ABSTRACT
L-type-voltage-dependent Ca2+ channels (L-VDCCs; CaV1.2, α1C), crucial in cardiovascular physiology and pathology, are modulated via activation of G-protein-coupled receptors and subsequently protein kinase C (PKC). Despite extensive study, key aspects of the mechanisms leading to PKC-induced Ca2+ current increase are unresolved. A notable residue, Ser1928, located in the distal C-terminus (dCT) of α1C was shown to be phosphorylated by PKC. CaV1.2 undergoes posttranslational modifications yielding full-length and proteolytically cleaved CT-truncated forms. We have previously shown that, in Xenopus oocytes, activation of PKC enhances α1C macroscopic currents. This increase depended on the isoform of α1C expressed. Only isoforms containing the cardiac, long N-terminus (L-NT), were upregulated by PKC. Ser1928 was also crucial for the full effect of PKC. Here we report that, in Xenopus oocytes, following PKC activation the amount of α1C protein expressed in the plasma membrane (PM) increases within minutes. The increase in PM content is greater with full-length α1C than in dCT-truncated α1C, and requires Ser1928. The same was observed in HL-1 cells, a mouse atrium cell line natively expressing cardiac α1C, which undergoes the proteolytic cleavage of the dCT, thus providing a native setting for exploring the effects of PKC in cardiomyocytes. Interestingly, activation of PKC preferentially increased the PM levels of full-length, L-NT α1C. Our findings suggest that part of PKC regulation of CaV1.2 in the heart involves changes in channel's cellular fate. The mechanism of this PKC regulation appears to involve the C-terminus of α1C, possibly corroborating the previously proposed role of NT-CT interactions within α1C.
KEYWORDS: calcium channel, cardiovascular, HL-1 cells, plasma membrane, protein kinase C, protein localization, Ser1928
Introduction
L-type voltage-dependent calcium channels (L-VDCC; CaV1.2) play a critical role in excitation-contraction coupling in cardiac, skeletal and smooth muscle,1-3 and in excitability and excitation-transcription coupling in neurons and cardiomyocytes.4-7 Ca2+ influx via these channels following membrane depolarization is the trigger for ryanodine receptor (RyR) activation and massive Ca2+ release from the sarcoplasmic reticulum (SR) leading to mechanic contraction.8-11
L-VDCCs are multi-subunit protein complexes containing, as a minimum, α1 (pore forming subunit), and β and α2δ as auxiliary subunits. The CACNA1C gene encodes α1C, the main subunit of CaV1.2 – the L-VDCC expressed in cardiac and smooth muscle and in the brain.12,13 α1C is alternatively spliced in different tissues. Among multiple splice variants, we have previously shown that the cardiac isoform contains exon 1A and is referred to as the “long-NT” (L-NT) isoform, while the smooth muscle isoform contains exon 1 and is referred to as the "short-NT (S-NT) isoform.14-17 Furthermore, an insertion of another exon, 9a, between exons 9 and 10 in a smooth muscle isoforms was found.18-20
Many hormones and transmitters modulate CaV1.2 channels via activation of G-Protein Coupled Receptors (GPCRs) and second messengers.21 A prominent modulatory pathway in the cardiovascular system is the enhancement of L-type Ca2+ currents by protein kinase C (PKC), via activation of Gq-coupled receptors by angiotensin II (AngII), acetylcholine (ACh), or directly by phorbol esters such as β-phorbol 12-myristate 13-acetate (PMA) 22,23 (reviewed in 24). As part of this signaling cascade, PKC is activated and was shown to be essential for Ca2+ current enhancement (discussed in 22,25-27). PKC inhibitors block AngII-induced vasoconstriction.28-31 Heterologous expression and reconstitution of the enhancing effects of PKC was so far successful only in Xenopus oocytes,20,22,32,33 while Cav1.2 expression and PKC activation in mammalian (HEK) cells yielded only a current decrease.34 In our previous studies using Xenopus oocytes expression system, we have demonstrated that PKC activation (either directly by PMA or indirectly via a Gq-coupled receptor) of the cardiac (L-NT) isoform (and a distinct smooth muscle isoform containing exon 9a, S-NT+9a) resulted in a macroscopic current increase. No increase in current was observed in smooth muscle (S-NT, -9a) isoforms used, or in L-NT-deletion mutants.22,23
In vitro studies, using GST-fused segments of α1C, and in vivo, in rat and mouse heart lysates, demonstrated that PKC phosphorylates α1C isoforms. α1C is a substrate for PKC on different residues, most prominently on Ser1928.35,36 Cardiac and neuronal α 1C is post-translationally modified, giving a full-length and a CT-truncated form, ∼240 kDa and 210 kDa, respectively.37-39 The latter is the result of a proteolytic cleavage around amino acid (a.a.) ∼180040 (heterologous expression yields only the full-length α1C protein). The cleaved distal-CT, dCT, is a ∼35 KDa protein. It appears to remain associated with the main α1C subunit, tonically inhibiting it.41 Ser1928 is located on the distal, truncated, part of the CT.
Initially, Ser1928 was considered to be the main residue phosphorylated by protein kinase A (PKA) following β-adrenergic stimulation, but, until very recently, without a defined function in PKA regulation (reviewed in42). Recent studies revealed important roles for phosphorylation of Ser1928 by PKA. First, it disrupts a physical interaction between α1C and β2-AR.43 Importantly, in smooth muscle and neurons (but not in the heart), phosphorylation of Ser1928 is essential for PKA regulation and has been speculated to also be important for PKC regulation of CaV1.2.44,45 Notably, Ser1928 is also significant in PKC modulation of CaV1.2. A mutation of Ser1928 to alanine significantly reduces the PMA- and Gq- induced increase in CaV1.2 channels expressed in Xenopus oocytes,23 revealing an important role for this residue in the modulation of CaV1.2 by PKC.
Despite extensive study, the mechanism by which PKC augments CaV1.2 current is unclear. We have previously studied the role of PKC in upregulation CaV1.2 currents in Xenopus oocytes. We hypothesize that the increase in current results from enhanced channel expression in the plasma membrane (PM). This work complements all of the previously published electrophysiological data in Xenopus oocytes. Here we examine PM expression of CaV1.2 using two distinct methodologies, and show that activation of PKC augments PM expression of α1C which may, in part, account for increased currents. In addition, we report a similar effect of PKC activation in a more “native” environment using HL-1 cells, which is a murine cardiomyocyte cell line that exhibits typical adult cardiomyocytes characteristics.46-48
Results
Increased α1C-L-NT PM expression following PKC activation
We wanted to quantify heterologously expressed α1C PM expression in Xenopus oocytes following PKC activation. To do so, we used 2 distinct methods: α1C immunocytochemistry in giant membrane patches (GMP)52 and surface biotinylation followed by Western blotting. We injected 5 ng/oocyte of RNAs of α1C, β2b, α2/δ (with or without m3R). Direct activation of PKC by application of 10 nM PMA (n = 90) for 5 minutes resulted in a significant increase of 125.76 ± 12.3% in PM expression of α1C in GMP compared with control (untreated oocytes, n = 88) (Fig. 1Aa). Treatment with DMSO alone (vehicle) did not significantly affect the amount of α1C in the PM (87.7 ± 21.57% of control, n = 5). Supporting evidence was obtained by surface biotinylation experiments. In all 4 experiments, activated PKC increased the amount of α1C in PM. However, the increase in PM expression was less prominent than observed in GMP because of some internalization of biotin in oocytes. There was an increase of 36.08 ± 8.76% (p < 0.05) in surface expression of PMA treated oocytes as compared with untreated oocytes (Fig. 1b).
Figure 1.
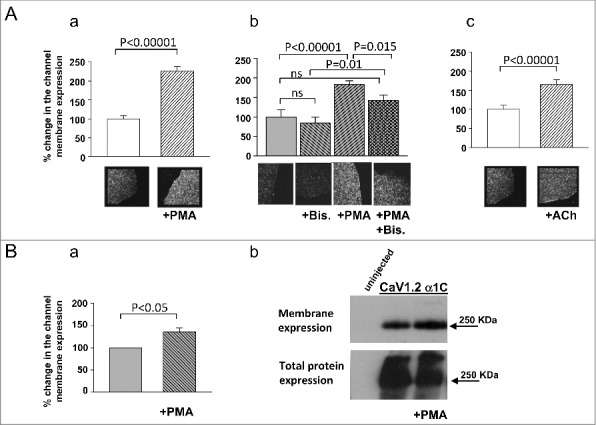
Plasma membrane expression of α1C is upregulated by PKC. (A) Giant oocyte membrane patches stained for α1C before and after PKC activation by PMA (Aa: PMA, n = 90; control, n = 88) or by ACh via m3R activation (Ac: ACh, n = 36; control n = 27), resulted in increased PM expression following PKC activation. Bis prevented the enhancing effects of PMA (Ab: control, n = 9; Bis, n = 8; PMA, n = 12; PMA+Bis, n = 11) (B) α1C content in Xenopus oocytes plasma membrane after PKC activation measured by surface biotinylation followed by Western blot. Bars show protein amounts normalized to channel expression without PKC activation.
To verify the involvement of PKC in this process, we applied PMA in the presence of the specific PKC inhibitor, Bis-indolylmaleimide (Bis). Oocytes were injected with 50 nl of 300 µM Bis, 2–4 h before the experiment. Bis did not significantly reduce the “basal” expression of α1C, yet significantly reduced the effect of PMA. Application of Bis resulted in a non significant decrease in α1C membrane expression to 85.08 ± 15.14% of untreated oocytes (Fig. 1Ab). Application of PMA in Bis-treated oocytes resulted in 67.3 ± 16.7% membrane expression (n = 11) as compared with oocytes treated with Bis alone (no PMA, vn = 8) (Fig. 1Ab), and this difference was statistically significant indicating that Bis did not completely inhibit the effect of PMA. However, a significant difference in the effect of PMA was observed between untreated (only PMA, n = 12) and Bis-treated (Bis and PMA, n = 11) groups: 84.5 ± 9.08% increase vs. 42.34 ± 14.24%, respectively (Fig. 1Ab). Thus, Bis significantly attenuated the effect of PMA. To further substantiate the role of PKC using a physiologic activator, we co-expressed a Gq-coupled receptor (m3R) along with CaV1.2. Activation of m3R by ACh leads to downstream activation of PKC.53-55 Application of ACh (10 µM) increased α1C PM content within 8 minutes by 66.14 ± 11.4% (n = 36) compared with untreated oocytes (n = 27) in GMP (Fig. 1Ac).
dCT and Ser1928 are essential for the enhanced PM expression
The role of dCT in this modulation was studied by expressing a dCT-deletion mutant in Xenopus oocytes, α1CΔ1821 (with β2b and α2/δ). PMA enhanced PM expression of α1CΔ1821 by 75.02 ± 25.31% (n = 6) as compared with untreated α1CΔ1821 oocytes. The effect of PMA on the dCT-deletion mutant was significant, yet significantly lower than the effect of PMA on full-length α1C in this set of experiments (217.16 ± 53.4% increase, n = 6). These results suggest an important role for dCT in modulation of CaV1.2 by PKC. To further study the role of dCT, we co-expressed dCT with α1CΔ1821. Interestingly, coexpression of dCT did not restore the full effect of PMA (Fig. 2A)
Figure 2.
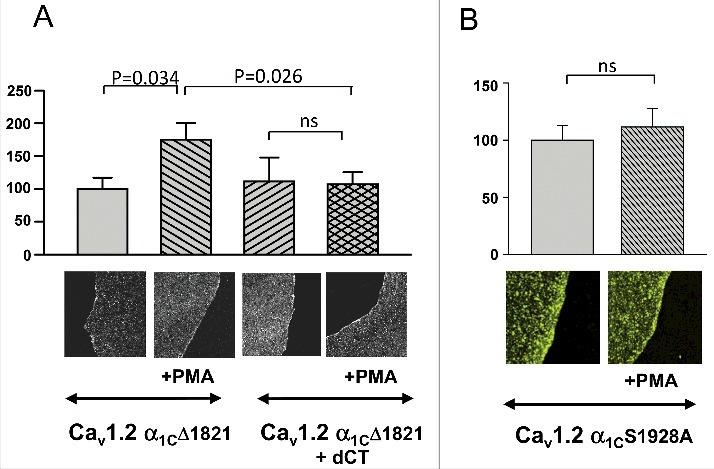
Involvement of dCT and Ser1928 in PKC regulation of α1C. Giant oocyte membrane patches were stained for α1C before and after PKC activation by PMA. Oocytes were injected with 5 ng channel RNA/oocyte. (A) Activation of PKC significantly enhanced PM expression in oocytes expressing α1CΔ1821 (n = 6), but not in oocytes coexpressing α1CΔ1821 and dCT (n = 5). (B) Activation of PKC failed to increase PM expression in oocytes expressing α1C S1928A (n = 28) as compared with control, wt α1C (n = 22).
Ser1928, located in the dCT of α1C, is an important phosphorylation site for PKC,35,36 and for PKA.39,57-59 We have previously shown that when this residue is mutated to alanine (S1928A), in Xenopus oocytes the extent of CaV1.2 currents enhancement by PMA was greatly attenuated as compared with wt α1C.23 We expressed wt α1C or S1928A-α1C (with β2b and α2/δ) and quantified PM α1C contents before and after a 5 minute application of PMA, by analyzing GMPs. The increase in PM content following PKC activation with PMA, was practically absent in oocytes expressing S1928A- α1C (111.5 ± 16.4% of control, p > 0.05; Fig. 2B; compare with the 225% of control for PMA-treated wild-type α1C; Fig. 1A). Thus, PM expression patterns resemble previously reported electrophysiological findings, and emphasize the importance of this specific residue, located in the dCT, in PKC regulation of the full-length cardiac α1C.
PM expression of α1C in HL-1 cells is upregulated by PKC
Prior to studying the effects of PKC on HL-1 cells, we examined α1C expression in these cells. Confocal images of intact HL-1 cells stained with α1C antibody revealed a diffused staining pattern that was not restricted to PM (Fig. 3A). Using Western blotting and immunocytochemistry, we were able to detect robust expression of α1C (Fig. 3), as previously reported by others.46 Interestingly, we detected 2 distinct bands corresponding to molecular weights of ∼250 kDa and ∼210 kDa. These bands likely represent the full- length and dCT-truncated α1C, respectively. This is supported by the comparison of the 2 bands detected in HL-1, with the bands of full-length and dCT-truncated α1C separately expressed in Xenopus oocytes and analyzed on the same blot (Fig. 3b). Previous studies have shown that the extent of truncation of α1C varies39,60,61 (also reviewed in42).
Figure 3.
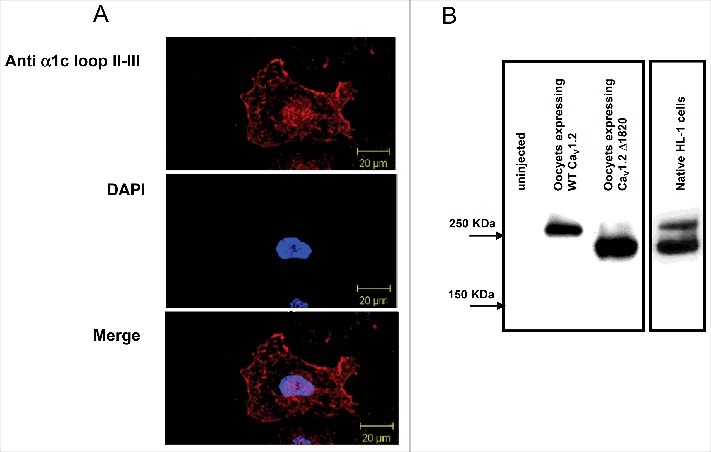
Native HL-1 cells express α1C. (A) Confocal images of HL-1 cells stained with α1C antibody. (B) Western blot of native HL-1 cells, with lysates of oocytes expressing either full-length of dCT-truncated α1C as a reference. HL-1 cells express 2 distinct molecular weights of α1C, probably corresponding to full-length and dCT-truncated channels.
Next, we examined the effects of PKC activation on α1C PM expression. PKC was activated by PMA and α1C expression was detected by immunofluorescence and surface biotinylation. The latter method enabled us to distinguish between the full-length and dCT-truncated forms of α1C in the PM. PMA application significantly increased PM content of α1C almost 2-fold, while DMSO did not change the PM expression, as detected by immunofluorescence. Interestingly, confocal images revealed a diffused expression pattern in untreated or DMSO-treated cells. PKC activation seemed to mobilize the channels and resulted in staining apparently much more confined to the PM, with a 84 ± 21% (n = 3) increase in the α1C labeling in the cell periphery (Fig. 4A). Surface biotinylation also demonstrated a 37 ± 8% increase in PM α1C content following activation of PKC by PMA (Fig. 5A). We have further separately analyzed the effect PKC had on the 2 distinct bands, presumably representing the full-length and truncated α1C. The intensity of the upper band was increased by 67 ± 11% (P < 0.01), whereas the lower band only by 31 ± 10% (P < 0.05) (Fig. 5b). Thus, the PM expression of the full-length α1C, was enhanced by PKC to a greater extent as compared with the non-truncated α1C. These findings highlight the role of the dCT and possibly the role of Ser1928 located on the dCT. Thus, quite remarkably, CaV1.2 PM expression in HL-1 cells, which resemble adult cardiomyocytes, is upregulated following PKC activation.
Figure 4.
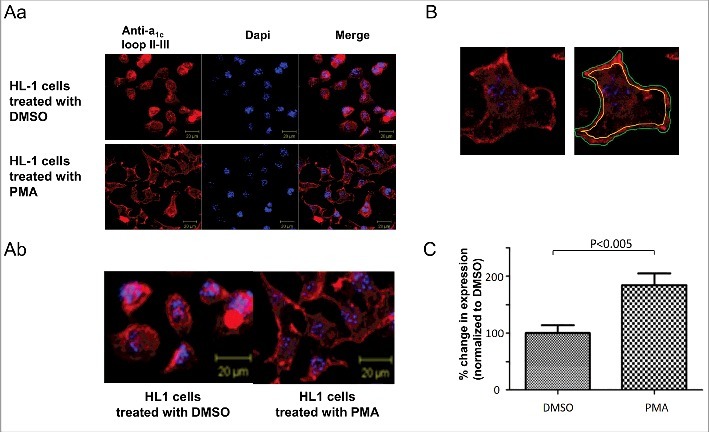
PKC increases α1C expression PM of HL-1 cells. (A) Hl-1 cells were treated with DMSO (vehicle) or PMA and visualized by staining α1C. Staining was much more confined to PM in PMA-treated cells. The lower panel is a zoom on a selected area from the upper panel images. (B) A representative cell depicting how PM content of α1C was quantified. (C) Quantification of PM content of α1C in DMSO vs. PMA treated HL-1 cells.
Figure 5.
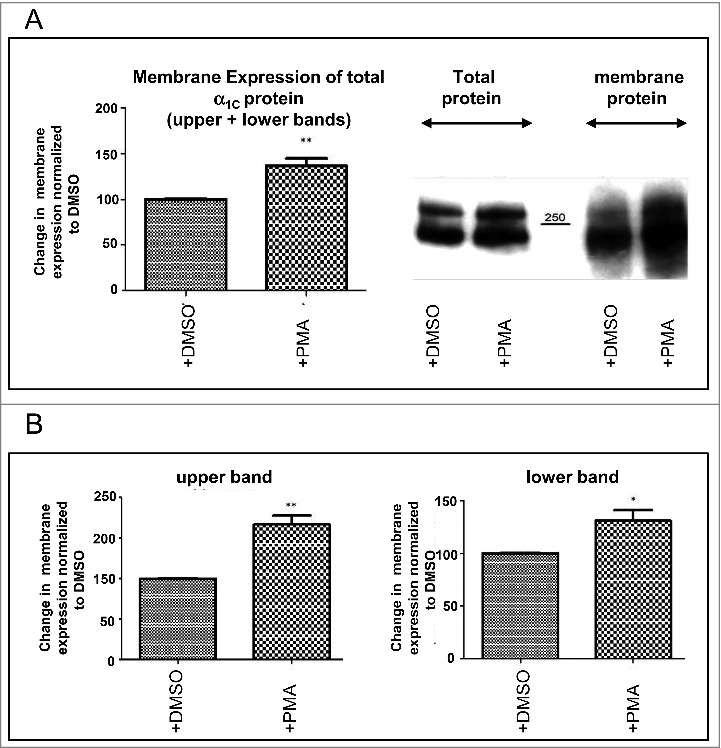
PKC increases the PM level of α1C in HL-1 cells. Quantification of the amount of α1C protein in HL-1 cells PM was done by biotinylation. (A) Quantification of total α1C protein. (B) Quantification of distinct molecular weights of α1C, corresponding to full-length and dCT-truncated α1C.
Discussion
CaV1.2 is the main calcium channel in the heart and smooth muscle, responsible for normal myocyte contraction.1-3 This channel was shown to be regulated both by PKA and PKC (reviewed in24,42,62). PKC was previously shown to enhance L-NT CaV1.2 currents in Xenopus oocytes.22,23,51,56 Furthermore, Ser1928, located on α1C-CT was identified as a crucial residue for this regulation.23 Here, we studied the regulation of PM expression of α1C by PKC in Xenopus oocytes and HL-1 cells. Despite previous notion that PKC enhances CaV1.2 currents due to a change in gating,63-65 here we report that activation of PKC increases α1C expression in PM, and intact CT is critical for the increased expression.
CaV1.2 is mobilized to and removed from the PM following various stimuli such as distinct cell signaling, G protein activation, phosphorylation and post-translational modifications (reviewed in66). It is well established that CaV1.2 channels in cardiomyocytes and smooth muscle cells form macromolecular complexes.63,67 Furthermore, it has been recently demonstrated that dynamin and cortactin co-localize next to the actin cytoskeleton. Activation of L-VDCC by GnRH enhanced Ca2+ influx and induced PM remodeling.68 The extensive trafficking mechanism of α1C to PM and intracellularly is tightly regulated, and PKC may be one such regulator that increases the amount of α1C in PM, thus enhancing macroscopic currents.
HL-1 cells are derived from AT-1 mouse atrial cardiomyocyte tumor lineage, and retain hallmark cardiomyocyte characteristics, including robust CaV1.2 expression.46 Immunofluorescent images revealed a diffused staining pattern that changed dramatically upon PKC activation and became much more restricted to the PM. In addition, biotinylation experiments demonstrated increased amount of α1C in PM of HL-1 cells. The increase in α1C content in the PM within several minutes may be attributed to enhanced trafficking to the PM rather than changes in internalization, thus supporting the hypothesis of clustering of CaV1.2 channels in cardiomyocytes and smooth muscle cells.63,67 Nevertheless, we still cannot rule out the possibility that PKC activation reduces the rate of internalization of α1C, which can also result in increased PM α1C content.
Interestingly, we were able to detect 2 distinct molecular sizes of α1C corresponding to full-length (∼250 kDa) and a dCT-truncated (∼210 kDa) α1C.39,40,69 in HL-1 cells. The extent of truncation is still disputed and probably differs among species (reviewed in42). Ser1928, a residue known to be phosphorylated by PKC,35,36 and crucial for current increase by PKC,23 is absent in dCT-truncated α1C. However, it has been suggested that the cleaved dCT remains associated with α1C via non-covalent bonds,39,40 and so the role of Ser1928 in the truncated dCT remains to be resolved. The PKC-induced increase in PM content of the lower molecular weight band (dCT-truncated α1C) was lower than full-length α1C, in HL-1 cells. In correlation, PKC-induced enhancement of PM content of a dCT-deletion mutant (α1C-Δ1800) expressed in oocytes was significantly lower than full-length α1C.
Xenopus oocyte is a very convenient model to study the effects of PKC by expressing various α1C mutants. This is the only heterologous expression system in which macroscopic CaV1.2 currents are enhanced following PKC activation; the amount of RNA injected for protein expression is accurately calculated and calibrated; the PM can be manually separated from the cell and its protein content can be quantified using specific antibodies. The significance of the distal part of the CT, which contains Ser1928 residue, in increased PM expression following PKC activation was confirmed in this preparation as well. Our present results, in which the effect of PKC on PM localization of CaV1.2 was very similar in HL-1 cells and in Xenopus oocytes, further validate the use of this model system for studies of mechanisms of modulation of the channel by PKC. Activation of PKC by PMA in oocytes was confirmed using a specific PKC inhibitor, Bis, that successfully and significantly reduced the effects of PKC. However, the robust activation of PKC by PMA was not completely blocked by PMA. It is conceivable that PMA affects additional cellular pathways that are not blocked by Bis. Still, when major phosphorylation sites are deleted or mutated (e.g. Ser1928), most of the effect of PKC is not observed, supporting a major involvement of PKC in PMA-induced changes in PM content of CaV1.2. It is not clear why coexpression of dCT with the truncated α1C did not restore the effect of PMA. It is possible that, when coexpressed with α1CΔ1821, dCT that contains Ser1928 phosphorylation site competes with other phosphorylation sites on α1CΔ1821, thereby eliminating the effect of PKC on α1CΔ1821. Taken together, our results suggest that dCT plays a role in PKC modulation, and Ser1928 is a crucial residue for this modulation.
In conclusion, the modulation of CaV1.2 by PKC (leading to enhanced macroscopic currents) involves increased α1C expression in PM. The dCT and phosphorylation of Ser1928 are crucial for this effect. As previously proposed the requirement for a L-NT isoform may indicate that a complex NT-CT interaction70,71 underlies the mechanism of the effect of PKC on α1C.
Materials and methods
Oocyte culture
All the experiments were performed in accordance with the Tel Aviv University Institutional Animal Care and Use Committee (permit no. M-13–002). Xenopus laevis frogs were maintained and operated, and oocytes were collected, defolliculated, and injected with RNA as described.49 Female frogs, maintained at 20 ± 2 °C on an 11 h light/13 h dark cycle, were anesthetized in a 0.15% solution of procaine methanesulfonate (MS222), and portions of ovary were removed through a small incision on the abdomen. The incision was sutured, and the animal was returned to a separate tank until it had fully recovered from the anesthesia, and afterwards was returned to a large tank where, together with the other postoperational animals, it was allowed to recover for at least 4 weeks until the next surgery. The animals did not show any signs of postoperational distress.
Oocytes were injected with equal amounts (by weight; 5 ng) of the mRNAs of CaV1.2α isoforms (original long-NT isoform: accession no. X15539) or its mutants with α2/δ (accession number M21948), with or without β2b (accession number X64297), with or without 1 ng of m3R, and incubated for 3–5 d at 20–22oC in NDE96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 2.5 mM Na pyruvate, 50 μg/ml gentamycine, 5 mM HEPES, pH 7.5).
HL-1 cell culture
HL-1 cells were maintained as described in Claycomb et al. 1998.46 Briefly, cells were plated in gelatin/fibronectin-coated culture flasks and maintained in Claycomb medium supplemented with 10% fetal bovine serum, 0.1 mM norepinephrine, 2 mM glutamine (Sigma) and 100 units/ml penicillin-streptomycin (Gibco), at 37°C in a humidified 5% CO2 environment. The media was replaced every 24–48 hours. The culture reaches confluency within 72 hours.
cDNA constructs and mRNA
cDNAs of α1C, α2/δ and β2b were as described.50 The rabbit heart α1C mutants used here were prepared in our laboratory as described.51 Rat m3R is in pGEM-HJ. The RNAs were prepared using a standard procedure described previously, which ensures capping of the 5′ end of the RNA and preferential inclusion of non-capped GTP in the rest of the RNA.49
Western blotting
Cultured HL-1 cells were washed 3 times in cold phosphate-buffered saline (PBS) to wash away remaining media and scraped with a cell scraper. Homogenization of the cells was done by gentle rotating the cells for 1 hour at 4°C in hypotonic lysis buffer (5 mM TRIS pH 7.4, 1 mM EDTA and Complete protease inhibitor mix (ThermoFisher)). The homogenate was spun at 1000 × g for 10 min at 4°C to separate debris and heavy molecules like DNA. Protein concentrations were determined using BCA protein assay kit (Sigma). Proteins were separated on a 6% SDS gel and transferred onto nitrocellulose membrane (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in TBST (Tris 20 mM, pH 7.6, 120 mM NaCl, 0.1% Tween), then incubated anti-α1C polyclonal antibody (Alomone, Jerusalem). After 3 washes with TBST, they were incubated with a horseradish-peroxidase-labeled anti-rabbit secondary antibody (Jackson Immunoresearch Laboratories). Membranes were washed 3 times with TBST, signals were developed with ECL+ (Amersham Pharmacia Biotech).
Surface biotinylation
HL-1 cells
HL-1 cells were cultured in 100 mm culture dishes (Corning) to confluency. Separate plates were treated with PMA (Alomone Laboratories) dissolved in DMSO to final concentration of 10 nM PMA and 1% v/v DMSO, or 1% v/v DMSO alone (vehicle) in supplemented Claycomb medium for 7 min with gentle rocking. Following treatment, culture medium was aspirated and the plates were washed with cold PBS containing 0.9 mM CaCl2 and 1 mM MgCl2 3 times to block any endocytosis. Then the dishes were incubated at room temperature in the presence of 1 mg/ml of the membrane-impermeable reagent EZ-Link Sulfo-NHS-LC-biotin (Thermo Scientific) in PBS. The cells were washed 3 times with quenching solution (PBS supplemented with 10 mM glycine (Sigma)) to stop the reaction. Following an additional wash in PBS, the cells were scraped into 1 ml hypotonic lysis buffer (5 mM TRIS pH 7.4, 1 mM EDTA, and complete protease inhibitor cocktail (ThermoFisher)). The scraped cells were collected into pre-chilled Eppendorf tubes and gently agitated on a rotator at 4°C for 1 hour. The lysate was spun at 1000 g for 10 min to sediment debris and heavy molecules like DNA. The supernatant was collected and a fraction kept aside as “total protein." The protein content was determined using the BCA protein estimation kit with bovine serum albumin as standard. PMA and DMSO samples were adjusted to similar protein concentration. The adjusted samples were spun at 40000 g in 4°C for 30 min to enrich the membrane fraction. The supernatant was discarded and the pellet was resuspended in lysis buffer supplemented with protease inhibitor complex. Immobilized Streptavidin-Agarose resin (Thermo Scientific) was added to this suspension, and the reaction mixture was gently rotated at 4°C for 16 h. The resin was spun down at 5000 g for 5 min and washed 3 times with ice-cold lysis buffer to remove any non-specific bound proteins. The beads were incubated in sample buffer to elute the “bound protein." The eluted proteins were separated on a 6% SDS gel. Since the total protein content in both samples is equal, we did not use any loading control. Proteins were transferred to nitrocellulose membranes for Western blotting. Band intensity was measured using ImageJ. The results were analyzed by Student's unpaired t-test using Prism software (GraphPad). Statistical significance limit was set at p < 0.05.
Oocytes
After mRNA injection, oocytes were incubated at 18°C for 4 d before experiments. Twenty oocytes from each group were washed with NDE96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 2.5 mM Na pyruvate, 50 μg/ml gentamycine, 5 mM HEPES, pH 7.5), and then transferred to biotinylation solution (NDE96 containing 1.5 mg/ml of freshly prepared EZ-Link Sulfo-NHS-LC-biotin). Oocytes were incubated on a rocker at room temperature for 20 min. Fresh biotinylation solution was added for further 20 min incubation. Oocytes were transferred to an ice-cold quenching solution (NDE96 with 50 mM glycine) and incubated for 10 min. Then, oocytes were washed in NDE96 for 10 min and transferred to a Eppendorf tube containing homogenization buffer (20 mM Hepes pH = 8.0, 5 mM EDTA, 5mM EGTA, 100 mM NaCl, protease inhibitor cocktail (ThermoFisher)). Oocytes were homogenized by pipetting on ice. Homogenates were cleared by centrifugation (700 g for 15 min). 1/60 from total volume of the supernatant was removed for measuring the total protein (bound+unbound). The supernatant was mixed with streptavidin bead slurry (ThermoFisher) and incubated on a rocker overnight at 4°C. Beads were separated by centrifugation and washed 3 times with homogenization buffer. Elution of the proteins from the beads was done by adding sample buffer and heating the samples at 65°C for 5 min. The eluted proteins were loaded on 6% SDS-PAGE. Proteins were transferred to nitrocellulose membranes for Western blotting.
HL-1 immunocytochemistry
HL-1 cells were fixed in 4% paraformaldehyde and rinsed in PBS 3 times. The cells were then permeabilized using 0.25% Triton X-100 (sigma) and rinsed in PBS. Cells were blocked in 10% normal donkey serum (Jackson Immunoresearch Laboratories) to eliminate non-specific binding. Cells were then incubated with anti-CaV1.2-ATTO-488 antibody (Alomone Labs) overnight at 4°C, rinsed in PBS to remove excess antibody and images were taken with a confocal laser scanning microscope (Zeiss 510 META). ImageJ (http://imagej.nih.gov/ij/) was used to quantify the expression of CaV1.2 in the membrane with and without PMA treatment. Fluorescence of the whole cell was measured in ‘integrated density’ using the free hand drawing tool. A second measurement was made at about 6–10 pixels inside the first one and this was considered as the fluorescence from cytoplasm. The intensity of whole cell was then subtracted from the intensity of the cytoplasm to give the intensity of the membrane. The intensity obtained for the membrane fraction was later corrected for background, by subtracting separate background measurements. The values obtained were analyzed using unpaired t-test using the Prism software.
Giant membrane patches
Giant excised patches of oocyte membrane were prepared as described.52 Oocytes were mechanically devitellinized using tweezers in a hypertonic solution (in mm: 6 NaCl, 150 KCl, 4 MgCl2, 10 Hepes, pH 7.6) and transferred onto a coverslip in EGTA-containing ND96 solution (in mm: 96 NaCl, 2 KCl, 1 MgCl2, 5 Hepes, 5 EGTA, pH 7.6) with their animal pole facing the coverslip, for 30 min. The oocytes were then removed with a jet of solution using a Pasteur pipette, leaving a giant membrane patch attached to the coverslip, with the cytosolic part facing the medium, and the extracellular surface facing the coverslip. The coverslip was washed thoroughly with fresh ND96 solution, and fixated using 4% formaldehyde in EGTA-containing ND96 solution for 30 min. Coverslips were mounted on a glass slide. Giant membrane patches were stained with primary antibody anti-α1C polyclonal antibody (Alomone, Jerusalem), followed by incubation with Cy3 fused secondary antibody. Fluorescent labeling was examined with the Zeiss 510 META confocal microscope, using a 63 × oil-immersion objective. Cy3 was excited by 488 nm laser and the intensities were measured at 560–569 nm window in the spectral mode. In each experiment, all oocytes from the different groups were studied using a constant set of imaging parameters. Net fluorescence intensity per unit area was obtained by subtracting an averaged background signal measured in the same way on the coverslip outside the oocytes.
Statistics and data presentation
The data are presented as mean±SEM, n = number of cells tested. Comparisons between 2 groups (e.g., control and PMA treated groups) were tested for statistically significant differences (P<0.05 or better) using 2-tailed unpaired t – test. Comparison between several groups was done using one-way analysis of variance (ANOVA) followed by Tukey's test, using the SigmaPlot software (SPSS Corp.).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by German-Israeli Foundation for Research and Development (GIF; S.W., N.D., H.H. and E.K., grant # I-1210–286.13/2012); the Fields Fund for Molecular Cardiology (N.D.).
References
- [1].Hughes BP, Both K, Harland L, Hunt J, Hurst KM, Lewis M, Barritt GJ. Identification of an mRNA species which encodes a voltage-operated Ca2+ channel in rat liver mRNA. Biochem Mol Biol Int. 1993;31:193-200. PMID:7505152 [PubMed] [Google Scholar]
- [2].Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569-74. doi: 10.1038/301569a0 [DOI] [PubMed] [Google Scholar]
- [3].Xiong Z, Sperelakis N. Regulation of L-type calcium channels of vascular smooth muscle cells. J Mol Cell Cardiol. 1995;27:75-91. doi: 10.1016/S0022-2828(08)80009-0. PMID:7760390 [DOI] [PubMed] [Google Scholar]
- [4].Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563-90. doi: 10.1146/annurev.neuro.31.060407.125631. PMID:18558867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marshall MR, Clark JP, Westenbroek R, Yu FH, Scheuer T, Catterall WA. Functional roles of a c-terminal signaling complex of CaV1 channels and A-kinase anchoring protein 15 in brain neurons. Journal of Biological Chemistry. 2011;286:12627-39. doi: 10.1074/jbc.M110.175257. PMID:21224388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brandmayr J, Poomvanicha M, Domes K, Ding J, Blaich A, Wegener JW, Moosmang S, Hofmann F. Deletion of the C-terminal phosphorylation sites in the cardiac β-subunit does not affect the basic β-adrenergic response of the heart and the Cav1.2 channel. Journal of Biological Chemistry. 2012;287:22584-92. doi: 10.1074/jbc.M112.366484. PMID:22589548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dixon RE, Yuan C, Cheng EP, Navedo MF, Santana LF. Ca2+ signaling amplification by oligomerization of L-type Cav1.2 channels. Proceedings of the National Academy of Sciences USA. 2012;109:1749-54. doi: 10.1073/pnas.1116731109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23-49. doi: 10.1146/annurev.physiol.70.113006.100455. PMID:17988210 [DOI] [PubMed] [Google Scholar]
- [9].Catterall WA. Regulation of cardiac calcium channels in the Fight-or-Flight response. Curr Mol Pharmacol. 2015;8:12-21. doi: 10.2174/1874467208666150507103417. PMID:25966697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annual Review of Physiology. 2005;67:69-98. doi: 10.1146/annurev.physiol.67.040403.114521. PMID:15709953 [DOI] [PubMed] [Google Scholar]
- [11].Wang CY, Yang F, He X, Chow A, Du J, Russell JT, Lu B. Ca2+ binding protein frequenin mediates GDNF-induced potentiation of Ca2+ channels and transmitter release. Neuron. 2001;32:99-112. doi: 10.1016/S0896-6273(01)00434-2. PMID:11604142 [DOI] [PubMed] [Google Scholar]
- [12].Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533-5. doi: 10.1016/S0896-6273(00)81057-0. PMID:10774722 [DOI] [PubMed] [Google Scholar]
- [13].Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230-3. doi: 10.1038/340230a0. PMID:2474130 [DOI] [PubMed] [Google Scholar]
- [14].Blumenstein Y, Kanevsky N, Sahar G, Barzilai R, Ivanina T, Dascal N. A novel long-N-terminus isoform of human L-type Ca2+ channel is up-regulated by protein kinase C. J. Biol. Chem. 2002;277:3419-23. doi: 10.1074/jbc.C100642200. PMID:11741969 [DOI] [PubMed] [Google Scholar]
- [15].Dai B, Saada N, Echetebu C, Dettbarn C, Palade P. A new promoter for a1C subunit of human L-type cardiac calcium channel Cav1.2. Biochem Biophys Res Commun. 2002;296:429-33. doi: 10.1016/S0006-291X(02)00894-X. PMID:12163037 [DOI] [PubMed] [Google Scholar]
- [16].Koch WJ, Ellinor PT, Schwartz A. cDNA cloning of a dihydropyridine-sensitive calcium channel from rat aorta. Evidence for the existence of alternatively spliced forms. J Biol Chem. 1990;265:17786-91. PMID:2170396 [PubMed] [Google Scholar]
- [17].Snutch TP, Tomlinson WJ, Leonard JP, Gilbert MM. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991;7:45-57. doi: 10.1016/0896-6273(91)90073-9. PMID:1648941 [DOI] [PubMed] [Google Scholar]
- [18].Biel M, Ruth P, Bosse E, Hullin R, Stuhmer W, Flockerzi V, Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990;269:409-12. doi: 10.1016/0014-5793(90)81205-3. PMID:2169433 [DOI] [PubMed] [Google Scholar]
- [19].Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human L-type voltage-gated calcium channel, Cav1.2 a1 subunit. J Biol Chem. 2004;279:44335-43. doi: 10.1074/jbc.M407023200. PMID:15299022 [DOI] [PubMed] [Google Scholar]
- [20].Weiss N, Legrand C, Pouvreau S, Bichraoui H, Allard B, Zamponi GW, De Waard M Jacquemond V. In vivo expression of G-protein b1g2 dimer in adult mouse skeletal muscle alters L-type calcium current and excitation-contraction coupling. J Physiol London. 2010;558:2945-60. doi: 10.1113/jphysiol.2010.191593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521-55. doi: 10.1146/annurev.cellbio.16.1.521. PMID:11031246 [DOI] [PubMed] [Google Scholar]
- [22].Weiss S, Doan T, Bernstein KE, Dascal N. Modulation of cardiac Ca2+ channel by Gq-activating neurotransmitters reconstituted in Xenopus oocytes. J. Biol. Chem. 2004;279:12503-10. doi: 10.1074/jbc.M310196200. PMID:14722109 [DOI] [PubMed] [Google Scholar]
- [23].Weiss S, Keren-Raifman T, Oz S, Ben Mocha A, Haase H, Dascal N. Modulation of distinct isoforms of L-type calcium channels by Gq-coupled receptors in Xenopus oocytes: Antagonistic effects of Gβγ and protein kinase C. Channels. 2012;6:426-37. doi: 10.4161/chan.22016. PMID:22990911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weiss S, Dascal N. Molecular aspects of modulation of L-type calcium channels by protein kinase C. Current Molecular Pharmacology. 2015;8:43-53. doi: 10.2174/1874467208666150507094733. PMID:25966700 [DOI] [PubMed] [Google Scholar]
- [25].Fish RD, Sperti G, Colucci WS, Clapham DE. Phorbol ester increases the dihydropyridine-sensitive calcium conductance in a vascular smooth muscle cell line. Circ Res. 1988;62:1049-54. doi: 10.1161/01.RES.62.5.1049. PMID:2452033 [DOI] [PubMed] [Google Scholar]
- [26].Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112-17. doi: 10.1073/pnas.0500360102. PMID:16040810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Callaghan B, Zhong J, Keef KD. Signaling pathway underlying stimulation of L-type Ca2+ channels in rabbit portal vein myocytes by recombinant Gbg subunits. Am J Physiol Heart Circ Physiol. 2006;291:H2541−2546. doi: 10.1152/ajpheart.00420.2006. PMID:16877561 [DOI] [PubMed] [Google Scholar]
- [28].Bauer J, Dau C, Cavarape A, Schaefer F, Ehmke H, Parekh N. ANG II- and TxA(2)-induced mesenteric vasoconstriction in rats is mediated by separate cell signaling pathways. Am J Physiol. 1999;277:H1−7. PMID:10409174 [DOI] [PubMed] [Google Scholar]
- [29].Lambert C. Mechanisms of angiotensin II chronotropic effect in anaesthetized dogs. Br J Pharmacol. 1995;115:795-800. doi: 10.1111/j.1476-5381.1995.tb15003.x. PMID:8548179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nagahama T, Hayashi K, Ozawa Y, Takenaka T, Saruta T. Role of protein kinase C in angiotensin II-induced constriction of renal microvessels. Kidney Int. 2000;57:215-23. doi: 10.1046/j.1523-1755.2000.00822.x. PMID:10620202 [DOI] [PubMed] [Google Scholar]
- [31].Fan QI, Vanderpool K, Marsh JD. A 27 bp cis-acting sequence is essential for L-type calcium channel alpha(1C) subunit expression in vascular smooth muscle cells. Biochim Biophys Acta. 2002;1577:401-11. doi: 10.1016/S0167-4781(02)00441-4. PMID:12359330 [DOI] [PubMed] [Google Scholar]
- [32].Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by α2δ and β subunits in Xenopus oocytes: contribution of changes in channel gating and α1 protein level. J Physiol (Lond). 1995;489:55-62. doi: 10.1113/jphysiol.1995.sp021029. PMID:8583415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singer-Lahat D, Gershon E, Lotan I, Hullin R, Biel M, Flockerzi V, Hofmann F, Dascal N. Modulation of cardiac Ca2+ channels in Xenopus oocytes by protein kinase C. FEBS Lett. 1992;306:113-18. doi: 10.1016/0014-5793(92)80980-U. PMID:1321730 [DOI] [PubMed] [Google Scholar]
- [34].McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci U S A. 2000;97:12334-38. doi: 10.1073/pnas.210384297. PMID:11035786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang L, Doshi D, Morrow J, Katchman A, Chen X, Marx SO. Protein kinase C isoforms differentially phosphorylate CaV1.2 α1c. Biochemistry. 2009;48:6674-83. doi: 10.1021/bi900322a. PMID:19527072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yang L, Liu G, Zakharov S, Morrow JP, Rybin VO, Steinberg SF, Marx SO. S1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207-14. doi: 10.1074/jbc.M410509200. PMID:15509562 [DOI] [PubMed] [Google Scholar]
- [37].Gao T, Puri TS, Gerhardstein BL, Chien AJ, Green RD, Hosey MM. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272:19401-07. doi: 10.1074/jbc.272.31.19401. PMID:9235939 [DOI] [PubMed] [Google Scholar]
- [38].Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel a1 subunits. J Cell Biol. 1993;123:949-62. doi: 10.1083/jcb.123.4.949. PMID:8227151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Jongh KS Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by adenosine 3',5'-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392-402. doi: 10.1021/bi953023c. PMID:8756695 [DOI] [PubMed] [Google Scholar]
- [40].Hulme JT, Yarov-Yarovoy V, Lin TW-C, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. The Journal of Physiology. 2006;576:87-102. doi: 10.1113/jphysiol.2006.111799. PMID:16809371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Ten Eick R, Hosey MM. C-terminal fragments of the a1C (Cav1.2) subunit associate with and regulate L-type calcium channels containing C-terminally truncated a1C subunits. J Biol Chem. 2001;276:21089-97. doi: 10.1074/jbc.M008000200. PMID:11274161 [DOI] [PubMed] [Google Scholar]
- [42].Weiss S, Oz S, Benmocha A, Dascal N. Regulation of cardiac L-type Ca2+ channel CaV1.2 via the β-adrenergic-cAMP-protein kinase A pathway: Old dogmas, advances, and new uncertainties. Circulation Research. 2013;113:617-31. doi: 10.1161/CIRCRESAHA.113.301781. PMID:23948586 [DOI] [PubMed] [Google Scholar]
- [43].Patriarchi T, Qian H, Di Biase V, Malik ZA, Chowdhury D, Price JL, Hammes EA, Buonarati OR, Westenbroek RE, Catterall WA, Hofmann F, Xiang YK, Murphy GG, Chen CY, Navedo MF, Hell JW. Phosphorylation of Cav1.2 on S1928 uncouples the L-type Ca2+ channel from the β2 adrenergic receptor. EMBO Journal. 2016;35:1330-45. doi: 10.15252/embj.201593409. PMID:27103070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Qian H, Patriarchi T, Price JL, Matt L, Lee B, Nieves-Cintron M, Buonarati OR, Chowdhury D, Nanou E, Nystoriak MA, Catterall WA, Poomvanicha M, Hofmann F, Navedo MF, Hell JW. Phosphorylation of Ser1928 mediates the enhanced activity of the L-type Ca2+ channel Cav1.2 by the beta2-adrenergic receptor in neurons. Sci Signal. 2017;10. doi: 10.1126/scisignal.aaf9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nystoriak MA, Nieves-Cintron M, Patriarchi T, Buonarati OR, Prada MP, Morotti S, Grandi E, Fernandes JD, Forbush K, Hofmann F, Sasse KC, Scott JD, Ward SM, Hell JW, Navedo MF. Ser1928 phosphorylation by PKA stimulates the L-type Ca2+ channel CaV1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci Signal. 2017;10. doi: 10.1126/scisignal.aaf9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2979-84. doi: 10.1073/pnas.95.6.2979. PMID:9501201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pelloux S, Robillard J, Ferrera R, Bilbaut A, Ojeda C, Saks V, Ovize M, Tourneur Y. Non-beating HL-1 cells for confocal microscopy: application to mitochondrial functions during cardiac preconditioning. Prog Biophys Mol Biol. 2006;90:270-98. doi: 10.1016/j.pbiomolbio.2005.06.009. PMID:16140363 [DOI] [PubMed] [Google Scholar]
- [48].White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. American Journal of Physiology – Heart and Circulatory Physiology. 2004;286:H823−H829. doi: 10.1152/ajpheart.00986.2003. PMID:14766671 [DOI] [PubMed] [Google Scholar]
- [49].Dascal N, Lotan I. Expression of exogenous ion channels and neurotransmitter receptors in RNA-injected Xenopus oocytes. In: Longstaff A,Revest P, eds Protocols in Molecular Neurobiology. Totowa: (NJ): Humana Press; 1992. p. 205-25. [Google Scholar]
- [50].Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553-57. doi: 10.1126/science.1716787. PMID:1716787 [DOI] [PubMed] [Google Scholar]
- [51].Shistik E, Ivanina T, Blumenstein Y, Dascal N. Crucial role of N terminus in function of cardiac L-type Ca2+ channel and its modulation by protein kinase C. J Biol Chem. 1998;273:17901-09. doi: 10.1074/jbc.273.28.17901. PMID:9651396 [DOI] [PubMed] [Google Scholar]
- [52].Singer-Lahat D, Dascal N, Mittelman L, Peleg S, Lotan I. Imaging plasma membrane proteins in large membrane patches of Xenopus oocytes. Pflugers Arch – Eur J Physiol. 2000;440:627-33. [DOI] [PubMed] [Google Scholar]
- [53].Steinberg SF. Cardiac actions of protein kinase C isoforms. Physiology (Bethesda). 2012;27:130-9. doi: 10.1152/physiol.00009.2012. PMID:22689788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995;268:247-51. doi: 10.1126/science.7716516. PMID:7716516 [DOI] [PubMed] [Google Scholar]
- [55].Nakashima S. Protein kinase C alpha (PKC alpha): regulation and biological function. J Biochem. 2002;132:669-75. doi: 10.1093/oxfordjournals.jbchem.a003272. PMID:12417014 [DOI] [PubMed] [Google Scholar]
- [56].Shistik E, Keren-Raifman T, Idelson GH, Dascal N, Ivanina T. The N-terminus of the cardiac L-type Ca2+ channel a1C subunit: The initial segment is ubiquitous and crucial for protein kinase C modulation, but it is not directly phosphorylated. J. Biol. Chem. 1999;274:31145-49. doi: 10.1074/jbc.274.44.31145. PMID:10531304 [DOI] [PubMed] [Google Scholar]
- [57].Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185-96. doi: 10.1016/S0896-6273(00)80358-X. PMID:9247274 [DOI] [PubMed] [Google Scholar]
- [58].Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J. Identification of PKA phosphorylation sites in the carboxyl terminus of L-type calcium channel a1 subunits. Biochemistry. 1996;35:9400-06. doi: 10.1021/bi960683o. PMID:8755718 [DOI] [PubMed] [Google Scholar]
- [59].Perets T, Blumenstein Y, Shistik E, Lotan I, Dascal N. A potential site of functional modulation by protein kinase A in the cardiac Ca2+ channel a1C subunit. FEBS Lett. 1996;384:189-92. doi: 10.1016/0014-5793(96)00303-1. PMID:8612821 [DOI] [PubMed] [Google Scholar]
- [60].Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiological Reviews. 2009;89:411-52. doi: 10.1152/physrev.00029.2007. PMID:19342611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res. 2009;104:1373-81. doi: 10.1161/CIRCRESAHA.108.191387. PMID:19461046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hofmann F, Flockerzi V, Kahl S, Wegener JW. L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiological Reviews. 2014;94:303-26. doi: 10.1152/physrev.00016.2013. PMID:24382889 [DOI] [PubMed] [Google Scholar]
- [63].Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611-22. doi: 10.1085/jgp.200609519. PMID:16702354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schuhmann K, Groschner K. Protein kinase-C mediates dual modulation of L-type Ca2+ channels in human vascular smooth muscle. FEBS Lett. 1994;341:208-12. doi: 10.1016/0014-5793(94)80458-3. PMID:8137940 [DOI] [PubMed] [Google Scholar]
- [65].Zhu WZ, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS One. 2009;4:e5407. doi: 10.1371/journal.pone.0005407. PMID:19404384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Huang J, Zamponi GW. Regulation of voltage gated calcium channels by GPCRs and post-translational modification. Curr Opin Pharmacol. 2016;32:1-8. doi: 10.1016/j.coph.2016.10.001. PMID:27768908 [DOI] [PubMed] [Google Scholar]
- [67].Navedo MF, Amberg GC. Local regulation of L-type Ca(2)(+) channel sparklets in arterial smooth muscle. Microcirculation. 2013;20:290-8. doi: 10.1111/micc.12021. PMID:23116449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Edwards BS, Dang AK, Murtazina DA, Dozier MG, Whitesell JD, Khan SA, Cherrington BD, Amberg GC, Clay CM, Navratil AM. Dynamin Is Required for GnRH Signaling to L-Type Calcium Channels and Activation of ERK. Endocrinology. 2016;157:831-43. doi: 10.1210/en.2015-1575. PMID:26696122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Domes K, Ding J, Lemke T, Blaich A, Wegener JW, Brandmayr J, Moosmang S, Hofmann F. Truncation of murine Cav1.2 at Asp1904 results in heart failure after birth. Journal of Biological Chemistry. 2011;286:33863-71. doi: 10.1074/jbc.M111.252312. PMID:21832054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Benmocha Guggenheimer A, Almagor L, Tsemakhovich V, Tripathy DR, Hirsch JA, Dascal N. Interactions between N and C termini of α1C subunit regulate inactivation of CaV1.2 L-type Ca2+ channel. Channels. 2016;10:55-68. doi: 10.1080/19336950.2015.1108499. PMID:26577286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by Gbg and calmodulin via interactions with N- and C-termini of a1C. J Biol Chem. 2000;275:39846-54. doi: 10.1074/jbc.M005881200. PMID:10995757 [DOI] [PubMed] [Google Scholar]


