Abstract
Background
The safety and tolerability of limited output transcranial electrical stimulation (tES) in clinical populations support a non-significant risk designation. The tolerability of long-term use in a healthy population had remained untested.
Objective
We tested the tolerability and compliance of two tES waveforms, transcranial direct current stimulation (tDCS) and modulated high frequency transcranial pulsed current stimulation (MHF-tPCS) compared to sham-tDCS, applied to healthy subjects for three to five days (17–20 minutes per day) per week for up to six weeks in a communal setting. MHF-tPCS consisted of asymmetric high-frequency pulses (7–11 kHz) having a peak amplitude of 10–20 mA peak, adjusted by subject, resulting in an average current of 5–7 mA.
Method
A total of 100 treatment blind healthy subjects were randomly assigned to one of three treatment groups: tDCS (n = 33), MHF-tPCS (n = 30), or sham-tDCS (n = 37). In order to test the role of waveform, electrode type and montage were fixed across tES and sham-tDCS arms: high-capacity self-adhering electrodes on the right lateral forehead and back of the neck. We conducted 1905 sessions (636 sham-tDCS, 623 tDCS, and 646 MHF-tPCS sessions) on study volunteers over a period of six weeks.
Results
Common adverse events were primarily restricted to influences upon the skin and included skin tingling, itching, and mild burning sensations. The incidence of these events in the active tES treatment arms (MHF-tPCS, tDCS) was equivalent or significantly lower than their incidence in the sham-tDCS treatment arm. Other adverse events had a rarity (<5% incidence) that could not be significantly distinguished across the treatment groups. Some subjects were withdrawn from the study due to atypical headache (sham-tDCS n = 2, tDCS n = 2, and MHF-tPCS n = 3), atypical discomfort (sham-tDCS n = 0, tDCS n = 1, and MHF-tPCS n = 1), or atypical skin irritation (sham-tDCS n = 2, tDCS n = 8, and MHF-tPCS n = 1). The rate of compliance, elected sessions completed, for the MHF-tPCS group was significantly greater than the sham-tDCS group’s compliance (p = 0.007). There were no serious adverse events in any treatment condition.
Conclusion
We conclude that repeated application of limited output tES across extended periods, limited to the hardware, electrodes, and protocols tested here, is well tolerated in healthy subjects, as previously observed in clinical populations.
Keywords: tDCS, MHF-tPCS, Tolerability, Compliance, Healthy population, Extended use
Introduction
Transcranial electrical stimulation (tES) using limited-output current intensities has been explored in healthy individuals as a tool to modulate cognitive performance [1–11]. Based on a wealth of prior evidence, limited-output tES is typically well tolerated and poses no significant risk to healthy populations [11–13]. However, the preponderance of evidence from healthy volunteers stems from studies testing ten or less tES treatment sessions [7]. The safety and tolerability of repeated use of tES for extended times (e.g. several sessions per week over several weeks) has been limited to studies in clinical populations.
In both normal and clinical populations, repeated use of tES has been proposed to increase efficacy through cumulative effects [14–16]. For example, repeated tES sessions have been demonstrated to increase clinical outcome in therapeutic studies [17,18]. With increasing research on tES to modulate cognition, as well as commercialization efforts, there have been concerns that the rate of testing has outpaced the data on tolerability [19–24].
In the context of reviewing tolerability, we include prior limited-output “tES” studies regardless of intent to directly modulate the cortex (e.g. transcranial random noise stimulation [25–28]) or cranial nerves (e.g. cranial TENS). Based on this historical data, repeated use of tES on healthy individuals is not expected to pose any significant risks as evidenced by: 1) repeated treatment sessions in clinical populations [29]; 2) acute studies applying a single or few treatment sessions in healthy subjects [1,30–37]; and 3) absence of any evidence for brain injury risk [38,39] though concerns about tradeoff in acute cognitive performance have been raised [40–42]. However, the dearth of data on the tolerability of repeated tES in healthy subject over an extended period of time has been cited as a limitation in informing human trials, as well as the use of tES for lifestyle and wellness applications [19–24]. Therefore, we monitored the tolerability of tES used repeatedly, three to five days per week, in a communal setting for up to six weeks by healthy volunteer subjects.
The tolerability of any tES technique is specific to: 1) session dose (electrical waveform properties and electrode montage) [43], and session repetition number/frequency; 2) electrode design [35,44]; and 3) subject exclusion and treatment protocols. We tested two limited output tES waveforms in addition to an active sham-tDCS waveform: transcranial direct current stimulation (tDCS) and modulated high-frequency transcranial pulsed current stimulation (MHF-tPCS). tDCS was applied at 2 mA, the highest dose commonly used. MHF-tPCS employs modulation designed for painless stimulation with peak intensity at 10–20 mA, adjusted by the subject. As our study was designed to evaluate the influence of different waveforms on tolerability and compliance, all other factors were fixed across study arms including electrode type and montage. Therefore, supporting both tPCS and tDCS, high-performance self-adhesive electrodes were positioned on the right temple and paraspinal area of the neck, allowing high-throughput and reliable electrode preparation, using simple landmarks (none neuro-navigated).
All tES and sham-tDCS sessions were conducted in a communal environment (“coffee shop” lounge setting). Adverse events, adverse reactions and subject-elected compliance were assessed for up to six weeks of repeated tES involving three to five sessions per week. The study included assessments on the effect of tES on State–Trait Anxiety Inventory which will be analyzed elsewhere.
Methods
Participants
The study was conducted in accordance to protocols and procedures approved by the Institutional Review Board of the City College of New York. All volunteer participants provided written informed consent to participate in the study. All subjects were between the ages of 18 and 40 (M = 23, SD = ±5). Transcranial electrical stimulation has been applied to both male and female participants in numerous published studies and no significant gender differences have been reported so both males and females were recruited for this study. The study included 100 healthy individuals (male = 63, female = 37) with no recent history of neurological or psychiatric conditions (past 36 months, see below). All subjects were recruited through local advertisement and financially compensated for their participation.
Screening and exclusion criteria
Participants were excluded if they presented with any skin disorder at or near stimulation locations that compromised skin integrity, such as eczema, rashes, blisters, open wounds, burns including sunburns, cuts (e.g. due to shaving), or other skin defects, as the goal of this study was not to determine if skin impairments influence the tolerability of tES [45]. Mild acne, even if treated by medication, that does not compromise the integrity of the skin and non-irritating skin disorders (for example, vitiligo) were not used as exclusion criteria if there were otherwise no skin lesions in or around the areas where electrodes are positioned. Subjects were excluded if they reported any communicable skin disorder, even if outside the stimulation area.
Participants were excluded if they were currently under treatment for neuropsychiatric disorders as the study aimed to: 1) not evaluate clinical treatment outcomes; 2) avoid unrelated adverse events during the six-week intervention; 3) avoid variations in adverse event reporting across patient populations [37,46]; 4) avoid any theoretical interactions with medical treatments. Participants with a history of neurological or psychiatric disorders must have been off any treatment medications for minimum of 3 years (36 months) to be considered for the study. Participants were excluded from consideration if they had suffered from any form of severe head trauma (for example, head injury or brain surgery) or had any medical devices implanted in the head (such as, a deep brain stimulator) or in the neck (such as, a vagal nerve stimulator).
Subjects were excluded if they suffered from chronic headaches or migraines (headaches or migraines that occurred for consecutive days and are longer than an hour) or had any change in the rate or severity of head pressure, headache, or migraine in the past two weeks. Specifically, two headaches above the subject’s typical rate for a two-week period, or two headaches in the past two weeks above the typical severity, or a single headache in the past two weeks with unusually high severity was considered for the exclusion criteria. Such subjects were excluded to minimize possible confounding of naturally occurring headaches with adverse events.
The exclusion criteria were evaluated by self-reported survey for each subject before enrollment in the study and periodically during the study. Before the beginning of the study, the subjects underwent a brief 2 min tES test session corresponding to the experimental arm they were assigned to. If subjects reported a high pain score or a desire not to proceed they were excluded. Based on the screening criteria, 8 subjects were excluded from the study from a total of 108 participants.
Experimental design and tES treatment conditions
The study consisted of a randomized single-blind between-subject design with two experimental conditions and one control condition. The three conditions (for tES waveforms see below) were sham-tDCS-tDCS (n = 37), tDCS (n = 33), and tPCS (n = 30). Electrodes were applied and stimulation was activated by trained research assistants. During recruitment, the subjects were informed that the study would test the tolerability and efficacy (“mental energy and mind states”) of different types of neuromodulation stimulation.
Over a six week period, subjects participated in three to five sessions per week (weekdays only) with a minimum of 16 hours between sessions. Subjects were required to complete a minimum of eight sessions in each two-week period throughout the study to continue participation. Except for screening and verbal questionnaires (which were conducted in private), all treatment sessions were conducted in a communal environment designed to provide a lounge or “coffee shop” feel. The experimental space for this study consisted of an open floor plan with both tables and lounge seating. Subjects were allowed to do work on their laptops, had access to magazines, or could engage in quiet discussions with one another.
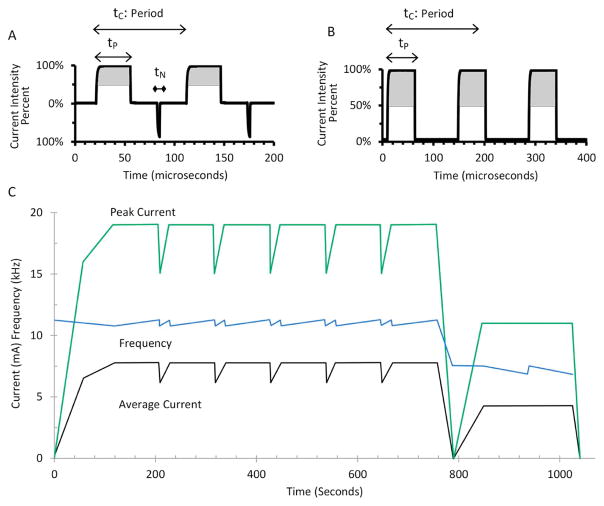
The sham-tDCS treatment delivered a 30 s linear ramp of current up to 2 mA and immediately back down to 0 mA over 30 s at the start of the session and again 20 minutes later at the end of the session. A Soterix Medical 1 × 1 tDCS was used to provide placebo stimulation (see below). The tDCS waveform was delivered with battery-driven, medical-grade tDCS devices with limited total energy (1 × 1 tDCS, Soterix Medical Inc., New York, NY). Current was linearly ramped up across 30 s to 2 mA, maintained at 2 mA for 20 minutes, and then linearly ramped down to 0 mA across 30 s. The MHF-tPCS waveforms were delivered with battery-powered, medical-grade transdermal electrical neurosignaling (TEN) devices (Thync, Inc., Los Gatos, CA) programmed to produce pulse-modulated (7–11 kHz), electrical currents producing average amplitudes of 5–7 mA for 17 minutes (Fig. 1C). The waveform from start of stimulation to 755 s is illustrated by Fig. 1A with a frequency of 11 kHz, positive pulse duration of 35 μs and negative pulse duration of 4 μs. Fig. 1B shows the waveform from 755 s until the end stimulation with a frequency of 7 kHz and positive pulse duration of 57 μs. A high-frequency carrier was chosen based on experimental and neuron modeling (e.g. cell diameter) studies showing relative minimization of neuromuscular and pain fiber stimulation [47–55] as well as retinal activation (phosphenes [56–60]). Moderate frequency-modulation was employed to circumvent any habituation to a particular stimulus frequency [61], though with a fixed progression compared to tRNS. Studies of both tES and spinal cord stimulation (SCS) indicated high frequency stimulation can produce characteristic physiological and clinical responses, compared to low (<100 Hz) waveforms [62–65]. Subjective adjustment of intensity is ubiquitous in clinical tPCS systems. Stimulators were placed on a station behind the subjects. Subjects in the MHF-tPCS arm were instructed to adjust an intensity dial on a GUI using an Apple iPod Touch connected to the TEN device over a Bluetooth low energy network such that it was comfortable to them. The peak intensity was scaled within the allowed adjustment range from 10 to 20 mA.
Figure 1.
MHF-tPCS waveform. A: Waveform from beginning of stimulation to 755 s (tN = 4 μs, tP = 35 μs, tC = 91 μs). B: Stimulation waveform from 755 s until the ends (tP = 57 μs, tC = 142 μs). C: Changes in frequency, average and peak current over a duration of 17 minutes. The adjustable range for the TENS device was from 50% to 100% of maximum current as indicated by the gray area in Fig. 2A,B. tN = Negative pulse duration. tP = Positive pulse duration. tC = Period (1/frequency).
Electrodes and montages
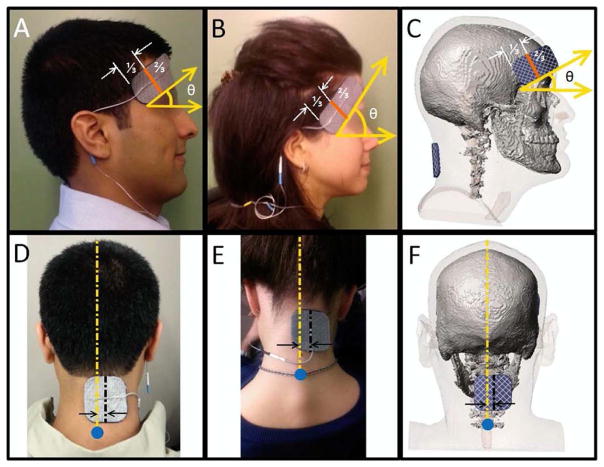
The electrodes were self-adhering hydrogel electrodes (Axelgaard PALS Platinum Blue Electrodes, Axelgaard Manufacturing Co. Ltd, Fallbrook, CA). A rectangular anode electrode (4 × 9 cm) was placed on the right temple of subjects using the temporal muscle during bite as a reference. If subjects presented with a significant amount of cosmetics, oil, or dirt in the electrode areas, the area was gently wiped, but not abraded, using a sterile wipe. The electrode placement was reinforced with a headband (Fig. 2). According to the international 10–20 electrode positioning system, the electrode spans approximately from F8 to FPz [66]. A square cathode electrode (5 × 5 cm) was positioned on the base of the neck, approximately above the cervical spine vertebra C7, 1 cm to the right of the midline. Adhesion was reinforced with light medical tape, when needed. Impedance was checked prior to stimulation and if >5 KOhm the electrode contact with the skin was checked and adjusted as needed. Electrodes were used for a single session and discarded afterwards.
Figure 2.
Electrode configurations and montages. Identical electrodes and montages were used across all treatment arms to allow for testing of the influence of variable waveforms on safety and tolerability. The rectangular anode electrode was placed on the subjects’ right temples after asking them to bite down for reference (panels A, B, C). A third of the electrode (landscape orientation reference) that is closest to the side at which the wire exits was placed over the temple. The other two thirds of the electrode was balanced toward the forehead at about a 45 degree angle (plane parallel to the floor reference shown by panel A, B, C) while avoiding as much of the subjects’ hairline as possible. As shown in panels D, E, F, the middle of square cathode electrode (dashed black line) was placed about 1 cm to the right of the subjects’ midline (vertical dashed yellow line) on the back of the neck. The electrode was placed above the cervical spine vertebra C7, which is marked with blue circle in panels D, E, F. The C7 bone is the last bone on the cervical vertebrae and generally protrudes, especially when bending the neck [67]. As needed, medical tape was used to ensure the edges of the cathode made good contact with the skin. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The single electrode type and montage, fixed across treatment arms, were selected based on experimental and engineering design. Off-hair placements allowed for precise and reliable assessment of skin tolerability. The supra-orbital (F8 to FPZ) and neck electrode positions were used together [68,69] and independently (with another location [70–72]) in both tDCS and tPCS; the supraorbital position in particular is used in >30% of tDCS and tPCS trials [73]. Axelgaard PALS Platinum Blue Electrodes combine features suitable for both transdermal DCS and PCS [74]. Key electrode design feature including: 1) construction of a knitted or woven stainless steel fabric that provides superior current spreading abilities for both tDCS and tPCS; 2) the PALS Blue electrodes use an e-beam cross-linked PVP hydrogel that provides for hypoallergenic skin contact and low volume resistivity; 3) high surface area and high profile (2.17 mm electrode to skin distance) enhance electrochemical buffering, especially relevant for DCS. The use of adhesive electrodes on exposed skin further supports consistent placement and preparation, even under high throughput (single center study with up to 3000 sessions). As emphasized throughout this manuscript, the relevance of tolerability data is limited to the extent of any dose or protocol variations. The adhesive electrode design tested here is not typical for tDCS as it cannot be used on scalp positions with hair.
Subject monitoring, adverse events, adverse reactions, and withdrawal criteria
A conservative approach was adopted for adverse events, adverse reaction assessments, and study withdrawal. Redundant methods of assessment were used with a bias toward detecting positive responses with either true or false. Subjects were withdrawn for atypical adverse events, even if not evidently hazardous, and without consideration if the event was related to study participation. Adverse events, adverse reactions, and study withdrawals were sub-classified into within-session or between-session occurrences. Subject status was rigorously monitored including: 1) a ‘screening bridge’ where all inclusion/exclusion criteria were reevaluated every two weeks along with a Short Form 36 health survey (SF-36); 2) detailed adverse event and adverse reaction questionnaires were administered before and after each treatment session; 3) visual inspection of the skin was conducted before and after each treatment session; 4) subjects were encouraged to verbally report adverse events or adverse reactions on an ongoing basis (e.g. painful or not typical sensations); 5) subjects were re-consented at the start of each session.
The withdrawal criteria are listed below:
-
Subjects experiencing any adverse event requiring medical intervention were excluded. Subjects were withdrawn if they experienced a serious adverse event defined based on International and US guidelines on serious adverse events from medical devices (including the Office of Human Research and Protection (OHRP) of the U.S. Department of Health And Human Services (HSS); FDA regulations at 21 CFR 312.32[a]; 1996 International Conference on Harmonization E-6 Guidelines for Good Clinical Practice; ISO/DIS 14155–Clinical investigations of medical devices in humans, good clinical practices, 2008). A severe adverse event related to stimulation was a documented event that:
Based upon scientific judgment determined to be caused or aggravated by the application of current to the head AND
Results in irreversible damage of brain tissue OR
Results in persistent disability or incapacity that produces an unwanted and substantial disruption of a person’s ability to conduct normal life functions, i.e., the adverse event resulted in an unwanted significant, persistent or permanent change, impairment, damage or disruption in the patient’s body function/structure, physical activities and/or quality of life OR
Results in inpatient hospitalization or prolongation of existing hospitalization, where emergency room visits that do not result in admission to the hospital should be evaluated for one of the other serious outcomes (e.g., life-threatening; required intervention to prevent permanent impairment or damage; other serious medically important event) OR
Results in death or is life-threatening where the patient was at substantial risk of dying at the time of the adverse event, or use was discontinued based on evidence tDCS might have resulted in death OR
Medical or surgical intervention was necessary to preclude permanent imminent impairment of a body function due to stimulation, or prevent permanent damage to a body structure due to stimulation.
Change of status relevant for inclusion or exclusion: throughout the study if any subject failed to meet study inclusion/exclusion criteria, including changes in medical diagnosis or treatment they were excluded from the study. The only withdrawals for changes of status occurred for atypical skin condition and atypical headache. One subject in the sham-tDCS arm presented hives on their arms and not at the electrode site. The subject was withdrawn on session 13 when the hives were discovered. The criterion for withdrawal was based on ‘atypical skin condition’ but not a serious adverse event.
“Atypical skin condition”: in addition to exclusion based on general skin health (for example, communicable diseases), a conservative approach for subject withdrawal was adopted based on minor skin irritation under the electrode areas, regardless of presumed associated with stimulation (for example, shaving irritation). Skin was visually inspected prior to and after each session by the investigator [75,76]. Prior to stimulation, moderate to severe erythema (that had persisted since the last session), but not slight erythema, was reviewed for withdrawal. Erythema after stimulation was not, in itself, criterion for withdrawal unless severe. Prior to stimulation, minor edema (for example, defined raising around electrode area) was reviewed for withdrawal. Moderate edema after stimulation (for example, area swollen/definite raising) was reviewed for withdrawal. Minor spotting (petechia) was not criterion for withdrawal. A blister (>1 mm) was a criterion for withdrawal. Review for withdrawal was based on skin irritation that appeared cumulative, namely the skin is altered from the prior session in a way that will influence skin response to the current session and next. Though not injurious [77], this conservative criterion was adopted as preventative. Within-session or between-session withdrawal depended on if the skin irritation was identified immediately before or after the session.
“Atypical headache”: headaches are expected in the normal population. Conservative criterion for exclusion was based on unusual or atypical intensity or frequency of headache (see above), regardless of causal link with stimulation. Within-session or between-session withdrawal depended on the time of the last headache.
“Atypical discomfort”: if during stimulation, subject expressed a desire to terminate the stimulation session for discomfort, stimulation was aborted and subjects were withdrawn from the study – regardless of their desire to continue with future sessions. If subjects indicated moderate discomfort (for example, based on their prior session’s experience) but desire to continue with the session, then stimulation was ramped down, electrodes were adjusted, and stimulation was re-started. If subjects were reluctant to go under stimulation because of discomfort between-session, then they were withdrawn. There were no such cases in this study.
Adverse events were assessed through self-reporting questionnaires. Hardcopy forms provided by research assistants were completed before and after each session to assess between-session effects (adverse events that persisted after the last treatment or occurred at a time since the last session) and within-session effects, respectively. For each evaluation subjects were queried with one open-form response and one adverse-event index. Lexical analysis mapped responses on the open form to any of the indexed adverse-events or classified as “anecdotal”. The lexical analysis was conducted using customized PHP software built in house which categorized and tallied all the different adverse events. In addition, the algorithm was designed to take into account positive and negative connotation of all the different adverse events. The open ended text was also checked manually for mistakes in the tallied reports or to find additional adverse events not detected by the algorithm. Itemized adverse-events encourage responsiveness [35] while the open form response allows for uncategorized response or individual terminology. The indexed events were based on commonly reported tDCS adverse events [30,32,37], selecting for items that were specific in etiology (for example, “skin tingling” as opposed to “discomfort”) and conducive to self-reporting (for example, skin redness was only accessed by the investigators). Indexed adverse reactions and adverse events were: 1) skin tingling; 2) skin itching; 3) skin burning sensation; 4) nauseous; 5) diffuse or migraine-like headache; 6) facial muscle twitching; 7) blurred vision; 8) short-lived localized head pain or pressure; 9) forgetfulness; 10) difficulty concentrating; 11) dizziness; and 12) difficulty breathing. Incidence of adverse events or reactions was coded in binary system (no = 0, yes = 1). For within-session evaluation participants scored the severity (1 = minimal; 4 = mild; 8 = moderate; 10 = severe) and duration (minutes) of each event.
Adverse events were categorized session-wise, aggregating across subjects (Table 1), and subject-wise with likelihood of adverse event (percent) collapsed across session for each subject (Fig. 3) with statistics only possible on the latter. The subject’s state anxiety level was measured using an abbreviated version of the State–Trait Anxiety Inventory containing six statements (STAI-6) according to scoring guidelines [78]. The STAI-6 questions are 1) I feel calm; 2) I am tense; 3) I feel upset; 4) I am relaxed; 5) I feel content; and 6) I am worried. Each question was scored from a value of 1 to 4 with 4 being a higher anxious state. For each session, the delta STAI-6 score was calculated by subtracting the total post-questionnaire STAI-6 score by the total pre-questionnaire STAI-6 score.
Table 1.
Summary of side effects incurred within and between sham or tES treatment sessions.
| Trial Arm | Assessment window |
Side Effects | tingling | itching | burning | nauseous | headache | twitching | blurred vision |
head pain |
forgetfulness | difficulty concentrating |
dizziness | difficulty breathing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham-tDCS | # Reports (%) | 447 (70.2%) | 188 (29.5%) | 175 (27.7%) | 16 (2.5%) | 25 (3.9%) | 10 (1.6%) | 7 (1.1%) | 24 (3.8%) | 6 (0.9%) | 16 (2.5%) | 8 (1.3%) | 5 (0.8%) | |
| Avg. Intensity (1–10) | 1.60 (±1.7) | 0.76 (±1.5) | 0.89 (±1.8) | 0.05 (±0.5) | 0.05 (±0.5) | 0.02 (±0.1) | 0.01 (±0.2) | 0.08 (±0.5) | 0.01 (±0.2) | 0.06 (±0.5) | 0.03 (±0.3) | <0.01 | ||
| Within Session | Avg. Duration (min) | 1.20 (±2.3) | 0.65 (±1.9) | 0.65 (±2.1) | 0.12 (±1.4) | 0.10 (±1.0) | 0.01 (±0.1) | 0.01 (±0.2) | 0.07 (±0.5) | 0.02 (±0.6) | 0.24 (±1.9) | 0.05 (±0.7) | 0(±0) | |
|
|
||||||||||||||
| When Reported | Avg. Intensity (1–10) | 2.3(±1.6) | 2.7(±1.5) | 3.4(±2.0) | 3.1(±2.0) | 4.5(±2.1) | 1.1(±0.3) | 1.5(±0.8) | 2.1(±1.7) | 1.6(±1.3) | 3.1(±1.9) | 2.3(±1.6) | 1(±0) | |
| Avg. Duration (min) | 1.7(±2.6) | 2.3(±2.9) | 2.4(±3.4) | 8.7(±9.0) | 11.3(±7.9) | 1.1(±0.9) | 1.8(±2.3) | 2.2(±2.1) | 5.0(±8.7) | 11.5(±7.5) | 6.0(±6.5) | 0(±0) | ||
|
| ||||||||||||||
| Between Sessions | # Reports (%) | 46 (7.3%) | 8 (1.3%) | 3 (0.5%) | 4 (0.6%) | 15 (2.4%) | 2 (0.3%) | 0 (0%) | 3 (0.5%) | 0 (0%) | 2 (0.3%) | 1 (0.2%) | 1 (0.2%) | |
|
| ||||||||||||||
| tDCS | # Reports (%) | 357 (57.7%) | 191 (30.9%) | 144 (23.3%) | 4 (0.6%) | 27 (4.4%) | 8 (1.3%) | 17 (2.7%) | 17 (2.7%) | 7 (1.1%) | 29 (4.7%) | 9 (1.5%) | 3 (0.5%) | |
| Avg. Intensity (1–10) | 1.42 (±2.1) | 0.9 (±1.9) | 1.02 (±2.4) | 0.01 (±0.1) | 0.05 (±0.4) | 0.01 (±0.1) | 0.03 (±0.3) | 0.05 (±0.5) | 0.02 (±0.2) | 0.10 (±0.5) | 0.02 (±0.3) | <0.01 | ||
| Within Session | Avg. Duration (min) | 4.03 (±6.4) | 2.2 (±5.1) | 2.07 (±5.1) | 0.01 (±0.2) | 0.15 (±1.5) | 0.01 (±0.1) | 0.01 (±0.1) | 0.08 (±0.7) | 0.06 (±0.9) | 0.59 (±3.2) | 0.05 (±0.8) | <0.01 | |
|
|
||||||||||||||
| When Reported | Avg. Intensity (1–10) | 2.7(±2.2) | 3.5(±2.2) | 4.8(±2.9) | 2.0(±1.4) | 3.0(±1.4) | 1.0(±0) | 3.0(±2.0) | 3.8(±2.4) | 2.0(±1.3) | 2.6(±1.6) | 2.4(±1.5) | 1(N/A) | |
| Avg. Duration (min) | 8.2(±7.0) | 8.2(±6.8) | 9.8(±6.9) | 3.0(±2.8) | 9.7(±7.3) | 1.0(±0) | 3.2(±3.5) | 4.4(±2.7) | 6.7(±8.4) | 15.3(±6.7) | 7.0(±8.8) | 1(N/A) | ||
|
| ||||||||||||||
| Between Sessions | # Reports (%) | 25 (4.0%) | 18 (2.9%) | 24 (3.9%) | 0 (0%) | 8 (1.3%) | 0 (0%) | 0 (0%) | 2 (0.3%) | 1 (0.2%) | 2 (0.3%) | 1 (0.2%) | 1 (0.2%) | |
|
| ||||||||||||||
| MHF-tPCS | # Reports (%) | 166 (25.8%) | 87 (13.5%) | 22 (3.4%) | 4 (0.6%) | 17 (2.6%) | 4 (0.6%) | 3 (0.5%) | 38 (5.9%) | 1 (0.2%) | 5 (0.8%) | 9 (1.4%) | 1 (0.2%) | |
| Avg. Intensity (1–10) | 0.78 (±1.7) | 0.40 (±1.3) | 0.09 (±0.6) | 0.01 (±0.2) | 0.06 (±0.5) | 0.04 (±0.5) | <0.01 | 0.16 (±0.7) | <0.01 | 0.03 (±0.3) | 0.03 (±0.3) | <0.01 | ||
| Within Session | Avg. Duration (min) | 1.28 (±3.3) | 0.72 (±2.6) | 0.12 (±1.0) | 0.01 (±0.2) | 0.06 (±0.7) | 0.01 (±0.3) | <0.01 | 0.31 (±1.8) | 0(±0) | 0.05 (±0.8) | 0.04 (±0.7) | 0(±0) | |
|
|
||||||||||||||
| When Reported | Avg. Intensity (1–10) | 3.2(±1.9) | 3.2(±2.3) | 3.1(±1.9) | 3.0(±2.8) | 2.9(±2.3) | 5.8(±4) | 1.0(±0) | 2.7(±1.4) | 1.0(N/A) | 3.6(±1.5) | 2.5(±2.1) | 1(N/A) | |
| Avg. Duration (min) | 5.7(±4.8) | 5.9(±5.2) | 4.5(±4.5) | 3.0(±2.0) | 4.0(±4.1) | 4.0(±4.2) | 2.5(N/A) | 5.5(±5.5) | N/A(N/A) | 7.3(±8.7) | 5.4(±6.7) | 0(N/A) | ||
|
| ||||||||||||||
| Between Sessions | # Reports (%) | 43 (6.7%) | 19 (2.9%) | 9 (1.4%) | 0 (0%) | 8 (1.2%) | 1 (0.2%) | 0 (0%) | 9 (1.4%) | 1 (0.2%) | 0 (0%) | 5 (0.8%) | 0 (0%) | |
All side effect incidences are from self-reported surveys administered daily, before (between session) and after (within session) treatment. Study withdrawal for atypical headache and atypical skin condition was scored automatically as between session headache since subjects did not complete the daily pre-treatment questionnaire the day following the adverse event or adverse reaction.
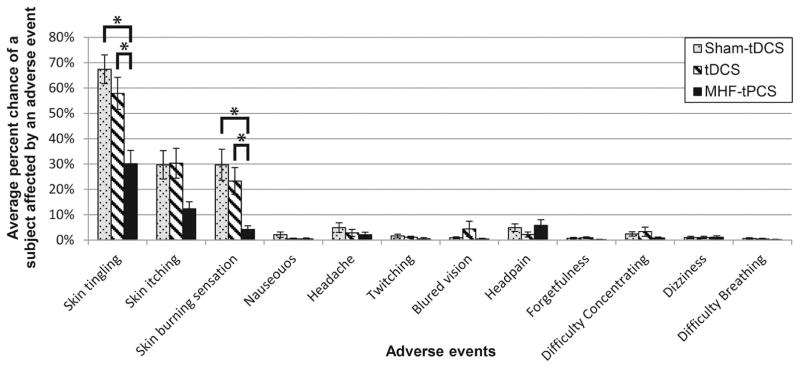
Figure 3.
Percent chance of reporting side effect for each subject: the average percent chance for a subject n = 37 sham-tDCS 33 tDCS, and 30 MHF-tPCS affected by a side effect within session. The percent is derived by calculating the total number of sessions a side effect was reported by a subject from the total number of sessions completed by the subject. The rate of reporting skin tingling for MHF-tPCS was lower than sham-tDCS (p = 9 × 10–05) and tDCS (p = 0.005). Furthermore, the rate of reporting skin burning sensation in MHF-tPCS was also lower than sham-tDCS (p = 0.006) and tDCS (p = 0.003). The error bars show the standard error of the mean. An asterisk indicates p < 0.01.
Blinding
Subjects were naïve to any brain stimulation. The subject consent indicated three stimulation types for any given session (“tDCS, tPCS, and sham”) but subjects were not informed what those waveforms entailed, or if they would be provided the same waveform throughout their participation. The subjects were questioned as to what waveform(s) they received in a follow-up survey. The subjects were asked to speculate if 1) they received a majority of one type of stimulation (sham, tDCS, or tPCS) and if so which; or 2) some combination of stimulation types; or 3) if they were not sufficiently aware of the meaning of the terms “sham/tDCS/tPCS” to speculate.
Data entry, validation, and aggregation were conducted by research assistants blind to the study arm.
Rationale and statistical tests
Primary end-points were: self-reported tolerability measures, compliance, and withdrawal rates.
Before applying any statistical test, the data sets were tested for a normal distribution. The normality was measured by the analysis of skewness and kurtosis. If the data were found to be normally distributed, then one-way ANOVA and t-test were used for the comparison. If the data were found not to be normally distributed, then Kruskal–Wallis test or Mann–Whitney rank–sum test were used for the comparison [79]. In order to correct for multiple comparisons, Benjamini–Hochberg procedure was used to further validate the significance of each p-value. The α-value was set to 0.05 for all the statistical tests and the Benjamini–Hochberg procedure. Table 1 shows mean ± standard deviation and Figs. 3 and 8 show error bars as standard error of the mean.
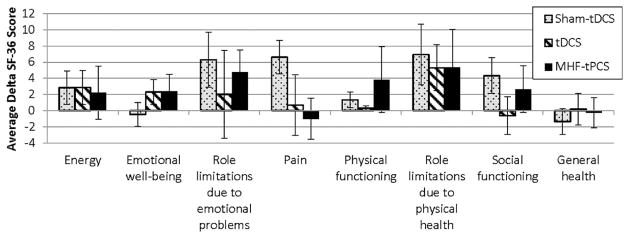
Figure 8.
Repeated use of tES had no significant detriment on quality of life as indicated by the SF-36 health survey. The change in scores (delta) obtained from the SF-36 administered before the first treatment session of the trial and at the end of the last treatment session of the trial are represented by histograms. The 36 questions in SF-36 health survey fall into the eight categories: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. Each question is scored on a scale of 0 to 100. For each of the questions and categories, a higher score defines a more favorable health state. The figure shows the average delta scores between the first and last session for subjects (sham-tDCS n = 37, tDCS n = 33 and MHF-tPCS n = 30). There were no significant differences found across the three treatment groups on any of the eight quality of life categories indicating that repeated use of tES had no significant detriment on the quality of life reflected by the SF-36 questions. The error bars indicate the standard effort of the mean.
Results
Compliance and withdrawal
A total of 1905 treatment sessions (sham-tDCS = 636, tDCS = 623, and MHF-tPCS = 646) were conducted on a total of 100 subjects (sham-tDCS = 37, tDCS = 33, and MHF-tPCS = 30). No severe adverse events were reported in any treatment condition during this study. The average number of sessions completed by subjects in each study arm were 17.2 ± 8.1 for sham-tDCS, 18.7 ± 7.8 for tDCS, and 21.5 ± 6.7 for MHF-tPCS treatment groups. A Mann–Whitney test indicated that the total number of sessions completed by subjects in the MHF-tPCS arm were significantly greater compared to the sessions completed by participants in the sham-tDCS group U = 318, p = 0.007, r = 0.33; no other completion comparisons were significant. Excluding subjects that withdrew, the average number of sessions completed by subjects in each study arm were 18.5 ± 7.5 for sham-tDCS, 21.6 ± 7.6 for tDCS, and 23.6 ± 5.3 for MHF-tPCS. Excluding withdrawn subjects, a Mann–Whitney test indicated that the number of sessions completed by subjects in tDCS (U = 235.5, p = 0.03, r = 0.30) and MHF-tPCS (U = 180.5, p = 0.0009, r = 0.45) arms were significantly greater than the number of sessions completed by subjects in sham-tDCS treatment group.
The data shown in Table 2 summarize treatment session counts and withdrawal rates. For “atypical discomfort”, there was one incident of a subject requesting a stimulation session to be stopped once initiated in the tDCS arm (after subject successfully completing 21 prior sessions) and one such incident in the MHF-tPCS arm (after subject successfully completing 20 prior sessions). In both cases, operators indicated that an electrode was not uniformly adhered to the skin. In both cases, subjects indicated a desire to remain enrolled in the study.
Table 2.
Summary of compliance indicated by treatment sessions, study completion and withdrawal rates for sham-tDCS, tDCS, and MHF-tPCS treatment groups.
| Group | Number of sessions | Total subjects | Finished trial | Subject that did not meet ongoing inclusion/exclusion criteria
|
Discomfort
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Atypical headache or migraine
|
Atypical skin condition
|
||||||||
| Between session | Within session | Between session | Within session | Between session | Within session | ||||
| Sham-tDCS | 636 | 37 | 33 | 2 | 0 | 2 | 0 | 0 | 0 |
| tDCS | 623 | 33 | 22 | 2 | 0 | 8 | 0 | 0 | 1 |
| MHF-tPCS | 646 | 30 | 25 | 3 | 0 | 1 | 0 | 0 | 1 |
| Total | 1905 | 100 | 80 | 7 | 0 | 11 | 0 | 0 | 2 |
Subjects who did not meet the ongoing inclusion/exclusion criteria were withdrawn for discomfort, atypical headache and atypical skin condition (see Methods). All other subjects were categorized as “finished trial”. Subjects elected how many sessions to complete over 6 weeks with the minimal requirement of completing four treatment sessions per seven days and a minimal enrollment commitment of 2 weeks.
Subjects’ self-reports of “atypical headache or migraine” (increased frequency or severity, see Methods) resulted in the study withdrawal of two subjects in the sham-tDCS group, two subjects in the tDCS group, and three subjects in the MHF-tPCS group. In all cases these withdrawals reflected adverse events occurring between, not during, sessions. The number of treatment sessions completed prior to withdrawal for the atypical headache or migraine events were: sham-tDCS = 10 and 3 sessions for the two subjects; tDCS = 19 and 10 sessions for the two subjects; and MHF-tPCS = 22, 9, and 8 sessions for the three subjects.
In some cases, inspection of the skin resulted in study discontinuation due to “atypical skin condition” (using the conservative thresholds described in Methods). Atypical skin conditions resulted in study discontinuation of two subjects from the sham-tDCS group, eight subjects from the tDCS group, and one subject from the MHF-tPCS group. One subject in the sham-tDCS arm was excluded after presenting hives on arms, not near electrodes, on his or her thirteenth treatment session. Of the remaining subjects withdrawn for atypical skin conditions, one subject in tDCS arm reported skin irritation under neck and forehead electrode while all the other subjects (across arms) reported skin irritation under the neck electrode only. For those subjects with skin irritation under the electrodes, the number of sessions completed prior to withdrawal for an atypical skin condition were: sham-tDCS = one subject was withdrawn after the first session; tDCS = eight subjects were withdrawn after the 13th, 9th, 14th, 13th, 8th, 12th, 8th, and 14th sessions; and MHF-tPCS = one subject was withdrawn from the study after the 21st session. In all cases, subjects indicated a desire to remain enrolled in the study.
After termination of stimulation, the percentage of subjects that were able to guess the treatment for sham, tDCS, and tPCS were 40%, 38% and 8%, respectively.
Tolerability results
Within-session tolerability was assessed by a questionnaire administered after each session. Between-session tolerability was accessed by a questionnaire administered prior to each session –for the period since the end of the last treatment session including the immediate post-stimulation period. Session-wise tolerability data are shown in an aggregated form in Table 1 collapsed across subjects (some subjects received more sessions hence no statistics on session-wise data is reported). Incidence of adverse events for all treatment groups within treatment sessions was < 3.5% with the exception of skin tingling, burning and itching sensations. In addition to indexed responses, if subjects experienced an interesting or peculiar sensation, then they could report it in an open form response. One subject in the MHF-tPCS arm reported phosphene (“light flash”) in one session, which was attributed to the electrode placed too close to the eye. The incidence of adverse events between treatment sessions was typically <5%.
Within-session subject-wise data (collapsing across sessions) supported a low (<7%) incidence rate for all adverse events except the adverse reactions of skin tingling, skin itching, and mild skin burning sensation (Fig. 3). A Mann–Whitney test indicated that the incidence of skin tingling in the MHF-tPCS (Mdn = 16.7%) treatment group was significantly lower than both sham-tDCS (Mdn = 81.8%, U = 245, p = 9 × 10−05, r = 0.48) and tDCS (Mdn = 62.5%, U = 290, p = 0.005, r = 0.35). In addition, the incidence of skin burning sensations in the MHF-tPCS (Mdn = 0%) group was also significantly lower than sham-tDCS (Mdn = 9%, U = 346.5, p = 0.006, r = 0.11) and tDCS (Mdn = 11.5%, U = 292, p = 0.003, r = 0.37). There were no other statistically significant differences in the incidence of adverse events across all treatment groups.
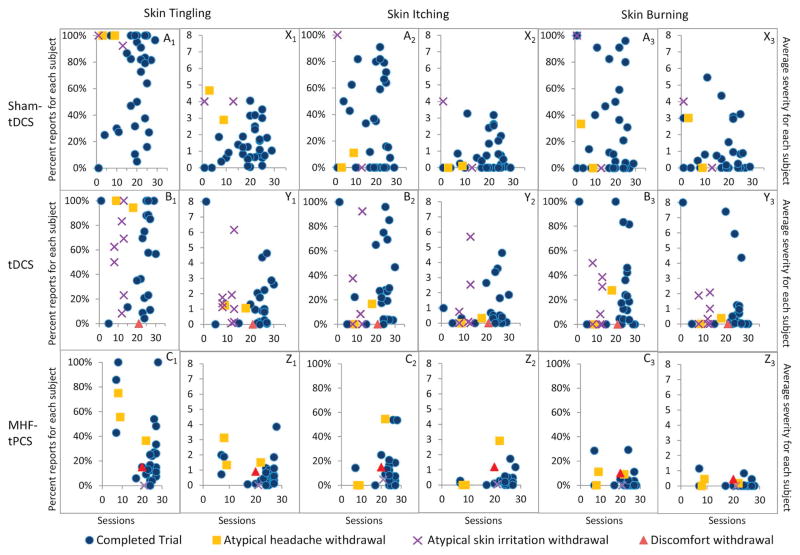
In exploratory analyses (Fig. 4), we considered the relation between skin tingling, itching, or burning sensations (common side effects) to compliance (number of sessions completed) and withdrawal rate. We found no evident correlations, which indicate adverse event severity of common adverse events (skin tingling, itching, and burning sensations) did not affect compliance rates. We next explored the relationship between within-session and between-session reporting (Table 3). Generally, reporting an adverse event within a stimulation session marginally increased the likelihood of reporting the same event in the following within-session period. Conversely, reporting any adverse event between-sessions increased the likelihood of reporting the same event during the next stimulation session period. These results are not fully controlled (e.g. account for carryover effects across many sessions) and do not address causality.
Figure 4.
Side effect incidence and severity does not affect compliance of individual subjects. The occurrence of common side effects (skin tingling = A1–C1, skin itching = A2–C2, and skin burning sensation = A3–C3) is plotted as a percentage against the total number of treatment sessions completed for each subject by experimental groups (sham-tDCS n = 37, tDCS n = 33, and MHF-tPCS n = 30). There was no correlation between percentage of side effects reported by subjects and the total number of treatment sessions they completed. The average severity of side effects (skin tingling = X1–Z1, skin itching = X2–Z2, and skin burning sensation = X3–Z3) is plotted against the number of sessions completed by each subject. There was no correlation between average side effect severity and the total sessions completed. Subject withdrawals for atypical headache, atypical skin irritation, and discomfort are indicated by the symbols purple X, yellow-square, and red-triangle, respectively. Since only a few subjects withdrew due to atypical headache, atypical skin condition, or discomfort in sham-tDCS, tDCS, and MHF-tPCS treatment groups, no trends can be inferred based on severity, incidence, or total sessions completed. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Likelihood for reporting the same adverse events consecutively within (during) and between (post-treatment), as well as between (post-treatment) and within (during the following treatment session) treatments.
| Δ% Tingling |
Δ% Itching |
Δ% Burning |
Δ% Nauseous |
Δ% Headache |
Δ% Twitching |
Δ% Blurred Vision |
Δ% Head Pain |
Δ% Forgetfulness |
Δ% Difficulty concentrating |
Δ% Dizziness |
Δ% Difficulty breathing |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham-tDCS | 18% | 46% | 6% | −0.6% | 2.6% | −1.6% | N/A | 2.9% | N/A | −2.5% | −1.3% | −0.8% |
| 7% | 1% | −0.4% | 12.2% | 10% | −0.3% | N/A | 3.8% | 0% | −0.3% | −0.2% | −0.2% | |
| tDCS | 32% | 37% | 63% | N/A | −4.4% | N/A | N/A | −2.7% | −1.1% | 45.5% | −1.4% | −0.5% |
| 5% | 7% | 17% | N/A | 6.4% | −0.2% | N/A | −0.3% | −0.2% | 3.3% | −0.2% | 0.2% | |
| MHF-tPCS | 40% | 2% | 19% | N/A | −2.7% | 99.5% | N/A | −5.3% | −0.2% | −0.8% | −1.4% | N/A |
| 18% | 2% | 22% | N/A | 4.8% | 25% | N/A | 15.3% | −0.2% | 0.0% | 33% | 0% |
The likelihood for a subject reporting the same incidence consecutively is shown for all the sessions (sham-tDCS n = 636, tDCS n = 623, MHF-tPCS n = 646). The top delta value shows the percent chance for a subject repeating the reporting of the same adverse event or adverse reaction from within a session to between sessions. The formula used to calculate the delta values shown in the upper quadrants was: (percentage of subjects reporting a within session adverse and the same adverse event between sessions) – (percentage of subjects not reporting a within session adverse event, but reporting a between session adverse event). The delta value shown in the bottom quadrant indicates the percent chance for a subject experiencing the same adverse event from between a session to the next within session. The formula used to calculate the delta value shown in the bottom quadrant was: (percentage of subjects reporting an adverse event between sessions and the same adverse event during the next within session period) – (percentage of subjects who did not report an adverse event between a session, but who reported an adverse event during the next within session period). The within to between session values were high for skin tingling (tDCS = 32%, MHF-tPCS = 40%), skin itching (sham-tDCS = 46%, tDCS = 37%) and mild burning sensations (tDCS = 63%) indicating the likelihood of subjects reporting the same incidence consecutively. However high values for twitching (MHF-tPCS = 99.5%), dizziness (MHF-tPCS = 33%), and difficulty concentrating (tDCS = 45.5%) cannot be deemed reliable due to small percent of reports for twitching (MHF-tPCS = 0.6%), dizziness (MHF-tPCS = 1.4%), and difficulty concentrating (tDCS = 4.7%).
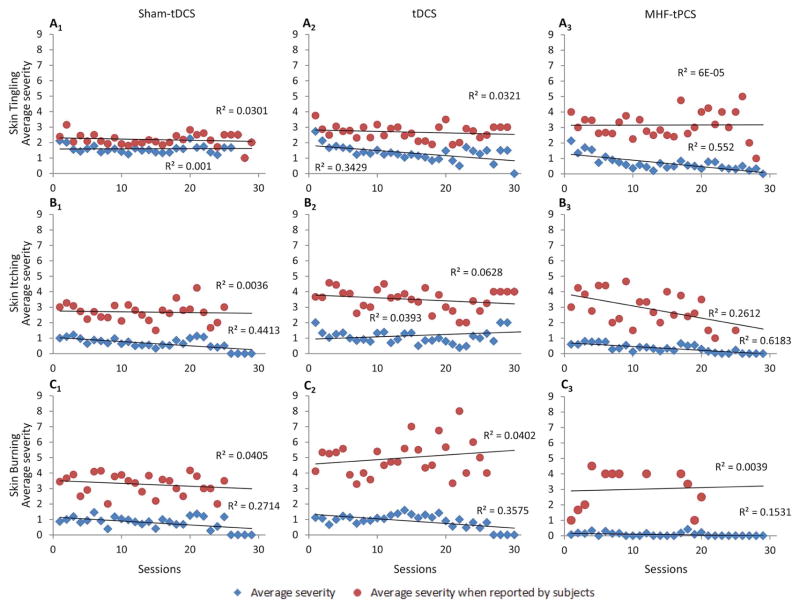
We further considered the change in severity of skin tingling, itching, or burning sensations over session numbers (Fig. 5). The severity was scored from one to ten by the subjects for the adverse event they reported. The trend when an adverse event was reported by subjects showed high variability (red circles Fig. 5). As a result, a second trend is shown where the adverse event severity was assumed to be zero when an adverse event severity was not reported by subjects (blue diamonds Fig. 5). The trend in the average adverse reaction severity either remained the same or decreased for all three treatment arms.
Figure 5.
Average adverse reaction severity remains stable or tends to decrease across treatment sessions. The average severities of common adverse reactions are plotted across the 30 treatment sessions for skin tingling (A1–A3), skin itching (B1–B3) and mild burning sensations (C1–C3) by treatment group. The average severity when an adverse reaction was reported by subjects (red circle) is higher and shows high variability compared to the grand average severity (blue diamonds) since the severity was assumed to be zero when an adverse reaction was not reported by subjects. Overall there was a general trend of decreasing average adverse reaction severity across the 30 treatment sessions as shown in panels A2, A3, B1, B3, C1, and C2. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
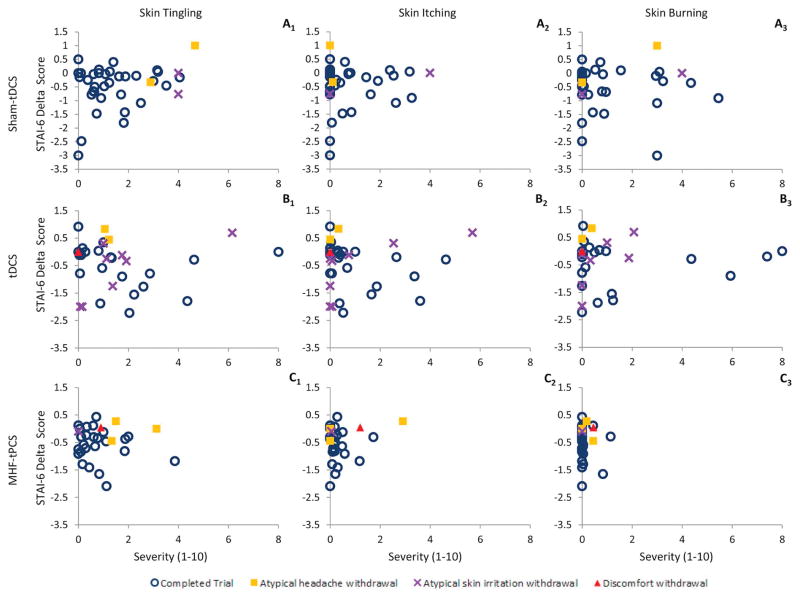
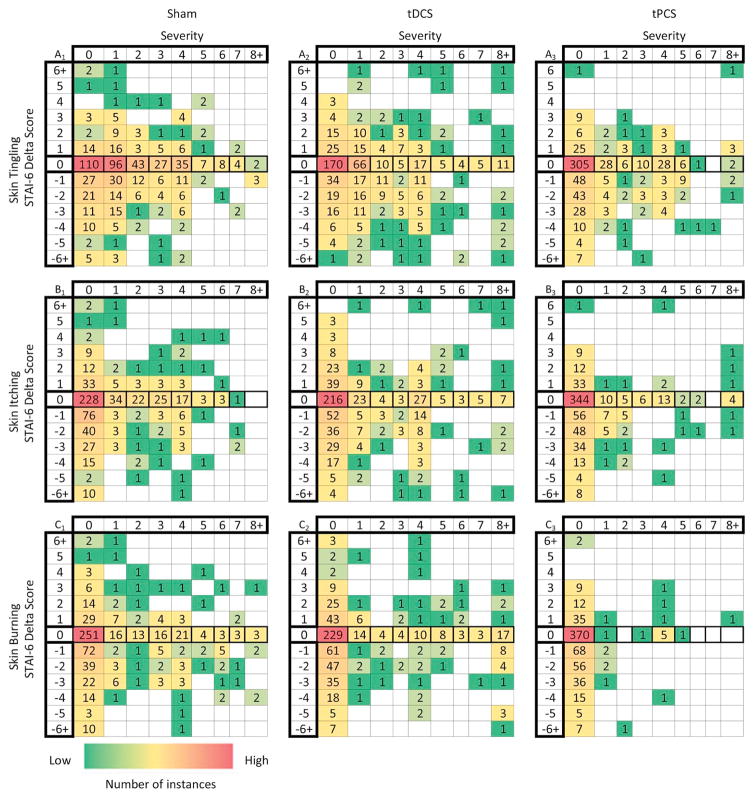
In exploratory analyses, we considered the relationship between severity of skin tingling, itching, or burning sensations and self-reported state anxiety levels (STAI-6 delta score) for each subject. We again found no evident correlations, which indicates the severity of common adverse reactions (skin tingling, itching, and burning sensations) did not affect state anxiety or the reporting of side effects (Fig. 6) in this subject-based correlation (n = 100). We next explored the session-based relationship (n = 1905) between state anxiety levels (STAI-6 delta) and adverse event severity (Fig. 7). Session based analyses did not show any significant correlation between state anxiety levels and adverse event severity. These findings are consistent with mild side effects that did not significantly affect compliance or side effect reporting.
Figure 6.
Side effect severity did not affect state anxiety levels as indicted by STAI-6 delta scores. The STAI-6 delta score (y-axis) reflects state anxiety changes and is plotted for each subject (sham-tDCS n = 37, tDCS n = 33, and MHF-tPCS = 30) against side effect (skin tingling = A1–C1, skin itching = A2–C2, and skin burning sensation = A3–C3) severity (x-axis) by treatment groups. Subject withdrawals for atypical headache, atypical skin irritation, and discomfort are indicated by a purple X, yellow-square, and red-triangle respectively. There was no significant trend between STAI-6 delta scores and side effect severity within treatment groups for skin tingling, itching, and burning sensations. Since the number of subjects withdrawing from the trial is small, no trends could be identified between withdrawal reason/severity and STAI-6 delta scores. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Figure 7.
Relationship between state anxiety (STAI-6 delta scores) and adverse event severity by treatment sessions. The heat-map grids shows the number of adverse event instances by session (sham-tDCS n = 636, tDCS n = 623, and MHF-tPCS n = 646) for each STAI-6 delta score corresponding to the reported severities for skin tingling (A1–A3), itching (B1–B3), and mild skin burning sensations (C1–C3). The STAI-6 delta score shows the overall stress level for each subject and is calculated based on 6 questions in both pre- and post-treatment questionnaires. There are high instances along zero severity and along zero STAI-6 delta scores since the majority of subjects did not experience a change in state anxiety levels or report a side effect after treatment. A few data points fell above a severity of 8 and a STAI-6 delta score of −6 or 6 and these data points are indicated in the columns or rows labeled with 8+, −6+ and 6+, respectively. No evident relationship was found between state anxiety (STAI-6 delta scores) and adverse event severity across the treatment sessions.
We administered a quality of life survey (SF-36 health survey [80]) bi-weekly and compared scores across all groups (Fig. 8). We did not find a significant difference between the three treatment groups for any of the eight health categories assessed: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. Given the SF-36 metrics [81] are gross, they are considered valid for severe quality of life changes across a population. Our observations indicate subjects’ general emotional and physical health was not negatively affected by sham-tDCS, tDCS, or MHF-tPCS during the length of the study.
Discussion
We found that tES is well tolerated and presents low-risk for repeated daily sessions in healthy volunteers, findings specific to the medical-grade technology and detailed protocols used here. To our knowledge, this is the longest duration study examining the tolerability of tES in healthy volunteers to date. Any mild adverse events that occur during or between active tES (tDCS or MHF-tPCS) sessions were comparable or lower to those observed for sham-tDCS waveforms.
General observations and compliance
In the present report, we describe the safety and tolerability outcomes from repeated application of transcranial electrical stimulation. All experimental conditions across arms were fixed except waveform (sham-tDCS, tDCS, MHF-tPCS). Based on prior trials [30,36,82], we developed a comprehensive adverse-event monitoring plan and implemented conservative (preventative) study withdrawal criteria. We typically could not distinguish between adverse effects and adverse event, i.e. whether side effects were either casual or causal, but for common adverse events we assessed dependence on waveform.
The average number of completed sessions in each arm were: sham-tDCS = 17.2 ± 8.1 (SD); tDCS = 18.7 ± 7.8; and MHF-tPCS: 21.5 ± 6.7. The compliance for MHF-tPCS was greater compared to sham-tDCS, regardless of whether withdrawn subjects were included or excluded in the analysis. The compliance to tDCS was comparable to sham-tDCS when including all subjects, and higher when excluding withdrawn subjects from the analysis. The severity of common adverse events was lowest in the MHF-tPCS treatment group. However, within each group we found no relationship between compliance and tolerability (severity of common adverse events). Conservatively, this supports the conclusion that active waveforms (tDCS or MHF-tPCS) do not reduce compliance.
Withdrawal and serious adverse events
Across 100 subjects in the three arms there were no serious adverse events reported with no subject requiring medical care as a result of participating in the study.
With almost 2000 sessions, we report only two cases of discontinuation due to during-stimulation adverse events (one tDCS subject after 21 completed sessions and one MHF-tPCS subject after 20 completed sessions). In both cases withdrawal was for “atypical discomfort” due to non-ideal electrode positions, but the subjects later indicated a desire to resume study participation and presented no other problems. There have been rare reports of mild electrical “shock” occurring with no injury during tES, which is associated with abrupt making or breaking of the stimulating circuit [30,36,82].
The remaining withdrawals occurred due to events occurring between treatment sessions. The number of subjects withdrawn for between-session headaches were: sham-tDCS = 2; tDCS = 2; and MHF-tPCS = 3. These low withdrawal numbers did not allow for assessment of causality due to treatment, especially since headaches occurring following tES occurred at rates similar to sham-tDCS. Notably, both tDCS and MHF-tPCS are investigated for the treatment of headache and migraine [29].
For withdrawals due to atypical skin irritation events, a causal link to study participation was evident by the location of irritation under the electrodes. Atypical skin irritation, either attributed to the electrodes or by stimulation, occurred in one subject from the sham-tDCS group, eight subjects in the tDCS group, and one subject in the MHF-tPCS group. Daily application of adhesive electrodes may have irritated the skin in some of these cases, but risk of atypical skin irritation appeared to be increased due to the tDCS waveform being transmitted by the specific electrodes used. The prevalence of irritation under the neck electrode suggests increased sensitivity of the skin on the neck region compared to the forehead; this may be due to difference in skin (hair follicle density) or the neck electrode being marginally smaller. While common in tPCS, the use of adhesive electrodes for tDCS is unusual; sponge electrodes are used in extended tDCS clinical trials with extremely rare occurrence of burns when proper equipment and protocols are employed [37]. We observed no skin injuries within-session. Instead, withdrawal was observed before stimulation (between-session), reflecting our conservative criteria [44] and preventing theoretical injury that might result from cumulative skin irritation. We emphasize that we made no observations of significant skin injury in this study. However, these findings reflect specific equipment and protocols, including stimulation across only healthy and intact skin.
Tolerability: skin tingling, itching, and burning sensations
Our dual on–off ramp sham-tDCS protocol was designed to mimic the sensation of tDCS [37]. During stimulation, mild tingling was the most common adverse event (sham-tDCS = 70.2 ± 1.8%, tDCS = 55.7 ± 2.0%, and MHF-tPCS = 25.8 ± 1.7%). The next most frequent adverse events during stimulation were mild burning or stinging sensations and itching. Mild burning sensations occurred in sham-tDCS 27.7 ± 1.8%, tDCS 23.3 ± 1.7%, and MHF-tPCS 3.4 ± 0.7% of the time and itching occurred in sham-tDCS 29.5 ± 1.8%, tDCS 30.9 ± 1.9%, and MHF-tPCS 13.5 ± 1.3% of the time. During stimulation, no other adverse events occurred at rates greater than 3.5%. Skin tingling, itching, and burning sensations are all cutaneous nociceptive signals caused by stimulation of cranial and cervical spinal nerve afferents that are linked to electrode electrochemical performance and skin current flow [83]. In the present trial, we found these sensations occurred during MHF-tPCS at significantly lower rates, which reflects the tolerability and comfort of the waveform/electrode combination used.
Although sensation is specific to waveform and electrode shape/design [44], the incidence rates we reported for tDCS and sham-tDCS using adhesive-electrodes are generally comparable to studies of single session tDCS in healthy subjects using sponge-electrodes. Poreisz et al. reported tDCS to elicit skin tingling, burning and itching sensations in 72.7%, 22.7%, and 36.4% of the cases respectively [32]. Kessler et al. reported skin tingling, burning and itching sensations due to tDCS occurred at rates of 76.9%, 54.2%, and 68.2% respectively [30].
We observed a trend toward decreased tingling over the first 2–3 sessions, possibly reflecting accommodation. We found there was no trend toward developing increased sensitivity to sensations across the duration of the trial (Fig. 5). The mild skin sensations reported were not associated with withdrawal, which is consistent with prior studies where sensation was not a reliable indicator of other theoretical risks [32].
Headache
The incidence of headache during stimulation (sham-tDCS = 3.9 ± 0.8%, tDCS 4.4 ± 0.8%, and MHF-tPCS 2.6 ± 0.6%) was comparable or moderately lower than reported by prior tES studies [32,37], which could be attributed to range of influences, including the communal (relaxed) environment and/or minimal headgear required (due to self-adhesive electrodes) to keep electrodes in place. The incidence of headache between-sessions was 2.4 ± 0.6% for sham-tDCS, 1.3 ± 0.5% for tDCS, and 1.2 ± 0.4% for MHF-tPCS treatment groups. These data illustrate that the theoretical risk of headache due to tES, including tDCS and MHF-tPCS, is low, especially considering the incidence rates of headache occurrence was equivalent between active tES treatment and sham-tDCS.
Other adverse events
Other adverse events occurred at a low incidence rate of < 5%. These rates were low across study arms, and any theoretical difference between arms is still lower. These data further suggest multiple tES sessions across several weeks do not present significant risks to healthy individuals when using medical-grade devices and proper protocols implementing limited outputs at current densities < 2 mA/cm2.
Limitations and implications for tolerability of daily extended-use tDCS and MHF-tPCS by healthy individuals
Specific to the protocols tested, the outcomes of this study support the tolerability of tDCS and tPCS over repeated sessions in healthy volunteers as compared to sham-tDCS procedures. Because our goal was to test the role of waveform, all other experimental conditions, including electrode design, were identical across treatment arms. This compromise represents a limitation of the study; we only evaluated one type of electrode, one which is not commonly used for tDCS procedures as it cannot be placed above the hairline. Thus, we speculate the tolerability of tDCS we observed could have been affected by the use of these electrodes, although they presented high tolerability rates for MHF-tPCS. Based on these observations, it is recommended that investigators choose electrodes that are optimal for the tES waveform being administered. Another limitation is that the sham-tDCS protocol was designed to produce skin sensation comparable to tDCS, and we discovered MHF-tPCS produced less skin sensations. Since differences were small and variable, this would not be expected to break naïve subject blinding as to the type of stimulation (correct guess of sham, tDCS, or tPCS), but this still [84] warrants consideration in designing future studies. A further limitation is that while tDCS intensity was fixed, consistent with established research protocols, MHF-tPCS intensity was adjusted, albeit within a controlled range (consistent with clinical tPCS systems). Since MHF-tPCS had equal or better tolerability than tDCS or sham-tDCS, conservatively this would mean that the lowest allowed MHF-tPCS dose was relativity better tolerated. In absolute terms, the entire tPCS protocol used was well tolerated compared to the tDCS treatment. Another limitation is that since stimulation was applied in a communal environment, we could not prevent or exclude subjects from sharing experiences.
The occurrence of common adverse reactions (itching, tingling, burning sensation) either decreased or remained stable over weeks. The use of adhesive electrodes produced cumulative skin irritation over the first two weeks in a minority of subjects. These results are broadly consistent with evidence of tolerability from single/limited sessions in healthy individuals [30,32,37] and extended-use in clinical populations, including investigational and FDA-cleared techniques [29]. Indeed, transcutaneous electrical stimulators, including transcutaneous electrical nerve stimulators or TENS, and electrical muscle stimulators, are indicated for a range of clinical/medical purposes (e.g., to relieve or treat pain or to improve range of motion) and for cosmetic/aesthetic purposes (e.g., to promote muscle toning or skin rejuvenation). These FDA-cleared devices often have current outputs as high as 120 mA and in the case of cosmetic/aesthetic TENS devices, can deliver current densities up to 46 mA/cm2 while having electrodes placed on the head or face. In contrast, tES current densities are typically < 2 mA/cm2 as was the case in the present study. Over the past 40 years, numerous studies have confirmed the safety and tolerability of electrical stimulation devices used daily for chronic time periods even at the higher current intensities and densities mentioned [85–87].
The safety and tolerability of any non-invasive electrical neuromodulation technique is specific to the dose, electrode preparation, and other protocol details. We used medical-grade stimulators with continuous impedance monitoring and waveform controls including limited outputs or limited-total-energy (LTE), high-capacity self-adhesive electrodes applied by trained operators, with rigorous monitoring and conservative withdrawal criterion. We emphasize the tolerability of any tES method is dependent on many factors including the protocols used, subject screening and monitoring, tES dose [12], and electrode design/montage [88,89]. The relevance of our results decreases as factors such as frequency of sessions (e.g. multiple daily sessions), withdrawal and exclusion criterion (e.g. demographics of our recruitment, medical history or use of none-prescription drugs not accessed in our protocol), procedure (e.g. trained operator vs. self-application), or environment (e.g. ambient lighting affects phosphene threshold, naturalistic environments with more variability than our testing facility) deviate from those tested here. Evidently, we cannot exclude rare or small differences in adverse events that our study was not powered to detect, or those adverse events that would not have been captured by our assessments. Using the protocols and methods described in this report, we found that extended use of tES in healthy subjects to pose low-risks and to be tolerable across multiple daily sessions.
Acknowledgments
Partial funding for this study was provided by a grant to the City College of New York (Grant # 76581-00 01) from Thync, Inc., Los Gatos, California, USA.
References
- 1.McIntire LK, McKinley RA, Goodyear C, Nelson J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 2014;7:499–507. doi: 10.1016/j.brs.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Wade S, Hammond G. Anodal transcranial direct current stimulation over premotor cortex facilitates observational learning of a motor sequence. Eur J Neurosci. 2015;41:1597–602. doi: 10.1111/ejn.12916. [DOI] [PubMed] [Google Scholar]
- 3.Del Felice A, Magalini A, Masiero S. Slow-oscillatory transcranial direct current stimulation modulates memory in temporal lobe epilepsy by altering sleep spindle generators: a possible rehabilitation tool. Brain Stimul. 2015;8:567–73. doi: 10.1016/j.brs.2015.01.410. [DOI] [PubMed] [Google Scholar]
- 4.Zhu FF, Yeung AY, Poolton JM, Lee TMC, Leung GKK, Masters RSW. Cathodal transcranial direct current stimulation over left dorsolateral prefrontal cortex area promotes implicit motor learning in a golf putting task. Brain Stimul. 2015;8:784–6. doi: 10.1016/j.brs.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Pisoni A, Turi Z, Raithel A, Ambrus GG, Alekseichuk I, Schacht A, et al. Separating recognition processes of declarative memory via anodal tDCS: boosting old item recognition by temporal and new item detection by parietal stimulation. PLoS ONE. 2015;10:e0123085. doi: 10.1371/journal.pone.0123085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinley RA, McIntire L, Bridges N, Goodyear C, Bangera NB, Weisend MP. Acceleration of image analyst training with transcranial direct current stimulation. Behav Neurosci. 2013;127:936–46. doi: 10.1037/a0034975. [DOI] [PubMed] [Google Scholar]
- 7.Elmasry J, Loo C, Martin D. A systematic review of transcranial electrical stimulation combined with cognitive training. Restor Neurol Neurosci. 2015;33:263–78. doi: 10.3233/RNN-140473. [DOI] [PubMed] [Google Scholar]
- 8.Miniussi C, Ruzzoli M. Transcranial stimulation and cognition. Handb Clin Neurol. 2013;116:739–50. doi: 10.1016/B978-0-444-53497-2.00056-5. [DOI] [PubMed] [Google Scholar]
- 9.Levasseur-Moreau J, Brunelin J, Fecteau S. Non-invasive brain stimulation can induce paradoxical facilitation. Are these neuroenhancements transferable and meaningful to security services? Front Hum Neurosci. 2013;7:449. doi: 10.3389/fnhum.2013.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause B, Cohen Kadosh R. Can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training. Dev Cogn Neurosci. 2013;6:176–94. doi: 10.1016/j.dcn.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin DM, Liu R, Alonzo A, Green M, Player MJ, Sachdev P, et al. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. Int J Neuropsychopharmacol. 2013;16:1927–36. doi: 10.1017/S1461145713000539. [DOI] [PubMed] [Google Scholar]
- 12.Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 2012;13:112–20. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin Neurophysiol. 2015;126:2181–8. doi: 10.1016/j.clinph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–32. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–9. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- 16.Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett. 2009;463:82–6. doi: 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 17.Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry. 2012;200:52–9. doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- 18.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–91. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 19.Widdows KC, Davis NJ. Ethical considerations in using brain stimulation to treat eating disorders. Front Behav Neurosci. 2014;8:351. doi: 10.3389/fnbeh.2014.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maslen H, Douglas T, Cohen Kadosh R, Levy N, Savulescu J. The regulation of cognitive enhancement devices: extending the medical model. J Law Biosci. 2014;1:68–93. doi: 10.1093/jlb/lst003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitz NS, Reiner PB. The challenge of crafting policy for do-it-yourself brain stimulation. J Med Ethics. 2013 doi: 10.1136/medethics-2013-101458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wexler A. The practices of do-it-yourself brain stimulation: implications for ethical considerations and regulatory proposals. J Med Ethics. 2015 doi: 10.1136/medethics-2015-102704. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton R, Messing S, Chatterjee A. Rethinking the thinking cap: ethics of neural enhancement using noninvasive brain stimulation. Neurology. 2011;76:187–93. doi: 10.1212/WNL.0b013e318205d50d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forum on Neuroscience and Nervous System Disorders, Board on Health Sciences Policy, Institute of Medicine, The National Academies of Sciences, Engineering, and Medicine. Non-invasive neuromodulation of the central nervous system: opportunities and challenges: workshop summary. Washington, DC: National Academies Press (US); 2015. [PubMed] [Google Scholar]
- 25.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–55. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaieb L, Kovacs G, Cziraki C, Greenlee M, Paulus W, Antal A. Short-duration transcranial random noise stimulation induces blood oxygenation level dependent response attenuation in the human motor cortex. Exp Brain Res. 2009;198:439–44. doi: 10.1007/s00221-009-1938-7. [DOI] [PubMed] [Google Scholar]
- 27.Paulus W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 2011;21:602–17. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- 28.Fertonani A, Pirulli C, Miniussi C. Random noise stimulation improves neuroplasticity in perceptual learning. J Neurosci. 2011;31:15416–23. doi: 10.1523/JNEUROSCI.2002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magis D, Sava S, d’Elia TS, Baschi R, Schoenen J. Safety and patients’ satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population. J Headache Pain. 2013;14:95. doi: 10.1186/1129-2377-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5:155–62. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales-Quezada L, Cosmo C, Carvalho S, Leite J, Castillo-Saavedra L, Rozisky JR, et al. Cognitive effects and autonomic responses to transcranial pulsed current stimulation. Exp Brain Res. 2015;233:701–9. doi: 10.1007/s00221-014-4147-y. [DOI] [PubMed] [Google Scholar]
- 32.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Raimundo RJS, Uribe CE, Brasil-Neto JP. Lack of clinically detectable acute changes on autonomic or thermoregulatory functions in healthy subjects after transcranial direct current stimulation (tDCS) Brain Stimul. 2012;5:196–200. doi: 10.1016/j.brs.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2. doi: 10.1016/s1388-2457(03)00235-9. author reply 2222–3. [DOI] [PubMed] [Google Scholar]
- 35.Russo R, Wallace D, Fitzgerald PB, Cooper NR. Perception of comfort during active and sham transcranial direct current stimulation: a double blind study. Brain Stimul. 2013;6:946–51. doi: 10.1016/j.brs.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Tadini L, El-Nazer R, Brunoni AR, Williams J, Carvas M, Boggio P, et al. Cognitive, mood, and electroencephalographic effects of noninvasive cortical stimulation with weak electrical currents. J ECT. 2011;27:134–40. doi: 10.1097/YCT.0b013e3181e631a8. [DOI] [PubMed] [Google Scholar]
- 37.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14:1133–45. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 38.Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol. 2009;120:1033–4. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–95. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sellers KK, Mellin JM, Lustenberger CM, Boyle MR, Lee WH, Peterchev AV, et al. Transcranial direct current stimulation (tDCS) of frontal cortex decreases performance on the WAIS-IV intelligence test. Behav Brain Res. 2015;290:32–44. doi: 10.1016/j.bbr.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremblay S, Lepage J-F, Latulipe-Loiselle A, Fregni F, Pascual-Leone A, Théoret H. The uncertain outcome of prefrontal tDCS. Brain Stimul. 2014;7:773–83. doi: 10.1016/j.brs.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar A, Dowker A, Cohen Kadosh R. Cognitive enhancement or cognitive cost: trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J Neurosci. 2014;34:16605–10. doi: 10.1523/JNEUROSCI.3129-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5:435–53. doi: 10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. J Neurosci Methods. 2010;190:188–97. doi: 10.1016/j.jneumeth.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiozawa P, da Silva ME, Raza R, Uchida RR, Cordeiro Q, Fregni F, et al. Safety of repeated transcranial direct current stimulation in impaired skin: a case report. J ECT. 2013;29:147–8. doi: 10.1097/YCT.0b013e318279c1a1. [DOI] [PubMed] [Google Scholar]
- 46.Harty S, Robertson IH, Miniussi C, Sheehy OC, Devine CA, McCreery S, et al. Transcranial direct current stimulation over right dorsolateral prefrontal cortex enhances error awareness in older age. J Neurosci. 2014;34:3646–52. doi: 10.1523/JNEUROSCI.5308-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph L, Butera RJ. High frequency stimulation selectively blocks different types of fibers in frog sciatic nerve. IEEE Trans Neural Syst Rehabil Eng. 2011;19:550–7. doi: 10.1109/TNSRE.2011.2163082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai C, de Groat WC, Roppolo JR. Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin–Huxley model. IEEE Trans Neural Syst Rehabil Eng. 2005;13:415–22. doi: 10.1109/TNSRE.2005.847356. [DOI] [PubMed] [Google Scholar]
- 49.Tai C, de Groat WC, Roppolo JR. Simulation analysis of conduction block in unmyelinated axons induced by high frequency biphasic electrical currents. IEEE Trans Biomed Eng. 2005;52:1323. doi: 10.1109/tbme.2005.847561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kilgore DKL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput. 2004;42:394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 51.Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman DK, Eddington DK, Rizzo JF, Fried SI. Selective activation of neuronal targets with sinusoidal electric stimulation. J Neurophysiol. 2010;104:2778–91. doi: 10.1152/jn.00551.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernhard CG, Skoglund CR. Selective activation of a transient reflex by restricting stimulation to certain frequencies. Acta Physiol Scand. 1942;4:125–35. doi: 10.1111/j.1748-1716.1942.tb01448.x. [DOI] [Google Scholar]
- 54.Carlson MD, Geha AS, Hsu J, Martin PJ, Levy MN, Jacobs G, et al. Selective stimulation of parasympathetic nerve fibers to the human sinoatrial node. Circulation. 1992;85:1311–17. doi: 10.1161/01.cir.85.4.1311. [DOI] [PubMed] [Google Scholar]
- 55.Plachta DTT, Gierthmuehlen M, Cota O, Espinosa N, Boeser F, Herrera TC, et al. Blood pressure control with selective vagal nerve stimulation and minimal side effects. J Neural Eng. 2014;11:036011. doi: 10.1088/1741-2560/11/3/036011. [DOI] [PubMed] [Google Scholar]
- 56.Antal A, Kincses TZ, Nitsche MA, Paulus W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia. 2003;41:1802–7. doi: 10.1016/s0028-3932(03)00181-7. [DOI] [PubMed] [Google Scholar]
- 57.Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. 2008;18:1839–43. doi: 10.1016/j.cub.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 58.Kanai R, Paulus W, Walsh V. Transcranial alternating current stimulation (tACS) modulates cortical excitability as assessed by TMS-induced phosphene thresholds. Clin Neurophysiol. 2010;121:1551–4. doi: 10.1016/j.clinph.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Turi Z, Ambrus GG, Janacsek K, Emmert K, Hahn L, Paulus W, et al. Both the cutaneous sensation and phosphene perception are modulated in a frequency-specific manner during transcranial alternating current stimulation. Restor Neurol Neurosci. 2013;31:275–85. doi: 10.3233/RNN-120297. [DOI] [PubMed] [Google Scholar]
- 60.Kar K, Krekelberg B. Transcranial electrical stimulation over visual cortex evokes phosphenes with a retinal origin. J Neurophysiol. 2012;108:2173–8. doi: 10.1152/jn.00505.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson MI. Transcutaneous electrical nerve stimulation (TENS): research to support clinical practice. Oxford: OUP Oxford; 2014. [Google Scholar]
- 62.Almay BG, Johansson F, von Knorring L, Sakurada T, Terenius L. Long-term high frequency transcutaneous electrical nerve stimulation (hi-TNS) in chronic pain. Clinical response and effects on CSF-endorphins, monoamine metabolites, substance P-like immunoreactivity (SPLI) and pain measures. J Psychosom Res. 1985;29:247–57. doi: 10.1016/0022-3999(85)90051-0. [DOI] [PubMed] [Google Scholar]
- 63.Darras BT, Jones HR, Jr, Ryan MM, Vivo DCD. Neuromuscular disorders of infancy, childhood, and adolescence: a clinician’s approach. Amsterdam: Elsevier; 2014. [Google Scholar]
- 64.Van Buyten J-P, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16:59–66. doi: 10.1111/ner.12006. [DOI] [PubMed] [Google Scholar]
- 65.Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123:851–60. doi: 10.1097/ALN.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 66. [accessed 20.06.16];EEG – Electroencephalography, Neuromonitoring blog Dr Richard Vogel. n.d http://neurologiclabs.com/neuromonitoring/eeg/
- 67.Highsmith JM., MD Cervical and thoracic spine. [accessed 19.04.15];Spine Universe. n.d http://www.spineuniverse.com/anatomy/cervical-thoracic-spine.
- 68.Siever D. Transcranial DC stimulation. NeuroConnections. n.d:33–40. [Google Scholar]
- 69.Mendonca ME, Santana MB, Baptista AF, Datta A, Bikson M, Fregni F, et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high-resolution computational models. J Pain. 2011;12:610–17. doi: 10.1016/j.jpain.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Reato D, Gasca F, Datta A, Bikson M, Marshall L, Parra LC. Transcranial electrical stimulation accelerates human sleep homeostasis. PLoS Comput Biol. 2013;9:e1002898. doi: 10.1371/journal.pcbi.1002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parasuraman R, McKinley RA. Using noninvasive brain stimulation to accelerate learning and enhance human performance. Hum Factors. 2014;56:816–24. doi: 10.1177/0018720814538815. [DOI] [PubMed] [Google Scholar]
- 72.Gladwin TE, den Uyl TE, Fregni FF, Wiers RW. Enhancement of selective attention by tDCS: interaction with interference in a Sternberg task. Neurosci Lett. 2012;512:33–7. doi: 10.1016/j.neulet.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 73.Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M. Classification of methods in transcranial electrical stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. 2013;219:297–311. doi: 10.1016/j.jneumeth.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Camel E, O’Connell M, Sage B, Gross M, Maibach H. The effect of saline iontophoresis on skin integrity in human volunteers. I. Methodology and reproducibility. Fundam Appl Toxicol. 1996;32:168–78. doi: 10.1006/faat.1996.0120. [DOI] [PubMed] [Google Scholar]
- 76.Draize J, Woodard G, Calvery H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;8:377–90. [Google Scholar]
- 77.Guarienti F, Caumo W, Shiozawa P, Cordeiro Q, Boggio PS, Benseñor IM, et al. Reducing transcranial direct current stimulation-induced erythema with skin pretreatment: considerations for sham-controlled clinical trials. Neuromodulation. 2015;18:261–5. doi: 10.1111/ner.12230. [DOI] [PubMed] [Google Scholar]
- 78.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State–Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31:301–6. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 79.Glantz SA. Primer of biostatistics. 7. New York: McGraw-Hill Medical; 2011. [Google Scholar]
- 80.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 81.36-item short form survey scoring instructions. RAND; n.d. [accessed 25.04.16]. Monica 1776 Main Street Santa, 90401-3208 C. http://www.rand.org/health/surveys_tools/mos/mos_core_36item_scoring.html. [Google Scholar]
- 82.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Jaberzadeh S, Bastani A, Zoghi M, Morgan P, Fitzgerald PB. Anodal transcranial pulsed current stimulation: the effects of pulse duration on corticospinal excitability. PLoS ONE. 2015;10:e0131779. doi: 10.1371/journal.pone.0131779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richardson JD, Fillmore P, Datta A, Truong D, Bikson M, Fridriksson J. Toward development of sham protocols for high-definition transcranial direct current stimulation (HD-tDCS) Neuro Regul. 2014;1:62. doi: 10.15540/nr.1.1.62. [DOI] [Google Scholar]
- 85.Xu K, Wang L, Mai J, He L. Efficacy of constraint-induced movement therapy and electrical stimulation on hand function of children with hemiplegic cerebral palsy: a controlled clinical trial. Disabil Rehabil. 2012;34:337–46. doi: 10.3109/09638288.2011.607213. [DOI] [PubMed] [Google Scholar]
- 86.Merring CA, Gobert DV. Recovery 9 years post stroke with standardized electrical stimulation. Occup Ther Health Care. 2011;25:108–18. doi: 10.3109/07380577.2011.556696. [DOI] [PubMed] [Google Scholar]
- 87.Quandt F, Hummel FC. The influence of functional electrical stimulation on hand motor recovery in stroke patients: a review. Exp Transl Stroke Med. 2014;6:9. doi: 10.1186/2040-7378-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dundas JE, Thickbroom GW, Mastaglia FL. Perception of comfort during transcranial DC stimulation: effect of NaCl solution concentration applied to sponge electrodes. Clin Neurophysiol. 2007;118:1166–70. doi: 10.1016/j.clinph.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 89.Kronberg G, Bikson M. Electrode assembly design for transcranial direct current stimulation: a FEM modeling study. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:891–5. doi: 10.1109/EMBC.2012.6346075. [DOI] [PubMed] [Google Scholar]