Abstract
A central assumption in ecological immunology is that immune responses are costly, with costs manifesting directly (e.g., increases in metabolic rate and increased amino acid usage) or as tradeoffs with other life processes (e.g., reduced growth and reproductive success). Across taxa, host longevity, timing of maturity, and reproductive effort affect the organization of immune systems. It is reasonable, therefore, to expect that these and related factors should also affect immune activation costs. Specifically, species that spread their breeding efforts over a long lifetime should experience lower immune costs than those that mature and breed quickly and die comparatively early. Likewise, body mass should affect immune costs, as body size affects the extent to which hosts are exposed to parasites as well as how hosts can combat infections (via its effects on metabolic rates and other factors). Here, we used phylogenetic meta-regression to reveal that, in general, animals incur costs of immune activation, but small species that are relatively long-lived incur the largest costs. These patterns probably arise because of the relative need for defense when infection risk is comparatively high and fitness can only be realized over a comparatively long period. However, given the diversity of species considered here and the overall modest effects of body mass and life history on immune costs, much more research is necessary before generalizations are appropriate.
1 | INTRODUCTION
Protection against infection often comes at a cost to hosts (Lochmiller & Deerenberg, 2000). Once exposed to an infectious threat, costs of immune activation can involve increases in resource use, such as elevated metabolic rate or amino acid assimilation (Brace, Sheikali, & Martin, 2015; Lochmiller & Deerenberg, 2000), or as tradeoffs with life-history traits such as growth or reproduction (Bonneaud et al., 2003). Although hosts can sometimes mitigate these costs by increasing resource intake (Ruiz, French, Demas, & Martins, 2010), in natural environments, resources are typically limited and thus must be distributed among competing physiological processes. A number of studies have demonstrated that costs of immune activation are present, marked, and variable among populations (Bonneaud et al., 2003; Cox & Calsbeek, 2010; Lee, 2006; Martin et al., 2017). However, the large-scale, evolutionary drivers of these observed costs remain under debate.
Exposing broad patterns of immune costs might refine and clarify the processes driving variation in susceptibility and perhaps even implicate species prone to serve as competent reservoirs (or infection risk-diluters) within communities. Potential drivers of immune system costs among species probably include life-history traits such as lifespan and time to reproductive maturity. Longer lived and slower to mature organisms likely experience overall greater exposure to parasites, and hence different trajectories of immune ontogeny, than fast-developing, prolifically breeding species. Some support from this expectation comes from the recurrent observation that some long-lived species favor specific immune defenses over nonspecific ones (Martin, Weil, & Nelson, 2007). The reasons for this pattern are thought to be twofold. First, longer lived organisms are more likely to encounter the same parasites multiple times during their lives, making it beneficial to control infections via specific defenses, which can be costly to develop (Ricklefs, 1992). Second, but nonexclusively, by favoring specific defenses (with memory), long-lived organisms might avoid recurrent collateral damage (costs) associated with nonspecific defenses (Ricklefs & Wikelski, 2002).
Body size might also influence immune costs experienced by organisms. Indeed, many aspects of physiology (Gillooly & Allen, 2007; Ricklefs & Wikelski, 2002; West, Brown, & Enquist, 1997) are influenced by host body size including traits of immune systems. Immune costs might also track body size. The surfaces of digestive and respiratory tracts and skin, for instance, which are the most common sites of parasite invasion, are larger in large than small organisms (Huang et al., 2013; West et al., 1997; Wiegel & Perelson, 2004). Metabolic rate (scaling coefficient of ¾) and number of cells (scaling coefficient of 1) also increase with body mass (Lindstedt & Calder III, 1981; Savage et al., 2004). Subsequently, large organisms should require more immune surveillance and protection, and probably more resources to provide protection, than small organisms.
Here, we investigated whether and how lifespan, time to reproductive maturity, and body size affect costs of immunity in hosts. We focused exclusively on functional costs of immunity such as changes in body mass, physical performance, food intake, growth rate, egg production, egg size, gonad size, breeding effort, survival probability, recruitment success, and dispersal behavior when exposed to an immune system stimulant (Bonneaud et al., 2003; Bonneaud, Mazuc, Chastel, & Westerdahl, 2004; Cox & Calsbeek, 2010; Klasing & Korver, 1997; Laugero & Moberg, 2000). We excluded changes in physiological traits (e.g., body temperature, metabolic rate, antibody production), as these traits are difficult to link to fitness. Sometimes increases are protective (e.g., fever, to a point), but sometimes decreases are protective (e.g., timely damping of inflammatory cytokine expression), and such patterns could even vary among taxa. We also focused solely on studies that used nonreplicating immune stimulants to induce immune responses, and excluded studies using live pathogens because live parasites could themselves affect (the costs of) host responses. In total, our analysis included 236 immune costs among 39 invertebrate and vertebrate species with lifespans and times to maturity spanning three orders of magnitude (21 days to 40 years and 8 days to 5 years, respectively), and body sizes spanning nine orders of magnitude (0.71 mg–200 kg). We hypothesized that costs of immunity would be the smallest in long-lived, long-developing, and/or small-bodied species (Tella, Scheuerlein, & Ricklefs, 2002; Wiegel & Perelson, 2004), and we used phylogenetic meta-analysis to test these hypotheses.
2 | METHODS
2.1 | Search methods and inclusion/exclusion criteria
Candidate studies were identified using Web of Science and Google Scholar based on the search terms: cost* and immun*, trade* and immun*, and fitness and immun*. Between November 2011 and January 2012, and approximately 179 candidate studies published between 1991 and 2010 identified with these search terms were selected by screening paper titles and abstracts. Studies were included in the meta-analysis if (1) immune responses were induced with a non-living pathogen or comparably immunogenic substance, (2) an unmanipulated or procedural (e.g., sham and saline) control group was used, (3) the study organism was not a domesticated species or human (as intentional selection or adaptation to captivity could have led to the evolution of damped immune costs), and (4) measured costs were functional (e.g., activity, food intake, growth rate, mass change, egg production, egg size, gonad size, breeding effort, individual survival, recruitment rate, and dispersal behavior). Studies that only reported statistical model outcomes or marginal means were excluded because effect sizes (see below) could not be calculated with such information. In total, 46 research articles met our inclusion criteria (see Supplementary Table S1).
From each research article, we extracted (1) the study species and its taxonomic affiliation, (2) the type of control (i.e., unmanipulated and procedural controls, including sham-injected and saline-injected individuals), and (3) the stimulant used to activate the immune system (e.g., LPS, vaccines, nylon thread). These stimulants were grouped depending on how they activated the immune system (i.e., innate or adaptive), or grouped as “mixed” if they were difficult to assign (e.g., vaccine treatments with multiple antigens and/or adjuvants). When possible, we also extracted average body mass, lifespan, and time to maturity for each study species from these papers. However, species characteristics were often not reported, so data were supplemented using other resources (Supplementary Table S2).
2.2 | Outcome extractions and experimental design modeling
Study outcomes were quantified as the standardized mean difference between a treatment (T) and control (C) group using effect sizes based on Hedges’ d (Hedges, 1981). Negative values for Hedges’ d thus represent costs of immune activation because they reflect a decline in performance or fitness in the treated group relative to the control. To calculate effect sizes, we extracted the means, standard deviations (SD), and sample sizes (N) of each group, and combined these study parameters into an effect size (d) using:
which has variance
When possible, these study parameters were extracted from tables, text, and supplemental materials, or extrapolated from plots manually. When standard errors were reported, they were converted to standard deviations. Several studies reported relevant outcomes based on counts or proportions (e.g., individuals surviving with or without immune activation). The outcomes of these studies were first quantified with an odds ratio effect size and then converted to Hedges’ d using the equation reported in LAJEUNESSE, KORICHEVA, GUREVITCH, and MENGERSEN (2013); less than 6% of effect sizes were derived from these conversions. Several outcomes were often extracted from a single study. This approach can be a problem for meta-analysis because multiple extractions derived from the same experimental design will share study parameters that are statistically dependent, which can increase type II error (Lajeunesse, 2011). In our meta-analysis, we modeled several forms of experimental dependencies to improve our variance estimates and hypothesis tests. For example, many studies used a design that compared several types of controls to a single treatment group. Typically, one control group consisted of unmanipulated (U) individuals, and a second group of individuals underwent a procedural treatment (P) intended to quantify administration effects of the stimulant (e.g., a sham or saline injection to control for injury effects). However, effect sizes derived from this design share a common treatment group and therefore do not represent independent study outcomes. We subsequently modeled the covariance between these two effects using a modified version of Gleser and Olkin’s (Olkin & Gleser, 2009) covariance (cov) equation for Hedges’ d effect sizes with a common control cov(dC-U, dC-P) = (1 + 0.5dC-UdC-P)/(NT + NC-U + NC-P), where both dC-U and dC-P used the pooled standard deviation:
When studies reported multiple outcomes as well as the correlation between these outcomes (e.g., outcomes based on two vaccines), we modeled their dependencies using the covariance equation between multivariate d for unequal sample sizes reported in Robinson, Lajeunesse, and Forbes (2012). Finally, when outcomes were reported as a time series (either pre- and post-effect designs, or through repeated measurements through time), we modeled dependencies by first estimating phi (an optimized autocorrelation value for the white noise in a weighted regression model using the nlme R package; Pinheiro, Bates, Debroy, Sarkar, & Team, 2009) using a simple ARMA (p, q) model assuming a first-order autoregressive structure (p = 1) and no moving average correlations (q = 1). When phi could not be estimated, we assumed phi had a small autoregressive structure between time series of 0.25. Phis were then converted into a variance/covariance matrix following Trikalinos and Olkin (2012).
2.3 | Traditional and phylogenetic analyses
We analyzed extracted effect sizes using multifactor mixed-model meta-analyses (for categorical predictors) and meta-regressions (for continuous predictors). All regression models assumed a maximum likelihood based between-study variance estimate (τ2) required for random-effects meta-analysis and included a block-diagonal sampling variance–covariance matrix. This approach provides the weights for each effect size used in the weighted regressions with the variances of d on main diagonal and the covariances used to model dependencies arising from various experimental designs (i.e., multiple control groups). The regression equations used to perform meta-analyses require that each variance–covariance matrix used to model dependencies be symmetric and positive definite; therefore when necessary, we used Higham’s method (Higham, 2002).
Phylogenetic analyses included two additional random effects: a random factor designating the multiple effect sizes derived from single species and a second, unstructured random effect matrix modeling the shared evolutionary history (i.e., phylogenetic correlations) of species (Lajeunesse, 2009). Our phylogenetic correlation matrix was derived from a composite phylogeny assuming a Brownian motion model of phenotypic evolution (Rohlf, 2001) using the vcv() function of the ape package in R (Paradis, Claude, & Strimmer, 2004). The taxonomic composition of this ultrametric tree was broad and included 39 species from three invertebrate (Insecta, Gastropoda, and Bivalvia) and three vertebrate classes (Aves, Mammalia, and Reptilia). The deep divergence times and topology were based on Hedges, Dudley, and Kumar (2006) for classes, Meredith et al., 2011 for mammals, Jetz, Thomas, Joy, Hartmann, and Mooers (2012) for birds, and Trautwein, Wiegmann, Beutel, Kjer, and Yeates (2012) for insects. In cases when divergence times were unavailable, we arbitrarily scaled branch-lengths distances using Grafen’s method using ρ to the power of 1.0 to model divergence times emerging from a Brownian motion model of evolution. Finally, all analyses were performed using the rma.mv() function from the metafor package in R (Viechtbauer, 2010) assuming the nlminb optimizer. Pseudo-R2 (proportional reduction in the total variance explained) for each model was estimated as follows: R2 = (Στ2base − Στ2predictors)/Στ2base, where Στ2base are all the estimated random effect variances from a model without predictors (base model) and τ2predictors are the variances of a model including predictors. Between-group Q-tests were used as omnibus tests for comparing differences among predictor categories (Hedges & Olkin, 2014) and continuous predictors (e.g., mass) were log10 transformed prior to analyses.
3 | RESULTS
3.1 | Is immune activation costly?
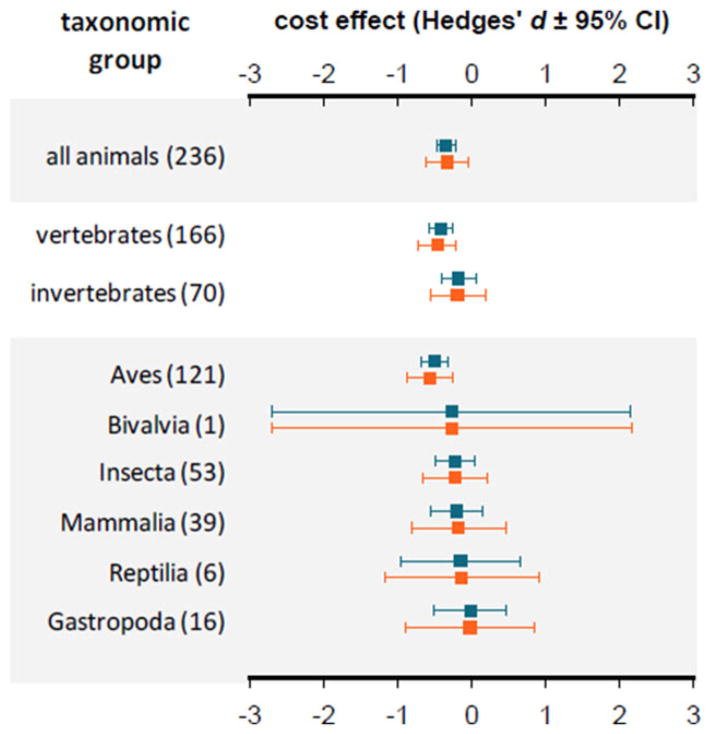
Overall, we detected significant functional costs of immune activation (k = 236 effect sizes; conventional grand mean d = − 0.34, 95% CI: − 0.47 to − 0.21; phylogenetic grand mean d = − 0.33, 95% CI: − 0.57 to − 0.09; Fig. 1); the mean effect size between immune challenged and unchallenged/control organisms was negative, revealing that immune activation tended to reduce performance relative to controls. Variation in these costs was also substantially beyond that predicted by sampling error (fixed-effect Q within = 1,253.5, df = 235, P < 0.001); thus, there is little evidence for a file drawer problem for null results. The file drawer problem refers to the possibility that many null results might not have been published, often because of concerns about the lack of statistical power to be confident that effects are genuinely absent (Rosenberg, 2005). Also, although the type of immune challenge and the type of costs were considered (e.g., lipopolysaccharide vs. nonpyrogens), these sources of variation were negligible when the phylogenetic history of taxa was modeled.
FIGURE 1.
Immune activation is costly among animals; all Hedges’ d effects were negative. Pooled effects are based on conventional (blue) and phylogenetic (orange) random-effects meta-analyses; the number of pooled effect sizes is provided in parentheses. Among all taxa, the conventional meta-analysis model was the better fit [Color figure can be viewed at wileyonlinelibrary.com]
3.2 | What factors predict immune costs?
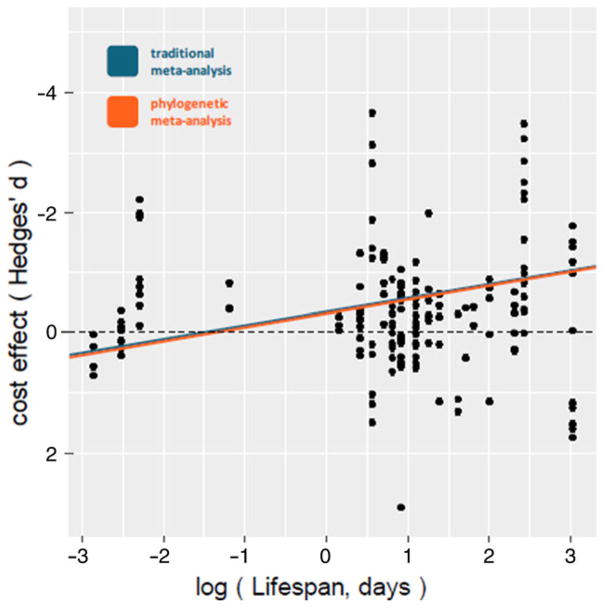
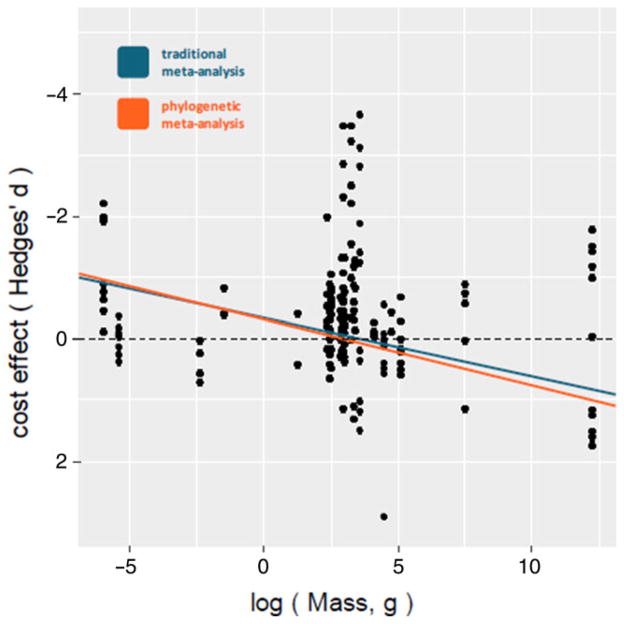
Contrary to our predictions, neither lifespan, nor time to maturity, nor body mass predicted costs of immune activation when modeled alone (lifespan: conventional β = − 0.02, SE = 0.05, 95% CI: − 0.13 to 0.08, P = 0.64; phylogenetic β = − 0.03, SE = 0.07, 95% CI: − 0.18 to 0.11, P = 0.64; time to maturity: conventional β = 0.05, SE = 0.05, 95% CI: − 0.04 to 0.16, P = 0.29; phylogenetic β = 0.03, SE = 0.08, 95% CI: − 0.11 to 0.19, P = 0.65; body mass: conventional β = 0.01, SE = 0.02, 95% CI: − 0.03 to 0.05, P = 0.58; phylogenetic β = 0.03, SE = 0.04, 95% CI: − 0.04 to 0.11, P = 0.40). However, when lifespan and body mass were modeled simultaneously, longer lived animals had larger immune costs than short-lived ones (Fig. 2) in conventional (β = − 0.23, SE = 0.11, 95% CI: − 0.42 to − 0.03, P = 0.02) but not phylogenetic models (β = − 0.23, SE = 0.13, 95% CI: − 0.49 to 0.03, P = 0.08). Body mass was a significant predictor of immune costs in both conventional (β = 0.1, SE = 0.04, 95% CI: 0.02–0.18, P = 0.01) and phylogenetic meta-regressions (β = 0.11, SE = 0.05, 95% CI: <0.001–0.21, P = 0.05), such that smaller animals experienced greater immune costs than larger ones (Fig. 3). The phylo-genetically informed model was the better-fit compared to the conventional model (LR c2 = 0.22, P = 0.318). In a separate meta-regression, we found no significant interaction between lifespan and body mass in either conventional (β = − 0.004, SE = 0.01, 95% CI: − 0.03 to 0.02, P = 0.70) or phylogenetic analyses (β = − 0.004, SE = 0.02, 95% CI: − 0.04 to 0.03, P = 0.79), so no interaction term was included in additional modeling efforts.
FIGURE 2.
Lifespan (mean days to death) was a significant positive predictor of immune activation cost (when body mass effects were controlled); longer-lived animals experience higher immune costs. Note the inverted y-axis to convey better increasing costs with increasing lifespan. Random-effects meta-regression lines are based on conventional (blue) and phylogenetically informed (orange) models derived from a meta-regression that simultaneously modeled mass and lifespan as cost predictors (nonsignificant intercept was not included in this model) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
Body mass (grams) corrected for lifespan is a significant predictor of immune cost such that costs are largest for the smallest animals. Higher costs are shown as more negative, indicating a decrease in function, performance, or fitness as a result of immune activation. Random-effects meta-regression lines are based on conventional (blue) and phylogenetic (orange) models, and are derived from a meta-regression that simultaneously modeled mass and lifespan as cost predictors (nonsignificant intercept was not included in this model) [Color figure can be viewed at wileyonlinelibrary.com]
When body mass was included in a meta-regression model testing whether time to maturity predicted costs of immune activation, neither time to maturity (conventional β = 0.03, SE = 0.08, 95% CI: − 0.13 to 0.19, P = 0.74; phylogenetic β= − 0.04, SE = 0.13, 95% CI: − 0.29 to 0.22, P = 0.77) nor body mass (conventional β= 0.02, SE = 0.03, 95% CI: − 0.05 to 0.08, P = 0.62; phylogenetic β= 0.05, SE = 0.06, 95% CI: − 0.05 to 0.16, P = 0.35) was predictive. The interaction between time to maturity and body mass was nonsignificant when tested in a separate model (conventional β= 0.01, SE = 0.01, 95% CI: − 0.01 to 0.03, P = 0.59; phylogenetic β= 0.003, SE = 0.02, 95% CI: − 0.03 to 0.04, P = 0.84), so it was excluded. The conventional meta-analysis model fit the data better than the phylogenetic model (among all animals: LR c2 = 10.78, P < 0.001; among taxonomic groups of vertebrates and invertebrates: LR c2 = 7.99, P < 0.001; and among taxonomic classes: LR c2 = 4.96, P < 0.001).
4 | DISCUSSION
Our phylogenetic meta-regression supported a central tenet of ecological immunology—immune activities impart functional costs to hosts (Bonneaud et al., 2003; Bowers, Bowden, Sakaluk, & Thompson, 2015). Contrary to our predictions, though, costs of immune activation were not strongly affected by lifespan, time to maturity, or body mass. Only when two traits were modeled together, there was some evidence that costs were related to these traits. In one case, small yet long-lived hosts tended to pay the largest costs of immune activation. In the other case, time to maturity did not significantly predict immune costs alone or when modeled with body mass. Moreover, there was no support for interactions between body mass and lifespan or time to maturity among taxa. In general and not surprisingly because of the distinctiveness of the immune systems of studied species, there was evidence for an influence of phylogeny on immune costs in multiple models. At first consideration, this outcome calls into question the existence of any influence of lifespan on immune costs independent of evolutionary history (Fig. 2). However, the pattern involving body mass, for a given lifespan, appears robust, as body mass remained a significant predictor in both corrected and uncorrected models. By contrast, phylogeny added little to the model for time to maturity and body mass as predictors of immune costs; there, the conventional model received comparable support to the evolutionarily informed one. Below, we discuss the implications of our results as well as promising avenues for future work.
4.1 | Body mass
Functional costs of an immune response were largest in small yet long-lived hosts. However, effects of body mass were modest at best (slope (β) ~0.11 ± 0.05 in both corrected and uncorrected models). In neither model did the confidence interval of body mass include zero, so although body mass effects were small, they were detectable, which is especially compelling given the very diverse group of organisms we considered. Indeed, our ability to detect an influence of body mass on immune costs among such distinct species warrants some discussion. One possible explanation for the pattern despite the immunological diversity inherent to the group is that all animals, once exposed to a parasite, tend to use nonspecific immune responses as a first line of defense. These responses are among the most resource-intensive and frequently result in tradeoffs with other physiological systems (Lochmiller & Deerenberg, 2000). In small animals, which already expend more energy per gram and experience more oxidative damage than large organisms (Speakman, 2005), the additional energy required to initiate an immune response and the resultant increase in oxidative damage may affect allocation of resources to fitness and performance severely. In other words, the higher costs we observed in small organisms might largely be a manifestation of a disproportionally more active metabolism in these species.
Another nonexclusive possibility involves risk of infection, which might also change with body size. Although larger animals have more total surface area at risk to parasite exposure (Wiegel & Perelson, 2004), the greater tissue volume of large hosts might act as a physically larger barrier for parasites to overcome. If so, parasite exposure in a small animal may have a greater potential for successful infection. Infection risk might be further exacerbated in small organisms because they have less cellular diversity; large organisms (especially across this scale of taxonomic diversity) will have more cell types than small ones. Less cell diversity could mean that invading parasites might be more likely to find a viable home; large hosts might thus experience some protection from infection just because of the relative inhospitability of their tissues. If this prediction holds true, the value of a single cell would much greater to a small than large host (particularly long-lived ones). Subsequently, high immune costs in small hosts might represent the need to protect individual integrity because bacterial/viral proliferation or macroparasite invasion might be more apt to subsume a greater proportion of the body mass compared to large organisms. Risk of infection, and damage from infection, could be further amplified by the higher per cell metabolic rate of small hosts, which would potentiate enemy replication in/on host tissues. Ultimately, it may be more beneficial for small animals to cope with the costs of a strong immune response than risk a potentially lethal infection (Siva-Jothy, Moret, & Rolff, 2005).
On the other hand, surveillance for parasites is a much greater challenge in large than small organisms. There are simply more and more diverse sites to hide in a large organism, and the vasculature is by and large comparable in efficacy among species. One way large hosts might circumvent this constraint and thus incur smaller induced immune costs could be via the assembly of robust constitutive defenses. We expect that strong constitutive defenses will be unviable for small-bodied organisms; their body sizes cannot support the storage of resources or the transport of robust barriers to prevent infection. Large-bodied organisms, on the other hand, could invest heavily in the development of enduring barriers to infection. There is some evidence that large-bodied animals have more robust constitutive immune defenses than small-bodied species. However, there is other evidence that indicates scale-invariance for some immune defenses (or even robust induced (inflammatory defenses)) in large animals. These opposing or absent patterns between immunity and body mass highlight the need for additional data to help explain the patterns we found.
Given the complexities inherent to the immune systems and the evolutionary ecology of host–parasite interactions, we ardently encourage future study of body mass relationships with immune costs and immune systems in general. Indeed, we cannot rule out taxonomic bias here as an influence on our outcomes. We used all available literature at the time and took the unprecented approach (in terms of its diversity) to compare immune costs among very diverse taxa. Still, many of the small animals we considered lack adaptive immunity. We thus cannot conclude with confidence that the associations found here arose because insects comprise many of the small bodied species and lack an adaptive immune system, which partly evolved to ameliorate the costs of repeated parasite exposure (Rolff, 2007). Moreover, in these animals that possess open circulatory systems, the rapidity of an immune response will impact the movement of parasites and their antigens throughout the body cavity (Siva-Jothy et al., 2005). In vertebrates, the vasculature and the molecules that coordinate the movements of defenses around the body are more sophisticated, not to mention integrated with the advantages of the adaptive immune system (Medzhitov & Janeway, 1998). We cannot rule out these issues as influential to our results.
Critically too, all of the above propositions for body mass effects on immune costs must be considered in light of the added influence of lifespan we detected here. Longer lifespan amplified the effects of small size on immune costs, whereas short lifespan ameliorated them. This complex pattern probably represents the consequence of strong selection for high-induced immune costs for small organisms evolved to live long lives. If (as argued above) investment in robust constitutive defenses is not an option for small organisms, small, long-lived organisms might have no recourse but to respond aggressively when exposed to an infectious threat. The mandate to live a long time might require a costly immune response with every exposure; otherwise, the host in question would never have the chance to gain fitness. Alternatively, small, short-lived species that have evolved short lifespans might be selected to avoid immune costs when at all possible. When such host species become infected, the most viable strategy might involve terminal investments (Bonneaud et al., 2004; Bowers et al., 2015) or the like. Of course, short-lived species would also have shorter generation times than long-lived ones, regardless of their size. This evolutionary advantage could have enabled short-lived, reproductively prolific species to evolve cost-compensatory mechanisms (particularly against collateral damage from immune responses), something unavailable to unrelated long-lived hosts. All of these possibilities require future investigation; as yet and surprisingly, we know very little about how body size affects the architecture and economics of immune systems.
4.2 | Life-history effects
There was surprisingly little evidence for independent effects of lifespan and time to maturity on immune activation costs. The only statistically significant results involved lifespan in conjunction with body size. However, in the better-supported phylogenetic model, lifespan effects were nonsignificant, so even this influence of life history was modestly supported. There are several possible, but nonexclusive, explanations of the absence of strong patterns between life-history traits and immune costs. The most obvious is that the two life-history traits we evaluated do not capture well the life-history differences among species that affect exposure to parasites and thus the relative fitness consequences parasites have for hosts. We chose lifespan and time to maturity because of prior support for their influences on immune systems in the literature, but also because they were among the few traits for which data were available and conducive to the breadth of host species we could compare. Other traits, such as total lifetime reproductive effort, might be more informative. However, we were unable to obtain such information, and even if such information could be attained, species in this data set might be so unique that comparisons of life-history traits might be difficult to execute effectively. Indeed, another possible explanation for the absence of strong life-history effects on immune costs might be the inclusion of so many diverse species. In an effort to identify drivers of broad patterns of immune variation, we chose to conduct one analysis on all available data. This approach might have obscured compelling and stronger patterns at finer levels of taxonomy. We are reticent to reexamine our data, though, because for most families/genera we have too few data. Only for birds, mammals, and insects do we have large sample sizes. Instead of conducting such additional meta-regressions, we encourage new efforts to describe immune costs in these and other species. Then, more nuanced study of life history (and body mass) might reveal stronger and more obvious effects on immune costs.
5 | CONCLUSION
Altogether, our meta-analysis revealed appreciable costs of immune activation among diverse animals, but effects of body mass were modest and effects of life history were (mostly) absent. What evidence of mass and life history we found suggested that small animals, disproportionately long-lived for their size, experience the largest costs of immune activation. However, given the diversity of species studied here (physiologically as well as evolutionarily), we are reticent to dwell on the implications of these results for disease risk or host–parasite evolution. We are intrigued that we were able, at all, to detect relationships between body mass and immune costs when the species studied have such distinct immune systems, parasite communities, and ecologies. Given the manifestation of a relationship between body mass, lifespan, and immune costs, it would be useful and probably insightful to continue investigations such as ours, either in the form of additional meta-analyses of more direct efforts to quantify and compare immune costs among species.
Supplementary Material
Acknowledgments
Funding information
Grant sponsor: NSF RCN in Ecoimmunology; Grant number: 0947177; Grant sponsor: NSF IOS; Grant numbers: 0920475, 1257773, 1656551, and 1054675; Grant sponsor: NSF DBI; Grant number: 1262545; Grant sponsor: NIH; Grant number: 1R15HD066378 01; Grant sponsor: ARC Future Fellowship; Grant number: FT140100131.
We thank Holly Kilvitis, Sarah Burgan, Stephanie Gervasi, and Doug Barron for comments on earlier drafts, and Jennifer Everhart for help collecting data. We also thank members of the NSF RCN in Ecoimmunology for multiple insightful discussions over the course of this project. Funding was provided by NSF RCN in Ecoimmunology grant (0947177), NSF IOS 0920475, 1257773, and 1656551 to L.B.M., NSF DBI 1262545 to M.J.L., and NIH grant 1R15HD066378 01 to J.L.G. K.L.B. was supported by ARC Future Fellowship FT140100131 and J.S.A. was supported by NSF IOS 1054675 to D.M.H.
Footnotes
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Bonneaud C, Mazuc J, Chastel O, Westerdahl H. Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the house sparrow. Evolution. 2004;58:2823–2830. doi: 10.1111/j.0014-3820.2004.tb01633.x. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. American Naturalist. 2003;161:367–379. doi: 10.1086/346134. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. American Naturalist. 2015;185:769–783. doi: 10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace AJ, Sheikali S, Martin LB. Highway to the danger zone: Exposure-dependent costs of immunity in a vertebrate ectotherm. Functional Ecology. 2015;29:924–930. [Google Scholar]
- Cox RM, Calsbeek R. Severe costs of reproduction persist in Anolis lizards despite the evolution of a single-egg clutch. Evolution. 2010;64:1321–1330. doi: 10.1111/j.1558-5646.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Gillooly JF, Allen AP. Changes in body temperature influence the scaling of and aerobic scope in mammals. Biology Letters. 2007;3:100–103. doi: 10.1098/rsbl.2006.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6:107–128. [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Elsevier; London: 2014. [Google Scholar]
- Hedges SB, Dudley J, Kumar S. TimeTree: A public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- Higham NJ. Computing the nearest correlation matrix—A problem from finance. IMA Journal of Numerical Analysis. 2002;22:329–343. [Google Scholar]
- Huang ZY, De Boer WF, Van Langevelde F, Olson V, Blackburn TM, Prins HH. Species’ life-history traits explain interspecific variation in reservoir competence: A possible mechanism underlying the dilution effect. PLoS One. 2013;8:e54341. doi: 10.1371/journal.pone.0054341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz W, Thomas G, Joy J, Hartmann K, Mooers A. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- Klasing KC, Korver DR. Leukocytic cytokines regulate growth rate and composition following activation of the immune system. Journal of Animal Science. 1997;75:58–67. [Google Scholar]
- Lajeunesse MJ. Meta-analysis and the comparative phylogenetic method. American Naturalist. 2009;174:369–381. doi: 10.1086/603628. [DOI] [PubMed] [Google Scholar]
- Lajeunesse MJ. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology. 2011;92:2049–2055. doi: 10.1890/11-0423.1. [DOI] [PubMed] [Google Scholar]
- Lajeunesse MJ. Recovering missing or partial data from studies: A survey of conversions and imputations for meta-analysis. In: Koricheva J, Gurevitch J, Mengersen K, editors. Handbook of meta-analysis in ecology and evolution. New Jersey, USA: Princeton University Press, Princeton; 2013. pp. 195–206. [Google Scholar]
- Laugero KD, Moberg GP. Effects of acute behavioral stress and LPS-induced cytokine release on growth and energetics in mice. Physiology & Behavior. 2000;68:415–422. doi: 10.1016/s0031-9384(99)00206-1. [DOI] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integrative and Comparative Biology. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lindstedt S, Calder W., III Body size, physiological time, and longevity of homeothermic animals. Quarterly Review of Biology. 1981;56:1–16. [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Martin LB, Kilvitis HJ, Brace AJ, Cooper L, Haussmann MF, Mutati A, … Ardia DR. Costs of immunity and their role in the range expansion of the house sparrow in Kenya. Journal of Experimental Biology. 2017;220:2228–2235. doi: 10.1242/jeb.154716. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007;88:2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr An ancient system of host defense. Current Opinion in Immunology. 1998;10:12–15. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- Meredith RW, Janecka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, … Stadler T. Impacts of the cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science. 2011;334:521–524. doi: 10.1126/science.1211028. [DOI] [PubMed] [Google Scholar]
- Olkin I, Gleser L. Stochastically dependent effect sizes. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. New York: Russel Sage Foundation; 2009. pp. 357–376. [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, Debroy S, Sarkar D, Team RC. R Package Version 3. 2009. nlme: Linear and nonlinear mixed effects models. [Google Scholar]
- Ricklefs RE. Embryonic development period and the prevalence of avian blood parasites. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4722–4725. doi: 10.1073/pnas.89.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends in Ecology & Evolution. 2002;17:462–468. [Google Scholar]
- Robinson SA, Lajeunesse MJ, Forbes MR. Sex differences in mercury contamination of birds: Testing multiple hypotheses with meta-analysis. Environmental Science & Technology. 2012;46:7094–7101. doi: 10.1021/es204032m. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ. Comparative methods for the analysis of continuous variables: Geometric interpretations. Evolution. 2001;55:2143–2160. doi: 10.1111/j.0014-3820.2001.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Rolff J. Why did the acquired immune system of vertebrates evolve? Developmental and Comparative Immunology. 2007;31:476–482. doi: 10.1016/j.dci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Rosenberg MS. The file-drawer problem revisited: A general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59:464–468. [PubMed] [Google Scholar]
- Ruiz M, French SS, Demas GE, Martins EP. Food supplementation and testosterone interact to influence reproductive behavior and immune function in Sceloporus graciosus. Hormones and Behavior. 2010;57:134–139. doi: 10.1016/j.yhbeh.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage VM, Gillooly J, Woodruff W, West G, Allen A, Enquist BJ, Brown J. The predominance of quarter-power scaling in biology. Functional Ecology. 2004;18:257–282. [Google Scholar]
- Siva-Jothy MT, Moret Y, Rolff J. Insect immunity: An evolutionary ecology perspective. Advances in Insect Physiology. 2005;32:1–48. [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. Journal of Experimental Biology. 2005;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Tella JL, Scheuerlein A, Ricklefs RE. Is cell-mediated immunity related to the evolution of life-history strategies in birds? Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:1059–1066. doi: 10.1098/rspb.2001.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein MD, Wiegmann BM, Beutel R, Kjer KM, Yeates DK. Advances in insect phylogeny at the dawn of the postgenomic era. Annual Review of Entomology. 2012;57:449–468. doi: 10.1146/annurev-ento-120710-100538. [DOI] [PubMed] [Google Scholar]
- Trikalinos TA, Olkin I. Meta-analysis of effect sizes reported at multiple time points: A multivariate approach. Clinical Trials. 2012;9:610–620. doi: 10.1177/1740774512453218. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- Wiegel FW, Perelson AS. Some scaling principles for the immune system. Immunology and Cell Biology. 2004a;82:127–131. doi: 10.1046/j.0818-9641.2004.01229.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.