Abstract
Enterovirus 71 (EV71) is a major pathogen that causes hand, foot and mouth disease (HFMD) as well as neurological complications, such as encephalitis. The chemokines involved in the migration of leukocytes have increasingly been implicated in infectious diseases of the central nervous system. Few studies have evaluated the levels of chemokines in HMFD children with EV71-related encephalitis. In the present study, we evaluated the cerebrospinal fluid (CSF) levels of the chemokines IL-8, RANTES, MIG, MCP-1 and IP-10 in 99 children with EV71-related encephalitis and 22 children with febrile convulsion (FC). We found that the concentrations of IL-8, RANTES, MIG and IP-10 were significantly higher in HFMD children with encephalitis compared to patients with FC. Additionally, these four chemokines were dramatically reduced during convalescence. Inversely, the level of MCP-1 was lower in encephalitis patients than FC patients and was not significantly reduced during convalescence. Additionally, MIG was strongly correlated with IP-10 in encephalitis patients. Furthermore, the area under the ROC curve (AUC) of CSF MIG and IP-10 in distinguishing encephalitis from FC were 0.869 and 0.876, and the corresponding sensitivities/specificities were 67.7%/100.0% and 67.7%/95.5%, respectively. In conclusion, our results indicate that chemokines play important roles in the pathogenesis of EV71 infection.
Introduction
Enterovirus 71 (EV71) is one of the major agents that causes hand, foot and mouth disease (HFMD) in infants and young children, which is characterized by fever, ulcers in the oral mucosa and vesicles on the hands and feet1. Although EV71 infection in most cases is self-limited, it can also induce severe neurological complications, such as aseptic meningitis (AM), encephalitis, brainstem encephalitis (BE), pulmonary edema (PE) and other rarer manifestations1. These neurological complications can sometimes lead to permanent neurologic sequelae and even death, and fatal PE or cardiorespiratory failure is thought be the main disease process in fatal cases1. It has also been postulated that dysregulation of inflammatory responses and cellular immunity are possibly responsible for this pathogenesis2–4. For instance, an in vitro study conducted by Lu et al. showed that EV71 inhibited the cellular type I IFN response by targeting a subunit of the IFN receptor, IFNAR12. An animal study revealed that both the lymphocyte and antibody responses reduced the mortality and tissue viral loads of EV71-infected mice3. In a clinical study, it was shown that elevated cytokine production (including IL-10, IL-13 and IFN-γ) and lymphocyte (including CD4 + T cells, CD8 + T cells, natural killer cells) depletion were responsible for EV71 infection4. Unfortunately, to date, there is no effective vaccines or therapy available for EV71 infection. Therefore, developing a better understanding of the neuropathogenesis of EV71 infection is of the utmost importance in developing a more effective clinical therapy for treating HMFD patients.
Chemokines are a group of small secreted proteins that have been shown to induce directed chemotaxis in response to inflammation5. The most investigated chemokines belong to the CC and CXC families, which are classified by the relative position of the first consensus cysteines (either separated by a non-conserved amino acid or next to each other). CC chemokines include regulated upon activation normal T cell expressed and secreted (RANTES, also termed CCL5) and monocyte chemoattractant protein-1 (MCP-1, also termed CCL2), while interferon-γ-inducible protein 10 (IP-10, also termed CXCL10), monokine induced by IFN-γ (MIG, also termed CXCL9) and interleukin-8 (IL-8, also termed CXCL8) belong to the CXC family5. Although they were initially characterized as being important in inflammation by targeting leukocytes, chemokines are now considered to be crucial mediators that play fundamental roles in both the physiological and pathological immune responses5. For example, the concentration of RANTES is higher in patients with a bacterial infection (including sepsis, community-acquired pneumonia, skin abscess) than controls with no infection, indicating that RANTES may be involved in the pathogenesis of bacteria6. In the same study, a unique pattern of immune response with high levels of IP-10 but low levels of MCP-1 were found in patients with sepsis6. IP-10, MIG and IL-8 were significantly elevated in the sputum of asthma patients compared to healthy controls7. Similarly, the plasma levels of IP-10, MCP-1, MIG and IL-8 were significantly higher in patients with PE than those with uncomplicated BE, indicating overexpression of the chemokine cascade, which appears to play an important role in the immune response to EV71 infection8. However, few studies have evaluated the levels of chemokines in the cerebrospinal fluid (CSF) of HMFD children. Additionally, encephalitis is the common neurological complication of EV71 infection; little is known about the roles of chemokines in encephalitis, and the key mediators underlying this inflammatory response remain unclear. Moreover, despite the severe consequences, early recognition of HFMD patients with neurological complications is important for selecting optimal intensive care, which might prevent disease progression.
Therefore, the purpose of this study was to (i) evaluate the CSF levels of the chemokines IL-8, RANTES, MIG, MCP-1 and IP-10 in HFMD patients with encephalitis; (ii) identify the possible interrelationships between the chemokines and CSF parameters; and (iii) investigate their potential role in HFMD as biomarkers reflecting pathogenesis, which may pave the way for new therapeutic targets.
Results
Patient characteristics and laboratory variables
This study included 99 HFMD patients with EV71-related encephalitis and 22 FC patients as controls. The baseline characteristics of the study population are listed in Table 1. In brief, there were no significant differences between encephalitis and FC patients regarding age, gender, and cell number in blood. However, encephalitis patients were more likely to have a longer duration of fever and hospital stay, have higher blood glucose, and have higher WBC and protein concentrations in CSF compared to children with FC.
Table 1.
Comparison of the demographic and clinical characteristics between patients with EV71-related encephalitis and those with simple FCs.
| Category, characteristic | Febrile convulsion (n = 22) | Encephalitis (n = 99) | P |
|---|---|---|---|
| Age (months) | 26.68 ± 16.84 | 29.26 ± 13.20 | 0.433 |
| Gender (male/female) | 13/9 | 60/39 | 0.895 |
| Hospital stays (days) | 6.23 ± 2.07 | 9.60 ± 2.45 | <0.001 |
| Fever duration before admission (days) | 1.61 ± 1.17 | 2.49 ± 1.55 | 0.007 |
| Fever duration during hospitalization (days) | 1.76 ± 1.19 | 2.44 ± 1.28 | 0.003 |
| Total fever duration (days) | 3.37 ± 1.66 | 4.92 ± 1.61 | <0.001 |
| Blood routine analysis | |||
| WBC (×109/L) | 13.10 ± 3.86 | 11.53 ± 3.82 | 0.084 |
| Neutrophil (%) | 56.69 ± 22.12 | 59.47 ± 16.01 | 0.793 |
| Lymphocyte (%) | 31.65 ± 18.42 | 33.38 ± 14.50 | 0.441 |
| Hemoglobin (g/L) | 119.77 ± 10.76 | 121.09 ± 9.07 | 0.553 |
| Platelet count (×109/L) | 299.23 ± 65.56 | 309.33 ± 73.81 | 0.555 |
| Blood glucose (mmol/L) | 5.27 ± 1.47 | 6.05 ± 1.62 | 0.043 |
| CSF variables | |||
| WBC (×106/L) | 4.14 ± 2.66 | 125.58 ± 123.68 | <0.001 |
| Neutrophil (%) | — | 49.54 ± 24.96 | — |
| Lymphocyte (%) | — | 45.03 ± 24.65 | — |
| Total protein (g/L) | 166.86 ± 78.11 | 267.34 ± 123.95 | <0.001 |
| Glucose (mmol/L) | 3.85 ± 0.75 | 5.11 ± 11.44 | 0.592 |
| Chloride (mmol/L) | 124.5 ± 4.46 | 122.07 ± 13.21 | 0.894 |
CSF chemokine profiles in the acute and recovery phases of EV71-related encephalitis
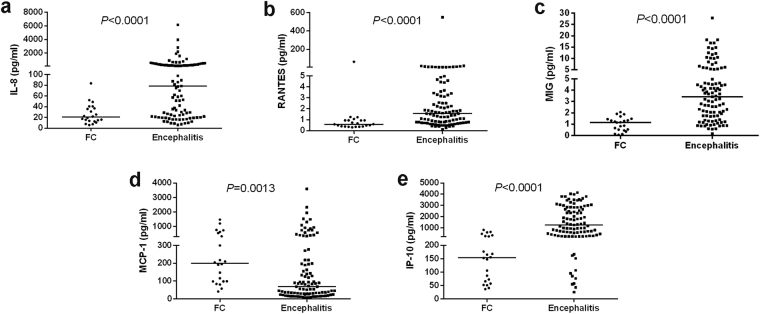
The CSF chemokine levels of IL-8 (78.40 (21.73–267.34) pg/ml), RANTES (1.56 (0.77–3.30) pg/ml), MIG (3.42 (1.72–5.34) pg/ml) and IP-10 (1238.82 (391.55–2812.31) pg/ml) were significantly higher in patients with encephalitis in the acute stage compared to those with FC (20.56 (13.28–37.98) pg/ml, 0.59 (0.38–0.94) pg/ml, 1.16 (0.50–1.46) pg/ml, 154.12 (63.48–264.60) pg/ml, respectively) (Fig. 1a,b,c,e). The CSF MCP-1 levels, on the contrary, were significantly lower in the encephalitis group (69.06 (29.37–217.59) pg/ml) compared to the FC group (199.84 (98.13–571.13) pg/ml) (Fig. 1d).
Figure 1.
CSF chemokine concentrations in EV71-related encephalitis patients and febrile convulsion (FC) patients in the acute stage. (a) IL-8; (b) RANTES; (c) MIG; (d) MCP-1; (e) IP-10.
Furthermore, we investigated the association between the chemokine levels in the acute phase and fever duration before admission. There was a positive correlation between RANTES and fever duration before admission (r = 0.469, p < 0.001). However, IL-8 (r = −0.398, p < 0.001) and MCP-1 (r = −0.294, p = 0.003) were negatively correlated with fever duration before admission. In addition, neither MIG (r = 0.114, p = 0.260) nor IP-10 (r = −0.139, p = 0.170) were associated with fever duration before admission.
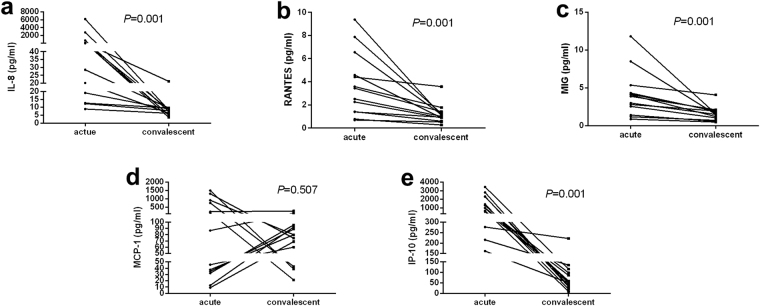
Compared with the acute stage, the CSF chemokine levels of IL-8 (77.54 (15.90–473.87) pg/ml vs. 7.89 (5.73–9.28) pg/ml), RANTES (3.44 (1.40–5.57) pg/ml vs. 1.00 (0.74–1.33) pg/ml), MIG (3.87 (1.97–4.83) pg/ml vs. 1.48 (0.86–1.95) pg/ml) and IP-10 (980.79 (406.20–2314.40) pg/ml vs. 56.07 (34.51–106.77) pg/ml) significantly decreased in the convalescent stage (Fig. 2a,b,c,e) of encephalitis in 13 patients, except for MCP-1 (86.60 (33.42–831.52) pg/ml vs. 79.86 (51.36–93.89) pg/ml) (Fig. 2d).
Figure 2.
CSF chemokine concentrations in EV71-related encephalitis patients in the acute and convalescent stage. (a) IL-8; (b) RANTES; (c) MIG; (d) MCP-1; (e) IP-10.
The association between the CSF chemokine levels and CSF cytology parameters in encephalitis patients
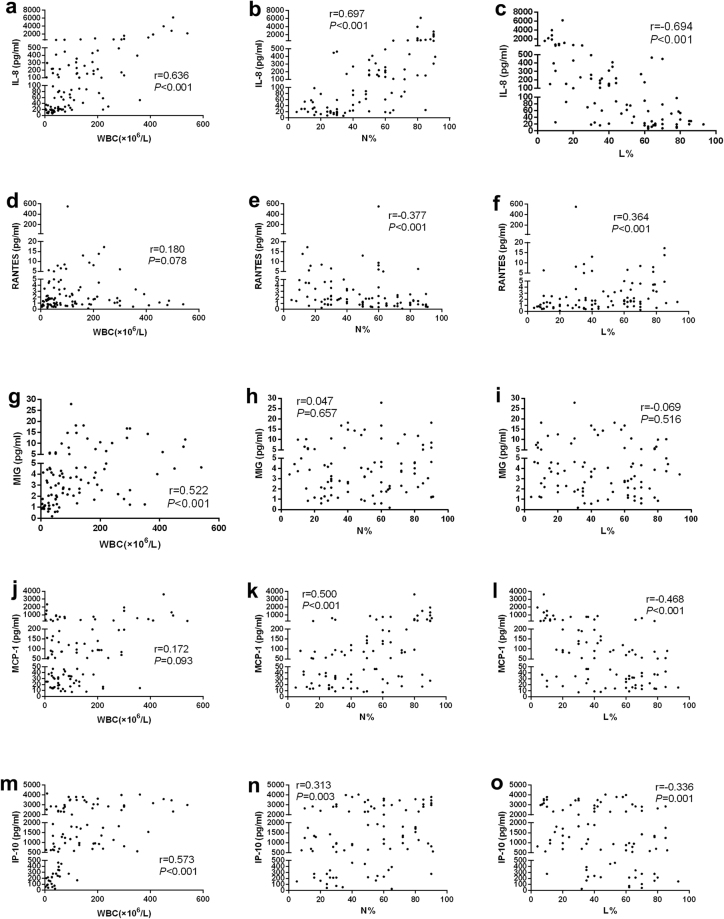
In encephalitis patients, all of the CSF cytology variables (including WBC, neutrophil percentages and lymphocyte percentages) were strongly associated with the CSF IL-8 levels (Fig. 3a–c). Although the CSF MIG and IP-10 concentrations correlated well with the CSF WBC (Fig. 3g,m), they showed poor or no correlation with the neutrophil and lymphocyte percentages (Fig. 3h,i,n,o). By contrast, a statistically significant correlation between the CSF RANTES, MCP-1 levels and neutrophil and lymphocyte percentages (Fig. 3e,f,k,l) were detected, but no correlation with the CSF WBC was found (Fig. 3d,j). We did not find any correlation between the CSF chemokine levels and cytology variables in FC patients (Supplementary Table S1).
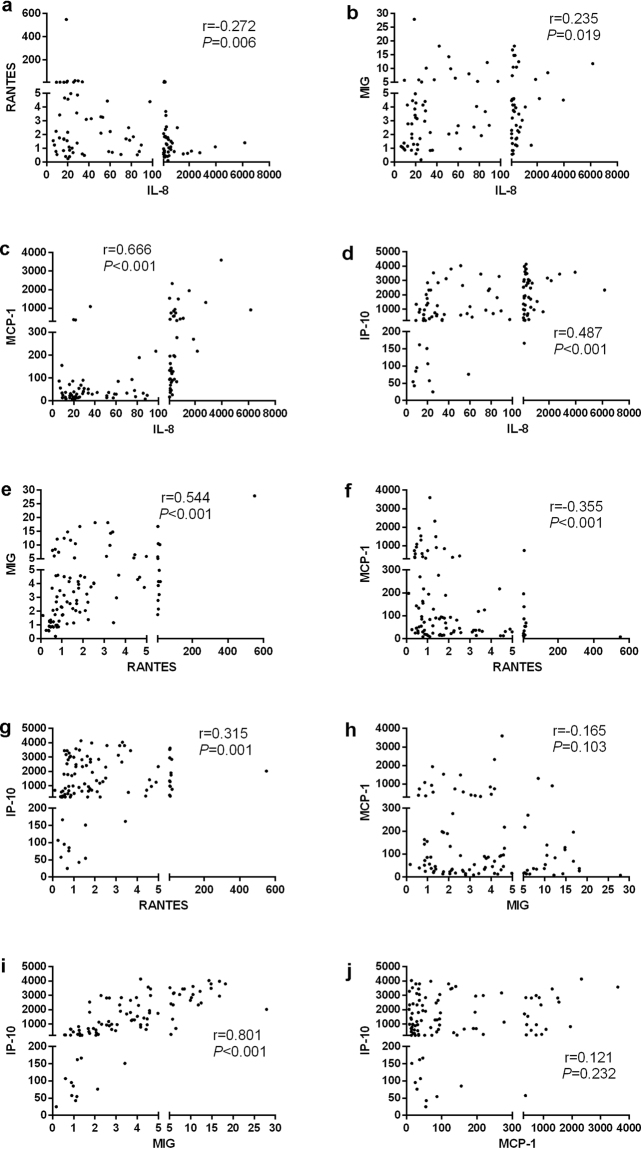
Figure 3.
Correlation analysis of CSF chemokine concentrations with CSF cytology variables in encephalitis patients depicted as scatter plots.
Clinical diagnostic values of CSF chemokines for HFMD patients with EV71-related encephalitis
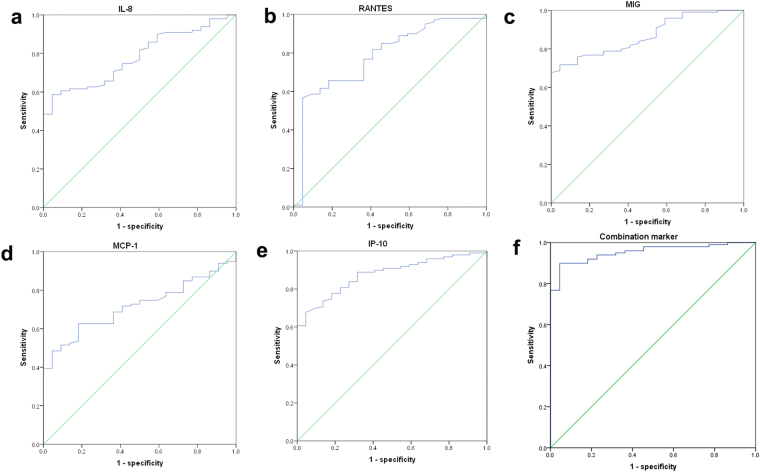
Given the marked variation of CSF chemokines in HFMD patients with encephalitis, the performance characteristics of CSF chemokines in differentiating encephalitis from FC were evaluated by ROCs. As shown in Table 2 and Fig. 4, ROC analyses showed that IP-10 and MIG yielded a significantly increased area under a ROC of 0.876 and 0.869, respectively, compared with IL-8 (0.781), RANTES (0.783), MCP-1 (0.716). The Youden-index-calculation provide a sensitivity of 67.7% and specificity of 95.5% for a cut-off of 621.79 pg/ml for IP-10 (Fig. 4e). When the cut-off point of MIG was 2.10 pg/ml, its sensitivity and specificity were 67.7% and 100%, respectively (Fig. 4c). Similarly, IL-8, RANTES and MCP-1 had 58.6%, 56.6% and 62.6% sensitivity, and 95.5%, 95.5% and 81.8% specificity, when the optimal cut-off values were 52.71 pg/ml, 1.26 pg/ml and 97.24 pg, respectively (Fig. a,b,d). Subsequently, a combination marker of these five chemokines was evaluated by ROC analysis, which had an area under the ROC curve of 0.953 (Table 2). Its sensitivity and specificity were 89.9% and 95.5%, respectively (Fig. 4f). Hence the diagnostic efficiency of the combination marker was superior to that of the single chemokine.
Table 2.
ROC analysis of CSF chemokines in HFMD patients with encephalitis.
| Variable | Area | Standard error | P value | 95% CI |
|---|---|---|---|---|
| IL-8/CXCL8 | 0.781 | 0.044 | <0.001 | 0.694–0.867 |
| RANTES/CCL5 | 0.783 | 0.054 | <0.001 | 0.678–0.888 |
| MIG/CXCL9 | 0.869 | 0.033 | <0.001 | 0.804–0.934 |
| MCP-1/CCL2 | 0.716 | 0.047 | 0.002 | 0.624–0.809 |
| IP-10/CXCL10 | 0.876 | 0.033 | <0.001 | 0.812–0.940 |
| Combination marker | 0.953 | 0.018 | <0.001 | 0.917–0.989 |
Figure 4.
ROC analysis of CSF chemokines in the encephalitis and FC groups. (a) IL-8; (b) RANTES; (c) MIG; (d) MCP-1; (e) IP-10; (f) combination marker of five chemokines.
The internal association between chemokines
To corroborate these observations, correlation and interrelation analysis was performed within these CSF chemokines. As shown in Fig. 5, IL-8 was positively correlated with MIG, MCP-1 and IP-10, whereas it was negatively correlated with RANTES. IL-8 was strongly associated with MCP-1 (Fig. 5c). Likewise, MIG, MCP-1 and IP-10 were associated with RANTES (Fig. 5e–g). Of note, there was a strong correlation between MIG and IP-10 (Fig. 5i). Furthermore, we did not find any correlation between MIG and MCP-1 (Fig. 5h). Similarly, there was no significant correlation between MCP-1 and IP-10 (Fig. 5j).
Figure 5.
Interrelation analysis between the chemokines in encephalitis patients depicted as scatter plots.
Discussion
Increasing evidence suggests that cellular and humoral immune dysfunction is involved in the exacerbation of HFMD8–10. Still, the effect of EV71 infection on the host’s adaptive immunity, especially chemokines, is not well understood. In the present study, the chemokines IL-8, RANTES, MIG, MCP-1 and IP-10 were found to be significantly changed in the CSF of HFMD patients in the acute stage of EV71-related encephalitis (Fig. 1). Our finding suggests that chemokines, which function in the selective recruitment of leukocyte populations, are involved in the pathology of the central nervous system (CNS) EV71 infection. The increased expression levels of IL-8, IP-10 and MIG are consistent with those of previous studies8,11. In addition, we found chemokines that were correlated with each other (Fig. 5), in accordance with the result of string bioinformatics analysis12, which showed that these chemokines act in cooperation with each other rather than alone (Supplementary Fig. S1). Likewise, a cytokine storm has been considered to be the main trigger of EV71-related cardiopulmonary collapse13. Taken together, this evidence implied that the dysregulation of the chemokine cascade in the CNS appears to be important regarding the elicitation of the inflammatory response to EV71 infection.
Chemokines are highly involved in the regulation of leukocyte migration and inflammation5, which may provide an explanation for the significant correlation between chemokines and CSF cytology. MIG and IP-10 are Th1 cell attractants, while MCP-1 is Th2 chemokine and RANTES can recruit both Th1 and Th2 cells5. Previous studies have shown that HFMD is a Th1-driven disease4,9. In line with this finding, we also showed that Th1 chemotactic factors (including MIG, IP-10 and RANTES), rather than MCP-1, are potent chemoattractants of Th2 and are significantly elevated in the acute phase. Thus, we assumed that increased CSF MIG, IP-10 and RANTES in children with encephalitis may result in enhancement of Th1 lymphocyte recruitment into the CNS. Accordingly, decreased expression of the Th2 chemokine MCP-1 in encephalitis patients may contribute to a diminished antagonizing effect on Th1 cytokine production and hence intensify Th1 predominance. Interestingly, we revealed that MIG, IP-10 and RANTES were decreased in the recovery phase, while no significant differences were observed for MCP-1. Together, these data imply that Th1 chemoattractants predominate after EV71 infection and contribute to host defense by promoting a protective Th1 response.
MIG and IP-10 are IFN-inducible CXC chemokines and are potent chemoattractants for activated T cells, memory T cells and natural killer cells by signaling through the CXCR3 receptor14. This could explain why MIG was strongly and positively correlated with IP-10, reflecting a temporal co-operation between CXCR3 ligands. An abundance of data demonstrated that MIG and IP-10 are induced in a diverse spectrum of neuroimmune diseases that influence the CNS14. Shen et al. observed that overexpression of IP-10 increased the MIG levels, reduced the viral burden in multiple tissues and increase the survival of mice after EV71 infection15. Consistently, IP-10 and MIG were significantly increased in the present study. Since chemokines are produced by CNS cells within hours of infection5, we suppose that the increased chemokine levels in patients with encephalitis may mediate early regulation and recruitment of protective immunity, reducing tissue damage. Likewise, it was reported that IP-10(−/−) mice infected with a neurotropic mouse hepatitis virus had reduced MIG levels that failed to control viral replication in the brain16. Kuang Y et al. showed that IP-10 enhanced the blood-brain barrier (BBB) and attenuation of rabies virus (RABV)17. This evidence suggests that early expression of IP-10 or MIG within the CNS after virus infection is important in initiating and maintaining a protective immune response. However, it has been implicated that herpes simplex virus (HSV), severe acute respiratory syndrome (SARS) and lymphocytic choriomeningitis virus (LCMV) can aggravate disease18–20. In detail, exaggerated activation of IP-10 and MIG have been proposed to be associated with adverse outcomes (intensive care unit admission or death) of SARS18. Increased secretion of IP-10 in the eye facilitates the spread of HSV to other restricted sites within the eye20. IP-10 regulates the severity of the LCMV-induced inflammatory process, resulting in fatal meningoencephalitis19. Collectively, these studies demonstrate that IP-10/MIG have subtle and perhaps divergent roles in different virus infection.
Of note, when analyzing the CSF levels of IP-10 and MIG, as well as of other potential biomarkers for encephalitis, we found that IP-10 had the highest potential for distinguishing encephalitis patients from FC patients with high sensitivity and specificity, followed by MIG. Similarly, a previous study showed that the CSF to plasma ratio for MIG tended to increase with the increasing severity of disease and that the CSF IP-10 levels in patients with EV71 BE were significantly higher than the plasma levels in control subjects8. Moreover, no established antiviral treatment is available for HFMD. Therefore, our results indicated that IP-10 and MIG might be novel biomarkers to diagnose encephalitis, which may guide patient treatment in clinical practice. Although, the significant difference of IP-10 and MIG had been determined in the CSF, the number of detected cases was not large enough to have power as a solo diagnostic biomarker for encephalitis. Moreover, we performed ROC analysis to analyze the diagnostic efficiency of the markers for encephalitis and showed that the sensitivities of CSF IP-10 and MIG were low (67.7% and 67.7%). However, the combination marker of the five chemokines had a significant elevation of the area under the ROC of 0.953, and its sensitivity in detecting encephalitis was 89.9%. Therefore, the combination of these five chemokines could be applied to clinical diagnosis of encephalitis, which may potentially serve to monitor disease progression, and guide clinical diagnosis and treatment.
The levels of MCP-1, however, presented a different pattern with low concentrations in patients with encephalitis compared to FC patients. Similarly, Wang et al. showed that the ratio of mean CSF to plasma levels for MCP-1 tended to fall with increasing disease severity8. These results suggest that MCP-1 may contribute to the overwhelming disease process. Inversely, it was shown that the plasma MCP levels elevated with increasing disease severity8. This could be explained by the difference in the immune response between the systemic and local inflammatory response. Interesting, although the MCP-1 levels decreased in convalescence, there was no statistically significant difference of the MCP-1 levels between the acute and recovery phases. This may be associated with the small paired sample size. To better elucidate this aspect, it would be interesting to determine the MCP-1 levels over a larger scale and at more than one time-point.
This study was limited by the small paired sample size and single time-point for most cases. Moreover, the control group did not include healthy children without underlying diseases because of ethical issues. Despite the limitations associated with the study group populations, the present findings enhance our understanding of EV71 pathogenesis and provide strategies for a better biomarker/therapeutic target for EV71 infection.
In conclusion, we detected that the CSF levels of four chemokines, IL-8, RANTES, MIG, and IP-10, were significantly elevated in HMFD patients with EV71-related encephalitis. Importantly, the results of the correlation and ROC curve analyses indicated that CSF IP-10 and MIG may have clinical diagnostic values in encephalitis. Additionally, further studies are needed to validate whether IP-10 and MIG could be therapeutic targets for EV71 infection. Thus, our findings provide important insights into the roles of some chemokines in the pathogenesis of EV71 infection.
Materials and Methods
Study subjects
This study was carried out at Children’s Hospital, Zhejiang University School of Medicine and Hangzhou Children’s Hospital between April and August 2013 and was approved by the Ethics Committees of both hospitals. All methods were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the children’s parents or guardians prior to enrolment.
Patients were identified and referred for enrolment by senior physicians after a careful examinations and evaluation. Diagnosis of HFMD was determined based on a popular or vesicular rash on the hands, feet, mouth or buttocks, which was usually accompanied with fever. Encephalitis was defined by the presence of an altered level of consciousness (including lethargy, drowsiness or coma, seizures or myoclonus) with CSF pleocytosis. In addition, patients with simple febrile convulsion (FC) were enrolled as controls. Diagnosis of FC was based on the criteria described previously21. In brief, simple FC was defined as generalized tonic-clonic seizures occurring in the first 48 hrs of a febrile illness and lasting for less than 15 min, but without any focal signs or recurring within 24 hrs, or no associated evidence of intracranial infection or a metabolic disorder21.
Sampling and data collection
At enrolment, throat swabs and rectal swabs were collected from all participants. Meanwhile, a lumbar puncture was taken to obtain an acute phase CSF sample from each patient. All clinical samples from the HFMD patients mentioned above were taken at the same time and within 3 days from HFMD symptom onset. Unfortunately, recovery phase CSF samples were only obtained from 13 HFMD patients with encephalitis. All samples were stored at −80 °C until analysis. All of the clinical data of the participants were collected by reviewing electronic medical records from both hospitals, including age, gender, hematologicalparameters, CSF cytology and CSF biochemical markers.
Real-time RT-PCR
The EV71 genome was detected in throat or rectal specimens by real-time RT-PCR as described previously21. In brief, total RNA was extracted from specimens using a Viral RNA Mini Kit (Qiagen, Hilden, Germany) and reversed transcribed into cDNA with the PrimeScript RT reagent kit (Da An Gene, Guangzhou, China). qPCR reactions based on TaqMan technology were performed using a 7500 real-time RT-PCR system (Applied Biosystems, Foster, CA, USA).
Cytometric bead array analysis of CSF chemokines
A cytometric bead array (CBA), was used to measure a panel of chemokines, namely IL-8, RANTES, MIG, MCP-1 and IP-10. In short, using 50 μl of standard and sample dilutions, the assay was performed according to the manufacturer’s recommendations with the BD CBA Human Chemokine Kit (BD Biosciences, San Diego, CA, USA), and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, CA, USA).
Statistical analysis
Statistical analysis was performed with SPSS software, version 22.0 (SPSS, Chicago, IL, USA). Variables are represented by the mean ± SD for normally distributed data or median and interquartile range for nonparametric distributed data. Accordingly, differences between groups were estimated by Student’s t-test for normal distributed data or the Mann-Whitney U test for nonparametric distributed data. Differences between the acute and recovery phases were analyzed by the paired t-test (normal distribution) or Wilcoxon rank sum test (nonparametric distribution). The correlation coefficient between the selected variables was calculated by Spearman’s rank correlation analysis. Receiver operator characteristic (ROC) curves were constructed to evaluate the performance characteristics of specific CSF chemokines in differentiating HFMD patients with EV71-related encephalitis from FC patients. A two-tailed P value < 0.05 was considered statistically significant.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
We thank all of the patients and guardians enrolled in this study. Additionally, we thank all of the doctors and nurses who participated in this study. The work was supported by grants from the Key Project of Science and Technology of Zhejiang Province (No. 2008C03003–1); the Medical and Health Science and Technology Plan of Zhejiang Province (No. 2016KYB171); the Education Department Project of Zhejiang Province (No. Y201534178).
Author Contributions
Jinling Liu and Shuxian Li were involved in the design and data analysis of this study and drafted the manuscript. Chunyan Cai and Yingchun Xu collected and analyzed the data. Yuan Jiang performed the CBA experiments. Zhimin Chen created the concept and design of this study. Zhimin Chen is the guarantor. All of the authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Jinling Liu and Shuxian Li contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19988-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong SS, Yip CC, Lau SK, Yuen KY. Human enterovirus 71 and hand, foot and mouth disease. Epidemiology and infection. 2010;138:1071–1089. doi: 10.1017/S0950268809991555. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, et al. Enterovirus 71 disrupts interferon signaling by reducing the level of interferon receptor 1. Journal of virology. 2012;86:3767–3776. doi: 10.1128/JVI.06687-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin YW, et al. Lymphocyte and antibody responses reduce enterovirus 71 lethality in mice by decreasing tissue viral loads. Journal of virology. 2009;83:6477–6483. doi: 10.1128/JVI.00434-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SM, et al. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. The Journal of infectious diseases. 2003;188:564–570. doi: 10.1086/376998. [DOI] [PubMed] [Google Scholar]
- 5.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. The New England journal of medicine. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 6.Cavalcanti NV, et al. Chemokine Patterns in Children with Acute Bacterial Infections. Scandinavian journal of immunology. 2016;84:338–343. doi: 10.1111/sji.12492. [DOI] [PubMed] [Google Scholar]
- 7.Takaku Y, et al. CXC chemokine superfamily induced by Interferon-gamma in asthma: a cross-sectional observational study. Asthma research and practice. 2016;2:6. doi: 10.1186/s40733-016-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SM, et al. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. The Journal of infectious diseases. 2008;198:1002–1006. doi: 10.1086/591462. [DOI] [PubMed] [Google Scholar]
- 9.Chang LY, et al. Status of cellular rather than humoral immunity is correlated with clinical outcome of enterovirus 71. Pediatric research. 2006;60:466–471. doi: 10.1203/01.pdr.0000238247.86041.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin TY, et al. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: implications for early recognition and therapy. Acta paediatrica. 2002;91:632–635. doi: 10.1111/j.1651-2227.2002.tb03292.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Comparative study of the cytokine/chemokine response in children with differing disease severity in enterovirus 71-induced hand, foot, and mouth disease. PloS one. 2013;8:e67430. doi: 10.1371/journal.pone.0067430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szklarczyk, D. et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic acids research (2016). [DOI] [PMC free article] [PubMed]
- 13.Lin TY, Hsia SH, Huang YC, Wu CT, Chang LY. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2003;36:269–274. doi: 10.1086/345905. [DOI] [PubMed] [Google Scholar]
- 14.Muller M, Carter S, Hofer MJ, Campbell IL. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity–a tale of conflict and conundrum. Neuropathology and applied neurobiology. 2010;36:368–387. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 15.Shen FH, et al. Enterovirus 71 infection increases expression of interferon-gamma-inducible protein 10 which protects mice by reducing viral burden in multiple tissues. The Journal of general virology. 2013;94:1019–1027. doi: 10.1099/vir.0.046383-0. [DOI] [PubMed] [Google Scholar]
- 16.Dufour JH, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. Journal of immunology. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 17.Kuang Y, Lackay SN, Zhao L, Fu ZF. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus research. 2009;144:18–26. doi: 10.1016/j.virusres.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang NL, et al. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clinical chemistry. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen JE, et al. Fulminant lymphocytic choriomeningitis virus-induced inflammation of the CNS involves a cytokine-chemokine-cytokine-chemokine cascade. Journal of immunology. 2009;182:1079–1087. doi: 10.4049/jimmunol.182.2.1079. [DOI] [PubMed] [Google Scholar]
- 20.Carr DJ, Chodosh J, Ash J, Lane TE. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. Journal of virology. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, et al. Cerebrospinal fluid Th1/Th2 cytokine profiles in children with enterovirus 71-associated meningoencephalitis. Microbiology and immunology. 2015;59:152–159. doi: 10.1111/1348-0421.12227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.