Abstract
Introduction
Drug-related problems (DRPs) are common in the elderly, leading to suboptimal therapy, hospitalisations and increased mortality. The integrated medicines management (IMM) model is a multifactorial interdisciplinary methodology aiming to optimise individual medication therapy throughout the hospital stay. IMM has been shown to reduce readmissions and drug-related hospital readmissions. Using the IMM model as a template, we have designed an intervention aiming both to improve medication safety in hospitals, and communication across the secondary and primary care interface. This paper presents the study protocol to explore the effects of the intervention with regard to healthcare use, health-related quality of life (HRQoL) and medication appropriateness in elderly patients.
Methods and analysis
A total of 500 patients aged ≥70 years will be included and randomised to control (standard care) or intervention group (1:1). The intervention comprises five steps mainly performed by pharmacists: (1) medication reconciliation at admission, (2) medication review during hospital stay, (3) patient counselling about the use of medicines, (4) a comprehensible and patient-friendly medication list with explanations in discharge summary and (5) postdischarge phone calls to the primary care level. The primary outcome is the difference between intervention and control patients in the rate of emergency medical visits (acute readmissions and visits to emergency department) 12 months after discharge. Secondary outcomes include length of index hospital stay, time to first readmission, mortality, hip fractures, strokes, medication changes, HRQoL and medication appropriateness. Patient inclusion started in September 2016.
Ethics and dissemination
The trial was approved by the Norwegian Centre for Research Data and the Norwegian Data Protection Authority. We aim to publish the results in international peer-reviewed open access journals, at national and international conferences, and as part of two PhD theses.
Trial registration number
Keywords: geriatric medicine, quality in healthcare, preventive medicine, adverse events, clinical pharmacology
Strengths and limitations of this study.
No randomised controlled trial investigating the effects of implementing an integrated medicines management-based intervention in the Norwegian healthcare setting has yet been published.
National healthcare registries will enable us to collect high-quality data for several outcomes including the primary outcome.
Collecting outcomes for a 1-year period after discharge allows us to measure sustainable effects of the intervention.
Including control and intervention patients from the same wards may introduce education and contamination bias.
As the intervention is complex this study will not allow for studying whether any of the specific steps are more or less responsible for any observed effects.
Introduction
Healthcare systems across the world are challenged by an ageing population. Ageing is frequently accompanied by morbidity, which increases the need for pharmacotherapy. The increased complexity of medication regimes combined with frailty, reduced cognitive function and changes in pharmacokinetics and pharmacodynamics increases the risk of adverse drug events and other drug-related problems (DRPs) in this population.1 2
A DRP is ‘an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes’.3 DRPs include inappropriate prescribing (drug, dose, dosage frequency and dosage form), drug interactions, adverse drug reactions, wrong administration, need for monitoring as well as non-adherence to medication therapy. DRPs occur frequently in the elderly,4 5 and are associated with an increased risk of hospitalisation, morbidity and mortality.6–8 For instance, adverse drug events alone contribute to 30%–40% of acute hospital admissions in the elderly,9 10 many of them being preventable.11–14
Communication barriers across primary and secondary care, multiple prescribers, fragmentation of care and frequent transitions across care levels make hospitalised elderly in particular risk of drug-induced harm.15 16 To improve the medicines management process in hospitals, pharmacist-dependent methods like medication reconciliation (MedRec), medication review and patient education have been developed and studied.17–20 The integrated medicines management (IMM) model is based on interdisciplinary collaboration where clinical pharmacists work together with physicians, nurses and patients aiming to optimise medication therapy by preventing and solving DRPs.21 22 In the IMM model different services like MedRec, medication review, patient counselling and dissemination of correct medication information at transition points are merged together in a systematic way.21 23 In Northern Ireland, the implementation of the IMM model in hospitals has led to a reduced length of hospital stay and an increased time to readmission compared to standard care.23 24 Also in Sweden, implementing IMM in single hospital settings has been associated with a reduction in readmissions and drug-related readmissions, improved communication of medication information at transition points and improved quality of medication therapy.21 25 26 In Norway, pharmaceutical care services in hospitals have since 2010 been based on the methodology embraced by the IMM model.27 However, no randomised controlled trial investigating the effects of implementing the IMM model in the Norwegian healthcare system has been published.
Based on the IMM model, we have designed an interdisciplinary collaboration structure aiming to optimise medication therapy in hospitals and to improve communication of medication-related issues between secondary and primary care. The aim of the study is to explore the effects of the intervention on healthcare use, health-related quality of life (HRQoL) and medication appropriateness in elderly patients.
Objectives
The primary objective is to investigate the effects of the intervention on rate of emergency medical visits (acute readmissions and visits to emergency departments (EDs)) 12 months after hospital discharge.
Secondary objectives include to investigate the effects on: self-reported HRQoL, acute readmissions, length of index hospital stay, time to first readmission, 30-day readmissions, general practitioner (GP) visit rate, mortality rate, medication appropriateness, medication-related readmissions, medication changes, hip fracture rate and stroke rate.
Methods and analysis
This protocol is developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement28 (see online supplementary file for SPIRIT 2013 checklist).
bmjopen-2017-020106supp001.pdf (58.5KB, pdf)
Study design
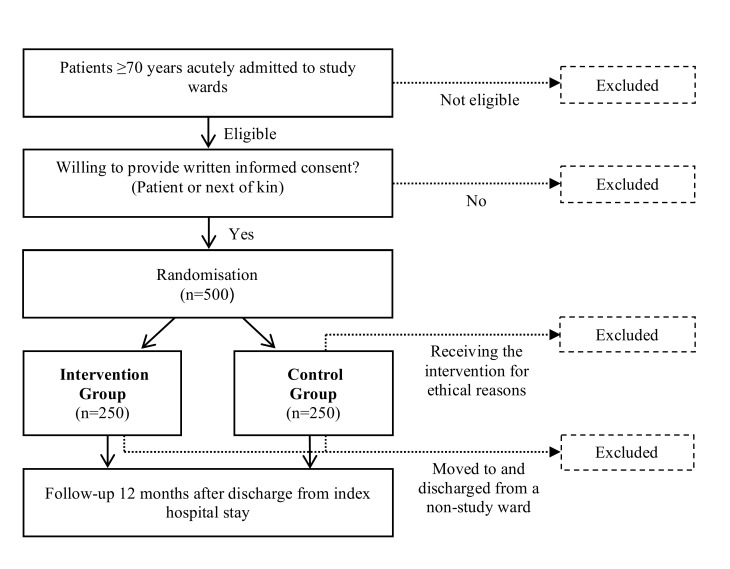
This is a non-blinded randomised controlled trial with an intervention group and a control group (1:1 ratio). The intervention group receives the intervention, while the control group receives standard care, see figure 1. Study enrolment started in September 2016.
Figure 1.
Flow chart of the study and study participants.
Settings
The study is carried out at two acute internal medicine wards at the University Hospital of North Norway (UNN); a geriatric internal medicine ward at UNN Tromsø and a general acute internal medicine ward at UNN Harstad. The geriatric ward cares for older patients with complex acute medical needs and has consultants specialised in geriatric medicine. The general medicine ward treats patients admitted for stroke, pulmonary, kidney and endocrine diseases as well as patients with geriatric concerns.
Study population
All acutely admitted patients are screened for eligibility and recruited by study pharmacists. Only eligible patients are invited to participate in the study. When written informed consent is obtained from patient or next of kin, the patient is included. Enrolment is only performed when a pharmacist is present. Readmitted study patients are not reincluded, but receive standard care.
Eligibility criteria
Inclusion criteria: age ≥70 years, acutely admitted and willing to provide written informed consent (patient or next of kin). Exclusion criteria: admitted to the study ward more than 72 hours before evaluation of eligibility, moved to and discharged from other wards during the index stay, inability to understand Norwegian (patient or next of kin), considered terminally ill or with a short life expectancy, planned discharged on the inclusion day, occupying a bed in a study ward but under the care of physicians from a non-study ward or if an intervention from a study pharmacist is considered necessary for ethical reasons (before randomisation or in control group).
Randomisation and blinding
After collecting baseline data, patients are randomised into the two study arms using a web-based service supplied by a third party. The randomisation block sizes are concealed and permuted. We stratify by study site. As pharmacists are only involved in intervention patients, blinding of group allocation is impossible for both the patients, pharmacists and medical team. However, the primary analysis will be performed by an investigator blinded for group allocation.
Standard care (control group)
Patients assigned to standard care receive treatment from a team consisting of physicians, nurses, nurse assistants, and sometimes occupational therapists and physiotherapists. Standard care may include elements as MedRec, medication review and patient counselling performed by physicians or nurses during the hospital stay. However, it is not standardised, structured or involving pharmacists. Study pharmacists are not involved in any clinical work concerning patients randomised to the control group.
Regarding MedRec at admission, this service is currently being implemented in hospitals nationwide as a part of the national patient safety programme. The local hospital procedure at UNN states that MedRec should be performed by a physician at admittance, but local data show that adherence to the procedure is low (data not published). Local procedures for communication of medication information at hospital discharge require that a discharge summary, including an updated medication list in addition to assessments, amendment and recommendations made during the hospital stay, is submitted electronically to the GP at discharge. For patients living in nursing homes or arecared for by the home care services, ward nurses call the home care services or nursing homes to inform about current medication therapy and to investigate the need for prescriptions or medications to be sent home with the patient. The GP is responsible for the follow-up of discharge summary recommendations as well as renewal and revision of prescribed medications.
Patients, for whom special home care is considered necessary, may be referred to a specialised patient care team before or at discharge. This team may include a pharmacist, which may supply pharmaceutical care services.
The intervention
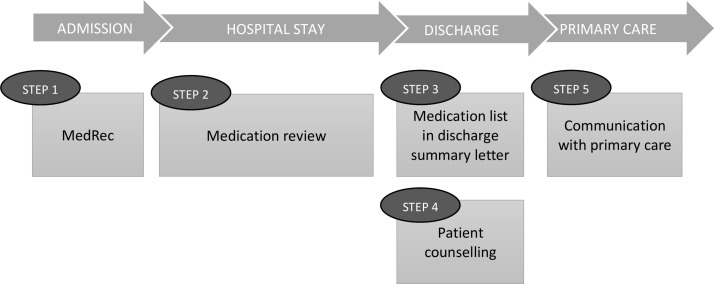
Patients randomised to the intervention group receive the IMM-based intervention including: (1) MedRec at admission, (2) medication review and monitoring during the hospital stay, (3) patient counselling designed to meet the needs of each individual patient, (4) MedRec at discharge together with an updated and structured medication list given to patients and submitted to primary care at discharge and (5) a follow-up phone call to the patient’s GP and nurses in home care service/nursing home to inform about and discuss current medication therapy and recommendations, see figure 2. Step 5 is in addition to the original IMM model. The study pharmacist is performing all steps in close collaboration with the hospital physician who has the medical responsibility for the patients.
Figure 2.
The intervention based on the IMM model (steps 1–4)21. Step 5 is added to the original model. IMM, integrated medicines management; MedRec, medication reconciliation.
Step 1: medication reconciliation
MedRec is performed using a standardised MedRec tool developed in Sweden and adapted to Norwegian circumstances/conditions.21 29 The tool facilitates information collection about the patient’s medication use and serves as documentation of information and information sources. It also includes questions about the patients practical handling and knowledge about medications, as well as medication adherence.21 29 Patients that handle their own medication are interviewed if possible. If not, information about medication use is collected from other relevant sources, that is, medication lists from GPs, national electronic medical records, local pharmacies, home care services, nursing homes or next of kin. These sources are also used to confirm medication information after patient interviews in case of uncertainties. Any adherence or medication information issues identified during MedRec is acted on during patient counselling or at hospital discharge (step 3).
During MedRec, the study pharmacists also perform a standardised symptom assessment to be used in step 2. This is done to identify possible adverse drug reactions, or possible targets for medication therapy improvements from a patient perspective. The assessment is performed to reveal if a patient recently has experienced any of the following 10 symptoms potentially related to medication therapy: dizziness, general fatigue, memory deficiency, sleeping difficulties, dry mouth, nausea, constipation, micturition difficulties, pain or cough. If the patient is incapable of answering the questions, information is obtained from relatives or associated healthcare workers.
Step 2: medication review
Medication review is based on information collected during MedRec, clinical and laboratory data and other relevant information. It is regularly updated during the hospital stay as long as the study pharmacists are present at the ward. A standardised tool, developed in Sweden and adapted to Norwegian circumstances/conditions, is applied to identify DRPs related to the following risk categories21: (1) medications requiring therapeutic drug monitoring, (2) potential inappropriate medications for elderly, (3) problems related to drug administration/dosage forms, (4) drug interactions, (5) dose or medications not suitable for the individual patient (eg, renal or liver failure), (6) lack of indication for drug therapy, (7) appropriate length of therapy for temporarily used medications, (8) suboptimal treated or untreated diagnosis or symptoms, (9) medications causing adverse drug reactions or change in laboratory measurements and (10) other needs for monitoring of treatments. Identified DRPs are discussed and solved in the interdisciplinary team and with the patient if possible. DRPs not dealt with or solved during the hospital stay are communicated to the GP as part of the discharge summary together with recommendations and monitoring needs. Identified DRPs are classified according to the validated Norwegian classification system.30
Step 3: patient counselling
For patients who will handle their own medication after discharge, a patient counselling session is arranged before discharge. The patients receive an updated medication list, which is discussed and explained. The pharmacists focuses on changes made during the hospital stay and reasons for these changes. Patients are also encouraged to ask questions about their medications. Any medication adherence, handling or information issues identified during the hospital stay is also focused on. If DRPs are identified during this counselling session, they are discussed with the responsible physician. This step does not replace the standard discharge meeting between the physician and the patient.
Step 4: structured and detailed medication list in discharge summaries
The discharge summary normally includes an updated overview of medications to be used after discharge. For intervention patients the study pharmacists draft this list in accordance with hospital procedures and recommendations from the national patient safety programme. They make sure it is reconciled, structured and correct according to amendments done and include information and explanations about medication changes made during the hospital stay as well as recommendations and follow-up issues. The responsible ward physician uses this draft when preparing the discharge summary.
Step 5: communication with primary care
Within a week after discharge, the pharmacists call the patient’s GP to inform about and discuss current medication therapy changes and recommendations stated in the discharge summary. The aim is to ensure that the changes and recommendations are implemented and acted upon.
One the day of discharge, for patients where the home care services or the nursing home administer the patient’s medications, the pharmacists call the responsible nurse to inform about medication changes, prescription and monitoring needs and other medication-related recommendations. Changes in multidosage dispensed medications are submitted to the local pharmacy responsible for dispensing the patient’s medications in agreement with the home care services.
This step is not carried out for patients with no change in medications during the hospital stay and/or no identified need for follow-up.
Outcomes
Primary outcome
The primary outcome is the rate of ‘acute readmissions and ED visits’ 12 months after discharge from the index hospital stay in the intervention group compared with the control group. An acute readmission is defined as any subsequent admission following the index admission excluding elective readmissions.
Secondary outcomes (intervention group compared with control group)
Change in self-reported HRQoL from discharge to 1, 6 and 12 months after hospital discharge.
Length of index hospital stay.
Time to first acute readmission after discharge from index hospital stay (up to 12 months follow-up).
The proportion of patients readmitted acutely within 30 days (a national quality indicator in Norway).
GP visit rate during 12 months’ follow-up.
Mortality rate during 12 months’ follow-up.
Change in total score of the Medication Appropriateness Index (MAI) from admission to discharge.
Change in potentially inappropriate medications prescribed identified by The Norwegian General Practice—Nursing Home criteria (NORGEP-NH), Screening Tool of Older Persons’ Prescriptions (STOPP) V.2 and Screening Tool to Alert doctors to Right treatment (START) V.2 from admission to discharge.
Change in potentially inappropriate medications prescribed using START V.2, STOPP V.2 and NORGEP-NH from discharge to 3 and 12 months.
Medication changes made during index hospital stay implemented by the GP at 3 and 12 months.
Medication-related first readmissions after index hospital stay.
Hip fracture rate during 12 months’ follow-up.
Stroke rate during 12 months’ follow-up.
Sample size calculation
Sample size calculation for the primary outcome is based on a Swedish randomised controlled trial applying the same composite endpoint.12 The Swedish trial investigated the effectiveness of interventions performed by ward-based pharmacists in reducing morbidity and use of hospital care among patients 80 years and older. They randomised 400 patients in a 1:1 relationship and found a 16% reduction in all-cause visits to the hospital in the intervention group. If we estimate a rate of acute hospital admissions and ED visits of 1.7 per year in our control group, we need to enrol 456 patients (228 in each group) to detect a 16% reduction in hospital visits with a significance level of 5% and a power of 80%. To compensate for dropouts, we aim to include 250 patients in each group.
Data collection and tool application
Baseline
Baseline data for all study patients is collected before randomisation to avoid collection bias. This include age, gender, smoking status, marital status, level of education, type and amount of help from home care services, and delivery of multidosage dispensed medications, medical diagnosis/medical history, weight, blood pressure, heart rate, relevant laboratory values (eg, blood creatinine, C reactive protein, haemoglobin and glucose) and medication use at time of hospital admission. The latter is denoted in the handwritten medication chart as standard procedure in our hospitals, while all other information is found in the electronic patient journal.
Hospital stay
For the intervention group only, we collect outcome data from the intervention (eg, discrepancies identified during MedRec, DRPs, physician agreement with regard to identified discrepancies or DRP, counselling issues, etc) during hospitalisation and track communication between pharmacist, patients and healthcare workers in the ward and in primary care. For all study patients, we collect the following data from the discharge summary: discharge diagnose(s), laboratory results, medication list including description of changes during the hospital stay and recommendations to the next care level.
After discharge
Data collection of outcomes after discharge is identical for all study patients.
National registries
Data on readmissions (dates, lengths and reasons), ED visits (dates and reasons), GP visits (dates and reasons), deaths (date and reason), strokes (dates), hip fractures (dates and reasons) and dispensed medications will be collected from six Norwegian Health registries. These registries are, respectively: The Norwegian Patient Registry (hospitalisations and ED visits), The Norwegian Health Economics Administration Registry (ED and GP visits), the National Cause of Death Registry, the Norwegian Stroke Registry, the Norwegian Hip Fracture Registry and the Norwegian Prescription Database (NorPD) holding information about all pharmacy dispensed medications in Norway. Linking data is possible through the unique personal identification number held by every Norwegian citizen. ED visits leading to a hospital stay will be counted as a hospital stay. We will collect data from all registries for the period 12 months before and 12 months after index hospital stay to enable adjustment for prestudy patterns.
Medication use
In addition to the data on prescriptions collected from NorPD, updated lists of medications in use are collected from GP offices or nursing homes as appropriate at 3 and 12 months after hospital discharge.
Inappropriate prescribing
The medications lists at hospital admission, at discharge and at 3 and 12 months after discharge will retrospectively be subjected to application of the following scoring tools to identify possible inappropriate prescribing by an investigator blinded for group allocation: NORGEP-NH,31 STOPP and START.32 The medication lists at admission and at discharge will be scored in accordance with the MAI by an experience pharmacist blinded to group allocation.33 34
Health-related quality of life
We use EuroQol 5 dimension (EQ-5D) and EuroQol visual analogue Scale (EQ-VAS) to measure HRQoL.35 This is performed by a study nurse blinded to group allocation. The measurement is performed at the end of the hospital stay and 1, 6 and 12 months after discharge. The study nurse calls patients and performs the interview by phone. Patients where next of kin provide informed consent are excluded from this measure. We collect information about need for home care services/nursing home at 1, 6 and 12 months to adjust for in the HRQoL analysis.
Medication-related readmissions
An interdisciplinary group of physicians and pharmacists will retrospectively assess whether the patient’s first readmission was related to his/her medications and whether it could have been prevented. This will be performed blinded to group allocation.
Data management
All data, except registry data, are entered manually into a Microsoft Access database. A random sample of patients will be drawn for control of data quality. Patient-ID is removed from all paper records and given consecutive study numbers. A list linking patient-IDs to study numbers is stored electronically on the hospital research server, separate from the Microsoft Access database. Only study personnel have access to the research server. Study papers used during work are kept at the hospital in accordance with hospital’s patient protection routines.
Statistical analysis
We will use IBM SPSS Statistics V.25 for data analysis. Data will be analysed according to intention-to-treat principle, and the reporting of results will follow the Consolidated Standards of Reporting Trials guidelines.36 All participants will be included in the analysis, regardless of whether the intervention was completed or not. A per-protocol analysis will also be performed. Descriptive statistics for both study arms and the total study population will be provided.
The primary analysis will be a Poisson regression of the rate of the composite endpoint during 12 months after discharge between the two study groups. Censoring of study participants will be accounted for, and adjustment for study site will be conducted. A two-sided alpha level of 5% will be used. We will perform a secondary analysis of the primary endpoint using the proportion of patients fulfilling the composite endpoint and a survival analysis of the time to reach the composite endpoint. In all analyses, adjustment for baseline variables will be conducted if appropriate.
We will analyse secondary outcomes applying appropriate statistical tests, for example, comparison between study arms by logistic regression analysis for binary responses and using Cox proportional hazards models for survival data. Continuous responses will be analysed using linear regression. A two-sided 5% significance level will be applied, with no adjustments for multiplicity.
The amount of data collected allows for different subgroup analyses and include: to assess whether the effect of the intervention varies by: (1) number of medications at admission or discharge; 0–5, 6–10, >10, (2) age groups 70–79, 80–89 and 90+, (3) patient responsibility for their own medication at discharge, (4) number and type of comorbidities at discharge, (5) number of hospital visits prior to inclusion, (6) length of hospital stay, (7) referred from home, home-care or nursing home or (8) able to self-provide informed consent or not.
Ethics and dissemination
The trial will be conducted in compliance with the protocol, the principles of Good Clinical Practice and the Declaration of Helsinki. Only patients who supply a written informed consent are included in the study. If patients are not able to consent, the next of kin is asked. If a patient is temporarily incapable of giving consent, for instance in the case of delirium, consent is first sought from the next of kin. If and when the patient is again considered able to consent he/she is asked to give the written consent themselves. Patients who refuse participation are excluded from the study.
We will not expose the patient for any new clinical intervention that may put the patient at risk. In fact, some of the elements/procedures included in the intervention have already been shown to reduce drug-related readmissions, and visits to the ED.19 20 Nevertheless, our intervention brings a new healthcare profession, the pharmacist, into the interdisciplinary team for whom the patient will have to relate to. We anticipate that patients feeling uncomfortable with this will refuse study participation.
We aim to publish study results in international peer-reviewed open access journals, at national and international conferences, and as part of two PhD theses.
Supplementary Material
Acknowledgments
We are extremely grateful to all participants in the study, employees at the departments where the study is performed, and our collaboration partners both at UNN Harstad, UNN Tromsø and the Hospital Pharmacy of North Norway Trust, in particular Kristian Svendsen. We would like to thank the clinical research department at UNN, and in particular Birthe Lund Angermo for help with data collection. We would also like to thank Inger Sperstad at UNN Tromsø for developing the Access Database and last but not least our funding body, the Northern Norway Regional Health Authority.
Footnotes
Contributors: JSJ, KH, KHH, BHG, SH, EK, LWS, KKV, LM and AGG were involved in study design. JSJ, KH, KHH and BHG drafted the manuscript. SH, EK, LWS, KKV, LM and AGG read and commented on the draft. JSJ, KH, KHH, BHG, SH, EK, LWS, KKV, LM and AGG all read and approved the final manuscript.
Funding: This work is supported by the Northern Norway Regional Health Authority grant number HST1314-16. The publication charges for this article have been funded by a grant from the publication fund of UiT—The Arctic University of Norway.
Disclaimer: The sponsor has no part in collection, management, analysis and interpretation of the data, as well as writing and reporting study conclusions.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study has approval from the Norwegian Centre for Research Data and the Norwegian Data Protection Authority to collect, store and link research data.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Alhawassi TM, Krass I, Bajorek BV, et al. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging 2014;9:2079–86. 10.2147/CIA.S71178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simonson W, Feinberg JL. Medication-related problems in the elderly : defining the issues and identifying solutions. Drugs Aging 2005;22:559–69. [DOI] [PubMed] [Google Scholar]
- 3. Europe PCN. PCNE Classification for Drug related problems V 5.01 2006. 2017. http://www.pcne.org/upload/files/16_PCNE_classification_V5.01.pdf (cited 6 Mar 2017).
- 4. Fialová D, Topinková E, Gambassi G, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA 2005;293:1348–58. 10.1001/jama.293.11.1348 [DOI] [PubMed] [Google Scholar]
- 5. Blix HS, Viktil KK, Reikvam A, et al. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur J Clin Pharmacol 2004;60:651–8. 10.1007/s00228-004-0830-4 [DOI] [PubMed] [Google Scholar]
- 6. Salvi F, Marchetti A, D’Angelo F, et al. Adverse drug events as a cause of hospitalization in older adults. Drug Saf 2012;35(Suppl 1):29–45. 10.1007/BF03319101 [DOI] [PubMed] [Google Scholar]
- 7. Jano E, Aparasu RR. Healthcare outcomes associated with beers’ criteria: a systematic review. Ann Pharmacother 2007;41:438–48. 10.1345/aph.1H473 [DOI] [PubMed] [Google Scholar]
- 8. Price SD, Holman CD, Sanfilippo FM, et al. Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother 2014;48:6–16. 10.1177/1060028013504904 [DOI] [PubMed] [Google Scholar]
- 9. Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 2001;31:199–205. 10.1046/j.1445-5994.2001.00044.x [DOI] [PubMed] [Google Scholar]
- 10. Gustafsson M, Sjölander M, Pfister B, et al. Drug-related hospital admissions among old people with dementia. Eur J Clin Pharmacol 2016;72:1143–53. 10.1007/s00228-016-2084-3 [DOI] [PubMed] [Google Scholar]
- 11. Howard RL, Avery AJ, Howard PD, et al. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care 2003;12:280–5. 10.1136/qhc.12.4.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009;169:894–900. 10.1001/archinternmed.2009.71 [DOI] [PubMed] [Google Scholar]
- 13. Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 2002;24:46–54. 10.1023/A:1015570104121 [DOI] [PubMed] [Google Scholar]
- 14. Winterstein AG, Sauer BC, Hepler CD, et al. Preventable drug-related hospital admissions. Ann Pharmacother 2002;36:1238–48. 10.1345/aph.1A225 [DOI] [PubMed] [Google Scholar]
- 15. Howard R, Avery A, Bissell P. Causes of preventable drug-related hospital admissions: a qualitative study. Qual Saf Health Care 2008;17:109–16. 10.1136/qshc.2007.022681 [DOI] [PubMed] [Google Scholar]
- 16. Witherington EM, Pirzada OM, Avery AJ. Communication gaps and readmissions to hospital for patients aged 75 years and older: observational study. Qual Saf Health Care 2008;17:71–5. 10.1136/qshc.2006.020842 [DOI] [PubMed] [Google Scholar]
- 17. Thomas R, Huntley AL, Mann M, et al. Pharmacist-led interventions to reduce unplanned admissions for older people: a systematic review and meta-analysis of randomised controlled trials. Age Ageing 2014;43:174–87. 10.1093/ageing/aft169 [DOI] [PubMed] [Google Scholar]
- 18. Walsh KA, O’Riordan D, Kearney PM, et al. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists' interventions in secondary care. Age Ageing 2016;45:201–9. 10.1093/ageing/afv190 [DOI] [PubMed] [Google Scholar]
- 19. Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open 2016;6:e010003 10.1136/bmjopen-2015-010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Renaudin P, Boyer L, Esteve MA, et al. Do pharmacist-led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta-analysis. Br J Clin Pharmacol 2016;82:1660–73. 10.1111/bcp.13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eriksson T. Results from a project to develop systematic patient focused clinical pharmacy services. The Lund Integrated Medicines Management model. Eur J Hosp Pharm 2014;21:121–4. 10.1136/ejhpharm-2013-000332 [DOI] [Google Scholar]
- 22. Bergkvist Christensen A, Holmbjer L, Midlöv P, et al. The process of identifying, solving and preventing drug related problems in the LIMM-study. Int J Clin Pharm 2011;33:1010–8. 10.1007/s11096-011-9575-1 [DOI] [PubMed] [Google Scholar]
- 23. Scullin C, Scott MG, Hogg A, et al. An innovative approach to integrated medicines management. J Eval Clin Pract 2007;13:781–8. 10.1111/j.1365-2753.2006.00753.x [DOI] [PubMed] [Google Scholar]
- 24. Scullin C, Hogg A, Luo R, et al. Integrated medicines management - can routine implementation improve quality? J Eval Clin Pract 2012;18:807–15. 10.1111/j.1365-2753.2011.01682.x [DOI] [PubMed] [Google Scholar]
- 25. Hellström LM, Bondesson A, Höglund P, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol 2011;67:741–52. 10.1007/s00228-010-0982-3 [DOI] [PubMed] [Google Scholar]
- 26. Torisson G, Minthon L, Stavenow L, et al. Multidisciplinary intervention reducing readmissions in medical inpatients: a prospective, non-randomized study. Clin Interv Aging 2013;8:1295–304. 10.2147/CIA.S49133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Major A-LS. [The IMM model to Norway]. Norsk farmaceutisk tidsskrift 2012;120:12–14. [Google Scholar]
- 28. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nilsson N, Lea M, Lao Y, et al. Medication discrepancies revealed by medication reconciliation and their potential short-term and long-term effects: a Norwegian multicentre study carried out on internal medicine wards. Eur J Hosp Pharm 2015;22:298–303. 10.1136/ejhpharm-2015-000686 [DOI] [Google Scholar]
- 30. Ruths S, Viktil KK, Blix HS. [Classification of drug-related problems]. Tidsskr Nor Laegeforen 2007;127:3073–6. [PubMed] [Google Scholar]
- 31. Nyborg G, Straand J, Klovning A, et al. The Norwegian General Practice-Nursing Home criteria (NORGEP-NH) for potentially inappropriate medication use: A web-based Delphi study. Scand J Prim Health Care 2015;33:134–41. 10.3109/02813432.2015.1041833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213–8. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samsa GP, Hanlon JT, Schmader KE, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol 1994;47:891–6. 10.1016/0895-4356(94)90192-9 [DOI] [PubMed] [Google Scholar]
- 34. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol 1992;45:1045–51. 10.1016/0895-4356(92)90144-C [DOI] [PubMed] [Google Scholar]
- 35. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 36. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020106supp001.pdf (58.5KB, pdf)