Key Points
Question
Is cerebral amyloid-β aggregation, a key characteristic of Alzheimer disease, associated with cognitive functioning in persons without dementia?
Findings
In this cross-sectional, multicenter study of 7041 persons without dementia, amyloid-positive persons with normal cognition had low episodic memory scores almost twice as often as persons without amyloid aggregation after the age of 70 years. Low memory scores emerged 10 to 15 years after the onset of amyloid positivity.
Meaning
Alzheimer disease manifests through low memory scores in elderly persons with normal cognition, but a low memory score has limited value as a screening tool for early Alzheimer disease.
Abstract
Importance
Cerebral amyloid-β aggregation is an early event in Alzheimer disease (AD). Understanding the association between amyloid aggregation and cognitive manifestation in persons without dementia is important for a better understanding of the course of AD and for the design of prevention trials.
Objective
To investigate whether amyloid-β aggregation is associated with cognitive functioning in persons without dementia.
Design, Setting, and Participants
This cross-sectional study included 2908 participants with normal cognition and 4133 with mild cognitive impairment (MCI) from 53 studies in the multicenter Amyloid Biomarker Study. Normal cognition was defined as having no cognitive concerns for which medical help was sought and scores within the normal range on cognitive tests. Mild cognitive impairment was diagnosed according to published criteria. Study inclusion began in 2013 and is ongoing. Data analysis was performed in January 2017.
Main Outcomes and Measures
Global cognitive performance as assessed by the Mini-Mental State Examination (MMSE) and episodic memory performance as assessed by a verbal word learning test. Amyloid aggregation was measured with positron emission tomography or cerebrospinal fluid biomarkers and dichotomized as negative (normal) or positive (abnormal) according to study-specific cutoffs. Generalized estimating equations were used to examine the association between amyloid aggregation and low cognitive scores (MMSE score ≤27 or memory z score≤−1.28) and to assess whether this association was moderated by age, sex, educational level, or apolipoprotein E genotype.
Results
Among 2908 persons with normal cognition (mean [SD] age, 67.4 [12.8] years), amyloid positivity was associated with low memory scores after age 70 years (mean difference in amyloid positive vs negative, 4% [95% CI, 0%-7%] at 72 years and 21% [95% CI, 10%-33%] at 90 years) but was not associated with low MMSE scores (mean difference, 3% [95% CI, −1% to 6%], P = .16). Among 4133 patients with MCI (mean [SD] age, 70.2 [8.5] years), amyloid positivity was associated with low memory (mean difference, 16% [95% CI, 12%-20%], P < .001) and low MMSE (mean difference, 14% [95% CI, 12%-17%], P < .001) scores, and this association decreased with age. Low cognitive scores had limited utility for screening of amyloid positivity in persons with normal cognition and those with MCI. In persons with normal cognition, the age-related increase in low memory score paralleled the age-related increase in amyloid positivity with an intervening period of 10 to 15 years.
Conclusions and Relevance
Although low memory scores are an early marker of amyloid positivity, their value as a screening measure for early AD among persons without dementia is limited.
This cross-sectional, multicenter amyloid biomarker study investigates whether amyloid-β aggregation is associated with cognitive functioning in persons without dementia.
Introduction
Cerebral amyloid-β aggregation is an early pathologic event in Alzheimer disease (AD), starting 2 to 3 decades before dementia onset.1,2 Approximately 25% of cognitively normal elderly individuals and 50% of patients with mild cognitive impairment (MCI) have biomarker evidence of amyloid pathology.1,3 These persons are at increased risk for developing AD-type dementia,1 but the extent to which amyloid-β aggregation is associated with cognition in persons without dementia is unclear. Understanding the association between amyloid pathology and cognitive functioning is important for a better understanding of the course of AD and for the design of AD-prevention trials.
Longitudinal cohort studies4,5,6 have revealed an association between the presence of amyloid pathology and long-term cognitive decline. To assess the role of cognitive screening as a tool to enrich AD clinical trials, the cross-sectional association also needs to be established. However, findings from cross-sectional studies in cognitively normal individuals4,7,8 and patients with MCI7,9,10 have been inconsistent. This variability may be caused by differences among studies in demographic and genetic factors, disease stage and reserve capacity, and methodologic approaches. Effects are mainly observed in the episodic memory and global cognition domains and tend to be small,11 indicating that large samples are needed to investigate the amyloid-cognition association.
We previously estimated the prevalence of amyloid positivity in persons without dementia and, in the present study, investigated the association between amyloid positivity and cognitive scores in this population by using individual participant data from 53 studies included in the multicenter Amyloid Biomarker Study.1 We also examined whether age, cognitive status, sex, educational level, and apolipoprotein E (APOE) genotype modify the association between amyloid status and global cognition or episodic memory; estimated temporal associations among amyloid positivity, low memory scores, and AD-type dementia; and tested the usefulness of cognitive scores as a screening instrument for amyloid positivity.
Methods
Participants
Participants were recruited from studies that participated in the multicenter Amyloid Biomarker Study1 on establishing the prevalence of amyloid pathology measured with a positron emission tomography (PET) or cerebrospinal fluid (CSF) biomarker. For details on study selection and data collection, see Jansen et al.1 Study inclusion began in 2012 and is ongoing. At time of analysis (January 2017), we included participant-level data from 2908 participants with normal cognition and 4133 patients with MCI from 53 studies that had data available on the Mini-Mental State Examination (MMSE) and/or an episodic memory score. Participants with normal cognition had no cognitive concerns for which medical help was sought and scored within the normal range on cognitive tests. The diagnosis of MCI was made according to published criteria, including subjectively experienced and objectively verified decline in memory or another cognitive domain. Characteristics of the included studies are given in eTable 1 in the Supplement. All participants gave written informed consent to participate, and data were deidentified. Study protocols were approved by the local ethics committees of all centers participating in the Amyloid Biomarker Study.
Cognitive Tests
Global cognition was assessed with the MMSE.12 The MMSE scores were available from 53 studies comprising a total of 2885 participants with normal cognition (38 studies) and 4133 patients with MCI (48 studies). A low MMSE score was defined as 27 or less.
Episodic memory was assessed with verbal word learning tests. Data were available from 31 studies including 2010 participants with normal cognition (21 studies) and 2615 patients with MCI (26 studies). Most studies provided raw scores (28 studies) that were transformed into z scores using the mean and SD of the center-specific group of cognitively unimpaired individuals or, in the absence of such a group, using published test-specific means. Three studies provided z scores that were calculated similarly. Episodic memory was assessed using 12 different verbal memory tests of immediate or delayed recall. When multiple memory test scores were provided for a participant, the delayed recall score was chosen. Tests of delayed recall were used for 2809 participants (21 studies) and tests of immediate recall for 1816 participants (10 studies). See eTable 2 in the Supplement for an overview of the memory tests used in each study. In our analyses, low episodic memory performance was defined as a z score of −1.28 or less, capturing performance in the 10th percentile or lower of the population mean.
Amyloid Assessment
In the sample selected for these analyses, amyloid pathology was assessed with amyloid-PET in 1224 participants with normal cognition (21 studies) and 956 patients with MCI (23 studies) and by amyloid-β1-42 level in CSF in 1684 participants with normal cognition (19 studies) and 3177 with MCI (29 studies). Measurement details have been described previously.1 The PET and CSF biomarkers were dichotomized as negative (normal) or positive (abnormal) according to study-specific cutoffs or visual reads.
Data Availability
Information on the level of education was available for 2558 participants (88.0%) with normal cognition (memory score: n = 1973; MMSE: n = 2536) and 3270 patients (79.1%) with MCI (memory score: n = 2456; MMSE: n = 3264). Information on APOE-ε4 carrier status (yes or no) was available for 2400 participants (82.5%) with normal cognition (memory score: n = 1764; MMSE: n = 2379) and 3292 patients (79.7%) with MCI (memory score: n = 2217; MMSE: n = 3286). The APOE genotype was available for 2347 participants (80.7%) with normal cognition (memory score: n = 1763; MMSE: n = 2326) and 3019 patients (73.0%) with MCI (memory score: n = 2215; MMSE: n = 3013).
Temporal Association Among Amyloid Positivity, Low Memory Scores, and AD-Type Dementia
To provide an estimation of the temporal associations among amyloid positivity, low memory scores, and AD-type dementia, we compared age-specific frequency estimates of low memory scores and amyloid positivity in participants with normal cognition with age-specific prevalence data of AD-type dementia in the general population (adopted from Jansen et al1).
Cognitive Screening as an Indication of Amyloid Status
To investigate the screening potential of low cognitive scores, we calculated the odds of low cognitive performance for amyloid positivity and examined improvement in area under the receiver operating characteristic curve when cognitive scores were added to a model with age and APOE-ε4 carrier status.
Statistical Analysis
Differences in clinical and demographic characteristics between the amyloid-positive and amyloid-negative subgroups were analyzed using analyses of variance for continuous variables and χ2 tests for categorical variables. Dichotomized MMSE and memory scores (low vs normal) were used as outcome variables in generalized estimating equations. Generalized estimating equations allow for the analysis of binary correlated data such that participant-level data from all studies could be modeled simultaneously while accounting for the clustering of participants within studies. We assumed a logit link function for binary outcome with an exchangeable correlation structure to account for within-study correlation. Age was included as a continuous measure and was centered at the median. Educational level was dichotomized at the median (high [≥14 years] vs moderate to low [<14 years]). For each outcome measure, we performed the following 6 models. The first model included amyloid pathology (present or absent), cognitive status (normal cognition or MCI), age, interactions among these 3 variables as predictors, and sex and educational level as covariates. In the second, third, and fourth models, we added sex (model 2), educational level (model 3), and APOE-ε4 carrier status (model 4) with up to 3-way interactions of these variables with amyloid pathology, cognitive status, and age. In the fifth model, we entered all variables and included up to 3-way interactions among age, sex, educational level, APOE-ε4 carrier status, cognitive status, and amyloid pathology using a forward selection method. In the sixth model, we examined whether APOE genotype (coded as ε4ε4, ε2ε4/ε3ε4, ε3ε3, or ε2ε2/ε2ε3) modified the association among amyloid, age, and cognition while correcting for sex and educational level. Terms were retained in the equation in case of a significant Wald statistic (P < .05). For an overview of significant terms in each of the models tested, see eTable 3 in the Supplement. We report estimates corrected for age, sex, and educational level in the text. Models unadjusted for sex and educational level yielded similar results and were used to display estimates in Figures 1, 2, 3 and Table 1 and Table 2. Associations did not change after correcting for multiple comparisons with the Bonferroni method. Secondary analyses are described in eAppendix 1 and eFigure3 in the Supplement. Analyses were conducted with SPSS statistical software, version 22.0 (IBM Corp), with a significance level set at P < .05 for unpaired, 2-sided tests.
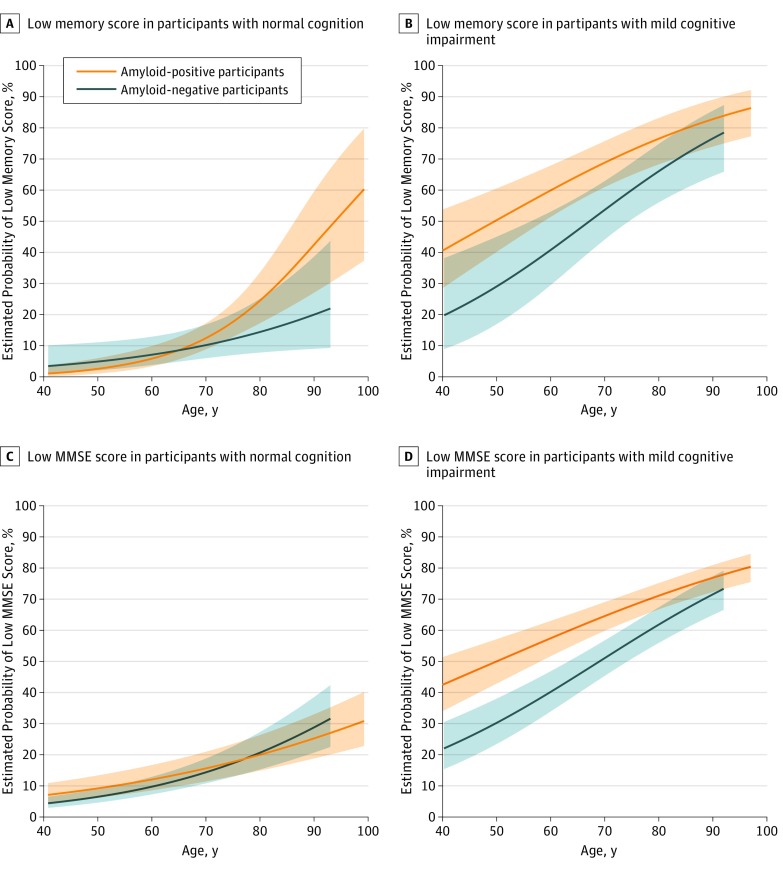
Figure 1. Frequencies of Low Memory and Low Mini-Mental State Examination (MMSE) Scores.
A and B, Model with terms for age, amyloid status, and cognitive status and up to 3-way interactions among these terms (3-way interaction P = .002). C, and D, Model with terms for age, amyloid status, cognitive status, and interactions between amyloid status and age (P = .03) and between amyloid status and cognitive status (P < .001). Adjustment for sex and educational level did not change either model. Low score was defined as 10th percentile or lower of z scores for the memory score and 27 or lower for MMSE scores. Shading indicated 95% CIs.
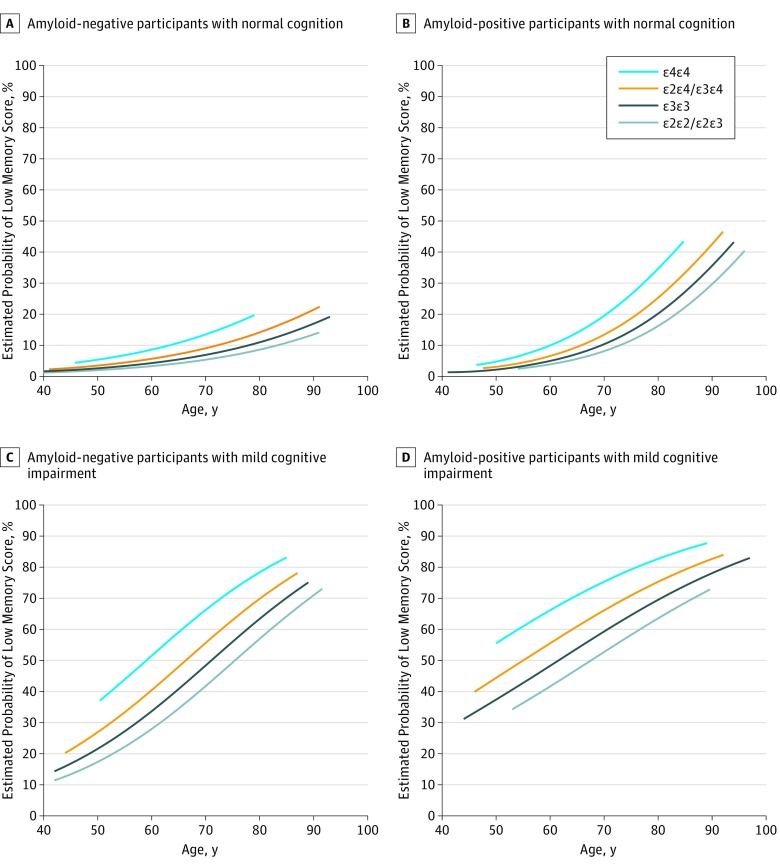
Figure 2. Frequency of Low Memory Score According to Apolipoprotein E (APOE) Genotype.
Model with terms for age, amyloid status, cognitive status, and up to 3-way interactions among these terms (3-way interaction P = .007), APOE genotype, and interactions among APOE genotype, amyloid status, and cognitive status (P = .02). Adjustment for sex and educational level did not change the model. Low score was defined as 10th percentile or lower of z scores.
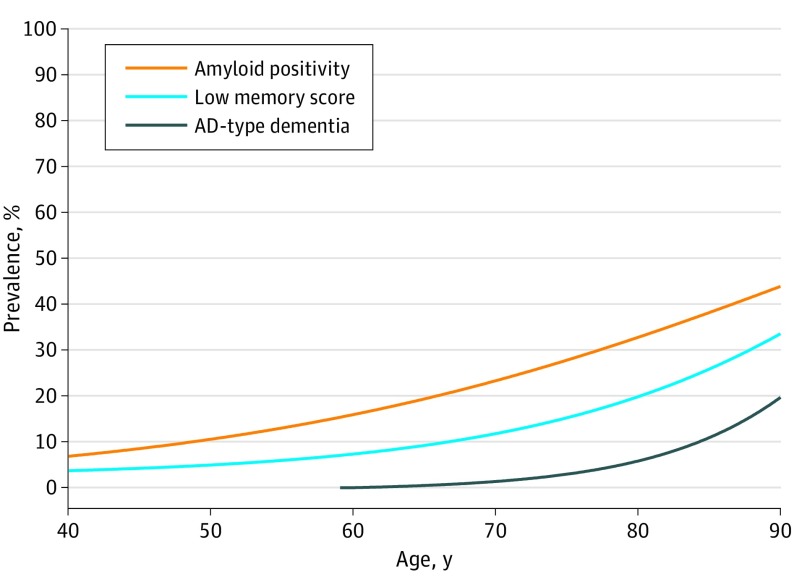
Figure 3. Prevalence of Amyloid Positivity and Low Memory Score in Participants With Normal Cognition and of Alzheimer Disease (AD)–Type Dementia in the General Population.
Prevalence estimates of amyloid positivity and low memory score were derived from separate models including age as a predictor. Prevalence estimates of AD-type dementia were derived from a meta-analysis of 14 studies.1 Low score was defined as 10th percentile or lower of z scores.
Table 1. Study Sample Characteristics by Cognitive Status and Amyloid Statusa.
| Characteristic | Normal Cognition | P Value | MCI | P Value | ||
|---|---|---|---|---|---|---|
| Amyloid
Negative (n = 2166) |
Amyloid
Positive (n = 742) |
Amyloid
Negative (n = 2000) |
Amyloid Positive
(n = 2133) |
|||
| Age, mean (SD), y | 65.6 (13.2) | 72.9 (9.5) | <.001 | 68.6 (8.9) | 71.7 (7.9) | <.001 |
| Age groups, y | ||||||
| <40 | 127 (5.9) | 2 (0.3) | NA | 1 (0.1) | 0 | NA |
| 40-49 | 91 (4.2) | 10 (1.3) | 29 (1.5) | 8 (0.4) | ||
| 50-59 | 311 (14.4) | 55 (7.4) | 309 (15.5) | 147 (6.9) | ||
| 60-69 | 714 (33.0) | 180 (24.3) | 719 (36.0) | 654 (30.7) | ||
| 70-79 | 714 (33.0) | 322 (43.4) | 736 (36.8) | 1004 (47.1) | ||
| 80-89 | 195 (9.0) | 160 (21.6) | 200 (10.0) | 308 (14.4) | ||
| ≥90 | 14 (0.6) | 13 (1.8) | 6 (0.3) | 11 (0.5) | ||
| Women | 1231/2154 (57.1) | 397/742 (53.5) | .08 | 898/2000 (45.0) | 1013/2133 (47.5) | .11 |
| Educational level | ||||||
| Mean (SD), y | 14.2 (3.7) | 14.7 (3.7) | .001 | 11.8 (4.2) | 12.7 (4.4) | <.001 |
| High level (≥14 y) | 1107/1906 (58.1) | 430/652 (66.0) | <.001 | 536/1597 (33.6) | 732/1673 (43.8) | <.001 |
| MMSE score | ||||||
| Mean (SD) | 29.1 (1.2) | 28.9 (1.3) | .001 | 27.2 (2.3) | 26.4 (2.6) | <.001 |
| MMSE score ≤27 | 208/2148 (9.7) | 88/737 (11.9) | .08 | 938/1999 (46.9) | 1312/2128 (61.7) | <.001 |
| Memory z score | ||||||
| Mean (SD) | 0.04 (0.95) | −0.16 (1.07) | <.001 | −1.27 (1.58) | −1.78 (1.47) | <.001 |
| Memory z score ≤1.28 | 130/1480 (8.9) | 76/530 (14.3) | <.001 | 594/1256 (47.3) | 878/1359 (64.6) | <.001 |
| APOE-ε4 positive | 402/1746 (23.0) | 324/654 (49.5) | <.001 | 421/1510 (27.9) | 1134/1782 (63.5) | <.001 |
| APOE genotype | ||||||
| ε2ε2/ε2ε3 | 263/1713 (15.4) | 26/634 (4.1) | NA | 172/1407 (12.2) | 61/1612 (3.8) | NA |
| ε3ε3 | 1052/1713 (61.4) | 293/634 (46.2) | 829/1407 (58.9) | 513/1612 (31.8) | ||
| ε2ε4/ε3ε4 | 376/1713 (21.9) | 263/634 (41.5) | 365/1407 (25.9) | 753/1612 (46.7) | ||
| ε4ε4 | 22/1713 (1.3) | 52/634 (8.2) | 41/1407 (2.9) | 285/1612 (17.7) | ||
Abbreviations: APOE, apolipoprotein E; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NA, not applicable.
Data are presented as number/total number (percentage) of study participants unless otherwise indicated. Amyloid-negative and amyloid-positive subgroups were compared among participants with normal cognition and among patients with MCI with t tests or χ2 tests.
Table 2. Estimated Frequencies of Low Cognitive Performance According to Amyloid Status and Agea.
| Age, y | Frequency, % (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Memory | MMSE | |||||||||||
| Normal Cognition | MCI | Normal Cognition | MCI | |||||||||
| Amyloid Negative | Amyloid Positive | P Value | Amyloid Negative | Amyloid Positive | P Value | Amyloid Negative | Amyloid Positive | P Value | Amyloid Negative | Amyloid Positive | P Value | |
| 50 | 4.9 (2.1-11.1) | 2.7 (1.2-6.1) | .17 | 29.1 (17.0-45.1) | 50.4 (40.1-60.6) | <.001 | 6.5 (4.7-9.0) | 9.3 (6.3-13.4) | .09 | 30.2 (23.4-38.0) | 50.0 (42.9-57.1) | <.001 |
| 55 | 5.9 (2.9-11.8) | 4.0 (2.0-7.7) | .24 | 34.8 (22.8-49.0) | 55.3 (45.9-64.2) | <.001 | 8.0 (5.9-10.8) | 10.6 (7.4-15.0) | .11 | 35.0 (28.4-42.3) | 53.7 (47.3-60.0) | <.001 |
| 60 | 7.1 (3.8-12.9) | 5.9 (3.4-9.9) | .47 | 40.9 (29.6-53.2) | 60.0 (51.5-68.0) | <.001 | 9.8 (7.3-13.0) | 12.1 (8.6-16.7) | .15 | 40.2 (33.9-46.8) | 57.4 (51.7-62.9) | <.001 |
| 65 | 8.5 (4.9-14.5) | 8.6 (5.6-12.9) | .97 | 47.3 (37.0-57.8) | 64.6 (56.6-71.9) | <.001 | 11.9 (9.0-15.6) | 13.8 (10.0-18.7) | .24 | 45.6 (39.6-51.6) | 61.0 (55.9-65.9) | <.001 |
| 70 | 10.2 (6.0-16.8) | 12.4 (8.9-17.2) | .20 | 53.8 (44.3-63.0) | 68.9 (61.1-75.8) | <.001 | 14.4 (10.9-18.8) | 15.7 (11.5-21.0) | .43 | 51.0 (45.3-56.7) | 64.5 (59.8-69.0) | <.001 |
| 75 | 12.2 (7.0-20.3) | 17.7 (12.9-23.8) | .002 | 60.1 (50.7-68.9) | 72.9 (65.0-79.7) | <.001 | 17.3 (13.0-22.7) | 17.7 (13.1-23.5) | .80 | 56.5 (50.8-62.0) | 67.9 (63.5-72.0) | <.001 |
| 80 | 14.4 (7.8-25.1) | 24.5 (17.3-33.6) | <.001 | 66.2 (56.2-74.9) | 76.6 (68.4-83.2) | <.001 | 20.7 (15.3-27.3) | 20.0 (14.9-26.4) | .73 | 61.8 (55.9-67.3) | 71.1 (66.7-75.1) | <.001 |
| 85 | 17.0 (8.5-21.3) | 32.9 (22.0-46.1) | <.001 | 71.7 (60.7-80.7) | 79.9 (71.4-86.4) | .004 | 24.5 (17.9-32.6) | 22.6 (16.8-29.5) | .39 | 66.8 (60.6-72.5) | 74.1 (69.6-78.0) | .001 |
| 90 | 20.0 (9.0-38.6) | 42.6 (27.0-59.7) | <.001 | 76.7 (64.6-85.6) | 82.9 (74.1-89.1) | .07 | 28.8 (20.7-38.5) | 25.3 (18.9-33.0) | .22 | 71.5 (64.9-77.3) | 76.8 (72.3-80.9) | .03 |
Abbreviations: MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination.
Data are estimated probabilities uncorrected for sex, educational level, and apolipoprotein E ε4 carrier status. The model for memory included terms for age, amyloid status, cognitive status, and interactions among these terms (3-way interaction P = .002). The model for MMSE included terms for age, amyloid status, cognitive status, and interactions between amyloid status and age (P = .02), and between amyloid status and cognitive status (P < .001). Adjustment for sex and educational level did not change the models. Low score was defined as 10th percentile or lower of z scores for the low memory score and 27 or lower for low MMSE scores.
Results
A total of 2908 persons with normal cognition (mean [SD] age, 67.4 [12.8] years; 1628 of 2896 participants [56.2%] were female) and 4133 patients with MCI (mean [SD] age, 70.2 [8.5] years; 1911 female [46.2%]) participated in the study. Sample characteristics of the participants with normal cognition and MCI according to amyloid status are given in Table 1. The amyloid-positive and amyloid-negative groups differed on all variables except sex in participants with normal cognition and MCI and except MMSE score in participants with normal cognition.
Association Between Amyloid Status and Episodic Memory Score
Amyloid positivity was differentially associated with low memory scores across age and diagnostic groups (3-way interaction; β = −0.54 [95% CI, −0.097 to −0.011]). In participants with normal cognition, low memory scores were more frequent in amyloid-positive than in amyloid-negative participants but only after 70 years of age (mean difference for amyloid-positive vs amyloid-negative participants at 72 years of age, 4% [95% CI, 0%-7%], P = .04; at 90 years of age, 21% [95% CI, 10%-33%], P < .001). At 80 years of age, the frequency of low memory scores in amyloid-positive participants with normal cognition was almost double that of their amyloid-negative counterparts (25% vs 14%) (Figure 1A and Table 2). In patients with MCI, amyloid-positive participants more often had low memory scores than amyloid-negative participants (mean difference, 16% [95% CI, 12%-20%], P < .001) (Figure 1B and Table 2), although this difference decreased with advancing age.
In participants with normal cognition and patients with MCI, sex, educational level, and APOE-ε4 carrier status were each associated with memory scores but did not moderate the association between amyloid status and memory scores (eFigure 1A in the Supplement). A low memory score was more frequent in men than in women (mean difference, 8% [95% CI, 3%-14%], P = .004), in participants with low rather than high educational level (mean difference, 8% [95% CI, 5%-11%], P < .001), and in APOE-ε4 carriers than in noncarriers (mean difference, 8% [95% CI, 4%-11%], P < .001).
Furthermore, APOE genotype was associated with low memory score independent of amyloid status, cognitive status, or age (Figure 2). Participants with the APOE-ε4ε4 genotype most often had a low memory score (mean difference vs ε2ε4/ε3ε4, 10% [95% CI, 4%-16%], P < .001; vs ε3ε3, 16% [95% CI, 9%-24%], P < .001; vs ε2ε2/ε2ε3, 21% [95% CI, 9%-24%], P < .001), followed by participants with the APOE-ε2ε4/ε3ε4 genotype (mean difference vs ε3ε3, 6% [95% CI, 3%-9%], P < .001; vs ε2ε2/ε2ε3, 11% [95% CI 4%-17%], P = .001) and the APOE-ε3ε3 and APOE-ε2ε2/ε2ε3 genotypes (mean difference, 5% [95% CI, −1% to 10%], P = .09).
Association Between Amyloid Status and MMSE Score
The association between amyloid positivity and a low score on the MMSE was dependent on age (β = 0.023 [95% CI, 0.009-0.037, P = .001 for interaction) and cognitive status (β = 0.348 [95% CI, 0.032-0.665], P = .03 for interaction; β = 0.006 [95% CI, −0.033 to 0.044], P = .10 for 3-way interaction). In participants with normal cognition, amyloid positivity was not associated with low MMSE scores at any age (mean difference, 3% [95% CI, −1% to 6%], P = .16) (Figure 1C and Table 2). In patients with MCI, the frequency of low MMSE scores was greater in amyloid-positive compared with amyloid-negative patients at all ages (mean difference, 14% [95% CI, 12%-17%], P < .001) (Figure 1D and Table 2).
When adjusting for sex, educational level, and APOE-ε4 carrier status, none of these factors modulated the association between amyloid status and MMSE score in participants with normal cognition or patients with MCI. APOE-ε4 carriership was more often associated with low MMSE scores regardless of cognitive status and age (mean difference, 4% [95% CI, 2%-7%], P < .001). Sex (β = −0.444 [95% CI, −0.681 to −0.207; P<.001 for interaction) and educational level (β = −0.554 [95% CI, −0.874 to −0.234], P<.001 for interaction) had an association with MMSE score that was dependent on cognitive status (eFigure 1B in the Supplement).
In addition, APOE genotype was associated with low MMSE score (P = .002) (eFigure 2 in the Supplement) independent of amyloid status, cognitive status, and age. Participants with the APOE-ε4ε4 genotype most often had a low MMSE score (mean difference vs ε2ε4/ε3ε4, 6% [95% CI, 0%-12%], P = .06; vs ε3ε3, 10% [95% CI, 3%-16%], P = .005; vs ε2ε2/ε2ε3, 10% [95% CI, 3%-16%], P = .005) followed by participants with the APOE-ε2ε4/ε3ε4 genotype (mean difference vs ε3ε3, 4% [95% CI, 1%-6%], P = .008; vs ε2ε2/ε2ε3, 4% [95% CI, 1%-8%], P = .14) and the APOE-ε3ε3 and APOE-ε2ε2/ε2ε3 genotypes (mean difference, 0% [95% CI, −4% to 4%], P = .99).
Amyloid Positivity, Low Memory Scores, and AD-Type Dementia
In participants with normal cognition, the age-related increase in low memory scores paralleled the age-related increase in amyloid positivity with an intervening period of 10 to 15 years. Subsequently, the age-related increase in low memory score was paralleled by an age-related increase in prevalence of AD-type dementia 10 to 15 years later (Figure 3).
Cognitive Screening as an Indication of Amyloid Status
The odds ratio of low memory score for amyloid positivity varied depending on age from 1.06 to 1.80 for participants with normal cognition and from 1.47 to 2.81 for patients with MCI. The odds ratio of low MMSE score varied from 0.77 to 1.19 for participants with normal cognition and from 1.32 to 2.15 for patients with MCI (eTable 4 in the Supplement). The receiver operating characteristic curve analyses showed that low memory or MMSE scores did not add to the estimation of amyloid positivity above that of age and APOE-ε4 carrier status (eTable 5 in the Supplement).
Discussion
In this cross-sectional analysis of adults without dementia enrolled from multiple studies, amyloid pathology was associated with low memory scores among cognitively normal individuals older than 70 years and in patients with MCI until old age, whereas it was associated with low MMSE scores in patients with MCI only. However, a low cognitive score had limited value as a screening measure for early AD. The association between amyloid pathology and cognition existed independent of sex, educational level, and APOE genotype, but these factors contributed to individual estimates of cognitive level. We further observed 10- to 15-year intervals between the onset of amyloid positivity and emergence of low memory scores in persons with normal cognition and between the age-related increase in low memory scores and prevalence of AD-type dementia.
Of note, the proportion of low memory scores in amyloid-positive participants with normal cognition increased rapidly after the age of 70 years. Possible explanations for this age association are that more extensive amounts of amyloid deposition are needed before becoming clinically manifest, that other pathology is also present after this age, or that cognitive vulnerability to amyloid pathology may be higher at older age. This finding agrees with a recent population-based study13 in cognitively normal individuals aged 50 to 69 years in which amyloid-PET levels were unrelated to cross-sectional and longitudinal measures of episodic memory. Previous contrasting findings from cross-sectional studies4,7,8 that examined the amyloid-cognition association may therefore, in addition to the previously identified task complexity,14 be explained by age of the individuals in the study sample.
We found no association of amyloid pathology with low MMSE score in participants with normal cognition. This result contrasts with a meta-analysis of biomarker and neuropathologic studies.11 However, that meta-analysis also included global cognition tests that have a larger memory component than the MMSE.
In patients with MCI, amyloid-positive patients had (depending on age) low memory and MMSE scores 5% to 20% more often than amyloid-negative patients. Unlike in participants with normal cognition, frequencies of low cognitive scores in amyloid-positive and amyloid-negative patients were more similar at older ages in patients with MCI. This finding is in line with a study in persons with dementia,15 suggesting that neuropathologic changes other than amyloid may contribute to cognitive impairment at more advanced age in patients with MCI but not in persons with normal cognition.
Sex and amyloid pathology independently predicted cognitive performance, suggesting that amyloid-related cognitive decline is equally prevalent in men and women, as has also been suggested in a large cross-sectional study16 of cognitively normal individuals from the general population not included in our study. High educational level was also associated with fewer cognitive deficiencies independent of amyloid status. This finding is consistent with earlier studies17,18 showing that cognitive reserve helps to preserve cognition in pathologic conditions and aging. Our study suggests that a high educational level does not counteract the effect of amyloid pathology on cognition but instead delays its expression, which agrees with earlier studies.19,20
Each APOE-ε4 allele increased the risk of low cognitive performance independent of amyloid status. Previous longitudinal studies examining the association among APOE-ε4, amyloid positivity, and cognition have had mixed results and identified mediating8,21 or interactive effects.22,23,24 Remarkably, our results indicate that the APOE genotype was also associated with cognitive performance in amyloid-negative participants. This finding may relate to subthreshold pathologic aggregation25,26 or to the association of APOE-ε4 genotype with non–amyloid-dependent processes in the brain that are associated with cognitive functioning.27
This study has implications for understanding the individual contributions of amyloid pathology and various AD risk factors to cognitive decline and the time course of AD. Therefore, these findings are important for the design of secondary AD prevention trials. Because amyloid positivity has become a requirement for enrollment in many AD prevention trials, cognitive testing would be an inexpensive and noninvasive alternative to screen for amyloid positivity. Our study showed that a low cognitive score for screening of amyloid positivity had only limited utility. Cognitive screening efficiency may be improved by more sensitive neuropsychological tests or test norms based on cognitively normal persons without amyloid pathology. The latter is supported by our finding that amyloid pathology appeared to explain a substantial part of the so-called age-related memory decline (Figure 3). However, cognitive measures can serve as an additional source of enrichment to optimize clinical trial design as has already been implemented in a prevention study.28
Limitations
This study has several methodologic limitations. There were several sources of variance among the pooled studies, including differences in amyloid assessment methods, participant selection, and other aspects of study design. However, the association of these differences with amyloid positivity was modest,1 and the association of amyloid status with cognitive performance was independent of biomarker modality. Furthermore, the type of memory test differed across studies but did not affect the association between amyloid and cognitive performance. Another potential limitation is that amyloid pathology might be associated with decline in other cognitive domains, such as executive functioning,8,11 that were not assessed in this study. Furthermore, the power to detect an association between amyloid positivity and MMSE score in participants with normal cognition was limited by the restricted range of MMSE scores in this group. In addition, because our findings were based on research or memory clinic populations, results may not be generalizable to the overall population or other settings. Last, our findings are based on cross-sectional data, which might not accurately reflect individual trajectories of amyloid-related cognitive decline.
Conclusions
Although low memory scores are an early marker of amyloid positivity, their value as a screening measure for early AD among persons without dementia is limited.
eTable 1. Sample Characteristics of Included Studies
eTable 2. Memory Test Details
eTable 3. Models Tested in Statistical Analyses
eTable 4. Cognitive Screening as Indication of Amyloid Status
eTable 5. Observed Probabilities of Low Cognitive Performance According to Amyloid Status and Age
eAppendix 1. Post Hoc Analyses
eAppendix 2. ADNI Protocol
eFigure 1. Frequencies of Low Memory Score According to Amyloid Status, Sex, Educational Level, and APOE-ε4 Carrier Status
eFigure 2. Frequencies of Low MMSE Score According to Amyloid Status, Sex, Educational Level, and APOE-ε4 Carrier Status
eFigure 3. Frequency of Low MMSE Score According to Amyloid Status and APOE Genotype
References
- 1.Jansen WJ, Ossenkoppele R, Knol DL, et al. ; Amyloid Biomarker Study Group . Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagan AM, Xiong C, Jasielec MS, et al. ; Dominantly Inherited Alzheimer Network . Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6(226):226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Johnson KA, Doraiswamy PM, et al. ; AV45-A05 Study Group . Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging. 2013;34(3):822-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnham SC, Bourgeat P, Doré V, et al. ; AIBL Research Group . Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 2016;15(10):1044-1053. [DOI] [PubMed] [Google Scholar]
- 7.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275-1283. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Wiste HJ, Weigand SD, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73(1):85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(pt 11):2837-2844. [DOI] [PubMed] [Google Scholar]
- 10.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29(10):1456-1465. [DOI] [PubMed] [Google Scholar]
- 11.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80(14):1341-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 13.Mielke MM, Machulda MM, Hagen CE, et al. Influence of amyloid and APOE on cognitive performance in a late middle-aged cohort. Alzheimers Dement. 2016;12(3):281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rentz DM, Amariglio RE, Becker JA, et al. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. ; Amyloid PET Study Group . Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 2015;72(5):511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81(4):314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138(Pt 3):761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amieva H, Mokri H, Le Goff M, et al. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain. 2014;137(Pt 4):1167-1175. [DOI] [PubMed] [Google Scholar]
- 20.Aguirre-Acevedo DC, Lopera F, Henao E, et al. Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: a retrospective cohort study. JAMA Neurol. 2016;73(4):431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80(19):1784-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mormino EC, Betensky RA, Hedden T, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing; Harvard Aging Brain Study . Amyloid and APOE ε4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82(20):1760-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim YY, Villemagne VL, Pietrzak RH, et al. ; Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group . APOE ε4 moderates amyloid-related memory decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2015;36(3):1239-1244. [DOI] [PubMed] [Google Scholar]
- 24.Seo EH, Kim SH, Park SH, Kang SH, Choo IH; Alzheimer’s Disease Neuroimaging Initiative . Independent and interactive influences of the APOE genotype and beta-amyloid burden on cognitive function in mild cognitive impairment. J Korean Med Sci. 2016;31(2):286-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu L, Boyle PA, Leurgans S, Schneider JA, Bennett DA. Disentangling the effects of age and APOE on neuropathology and late life cognitive decline. Neurobiol Aging. 2014;35(4):819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11(4):575-580. [DOI] [PubMed] [Google Scholar]
- 27.Dowell NG, Evans SL, Tofts PS, King SL, Tabet N, Rusted JM. Structural and resting-state MRI detects regional brain differences in young and mid-age healthy APOE-e4 carriers compared with non-APOE-e4 carriers. NMR Biomed. 2016;29(5):614-624. [DOI] [PubMed] [Google Scholar]
- 28.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Sample Characteristics of Included Studies
eTable 2. Memory Test Details
eTable 3. Models Tested in Statistical Analyses
eTable 4. Cognitive Screening as Indication of Amyloid Status
eTable 5. Observed Probabilities of Low Cognitive Performance According to Amyloid Status and Age
eAppendix 1. Post Hoc Analyses
eAppendix 2. ADNI Protocol
eFigure 1. Frequencies of Low Memory Score According to Amyloid Status, Sex, Educational Level, and APOE-ε4 Carrier Status
eFigure 2. Frequencies of Low MMSE Score According to Amyloid Status, Sex, Educational Level, and APOE-ε4 Carrier Status
eFigure 3. Frequency of Low MMSE Score According to Amyloid Status and APOE Genotype
Data Availability Statement
Information on the level of education was available for 2558 participants (88.0%) with normal cognition (memory score: n = 1973; MMSE: n = 2536) and 3270 patients (79.1%) with MCI (memory score: n = 2456; MMSE: n = 3264). Information on APOE-ε4 carrier status (yes or no) was available for 2400 participants (82.5%) with normal cognition (memory score: n = 1764; MMSE: n = 2379) and 3292 patients (79.7%) with MCI (memory score: n = 2217; MMSE: n = 3286). The APOE genotype was available for 2347 participants (80.7%) with normal cognition (memory score: n = 1763; MMSE: n = 2326) and 3019 patients (73.0%) with MCI (memory score: n = 2215; MMSE: n = 3013).