Abstract
BACKGROUND
Ovarian cancer is the fifth leading cause of cancer death among women in the United States. This study reports ovarian cancer survival by state, race, and stage at diagnosis using data from the CONCORD-2 study, the largest and most geographically comprehensive, population-based survival study to date.
METHODS
Data from women diagnosed with ovarian cancer between 2001 and 2009 from 37 states, covering 80% of the US population, were used in all analyses. Survival was estimated up to 5 years and was age standardized and adjusted for background mortality (net survival) using state-specific and race-specific life tables.
RESULTS
Among the 172,849 ovarian cancers diagnosed between 2001 and 2009, more than one-half were diagnosed at distant stage. Five-year net survival was 39.6% between 2001 and 2003 and 41% between 2004 and 2009. Black women had consistently worse survival compared with white women (29.6% from 2001–2003 and 31.1% from 2004–2009), despite similar stage distributions. Stage-specific survival for all races combined between 2004 and 2009 was 86.4% for localized stage, 60.9% for regional stage, and 27.4% for distant stage.
CONCLUSIONS
The current data demonstrate a large and persistent disparity in ovarian cancer survival among black women compared with white women in most states. Clinical and public health efforts that ensure all women who are diagnosed with ovarian cancer receive appropriate, guidelines-based treatment may help to decrease these disparities. Future research that focuses on the development of new methods or modalities to detect ovarian cancer at early stages, when survival is relatively high, will likely improve overall US ovarian cancer survival.
Keywords: cancer registries, ovarian cancer, population-based survival, surveillance, survival
INTRODUCTION
Ovarian cancer is the eighth most commonly diagnosed and fifth leading cause of cancer death in the United States.1 Ovarian cancer is a heterogeneous disease, consisting of epithelial and nonepithelial types and subtypes. Because of their similarity to epithelial ovarian cancer in terms of histology, pathogenesis, and clinical disease course, primary fallopian tube and primary peritoneal cancers are often included in analyses of ovarian cancer.2
Population-based ovarian cancer incidence and mortality (the number of new cases and deaths in a given time period, respectively) are presented annually in several reports.1,3,4 These reports indicate that age-standardized ovarian cancer incidence and death rates are highest among white women in the United States.1,3,4 Population-based survival is less often reported and reflects the average survival for all patients with cancer in the population, regardless of their age, sex, race, health status, clinical disease characteristics (eg, stage of disease), socioeconomic status, residence at diagnosis, or access to care.5 Therefore, population-based cancer survival provides an indicator of the overall effectiveness of the health care system to deliver cancer screening (if available), early diagnosis, and evidence-based treatment services and follow-up care to all individuals in the population being served.5 Population-based survival estimates also allow cancer-control practitioners to identify target populations for educational interventions and environmental and health-systems changes that could help patients with cancer lead longer, healthier lives.
In the United States, information on population-based ovarian cancer survival has come from individual state reports and from the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program, which covers from 9% to 26% of the US population.3 Additional nonpopulation-based survival reports come from individual hospitals or institutions or the from National Cancer Database, which consists only of hospitals accredited by the Commission on Cancer.6 More recently, the Centers for Disease Control and Prevention’s (CDC’s) National Program of Cancer Registries (NPCR) has begun to collect high-quality survival data.7 Combined, SEER and NPCR data represent the official US federal government statistics on cancer.8
The CONCORD-2 study collaborated with SEER and NPCR cancer registries, along with other population-based cancer registries around the world, to establish surveillance of cancer survival for 10 common cancers from 67 countries.9 CONCORD-2 findings indicated that, for women who were diagnosed between 1995 and 2009, international differences in ovarian cancer 5-year, age-standardized net survival were wide, even after adjustment for differences in mortality from other causes of death, with survival in the United States improving and among the highest in the world.9
The objective of this report is to extend the CONCORD-2 study, as well as official US government annual reports of cancer incidence and mortality, to provide population-based ovarian cancer survival estimates for 37 US states. This is the largest population-based ovarian cancer survival study in the United States to date, covering 80% of the US population, and provides critical information for directing the state-specific ovarian cancer efforts of the National Comprehensive Cancer Control Program (NCCCP).
MATERIALS AND METHODS
Detailed descriptions of data sources, evaluation methods, and statistical analyses are provided in this Supplement of Cancer.10 A brief description follows.
Data Source and Variables
We used data from the 37 NPCR or SEER state-wide cancer registries that participated in the CONCORD-2 study,9 covering approximately 80% of the US population, and consented to the inclusion of their data in the more detailed analysis reported here. We analyzed individual tumor records for females (ages 15–99 years) who were diagnosed between 2001 and 2009 (and followed through to December 31, 2009) with cancer of the ovary, fallopian tube, peritoneum, and retroperitoneum (henceforth referred to as ovarian cancer; International Classification of Diseases for Oncology, third edition, codes11 for the tumors included in this analysis are C48.0-C48.2, C56.9, C57.0-C57.4, and C57.7-C57.9).9 Malignant tumors of uterine ligaments and those from other and unspecified female genital organs were included in this analysis according to the CONCORD-2 protocol to allow for a comparison of survival data worldwide across all registries in the CONCORD-2 study.9 We included only the first primary, malignant cancer of the ovary, regardless of whether a woman had a previous cancer from a different site. Any subsequent ovarian cancer diagnoses between 2001 and 2009 were excluded. All benign and borderline tumors of the ovary were excluded; all malignant ovarian tumors (including epithelial and nonepithelial tumors) were included.
We grouped patients by year of diagnosis into 2 calendar periods (2001–2003 and 2004–2009) to reflect changes in staging methods used by US cancer registries to collect SEER Summary Stage 2000 (SS2000) at diagnosis. SS2000 is the long-standing staging system routinely used by all US cancer registries and broadly categorizes malignant tumors into localized, regional, distant, and unstaged to allow overall population-based reporting of staging trends.12 Between 2001 and 2003, cancer registries directly coded SS2000 from the medical record; whereas, between 2004 and 2009, all registries derived SS2000 using a series of data elements collected from the Collaborative Staging System.13 The derived SS2000 maintains the same stage categorization but generally results in fewer cases staged as unknown because of the collection of a series of individual data elements related to disease extent and the use of rule-based algorithms to assign a stage from those elements.
All cancer registry data used in this analysis are high-quality, as assessed by the US Cancer Statistics Working Group1 and the North American Association of Central Cancer Registries (NAACCR).14 All SEER and NPCR registries use the same standardized procedures to collect the majority of information on cancer cases.10 However, SEER registries conduct both active and passive follow-up to ascertain vital status, whereas most NPCR registries only conduct passive follow-up through linkages with their state vital records and the National Death Index to obtain information on deaths that occurred within their state and elsewhere within the United States.14
Survival Analyses
We analyzed ovarian cancer survival by state, race (all races combined, black, and white), SS2000, and calendar period of diagnosis (2001–2003 and 2004–2009). The all races combined category includes all ovarian cancer cases in the data set (black, white, and women of other or unspecified race). We estimated net survival up to 5 years after diagnosis with 95% confidence intervals (CIs) using the Pohar Perme estimator.15 Net survival is interpreted as the probability of survival up to a given time since diagnosis, after controlling for other causes of death (background mortality). To control for wide differences in background mortality among participating registries, we constructed life tables based on published methods16 of all-cause mortality in the general population of each state from the number of deaths and the populations, by single year of age, sex, calendar year, and, where possible, by race (white, black), using a flexible Poisson model.17
We estimated net survival using the cohort approach for patients diagnosed between 2001 and 2003, because all patients were followed for at least 5 years by December 31, 2009. We used the complete approach to estimate net survival for patients diagnosed between 2004 and 2009, because 5 years of follow-up data were not available for all patients from that calendar period. Net survival was estimated for 5 age groups (ages 15–44, 45–54, 55–64, 65–74, and 75–99 years). We obtained age-standardized survival estimates using the International Cancer Survival Standard weights.18 If 2 or more of the 5 age-specific estimates could not be obtained, then only the pooled, unstandardized survival estimate for all ages combined was presented. Unstandardized survival estimates are italicized in the supporting tables, which provide state-specific data. Trends, geographic variations, and differences in age-standardized survival by race are presented graphically and in funnel plots.19 Funnel plots of net survival in the United States by race and state indicate how much a particular survival estimate deviates from the pooled US estimate, given its level of precision. More information on these methods can be found in this Supplement.10
RESULTS
Of the 172,849 cancer cases included in this analysis, 56,390 women were diagnosed between 2001 and 2003, and 116,459 were diagnosed between 2004 and 2009 (Table 1). Over 85% of diagnoses were among white women during both time periods (49,893 from 2001–2003 and 101,717 from 2004–2009), and more than one-half of all cases (53.5% from 2001–2003 and 56.8% from 2004–2009) were diagnosed at distant stage, with minimal variability in stage distribution by race. State-specific patterns mirrored national patterns, in that there were much higher numbers of cases among white women compared with black women, and cases were most often diagnosed at distant stage (Supporting Table 1).
TABLE 1.
Ovarian Cancer: The Number of Cases Diagnosed Among Females Ages 15 to 99 Years by Stage at Diagnosis and Calendar Period Between 2001 and 2009
| SS2000a | 2001–2003 | 2004–2009 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| All Races | White | Black | All Races | White | Black | |
| No. of patients | 56,390 | 49,893 | 4262 | 116,459 | 101,717 | 9440 |
| Localized, % | 15.9 | 15.7 | 15.4 | 14.2 | 13.9 | 14.5 |
| Regional, % | 18.9 | 18.9 | 16.4 | 18.9 | 19.0 | 15.7 |
| Distant, % | 53.5 | 54.1 | 51.0 | 56.8 | 57.4 | 55.3 |
| Unknown, % | 11.7 | 11.3 | 17.2 | 10.1 | 9.7 | 14.5 |
Abbreviations: SS2000, Surveillance, Epidemiology, and End Results (SEER) Summary Stage 2000.
Stage at diagnosis is SS2000.
Table 2 provides details ovarian cancer age-standardized net survival at 1, 3, and 5 years by time period and race. In both calendar periods, survival decreased with increasing time since diagnosis. Between 2004 and 2009, survival was 73.3% (95% CI, 73%–73.6%) at 1 year, 52.8% (95% CI, 52.4%–53.1%) at 3 years, and 41.0% (95% CI, 40.5%–41.5%) at 5 years. Five-year survival was at least 10% lower in black women compared with white women in both calendar periods (2001–2003: 29.6% [95% CI, 28.1%–31.1%] vs 40.1% [95% CI, 39.6%–40.6%], respectively; 2004–2009: 31.1% [95% CI, 29.5%–32.7%] vs 41.7% [95% CI, 41.2%–42.2%], respectively). The racial gap appeared within the first year after diagnosis and persisted between the 2 calendar periods. Similar patterns were observed in most states (Supporting Table 2).
TABLE 2.
Ovarian Cancer: Age-Standardized Net Survival (%) at 1, 3, and 5 Years Among Females Ages 15 to 99 Years by Race and Calendar Period Between 2001 and 2009
| Years | 2001–2003 | 2004–2009 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
|
|
|
|
|
|
|||||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | |
| 1 | 72.3 | 71.9–72.7 | 73.3 | 72.9–73.7 | 59.8 | 58.2–61.3 | 73.3 | 73.0–73.6 | 74.2 | 73.9–74.5 | 62.5 | 61.4–63.6 |
| 3 | 50.9 | 50.4–51.3 | 51.6 | 51.1–52.1 | 39.4 | 37.8–41.0 | 52.8 | 52.4–53.1 | 53.6 | 53.2–54.0 | 41.6 | 40.3–42.9 |
| 5 | 39.6 | 39.1–40.0 | 40.1 | 39.6–40.6 | 29.6 | 28.1–31.1 | 41.0 | 40.5–41.5 | 41.7 | 41.2–42.2 | 31.1 | 29.5–32.7 |
Abbreviations: CI, confidence interval; NS, net survival.
Table 3 provides details on 5-year, age-standardized net survival by race, stage at diagnosis, and calendar period. For all races combined, stage-specific survival improved between calendar periods; however, black women had lower survival compared with white women for each stage at diagnosis. In the most recent calendar period, survival was highest for localized stage at diagnosis (86.4%; 95% CI, 84.8%–87.9%), followed by regional stage at diagnosis (60.9%; 95% CI, 59.7%–62.2%), and distant stage at diagnosis (27.4%; 95% CI, 26.9%–28%). Similar patterns were observed in most states (Supporting Table 3).
TABLE 3.
Ovarian Cancer: Five-Year Age-Standardized Net Survival (%) Among Females Ages 15 to 99 Years by Stage at Diagnosis, Race, and Calendar Period Between 2001 and 2009
| SS2000a | 2001–2003 | 2004–2009 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
|
|
|
|
|
|
|||||||
| NS (%) | 95% CI% | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | |
| All stages | 39.6 | 39.1–40.0 | 40.1 | 39.6–40.6 | 29.6 | 28.1–31.1 | 41.0 | 40.5–41.5 | 41.7 | 41.2–42.2 | 31.1 | 29.5–32.7 |
| Localized | 84.8 | 83.3–86.2 | 84.9 | 83.3–86.4 | 78.8 | 71.9–85.7 | 86.4 | 84.8–87.9 | 86.9 | 85.2–88.5 | 80.9 | 74.8–86.9 |
| Regional | 53.9 | 52.8–55.1 | 54.7 | 53.5–55.9 | 38.6 | 34.3–42.9 | 60.9 | 59.7–62.2 | 61.6 | 60.3–62.9 | 45.3 | 40.7–49.9 |
| Distant | 25.2 | 24.7–25.7 | 25.8 | 25.2–26.4 | 16.3 | 14.7–18.0 | 27.4 | 26.9–28.0 | 28.0 | 27.4–28.7 | 18.6 | 16.8–20.5 |
| Unknown | 33.1 | 31.9–34.4 | 33.7 | 32.4–35.1 | 26.8 | 23.3–30.2 | 32.6 | 31.3–33.9 | 33.6 | 32.2–35.0 | 25.7 | 22.0–29.4 |
Abbreviations: CI, confidence interval; NS, net survival.
Stage at diagnosis is the Surveillance, Epidemiology, and End Results (SEER) Summary Stage 2000 (SS2000).
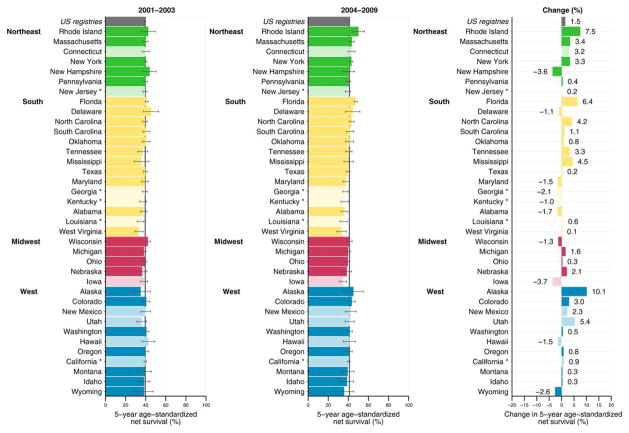
Figure 1 illustrates the absolute change in ovarian cancer survival between 2001 and 2003 and between 2004 and 2009. Overall, 5-year net ovarian cancer survival increased 1.5% between 2001 and 2003 and between 2004 and 2009. Among states, 27 had increases and 10 had decreases in survival between the 2 calendar periods. In approximately one-half of states (18 of 37 states), the increase or decrease was ≤ 1.5%.
Figure 1.
Ovarian cancer 5-year, age-standardized net survival (%) among females ages 15 to 99 years and absolute change in net survival (%) are illustrated by calendar period between 2001 and 2009. Note that states are ranked within Census Region by the survival estimate for the years 2004 to 2009. Dark colors are registries affiliated with the National Program of Cancer Registries; and pale colors are registries affiliated with the Surveillance, Epidemiology, and End Results program. An asterisk denotes registries affiliated with both programs.
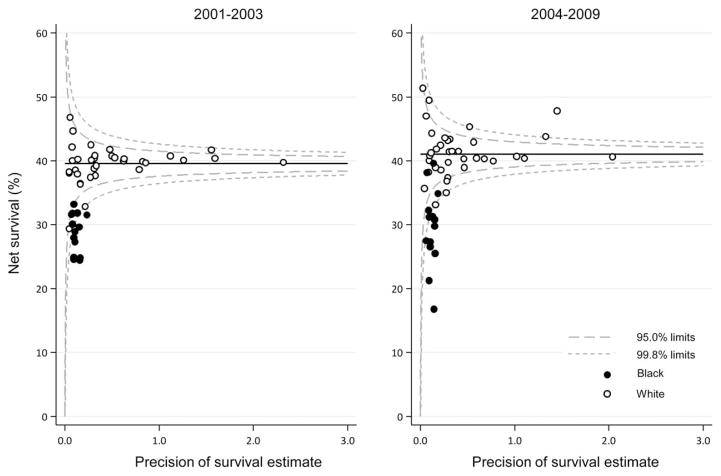
Funnel plots illustrating 5-year, age-standardized net ovarian cancer survival by race are presented in Figure 2. Between 2001 and 2003, the age-standardized estimates for white women ranged from 29.3% to 46.8%; and, between 2004 and 2009, the range was from 33.1% to 51.4%. In the first calendar period, all survival estimates for white women were within the control limits (no more than 2 or 3 standard deviations below or above the target of the pooled US all races combined estimate of 39.6%, after controlling for precision). The same pattern was observed between 2004 and 2009, with estimates within the control limits around the pooled US estimate of 41.0%; however, in this time period, survival for white women in 1 state improved to a level above these limits. Survival among black women ranged from 24.4% to 33.1% between 2001 and 2003 and from 16.5% to 41.7% between 2004 and 2009. In both calendar periods, survival for black women was consistently lower in all states compared with survival for pooled US all races combined estimate, and most estimates were outside the control limits.
Figure 2.
Ovarian cancer 5-year, age-standardized net survival (NS) (%) among females ages 15 to 99 years is illustrated by state, race, and calendar period of diagnosis. Note that the pooled US survival estimates for each calendar period are indicated by horizontal (solid) lines with corresponding 95% and 99.8% control limits (dashed lines).
DISCUSSION
Findings from this large, population-based study indicate that US net survival from ovarian cancer is moderate overall: approximately 40% to 41% at 5 years. We have also demonstrated a modest increase in survival in the most recent time period examined. The data presented here indicate a consistent and persistent disparity in ovarian cancer survival among black women compared with white women.
Our overall US survival estimates are somewhat lower than those from US analyses that include only SEER registries, which reportedly are 44% survival at 5 years. With regard to trends, our findings are generally consistent with a recent SEER registry analysis that reported increases in ovarian cancer survival since 1990. NPCR cancer registries contain an older and more rural population than SEER alone, which is a sample population that tends to be more urban and affluent than the general population.8 Several smaller studies have demonstrated that older populations have lower survival from ovarian cancer, potentially because of the existence of comorbidities or lack of access to care and other resources.20,21 Therefore, the greater inclusion of data from these older individuals likely underlies the somewhat lower survival estimates we report in this comprehensive study. Given the passive, follow-up only procedures of NPCR registries, NPCR registries may miss some deaths, particularly for patients who leave the United States between the time of their diagnosis and death or those who have incomplete demographic variables available for matching; this may result in a slight overestimation of survival rates.14 Therefore, the lower survival estimates observed here are likely true reflections of the broader inclusion of the population with ovarian cancer and not because of differences in vital status follow-up procedures. Internationally, the ovarian cancer survival estimates reported here are similar to the estimates from countries with relatively higher estimates of ovarian cancer survival from the CONCORD-2 study and are slightly higher than those reported from Canada (37.5% 5-year survival from 2005 to 2009).9
Our finding that black women have consistently lower ovarian cancer survival than white women likely reflects a true and widespread racial disparity in ovarian cancer survival, given our inclusion of 80% of the US population. There is general inconsistency among published studies with regard to race-specific ovarian cancer survival. Some articles report lower survival among black women compared with white women,6,22–26 whereas others report no difference.27–29 In addition, a meta-analysis of pooled 5-year survival results from 8 studies (106,704 women) detected no difference in survival between black and white women.30 Because ovarian cancer is diagnosed in much greater numbers among white women than among black women, a large study sample size is important in ovarian cancer studies that are stratified by race. Many smaller studies may not have had enough power to detect a difference in survival between black and white women. Our data include almost 14,000 cases of ovarian cancer among black women, providing sufficient power for detecting racial differences.
The finding that ovarian cancer 5-year net survival is moderate and has not changed considerably over time is likely because most cases continue to be diagnosed at distant stage. The preponderance of late-stage diagnoses contributes to the description of ovarian cancer as a particularly deadly disease31; however, patients who have localized and regional stage diagnoses have relatively good survival overall,32,33 and stage-specific ovarian cancer survival is similar to that of breast and uterine cancers.3 The differences in stage distribution observed among these 3 cancers is likely because of the availability of early detection methods for breast cancer34 and the presence of gynecologic-specific symptoms, such as postmenopausal vaginal bleeding for uterine cancer.35 Although US studies have been conducted to develop effective early detection methods for ovarian cancer, none have been identified that can provide a shift to earlier stage at diagnosis36,37 or an overall mortality benefit,38 and some have reportedly caused significant harms to women (mainly associated with unnecessary surgery).38 Recent reports from a UK ovarian cancer screening trial have provided more encouraging results with regard to the detection of ovarian cancer at earlier stage39,40; however, a reduction in mortality from ovarian cancer has not yet been observed in that trial.41 Continued follow-up of these trial participants, as well as positive evidence from other screening studies, is necessary before any changes in the current state of ovarian cancer screening among US women may be considered. In lieu of evidence-based screening, symptom recognition may assist with early detection of ovarian cancer.42 Several studies have examined and defined the presence of a specific set of symptoms that occur in a majority of women before an ovarian cancer diagnosis.43,44 These symptoms, including bloating, pelvic pain, change in urination frequency and/or intensity, and early satiety after eating, often go unrecognized by women because of their nongynecologic nature. They can also be associated with other existing conditions, and women may not immediately seek care for such symptoms, which can prolong the time to diagnosis.45,46 Increased public education regarding ovarian cancer symptom recognition and prompt care-seeking for those symptoms may help with increasing early stage diagnoses, resulting in increases in ovarian cancer survival.
Clinical Implications
Although effective early detection methods that reduce ovarian cancer mortality have yet to be developed, guidelines-based treatment protocols for ovarian cancer are well established, and adherence to these protocols leads to better surgical outcomes and improved survival among all patients with ovarian cancer in the United States. Several patient factors are consistently associated with not receiving recommended treatment, including older age, black race, the presence of comorbid conditions, and low socioeconomic status.20,47–50 In contrast, receiving treatment at a high-volume facility, at an NCI-designated cancer center, or by a gynecologic oncologist have consistently been associated with receiving guidelines-based, recommended treatment.47,51–54 The consistent finding that black women do not receive guidelines-based treatment compared with white women, even when treated within the same hospital,26 likely contributes to the lower survival observed in this study among black women. It is unclear why black women are not receiving guidelines-based treatment; however, it is possible that difficulty in accessing particular hospitals or physicians may play a role. Geographic disparities in ovarian cancer care have been well documented,55–58 and a recent study in 1 urban NCI-designated cancer center indicated that, the farther patients with gynecologic cancer were required to travel to get to treatment (those traveling >10 miles but <50 miles), the less likely they were to complete recommended care.57 Patient influences could be another potential reason why black women may not be receiving guidelines-based care. Fatalistic attitudes and mistrust of the medical system have been identified as more prevalent among black patients with prostate cancer compared with white patients.59 These 2 factors, along with negative beliefs about surgery, are thought to explain almost one-third of the observed racial disparities in lung cancer treatment among black patients.60 Telemedicine, which would allow specialists to consult on patient cases remotely by telephone and/or video conferencing, is an emerging area that may improve access to quality care in rural or under-served areas and/or may assist patients with receiving care primarily from a chosen and trusted physician.61 The utility of this method for ovarian cancer in the United States is unknown, however, and effective delivery of ovarian cancer surgical care, which is a key mediator of improved survival, is still being studied in telemedicine models.61
Cancer-Control Implications
Public health efforts that educate women about ovarian cancer and allow women to navigate an ovarian cancer diagnosis more easily may assist with improvements in ovarian cancer survival. The CDC’s NCCCP operates in all 50 states, the District of Columbia, 7 tribal governments and organizations, and 7 territories and US-associated Pacific Island jurisdictions to support the development and implementation of evidence-based initiatives to prevent and control cancer.62 Recent studies have documented prior ovarian cancer activities of the NCCCP; nearly one-half of programs are undertaking activities related to ovarian cancer, which largely center on education, primary prevention, and implementation of interventions to improve ovarian cancer survivors’ well being.63,64 Primary prevention activities include promoting smoking cessation and smoke-free environments, because smoking is a risk factor for some types of ovarian cancer,65 as well as the promotion of breastfeeding among women who have the opportunity, which several studies have suggested reduces risk for epithelial ovarian cancer.66 Several NCCCP grantees have partnered with the CDC’s Inside Knowledge: Get the Facts about Gynecologic Cancer campaign67 to increase knowledge of other risk factors, symptoms, and recommendations for treatment of ovarian cancer among the public and providers. These specific educational efforts are designed to reach and capture traditionally underserved populations in the United States, including black women. Survivor interventions include developing patient navigation programs to assist cancer patients in seeking referrals and follow-up services, scheduling transportation to appointments, and improved communication with their providers, among other activities. These efforts may assist patients who have ovarian cancer with attending all scheduled medical appointments, which may result in longer disease-free intervals68 and improved survival. Taken together, these public health efforts have the potential to improve ovarian cancer survival among all women diagnosed in the United States, especially if they are adopted more widely by a majority of NCCCP grantees.
This study is subject to some limitations. First, the clinical utility of our analysis is limited, because the results were not stratified by histologic type of ovarian cancer. Because of the heterogeneity of ovarian cancer, survival varies widely both by histologic type (epithelial vs nonepithelial)69 and subtype (eg, serous adenocarcinoma vs clear cell adenocarcinoma).70 Our analysis masked these differences. Second, our high-level analysis does not consider factors known to influence survival from ovarian cancer, including age, patient comorbidity status, and treatment.20,53 Furthermore, we include only SS2000, the registry staging system, as opposed to International Federation of Gynecology and Obstetrics stage, which is more commonly used in the clinic. Balanced with these limitations are several strengths. Our study was designed to be particularly useful for public health efforts. This high-level analysis by state provides necessary data for resource allocation within health departments and actionable items for the NCCCP in their efforts to help reduce the ovarian cancer burden. It also reveals additional health inequities for all public health practitioners and stakeholders to address and demonstrates the need for continued funding for ovarian cancer, because increases in survival have been modest in recent years. In addition, the rigorous quality-control and statistical methods used ensure that only the highest quality data were included in this analysis.10 Almost all cases included in this analysis were microscopically confirmed, further ensuring the high quality of the data. Finally, our study includes data from a majority of US states, making it, to our knowledge, the largest and most geographically comprehensive US ovarian cancer survival analysis to date. The inclusion of this large number of states allowed for an adequate sample size to detect differences among racial populations.
Conclusion
Ovarian cancer survival is moderate across the United States; however, black women have consistently lower survival from this disease than white women. Future research focusing on the development of new screening methods or modalities that lead to a greater number of earlier stage diagnoses will likely improve overall ovarian cancer survival. In the meantime, clinical efforts that ensure all women who are diagnosed with ovarian cancer receive appropriate, guidelines-based treatment and public health efforts that educate women about the risks factors, signs, and symptoms of ovarian cancer may help to decrease current disparities in US ovarian cancer survival.
Supplementary Material
Acknowledgments
This Supplement edition of Cancer has been sponsored by the U.S. Centers for Disease Control and Prevention (CDC), an Agency of the Department of Health and Human Services.
FUNDING SUPPORT
Rhea Harewood and Melissa Matz were supported by grants from the US. Centers for Disease Control and Prevention (CDC; 12FED03123, ACO12036).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the CDC.
The CONCORD-2 study was approved by the Ethics and Confidentiality Committee of the UK’s statutory National Information Governance Board (now the Health Research Authority) (ref ECC 3-04(i)/2011) and by the National Health Service Research Ethics Service (Southeast; 11/LO/0331).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Sherri L. Stewart: Writing–original draft, and supervision. Rhea Harewood: Data validation, formal analysis, and visualization. Melissa Matz: Writing–review and editing. Sun Hee Rim: Writing–review and editing. Susan A. Sabatino: Writing–review and editing. Kevin C. Ward: Writing–review and editing. Hannah K. Weir: Conceptualization, writing–review and editing, project administration, and funding acquisition.
References
- 1.US Cancer Statistics (USCS) Working Group. US Cancer Statistics: 1999–2012 Incidence and Mortality Web-Based Report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2015. [Accessed August 9, 2017]. Available at: www.cdc.gov/uscs. [Google Scholar]
- 2.Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995–2004. Cancer Epidemiol Biomarkers Prev. 2009;18:132–139. doi: 10.1158/1055-9965.EPI-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2015. [Accessed August 9, 2017]. Available at: http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 4.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemani C, Coleman MP. Public health surveillance of cancer survival in the US and world-wide: the contribution of the CONCORD programme. Cancer. 2017;123:4977–4981. doi: 10.1002/cncr.30854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Averette HE, Janicek MF, Menck HR. The National Cancer Data Base report on ovarian cancer. American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1995;76:1096–1103. doi: 10.1002/1097-0142(19950915)76:6<1096::aid-cncr2820760626>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries’ (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014;49:198–209. doi: 10.1093/jncimonographs/lgu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wingo PA, Jamison PM, Hiatt RA, et al. Building the infrastructure for nationwide cancer surveillance and control—a comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) Program (United States) Cancer Causes Control. 2003;14:175–193. doi: 10.1023/a:1023002322935. [DOI] [PubMed] [Google Scholar]
- 9.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allemani C, Harewood R, Johnson C, et al. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123:4982–4993. doi: 10.1002/cncr.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz AG, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology (ICD-O) 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 12.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER Summary Staging Manual-2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01–4969. [Google Scholar]
- 13.National Cancer Institute; Surveillance, Epidemiology, and End Results (SEER) Program. Collaborative Stage. Bethesda, MD: National Cancer Institute; 2004. [Accessed April 1, 2016]. Available at: http://seer.cancer.gov/tools/collabstaging/ [Google Scholar]
- 14.Weir HK, Stewart SL, Allemani C, et al. Population-based cancer survival (2001–2009) in the United States: findings from the CONCORD-2 study. Cancer. 2017;123:4963–4968. doi: 10.1002/cncr.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pohar Perme M, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 16.Spika D, Bannon F, Bonaventure A, et al. Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods [serial online] BMC Cancer. 2017;17:159. doi: 10.1186/s12885-017-3117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rachet B, Maringe C, Woods LM, Ellis L, Spika D, Allemani C. Multivariable flexible modelling for estimating complete, smoothed life tables for sub-national populations [serial online] BMC Public Health. 2015;15:1240. doi: 10.1186/s12889-015-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Quaresma M, Coleman MP, Rachet B. Funnel plots for population-based cancer survival: principles, methods and applications. Stat Med. 2014;33:1070–1080. doi: 10.1002/sim.5953. [DOI] [PubMed] [Google Scholar]
- 20.O’Malley CD, Shema SJ, Cress RD, et al. The implications of age and comorbidity on survival following epithelial ovarian cancer: summary and results from a Centers for Disease Control and Prevention study. J Womens Health (Larchmt) 2012;21:887–894. doi: 10.1089/jwh.2012.3781. [DOI] [PubMed] [Google Scholar]
- 21.Chan JK, Urban R, Cheung MK, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer. 2006;95:1314–1320. doi: 10.1038/sj.bjc.6603457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JD, Chen L, Tergas AI, et al. Trends in relative survival for ovarian cancer from 1975 to 2011. Obstet Gynecol. 2015;125:1345–1352. doi: 10.1097/AOG.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnholtz-Sloan JS, Tainsky MA, Abrams J, et al. Ethnic differences in survival among women with ovarian carcinoma. Cancer. 2002;94:1886–1893. doi: 10.1002/cncr.10415. [DOI] [PubMed] [Google Scholar]
- 24.McGuire V, Herrinton L, Whittemore AS. Race, epithelial ovarian cancer survival, and membership in a large health maintenance organization. Epidemiology. 2002;13:231–234. doi: 10.1097/00001648-200203000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Chan JK, Zhang M, Hu JM, Shin JY, Osann K, Kapp DS. Racial disparities in surgical treatment and survival of epithelial ovarian cancer in United States. J Surg Oncol. 2008;97:103–107. doi: 10.1002/jso.20932. [DOI] [PubMed] [Google Scholar]
- 26.Parham G, Phillips JL, Hicks ML, et al. The National Cancer Data Base report on malignant epithelial ovarian carcinoma in African-American women. Cancer. 1997;80:816–826. [PubMed] [Google Scholar]
- 27.O’Malley CD, Cress RD, Campleman SL, Leiserowitz GS. Survival of Californian women with epithelial ovarian cancer, 1994–1996: a population-based study. Gynecol Oncol. 2003;91:608–615. doi: 10.1016/j.ygyno.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 29.Morgan MA, Behbakht K, Benjamin I, Berlin M, King SA, Rubin SC. Racial differences in survival from gynecologic cancer. Obstet Gynecol. 1996;88:914–918. doi: 10.1016/s0029-7844(96)00342-0. [DOI] [PubMed] [Google Scholar]
- 30.Terplan M, Smith EJ, Temkin SM. Race in ovarian cancer treatment and survival: a systematic review with meta-analysis. Cancer Causes Control. 2009;20:1139–1150. doi: 10.1007/s10552-009-9322-2. [DOI] [PubMed] [Google Scholar]
- 31.Clinton K. Mortality rates by stage-at-diagnosis. Semin Surg Oncol. 1994;10:7–11. doi: 10.1002/ssu.2980100104. [DOI] [PubMed] [Google Scholar]
- 32.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol. 2015;126:491–497. doi: 10.1097/AOG.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(suppl 1):S108–S115. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helvie MA, Chang JT, Hendrick RE, Banerjee M. Reduction in late-stage breast cancer incidence in the mammography era: implications for overdiagnosis of invasive cancer. Cancer. 2014;120:2649–2656. doi: 10.1002/cncr.28784. [DOI] [PubMed] [Google Scholar]
- 35.Leminen A, Paavonen J, Forss M, Wahlstrom T, Vesterinen E. Adenocarcinoma of the uterine cervix. Cancer. 1990;65:53–59. doi: 10.1002/1097-0142(19900101)65:1<53::aid-cncr2820650112>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Partridge E, Kreimer AR, Greenlee RT, et al. Results from 4 rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775–782. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visintin I, Feng Z, Longton G, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–1072. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 38.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal AN, Fraser L, Manchanda R, et al. Results of annual screening in phase I of the United Kingdom familial ovarian cancer screening study highlight the need for strict adherence to screening schedule. J Clin Oncol. 2013;31:49–57. doi: 10.1200/JCO.2011.39.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKC-TOCS) Lancet Oncol. 2009;10:327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goff BA. Ovarian cancer: screening and early detection. Obstet Gynecol Clin North Am. 2012;39:183–194. doi: 10.1016/j.ogc.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221–227. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 44.Ryerson AB, Eheman C, Burton J, et al. Symptoms, diagnoses, and time to key diagnostic procedures among older U.S. women with ovarian cancer. Obstet Gynecol. 2007;109:1053–1061. doi: 10.1097/01.AOG.0000260392.70365.5e. [DOI] [PubMed] [Google Scholar]
- 45.Trivers KF, Rodriguez JL, Hawkins NA, Cooper CP, Polonec L, Gelb CA. Intention to seek care for symptoms associated with gynecologic cancers, HealthStyles survey, 2008 [serial online] Prev Chronic Dis. 2011;8:A144. [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper CP, Polonec L, Stewart SL, Gelb CA. Gynaecologic cancer symptom awareness, concern and care seeking among US women: a multi-site qualitative study. Fam Pract. 2013;30:96–104. doi: 10.1093/fampra/cms040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long B, Chang J, Ziogas A, Tewari KS, Anton-Culver H, Bristow RE. Impact of race, socioeconomic status, and the health care system on the treatment of advanced-stage ovarian cancer in California. Am J Obstet Gynecol. 2015;212:468, e461–e469. doi: 10.1016/j.ajog.2014.10.1104. [DOI] [PubMed] [Google Scholar]
- 48.Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11–17. doi: 10.1016/j.ygyno.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodeib M, Chang J, Liu F, et al. Socioeconomic status as a predictor of adherence to treatment guidelines for early stage ovarian cancer. Gynecol Oncol. 2015;138:121–127. doi: 10.1016/j.ygyno.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol. 2015;125:833–842. doi: 10.1097/AOG.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Impact of National Cancer Institute Comprehensive Cancer Centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015;220:940–950. doi: 10.1016/j.jamcollsurg.2015.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan JK, Sherman AE, Kapp DS, et al. Influence of gynecologic oncologists on the survival of patients with endometrial cancer. J Clin Oncol. 2011;29:832–838. doi: 10.1200/JCO.2010.31.2124. [DOI] [PubMed] [Google Scholar]
- 53.Stewart SL, Rim SH, Richards TB. Gynecologic oncologists and ovarian cancer treatment: avenues for improved survival. J Womens Health (Larchmt) 2011;20:1257–1260. doi: 10.1089/jwh.2011.3053. [DOI] [PubMed] [Google Scholar]
- 54.Cress RD, Bauer K, O’Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol. 2011;121:94–99. doi: 10.1016/j.ygyno.2010.12.359. [DOI] [PubMed] [Google Scholar]
- 55.Stewart SL, Cooney D, Hirsch S, et al. The effect of gynecologic oncologist availability on ovarian cancer mortality. World J Obstet Gynecol. 2014;3:71–77. doi: 10.5317/wjog.v3.i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shalowitz DI, Vinograd AM, Giuntoli RL., 2nd Geographic access to gynecologic cancer care in the United States. Gynecol Oncol. 2015;138:115–120. doi: 10.1016/j.ygyno.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Temkin SM, Fleming SA, Amrane S, Schluterman N, Terplan M. Geographic disparities amongst patients with gynecologic malignancies at an urban NCI-designated cancer center. Gynecol Oncol. 2015;137:497–502. doi: 10.1016/j.ygyno.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Delva F, Soubeyran P, Rainfray M, Mathoulin-Pelissier S. Referral of elderly cancer patients to specialists: action proposals for general practitioners. Cancer Treat Rev. 2012;38:935–941. doi: 10.1016/j.ctrv.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Bustillo NE, McGinty HL, Dahn JR, et al. Fatalism, medical mistrust, and pretreatment health-related quality of life in ethnically diverse prostate cancer patients. Psychooncology. 2017;26:323–329. doi: 10.1002/pon.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin JJ, Mhango G, Wall MM, et al. Cultural factors associated with racial disparities in lung cancer care. Ann Am Thorac Soc. 2014;11:489–495. doi: 10.1513/AnnalsATS.201402-055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shalowitz DI, Smith AG, Bell MC, Gibb RK. Teleoncology for gynecologic cancers. Gynecol Oncol. 2015;139:172–177. doi: 10.1016/j.ygyno.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 62.Major A, Stewart SL. Celebrating 10 years of the National Comprehensive Cancer Control Program, 1998 to 2008 [serial online] Prev Chronic Dis. 2009;6:A133. [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart SL, Rim SH, Trivers KF. Summary and impact of ovarian cancer research and programmatic activities at the Centers for Disease Control and Prevention. J Womens Health (Larchmt) 2010;19:1427–1432. doi: 10.1089/jwh.2010.2164. [DOI] [PubMed] [Google Scholar]
- 64.Stewart SL, Townsend JS, Puckett MC, Rim SH. Adherence of primary care physicians to evidence-based recommendations to reduce ovarian cancer mortality. J Womens Health (Larchmt) 2016;25:235–241. doi: 10.1089/jwh.2015.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faber MT, Kjaer SK, Dehlendorff C, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control. 2013;24:989–1004. doi: 10.1007/s10552-013-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li DP, Du C, Zhang ZM, et al. Breastfeeding and ovarian cancer risk: a systematic review and meta-analysis of 40 epidemiological studies. Asian Pac J Cancer Prev. 2014;15:4829–4837. doi: 10.7314/apjcp.2014.15.12.4829. [DOI] [PubMed] [Google Scholar]
- 67.Rim SH, Polonec L, Stewart SL, Gelb CA. A national initiative for women and healthcare providers: CDC’s Inside Knowledge: Get the Facts About Gynecologic Cancer campaign. J Womens Health (Larchmt) 2011;20:1579–1585. doi: 10.1089/jwh.2011.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jabson JM. Treatment summaries, follow-up care instructions, and patient navigation: could they be combined to improve cancer survivor’s receipt of follow-up care? J Cancer Surviv. 2015;9:692–698. doi: 10.1007/s11764-015-0444-0. [DOI] [PubMed] [Google Scholar]
- 69.Matz M, Coleman MP, Carreira H, Salmeron D, Chirlaque MD, Allemani C. Worldwide comparison of ovarian cancer survival: histological group and stage at diagnosis (CONCORD-2) Gynecol Oncol. 2017;144:396–404. doi: 10.1016/j.ygyno.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schnack TH, Hogdall E, Nedergaard L, Hogdall C. Demographic clinical and prognostic factors of primary ovarian adenocarcinomas of serous and clear cell histology—a comparative study. Int J Gynecol Cancer. 2016;26:82–90. doi: 10.1097/IGC.0000000000000585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.