ABSTRACT
The Arabidopsis K+ channel KAT1 complements in K+-limited medium the growth of the K+ uptake defective Saccharomyces cerevisiae mutant strain CY162, while another K+ channel, AKT2, does not. To gain insight into the structural basis for this difference, we constructed 12 recombinant chimeric channels from these two genes. When expressed in CY162, only three of these chimeras fully rescued the growth of CY162 under K+-limited conditions. We conclude that the transmembrane core region of KAT1 is important for its activity in S. cerevisiae. This involves not only the pore region but also parts of its voltage-sensor domain.
KEYWORDS: AKT2, KAT1, membrane transport, potassium channels, transporter
Introduction
In plants, voltage-dependent K+ channels play an important role in K+ uptake from the soil, long-distance K+ transport in the xylem and phloem, and K+ fluxes in guard cells during stomatal movements, for instance.1-3 The genome of the model plant Arabidopsis thaliana contains seven genes coding for voltage-dependent K+ channel subunits. A functional plant K+ channel is built of four α-subunits. A single α-subunit shows a topology of six transmembrane domains, named S1-S6, which are flanked by cytosolic N- and C-termini, and a P (pore) loop between S5 and S6 that contribute to the channel pore and ion selectivity filter.4 The voltage-sensing property of voltage-gated K+ channels is associated with the first four transmembrane domains and mainly with the S4 helix.5 The highest conservation among plant voltage-gated K+ channels is found in the P loop, which contains the hallmark motif GYGD, the signature of highly selective K+ channels.6 The long C-terminal region harbors a putative cyclic nucleotide-binding domain (cNBD) and at the extreme C-terminus the KT region, which is involved in channel tetramerization,7 and the so-called KHA region (rich in hydrophobic and acidic residues), which might be involved in channel clustering in the membrane.8,9 An ankyrin domain, hypothesized to be a site of interaction with regulatory proteins,10 is present in many plant K+ channels between the cNBD and the KHA region.
According to their gating characteristics, the family of plant voltage-gated K+ channels segregates into at least three functionally distinct subfamilies6,11,12: (1) hyperpolarization-activated, inward-rectifying K+ channels, such as KAT1 and AKT1, mediate K+ uptake, (2) depolarization-activated, outward-rectifying K+ channels, such as SKOR and GORK, mediate K+ release, (3) weak-rectifying K+ channels can mediate both K+ uptake and release.
In Arabidopsis, the only member of the third subfamily is AKT2/AKT3. AKT313 is shorter than AKT214 and lacks the first 15 amino acids encoded by the AKT2 gene15; but both show the same electrophysiological properties when expressed in Xenopus oocytes.15,16 The weak-rectification of AKT2 is due to a bi-modal gating behavior17 regulated by phosphorylation.18-21 This peculiar feature allows to tap a ‘potassium battery’ to energize membrane transport processes in plant vascular tissues.22
KAT1 is the best characterized plant K+ channel and contributes to K+ uptake in guard cells for controlling stomata aperture.23,24 The two K+ channels KAT1 and AKT2 showed a different ability to complement the growth of a yeast mutant defective in K+ uptake systems. KAT1 could complement the growth of such a yeast mutant,25 while AKT2 could not.14 On the other hand, both KAT1 and AKT2 proteins were functional in Xenopus oocytes and showed activation upon membrane hypolarization.15,26 To investigate the molecular basis of the difference in yeast complementation, we constructed a series of chimeric KAT1/AKT2 channels, expressed the proteins in the S. cerevisiae mutant strain CY162 and in Xenopus laevis oocytes and analyzed the properties of the chimeric channels.
Results
Functional characterization of KAT1-AKT2 chimeras in S. cerevisiae
To ascertain the region that is essential for the complementation properties of KAT1 in S. cerevisiae, we generated 12 KAT1-AKT2 chimera constructs as shown in detail in Fig. 1. These chimeras and KAT1 and AKT2 were expressed in the S. cerevisiae strain CY162, which lacks the endogenous potassium transporters TRK1 and TRK2 and which cannot grow on low K+ medium. Growth complementation tests were carried out in K+-limited medium. In this medium, cells being transformed only with the empty vector could not grow (Fig. 2). This growth deficit was not complemented by AKT2, but cells expressing KAT1 could grow well. Similar to KAT1, only the three chimeras C-4, C-6 and C-12 rescued the growth of S. cerevisiae CY162 under K+ depleted conditions. Additionally, the chimera C-7 showed slight growth in liquid culture (Fig. 2B). All other chimeras did not confer the K+-uptake deficient yeast any ability to grow on solid or in liquid low K+ medium (Fig. 2).
Figure 1.
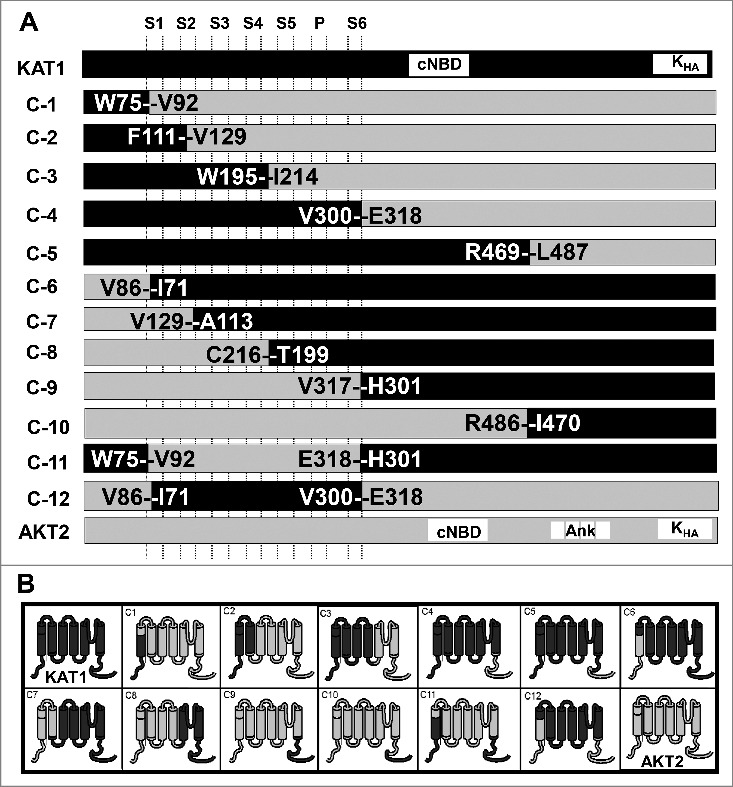
A. Schematic diagram of KAT1, AKT2 and chimeric constructs. The diagram shows the fusion sites of the chimeric constructs. S1-S6 (transmembrane segments), P (pore), cNBD (cyclic nucleotide-binding domain), Ank (Ankyrin domain), KHA (an interaction domain rich in hydrophobic and acidic residues), and C-1 – C-12 (chimeras). B. Schematic structure of the chimeric subunits. Parts originating from KAT1 are displayed in dark grey and parts from AKT2 in light grey.
Figure 2.
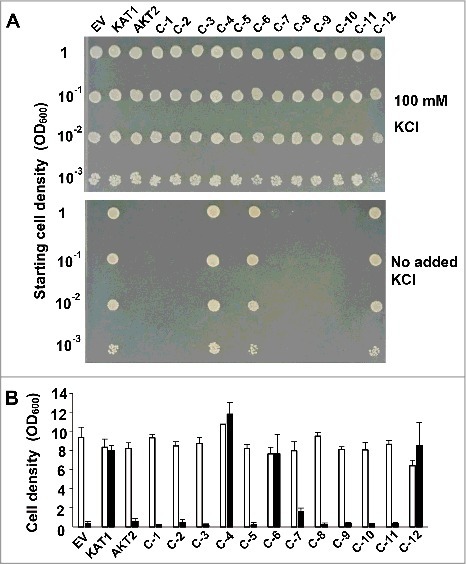
Complementation test of the K+ uptake-deficient S. cerevisiae CY162 transformed with pYES2-KAT1, pYES2-AKT2 or pYES2-chimeras. (A) CY162 cells expressing KAT1, AKT2 or chimeras were spotted on YNB-based medium agar plates in dilution series from OD600 = 1 to10−3. The media were supplemented with 100 mM KCl (upper) or without 100 mM KCl (bottom). Non-supplemented YNB medium contains approximately 7 mM K+. The image is representative for three individual experiments. (B) CY162 cells expressing KAT1, AKT2 or chimeras were grown in YNB-based liquid medium, starting from an OD600 of 0.1. The graph shows the average of three individual experiments. Error bars represent ± SD.
Expression of chimeric channels in Xenopus laevis oocytes
In the complementation growth experiment, only 3 chimeras could fully rescue the growth of S. cerevisiae CY162 in K+ depleted medium. Next, we characterized the activity of the chimeric channels by two-electrode voltage clamp analysis in Xenopus laevis oocytes (Fig. 3A). The chimeras C-4, C-6, C-12, which complemented the yeast, were also functional in oocytes. C-4 harbors the N-terminal to S6 region of KAT1 and the whole C-terminal part of AKT2, C-6 is built of the KAT1 core and C-terminus and the entire cytosolic N-terminal region from AKT2, and C-12 is the combination of C-4 and C-6 with the KAT1 core region and N-terminal and C-terminal parts of AKT2. Upon voltage stimulation, all 3 chimeras carried K+ currents that were activated by hyperpolarization and with increasing external K+ concentration (Fig. 3B). Chimeras C-4 and C-12 showed inward-rectifying behavior like KAT1. The chimera C-6 carried inward and outward K+ currents and resembled in these features AKT2. Both, C-6 and AKT2, were significantly active at 0 mV. However, the chimera C-6 deactivated at slightly more positive voltages, while AKT2 did not in this voltage range. Thus, chimeras C-4, C-6, and C-12 showed KAT1-like gating, albeit the activation threshold was shifted along the voltage axis. While KAT1 activated at approximately -70 mV, C-4 did at more negative voltages (activation approximately at -130 mV), C-12 at slightly more positive voltages (activation approximately at -50 mV) and C-6 at significantly more positive voltages (activation approximately at +10 mV). C-12, which is a combination of C-4 and C-6 is thus also functionally in between the two parental chimeras. Also C-7, which conferred slight growth rescue to the S. cerevisiae mutant CY162 (Fig. 2), was functional in oocytes. This chimera, however, exhibited non-rectifying currents which were more similar to those of AKT2 than to those of KAT1. Interestingly, also the chimeras C-8, C-9 and C-10, which failed to rescue S. cerevisiae CY162, carried K+ currents in oocytes (Fig. 4). These chimeras showed non-rectifying AKT2-like currents, which resembled those of C-7. No currents distinguishable from the background were observed with the other chimeric channels (C-1, C-2, C-3, C-5 and C-11), suggesting that these channels were functionally disrupted (Fig. 4). This result fits well with their inability to rescue the growth of S. cerevisiae CY162 (Fig. 2).
Figure 3.
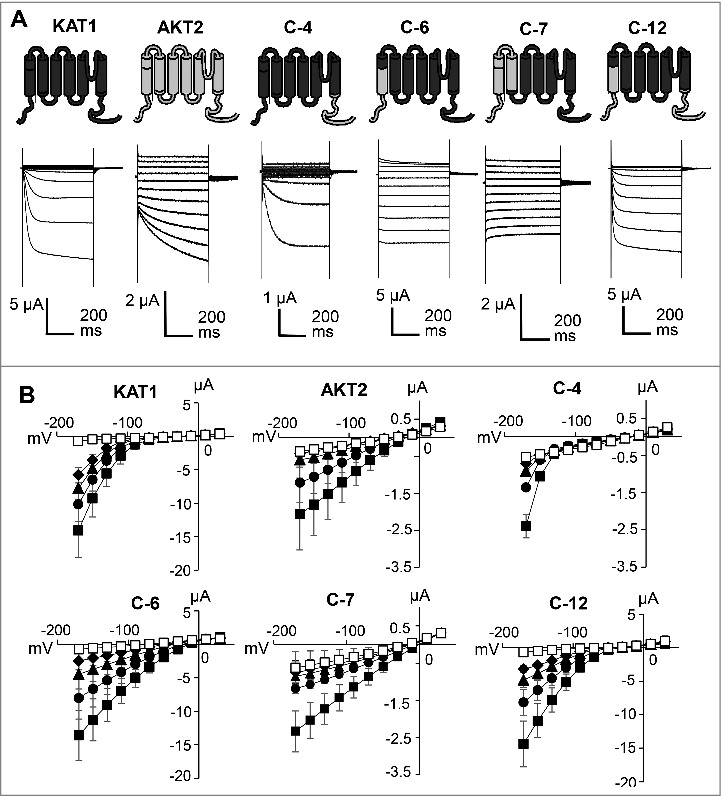
Functional characterization of KAT1, AKT2, C-4, C-6, C-7 and C-12 in Xenopus oocytes. A. Representative currents recorded from oocytes expressing the corresponding channels in the presence of 120 mM KCl. B. Current-voltage curves of the currents measured at the end of the 500-ms test pulses in the prescence of 120 mM K+ (▪), 60 mM K+ + 60 mM Na+ (•), 30 mM K+ + 90 mM Na+ (▴), 15 mM K+ + 105 mM Na+ (♦) or 120 mM Na+ (□) (Mean ± SD, n = 3). Experiments were repeated for more than three independent batches of oocytes. Data from a representative batch of oocytes are shown.
Figure 4.
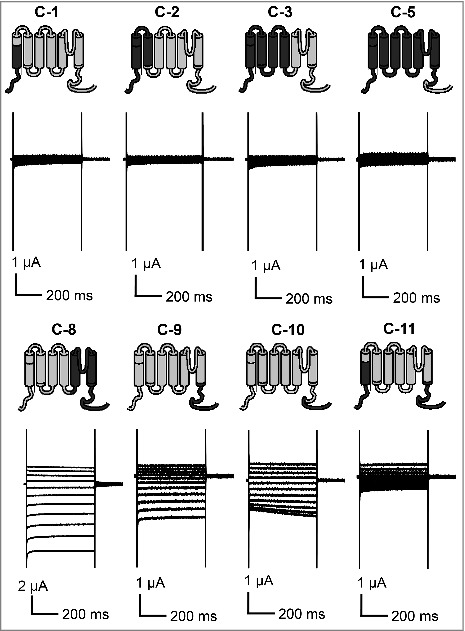
Current recordings for C-1, C-2, C-3, C-5, C-8, C-9, C-10 and C-11 in Xenopus oocytes in the presence of 120 mM KCl. Representative currents recorded from oocytes expressing the corresponding channels are shown. The data are representative for at least 3 repeats.
Discussion
Since about two decades, it is not clear, why KAT1 and AKT2 exhibit different functional fingerprints in the yeast and the oocyte expression system. In oocytes, both channels carry inward K+ currents at hyperpolarizing voltages. In yeast, however, only KAT1 is capable of complementing the potassium uptake deficiency of a mutant strain. This suggests that the K+-uptake screens in yeast are more rigid than the unbiased electrophysiological analysis in oocytes. This conclusion is also supported in this study here. From the 12 chimeras tested, 7 were functional in oocytes, but among those only 4 in yeast. The results obtained with the chimeras allow some conclusions on the structural determinants of KAT1 which allow yeast complementation.
If in the KAT1 channel the cytosolic C-terminus is replaced by that of AKT2, the essential properties of the chimera C-4 were KAT1-like with a negatively shifted activation threshold (Fig. 3). If additionally, the cytosolic N-terminus and part of the first transmembrane domain S1 were transplanted, the chimera C-12 activated at more positive voltages. This positive shift of the activation threshold induced by the exchange of the N-terminal region was even more pronounced if in C-12 the C-terminus was swapped back to that of KAT1 resulting in the chimera C-6. These results indicate that the replacements of the cytosolic N- and C-termini modified the sensitivity of KAT1 towards voltage in two opposite directions. These alterations, however, did not affect the ability of the chimeras to complement the K+-uptake deficient yeast strain. Interestingly, the chimera C-6, with its positively shifted activation threshold, carried currents in oocytes that were very similar to those of AKT2. Thus, the activity of the channel at 0 mV or even at positive voltages cannot be the reason why KAT1 and C-6 can complement the yeast, while AKT2 cannot. The difference between the two plant K+ channels has to be located in the transmembrane region from S1 to S6.
The chimera C-7, which differs from C-6 by the additional replacement of the S1-S2 linker and the second transmembrane domain S2, is only poorly capable of complementing the K+-uptake deficient yeast, whereas C-6 showed full complementation (Fig. 2). This difference points to the importance of the S1 and in particular the S2 region for KAT1 functioning in yeast. In oocytes, C-7 did not show any sign of rectification, unlike the inward rectifying C-6 (Fig. 3) indicating fundamental alterations in gating. The voltage sensing domain of KAT1 was shown to comprise pairs of negatively charged residues in S2 and S3 and positively charged residues in S4.27 The electrostatic interaction between certain negatively charged residues in S2 and positive residues in S4 controls the gating of KAT1.28 In the case of C-7, it is likely that the substitution of S2 from KAT1 to that of AKT2 caused some malfunction in gating, resulting in deficient channel activity in yeast and altered rectification in oocytes. Further replacements of transmembrane regions from KAT1 by those of AKT2 in the chimeras C-8, C-9, and C-10 still resulted in non-rectifying channels in oocytes. These chimeras, however, were not able to rescue yeast growth anymore. Thus, the capability of complementing the yeast strain CY162 is correlated with the presence of the core S2-S6 region from KAT1. This feature therefore does not only depend on the permeation properties of the channel / chimera, which is determined by the S5-P-S6 region,20 but also on parts of the voltage-sensing module built by the transmembrane domains S1-S4.
It should be noted that the chimeras C-4 and C-5 are very similar. At the functional level, however, they were strongly different. C-4 was functional in yeast and oocytes, C-5 was not. The functional C-4 contained the entire cytosolic C-terminal region of KAT1, whereas the non-functional C-5 had a chimeric C-terminal region, which might negatively interfere with channel assembly. It has been reported that the interaction between the cNBD and the KT/KHA domains is important for subunit assembly and the formation of functional channels.7,8 In C-5, the fusion site of KAT1 and AKT2 is located directly downstream the cNBD, which confers an “extended” amino acid sequence between cNBD and KHA, in particular due to the addition of an ankyrin domain. The ankyrin domain, which is present in AKT2 but not in KAT1, is proposed to be important for the interaction of the channel with kinases.29 Insertion of this domain possibly resulted in a distortion of the proper interaction between cNBD and KHA/KT and in the aberration of functional channel assembly in S. cerevisiae and oocytes.
Material and methods
Construction of chimeric channels
Twelve KAT1-AKT2 chimeras listed in Fig. 1 were constructed by a recombinant polymerase chain reaction method. The DNA fragment was digested with HindIII and BamHI restriction enzymes, and the fragment was cloned into pYES2 expression vector (Invitrogen Inc., San Diego. CA). The DNA insertion was verified by DNA sequencing.
Complementation growth test of S. cerevisiae CY162
Transformation of Saccharomyces cerevisiae strain CY162 (TRK1 and TRK2 deficient) with the pYES2-KAT1, pYES2-AKT2 or chimeric constructs was conducted by electroporation according to instructions of MicroPulser™ (BIO-RAD). Transformants were selected on YNB-based synthetic medium which consists of yeast nitrogen base (0.67%), glucose (2%) and all required amino acids except uracil, with the addition of 100 mM KCl and agar (2%). The plates were incubated at 30°C for 3 days. Positive colonies were streaked on synthetic medium containing the same composition as above, but sucrose (2%) and galactose (2%) were used instead of glucose. Colony PCR was performed again to confirm the yeast transformants harboring the plasmid construct. The yeast cells harboring the constructs were grown in YNB based liquid medium supplemented with sucrose (2%), galactose (2%) and 100 mM KCl. Five µl of the cell culture was dropped with dilution series at OD600 = 1 to 10−3 on YNB-based medium supplemented with 100 mM KCl or without KCl. The plates were incubated for 3 days at 30 ˚C.
Expression of chimera channels in Xenopus oocytes
pYES2 plasmids harboring KAT1, AKT2 or chimeras were linearized by NotI. Capped complementary cRNAs were synthesized by using the mMESSAGE mMACHINE T7 Kit (Ambion, Austin TX). cRNAs were injected into Xenopus laevis oocytes prepared as described previously.30 The injected oocytes were then incubated at 18˚C in Barth's solution (88 mM NaCl, 1 mM KCl, 0.41 mM CaCl2, 0.33 mM Na(NO3)2, 1 mM MgSO4, 2.4 mM NaHCO3, 5 mM HEPES-NaOH, pH 7.3). After 1 or 2 days of incubation, two-electrode voltage clamping, data acquisition and analysis were performed with an AxoClamp 2B voltage clamp amplifier (Axon Instruments, Foster City CA) and an Axon Digidata 1550 Low-Noise Data Acquisition System (Molecular Devices). Starting from a holding potential of 0 mV, oocytes were subjected to a protocol of 500 ms voltage pulses in 20 mV steps, ranging from +30 mV to −170 mV. The recording solutions contained 1 mM MgCl2, 1 mM CaCl2, 1mM LaCl3, 10 mM HEPES (pH 7.3 adjusted with NaOH), and indicated concentrations of KCl and NaCl.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Grants-in-Aid for Scientific Research (16H06558 and 16H04906) to N.U. from the Ministry of Education, Culture, Sports, Science, and Technology. I.D. acknowledges support by the Comisión Nacional Cientíıfica y Tecnológica of Chile (FONDECYT grant no. 1150054).
References
- [1].Chérel I. Regulation of K+ channel activities in plants: From physiological to molecular aspects. J Exp Bot. 2004;55:337-51. doi: 10.1093/jxb/erh028. PMID:14739260 [DOI] [PubMed] [Google Scholar]
- [2].Véry A-A, Sentenac H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol. 2003;54:575-603. doi: 10.1146/annurev.arplant.54.031902.134831. PMID:14503004 [DOI] [PubMed] [Google Scholar]
- [3].Sharma T, Dreyer I, Riedelsberger J. The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front Plant Sci. 2013;4:224. doi: 10.3389/fpls.2013.00224. PMID:23818893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dreyer I, Antunes S, Hoshi T, Muller-rber B, Palme K, Pongs O. Plant K+ Channel α-Subunits assemble indiscriminately. Biophys J. 1997;72:2143-50. doi: 10.1016/S0006-3495(97)78857-X. PMID:9129816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Uozumi N, Nakamura T, Schroeder J I and Muto S. Determination of transmembrane topology of an inward rectifying potassium channel from Arabidopsis thaliana based on functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1998;95:9773-78. doi: 10.1073/pnas.95.17.9773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H. Five-group distribution of the Shaker-like K+ channel family in higher plants. J Mol Evol. 2003;56:418-34. doi: 10.1007/s00239-002-2413-2. PMID:12664162 [DOI] [PubMed] [Google Scholar]
- [7].Dreyer I, Porée F, Schneider A, Mittelstädt J, Bertl A, Sentenac H, Thibaud J-B, Mueller-Roeber B. Assembly of plant Shaker-like Kout channels requires two distinct sites of the channel alpha-subunit. Biophys J. 2004;87:858-72. doi: 10.1529/biophysj.103.037671. PMID:15298894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Daram P, Urbach S, Gaymard F, Sentenac H, Chérel I. Tetramerization of the AKT1 plant potassium channel involves its C-terminal cytoplasmic domain. EMBO J. 1997;16:3455-63. doi: 10.1093/emboj/16.12.3455. PMID:9218788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ehrhardt T, Zimmermann S, Müller-Röber B. Association of plant K+in channels is mediated by conserved and does not affect subunit assembly. FEBS Lett. 1997;409:166-70. doi: 10.1016/S0014-5793(97)00502-4. PMID:9202139 [DOI] [PubMed] [Google Scholar]
- [10].Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science (80-). 1992;256:663-5. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- [11].Gambale F, Uozumi N. Properties of Shaker-type potassium channels in higher plants. J Membr Biol. 2006;210:1-19. doi: 10.1007/s00232-006-0856-x. PMID:16794778 [DOI] [PubMed] [Google Scholar]
- [12].Dreyer I, Blatt MR. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci. 2009;14:383-90. doi: 10.1016/j.tplants.2009.04.001. PMID:19540150 [DOI] [PubMed] [Google Scholar]
- [13].Ketchum KA, Slayman CW. Isolation of an ion-channel gene from Arabidopsis thaliana using the H5 signature sequence from voltage-dependent K+ channels. FEBS Lett. 1996;378:19-26. doi: 10.1016/0014-5793(95)01417-9. PMID:8549795 [DOI] [PubMed] [Google Scholar]
- [14].Cao YW, Ward JM, Kelly WB, Ichida AM, Gaber RF, Anderson JA, Uozumi N, Schroeder JI, Crawford NM. Multiple genes, tissue-specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995;109:1093-106. doi: 10.1104/pp.109.3.1093. PMID:8552711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB. A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell. 2000;12:837-51. PMID:10852932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marten I, Hoth S, Deeken R, Ache P, Ketchum KA, Hoshi T, Hedrich R. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc Natl Acad Sci U S A. 1999;96:7581-6. doi: 10.1073/pnas.96.13.7581. PMID:10377458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dreyer I, Michard E, Lacombe B, Thibaud JB. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 2001;505:233-9. doi: 10.1016/S0014-5793(01)02832-0. PMID:11566182 [DOI] [PubMed] [Google Scholar]
- [18].Chérel I, Michard E, Platet N, Mouline K, Alcon C, Sentenac H, Thibaud J-B. Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell. 2002;14:1133-46. doi: 10.1105/tpc.000943. PMID:12034902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Michard E, Dreyer I, Lacombe B, Sentenac H, Thibaud JB. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. 2005;44:783-97. doi: 10.1111/j.1365-313X.2005.02566.x. PMID:16297070 [DOI] [PubMed] [Google Scholar]
- [20].Michard E, Lacombe B, Porée F, Mueller-Roeber B, Sentenac H, Thibaud J-B, Dreyer I. A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J Gen Physiol. 2005;126:605-17. doi: 10.1085/jgp.200509413. PMID:16316977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sandmann M, Skłodowski K, Gajdanowicz P, Michard E, Rocha M, Gomez-Porras JL, González W, Corrêa LGG, Ramírez-Aguilar SJ, Cuin TA, et al.. The K+ battery-regulating Arabidopsis K+ channel AKT2 is under the control of multiple post-translational steps. Plant Signal Behav. 2011;6:558-62. doi: 10.4161/psb.6.4.14908. PMID:21445013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dreyer I, Gomez-porras JL, Riedelsberger J. The potassium battery : a mobile energy source for transport processes in plant vascular tissues. New Phytol. 2017. doi: 10.1111/nph.14667. PMID:28643868 [DOI] [PubMed] [Google Scholar]
- [23].Nakamura RL, McKendree WL, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371-4. doi: 10.1104/pp.109.2.371. PMID:7480337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dietrich P, Sanders D, Hedrich R. The role of ion channels in light-dependent stomatal opening. J Exp Bot. 2001;52:1959-67. doi: 10.1093/jexbot/52.363.1959. PMID:11559731 [DOI] [PubMed] [Google Scholar]
- [25].Anderson JA, Huprikar SS, Kochian L V, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992;89:3736-40. doi: 10.1073/pnas.89.9.3736. PMID:1570292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schachtman D, Schroeder J, Lucas W, Anderson J, Gaber R. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science (80-). 1992;258:1654-8. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- [27].Sato Y, Sakaguchi M, Goshima S, Nakamura T, Uozumi N. Molecular dissection of the contribution of negatively and positively charged residues in S2, S3, and S4 to the final membrane topology of the voltage sensor in the K+ channel, KAT1. J Biol Chem. 2003;278:13227-34. doi: 10.1074/jbc.M300431200. PMID:12556517 [DOI] [PubMed] [Google Scholar]
- [28].Lefoulon C, Karnik R, Honsbein A, Gutla PV, Grefen C, Riedelsberger J, Poblete T, Dreyer I, Gonzalez W, Blatt MR. Voltage-sensor transitions of the inward-rectifying K+ channel KAT1 indicate a latching mechanism biased by hydration within the voltage sensor. Plant Physiol. 2014;166:960-75. doi: 10.1104/pp.114.244319. PMID:25185120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee SC, Lan W-Z, Kim B-G, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc Natl Acad Sci U S A. 2007;104:15959-64. doi: 10.1073/pnas.0707912104. PMID:17898163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Uozumi N, Gassmann W, Cao Y, Schroeder J I Identification of strong modifications in cation selectivity in an Arabidopsis inward rectifying potassium channel by mutation selection in yeast. J. Biol. Chem. 1995;270:24276-81. doi: 10.1074/jbc.270.41.24276 [DOI] [PubMed] [Google Scholar]


