Abstract
Collagenases are useful in enzymatic wound debridement. Clostridial collagenase, marketed as Collagenase Santyl Ointment (CSO), is FDA approved for such use. Building on the scientific premise that collagenases as well as collagen degradation products may regulate immune cell function, we sought to investigate the potential role of CSO in wound inflammation. We tested the hypothesis that in addition to enacting debridement, CSO contributes to the resolution of persistent wound inflammation. Wound macrophages were isolated from PVA sponges loaded with CSO or petrolatum and implanted in mice. Significant increase in pro-reparative and decrease in pro-inflammatory polarization was noted in macrophages of acute as well as diabetic wounds. Wound macrophages from CSO-treated group displayed increased production of anti-inflammatory cytokines IL-10 and TGF-β, and decreased levels of pro-inflammatory cytokines TNF-α and IL-1β. The active ingredient of CSO, CS-API, induced the expression of mϕheal /M(IL-4) polarization markers ex vivo. CS-API treatment attenuated transactivation of NF-κB and significantly induced STAT6 phosphorylation. A significant role of a novel PGE2-EP4 pathway in CS-API induced STAT6 activation and the mϕheal /M(IL-4) polarization was identified. Taken together, findings of this work reposition CSO as a potential agent that may be effective in resolving wound inflammation, including diabetic wounds.
Introduction
Chronic wounds are a major socioeconomic threat costing billions of dollars annually to the US healthcare system1. A critical aspect of chronic wound management is maintaining a clean and well-vascularized wound bed that can then successfully progress through the stages of wound healing2. Debridement of devitalized tissue from the wound bed is an essential component of wound bed preparation. Collagenase Santyl Ointment (CSO) is an FDA-approved clostridial collagenase based prescription ointment for effective enzymatic debridement of chronic wounds and burns3. CSO has been shown to be an effective adjunct to wound therapy4,5. While controlled expression of metalloproteinases, e.g. collagenases, is required for wound healing, persistent hyperactivity of endogenous metalloproteinases may be detrimental for healing6,7. Interestingly, CSO may have roles at the wound site that are beyond its role in debridement8. However, whether CSO activity at the wound site may modify the function of wound resident and immune cells remains unknown.
Unresolved persistent inflammation is a hallmark of chronic wounds9,10. Macrophages (mϕ) are one of the central players orchestrating the inflammatory response at the wound site11,12. Selective depletion of macrophages in the initial inflammatory phase of wound healing compromised angiogenesis and epithelialization13,14. Depletion of macrophages in the remodeling phase resulted in severe hemorrhage in the wound tissue13 pointing towards a key role of these cells in tissue repair. The removal of dead and dying cellular debris by mϕ from wounds is required for resolution of inflammation15. Mϕ show diversity and plasticity in structure and function16. The ‘classically activated’ mϕ are pro-inflammatory in nature and play a critical role in host defense against infection. The ‘alternatively activated’ mϕ are associated with tissue repair/remodeling and resolution of inflammation16,17. Recent studies indicate that these two phenotypes represent the two extremes of the full spectrum of mϕ functions/phenotypes18. Given the current ambiguity in macrophage nomenclature specifically for tissue macrophages18,19, and proposed misfit of wound macrophage (wmϕ) with the M1/M2 nomenclature16,20,21, for this work we classify in vivo wmϕ based on the pro-inflammatory (mϕinf) or pro-resolution/healing (mϕheal) polarization states. The in vitro polarized M1/M2 have been referred to as M(LPS + IFNγ)/M(IL-4) as per revised guidelines18. Microenvironmental cues primarily dictate the phenotype and function of mϕ22. The repertoire of molecules and factors that trigger mϕ polarization have been well-defined although the field remains highly dynamic23. In this work, we sought to understand whether clostridial collagenase, the enzyme used as the wound debridement agent in CSO, may influence the fate of wound inflammation by inducing mϕ polarization.
Results
CSO caused a pro-resolution milieu in acute and chronic diabetic wounds
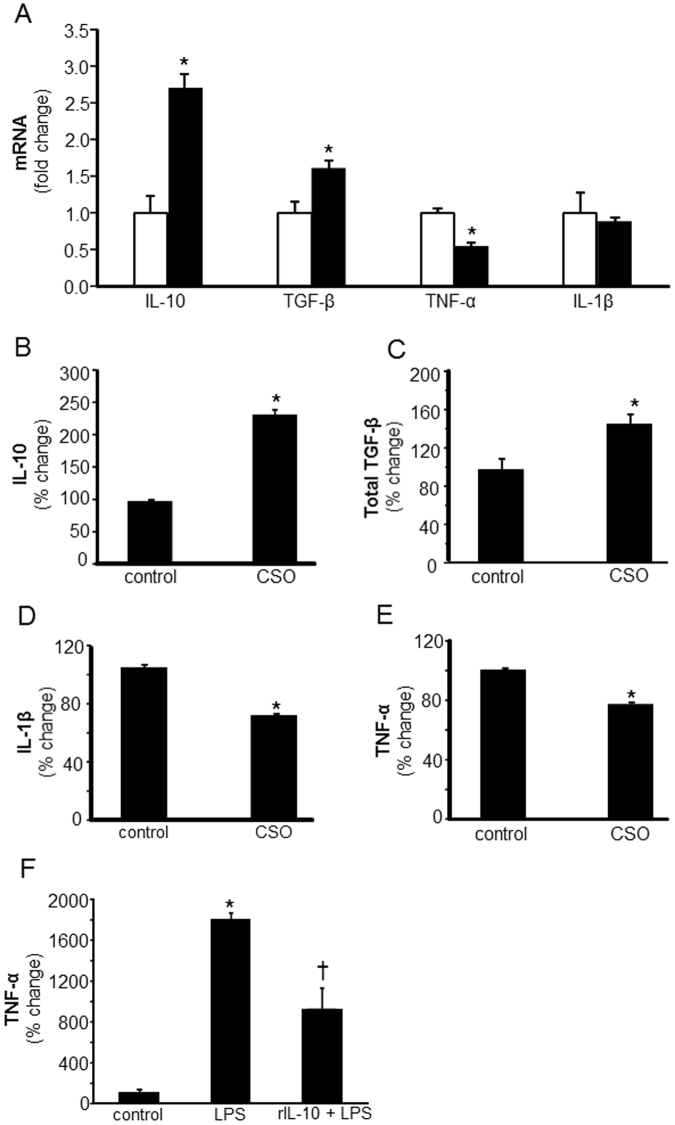
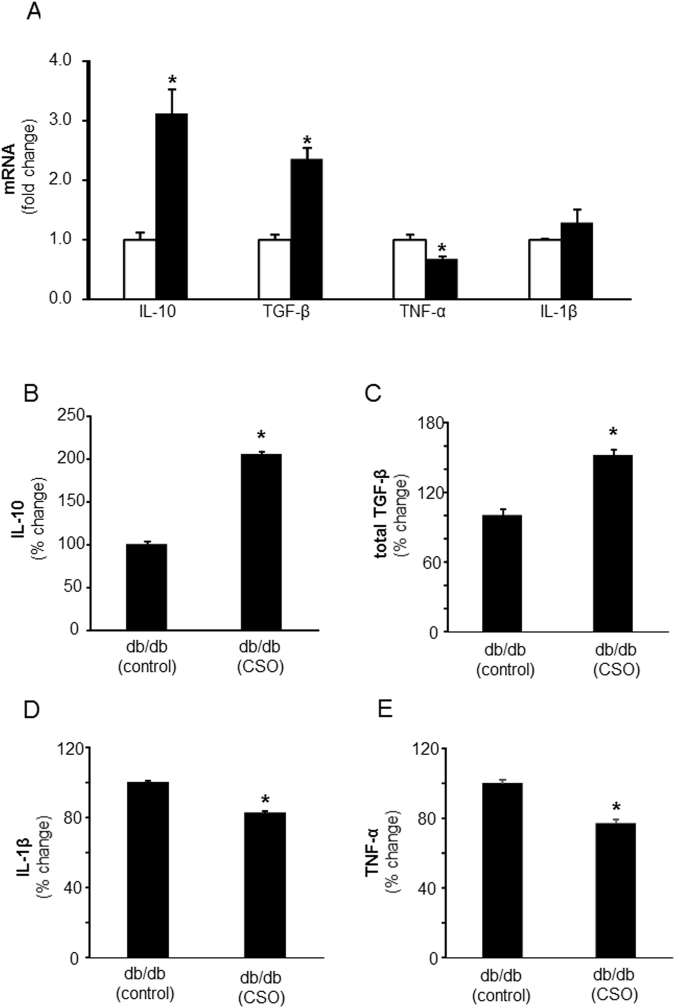
Mounting of a robust inflammatory response followed by a timely resolution are critical components of the healing process10,16. Wound mϕ are key players in shaping the wound microenvironment in the inflammatory phase14,16,24. To study the effect of CSO on wound microenvironment, mϕ were isolated from wounds treated with CSO or petrolatum (vehicle). Major pro- and anti-inflammatory cytokine/growth factors released by wound mϕ were assessed following potent pro-inflammatory stimulus (lipopolysaccharide, LPS) treatment. Potent upregulation of anti-inflammatory cytokine IL-10 mRNA and protein was noted in CSO-treated group as compared to the control group (Fig. 1A,B). A significant increase in TGF-β mRNA and protein was also observed in CSO-treated group (Fig. 1A,C). The increase in IL-10 and TGF-β was associated with a significant reduction in the levels of TNF-α mRNA and TNF-α/IL-1β proteins (Fig. 1A,D,E). Treatment of bone marrow derived macrophages (BMDM) with recombinant IL-10 significantly inhibited LPS-induced TNF-α release (Fig. 1F) suggesting that increased IL-10 in response to CSO may be implicated in lowering of LPS-induced TNF-α release. To determine whether CSO treatment influenced overall cell flux at the wound-site, we performed multiplexed flow cytometry analysis. While no significant changes in F4/80+ (wmϕ), Ly6G (PMN), or Treg (CD4+ CD25+) cell populations were noted in the CSO treated group, a significant reduction in pro-inflammatory monocytes (CCR2+Ly6Chi cells) was observed following CSO treatment (Fig. S1). This finding is supportive of the contention that CSO facilitates an anti-inflammatory pro-resolution milieu at the wound-site. Resolution of wound inflammation is known to be impaired in diabetic wounds15,25. Interestingly, CSO, in diabetic wounds, promoted the secretion of pro-resolving IL-10 and TGF-β while significantly downregulating TNF-α and IL-1β protein (Fig. 2A–E) demonstrating that CSO may foster pro-resolving milieu in the wound microenvironment even under diabetic conditions.
Figure 1.
CSO modifies cytokines release from murine wound macrophages contributing towards a pro-resolution milieu in acute wounds. Day 7 wound mϕ were harvested from PVA sponges coated with CSO (300 mg), implanted subcutaneously in C57bl/6 mice. (A) Total RNA was isolated and mRNA expression of IL-10, TGF-β, TNF-α and IL-1β was measured using RTPCR. Data are expressed as mean ± SEM (n = 5); *p < 0.05 compared to day 7 wound mϕ treated with equivalent amounts of white petrolatum (control). CSO, solid bars; control, blank bars. (B–E) The isolated wound mϕ treated in vivo with CSO (300 mg) were cultured in presence of LPS (1 µg/ml). After 24 h of culture the media was collected and expression of cytokines were measured using ELISA: (B) IL-10, (C) total TGF-β, (D) IL-1β and (E) TNF-α. Data are expressed as % compared to white petrolatum (control) treated group, mean ± SEM (n = 5); *p < 0.05 compared to day 7 wound mϕ treated with equal amounts of white petrolatum (control) treated with LPS. (F) Bone marrow derived macrophages (BMDM) from C57bl/6 mice were treated with recombinant mouse IL-10 for 1 h followed by activation with LPS (1 mg/ml, for 24 h). The release of TNF-α protein in culture media by BMDM was determined using ELISA. Data are mean ± SEM (n = 4); *p < 0.05 compared to control; †p < 0.05, compared to LPS treated group.
Figure 2.
CSO modifies cytokines release from murine wound macrophages contributing towards a pro-resolution milieu in chronic diabetic wounds. Day 7 wound mϕ were harvested from PVA sponges coated with CSO (300 mg) implanted subcutaneously in db/db mice. (A) Total RNA was isolated and mRNA expression of IL-10, TGF-β, TNF-α and IL-1β was measured using RTPCR. Data are expressed as mean ± SEM (n = 5); *p < 0.05 compared to day 7 wound mϕ treated with equivalent amounts of white petrolatum (control). CSO, solid bars; control, blank bars. (B–E) The isolated wound mϕ treated in vivo with CSO (300 mg) were cultured in presence of LPS (1 µg/ml). After 24 h of culture the media was collected and expressions of cytokines were measured using ELISA: (B) IL-10, (C) total TGF-β, (D) IL-1β and (E) TNF-α. Data are expressed as % compared to white petrolatum (control) treated group, mean ± SEM (n = 5); *p < 0.05 compared to day 7 wound mϕ treated with equal amounts of white petrolatum (control) treated with LPS.
CSO induced pro-resolution/healing macrophage (mϕheal) phenotype and attenuated pro-inflammatory (mϕinf) phenotype in acute and chronic diabetic wounds
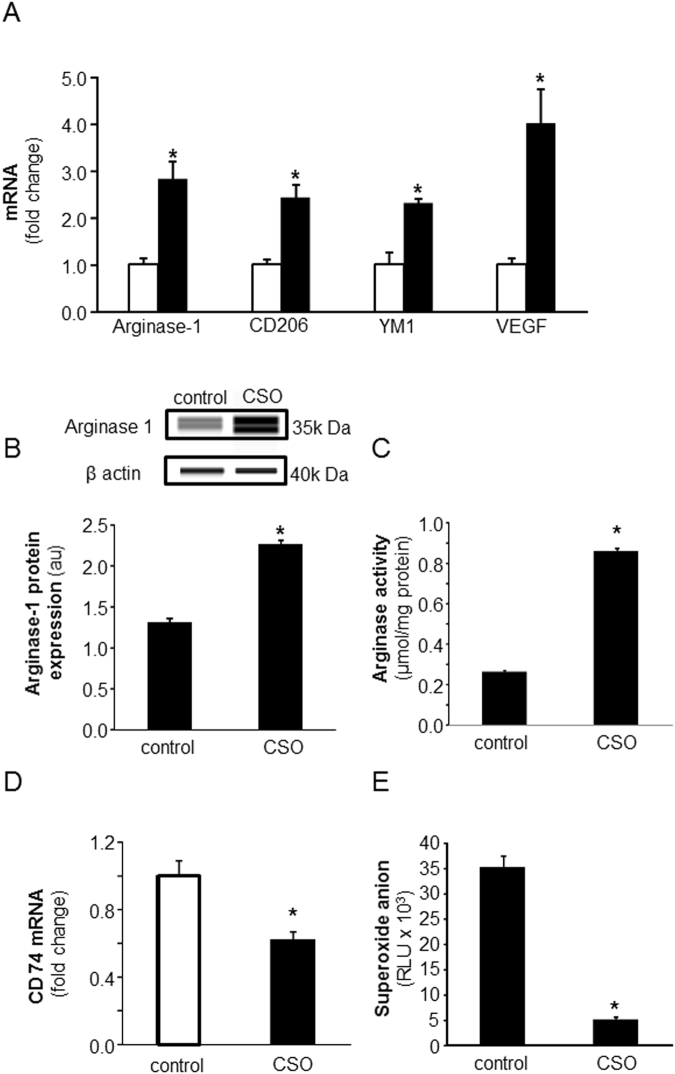
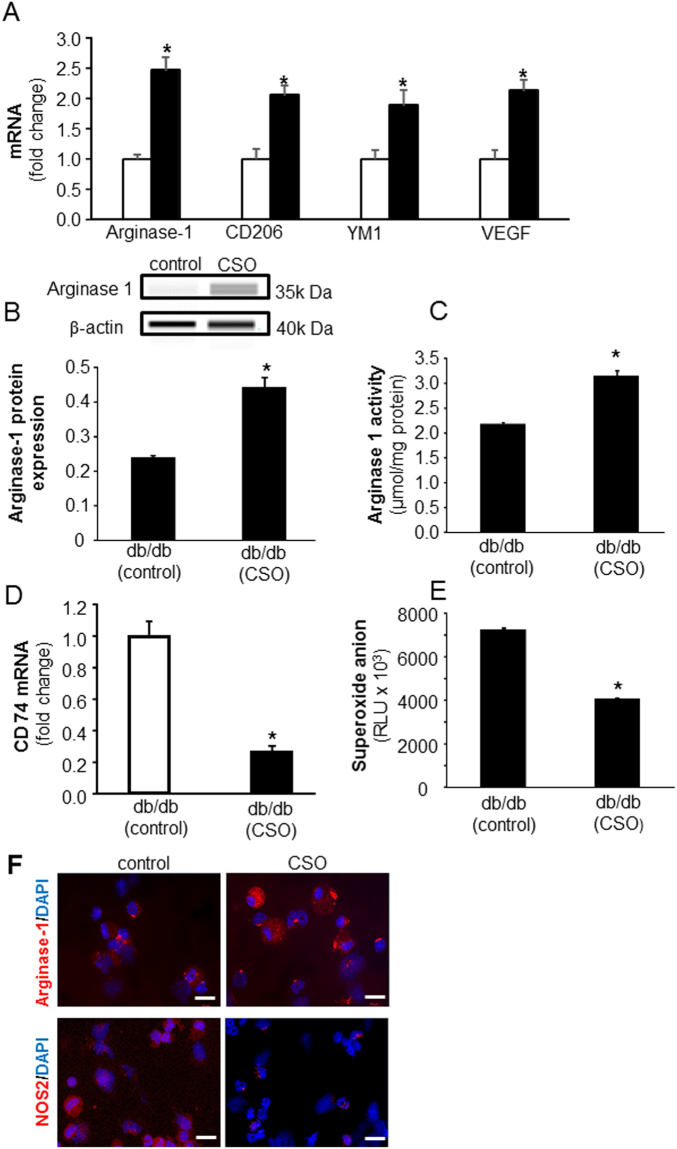
Although debatable, mϕ are commonly classified as pro-inflammatory (mϕinf) aka M1 or M(LPS + IFNγ), and pro-reparative (mϕheal) aka M2 or M(IL-4) polarization states16,19,26. To determine if CSO-induced anti-inflammatory milieu at the wound-site is a result of the presence of more pro-reparative mϕheal in CSO-treated wounds, the expression of mϕinf and mϕheal polarization state markers was analyzed using real-time PCR. Analysis of recognized M(IL-4) polarization markers (Arginase-1, CD206, Ym-1, VEGF) revealed a significant increase in M(IL-4) markers in day 7 wound mϕ in the CSO treated group as compared to vehicle-treated (Fig. 3A). Protein expression of Arginase-1, a classical marker for M(IL-4) mϕ27, was measured using capillary electrophoresis immunoassay and found to be significantly induced in CSO treatment group (Fig. 3B). Consistent with Arginase-1 mRNA and protein expression, significant increase in the activity of this protein in the CSO-treated group was noted (Fig. 3C). The recognized M(LPS + IFNγ) marker CD74 and PMA-induced superoxide production, on the other hand, were significantly attenuated in wound mϕ exposed to CSO (Fig. 3D,E). Notably, a similar effect of augmentation on mϕheal markers and attenuation in mϕinf polarization was also observed in diabetic wounds that are known to have high mϕinf wound mϕ populations21,25 (Fig. 4A–E). Immunocytochemistry (ICC) was performed to determine CSO-induced changes in expression of Arginase-1 and NOS2 protein expression. The data demonstrate that CSO treatment induced Arginase-1 while attenuating NOS2 in majority of the wound mϕ (Fig. 4F).
Figure 3.
CSO is a potent inducer of mϕheal macrophage polarization while attenuating M1 phenotype in acute wounds. (A) Day 7 wound mϕ were harvested from PVA sponges coated with CSO (300 mg) implanted subcutaneously in C57bl/6 mice. Total RNA was isolated and mRNA expression of Arginase-1, CD206, YM1 and VEGF was measured using RTPCR. CSO, solid bars; control, blank bars. (B) Total protein isolated from day 7 wound mϕ of C57bl/6 mice, treated with CSO in vivo, was subjected to capillary electrophoresis immunoassay to measure expression of Arginase-1 protein. (C) Arginase activity of in vivo CSO treated (300 mg) day 7 wound mϕ of C57bl/6 mice were measured by Arginase activity assay kit (Colorimetric). (D) mRNA expression of CD74 was measured in day 7 wound mϕ of C57bl/6 mice treated with CSO (300 mg). (E) PMA-induced superoxide anion production in day 3 wound mϕ of C57bl/6 mice treated in vivo with CSO (300 mg) was measured. For all figure parts, data are expressed as mean ± SEM (n = 3–6); *p < 0.05 compared to wound mϕ treated with equal amounts of white petrolatum (control).
Figure 4.
CSO is a potent inducer of mϕheal macrophage polarization while attenuating M1 phenotype in chronic diabetic wounds. (A) Wound mϕ were harvested from PVA sponges coated with CSO (300 mg) implanted subcutaneously in db/db mice on day 7 post-implantations. Total RNA was isolated and mRNA expression of Arginase-1, CD206, YM1 and VEGF was measured using RTPCR. CSO, solid bars; control, blank bars. (B) Total protein isolated from day 7 wound mϕ of db/db mice, treated with CSO in vivo, was subjected to capillary electrophoresis immunoassay to measure expression of Arginase 1 protein. (C) Arginase activity of in vivo CSO treated (300 mg) day 7 wound mϕ of db/db mice were measured by Arginase activity assay kit (Colorimetric). (D) mRNA expression of CD74 was measured in day 7 wound mϕ of db/db mice treated with CSO (300 mg). (E) PMA-induced superoxide anion production in day 3 wound mϕ of db/db mice treated in vivo with CSO (300 mg) was measured. For all figure parts, data are expressed as mean ± SEM (n = 3–6); *p < 0.05 compared to wound mϕ treated with equal amounts of white petrolatum (control). (F) Immunocytochemistry (ICC) images of Arginase-1 (red) and NOS2 (red) protein expression in d7 wound mϕ from db/db animals. Day 7 wound mϕ were harvested from PVA sponges coated with CSO (300 mg) implanted subcutaneously. Counter staining was performed using DAPI (nuclear, blue). Scale bar, 20 μm.
The active constituent of CSO facilitated mϕheal polarization
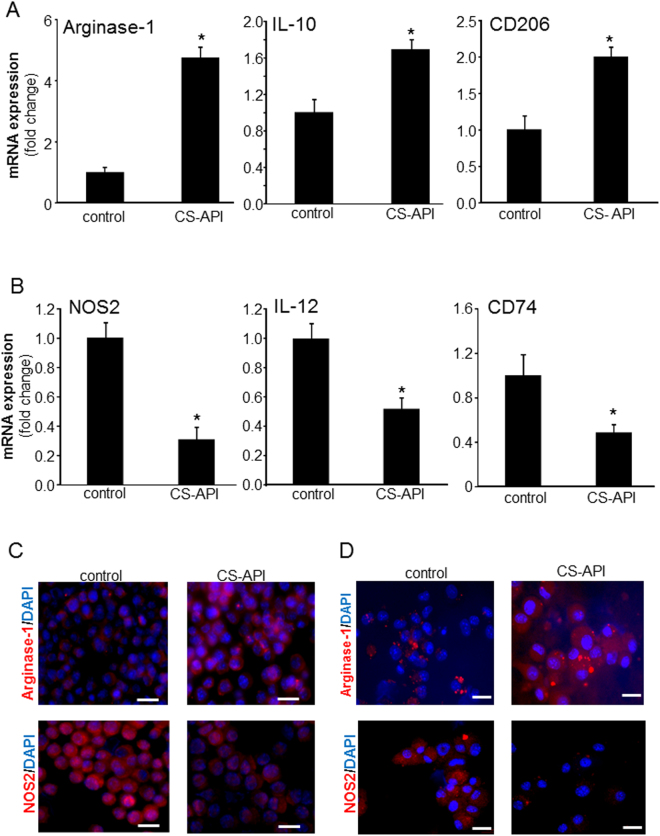
The active ingredients in CSO are clostridial collagenases that are produced by Clostridium histolyticum bacteria via a proprietary fermentation process3,8,28,29. This collagenase material contains two distinct collagenases (col), col G (114 kDa) and col H (110 kDa), and a lesser amount of a non-specific neutral protease (a metalloproteinase, 35 kDa) (data not shown). The active constituent of CSO, henceforth called as CS-API, was used to directly treat isolated wound mϕ ex vivo to examine if there is a direct effect of CS-API on mϕ polarization. CS-API exists in a powder form as opposed to CSO that is in ointment form suited for wound applications and not suitable for cell culture application. Based on the cell viability data (>90% viable, data not shown), 250 ng/ml dose was selected for ex vivo wound mϕ studies. CS-API directly acted on the wound mϕ and caused polarization to mϕheal phenotype. Substantial induction in M(IL-4) marker genes, Arginase-1, IL-10 and CD206 in wound mϕ was noted as compared to control group (Fig. 5A). Such induction was concomitant with potent down-regulation of M(LPS + IFNγ), genes, e.g. NOS2, IL-12 and CD74 (Fig. 5B). The balance of Arginase-1 and NOS2 protein expression in day 3 wound mϕ and BMDM were determined using ICC (Fig. 5C,D). Increased expression in Arginase-1 and attenuation in the expression of NOS2 by CS-API treatment was noted in both activated wound mϕ as well as naïve BMDM (Fig. 5C,D). Next, we determined whether CS-API was effective in influencing polarization in human macrophages. Treatment of human blood monocyte derived macrophages (hMDM) with CS-API resulted in macrophage polarization toward a pro-reparative phenotype as observed with mice wound mϕ and BMDMs. Thus, the anti-inflammatory effect of CSO on macrophage polarization may be generalized across these species (Fig. S2A). Interestingly, CS-API treatment of hMDM, previously polarized towards pro-reparative phenotype, did not further induce the pro-reparative markers IL-10 or Arginase (Fig. S2B). These data recognize that the active component of CSO i.e., clostridial collagenases, directly influence mϕ response and function across rodents and humans at the wound-site.
Figure 5.
Ex vivo treatment with active pharmaceutical ingredient (CS-API) directly induces mϕheal macrophage polarization while inhibiting the mϕinf phenotype. (A,B) Day 3 wound mϕ of C57bl/6 mice were harvested from PVA sponges and treated ex vivo with CS-API (250 ng/mL) for 24 hours (A) mRNA expression of Arginase-1, IL-10 and CD206 was measured using RTPCR. Data are expressed as mean ± SEM (n = 4); *p < 0.05 compared to day 3 wound mϕ treated with vehicle (control). (B) mRNA expression of NOS2, IL-12 and CD74 was measured using RTPCR. Data are expressed as mean ± SEM (n = 4); *p < 0.05 compared to day 3 wound mϕ treated with vehicle (control). (C,D) Immunocytochemistry (ICC) images of Arginase-1 (red) and NOS2 (red) protein expression in (C) day 3 wound mϕ of C57bl/6 and (D) BMDM from db/db animals treated ex vivo with CS-API (250 ng/ml) for 24 h. Counter staining was performed using DAPI (nuclear, blue). Scale bar, 20 μm.
Molecular mechanisms of CSO mediated promotion of wound mϕheal polarization
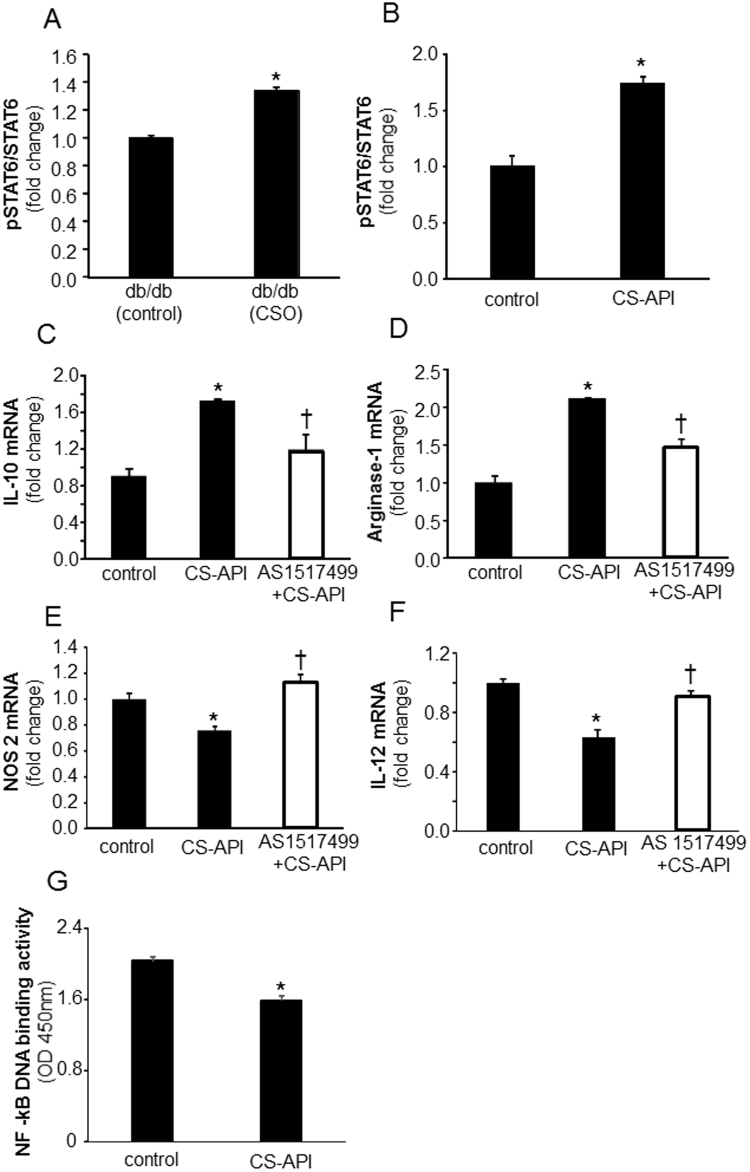
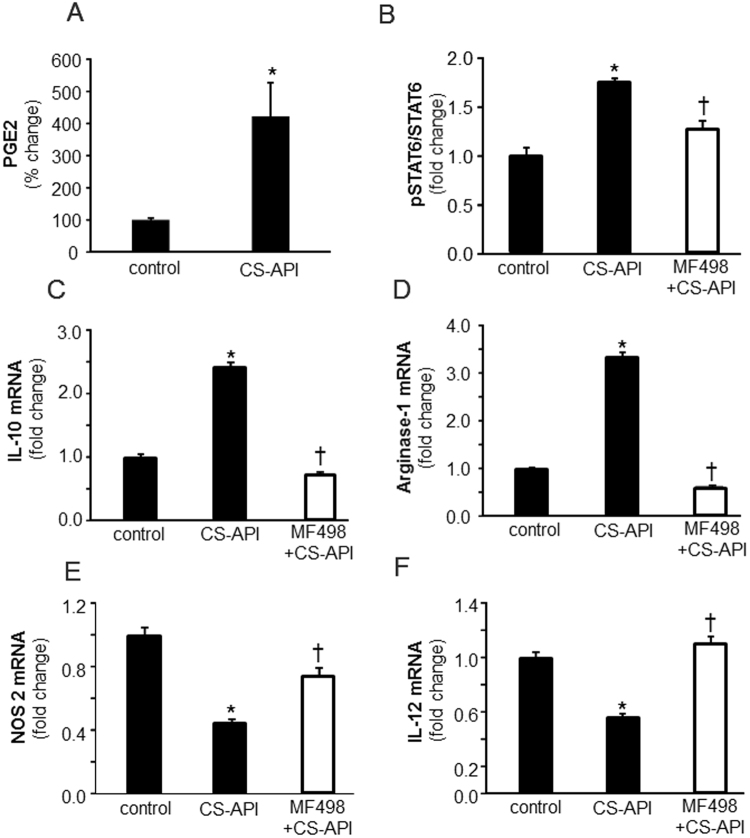
Molecular regulation of mϕ polarization is complex and involves a network of signaling molecules, transcription factors, epigenetic mechanisms, as well as posttranscriptional regulators. Canonical IRF/STAT signaling pathways are activated by IFNs and TLR signaling to skew mϕ function towards M(LPS + IFNγ) phenotype via STAT1, while IL-4 and IL-13 treatments skew toward the M(IL-4) polarization/activity via STAT630. CSO as well as its active constituent CS-API potently increased phosphorylation of STAT6 both in vivo in diabetic wound mϕ and in vitro in an established mouse mϕ cell line RAW 264.7 (Fig. 6A,B). To determine if the robust increase in STAT6 phosphorylation by CS-API is the underlying cause for the induction in mϕheal mϕ polarization, RAW 264.7 mϕ were treated with a specific pharmacological inhibitor of STAT6, AS1517499. AS1517499, or 4-(benzylamino)-2-pyrimidine-5-carboxamide, is a potent and selective inhibitor of STAT631. Pretreatment of mϕ with STAT6 inhibitor abrogated the effect of CS-API on mϕ polarization, eliminating or reducing the shift towards mϕheal phenotype for different markers (Fig. 6C–F), pointing towards a key role of STAT6 in CS-API and CSO mediated mϕheal polarization of mϕ at the wound-site. In addition to a key role of STAT6 pathway, a moderate attenuation in LPS inducible NF-κB activation was noted in CS-API treated cultured macrophages (Fig. 6G). In our efforts to further understand the underlying mechanisms, the involvement of the IL-4Rα pathway was studied by blocking IL-4Rα. Although such blocking of IL-4Rα abrogated IL-4 induced Arginase expression, no reduction was noted in CS-API-induced Arginase in the RAW 264.7 mϕ (Fig. S6) ruling involvement of the IL-4-IL-4Rα pathway in CS-API mediated effect on macrophage polarization as unlikely. The classical pathway of STAT6 activation occurs via IL-4R. However, alternatives to this canonical pathway for STAT6 activation have been reported32–40. To determine if alternative pathways of STAT6 activation are involved in CSO mediated activation of STAT6 → mϕheal polarization, we screened specific signaling pathways known to induce mϕheal phenotype. These studies led us to identify a novel PGE2-EP4 alternative pathway for CSO-STAT6 activation → mϕheal phenotype following CS-API treatment (Fig. 7). Treatment of cultured mϕ with CS-API resulted in marked increase in PGE2 production (Fig. 7A). Our laboratory has earlier reported that PGE2 in mϕ acts via an EP4–cAMP mediated pathway41. Inhibition of PGE2 receptor EP4 using specific pharmacological inhibitor (MF498), resulted in significant reduction in CS-API mediated STAT6 activation (Fig. 7B) as well as a significant induction of mϕheal phenotype (Fig. 7C–F).
Figure 6.
CS-API activates STAT6 while inhibiting NF-κB pathways in macrophage polarization. (A) Wound mϕ were harvested from PVA sponges coated with CSO (300 mg) implanted subcutaneously in db/db mice on day 7 post-implantations. Phosphorylation of STAT6 was measured using a cell-based ELISA kit. Data are expressed as mean ± SEM (n = 5); *p < 0.05 compared to white petrolatum (control) treated group. (B) Cultured mouse mϕ were treated with CS-API (250 ng/ml) for 24 hours. Phosphorylation of STAT6 was measured using a cell-based ELISA kit. Data are expressed as mean ± SEM (n = 5); *p < 0.05 compared to control. (C–F) Cultured mouse mϕ were treated with STAT6 inhibitor AS 1517499 (5 nM) for 1 hour followed by CS-API (250 ng/mL) treatment for 24 hours. mRNA expression of (C) IL-10 (D) Arginase-1 (E) NOS2 and (F) IL-12 was measured. Data are expressed as mean ± SEM (n = 3–4); *p < 0.05 compared to control, †p < 0.05, compared to CS-API treated group. (G) Cultured mouse mϕ were treated with CS-API (250 ng/ml) for 24 hours. DNA binding activity of LPS (1 µg/ml, 3 h) inducible NF-κB was measured using an ELISA-based (Trans-AM) method. Data are expressed as mean ± SEM (n = 5); *p < 0.05 compared to control.
Figure 7.
PGE2-EP4 pathway in CS-API mediated STAT6 phosphorylation and macrophage polarization. (A) Cultured mouse mϕ were treated with CS-API (250 ng/ml) for 24 hours. PGE2 level in the media was measured using ELISA. Data are expressed as mean ± SEM (n = 3); *p < 0.05 compared to control. (B) Cultured mouse mϕ were treated with EP4 inhibitor MF498 (50 nM) for 1 hour followed by CS-API (250 ng/ml) for 24 hours. Phosphorylation of STAT6 was measured using cell-based ELISA. Data are expressed as mean ± SEM (n = 3); *p < 0.05 compared to control, †p < 0.05, compared to CS-API treated group. (C–F) Cultured mouse mϕ were treated with EP4 inhibitor MF498 (50 nM) for 1 hour followed by CS-API (250 ng/ml) for 24 hours. mRNA expression of (C) IL-10 (D) Arginase-1 (E) NOS2 and (F) IL-12 was measured. Data are expressed as mean ± SEM (n = 3); *p < 0.05 compared to control, †p < 0.05, compared to CS-API treated group.
Discussion
Mϕ are critical mediators of innate immune response and key facilitators of mounting/resolving the wound inflammatory response16,42. In response to altering wound microenvironment, considerable plasticity in these cells allows them to acquire and exhibit diverse functional/activation/polarization states20,43,44. The factors or cues in the wound microenvironment that drive such diversity are vaguely understood at present. This study provides maiden evidence demonstrating that a commonly used enzymatic wound debridement agent CSO that contains clostridial collagenases as the active ingredient is a powerful inducer of mϕheal functional state in acute and chronic diabetic wounds. CSO treatment to wounds also promoted a pro-resolving environment that may be beneficial for chronic wounds known to have persistent inflammation as one of the major underlying factors driving the chronicity of these wounds.
The last decade has witnessed a considerable development in understanding of intrinsic mechanisms that regulate the inflammatory response. Such understanding has led to the design of therapies that promote mechanisms favoring resolution of inflammation. Remarkably, a widely used enzymatic debridement agent, CSO, promoted a pro-resolving wound environment by augmenting production of IL-10 by wound-site mϕ in both acute and diabetic chronic wounds. IL-10 is one of the most prominent pro-resolution cytokines that is released by cells of monocytic lineage45. The pro-resolution property of IL-10 is attributed to its potent inhibitory effect on the production of a large spectrum of pro-inflammatory cytokines such as IL- 1β, IL-6, and TNF-α46,47. Forced overproduction of IL-10 in wounds promoted regenerative adult wound healing by decreasing the inflammatory response48. Presence of multifold high levels of pro-inflammatory cytokines is a hallmark of chronic wounds10,49 including diabetic ulcers50,51. We and others have reported that mϕ from diabetic wounds produce significantly attenuated levels of IL-10 as compared to mϕ from non-diabetic wounds. CSO significantly improved the production of IL-10 in diabetic wound mϕ underscoring the potential to promote resolution of inflammation in chronic diabetic wounds.
Hallmarks of mϕ cell biology include plasticity and dynamicity such that these cells can exist within an inflammatory environment in diverse activation and function states16,52. Such diverse activation forms are part of a continuum, the two terminals of this continuum are defined by the classically activated or inflammatory mϕ, and reparative or alternatively activated mϕ17,52–54. The classically activated mϕ produces factors that favor a pro-inflammatory milieu (mϕinf). These factors include IL–12, TNF-α, IL-6, IL-1β, and nitric oxide (NO) in response to microbial pathogens or LPS55. The alternatively activated mϕ are known to favor a pro-resolution and reparative (mϕheal) milieu by producing IL-10, TGF-β, Arginase-1 (Arg1), CD206 (mannose receptor) and Clec7a (dectin-1)55. The ratio of nitric oxide synthase iNOS to Arg 1 is commonly used to determine the functional status of mϕ22,56,57. The M(LPS + IFNγ) & M(IL-4) paradigm represents two contrasting pathways for utilization of an amino acid. Arginine, via increased iNOS, in M(LPS + IFNγ) mϕ is metabolized to: arginine →NO + citrulline. In contrast, for M (IL-4) mϕ, arginine → ornithine + urea58. NO, a product of iNOS, is a reactive radical, the toxicity of which is greatly enhanced upon reaction with superoxide to form peroxynitrite (ONOO−)59. Ornithine, generated as a result of arginase metabolism, serves as a substrate for multiple enzymes including: (i) ornithine decarboxylase (ODC), a rate-limiting enzyme in the synthesis of polyamines, and (ii) ornithine aminotransferase (OAT), an enzyme that catalyzes the conversion to proline27. Although the role of Arginase in fibrosis has been questioned in regulation of fibrosis60, polyamines are essential for cell growth and development and have been shown to exert inhibitory effect against pro-inflammatory cytokines27. Proline is essential for the synthesis and maturation of collagen. A critical role of Arginase-expressing and ornithine-producing mϕheal has been reported in facilitating wound healing58. A potent increase in Arginase-expressing mϕheal in wounds treated with CSO indicates an alternative action of this proven debridement agent in facilitating wound healing. It is now recognized that multiple subtypes of the anti-inflammatory mϕheal exist at the repair-site54. In addition to mϕheal phenotype that may promote collagen synthesis, collagenolytic subtypes may exist61,62. The current study did not subcategorize the mϕheal at the wound-site.
STAT6 (signal transducers and activators of transcription 6) a member of STAT family of transcription factors63, directly regulates transcription of many genes associated with M(IL-4) mϕ phenotype17,64. This study noted that CS-API potently activated STAT6 and inhibited NF-κB as a central mechanism to promote M(IL-4) or mϕheal while attenuating mϕinf polarization. Abrogating the activation of STAT6 by pharmacological inhibitors resulted in the loss of function of CS-API in facilitating mϕheal polarization recognizing STAT6 as one of the primary pathways for CSO action on influencing mϕ polarization. STAT6 activation occurs via IL-4R mediated canonical65,66 as well as non-canonical pathways32–40. Our laboratory has earlier reported that PGE2 in mϕ acts via an EP4–cAMP pathway41. PGE2 is known to drive mϕ towards mϕheal polarization via a cAMP/CREB pathway67–69. Inhibition of PGE2 receptor EP4 using specific pharmacological inhibitor (MF498), resulted in significant reduction in CS-API mediated STAT6 activation and a significant induction of mϕheal phenotype. These new data implicate a novel alternative PGE2-EP4 pathway for STAT6 activation and a significant induction of mϕheal phenotype following CS-API treatment.
The NF-κB family of transcription factors regulate the expression of numerous genes implicated in immunity and inflammation70.We have previously reported a novel miR-21-NFκB-PTEN mediated pathway in the regulation of wound macrophage polarization46.
The principle active ingredients in CSO are collagenases derived from a specific propriety strain of Clostridium histolyticum3,8. First reported in 1953, clostridial collagenases are encoded by col G and col H genes for class I and class II type of collagenases, respectively71. Unlike mammalian matrix metalloproteinases (MMPs) that cleave native collagen into specific three-quarter and one-quarter fragments, clostridial collagenases breakdown repeating Gly-X-Y collagen sequences at distinct Y-Gly bond containing sites71. A recent study has identified novel collagen fragments, and collagen-associated peptides derived from thrombospondin-1, multimerin-1, fibronectin, and tenascin-C, generated from CSO-digested human dermal capillary endothelial and fibroblastic extracellular matrix (ECM)8. It is plausible that such degradation products of CSO are implicated in the mechanism of action reported in this study. Additionally, a direct effect of CS-API on mϕ receptors/pathways leading to induction of mϕheal polarization may not be ruled out. Taken together, this work sheds new light on what is currently utilized as a standard wound debridement agent in clinics. Findings of this work reposition CSO as a potential agent that may be effective in resolving wound inflammation, including wounds of diabetics, via STAT6 and NF-κB pathways.
Materials and Methods
Isolation of murine wound mϕ and bone marrow-derived macrophages (BMDM)
All animal studies have been approved by, and all methods were performed in accordance with the guidelines and regulations set by The Ohio State University’s Institutional Animal Care and Use Committee (IACUC). Male C57BL/6 mice (8–12 weeks old) were obtained from Harlan Laboratories (Envigo). Mice homozygous for spontaneous mutation of the leptin receptor (Leprdb) (BKS.Cg-m +/+ Leprdb/J, or db/db; stock no 000642) were procured from Jackson Laboratories. For wound mϕ isolation, circular (8 mm) sterile PVA sponges loaded with (300 mg/sponge) CSO (Smith & Nephew, Inc., Fort Worth, TX) or white petrolatum vehicle (control) (Fougera Pharmaceuticals Inc., NY) were implanted subcutaneously on the backs of 8 to 12 week-old mice15. The incisional wounds were sutured and closed after implantation of the PVA sponges. Sponge-infiltrated wound mϕ were isolated after 3 or 7 days post-implantation, as previously described15,25,41. In brief, following induction of anesthesia, subcutaneously implanted PVA sponges were harvested and wound cell suspension was generated by repeated compression of the sponges. The cell suspension was then subjected to magnetic cell sorting using mouse anti-CD11b tagged microbeads (Miltenyi Biotec, Auburn, CA). This procedure yields a purified (>95%) population of wound mϕ as determined by F4/80 staining15,25,41. Murine bone marrow-derived macrophages (BMDM) were isolated as previously described41.
ELISA
Levels of cytokines secreted by wound mϕ were measured using commercially available ELISA kits41,46. For the assay, wound mϕ isolated from CSO or vehicle treated groups were seeded in 6-well or 12-well plates followed by activation with LPS (1 µg/ml) for 24 h. After 24 h, the media was collected and the cytokine levels in culture media were measured using commercially available ELISA kits (R & D Systems, Minneapolis, MN) as per manufacturer’s instructions.
Isolation of RNA, reverse transcription and quantitative RT-PCR (qRT-PCR)
mirVana RNA isolation kit (Ambion, Austin, TX), was used according to the manufacturer’s instructions to extract total RNA as described72. Gene expression was measured by real-time qPCR assay using DNA intercalation dye SYBR Green-I (Applied Biosystems, CA) as described previously15,25,41,46,73,74.
Arginase activity assay
Arginase activity was determined with Arginase activity assay kit per manufacturer’s recommendation (Abcam, Cambridge, MA). In this assay, Arginase reacts with arginine through an intermediary product reacts with the OxiRed™ Probe to generate a colored product that can be read at OD 570 nm. For the assay, cell lysates or standards are mixed with a reaction cocktail provided with the kit followed by incubation at 37 °C for 30 mins. The colored product was read at 570 nm with a Plate Reader. The activity was normalized using equal amount of proteins in the cell lysate. The activity was expressed in μmol/mg of protein.
Superoxide anion production
Superoxide anion production was measured using a standard luminol based LumiMax Superoxide anion detection kit (Agilent technologies, Santa Clara, CA)72. The principle of the assay is that superoxide anion oxidizes luminol in a reaction that produces photons of light that are readily measured with a standard luminometer.
Capillary Electrophoresis Immunoassay
Capillary electrophoresis immunoassay was performed using the Simon (Protein Simple, Santa Clara, CA) according to the manufacturer’s protocol. The sample loading, electrophoresis and immunodetection was performed using a fully automated capillary electrophoresis system as described75. Capillary electrophoresis immunoassay was carried out at room temperature, and using default instrument settings (stacking/sample load time, 12 sec/8 sec; separation time, 40 min; blocking time, 15 min; primary antibody, 120 min; secondary antibody, 60 min). Arginase-1 (BD Biosciences, CA; dilution 1:30), and β-actin (Sigma Aldrich, MO; dilution 1:100) primary antibody were used for immuno-detection. The digital images were analyzed using Compass software (Protein Simple).
Immunocytochemistry
Cytospin smears from wound mϕ suspension were fixed in cell fixation buffer (BD Cytofix, BD Biosciences, CA) for 10 min. Following fixation, wound mϕ were washed, blocked in 10% NGS for 30 min and were incubated in primary antibodies for Arginase-1 (1:100; BD Biosciences) and iNOS (1:100; Abcam,). Fluorescence tagged secondary antibody detection was performed with Alexa Fluor 568 secondary antibody (1:200, Life Technologies) as described previously25.
Identification of wound cell populations by flow cytometry
Cytometry markers used to identify leukocyte subsets included: Alexa Fluor® 488-F4/80, PerCP-Ly6G, APC-Ly6C, PE-CCR2 (Biolegend), PE-CD4 and FITC-CD25 (Miltenyi). Cells were surface-stained for 30 minutes in staining buffer (1 × DPBS/1% BSA) as described previously43. Cells were then gated using forward and side scatter characteristics for leukocytes with at least 2,000 gated events recorded using BDTM LSR II flow cytometry (BD Biosciences) and analyzed using FlowJo and BD FACSDIVA™ software.
Cell based ELISA study
STAT6/pSTAT6 levels in mϕ were measured using commercially available cell-based ELISA kits as per manufacturer’s instructions. (R & D Systems and Abcam).
DNA binding of NF-κB
Nuclear protein extracts from mϕ were prepared using the nuclear protein extraction kit (Active Motif, Carlsbad, CA). Binding of NF-κB family of proteins to their consensus sites was determined using an ELISA-based Trans-AM NF-κB kit (Active Motif, Carlsbad, CA) following manufacturer’s instructions as previously described46.
Statistics
Data are reported as mean ± SEM as indicated in the respective figure legends. Student’s t test (two-tailed) was used to determine significant differences. Comparisons among multiple groups were tested using ANOVA, and p < 0.05 was considered statistically significant.
Disclosures
The authors declare that CSO, CS-API and partial research funding was provided by Smith & Nephew, Inc. ER and LS are current employees of Smith & Nephew, Inc (R&D, Fort, Worth).
Electronic supplementary material
Acknowledgements
This work was also supported in part by NIGMS (R01 GM077185, R01 GM108014) and NINR (R01 NR015676, R01 NR013898) and NIDDK (R56 DK076566).
Author Contributions
S.R., C.K.S., E.R. and K.G. conceived and designed the work. A.D., S.D., C.K.S., S.R., S.K., E.R., S.C., E.J., L.S., K.G. collected, analyzed data for this work and participated in the preparation of the manuscript. S.R., C.K.S., A.D. and S.D. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Amitava Das and Soma Datta contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19879-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sen CK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei C, Granick MS. Surgical management of chronic wounds T. Wounds. 2008;20:62–66. [PubMed] [Google Scholar]
- 3.Shi L, Carson D. Collagenase Santyl ointment: a selective agent for wound debridement. J Wound Ostomy Continence Nurs. 2009;36:S12–16. doi: 10.1097/WON.0b013e3181bfdd1a. [DOI] [PubMed] [Google Scholar]
- 4.Mosher BA, Cuddigan J, Thomas DR, Boudreau DM. Outcomes of 4 methods of debridement using a decision analysis methodology. Adv Wound Care. 1999;12:81–88. [PubMed] [Google Scholar]
- 5.Rao DB, Sane PG, Georgiev EL. Collagenase in the treatment of dermal and decubitus ulcers. J Am Geriatr Soc. 1975;23:22–30. doi: 10.1111/j.1532-5415.1975.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 6.McCarty SM, Percival SL. Proteases and Delayed Wound Healing. Adv Wound Care (New Rochelle) 2013;2:438–447. doi: 10.1089/wound.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarty SM, Cochrane CA, Clegg PD, Percival SL. The role of endogenous and exogenous enzymes in chronic wounds: a focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012;20:125–136. doi: 10.1111/j.1524-475X.2012.00763.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheets AR, et al. Identification and Characterization of Novel Matrix-Derived Bioactive Peptides: A Role for Collagenase from Santyl(R) Ointment in Post-Debridement Wound Healing? PLoS One. 2016;11:e0159598. doi: 10.1371/journal.pone.0159598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep. 2007;7:242–248. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- 10.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 11.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 12.Leibovich SJ, Wiseman DM. Macrophages, wound repair and angiogenesis. Prog Clin Biol Res. 1988;266:131–145. [PubMed] [Google Scholar]
- 13.Lucas T, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 14.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna S, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das A, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 18.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirza RE, Koh TJ. Contributions of cell subsets to cytokine production during normal and impaired wound healing. Cytokine. 2015;71:409–412. doi: 10.1016/j.cyto.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon S. Alternative activation of macrophages. Nature reviews. Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 23.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goren I, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das A, et al. Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing in Diabetes. J Immunol. 2016;196:5089–5100. doi: 10.4049/jimmunol.1502270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S. miRNA in Macrophage Development and Function. Antioxid Redox Signal. 2016;25:795–804. doi: 10.1089/ars.2016.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L, Ramsay S, Ermis R, Carson D. pH in the bacteria-contaminated wound and its impact on clostridium histolyticum collagenase activity: implications for the use of collagenase wound debridement agents. J Wound Ostomy Continence Nurs. 2011;38:514–521. doi: 10.1097/WON.0b013e31822ad034. [DOI] [PubMed] [Google Scholar]
- 29.Shi L, Ermis R, Garcia A, Telgenhoff D, Aust D. Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration. Int Wound J. 2010;7:87–95. doi: 10.1111/j.1742-481X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. The Journal of clinical investigation. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagashima S, et al. Synthesis and evaluation of 2-{[2-(4-hydroxyphenyl)-ethyl]amino}pyrimidine-5-carboxamide derivatives as novel STAT6 inhibitors. Bioorg Med Chem. 2007;15:1044–1055. doi: 10.1016/j.bmc.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Masuda A, et al. Interleukin-15 induces rapid tyrosine phosphorylation of STAT6 and the expression of interleukin-4 in mouse mast cells. J Biol Chem. 2000;275:29331–29337. doi: 10.1074/jbc.M910290199. [DOI] [PubMed] [Google Scholar]
- 33.Masuda A, Matsuguchi T, Yamaki K, Hayakawa T, Yoshikai Y. Interleukin-15 prevents mouse mast cell apoptosis through STAT6-mediated Bcl-xL expression. J Biol Chem. 2001;276:26107–26113. doi: 10.1074/jbc.M011475200. [DOI] [PubMed] [Google Scholar]
- 34.Patel BK, et al. Stat6 and Jak1 are common elements in platelet-derived growth factor and interleukin-4 signal transduction pathways in NIH 3T3 fibroblasts. J Biol Chem. 1996;271:22175–22182. doi: 10.1074/jbc.271.36.22175. [DOI] [PubMed] [Google Scholar]
- 35.Hua K, Deng J, Harp JB. Interleukin-4 inhibits platelet-derived growth factor-induced preadipocyte proliferation. Cytokine. 2004;25:61–67. doi: 10.1016/j.cyto.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Hundley TR, et al. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 37.Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci USA. 1998;95:5590–5594. doi: 10.1073/pnas.95.10.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghilardi N, et al. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fasler-Kan E, Pansky A, Wiederkehr M, Battegay M, Heim MH. Interferon-alpha activates signal transducers and activators of transcription 5 and 6 in Daudi cells. Eur J Biochem. 1998;254:514–519. doi: 10.1046/j.1432-1327.1998.2540514.x. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Jiang M, Pernis AB. IFN-alpha activates Stat6 and leads to the formation of Stat2:Stat6 complexes in B cells. J Immunol. 1999;163:3834–3841. [PubMed] [Google Scholar]
- 41.Ganesh K, et al. Prostaglandin E(2) induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J Immunol. 2012;189:2563–2573. doi: 10.4049/jimmunol.1102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porcheray F, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clinical & Experimental Immunology. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crane MJ, et al. The monocyte to macrophage transition in the murine sterile wound. PLoS One. 2014;9:e86660. doi: 10.1371/journal.pone.0086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pretolani M. Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy. 1999;29:1164–1171. doi: 10.1046/j.1365-2222.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 46.Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192:1120–1129. doi: 10.4049/jimmunol.1300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 48.Peranteau WH, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 49.Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen. 1996;4:234–239. doi: 10.1046/j.1524-475X.1996.40211.x. [DOI] [PubMed] [Google Scholar]
- 50.Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis. 2004;32:88–94. doi: 10.1016/j.bcmd.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 52.Xue J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 54.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh CL, et al. Traumatic brain injury induces macrophage subsets in the brain. Eur J Immunol. 2013;43:2010–2022. doi: 10.1002/eji.201243084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4 + T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- 57.Stout RD, et al. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 58.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/CritRevImmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 59.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 60.Barron L, et al. Role of arginase 1 from myeloid cells in th2-dominated lung inflammation. PLoS One. 2013;8:e61961. doi: 10.1371/journal.pone.0061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madsen DH, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202:951–966. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohani MG, et al. MMP-10 Regulates Collagenolytic Activity of Alternatively Activated Resident Macrophages. J Invest Dermatol. 2015;135:2377–2384. doi: 10.1038/jid.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- 64.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature reviews. Immunology. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/S1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 66.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 67.Luan B, et al. CREB pathway links PGE2 signaling with macrophage polarization. Proc Natl Acad Sci USA. 2015;112:15642–15647. doi: 10.1073/pnas.1519644112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montero J, Gomez-Abellan V, Arizcun M, Mulero V, Sepulcre MP. Prostaglandin E2 promotes M2 polarization of macrophages via a cAMP/CREB signaling pathway and deactivates granulocytes in teleost fish. Fish Shellfish Immunol. 2016;55:632–641. doi: 10.1016/j.fsi.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 69.Corraliza IM, Soler G, Eichmann K, Modolell M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem Biophys Res Commun. 1995;206:667–673. doi: 10.1006/bbrc.1995.1094. [DOI] [PubMed] [Google Scholar]
- 70.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 71.Zhang YZ, Ran LY, Li CY, Chen XL. Diversity, Structures, and Collagen-Degrading Mechanisms of Bacterial Collagenolytic Proteases. Appl Environ Microbiol. 2015;81:6098–6107. doi: 10.1128/AEM.00883-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roy S, et al. Particulate beta-glucan induces TNF-alpha production in wound macrophages via a redox-sensitive NF-kappabeta-dependent pathway. Wound Repair Regen. 2011;19:411–419. doi: 10.1111/j.1524-475X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elgharably H, et al. A modified collagen gel dressing promotes angiogenesis in a preclinical swine model of chronic ischemic wounds. Wound Repair Regen. 2014;22:720–729. doi: 10.1111/wrr.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elgharably H, et al. A modified collagen gel enhances healing outcome in a preclinical swine model of excisional wounds. Wound Repair Regen. 2013;21:473–481. doi: 10.1111/wrr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordillo GM, et al. Dicer knockdown inhibits endothelial cell tumor growth via microRNA 21a-3p targeting of Nox-4. J Biol Chem. 2014;289:9027–9038. doi: 10.1074/jbc.M113.519264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.