 3-kinase (PI3K) and Ral-GDS leading to inhibition of MAPK and PI3K signaling and mitotic arrest. [73]
Rigosertib in intravenous formulation has been used as monotherapy in patients with high-risk MDS, who failed hypomethylating therapy [74]. However, in combination with hypomethylating agents, oral rigosertib is the preferred formulation currently under investigation. Preliminary results from a phase I study in patients with MDS and AML previously untreated or refractory to HMA, combination therapy with oral rigosertib and azacitidine showed overall response rates of 50% with CR 8.3% in 12 evaluable patients. The most frequent adverse event was constipation, diarrhea, nausea, pneumonia, and hypotension. The phase II study is ongoing with a recommended dose of 560/280mg BID of rigosertib. [75]
Hedgehog Pathway Inhibitors
The hedgehog pathway is an important regulator of normal hematopoiesis and is dysregulated in myelodysplastic syndromes. [76, 77] In the canonical hedgehog pathway, the transmembrane receptor Patched-1 (PTCH1) inhibits the G-protein coupled receptor smoothened (SMO) to prevent activation of a signaling cascade via SUFU and GLI proteins. Binding of one or more of hedgehog ligands (Sonic hedgehog (SHH), Desert Hedgehog (DHH), and Indian Hedgehog (IHH)) to PTCH1 removes the inhibitory effect on SMO, which leads to activation of GLI transcription factors and expression of genes involved with cellular proliferation and survival. [78] An RNA-interference screen of 270 genes located with the CDRs of chromosome 5 and 7 performed on several AML cell lines treated with azacitidine identified SHH, SMO, and GLI-3 as potential sensitizers to hypomethylating therapy. Additional in vitro studies showed synergy between azacitidine and the SMO inhibitor LDE225 (erismodegib). Interestingly, concurrent dosing with azacitidine have greater synergy whereas sequential dosing was more antagonistic. [79] Combination of azacitidine and SMO inhibitors are currently being investigated. Preliminary results from a phase I/Ib study of 36 patients with AML, MDS, or CMML receiving 2 cycles of azacitidine 75mg m2 ×7 days and the oral SMO inhibitor sonidegib 200mg daily showed CR/CRi in 2/5 untreated MDS/CMML patients, OS 77% at 6 months (95% CI 44–97%), and median progression free survival of 4 months. All patients experienced at least one hematologic grade 3 and 4 toxicity and non-hematologic toxicities, most commonly hyponatremia and fatigue occurred in 24 and 28% of patients respectively. [79]
VII. Future directions
Isocitrate Dehydrogenase Inhibitors
Mutations in isoforms IDH-1 and IDH-2 are common in hematologic disorders, occurring at prevalence of 5–20% in de novo AML and 7.5% in de novo MDS. [80, 81] The mutations confer a gain-of-function activity leading to the conversion of α-ketoglutarate to β-hydroxyglutarate (2-HG). [82] The current model of IDH-mutant leukemogenesis is that 2-HG inhibits TET2, which results in impaired global hydroxymethylation of DNA and dysregulated methylation patterns. [80, 83, 84] Several compounds targeting IDH1 and/or IDH2 are currently developed. AG-221, an oral, reversible inhibitor of IDH2 is currently being investigated in phase I dose escalation studies in patients with IDH2 mutation-positive AML or MDS. Preliminary results have demonstrated reduction in plasma 2-HG up to 98% in subjects with the IDH2-R140Q mutation, overall response rates of 56% (25/45 patients), and CR of 22% (10/45 patients) with durable responses up to 8.8 months. IDH inhibitors target distinct mechanisms in epigenetic regulation and therefore have the potential for synergism with hypomethylating agents. [85] Combination therapy with IDH inhibitors and hypomethylating agents are currently investigation.
Inhibitors of Apoptosis Proteins (IAP) Inhibitors
The ability to evade programed cell death is one of the defining traits of cancer. [86] The inhibitor of apoptosis proteins (IAP) are frequently overexpressed in cancer cells including AML and associated with worse overall prognosis. [87, 88]
IAPs bind caspases and have E3 ubiquitin ligase activity responsible for ubiquitination and proteasomal degradation of the apoptosis signaling proteins. [89] There have been two approaches used in the development of IAP inhibitors: XIAP antisense oligonucleotides and small-molecule inhibitors mimicking the endogenous IAP antagonist Smac. AEG35156 is an antisense oligonucleotide (ASO), which targets XIAP mRNA leading to its degradation. Despite promising data from preclinical and clinical studies, a phase II study of 40 patients with AML refractory to initial cytarabine, randomized to receive reinduction cytarabine and idarubicin with and without AEG35156 was terminated early due to inferior (although not statistically significant) overall response rates between AEG35156 and control arms (41% and 69%, p=0.18, respectively). [90, 91] Second mitochondria-derived activators of caspases (Smac) proteins are released from the mitochondria during apoptosis and neutralize the inhibitory activity of IAPs. [92, 93] SMAC mimetics bind select baculovirus IAP repeat (BIR) domains leading to autoubiquitination and proteasomal degradation of c-IAP1 and c-IAP2, resulting in apoptosis and necroptosis. [94] In vitro studies have shown synergistic cell death with hypomethylating agents used in combination with the SMAC mimetics BV6 in AML cell lines and Birinapant in primary AML mononuclear cells. [89, 95, 96] In a xenograft mouse model of human AML, combination therapy with birinapant and azacitidine led to significantly longer median survival than with azacitidine or birinapant alone (30 days, 26 days, and 22 days, respectively p=0.04 vs. AZA, and P<0.001 vs. birinapant). The synergistic effect was in part mediated by cIAP1 since siRNA knockdown in OCI-AML cells resulted in left shifting of the Aza dose-response curve. [96] Preliminary results from a phase II study of birinapant in combination with azacitidine in patients naïve to hypomethylating therapy showed promising results with an OR of 5/6 (83%) and a CR of 3/6 (50%). Grade 3/4 adverse events included neutropenia, thrombocytopenia, fatigue, anemia, increased lipase and syncope. [97]
Immunotherapies
Proof of principle of the importance of immunotherapy approaches remains that allogeneic transplantation, even in non-myeloablative settings, remains the only curative option for myelodysplastic syndromes. The revival of immunotherapies initiated in solid tumors with the impressive efficacy of immune checkpoint blockers and adoptive T cell therapies led to the current development of combinations therapies with HMA. From the biological standpoint, exposure to HMA increases the expression of programmed death ligand 1 (PD-L1), programmed death ligand 2 (PD-L2), programmed death 1 (PD-1), and cytotoxic T lymphocyte-associated antigen 4 (CTLA4) in leukemia cell lines and in peripheral blood mononuclear cells of patients with CMML, AML and MDS during the first cycle of therapy. [98] In sorted peripheral blood CD4+ and CD8+ T cells from patients with MDS, CMML and AML treated with azacitidine, PD-1 promoter demethylation correlated with increased PD-1 expression and significantly worse overall response rate. [99] The cancer/ testes antigens (CTA), which are well-known targets of adoptive T cell therapies, have also been shown to enhance expression after treatment with HMA. In particular, pretreatment with azacitidine led to upregulation of PRAME and de Novo expression of SSX2. [100] HMA leads to global demethylation of promotor regions and subsequent overexpression of checkpoint inhibitor receptors as well as other targets of immunotherapy necessary for leukemogenesis. Several combination studies are currently ongoing
Discussion
The development of HMA based combinations is an intense field of research for both AML and MDS. To date, despite potential strong in vitro data and, in some cases promising phase I and early phase II results, no single study has demonstrated a response of survival advantage of a combination as compared to single agent hypomethylating agent. Thus, HMA monotherapy remains the standard of care for higher risk MDS patients.
Nevertheless, several important points have been learned from the last decade and should be considered for the design and interpretation of future studies. The first question to ask is if we need a combination therapy for every patient. TET2 and IDH mutations predict a favorable response to treatment with HMAs in patients with MDS and AML with response rates as high as 82% and 71% respectively. [101, 102] Achieving additional benefit with IDH inhibitor and HMA combination therapy would be difficult and may not warrant the additional toxicity.
A pragmatic way to consider combination would be to add another drug, only after failure or sub-optimal response of HMA typically after 4 or 6 cycles. Several trials are currently exploring these add-on strategies but none has been fully published to date.
Another question to address is which endpoint should be used to assess efficacy. The use of HMA is associated with a new paradigm with a potential disconnect between the quality of (bone marrow) response and the survival benefit. Most of the studies have used response rate according to IWG2006 as primary criteria assuming that it can still be considered as a surrogate marker of survival. However, there are problems with this approach. For example, there is trend for a better relapse free survival in patients treated with AZA+VOR but no improvement in overall response and conversely, the better response rate of IDH or TET2 mutated patients does not translate in an improved survival. Progression free survival may be a more valuable primary outcome than response rates since there is a relatively short PFS with AZA alone and a strong correlation between PFS and OS in this group of patients with HMA failure having particularly dreadful outcomes. [103]
The large number of possible combinations to be tested and determining which combinations to use for specific populations present major challenges. Targeting specific mutations like IDH with selective inhibitors, or to a lesser extent using lenalidomide in patients with 5q, seem obvious potential strategies. However, the issue of potential cumulative toxicities may counterbalance the clinical benefit, as demonstrated with AZA LEN combinations. Moreover, based on our current understanding of the genetic landscape of MDS, we know that less than a third of the recurrent genetic or cytogenetic drivers can be easily drugged. [104]
Epigenetic marks as well may be targeted, supported by the robust in vitro evidences of the role of DNA methylation in MDS; however, there again, cumulative clinical toxicities of the combinations may strongly outweigh the clinical benefit. Moreover, the methylomic studies of the recent E1905 trial demonstrate a potential pharmacodynamics antagonism (Entinostat blocks the cell cycle thus limits integration of AZA in DNA and thus decrease demethylation) that was not captured by the prior in vitro and early phase studies. An evaluation of the sequence of the drugs for a specific combination should so be systematically and carefully performed.
The next generation hypomethylating agents, including guadecitabine and ASTX727, increase bioavailability by targeting degradation by cytidine deaminases, thereby increasing the duration of exposure. The subcutaneously injected Guadecitabine or SGI-110, a dinucleotide of decitabine and deoxyguanosine, has been shown have nearly a 4-fold longer exposure window and lower than a third the peak serum concentration at 1 hour after administration when compared to standard decitabine dosing 20mg/m2. [105] The first oral hypomethylting agent ASTX727, a combination of the cytidine deaminase inhibitor E7727 and decitabine at a dose of has been shown to exceed IV DAC 20 mg/m2 AUC levels at day 5 and achieve similar LINE-1 demethylation when compared to decitabine monotherapy. [106] Next generation hypomethylating agents have the potential to enhance efficacy and improve the side effect profile by enabling prolonged drug exposure at lower serum drug concentrations.
In conclusion, 10 years after the publication of the first combination study and the FDA registration of hypomethylating agents, the hematology community is still struggling to optimize combination therapy for higher risk MDS. Our better understanding of the genomic and epigenomic complexity of the disease clearly points out the limits of a “one-size-fits-all” approach while also underscoring the need for large-scale academic collaborations in subgroups of patients that may represent 1 to 10% of the MDS at diagnosis. The emergence of new hypomethylating agents as well as new classes of drugs (IDH inhibitors, immunotherapies) may finally change the scope of the question by challenging the place of first generation hypomethylating agents as the backbone of treatment in higher risk MDS rather than considering combinations.
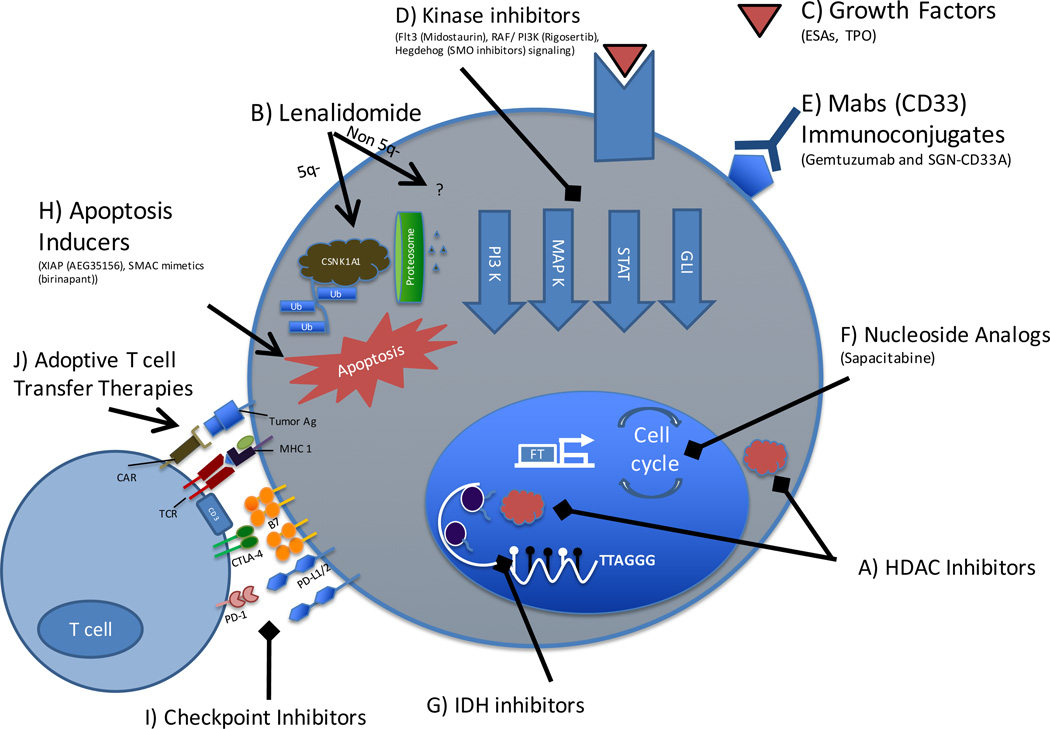
Figure 1. Overview of current therapies used in combination with hypomethylating agents in MDS.
A) HDAC inhibitors remove acetyl molecules on histone and non-histone proteins to regulate transcription and multiple physiologic processes. B) Lenalidomide activates the CRBN-CRL4 E3 ubiquitin ligase leading to degradation of casein kinase 1A1 (CSNK1A1) and p53 mediated apoptosis in patients with 5q- haploinsufficiency. The mechanism of lenalidomide in patients without the 5q- deletion is less clear. C) ESAs and TPO mimetics bind to growth factor receptors to promote production of RBCs and platelets, and potentially mitigate treatment associated cytopenias. D) Kinase and multikinase inhibitors target cell signaling pathways essential to leukemogenesis. E) The antibody drug conjugates gemtuzumab ozogamicin and SGN-CD33A target the myeloid marker CD33, and are linked to cytotoxic agents. F) The oral nucleoside analog sapacitabine is incorporated into DNA leading to single-strand breaks and G2/M cell cycle arrest. G) IDH inhibitors decrease conversion of α-ketoglutarate to β-hydroxyglutarate, a metabolite responsible for impairing TET2 mediated hydroxymethylation; H) The inducers of apoptosis, AEG35156 and SMAC degrade the inhibitor of apotosis proteins (IAP) thereby promoting programmed cell death. I) Immune checkpoint proteins (e.g. PD-1, CTLA4, PD-L1, and PD-L2) are upregulated after pretreatment with hypomethylating therapy and are therefore potential targets for checkpoint inhibitor therapies. J) Upregulation of tumor antigens (e.g. PRAME and SSX2) with hypomethylating therapy also has the potential to serve as targets for chimeric antigen receptor (CAR) or T cell receptor (TCR) based adoptive T cell therapies.
Acknowledgments
Disclosures
BB, AZ no disclosures
TP, SG received research funding from Celgene
References
- 1.Ades L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383:2239–2252. doi: 10.1016/S0140-6736(13)61901-7. [DOI] [PubMed] [Google Scholar]
- 2.Cogle CR, Craig BM, Rollison DE, List AF. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: High number of uncaptured cases by cancer registries. Blood. 2011;117:7121–7125. doi: 10.1182/blood-2011-02-337964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SEER cancer statistics review, 1975–2011 [Internet] Bethesda, MD: National Cancer Institute; 2014. Apr, [cited 2016]. April 2014. - Available from: http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 4.Schanz J, Tuchler H, Sole F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–829. doi: 10.1200/JCO.2011.35.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcovati L, Della Porta MG, Strupp C, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based prognostic scoring system (WPSS) Haematologica. 2011;96:1433–1440. doi: 10.3324/haematol.2011.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Della Porta MG, Tuechler H, Malcovati L, et al. Validation of WHO classification-based prognostic scoring system (WPSS) for myelodysplastic syndromes and comparison with the revised international prognostic scoring system (IPSS-R). A study of the international working group for prognosis in myelodysplasia (IWG-PM) Leukemia. 2015;29:1502–1513. doi: 10.1038/leu.2015.55. [DOI] [PubMed] [Google Scholar]
- 8.Blau O, Blau IW. Some aspects of allogeneic stem cell transplantation in patients with myelodysplastic syndrome: Advances and controversy. Stem Cells Cloning. 2014;7:101–108. doi: 10.2147/SCCAA.S50514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mufti GJ, Potter V. Myelodysplastic syndromes: Who and when in the course of disease to transplant. Hematology Am Soc Hematol Educ Program. 2012;2012:49–55. doi: 10.1182/asheducation-2012.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Zeidan AM, Linhares Y, Gore SD. Current therapy of myelodysplastic syndromes. Blood Rev. 2013;27:243–259. doi: 10.1016/j.blre.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollenbach PW, Nguyen AN, Brady H, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One. 2010;5:e9001. doi: 10.1371/journal.pone.0009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christman JK. 5-azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 13. [cited 2016 1/24/2016];FDA approval for azacitidine - national cancer institute[Internet] Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-azacitidine.
- 14. [cited 2016 1/24/2016];FDA approval for decitabine - national cancer institute[Internet] Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-decitabine.
- 15.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 16.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the cancer and leukemia group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 17.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 19.Craddock C, Quek L, Goardon N, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013;27:1028–1036. doi: 10.1038/leu.2012.312. [DOI] [PubMed] [Google Scholar]
- 20.Eberharter A, Becker PB. Histone acetylation: A switch between repressive and permissive chromatin. second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santini V, Melnick A, Maciejewski JP, et al. Epigenetics in focus: Pathogenesis of myelodysplastic syndromes and the role of hypomethylating agents. Crit Rev Oncol Hematol. 2013;88:231–245. doi: 10.1016/j.critrevonc.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 22.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spange S, Wagner T, Heinzel T, Kramer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41:185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: Mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008;269:7–17. doi: 10.1016/j.canlet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2’-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffoux E, Cras A, Recher C, et al. Phase 2 clinical trial of 5-azacitidine, valproic acid, and all-trans retinoic acid in patients with high-risk acute myeloid leukemia or myelodysplastic syndrome. Oncotarget. 2010;1:34–42. doi: 10.18632/oncotarget.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Issa JP, Garcia-Manero G, Huang X, et al. Results of phase 2 randomized study of low-dose decitabine with or without valproic acid in patients with myelodysplastic syndrome and acute myelogenous leukemia. Cancer. 2015;121:556–561. doi: 10.1002/cncr.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: Overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 30.Prebet T, Vey N. Vorinostat in acute myeloid leukemia and myelodysplastic syndromes. Expert Opin Investig Drugs. 2011;20:287–295. doi: 10.1517/13543784.2011.542750. [DOI] [PubMed] [Google Scholar]
- 31.Kirschbaum M, Gojo I, Goldberg SL, et al. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol. 2014;167:185–193. doi: 10.1111/bjh.13016. [DOI] [PubMed] [Google Scholar]
- 32.Tan P, Wei A, Mithraprabhu S, et al. Dual epigenetic targeting with panobinostat and azacitidine in acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood Cancer J. 2014;4:e170. doi: 10.1038/bcj.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekeres M, Othus M, List A, Odenike O, Stone R, Gore S. Additional analysis of a randomized phase II study of azacitidine monotherapy in higher-risk myelodysplastic syndromes (MDS and chronic myelomonocytic leukemia (CMML): North american intergroup study SWOG S1117. [abstract] Blood. 2015;126:908. doi: 10.1200/JCO.2015.66.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekeres M, Othus M, List A. A randomized phase II study of azacitidine combined with lenalidomide or with vorinostat vs. azacitidine monotherapy in higher-risk myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML): North american intergroup study SWOG S1117. [abstract] Blood. 2014;124 doi: 10.1200/JCO.2015.66.2510. LBA-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Manero G, Sekeres MA, Egyed M, et al. Panobinostat plus azacitidine in adult patients with MDS, CMML, or AML: Results of a phase 2b study. Blood. 2015;126:2861. [Google Scholar]
- 36.Garcia-Manero G, Berdeja J, Komrokji R, et al. A randomized, placebo-controlled, phase II study of pracinostat in combination with azacitidine (AZA) in patients with previously untreated myelodysplastic syndrome (MDS). [abstract] Blood. 2015;126:911. [Google Scholar]
- 37.Prebet T, Sun Z, Figueroa ME, et al. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: Results of the US leukemia intergroup trial E1905. J Clin Oncol. 2014;32:1242–1248. doi: 10.1200/JCO.2013.50.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prebet T, Sun Z, Ketterling RP, et al. Azacitidine with or without entinostat for the treatment of therapy-related myeloid neoplasm: Further results of the E1905 north american leukemia intergroup study. Br J Haematol. 2015 doi: 10.1111/bjh.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillermo G, Selina L, Virginia K, et al. Combination therapy with mocetinostat, an oral, spectrum-selective histone deacetylase (HDAC) inhibitor, and 5-azaciditine: Indication of clinical activity in MDS. Blood. 2013;122:1550. [Google Scholar]
- 40. [cited 2016 1/24/2016];FDA approval for lenalidomide - national cancer institute [Internet] Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-lenalidomide.
- 41.Ghosh N, Grunwald MR, Fasan O, Bhutani M. Expanding role of lenalidomide in hematologic malignancies. Cancer Manag Res. 2015;7:105–119. doi: 10.2147/CMAR.S81310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fink E. Lenalidomide induces ubiquitination and degradation of CSNK1A1 in MDS with del (5q); San Fracison, CA. Abstract 4 Presented at Th 56th ASH Annual Meeting.2014. Dec 6–9, [Google Scholar]
- 43.Kronke J, Fink EC, Hollenbach PW, et al. Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature. 2015;523:183–188. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 45.Giagounidis AA, Germing U, Haase S, et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia. 2004;18:113–119. doi: 10.1038/sj.leu.2403189. [DOI] [PubMed] [Google Scholar]
- 46.Mittelman M, Filanovsky K, Rosenbaum H, et al. Azacitidine and lenalidomide combination in higher-risk myelodysplastic syndromes-preliminary results of the vilen-01 protocol. Blood. 2013;122:1576. [Google Scholar]
- 47.Platzbecker U, Braulke F, Kundgen A, et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: A phase I study. Leukemia. 2013;27:1403–1407. doi: 10.1038/leu.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ades L. A phase I-II study of the efficacy and safety of lenalidomide (LEN) combined to azacitidine (AZA) in higher risk MDS and AML with del 5q- a study by the groupe francophone des myelodysplasies (GFM); Orlando, FL. Abstract 2892 Presented at ASH 57th Annual Meeting.2015. Dec 5–8, [Google Scholar]
- 49.Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120:4945–4951. doi: 10.1182/blood-2012-06-434639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narayan R, Garcia JS, Percival MM, et al. Sequential azacitidine plus lenalidomide in previously treated elderly patients with acute myeloid leukemia and higher risk myelodysplastic syndrome. Leuk Lymphoma. 2015:1–7. doi: 10.3109/10428194.2015.1091930. [DOI] [PubMed] [Google Scholar]
- 51.DiNardo CD, Daver N, Jabbour E, et al. Sequential azacitidine and lenalidomide in patients with high-risk myelodysplastic syndromes and acute myeloid leukaemia: A single-arm, phase 1/2 study. Lancet Haematol. 2015;2:e12–e20. doi: 10.1016/S2352-3026(14)00026-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabrilove J, Paquette R, Lyons RM, et al. Phase 2, single-arm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodysplastic syndromes. Br J Haematol. 2008;142:379–393. doi: 10.1111/j.1365-2141.2008.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelaidi C, Beyne-Rauzy O, Braun T, et al. High response rate and improved exercise capacity and quality of life with a new regimen of darbepoetin alfa with or without filgrastim in lower-risk myelodysplastic syndromes: A phase II study by the GFM. Ann Hematol. 2013;92:621–631. doi: 10.1007/s00277-013-1686-4. [DOI] [PubMed] [Google Scholar]
- 54.Brierley CK, Steensma DP. Thrombopoiesis-stimulating agents and myelodysplastic syndromes. Br J Haematol. 2015;169:309–323. doi: 10.1111/bjh.13285. [DOI] [PubMed] [Google Scholar]
- 55.Itzykson R, Thepot S, Beyne-Rauzy O, et al. Does addition of erythropoiesis stimulating agents improve the outcome of higher-risk myelodysplastic syndromes treated with azacitidine? Leuk Res. 2012;36:397–400. doi: 10.1016/j.leukres.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 56.Boehrer S, Beyne-Rauzy O, Prebet T, Park S. Interim results of A randomized phase II trial of azacitidine (AZA) +/− epo in lower risk myelodysplastic syndrome (MDS) resistant to an erythropoietic stimulating agent (ESA) alone. Blood (ASH Annual Meeting Abstracts) 2010;116:784. [Google Scholar]
- 57.Fili C, Malagola M, Follo MY, et al. Prospective phase II study on 5-days azacitidine for treatment of symptomatic and/or erythropoietin unresponsive patients with low/INT-1-risk myelodysplastic syndromes. Clin Cancer Res. 2013;19:3297–3308. doi: 10.1158/1078-0432.CCR-12-3540. [DOI] [PubMed] [Google Scholar]
- 58.Tobiasson M, Dybedahl I, Holm MS, et al. Limited clinical efficacy of azacitidine in transfusion-dependent, growth factor-resistant, low- and int-1-risk MDS: Results from the nordic NMDSG08A phase II trial. Blood Cancer J. 2014;4:e189. doi: 10.1038/bcj.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenberg PL, Garcia-Manero G, Moore M, et al. A randomized controlled trial of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving decitabine. Leuk Lymphoma. 2013;54:321–328. doi: 10.3109/10428194.2012.713477. [DOI] [PubMed] [Google Scholar]
- 60.Kantarjian HM, Giles FJ, Greenberg PL, et al. Phase 2 study of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving azacitidine therapy. Blood. 2010;116:3163–3170. doi: 10.1182/blood-2010-03-274753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giagounidis A, Mufti GJ, Fenaux P, et al. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014;120:1838–1846. doi: 10.1002/cncr.28663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Svensson T, Chowdhury O, Garelius H, et al. A pilot phase I dose finding safety study of the thrombopoietin-receptor agonist, eltrombopag, in patients with myelodysplastic syndrome treated with azacitidine. Eur J Haematol. 2014;93:439–445. doi: 10.1111/ejh.12383. [DOI] [PubMed] [Google Scholar]
- 63.Stone RM, Mandrekar S, Sanford BL, Geyer S. The multi-kinase inhibitor midostaurin (M) prolongs survival compared with placebo (P) in combination with daunorubicin (D)/cytarabine (C) induction (ind), high-dose C consolidation (consol), and as maintenance (maint) therapy in newly diagnosed acute myeloid leukemia (AML) patients (pts) age 18–60 with FLT3 mutations (muts): An international prospective randomized (rand) P-controlled double-blind trial (CALGB 10603/RATIFY [alliance]) Blood (ASH Annual Meeting Abstracts) 2015 [Google Scholar]
- 64.Strati P, Kantarjian H, Ravandi F, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90:276–281. doi: 10.1002/ajh.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jilani I, Estey E, Huh Y, et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol. 2002;118:560–566. doi: 10.1309/1WMW-CMXX-4WN4-T55U. [DOI] [PubMed] [Google Scholar]
- 66.Laszlo GS, Estey EH, Walter RB. The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev. 2014;28:143–153. doi: 10.1016/j.blre.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 68.Daver N, Kantarjian H, Ravandi F, et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2015 doi: 10.1038/leu.2015.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kung Sutherland MS, Walter RB, Jeffrey SC, et al. SGN-CD33A: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122:1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- 70.Fathi AT, Erba HP, Lancet JE, et al. SGN-CD33A plus hypomethylating agents: A novel, well-tolerated regimen with high remission rate in frontline unfit AML. Blood. 2015;126:454. [Google Scholar]
- 71.Hanaoka K, Suzuki M, Kobayashi T, et al. Antitumor activity and novel DNA-self-strand-breaking mechanism of CNDAC (1-(2-C-cyano-2-deoxy-beta-D-arabino-pentofuranosyl) cytosine) and its N4-palmitoyl derivative (CS-682) Int J Cancer. 1999;82:226–236. doi: 10.1002/(sici)1097-0215(19990719)82:2<226::aid-ijc13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 72.Ravandi F, Kadia TM, Borthakur G, et al. Pooled analysis of elderly patients with newly diagnosed AML treated with sapacitabine and decitabine administered in alternating cycles. [abstract]. American Society of Hematology (ASH) 54th Annual Meeting; 2012. Abstract 2630. [Google Scholar]
- 73.Athuluri-Divakar SK, Vasquez-Del Carpio R, Dutta K, et al. A small molecule RAS-mimetic disrupts RAS association with effector proteins to block signaling. Cell. 2016;165:643–655. doi: 10.1016/j.cell.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Manero G, Fenaux P, Al-Kali A, et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): A randomised, controlled, phase 3 trial. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)00009-7. [DOI] [PubMed] [Google Scholar]
- 75.Navada SC, Garcia-Manero G, Wilhelm F, et al. A phase I/II study of the combination of oral rigosertib and azacitidine in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) Blood. 2014;124:3252. [Google Scholar]
- 76.Merchant A, Joseph G, Wang Q, Brennan S, Matsui W. Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood. 2010;115:2391–2396. doi: 10.1182/blood-2009-09-241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou J, Hong Y, Tong Y, et al. Sonic hedgehog produced by bone marrow-derived mesenchymal stromal cells supports cell survival in myelodysplastic syndrome. Stem Cells Int. 2015;2015:957502. doi: 10.1155/2015/957502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan AA, Harrison CN, McLornan DP. Targeting of the hedgehog pathway in myeloid malignancies: Still a worthy chase? Br J Haematol. 2015;170:323–335. doi: 10.1111/bjh.13426. [DOI] [PubMed] [Google Scholar]
- 79.Tibes R, Al-Kali A, Oliver GR, et al. The hedgehog pathway as targetable vulnerability with 5-azacytidine in myelodysplastic syndrome and acute myeloid leukemia. J Hematol Oncol. 2015;8:114,015-0211-8. doi: 10.1186/s13045-015-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKenney AS, Levine RL. Isocitrate dehydrogenase mutations in leukemia. J Clin Invest. 2013;123:3672–3677. doi: 10.1172/JCI67266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin J, Hu C, Yu M, et al. Prognostic value of isocitrate dehydrogenase mutations in myelodysplastic syndromes: A retrospective cohort study and meta-analysis. PLoS One. 2014;9:e100206. doi: 10.1371/journal.pone.0100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Losman JA, Looper RE, Koivunen P, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DiNardo C, Stein E, Altman J, Collins R, Deangelo DJ. AG-221, an oral, selective, first-in-class, potent inhibitor of the IDH2 mutant enzyme, induced durable responses in a phase 1 study of IDH2 mutation-positive advanced hematologic malignancies. haematol eur hematol assoc annu meet 2015;100(s1):569 [abstr)]; European Hematology Association Annual Meeting; 2015. p. 569. [Google Scholar]
- 86.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 87.Tamm I, Kornblau SM, Segall H, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 88.Tamm I, Richter S, Scholz F, et al. XIAP expression correlates with monocytic differentiation in adult de novo AML: Impact on prognosis. Hematol J. 2004;5:489–495. doi: 10.1038/sj.thj.6200549. [DOI] [PubMed] [Google Scholar]
- 89.Fulda S. Inhibitor of apoptosis (IAP) proteins in hematological malignancies: Molecular mechanisms and therapeutic opportunities. Leukemia. 2014;28:1414–1422. doi: 10.1038/leu.2014.56. [DOI] [PubMed] [Google Scholar]
- 90.Schimmer AD, Estey EH, Borthakur G, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27:4741–4746. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schimmer AD, Herr W, Hanel M, et al. Addition of AEG35156 XIAP antisense oligonucleotide in reinduction chemotherapy does not improve remission rates in patients with primary refractory acute myeloid leukemia in a randomized phase II study. Clin Lymphoma Myeloma Leuk. 2011;11:433–438. doi: 10.1016/j.clml.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 92.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 93.Wu G, Chai J, Suber TL, et al. Structural basis of IAP recognition by smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 94.Varfolomeev E, Blankenship JW, Wayson SM, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 95.Steinhart L, Belz K, Fulda S. Smac mimetic and demethylating agents synergistically trigger cell death in acute myeloid leukemia cells and overcome apoptosis resistance by inducing necroptosis. Cell Death Dis. 2013;4:e802. doi: 10.1038/cddis.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carter BZ, Mak PY, Mak DH, et al. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J Natl Cancer Inst. 2014;106:djt440. doi: 10.1093/jnci/djt440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ritchie E, Agrawal A, Patel K, Rosen S. A phase 2 study of brinipant in combination with 5-azacitidine in patient with myelodysplastic syndrome who are naive to 5-azacitidine: A preliminary analysis of phase 2a; Orlando, FL. Abstract 2904 Presented at ASH 57th Annual Meeting.2015. Dec 5–8, [Google Scholar]
- 98.Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–1288. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orskov AD, Treppendahl MB, Skovbo A, et al. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget. 2015;6:9612–9626. doi: 10.18632/oncotarget.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atanackovic D, Luetkens T, Kloth B, et al. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am J Hematol. 2011;86:918–922. doi: 10.1002/ajh.22141. [DOI] [PubMed] [Google Scholar]
- 101.Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25:1147–1152. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 102.Emadi A, Faramand R, Carter-Cooper B, et al. Presence of isocitrate dehydrogenase mutations may predict clinical response to hypomethylating agents in patients with acute myeloid leukemia. Am J Hematol. 2015;90:E77–E79. doi: 10.1002/ajh.23965. [DOI] [PubMed] [Google Scholar]
- 103.Prebet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papaemmanuil E, Gerstung M, Malcovati L, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616,27. doi: 10.1182/blood-2013-08-518886. quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Issa JP, Roboz G, Rizzieri D, et al. Safety and tolerability of guadecitabine (SGI-110) in patients with myelodysplastic syndrome and acute myeloid leukaemia: A multicentre, randomised, dose-escalation phase 1 study. Lancet Oncol. 2015;16:1099–1110. doi: 10.1016/S1470-2045(15)00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Savona M. Results of first in human (FIH) phase 1 pharmacokinetic (PK) guided dose-escalation study of ASTX727, a combination of the oral cytidine deaminase inhibitor (CDAi) E7727 with oral decitabine in subjects with myelodysplastic syndrome (MDS); Orlando, FL. Abstract 1683 Presented at ASH 57th Annual Meeting December 5th-8th, 2015. [Google Scholar]
- 107.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 108.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]