Abstract
The canonical model for netrin1 function proposed that it acted as a long-range chemotropic axon guidance cue. In the developing spinal cord, floor-plate (FP)-derived netrin1 was thought to act as a diffusible attractant to draw commissural axons to the ventral midline. However, our recent studies have shown that netrin1 is dispensable in the FP for axon guidance. We have rather found that netrin1 acts locally: netrin1 is produced by neural progenitor cells (NPCs) in the ventricular zone (VZ), and deposited on the pial surface as a haptotactic adhesive substrate that guides Dcc+ axon growth. Here, we further demonstrate that this netrin1 pial-substrate has an early role orienting pioneering spinal axons, directing them to extend ventrally. However, as development proceeds, commissural axons choose to grow around a boundary of netrin1 expressing cells in VZ, instead of continuing to extend alongside the netrin1 pial-substrate in the ventral spinal cord. This observation suggests netrin1 may supply a more complex activity than pure adhesion, with netrin1-expressing cells also supplying a growth boundary for axons. Supporting this possibility, we have observed that additional domains of netrin1 expression arise adjacent to the dorsal root entry zone (DREZ) in E12.5 mice that are also required to sculpt axonal growth. Together, our studies suggest that netrin1 provides “hederal” boundaries: a local growth substrate that promotes axon extension, while also preventing local innervation of netrin1-expressing domains.
Introduction
Neural circuits are formed during development when axons navigate along defined pathways towards their synaptic targets. Axons use both spatial (Butler and Tear, 2007) and temporal (Phan et al., 2010) guidance signals to orient their trajectories. These cues have been classified as having short-range or long-range activities depending on the distance over which they elicit a response (Dickson, 2002; Tessier-Lavigne and Goodman, 1996). A number of putative long-range cues have been identified, including the netrins (Kennedy et al., 1994; Serafini et al., 1994), semaphorins (Kolodkin et al., 1993; Luo et al., 1993), slits (Brose et al., 1999; Kidd et al., 1999) and morphogens, such as the bone morphogenetic proteins (Augsburger et al., 1999) and sonic hedgehog (Shh) (Charron et al., 2003).
Long-range guidance cues have been the focus of studies in both developing and regenerating neural circuits, based on observations dating back over a century (De Carlos and Borrell, 2007). Cajal first suggested that guidance cues act from intermediate targets, also known as “guidepost” cells, to steer axons in a stepwise manner as they grow toward their synaptic partners (Ramón y Cajal, 1995). Guidepost cells were first identified in grasshoppers (Bentley and Caudy, 1983; Ho and Goodman, 1982), and then in vertebrates (Placzek et al., 1990; Tessier-Lavigne et al., 1988), leading to the textbook model of chemotropic axon guidance whereby an intermediate target orients axons either towards or away from it by secreting a graded attractant or repellent (Tessier-Lavigne and Goodman, 1996). In landmark studies, netrin1 was the first chemotropic guidance cue identified in developing vertebrates, and was proposed to act from the floor plate (FP) to guide spinal commissural axons towards the ventral midline (Evans and Bashaw, 2010; Kennedy et al., 1994; Serafini et al., 1996; Serafini et al., 1994). Short-range cues have also been identified; they include ephrin/Eph signaling (Kao et al., 2012) and factors in the extracellular matrix (ECM) (Barros et al., 2011). Short-range cues have been generally thought to provide local permissive or non-permissive substrates for axon outgrowth, such as the ability of laminin to support the outgrowth of retinal ganglion axons in the optic tract (de Curtis and Reichardt, 1993).
More recent studies have questioned the prevalence of long-range mechanisms in axon guidance. In particular, netrin1, which has homology with the laminin family, has now been shown to act over a shorter range than originally postulated. Membrane-bound netrin1 can rescue the loss of netrinA/B in the Drosophila nerve cord (Brankatschk and Dickson, 2006; Keleman and Dickson, 2001) and visual system (Timofeev et al., 2012). Other studies have suggested a local, adhesive role for netrin, attaching growth cones to source cells in the developing medulla (Akin and Zipursky, 2016). Moreover, our recent studies in the spinal cord (Varadarajan et al., 2017), with those in the hindbrain (Dominici et al., 2017), have revisited the role of FP-derived netrin1 in axon guidance. In the mouse spinal cord, both neural progenitor cells (NPCs) in the ventricular zone (VZ), and FP cells express netrin1 (Serafini et al., 1996) and netrin1 protein decorates both commissural axons and the pial (basal) margin of the spinal cord (Kennedy et al., 2006; Varadarajan et al., 2017). We used conditional genetic approaches in mouse to remove netrin1 expression from either the VZ or the FP. In the absence of netrin1, spinal axons aberrantly innervate the VZ and commissural axons either stall or are dramatically defasciculated. These phenotypes are only observed when netrin1 is ablated from the VZ, but not the FP in both the hindbrain and spinal cord (Dominici et al., 2017; Varadarajan et al., 2017). Thus, the key source of the netrin1 that supplies guidance activities comes from NPCs in the VZ, rather than the FP, as previously suggested.
These studies further suggested that the bipolar geometry of NPCs permits them to establish a local netrin1+ substrate along the pial surface of the spinal cord (Dominici et al., 2017; Varadarajan et al., 2017). This pial-netrin1 substrate promotes commissural axon outgrowth, directing their extension in a fasciculated, i.e. bundled, manner around the VZ and towards the ventral midline. Thus, netrin1 promotes directed axon growth not by long-range chemotaxis, but rather by haptotaxis, the directed growth of cells along an adhesive surface (Carter, 1965; Kennedy et al., 1994). However, these studies were limited to a specific developmental time-window, when commissural axons are in the process of crossing the FP, leaving the extent to which netrin1 behaves as a haptotactic substrate unresolved.
Here, we have examined the role of VZ-derived netrin1, first during early axogenesis at embryonic (E) stage 10.5, when the commissural axons are pioneering their trajectories towards the FP (Bovolenta and Dodd, 1990). We show that netrin1 is required from the earliest stages to orient the trajectory of pioneering commissural axons to grow ventrally in a fasciculated manner around the VZ. Second, we assessed the role of netrin1 at E12.5, the stage by which commissural axons have crossed the FP, and the central branches of sensory axons are about to invade the spinal cord to form the dorsal funiculus (Watanabe et al., 2006). We find that netrin1 continues to be required for commissural axon fasciculation while simultaneously specifying new boundaries for axon growth. Bilaterally symmetric domains expressing netrin1 arise adjacent to the dorsal root entry zone (DREZ) (Watanabe et al., 2006). Here we show that these domains shape the trajectory of axons, by apparently directing axon extension around the edge of a boundary of netrin1 expressing cells. Together, these data suggest that netrin1 reiteratively shapes axonal trajectories at different time points during development by specifying “hederal” guidance boundaries that, like a wall supporting a growing hedera (ivy plant), promotes fasciculated growth along the substrate while preventing penetration of the substrate.
Materials and Methods
Generation of mutant mice
Netrin1lacZ/lacZ mice (Serafini et al., 1996) were bred into 129/Sv backgrounds and maintained as heterozygous mating pairs. This mouse was made using the pGT1.8M vector, which contains a signal sequence that results in the β-gal fusion protein being inserted into the membrane of the endoplas ic reticulu oriented such that the β-gal domain is present, and active in the cytosol (Skarnes et al., 1995).
Embryos were collected from timed matings. The presence of a vaginal plug was considered embryonic day E0.5. Heads were used to isolate the mRNA and cDNA and were amplified by PCR to identify the genotypes of each embryo. All analyses were done using littermate controls. ImageJ was used to quantif the ean intensit of netrin1::βgal immunostaining. Pixel density was measured within a specified region of interest (ROI) and intensity quantifications were not normalized for area. Note that the tissue from different ages was not batch processed and was thus stained on different slides. Therefore, mean intensity plotted depicts the relative decrease along the dorsal-ventral axis at each given age, and does not serve to compare absolute values between ages for a given zone. Hence we have not included a statistical analysis. All animal procedures were carried out in accordance with University of California Los Angeles IACUC guidelines.
Tissue processing
Spinal cords were fixed using 4% paraformaldehyde for 2 hours at 4°C. After fixation, the tissue was cryoprotected in a 30% sucrose solution overnight, following which the tissue was mounted in optimal cutting temperature (OCT) and cryosectioned at 30µm. Sections were collected on slides and processed for immunohistochemistry or in situ hybridization as previously described (Varadarajan et al., 2017).
Immunohistochemistry
The following primary antibodies were used overnight at 4°C: Rabbit: neurofilament (Cell Signaling Technology #C28E10, 1:200), Sox2, Goat: Sox2 (Santa Cruz Biotechnology #17320, 1:2000), human-Robo3 (R&D #AF3076, 1:200), β-galactosidase (1:2000), netrin1 (R&D #AF1109, 1:500); Mouse: neurofilament (DSHB #3A10, 1:100), Sox2 (Santa Cruz Biotechnology E4 #365823, 1:1000), Nkx2.2 (DSHB #74.5 A5-s, 1:100), mAb Tag1 (DSHB #4D7, 1:100) (Dodd et al., 1988); Guinea Pig: Olig2 (Novitch et al., 2001) (1:20,000). Secondary antibodies were incubated for 2 hours at RT. Netrin1 antibody signal was boosted using antigen retrieval methods as described previously (Varadarajan et al., 2017). Note that many antibodies, including Tag1, do not survive the antigen retrieval process.
In situ hybridization
Netrin1 digioxigenin probes (Serafini et al., 1996) were used in in situ hybridization experiments on 12µm thin sections, as described previously (Varadarajan et al., 2017). NBT/BCIP and anti-DIG antibody conjugated to an alkaline phosphatase (Roche) were used to visualize the mRNA.
Results
Axons navigate circumferentially around the ventricular zone
The spinal cord is organized into layers from its earliest genesis: Sox2+ neuroepithelial progenitors within the VZ give rise to postmitotic neurons that migrate laterally to form the mantle layer and extend axons in the lateral marginal zone (Butler and Bronner, 2015). Spinal axons can be broadly categorized by their protein complement: neurofilament (NF) is generally present in spinal axons, with Robo3 additionally being present in the commissural axons projecting towards FP at the ventral midline (Sabatier et al., 2004). The commissural axons that arise from the dorsal-most spinal neurons (dI1s) are also Tag1+ (Dodd et al., 1988).
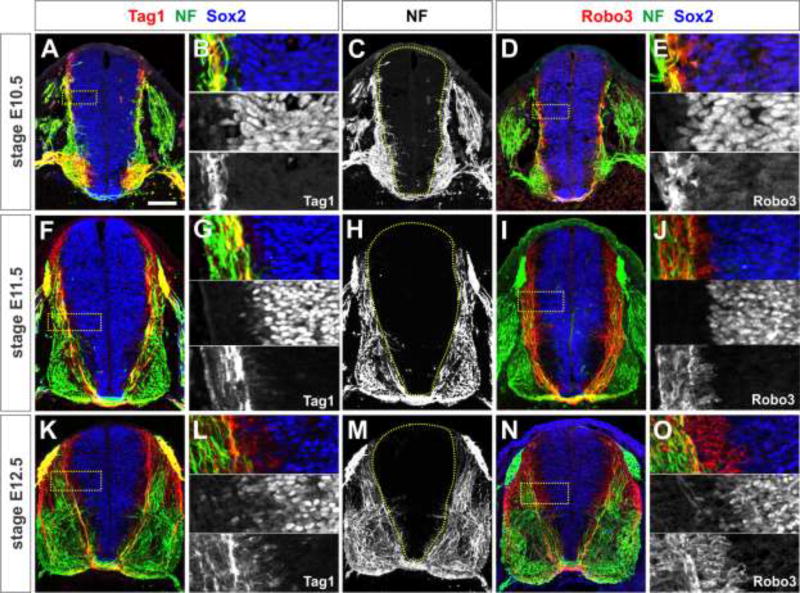
Both NF+ axons, and the Tag1+ and Robo3+ populations of commissural axons respect the boundary of the VZ (Fig. 1). From the earliest stages of spinal axogenesis in E10.5 mice, NF+, Tag1+ and Robo3+ axons are located adjacent to, but segregated from, the cell bodies of Sox2+ NPCs in the VZ (Bylund et al., 2003; Graham et al., 2003) (Fig. 1A–E). By E11.5, when most dorsal commissural axons have reached and crossed the ventral midline, there is an inverse relationship between the neural-progenitor rich VZ and the axon-rich mantle zone (Fig. 1F–J). In particular, Tag1+ and Robo3+ commissural axons grow precisely around the edge of the VZ. This relationship is maintained in E12.5 spinal cords (Fig. 1K–O). Together, these observations suggest that the VZ represents a boundary for commissural axon growth from the earliest stages of axogenesis.
Figure 1. All spinal axons project precisely around the ventricular zone (VZ).
(A–O) Thoracic level sections of E10.5 (A–E), E11.5 (F–J) or E12.5 (K–O) mouse spinal cords labeled with antibodies against Tag1 (red, A, B, F, G, K, L), Robo3 (red, D, E, I, J, N, O), neurofilament (NF, green) and Sox2 (blue).
(A–E) In E10.5 mouse embryos, the earliest axon projections of Tag1+ (A, B) and Robo3+ (D, E) commissural axons avoid the Sox2+ ventricular zone (VZ, dotted line, C). This behavior is also generally observed for NF+ spinal axons (C). High magnification images (inset in A and D shown in B and E respectively) show a precise inverse relationship between Sox2+ progenitors in the VZ and axons in the mantle zone (B, E).
(F–J) All three classes of spinal axons continue to avoid the Sox2+ VZ in E11.5 spinal cords as the commissural axons complete their trajectory to the floor plate (FP) at the ventral midline.
(K–O) This spinal architecture is maintained in E12.5 mouse embryos. Scale bar: 100 µm
Netrin1 is expressed by NPCs in the spinal VZ during axogenesis
Our recent studies demonstrated a role for VZ-derived netrin1 directing the trajectories of spinal neural circuits (Varadarajan et al., 2017). However, the extent to which netrin1 acts to establish and/or maintain spinal architecture during axogenesis remains unresolved. Here, we have assessed the distribution of both netrin1 transcript (Fig. 2A, G, K) and netrin1 protein (Fig. 2D, J, N) from embryonic (E) stage 10.5 when axogenesis commences in the spinal cord, to E12.5, when spinal circuitry is being established. In these studies, we used a genetically encoded marker, β-galactosidase (β-gal) reporter line to provide a highly sensitive readout of the location of any cell expressing the netrin1 gene (netrin1::β-gal; Fig. 2C, I, M).
Figure 2. Distribution of netrin1 transcript and netrin1 protein in the spinal cord during commissural axogenesis.
(A–N) Transverse sections from E10.5 (thoracic: A–D, F; lumbar, E, E’), E11.5 (G–J) or E12.5 (K–N) control (A, D, G, J, K, N), netrin1lacZ/+ (C, E, F, I, M) or netrin1lacZ/lacZ (B, H, L) mouse spinal cords, processed for netrin1 in situ hybridization (A, B, G, H, K and L) or labeled with antibodies against β-galactosidase (β-gal, red C, E–F, I and M), netrin1 (D, J and N), Olig2 (green, E, F) and NF (blue, E). The presence of β-gal in netrin1lacZ/+ embryos can be used as a reporter to assess the domain of cells producing netrin1 transcript. Panels D, J and N were performed with antigen retrieval.
(A–D) At stage E10.5, netrin1 is expressed at high levels by FP cells and at lower levels by NPCs in the VZ. Netrin1 protein is observed broadly in the spinal cord, present in both the VZ and on the pial surface (blue chevrons, D). However, it is excluded from the dorsal-most spinal cord (compared above dotted lines, C, D). Only trace levels of netrin1 expression remains in netrin1lacZ/lacZ spinal cords (arrowhead, B, H and L).
(E, F) Netrin1::β-gal expression is excluded from Olig2+ motor neuron progenitors and the early post-mitotic motor column in lumbar (E, E’) and thoracic (F) sections.
(G–J) B E11.5, netrin1::β-gal continues to be expressed in a graded manner, in a domain that extends from the FP into the dorsal spinal cord (dotted line, I), ending at the dorsal root entry zone (DREZ, blue dotted lines, I, J). As described previously (Varadarajan et al., 2017), netrin1 decorates both the pial surface (blue chevrons, J) as well as commissural axons (yellow chevrons, J).
(K–N) The graded expression of netrin1::β-gal is maintained in the VZ of E12.5 spinal cords. Netrin1 is now also observed in a lateral region of the spinal cord, immediately adjacent to the DREZ (yellow chevrons, K and yellow dotted lines, M,) and on the pial surface (blue chevrons, N).
(O) The spinal cord was divided into four roughly equal zones, zones 1–4, for quantification purposes.
(P) The intensity of netrin1::β-gal levels was measured in zones 1–4. While there are differences in the mean β-gal intensity within a given zone as time progresses, the respective intensity of the β-gal staining in the zones - high FP expression (zone 4), lower intermediate expression (zone 2/3), no dorsal expression (zone 1) - does not change over time (See Methods for more details) (E10.5: n= 45 sections from 2 embryos; E11.5: n= 61 sections from 3 embryos; E12.5: n= 36 sections from 2 embryos).
Scale bar: 65 µm
As described previously (Serafini et al., 1996), netrin1::β-gal is expressed in two distinct regions in the E10.5 spinal cord; it is present at high levels in the FP and at lower levels in a domain in the intermediate VZ (Fig 2A, C). Strikingly, this early bifurcation in the netrin1::β-gal expression pattern may be transiently regulated by motor NPCs in the ventral spinal cord, since netrin1::β-gal expression is markedly lower in the Olig2+ domain (Novitch et al., 2001) (Fig. 2E, F). Netrin1 protein also broadly decorates the ventral and intermediate spinal cord (Fig. 2D) (Kennedy et al., 2006; Varadarajan et al., 2017). It is observed at low levels in the VZ preceding the onset of axogenesis, and is present on the pial surface at the circumference of spinal cord (blue chevrons, Fig. 2D) as neurons start extending axons in the mantle layer (Fig. 3E). Neither netrin1 transcript or netrin1 protein is observed in the dorsal-most spinal cord (compare dotted lines, Fig. 2C, D).
Figure 3. Netrin1 orients the initiation of axonal growth around the circumference of the spinal cord.
(A–T) Lumbar (A, B, E–H) or thoracic (C, D, I–T) level transverse sections of E10.5 netrin1+/+(E, F, K–O), netrin1lacZ/+ (A–D) or netrin1lacZ/lacZ (G–J, P–T) mouse spinal cords labeled with antibodies against NF (green), β-gal (red, A–D, G–J), Robo3 (red, K–L, N, P–Q and S), netrin1 (E–F) and Tag1 (blue, K–L, O, P–Q and T).
(A–F) Ventrally-directed NF+ axon growth starts at a position co-incident with the dorsal-most border of both netrin1::β-gal expression in the VZ domain (chevrons, B, D) and the netrin1 substrate on the pial surface (chevrons, F). Inset in E shown in F.
(G–J) In contrast, NF+ axons grow more randomly in netrin1 mutant spinal cords (I), robustly extending both into zone 1, i.e. above the netrin1::β-gal dorsal boundary (brackets, H, J) and into zone 2, i.e. medially into the VZ (chevrons, H, J).
(K–O) At E10.5, control NF+, Robo3+ and Tag1+ commissural axons are in the process of pioneering their path to the FP at the ventral midline. The motor column and DRGs also express Tag1 at this stage.
(P–T) In netrin1 mutants, NF+ and Robo3+ axons project randomly along the dorsal-ventral axis of the spinal cord (chevrons, Q, R, S). The intermediate spinal cord in K, P is shown magnified in L, Q. Tag1+ (T) and Robo3+ (S) commissural axons additionally show an axon growth defect, either stalled growth (Serafini et al., 1996) or a delay in the initiation of axon growth.
(U) Quantification showed that NF+ axon growth co enced at the netrin1::β-gal dorsal border in >70% of control sections (n=35 sections, 2 mice), whereas there was growth above this border in >60% of sections taken from netrin1 mutant sections (n= 55 sections from 4 mice).
(V) Up to 4-fold more NF+ Robo3+netrin1 mutant growth cones (shown in the inset panel) are observed extending towards the lumen. n as for (W).
(W) Up to 4-fold more NF+ or NF+ Robo3+ axons project into the VZ in netrin1 mutant spinal cords (n=62 sections from 4 embryos) compared to control littermates (n=34 sections from 3 embryos).
(X) The NF+ and NF+ Robo3+ mis-projecting axons were also assigned to four zones within the spinal cord (Fig. 2O), to assess the fold change in axon extension into the netrin1lacZ/lacZ VZ along the dorsal-ventral axis. n as for (W).
For the quantification, probability of similarity between control and mutant, *** p< 0.0005, Student’s t-test.
Scale bar: 130 µm
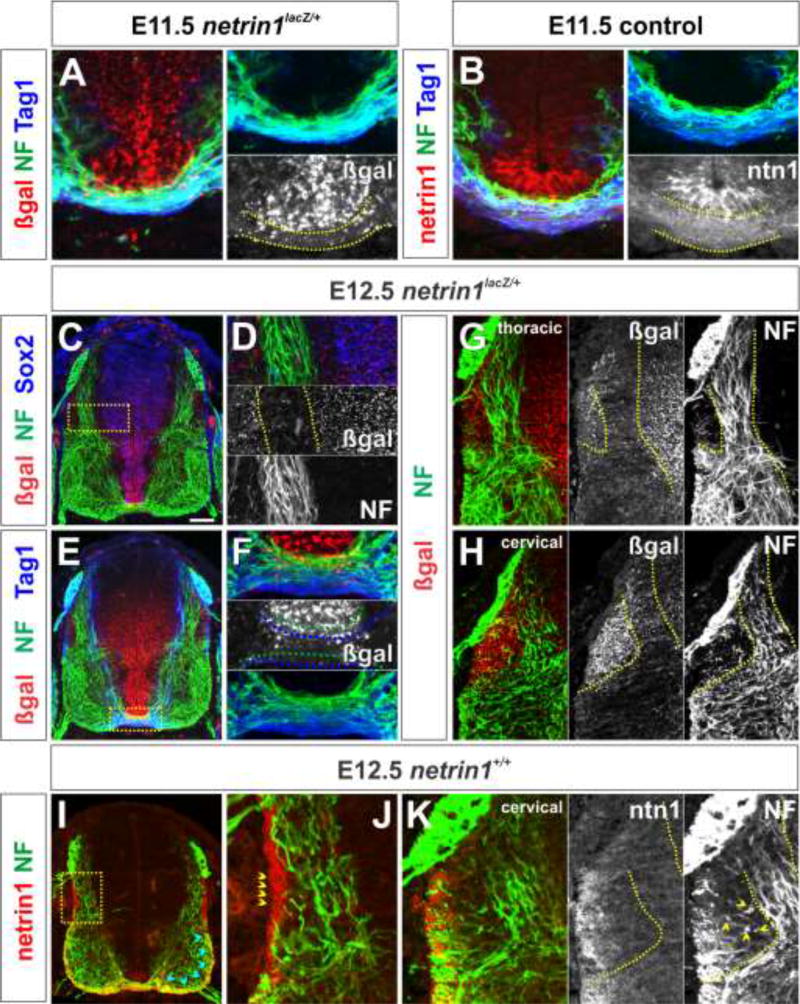
By E11.5, commissural axons are in the process of extending towards and across FP at the ventral midline (Altman and Bayer, 1984) (Fig. 4A, B). Our recent studies (Varadarajan et al., 2017) showed that netrin1::β-gal is now present in a broad swathe throughout the VZ, from the FP to a boundary in the dorsal spinal cord at the same level as the dorsal root entry zone (DREZ, blue dotted region, Fig 2I). In contrast, netrin1 protein has a strikingly different distribution, present on both the pial surface (blue chevrons, Fig. 2J) and on commissural axons (yellow chevrons, Fig, 2J). Our previous studies resolved this discrepancy, suggesting that the netrin1 protein produced by NPCs in the VZ is deposited on the pial surface using their radial processes (Varadarajan et al., 2017). However, these studies did not address how the pattern of netrin1 is maintained as development proceeds.
Figure 4. Spinal axons project precisely around domains of netrin1 expression.
(A–K) Thoracic (A, C–F, G, I–J), lumbar (B) or cervical (H, K) level transverse sections of E11.5 (A) or E12.5 (C–H) netrin1lacZ/+ and E11.5 (B) or E12.5 (I–K) netrin1+/+ mouse spinal cords labeled with antibodies against β-gal (red, A, C–H), netrin1 (red, B, I–K: panel B and I was processed without antigen retrieval), NF (green), Sox2 (blue, C) and Tag1 (blue, A–B, E).
(A, B) By E11.5, NF+ and Tag1+ axons have projected across the FP, at the ventral midline extending precisely underneath the do ain of either βgal, i.e. netrin1 transcript (A) or netrin1 protein present in FP cells (B).
(C–F) By E12.5, NF+ and Tag1+ axons project specifically within the domain where netrin1::β-gal staining is lowest (D), resulting in axons growing around a continuous border of netrin1::β-gal+ cells. This border spans from the dorsal VZ (dotted lines, D) to the apical FP (dotted lines, F). Commissural axons are most fasciculated as the project beneath the do ain of netrin1::β-gal at the FP, and they appear to sort into either Tag1+ (blue dotted lines, F) or NF+ (green dotted lines, F) fasciculated bundles.
(G, H) At E12.5, NF+ spinal axons are also progressively excluded from the DREZ domain of netrin1::β-gal, with NF+ axons curving more markedly as development proceeds to circumvent this region (dotted lines, G, H). DAPI staining (data not shown) reveals no cell density differences between this region and the rest of the mantle layer.
(I–K) Netrin1 protein continues to decorate the pial surface of the spinal cord. NF axons appear to show two responses to pial-netrin1: NF+ axons grow adjacent to pial-netrin1 in the dorsal spinal cord (yellow chevrons, J), but within the pial-netrin in the ventral spinal cord (blue chevrons, I). There is no obvious functional correspondence between the path of NF+ axons around the DREZ-domain and the distribution of netrin1 protein (K). Inset in I shown in J.
Scale bar: 100µm.
To assess this question, we examined the distribution of netrin1 transcript and netrin1 protein in E12.5 spinal cords. Netrin1::β-gal continues to be expressed in the VZ, up to the DREZ boundary (blue dotted region, Fig 2M). Quantification of the extent and intensity of netrin1::β-gal was measured in E10.5-E12.5 embryos within four spatially equivalent zones of the spinal cord (Fig. 2O). This analysis suggested that the relative intensity (see Methods) of β-gal staining in zones 1–4, i.e. high FP expression (zone 4), lower intermediate expression (zone 2/3) and no dorsal expression (zone 1), remains constant (Fig. 2P). Thus, netrin1 expression appears to be stably maintained over time, i.e. this distribution pattern does not result from the perdurance of β-gal. This conclusion is supported b the absence of β-gal tracing into postmitotic spinal neurons. Additionally, as previously reported (Watanabe et al., 2006), bilaterall s etric do ains of netrin1::β-gal expression appear immediately adjacent to DREZ (chevrons, Fig. 2K; dotted lines, Fig. 2M). Netrin1 protein continues to decorate both the pial surface (blue chevrons, Fig. 2N) and commissural axons (yellow chevrons, Fig. 2N). However, netrin1 is now present on two commissural axon fascicles: a medial fascicle bordering the VZ, as well as a second more lateral fascicle that projects across the motor column. Together, these data show that netrin1 continues to be present on both the pial surface and commissural axons while axons are navigating their circumferential trajectories in the spinal cord.
Axons initiate ventrally directed growth adjacent to the pial-netrin1 substrate
Our analysis of the distribution of netrin1 transcript and netrin protein suggests that spinal axon growth initiates specifically in the regions where netrin1 is present on the pial surface of the spinal cord (Fig. 3A–F). Thus, neurons start to extend NF+ axons 70% of the time at the netrin1::β-gal dorsal border (chevrons, Fig. 3B, D, U) on the pial-netrin1 substrate (Fig. 3E, F). We assessed whether netrin1 was required to mediate early axogenesis by examining E10.5 netrin1 mutant embryos. This netrin1 allele stems from lacZ having been inserted into the netrin1 genomic locus (Serafini et al., 1996), thereby generating a hypomorphic allele. However while there are trace amounts of netrin1 expression in the netrin1lacZ/lacZ FP (chevrons, Fig. 2B, H, L) there is no detectable netrin1 transcript in the netrin1lacZ/lacZ VZ at any stage or any detectable netrin1 protein (Poliak et al., 2015; Varadarajan et al., 2017).
Defects are observed from the earliest stages in axogenesis in E10.5 netrin1 mutant embryos (Fig. 3G–J) compared to control littermates (Fig. 3A–F). NF+ axon growth now initiates only ~40% of the ti e on the netrin1::β-gal dorsal border in netrin1lacZ/lacZ embryos (Fig. 3U). The orientation of axons is highly aberrant, with a significant increase in the number of NF+ Robo3+ commissural axons growing into the dorsal-most spinal cord towards the RP (compare brackets, Fig. 3C, D, I, J). Quantification demonstrated that NF+ axons, i.e. spinal axons, project medially into all four zones of the spinal cord in mutants (Fig. 3P, Q, R, W, X), with up to a ~3 fold increase in axons invading the VZ (chevrons, Fig. 3Q) compared to controls (Fig. 3K–M). NF+ Robo3+ axons, i.e. the subset of NF+ axons that are specifically commissural axons, also extend medially into the VZ in all zones of the spinal cord (Fig. 3P, S, W, X). These commissural axons constitute ~50% of the total misprojecting NF+ axons (Fig. 3W). For example, there is a ~9-fold and ~4-fold increase in the number of NF+ Robo3+ axons projecting into zone 1 (chevrons, Fig. 3R, S, X) and zone 2/3 (Fig. 3Q, X) respectively. These neurites appear to be actively extending axons, rather than trailing processes, because numerous Robo3+ growth cones can be observed at the tips of the medially projecting NF+ axons (chevrons, Fig. 3V). Both Robo3+ (Fig. 3S) and Tag1+ (Fig. 3T) axon extension is reduced compared to control littermates (Fig. 3N, O), either because growth is stalled (Serafini et al., 1996), or because the initiation of growth is slowed.
Taken together, these data suggest that netrin1 is required to anchor ventrally directed axon growth from the earliest stages of axogenesis.
Spinal axons continue to avoid netrin1-expressing domains as development proceeds
Our recent studies showed that in E11.5 spinal cords, Robo3+ and Tag1+ commissural axons project in a fasciculated manner around a continuous boundary of netrin1 expressing cells (Varadarajan et al., 2017). Commissural axons grow around the netrin1::β-gal+ VZ and then beneath the netrin1::β-gal+ cells in the FP ((Varadarajan et al., 2017), see also dotted lines, Fig. 4A). The extent of axon fasciculation and level of netrin1 may be correlated; axons are most tightly fasciculated as they pass beneath the concentrated domain of netrin1 in the FP (Fig. 4B).
The netrin1::β-gal+ VZ boundary is maintained in E12.5 embryos, with NF+ (Fig. 4C–F), Robo3+ (Fig. 5K, O), and Tag1+ (Fig. 4E, F) axons continuing to project precisely around the VZ (Fig. 4D) and under the FP (Fig. 4F). Commissural axons continue to be at their most fasciculated as the project beneath the do ain of netrin1::β-gal at the FP. There may be topographic organization to the axons crossing the FP: they are segregated into either Tag1+ (blue dotted lines, Fig. 4F) or NF+ (green dotted lines, Fig. 4F) fasciculated bundles. Similar to E11.5, netrin1 protein continues to decorate the pial surface ventral to the DREZ (blue dotted line, Fig. 2N, yellow chevrons Fig. 4J). However by E12.5, NF+ axons show multiple responses to this domain: pre-crossing NF+ axons in the dorsal spinal cord project immediately adjacent to the pial-netrin1 substrate (Fig. 4I, J), while post-crossing NF+ axons in the ventral spinal cord project within the pial-netrin1 substrate in the ventral funiculus (blue chevrons, Fig. 4I).
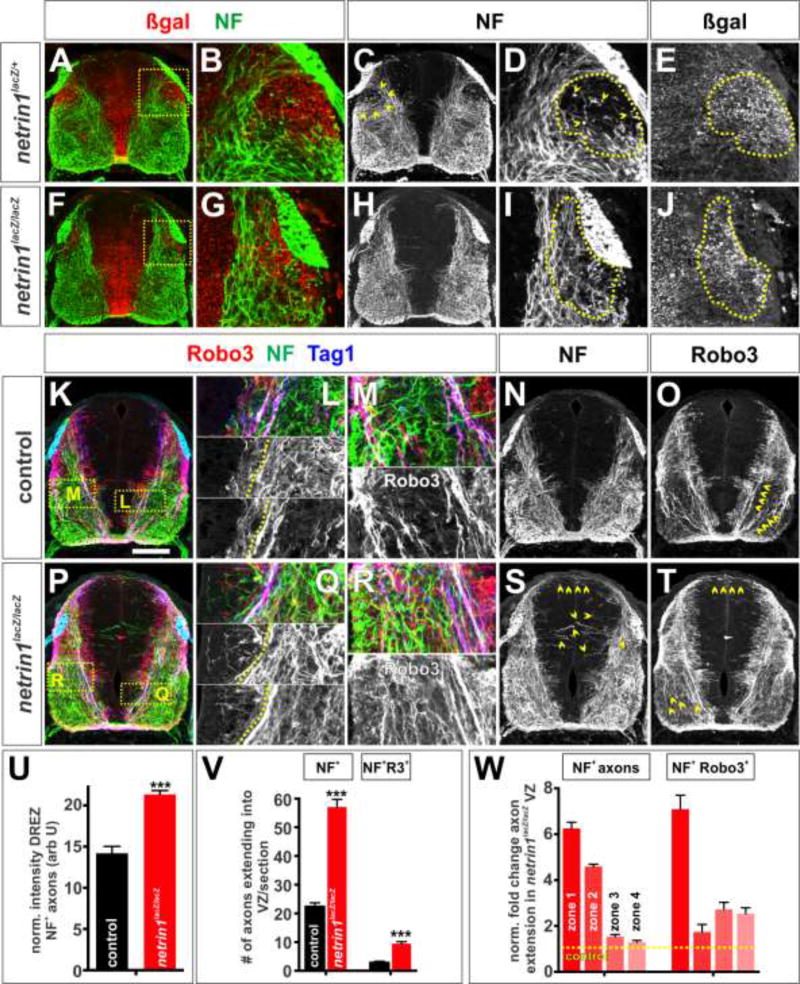
Figure 5. Netrin1 maintains axonal fasciculation and establishes additional boundaries as development proceeds.
(A–T) Brachial (A–J) or thoracic (K–T) level transverse sections of E12.5 netrin1+/+ (K–O), netrin1lacZ/+ (A–E) or netrin1lacZ/lacZ (F-J, P–T) mouse spinal cords labeled with antibodies against NF (green), β-gal (red, A–B, F–G), Robo3 (red, K–M, O–R, T) and Tag1 (blue).
(A–E) In brachial sections, NF+ axons project around the lateral netrin1::β-gal domain below the DREZ (arrowheads, C, dotted lines, D, E). Insets in A and F shown in B, D, E and G, I, J respectively.
(F–J) The sculpting is reduced in netrin1 mutants, with many NF+ axons extending through the putative boundary below the DREZ (dotted lines, I)
(K–O) Control E12.5 NF+ Robo3+ Tag1+ axons continue to observe the VZ boundary (dotted line, L) while the Robo3+ (K, M and O) and Tag1+ (K) axons form two major fascicles, one adjacent to the VZ and the other lateral to it (magnified, M; arrowheads, O). While Robo3+ staining is observed in the DREZ-domain, it appears to label neuronal soma in this region, not axons. However, this staining makes it challenging to assess how the trajectory of Robo3+ axons is affected by the netrin1+ DREZ domain.
(P–T) Netrin1 mutant E12.5 NF+, Tag1+ and Robo3+ axons continue to extend robustly into the VZ (left of the dotted line, Q). The Robo3+ (P, R and T) and Tag1+ (R) fascicles are almost completely defasiculated (magnified, R; arrowheads, T).
(U) Quantification of NF+ intensity levels, normalized for area, in the E12.5 D EZ β-gal zone suggest that there is ~33% increase in axons in the mutants (netrin1lacZ/lacZ, n=131 hemisections from 4 embryos) compared to control (netrin1lacZ/+, n=72 hemisections from 2 embryos) embryos.
(V) Up to 3-fold more NF+ and NF+ Robo3+ axons project into the VZ in E12.5 netrin1 mutant embryos (n=54 sections from 4 embryos) compared to control littermates (n= 29 sections from 3 embryos).
(W) Quantification of fold change in NF+ and NF+ Robo3+axon extension into the netrin1lacZ/lacZVZ along the dorsal-ventral axis. n as for (V).
Probability of similarity between control and mutant, *** p< 0.0005 Student’s t-test.
Scale bar: 145 µm
By E12.5, an additional domain of netrin1 expression has emerged adjacent to the DREZ (Fig. 2K, M). This DREZ-netrin1 domain also appears to sculpt axon growth: first, it may subdivide Tag1+ Robo3+ netrin1+ commissural axons into two fascicles (Laumonnerie et al., 2015). DREZ-netrin1 anchors the point where the fascicles emerge; the medial fascicle continues to grow around the VZ, while the lateral fascicle arises lateral to the DREZ-boundary (yellow chevrons, Fig. 2N, Fig. 5O). Second, it appears to shape NF+ trajectories. NF+ axons start to avoid this region as soon as it appears at thoracic/lumbar levels (Fig. 4G). As development proceeds i.e. in cervical sections, the netrin1::β-gal+ DREZ-domain expands and NF+ axon trajectories in the transverse plane markedly curve to extend around it (Fig. 4H, Fig. 5B). The few axons observed in the DREZ-netrin1 domain appear to be extending longitudinally along the spinal cord (chevrons, Fig. 4K, Fig. 5D). Notably, the domain of netrin1 expression in the DREZ domain appears to demarcate the boundary along which NF+ axons grow, rather than the presence of netrin1 protein at the pial surface (dotted lines, Fig. 4K).
Netrin1 provides additional boundaries for axon growth as development proceeds
Our observations suggest that netrin1 maintains axon fasciculation while also providing boundaries for axon growth. We further assessed this hypothesis by examining E12.5 netrin1lacZ/lacZ embryos. By this stage, the Tag1+ Robo3+ netrin1+ commissural axons have resolved into medial and lateral fascicles in control littermates (chevrons, Fig. 2N, Fig. 5K, M, O). These bundles are highly defasciculated in E12.5 netrin1 mutants (Laumonnerie et al., 2015), projecting randomly throughout the ventral spinal cord (chevrons, Fig. 5P, R, T). NF+ Robo3+ axons continue to extend into the VZ (compare to the left of dotted line, Fig. 5 L, Q; chevrons, Fig. 5S). Quantification demonstrated that NF+ Robo3+ axons project aberrantly into all four zones of the spinal cord (Fig. 5V, W), as seen in both E10.5 (Fig. 3X) and E11.5 (Varadarajan et al., 2017) spinal cords. However the frequency of NF+ Robo3+ axons invading the VZ boundary declines compared to E10.5 and E11.5 embryos suggesting that this phenotype might ultimately be transient for commissural axons. Finally, we assessed the ability of the bilaterally symmetric netrin1+ DREZ domain to shape the trajectory of NF+ axons. While axons grow around this region in control littermates (Fig. 5A–E), NF+ axons no longer respect this putative boundary in netrin1 mutants; rather they profusely extend across it (Fig. 5F–J, U).
Taken together, this analysis supports the hypothesis that netrin1 is a major architect of spinal axon growth. Axon growth appears to be shaped by both the contours of the region producing netrin1 transcript and the presence of netrin1 protein at the pial surface and on axons.
Discussion
Netrin1 boundaries reiteratively direct the formation of spinal neural circuits
Netrin1 was identified in tour de force biochemical screens of soluble factors from chicken brain extracts that promote axon outgrowth (Kennedy et al., 1994; Serafini et al., 1994). Through these experiments, netrin1 became the canonical example of a diffusible chemoattractant, proposed to act from the FP to guide commissural axons towards the ventral midline. However, our recent studies have rather suggested that the NPCs are the key source of netrin1 that provide guidance cues in the spinal cord. We showed that NPCs establish netrin1 as a haptotactic surface at the pial surface of the spinal cord to direct axons towards the ventral midline (Varadarajan et al., 2017). Similar results were found in the hindbrain (Dominici et al., 2017).
Here, we provide further evidence of the importance of netrin1 specifying spinal neural circuitry. Our current studies demonstrate that netrin1 supplies a haptotactic surface from the beginning of axogenesis that directs fasciculated axon growth (Fig. 6A). However, axons respond in additional, more complex ways to netrin1 as development progresses. Axons have to choose whether to extend alongside or within a netrin1 haptotactic surface and/or use netrin1-expressing cells as a boundary for growth (Fig. 6 B, C).
Figure 6. Netrin1 specifies “hederal” boundaries.
(A) Netrin1 first orients ventrally-directed growth of axons in E10.5 embryos. Netrin1 protein (green) deposited on the pial surface (Varadarajan et al., 2017) promotes axon extension while cells expressing netrin1 (red) in the VZ are refractory to axon growth.
(B, C) As development progresses in E11.5 and E12.5 embryos, pre-crossing commissural axons grow adjacent to pial-netrin1 in the dorsal spinal cord (dark green) while avoiding pial-netrin1 in the ventral spinal cord (light green). After crossing the FP, commissural axons turn rostrally to extend in the pial-netrin1 in the ventral funiculus (light green). Concomitantly, netrin1 expressing cells (red) appear to provide guidance boundaries in the VZ and in a domain adjacent to the DREZ (C). These boundaries promote axon fasciculation while preventing innervation, such that axons grow along the edges of netrin1 expression. Together, these activities sculpt axonal trajectories within the spinal cord.
Netrin1 mediates the orientation of axogenesis
Netrin1 is present at the pial surface prior to the onset of axogenesis (Fig. 6A), with a sharp boundary of netrin1 deposition in the dorsal spinal cord (Fig. 3E, F). This netrin1-pial substrate is required to orient the first trajectories of pioneering axons. Neurons extend ventrally oriented fasciculated axons specifically on pial-netrin1, from the earliest stages of axogenesis (Fig. 3A, B). In the absence of netrin1, neurons initiate NF+ axon extension. However, this growth is randomized; defasciculated axons grow in all directions, including into the VZ. The NF+ axon population includes a subset of Robo3+ commissural axons; these NF+ Robo3+ axons are also defasciculated in netrin1 mutants. However Robo3+ Tag1+ commissural axons, i.e. the dorsal-most commissural axons (Dodd et al., 1988), show an alternative phenotype: their growth is generally slowed or stalled (Serafini et al., 1996). The initiation of Tag1+ axon growth appears to be the most compromised, perhaps explaining why Tag1+ axon growth is not as disordered as NF+ Robo3+ axon growth. Thus from early axogenesis, spinal axons have distinct responses to the pial-netrin1 substrate: pial-netrin1 is an anchor that orients the directed growth of NF+ and NF+ Robo3+ axons, while also being a growth promoting substrate for Tag1+ Robo3+ axons.
Axons alter their responses to pial-netrin1 depending on location
As development proceeds, spinal axons appear to modulate their response to pial-netrin1. By E11.5, commissural axons have detached from the pial surface in the ventral spinal cord (light green, Fig. 6B) to extend in an increasingly fasciculated manner around the VZ to the FP. After crossing the FP, commissural axons now grow within the pial-netrin1 substrate, to extend along ventral funiculus. This behavior is most pronounced in thoracic E12.5 sections, where precrossing NF+ commissural axons grow immediately adjacent to dorsal pial-netrin1 (Fig. 4J) while post-crossing NF+ axons grow within the ventral pial-netrin1 (blue chevrons, Fig. 4I).
For technical reasons (see Methods), it has been easiest to determine the trajectories of NF+ axons in relationship to pial-netrin1. However, the behavior of Robo3+ Tag1+ commissural axons is consistent with the model that all dorsal spinal axons grow adjacent to pial netrin1 (dark green, Fig. 6B). Axons then modulate their response to netrin1 in the ventral spinal cord. Robo3+ Tag1+ commissural axons are first refractory to ventral pial-netrin1, as they first grow adjacent to the motor column, but are then putatively attracted to pial-netrin1 once on the contralateral side of the spinal cord. This changing response suggests further regulation exists to permit axons to decide how and when to respond appropriately to pial-netrin1. Candidate regulators include the previously identified factors that regulate commissural axon orientation, such as the BMPs (Butler and Dodd, 2003), Shh (Charron et al., 2003), Wnts (Lyuksyutova et al., 2003) and Eph/ephrins (Kadison et al., 2006).
Axons grow around netrin1-expressing domains, or hederal boundaries
Our studies are also consistent with the model that netrin1-expressing domains have a key role shaping the trajectory of spinal axons. We have identified two examples of this phenomenon in the spinal cord. First, commissural axons grow around a boundary of netrin1-expressing cells in VZ from early axogenesis. Our previous studies used manipulations of the notch signaling pathway to make ectopic boundaries of netrin1 expression in the VZ. NF+ axons deviated from their normal trajectories to follow the ectopic netrin1 (on): netrin1 (off) boundaries, with no obvious disruption in the distribution of pial-netrin1 (Varadarajan et al., 2017). Second, we have observed that a subset of NF+ axons extends precisely around a domain of netrin1 expression that emerges at E12.5 adjacent to DREZ (this study). Strikingly, there is no obvious correlation between the deposition of netrin1 protein and the pattern of axon growth in this region. Directed NF+ axon growth around the DREZ-domain is lost in netrin1 mutants. These boundary activities appear to be a co bination of a “go” signal – axons grow along the boundary of the netrin1 expressing cells - and a “no go” activit - axons do not grow into netrin1-expressing regions. We propose to refer collectively to these activities as “hederal” guidance boundaries, taken from the analogy of a wall supporting the growth of ivy (genus: hedera). The wall provides a substrate for growth, but is not itself penetrated by the ivy. It remains unresolved how widely hederal boundaries act to establish neural circuits, however further putative examples have been observed in both the central and peripheral nervous systems (Burrill and Easter, 1994; Poliak et al., 2015).
The mechanism that mediates hederal boundaries remains unresolved. It is notable that the VZ and DREZ boundaries can affect the patterns of netrin1+ axon fasciculation. We previously showed that netrin1 accumulation in axons is dramatically reduced in Dcc−/− (Varadarajan et al., 2017), suggesting that Dcc may play an important role in specifying how axons interpret the hederal boundary. One possibility is that Dcc+ commissural axons accumulate netrin1 when growing adjacent to the pial-netrin1 haptotactic surface. Netrin1+ Dcc+ axons are then able to extend in directed, fasciculated manner around the VZ (Varadarajan et al., 2017). Supporting this hypothesis, the occasional axons that grow into the VZ in control spinal cords are always netrin1− (data not shown). Similarly the DREZ-domain may control the fasciculation pattern of netrin1+ Tag1+ Robo3+ commissural axons at later stages. The appearance of the netrin1-DREZ domain coincides with these commissural axons splitting into two fascicles. However, the NF+ axons that grow around the netrin1-DREZ domain do not obviously accumulate netrin1 protein (Fig 4K), suggesting that this response is mechanistically distinct. The NF+ Robo3+ and Tag1+ Robo3+ populations of commissural axons also fasciculate in a distinct topological manner, as they extend towards and across the FP (Fig. 4F), additionally suggesting that these populations have differential sensitivities to netrin1.
In summary, our studies demonstrate that netrin1 has a reiterative role defining the spatial boundaries that guide spinal axon trajectories. Similar growth-boundaries are likely to direct to axon growth outside of the spinal cord. Motor axons notabl grow alongside netrin1::βgal boundaries as they innervate the limb (Poliak et al., 2015). Netrin1 has also been implicated in the neuronal responses after spinal cord injury (Manitt et al., 2006); a mechanistic understanding of its capacity to direct axon growth is critical if it is to be deployed correctly in therapeutic regenerative contexts.
Highlights.
Multiple netrin1-mediated hederal boundaries arise in the early spinal cord
A haptotactic netrin1+ substrate directs pioneering spinal axons to grow ventrally
Netrin1-expressing, not netrin1+, domains, may promote fasciculation and prevent local innervation
Spinal axons grow on different netrin1+ substrates as development proceeds
Acknowledgments
We thank Keith Phan and Anna Maglunog for technical help and reagents; Marc Tessier-Lavigne for generous gifts of mutant mice. We would also like to thank Sandy Alvarez, Bennett Novitch and Caroline Pearson for helpful discussions and comments on the manuscript. This work was supported by grants from the March of Dimes (1-FY07-458), the NIH (NS063999 and NS085097) and the UCLA Broad Stem Cell Research Center to Samantha Butler and a UCLA Graduate Division Dissertation Year Fellowship to Supraja Varadarajan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akin O, Zipursky SL. Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system. eLife. 2016;5:e20762. doi: 10.7554/eLife.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. The development of the rat spinal cord. 1984 doi: 10.1007/978-3-642-69537-7. [DOI] [PubMed] [Google Scholar]
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–141. doi: 10.1016/s0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- Barros CS, Franco SJ, Muller U. Extracellular matrix: functions in the nervous system. Cold Spring Harbor perspectives in biology. 2011;3:a005108. doi: 10.1101/cshperspect.a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D, Caudy M. Pioneer axons lose directed growth after selective killing of guidepost cells. Nature. 1983;304:62–65. doi: 10.1038/304062a0. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Dodd J. Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development. 1990;109:435–447. doi: 10.1242/dev.109.2.435. [DOI] [PubMed] [Google Scholar]
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Easter SS., Jr Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio) J Comp Neurol. 1994;346:583–600. doi: 10.1002/cne.903460410. [DOI] [PubMed] [Google Scholar]
- Butler SJ, Bronner ME. From classical to current: analyzing peripheral nervous system and spinal cord lineage and fate. Dev Biol. 2015;398:135–146. doi: 10.1016/j.ydbio.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- Butler SJ, Tear G. Getting axons onto the right path: the role of transcription factors in axon guidance. Development. 2007;134:439–448. doi: 10.1242/dev.02762. [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Carter SB. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965;208:1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- De Carlos JA, Borrell J. A historical reflection of the contributions of Cajal and Golgi to the foundations of neuroscience. Brain research reviews. 2007;55:8–16. doi: 10.1016/j.brainresrev.2007.03.010. [DOI] [PubMed] [Google Scholar]
- de Curtis I, Reichardt LF. Function and spatial distribution in developing chick retina of the laminin receptor alpha 6 beta 1 and its isoforms. Development. 1993;118:377–388. doi: 10.1242/dev.118.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P, Chedotal A. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature. 2017;545:350–354. doi: 10.1038/nature22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ. Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol. 2010;20:79–85. doi: 10.1016/j.conb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Ho RK, Goodman CS. Peripheral pathways are pioneered by an array of central and peripheral neurones in grasshopper embryos. Nature. 1982;297:404–406. doi: 10.1038/297404a0. [DOI] [PubMed] [Google Scholar]
- Kadison SR, Makinen T, Klein R, Henkemeyer M, Kaprielian Z. EphB receptors and ephrin-B3 regulate axon guidance at the ventral midline of the embryonic mouse spinal cord. J Neurosci. 2006;26:8909–8914. doi: 10.1523/JNEUROSCI.1569-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TJ, Law C, Kania A. Eph and ephrin signaling: lessons learned from spinal motor neurons. Semin Cell Dev Biol. 2012;23:83–91. doi: 10.1016/j.semcdb.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–617. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Laumonnerie C, Tong YG, Alstermark H, Wilson SI. Commissural axonal corridors instruct neuronal migration in the mouse spinal cord. Nature communications. 2015;6:7028. doi: 10.1038/ncomms8028. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Manitt C, Wang D, Kennedy TE, Howland DR. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J Neurosci Res. 2006;84:1808–1820. doi: 10.1002/jnr.21070. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Phan KD, Hazen VM, Frendo M, Jia Z, Butler SJ. The bone morphogenetic protein roof plate chemorepellent regulates the rate of commissural axonal growth. J Neurosci. 2010;30:15430–15440. doi: 10.1523/JNEUROSCI.4117-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek M, Tessier-Lavigne M, Jessell T, Dodd J. Orientation of commissural axons in vitro in response to a floor plate-derived chemoattractant. Development. 1990;110:19–30. doi: 10.1242/dev.110.1.19. [DOI] [PubMed] [Google Scholar]
- Poliak S, Morales D, Croteau LP, Krawchuk D, Palmesino E, Morton S, Cloutier JF, Charron F, Dalva MB, Ackerman SL, Kao TJ, Kania A. Synergistic integration of Netrin and ephrin axon guidance signals by spinal motor neurons. eLife. 2015;4 doi: 10.7554/eLife.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histology of the nervous system of man and vertebrates. Oxford University Press; New York: 1995. [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Skarnes WC, Moss JE, Hurtley SM, Beddington RS. Capturing genes encoding membrane and secreted proteins important for mouse development. Proc Natl Acad Sci U S A. 1995;92:6592–6596. doi: 10.1073/pnas.92.14.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Timofeev K, Joly W, Hadjieconomou D, Salecker I. Localized netrins act as positional cues to control layer-specific targeting of photoreceptor axons in Drosophila. Neuron. 2012;75:80–93. doi: 10.1016/j.neuron.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan SG, Kong JH, Phan KD, Kao TJ, Panaitof SC, Cardin J, Eltzschig H, Kania A, Novitch BG, Butler SJ. Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron. 94:790–799. e793. doi: 10.1016/j.neuron.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Tamamaki N, Furuta T, Ackerman SL, Ikenaka K, Ono K. Dorsally derived netrin 1 provides an inhibitory cue and elaborates the 'waiting period' for primary sensory axons in the developing spinal cord. Development. 2006;133:1379–1387. doi: 10.1242/dev.02312. [DOI] [PubMed] [Google Scholar]