Abstract
Background
Both genetic and environmental factors play a role in the development of autism spectrum disorder (ASD). This case-control study examined the association between childhood ASD and single-nucleotide polymorphisms (SNPs) in genes involved with vitamin B12 and folate metabolism.
Material/Methods
Genotypes of transcobalamin 2 (TCN2) rs1801198, methionine synthase (MTR) rs1805087, methionine synthase reductase (MTRR) rs1801394, and methylene tetrahydrofolate reductase (MTHFR) rs1801133 were examined in 201 children with ASD and 200 healthy controls from the Han Chinese population.
Results
Our results showed no association of all examined SNPs with childhood ASD and its severity.
Conclusions
None of the examined SNPs were a risk factor for the susceptibility to childhood ASD and severity of the disease in a Han Chinese population.
MeSH Keywords: Autistic Disorder; Child Development Disorders, Pervasive; Polymorphism, Single Nucleotide
Background
Autism spectrum disorder (ASD) is an early-onset neurodevelopmental disorder characterized by struggles in social relationships, deficiency in language and speech, and stereotypical behaviors [1]. Due to increasing prevalence and no current effective treatments, ASD brings huge economic and emotional burdens to affected families and societies [2]. Studies in this field are of great clinical benefit.
Both genetic and environmental factors play a role in the development of ASD [3–7]. Family and twin studies provide strong evidence supporting the contribution of genetics in the development of the disease [7,8]. Molecular genetic studies have discovered that ASD is possibly caused by diverse genetic variants such as gene mutations, single-nucleotide polymorphisms (SNPs), chromosomal abnormalities, and copy number variations (CNVs). Owing to the high heterogeneity of ASD, single genetic variants are found in only a small proportion of ASD cases [9]. The interactions between genetic predisposition and environmental factors have been proposed as the major mechanisms in the etiology of ASD [5,10].
Vitamin B12 (cobalamin) and folate participate in the methylation cycle as well as in DNA and RNA biosynthesis. Metabolic abnormalities of vitamin B12 and folate have been associated with the risk of ASD. Deficiency of vitamin B12 has been associated with many psychiatric and neurological disorders [11]. ASD patients were found to have lower serum vitamin B12 compared to healthy controls [12]. A clinical trial revealed that methyl B12 supplementation improved symptoms and reduced oxidative stress in a subgroup of autistic children [13]. Furthermore, children with brain folate deficiency had a higher risk of being diagnosed with ASD [14]. Folic acid supplementation at about the time of conception have been linked to a lower incidence of ASD in offspring [15,16].
Enzymes and transporter proteins play important roles in metabolism of vitamin B12 and folate. Transcobalamin II (TCN2) is the transporter protein that carries vitamin B12 (cobalamin) into cells within target tissues. Using cobalamin as a cofactor, methionine synthase (MTR) converts homocysteine into methionine and transfers methyl groups from 5-methyltetrahydrofolate to homocysteine, producing tetrahydrofolate for nucleic acid synthesis and methionine for methylation reactions. Methionine synthase reductase (MTRR) is responsible for the regeneration of MTR functions. It converts 5,10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate and regulates the intracellular flow of folate. Methylene tetrahydrofolate reductase (MTHFR) catalyzes the irreversible conversion of 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate (Figure 1). Polymorphisms in genes related to the metabolism of vitamin B12 and folate have been examined individually or in combination in previous studies [17–22], but the results are still ambiguous or inclusive. Particularly, the polymorphisms in these genes have not been well defined in the Chinese population.
Figure 1.
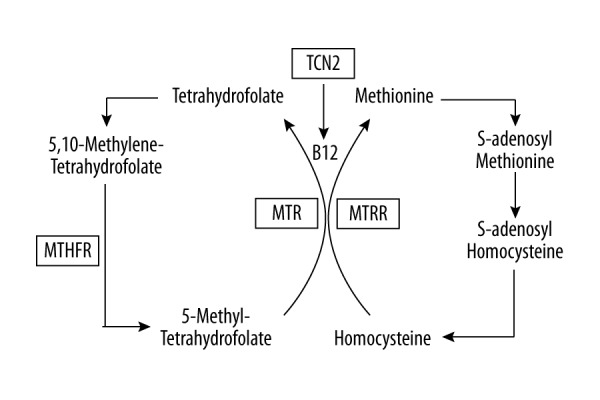
Enzymes (TCN2, MTR, MTRR, and MTHFR) are important in B12 and folate metabolism. TCN2 transports vitamin B12 (cobalamin) to cells. MTR converts homocysteine into methionine and 5-methyltetrahydrofolate to tetrahydrofolate. MTRR is essential for regenerating functional MTR. MTHFR converts 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate. TCN2 – transcobalamin; MTR – methionine synthase; MTRR – methionine synthase reductase; MTHFR – methylene tetrahydrofolate reductase.
The present study examined genotypes of SNPs TCN2 rs1801198, MTR (rs1805087), MTR (rs1801394), and MTHFR rs1801133 among children with ASD and healthy controls from the Han Chinese population. The purpose of our study was to examine the association of these SNPs with the risk of childhood ASD.
Material and Methods
Patients with ASD and controls
All patients and controls were recruited from Xiaoshan District of Zhejiang Province from September 2012 to June 2016. A total of 201 Han Chinese children with ASD and 200 healthy children were included, with detailed information described previously [23]. According to the Childhood Autism Rating Scale (CARS), patients with scores of <36 were classified as mild-to-moderate and ≥36 as severe. The Medical Ethics Committee of Zhejiang Xiaoshan Hospital approved this study. Informed consent was signed by parents or guardians of all children.
Genotyping of SNPs
DNA was extracted from whole blood cells using the Qiagen Blood DNA mini kit (Qiagen China, Shanghai, China). TaqMan probes were obtained from Applied Biosystems (Beijing, China). Assay IDs for rs1801198, rs1801394, rs1805087, and rs1801133 were C__325467_10, C__3068176_10, C__12005959_10, and C__1202883_20, respectively. Genotypes of SNPs were examined using a TaqMan probe-based real-time PCR approach using the protocol described in previous studies [2,24].
Statistical analysis
All data were analyzed using SAS 9.3 software (SAS Institute Inc., Cary, NC). Hardy-Weinberg equilibrium was examined by the chi-square test. The association between SNPs and the risk of ASD was tested by the logistic regression model. Data were presented as odds ratios (ORs) and 95% confidence intervals (CIs). A P value <0.05 was considered to be statistically significant.
Results
Our data revealed that genotypic distributions of TCN2 rs1801198, MTR rs1805087, MTRR rs1801394, and MTHFR rs1801133 were in Hardy-Weinberg genetic equilibrium (Table 1).
Table 1.
Hardy-Weinberg equilibrium tests (P values) of SNPs in case and control groups.
| SNPs | Cases | Control |
|---|---|---|
| rs1801198 | 0.0776 | 0.6458 |
| rs1801394 | 0.9758 | 0.7129 |
| rs1805087 | 0.2048 | 0.0730 |
| rs1801133 | 0.5873 | 0.0991 |
Logistic regression analysis showed no significant differences in the genotypic distributions and allele frequencies of all examined SNPs between case and control groups (Table 2).
Table 2.
Correlation between SNP genotypes and allele frequencies with childhood ASD.
| SNPs | Genotype/Allele | Cases n (%) | Controls n (%) | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| rs1801198 | ||||||
| C/C | 43 (21.4) | 38 (19.0) | 1 | |||
| C/G | 113 (56.2) | 102 (51.0) | 0.98 | 0.59–1.63 | 0.9353 | |
| G/G | 45 (22.4) | 60 (30.0) | 0.66 | 0.37–1.19 | 0.1667 | |
| C | 199 (49.5) | 178 (44.5) | 1 | |||
| G | 203 (50.5) | 222 (55.5) | 0.82 | 0.62–1.08 | 0.1564 | |
| rs1801394 | ||||||
| A/A | 121 (60.2) | 123 (61.8) | 1 | |||
| A/G | 70 (34.8) | 68 (34.2) | 1.05 | 0.69–1.59 | 0.8313 | |
| G/G | 10 (5.0) | 8 (4.0) | 1.27 | 0.49–3.33 | 0.6259 | |
| A | 312 (77.6) | 314 (78.9) | 1 | |||
| rs1805087 | ||||||
| A/A | 168 (83.6) | 155 (77.5) | 1 | |||
| A/G | 33 (16.4) | 45 (22.5) | 0.68 | 0.41–1.12 | 0.1252 | |
| A | 369 (91.8) | 355 (88.8) | 1 | |||
| G | 33 (8.2) | 45 (11.2) | 0.71 | 0.44–1.13 | 0.1477 | |
| rs1801133 | ||||||
| A/A | 32 (15.9) | 42 (21.1) | 1 | |||
| A/G | 101 (50.3) | 86 (43.2) | 1.54 | 0.90–2.65 | 0.1179 | |
| G/G | 68 (33.8) | 71 (35.7) | 1.26 | 0.71–2.22 | 0.4295 | |
| A | 165 (41.0) | 170 (42.7) | 1 | |||
| G | 237 (59.0) | 228 (57.3) | 1.07 | 0.81–1.42 | 0.6324 | |
The role of these SNPs in childhood ASD was further analyzed using dominant and recessive models. Our study showed that MTRR rs1801394 and MTHFR rs1801133 were not significantly associated with childhood ASD in any of the models (Table 3).
Table 3.
SNP genotype distributions and corresponding risk assessments for ASD using genetic models of inheritance.
| SNPs/Models | Genotype | Cases n (%) | Controls n (%) | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| rs1801198 | ||||||
| Dominant | C/C | 43 (21.4) | 38 (19.0) | 1 | ||
| G/G+C/G | 158 (78.6) | 162 (81.0) | 0.86 | 0.53–1.41 | 0.5513 | |
| Recessive | C/C+C/G | 156 (77.6) | 140 (70.0) | 1 | ||
| G/G | 45 (22.4) | 60 (30.0) | 0.67 | 0.43–1.05 | 0.0838 | |
| rs1801394 | ||||||
| Dominant | A/A | 121 (60.2) | 123 (61.5) | 1 | ||
| G/G+A/G | 80 (39.8) | 77 (38.5) | 1.06 | 0.71–1.58 | 0.7896 | |
| Recessive | AA+AG | 191 (95.0) | 192 (96.0) | 1 | ||
| G/G | 10 (5.0) | 8 (4.0) | 1.26 | 0.49–3.25 | 0.6379 | |
| rs1801133 | ||||||
| Dominant | A/A | 32 (15.9) | 42 (21.0) | 1 | ||
| G/G+A/G | 169 (84.1) | 158 (79.0) | 1.40 | 0.84–2.33 | 0.1910 | |
| Recessive | AA+AG | 133 (66.2) | 129 (64.5) | 1 | ||
| G/G | 68 (33.8) | 71 (35.5) | 0.93 | 0.62–2.22 | 0.1410 | |
One hundred and twenty-two patients had mild-to-moderate ASD and 79 had severe ASD among all these children with ASD. There was no significant association between the examined SNPs and severity of childhood ASD (Table 4).
Table 4.
Correlation between SNP genotypes and allele frequencies and severity of childhood ASD.
| SNPs | Genotype/Allele | Moderate-severe n (%) | Severe n (%) | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| rs1801198 | ||||||
| C/C | 25 (20.5) | 18 (22.8) | 1 | |||
| C/G | 71 (58.2) | 42 (53.2) | 0.82 | 0.40–1.68 | 0.5906 | |
| G/G | 26 (21.3) | 19 (24.0) | 1.02 | 0.44–2.37 | 0.9726 | |
| C | 121 (49.6) | 78 (49.4) | 1 | |||
| G | 123 (50.4) | 80 (50.6) | 1.01 | 0.68–1.51 | 0.9651 | |
| rs1801394 | ||||||
| A/A | 75 (61.5) | 46 (58.2) | 1 | |||
| A/G | 40 (32.8) | 30 (38.0) | 1.22 | 0.67–2.23 | 0.5104 | |
| G/G | 7 (5.7) | 3 (3.8) | 0.70 | 0.17–2.84 | 0.6162 | |
| A | 190 (77.9) | 122 (77.2) | 1 | |||
| G | 54 (22.1) | 36 (22.8) | 1.04 | 0.64–1.68 | 0.8777 | |
| rs1805087 | ||||||
| A/A | 100 (82.0) | 68 (86.1) | 1 | |||
| A/G | 22 (18.05) | 11 (13.9) | 0.74 | 0.34–1.62 | 0.4436 | |
| A | 147 (93.0) | 222 (91.0) | 1 | |||
| G | 11 (7.0) | 22 (9.0) | 0.76 | 0.36–1.60 | 0.4648 | |
| rs1801133 | ||||||
| A/A | 22 (18.0) | 10 (12.7) | 1 | |||
| A/G | 59 (48.4) | 42 (53.2) | 1.57 | 0.67–3.65 | 0.2986 | |
| G/G | 41 (33.6) | 27 (34.2) | 1.45 | 0.59–3.53 | 0.4151 | |
| A | 103 (42.2) | 62 (39.2) | 1 | |||
| G | 141 (57.8) | 96 (60.8) | 1.13 | 0.75–1.70 | 0.5541 | |
Discussion
In this current case-control study, we analyzed polymorphisms of SNPs in genes related to vitamin B12 and folate metabolism among children with ASD and healthy controls from the Chinese Han population. There were no significant differences in genotypic distributions and allele frequencies of SNPs rs1801198 in TCN2, rs1805087 in MTR, rs1801394 in MTR, and rs1801133 in MTHFR between children with ASD and healthy controls. No significant correlation was observed between the examined SNPs and the severity of the disease.
The TCN2 gene is positioned at chromosome 22. SNP rs1801198 (C776G) affects the folding of the protein and thus alters its binding affinity for B12 [25]. This polymorphism in the TCN2 gene has been associated with lower circulating levels of vitamin B12 and increased circulating levels of homocysteine [26]. The disturbed methionine-homocysteine metabolism caused by SNP rs1801198 (C776G) in the TCN2 gene may lead to the development of ASD. A previous study reported a significant difference in the genotypic distribution and allele frequency of rs1801198 between ASD patients and healthy controls [27]. SNP rs1801198 has been associated with peripheral neuropathy [28]. In contrast, our data reveal that rs1801198 in the TCN2 gene is not a risk factor for childhood ASD.
The MTR gene is located at chromosome 1. The polymorphism rs1805087 (A2756G) in the MTR gene influences the activity of its encoding enzyme, leading to elevated circulating homocysteine [29]. Haghiri et al. [17] performed a case-control study with 108 autistic children and 130 healthy controls from an Iranian population. Although no significant difference in the genotypic distribution of rs1805087 (A2756G) was observed between cases and controls, the G allele of the rs1805087 was associated with a higher risk for ASD compared to the A allele. In addition, homozygosity for the A allele was associated with greater severity of dementia [18]. The present study found no significant association between rs1805087 and the risk of ASD in the Han Chinese population.
The MTRR gene is located at chromosome 5. The rs1801394 (A66G) in the MTRR gene leads to reduced affinity for the substrate [30,31]. A case-control study found that the A allele of rs1801394 in the MTRR gene was associated with reduced risk of ASD [21]. Our data showed no significant association between SNP rs1801394 and childhood ASD or its severity. rs1801394 in the MTRR gene has been identified as a risk factor for several neuropsychiatric disorders [32].
The MTHFR gene is located on chromosome 1. rs1801133 (C677T) in the MTHFR gene influences enzymatic activity and serum homocysteine levels [33,34]. Synergistic interactions between MTHFR C677T and MTRR A66G increase homocysteine, which is considered a significant risk factor for autism [35]. A previous study was conducted with 186 cases and 186 controls from the Han Chinese population. The results found that both the TT genotype and the T-allele of rs1801133 were associated with a significantly increased risk for childhood ASD [22]. A recent meta-analysis analyzed geographical and ethnic distributions of MTHFR C677T among Chinese populations. MTHFR C677T was found to be significantly correlated with the risk of ASD [36]. The SNP rs1801133 was associated with the risk for autism in Indians [21] and North Americans [20]. In contrast, no association between rs180113 and ASD was observed zin our study, and no such association was found in Brazilian [37], Turkish [38], or Egyptian [39] populations.
The limitations of the current study should be addressed, including its case-control design and its relatively small sample size. Only 4 SNPs on related genes were examined in this study. In addition, metabolites of vitamin B12 and folate are involve in redox and DNA methylation relevant to the development of ASD [10]. Measurement of metabolites of folate was able to separate ASD subjects from controls and to predict severity of the disease [40]. However, the levels of vitamin B12, folate, and their related metabolites were not examined in these children. Metabolic disorders of the vitamins might interact with certain polymorphisms in these genes to increase risk of ASD. The role of such interactions was not examined in this study.
Conclusions
Our study reveals that TCN2 rs1801198, MTR rs1805087, MTRR rs1801394, and MTHFR are not risk factors for susceptibility to childhood ASD or the severity of the disease in the Han Chinese population.
Abbreviations
- ASD
autism spectrum disorder
- CARS
child autism rating scale
- CNV
copy number variations
- MTR
methionine synthase
- MTRR
methionine synthase reductase
- MTHFR
methylene tetrahydrofolate reductase
- SNP
single-nucleotide polymorphism
- TCN2
transcobalamin 2
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by grants from the Health and Family Planning Commission of Zhejiang Province (2014KYB225); the Science and Technology Commission of Hangzhou City (20140633B48); the Science Technology Department of Zhejiang Province (2017C33205), the Hangzhou Municipal Science and Technology Commission (20163501Y82); and the Health and Family Planning Commission of Hangzhou (2016B26)
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition. Arlington: VAPP; 2013. [Google Scholar]
- 2.Baxter AJ, Brugha TS, Erskine HE, et al. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45:601–13. doi: 10.1017/S003329171400172X. [DOI] [PubMed] [Google Scholar]
- 3.Vijayakumar NT, Judy MV. Autism spectrum disorders: Integration of the genome, transcriptome and the environment. J Neurol Sci. 2016;364:167–76. doi: 10.1016/j.jns.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Homberg JR, Kyzar EJ, Scattoni ML, et al. Genetic and environmental modulation of neurodevelopmental disorders: Translational insights from labs to beds. Brain Res Bull. 2016;125:79–91. doi: 10.1016/j.brainresbull.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13. doi: 10.1186/s13229-017-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaugler T, Klei L, Sanders SJ, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–85. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–95. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16:551–63. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- 10.Deth R, Muratore C, Benzecry J, et al. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Briani C, Dalla Torre C, Citton V, et al. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients. 2013;5:4521–39. doi: 10.3390/nu5114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bala KA, Dogan M, Mutluer T, et al. Plasma amino acid profile in autism spectrum disorder (ASD) Eur Rev Med Pharmacol Sci. 2016;20:923–29. [PubMed] [Google Scholar]
- 13.Bertoglio K, Jill James S, Deprey L, et al. Pilot study of the effect of methyl B12 treatment on behavioral and biomarker measures in children with autism. J Altern Complement Med. 2010;16:555–60. doi: 10.1089/acm.2009.0177. [DOI] [PubMed] [Google Scholar]
- 14.Moretti P, Peters SU, Del Gaudio D, et al. Brief report: autistic symptoms, developmental regression, mental retardation, epilepsy, and dyskinesias in CNS folate deficiency. J Autism Dev Disord. 2008;38:1170–77. doi: 10.1007/s10803-007-0492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suren P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570–77. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu XF, Li M, Zheng Y. [Association between maternal folate supplementation during pregnancy and the risk of autism spectrum disorder in the offspring: A meta analysis]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:286–91. doi: 10.7499/j.issn.1008-8830.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haghiri R, Mashayekhi F, Bidabadi E, Salehi Z. Analysis of methionine synthase (rs1805087) gene polymorphism in autism patients in Northern Iran. Acta Neurobiol Exp (Wars) 2016;76:318–23. doi: 10.21307/ane-2017-030. [DOI] [PubMed] [Google Scholar]
- 18.Bosco P, Gueant-Rodriguez RM, Anello G, et al. Association of IL-1 RN*2 allele and methionine synthase 2756 AA genotype with dementia severity of sporadic Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1036–38. doi: 10.1136/jnnp.2003.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bathum L, von Bornemann Hjelmborg J, Christiansen L, et al. Methylenetetrahydrofolate reductase 677C>T and methionine synthase 2756A>G mutations: No impact on survival, cognitive functioning, or cognitive decline in nonagenarians. J Gerontol A Biol Sci Med Sci. 2007;62:196–201. doi: 10.1093/gerona/62.2.196. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Solehdin F, Cohen IL, et al. Population- and family-based studies associate the MTHFR gene with idiopathic autism in simplex families. J Autism Dev Disord. 2011;41:938–44. doi: 10.1007/s10803-010-1120-x. [DOI] [PubMed] [Google Scholar]
- 21.Mohammad NS, Jain JM, Chintakindi KP, et al. Aberrations in folate metabolic pathway and altered susceptibility to autism. Psychiatr Genet. 2009;19:171–76. doi: 10.1097/YPG.0b013e32832cebd2. [DOI] [PubMed] [Google Scholar]
- 22.Guo T, Chen H, Liu B, et al. Methylenetetrahydrofolate reductase polymorphisms C677T and risk of autism in the Chinese Han population. Genet Test Mol Biomarkers. 2012;16:968–73. doi: 10.1089/gtmb.2012.0091. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Mo W, Zhang Z, et al. Single nucleotide polymorphisms in SLC19A1 and SLC25A9 are associated with childhood autism spectrum disorder in the Chinese Han population. J Mol Neurosci. 2017;62:262–67. doi: 10.1007/s12031-017-0929-6. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Liu J, Yang A, et al. Lack of association between polymorphisms in dopa decarboxylase and dopamine receptor-1 genes with childhood autism in Chinese Han population. J Child Neurol. 2016;31:560–64. doi: 10.1177/0883073815601496. [DOI] [PubMed] [Google Scholar]
- 25.Stanislawska-Sachadyn A, Woodside JV, Sayers CM, et al. The transcobalamin (TCN2) 776C>G polymorphism affects homocysteine concentrations among subjects with low vitamin B(12) status. Eur J Clin Nutr. 2010;64:1338–43. doi: 10.1038/ejcn.2010.157. [DOI] [PubMed] [Google Scholar]
- 26.Gueant JL, Chabi NW, Gueant-Rodriguez RM, et al. Environmental influence on the worldwide prevalence of a 776C->G variant in the transcobalamin gene (TCN2) J Med Genet. 2007;44:363–67. doi: 10.1136/jmg.2006.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:947–56. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawaengsri H, Bergethon PR, Qiu WQ, et al. Transcobalamin 776C-->G polymorphism is associated with peripheral neuropathy in elderly individuals with high folate intake. Am J Clin Nutr. 2016;104:1665–70. doi: 10.3945/ajcn.116.139030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leclerc D, Campeau E, Goyette P, et al. Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet. 1996;5:1867–74. doi: 10.1093/hmg/5.12.1867. [DOI] [PubMed] [Google Scholar]
- 30.Harmon DL, Shields DC, Woodside JV, et al. Methionine synthase D919G polymorphism is a significant but modest determinant of circulating homocysteine concentrations. Genet Epidemiol. 1999;17:298–309. doi: 10.1002/(SICI)1098-2272(199911)17:4<298::AID-GEPI5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Olteanu H, Munson T, Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry. 2002;41:13378–85. doi: 10.1021/bi020536s. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell ES, Conus N, Kaput J. B vitamin polymorphisms and behavior: Evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci Biobehav Rev. 2014;47:307–20. doi: 10.1016/j.neubiorev.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Burda P, Schafer A, Suormala T, et al. Insights into severe 5,10-methylenetetrahydrofolate reductase deficiency: Molecular genetic and enzymatic characterization of 76 patients. Hum Mutat. 2015;36:611–21. doi: 10.1002/humu.22779. [DOI] [PubMed] [Google Scholar]
- 34.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–13. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 35.Shaik Mohammad N, Sai Shruti P, Bharathi V, et al. Clinical utility of folate pathway genetic polymorphisms in the diagnosis of autism spectrum disorders. Psychiatr Genet. 2016;26:281–86. doi: 10.1097/YPG.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Fan S, Zhi X, et al. Geographical and ethnic distribution of MTHFR gene polymorphisms and their associations with diseases among Chinese population. Clin Genet. 2016 doi: 10.1111/cge.12929. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.dos Santos PA, Longo D, Brandalize AP, Schuler-Faccini L. MTHFR C677T is not a risk factor for autism spectrum disorders in South Brazil. Psychiatr Genet. 2010;20:187–89. doi: 10.1097/YPG.0b013e32833a2220. [DOI] [PubMed] [Google Scholar]
- 38.Sener EF, Oztop DB, Ozkul Y. MTHFR gene C677T polymorphism in autism spectrum disorders. Genet Res Int. 2014;2014:698574. doi: 10.1155/2014/698574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meguid NA, Dardir AA, Khass M, Hossieny LE, Ezzat A, El Awady MK. MTHFR genetic polymorphism as a risk factor in Egyptian mothers with Down syndrome children. Dis Markers. 2008;24:19–26. doi: 10.1155/2008/214027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howsmon DP, Kruger U, Melnyk S, et al. Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLoS Comput Biol. 2017;13:e1005385. doi: 10.1371/journal.pcbi.1005385. [DOI] [PMC free article] [PubMed] [Google Scholar]


