Abstract
Women with fibromyalgia (FM) often complain of whole-body pain, and muscle fatigue, which may be related to autonomic dysfunction. Therefore, the purpose of the present study was to investigate the effects of resistance exercise training (RET) on disease impact, pain catastrophizing, and autonomic modulation in women with FM. Women with FM (n=26) and healthy control women (HC: n=9), aged 19–65 yrs, were compared at rest. Women with FM were randomly assigned to a resistance-training group (FM-RT: n=14) or a non-exercising control group (FM-CON: n=12). Women in the FM-RT group underwent 8-weeks of RET on 4 different exercises, 2 times per week, 3 sets of 8–12 repetitions at 50%-60% of the pre-determined 1-repetition max (1RM). Autonomic modulation was assessed using heart rate variability and heart rate complexity. Healthy control women had a lower resting heart rate, decreased normalized low-frequency power, and increased normalized high-frequency power compared to the FM groups at rest. After the 8-week intervention, significant increases (p ≤ 0.05) in 1RM were observed for both chest press and leg extension for women in the RT group. Disease impact was significantly reduced (p ≤ 0.05) for participants in the FM-RT group (FM-RT: 59±12 to 41±24 units; FM-CON: 72±7 to71±8 units), but pain catastrophizing was unaltered. There were no significant changes in autonomic modulation after the RET intervention. These data demonstrate that while women with FM may still have autonomic dysfunction after undergoing a RET program, disease impact was significantly reduced.
Keywords: Heart rate variability, heart rate complexity, strength training, fibromyalgia impact, widespread pain index
INTRODUCTION
Fibromyalgia (FM) is an idiopathic disease characterized by chronic, widespread pain and a variety of symptoms (37). These symptoms include, but are not limited to, reduced muscular strength and muscular endurance (26), muscular fatigue (2), and elevated levels of distress resulting in pain-related catastrophizing (13). Researchers suggest exercise training assists symptomatic relief in women with FM. However, much of the data have focused on aerobic training, while only a few studies have investigated resistance exercise training (RET) as an exercise intervention.
While the etiology of FM is unknown, autonomic dysfunction has been considered a primary factor contributing to symptom severity, including pain (21), orthostatic intolerance (29), and intolerance to cold (36). Studies evaluating autonomic modulation in individuals with FM, via heart rate variability (HRV), have reported reduced parasympathetic activity at rest compared to healthy controls (7, 22). On the contrary, other reports have demonstrated no difference in resting measures of autonomic modulation in women with FM compared to healthy controls (15, 16).
Heart rate complexity (HRC), another measure of autonomic modulation, has been suggested to be a more sensitive assessment of parasympathetic modulation compared to HRV (14). Although the two methods are similar, HRV is a linear measure of heart rate kinetics, while HRC is a non-linear tool to measure vagal modulation. Chervin et al. (2009) reported women with FM to have decreased HRC compared to age-matched, healthy controls (5). Their findings further support the notion that women with FM may have reduced parasympathetic activity at rest.
Researchers that have investigated the effects of RET on healthy populations suggest that it may not affect HRV (8). In addition, work by Heffernan et al. (2007) observed no change in HRV, but did report significant increases in HRC after a period of RET. While these researchers have reported no change in HRV in healthy individuals, data in women with FM that had autonomic dysfunction at rest had increased parasympathetic activity with RET when measured via HRV (11). It is clear more information is needed.
To our knowledge no study has investigated the effects of RET on HRC in women with FM. In addition, it has been suggested that pain catastrophizing, a propensity for an exaggerated pain experience, may be elevated to a greater degree in women with FM compared to other pain conditions (10, 13). This may lead to the avoidance of physical activity, therefore inducing deconditioning, and consequently more pain (9). This cascade of events greatly increases the likelihood of pain catastrophizing in the FM population. However, research thus far has yet to extensively evaluate the effects of RET on pain catastrophizing in women with FM. Therefore, the purpose of this study was to assess the effects of 8 weeks of RET on disease impact, pain catastrophizing, and autonomic modulation in women with FM. We hypothesized that women with FM would have autonomic dysfunction at rest compared to a group of healthy control women. We also hypothesized that 8 weeks of RET would increase maximal strength, decrease symptoms including pain, and symptom severity, as well as decrease pain catastrophizing and the impact of the disease on quality of life. Furthermore, we hypothesized the 8 weeks of RET would improve autonomic modulation measured via HRC, but not HRV.
METHODS
Participants
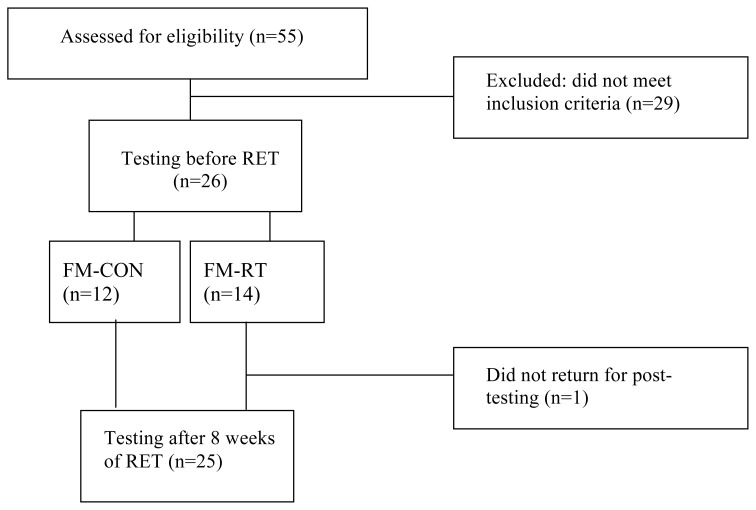
Based on our preliminary data, an effect size of 0.89 was determined on the variable of Sample Entropy (SampEn), with a power of 80% and an alpha of 0.05, thereby 21 participants would be needed. Therefore, thirty-five women (19–65 yrs), 26 women with FM and 9 healthy controls (HC), were recruited through fliers and newspaper advertisements in the local community. Exclusion criteria included having engaged in any form of exercise within the past year, smoking within the past year, history of cardiovascular, pulmonary or metabolic diseases and using any medications that may affect heart rate or blood pressure (BP). Medications that were reported being used by the women in the present study included painkillers (n=3) and sleep aids (n=5). All medications were stopped 12 hours prior to testing in order to remove any effect they may have on heart rate or BP. There were 55 women with FM that were interviewed for this study (Figure 1). Of those 55 women, 26 women, aged 19–65 years, met the necessary criteria for inclusion in the study. Women with FM were randomized to either a resistance-training group (FM-RT; n=14) or a control group (FM-CON; n=12). All participants gave written consent, and the study was approved by the Institutional Review Board at Kent State University.
Figure 1.
Timeline of participant progression for women with fibromyalgia. FM-CON: Women with fibromyalgia in control group, FM-RT: Women with fibromyalgia in resistance training group; RET: resistance exercise training
Protocol
The HC group was assessed only for autonomic modulation at baseline in order to determine if the women with FM had autonomic dysfunction before beginning the RET or the control (11). Women with FM were tested for all variables before and after 8 weeks of either RET (FM-RT) or a non-exercising control (FM-CON) period. There were three initial visits for each of the testing periods. The initial testing consisted of questionnaires, anthropometrics and maximal strength, which was verified during the second visit. The last test day included measures of autonomic modulation at rest. All measurements were collected at the same time of day over the course of the week to control for circadian rhythm as it has been suggested that time of day may alter autonomic modulation (3). Autonomic modulation testing after the 8 weeks of RET was completed within 72 hours of the participants’ last training session. Participants in the control group were asked to maintain their normal diet and current activities of daily living throughout the 8 weeks.
The American College of Rheumatology revised the diagnostic criteria for FM in order to focus on the Widespread Pain Index (WPI) and Symptom Severity (SS) scale as a means to verify diagnosis of FM, and to determine the extent of whole-body pain and the severity of symptoms (37) in those individuals with FM. The WPI assesses whole-body pain by asking participants to circle where they hurt using 19 different areas for reference. The SS utilizes 6 categories of symptoms, including: somatic symptoms, waking up unrefreshed, cognition, fatigue, sleep problems, and mood. This revised diagnostic criteria for FM requires a WPI ≥ 7 and SS ≥ 5 or a WPI 3–6 and SS ≥ 9 for diagnosis of FM (37).
The Fibromyalgia Impact Questionnaire (FIQ) consists of 20 questions directed towards the ability to complete activities of daily living, number of days of pain, number of days of missed work due to FM, and symptom severity (4). The greater the FIQ score, the greater the impact on week-to-week life in those with FM. Marques et al. (2004) determined the average individual with FM scores a 50 on the FIQ, while a more severely impacted individual with FM will score 70 or above (20). The FIQ is a reliable and valid assessment tool (4).
The Pain Catastrophizing Scale (PCS) is a 13-item self-reported measure of pain catastrophizing (34). Sullivan et al. (1995) defined pain catastrophizing as how an individual experiences their pain and has been suggested to have three primary parts: focused attention on pain, magnification of pain, and the feeling of helplessness. Higher scores are associated with higher levels of pain catastrophizing. Reported data demonstrate that the PCS is valid and reliable in both clinical and nonclinical populations (23, 34).
Body weight was measured on a scale (My Weigh, Elite Scale, Vancounver, BC, Canada), calibrated to the nearest 0.1kg prior to each use. Height was measured to the nearest 0.05cm on a wall-mounted stadiometer (Charder, HM210D, Taichung City, Taiwan). BMI was calculated from these measurements and reported in kg/m2.
The 1-repetition maximum (1RM) was determined for the leg extension and chest press (Hammer Strength; Life Fitness, Schiller Park, Illinois, USA). All measurements were assessed within 3–5 attempts, with three minutes between each attempt to allow ample time for muscle recovery. Seventy-two hours later the 1RM was re-assessed to verify the original 1RM. The higher of the two measurements was taken as the participants’ 1RM.
All participants abstained from food and caffeine for at least 12 hours, and strenuous physical activity for at least 24 hours prior to testing autonomic modulation. Participants were instructed to breathe with a metronome set at 12 breaths/min while resting measures were recorded using an electrocardiograph (ECG) during the last five minutes of a 25-min supine resting period.
Autonomic modulation using HRV was collected following parameters outlined by the European Task Force on HRV (35). ECG signals were recorded for a 5-minute time period using a modified three lead, CM5 configuration at a rate of 1000 Hz that was interfaced with a Biopac acquisition system [Biopac Systems, Santa Barbara, California, USA]. The R-R intervals from the ECG recordings were used to calculate HRV and HRC. WinCPRS software [Absolute Aliens, Turku, Finland] was used to import the ECG and for the subsequent, offline analysis. Each ECG was visually inspected for ectopics, noise, and artifacts prior to analysis. If any ectopics were noted during visual inspection, data were interpolated appropriately by research personnel. Since the sample was only a 5-minute time period, Fast Fourier transformation was used to produce the power spectrum. Total power of HRV was used as an index of total activity from the autonomic nervous system. Low-frequency (LF) power (0.04–0.15 Hz) has been suggested to be indicative of both sympathetic and parasympathetic modulations (19, 27, 35), while high-frequency (HF) (0.15–0.4 Hz) power is mediated by changes in parasympathetic modulation (35). The LF/HF ratio was used as a measure of sympathovagal dominance (1). Power spectra were calculated in absolute (ms2) and normalized units (nu) to evaluate the power of each component in relation to total power. Normalized units are determined by dividing the total power by the appropriate component and then dividing by 100 (35). Researchers utilizing power spectral analysis suggest normalized values of LF (LFnu) and HF (HFnu) are surrogates for sympathetic and parasympathetic modulation, respectively (35).
Autonomic modulation was also determined using HRC. Sample entropy was used as a method for examining the R-R interval over the 5-min time period following removal of the linear trend. Sample Entropy has been defined as the probability of matches or sequences being similar over a short period of time, which has a range of 0–2 (32). A value closer to 0 indicates a more predictive signal, while a value closer to 2 is considered more chaotic (32). Palazzolo et al. (1998) observed significant reductions in cardiovascular complexity after the administration of atropine– a medication used to block cholinergic activity (24). SampEn is a non-linear measure of autonomic modulation that is strongly influenced by the vagus nerve, and therefore sensitive to changes in the complexity of the cardiovasculature (24).
Supervised RET was performed twice a week for eight weeks. Each session was separated by at least 48 hours. The RET regime consisted of 3 sets of 8–12 repetitions, followed by a 90-s rest period between each set. Four exercises were performed: chest press, leg extension, leg curl, and seated row. The initial training intensity was 50% and 60% of the 1RM for the upper- and lower-body, respectively. The training intensity for the leg curl and seated row was defined as being 5% lower than the 1RM of their antagonist muscle group. When the participants were able to complete 12 repetitions on all 3 sets, over two consecutive training days, the resistance was increased by 2–10% as recommended by the American College of Sports Medicine (17). Each training session lasted approximately 30 minutes.
Statistical Analysis
Participant characteristics between the groups (FM versus HC) were assessed with an independent samples t-test. The absolute values of total power, LF and HF were assessed for their distribution via the Kolmogorov-Smirnoff test, a test of sample distribution, which revealed that the data were not normally distributed. Therefore, they were subsequently transformed to their natural log (Ln total power, Ln low-frequency power, and Ln high-frequency power) in order to meet the assumptions of parametric testing. Autonomic modulation between women with FM and the HC group was performed using independent samples t-test. In women with FM, a 2 × 2 (group [FM-RT, FM-CON] × time [before and after]) repeated measures analysis of variance (ANOVA) was used to analyze mean differences for all relevant variables. In the event of a significant interaction, paired t-tests were used for post-hoc comparisons. Significance was set a priori at p≤0.05. All data are expressed as mean ± standard deviation (SD) except in figures where standard error of the mean (SEM) was used. All statistical analyses were performed using SPSS version 23 (IBM SPSS, Armonk, NY, USA).
RESULTS
Women with FM were similar (p>0.05) for age, height, weight, and BMI before and after RET or the control (Table 1). One participant withdrew from the FM-RT group due to scheduling conflicts. Adherence to the exercise regime in the FM-RT group was 92%.
Table 1.
Participant characteristics for women with fibromyalgia and healthy controls (N=34).
| Variable | FM (n=25) | HC (n=9) |
|---|---|---|
| Age (yrs) | 52 ± 13 | 49 ± 8 |
| Height (m) | 1.63 ± 0.05 | 1.62 ± 0.08 |
| Weight (kg) | 86 ± 17 | 81 ± 22 |
| BMI (kg/m2) | 32.5 ± 4.4 | 30.9 ± 3.9 |
| SS | 10 ± 3 | - |
| WPI | 11 ± 3 | - |
Values are expressed as mean ± SD; BMI: Body mass index; FM: Fibromyalgia: HC: Healthy Control; SS: Symptom Severity; WPI: Widespread Pain Index
Heart rate and normalized LF power were significantly lower (p≤0.05) in the HC compared to the women with FM (Table 2). The HC group had greater Ln total power, greater absolute and normalized HF power, as well as lower levels of normalized LF power, compared to the women with FM. There was no difference in SampEn between the groups.
Table 2.
Baseline autonomic modulation in women with fibromyalgia and healthy controls (N=34).
| Variable | FM (n=25) | HC (n=9) |
|---|---|---|
| Heart rate | 73 ± 11 | 63 ± 6* |
| Total power (Ln ms2) | 6.8 ± 0.7 | 7.6 ± 1.0* |
| Low-frequency (Ln ms2) | 5.2 ± 0.9 | 5.5 ± 1.3 |
| Low-Frequency (nu) | 44.2 ± 15.5 | 31.5 ± 18.8* |
| High-frequency (Ln ms2) | 5.3 ± 0.8 | 6.4 ± 1.8* |
| High-frequency (nu) | 53.4 ± 15.3 | 68.1 ± 18.4* |
| LF/HF | 1.04 ± 0.69 | 0.6 ± 0.54 |
| SampEn | 1.3 ± 0.5 | 1.4 ± 0.2 |
Values are expressed as mean ± SD; FM: Fibromyalgia; HC: Healthy control; HF: High frequency; LF: Low frequency;
p≤0.05 significant group difference
In the women with FM, there were no differences in muscle strength prior to the intervention (Table 3). There was a significant group × time interaction for the chest press (F1, 34=15.2, p≤0.05, Effect size (ES)=1.2) and the leg extension (F1, 34=20.711, p≤0.05, ES=1.0) (Table 3). The women with FM that underwent RET had a 35% increase in their maximal chest press and a 39% increase in their leg extension compared to a non-significant decrease of maximal strength in the women with FM that did not (FM-CON).
Table 3.
Maximal strength for women with fibromyalgia (N=25).
| Variable | FM-RT (n=13) | FM-CON (n=12) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Chest press (kg) | 34 ± 10 | 46 ± 12*† | 22 ± 12 | 24 ± 12 |
| Leg extension (kg) | 36 ± 12 | 50 ± 17*† | 22 ± 10 | 20 ± 10 |
Values are expressed as mean ± SD; FM-CON: Control; FM-RT: Resistance Trained;
p=0.05 significant group difference:
p=0.05 versus before training.
There were no differences between the women with FM at baseline for either the FIQ or PCS (Table 4). The FIQ was different between the women with FM across time, demonstrated by a significant (F1, 34=17.9, p≤ 0.05, ES=0.75) group × time interaction such that it was decreased in the FM-RT group but not in the FM-CON group. There was no effect of RET on the PCS (F1, 34 =0.14, p≤0.05, ES=0.53) in the FM-RT group.
Table 4.
Participant disease impact and pain catastrophizing in women with fibromyalgia (N=25).
| Variable | FM-RT (n=13) | FM-CON (n=12) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| FIQ (units) | 59 ± 12 | 41 ± 24*† | 72 ± 7 | 71 ± 8 |
| PCS (units) | 18 ± 13 | 11 ± 12 | 28 ± 14 | 20 ± 15 |
Values are expressed as mean ± SD; CON: Control; FIQ: Fibromyalgia Impact Questionnaire; PCS: Pain Catastrophizing Scale; RT: Resistance Trained;
p≤0.05 significant group difference;
p≤0.05 versus before training.
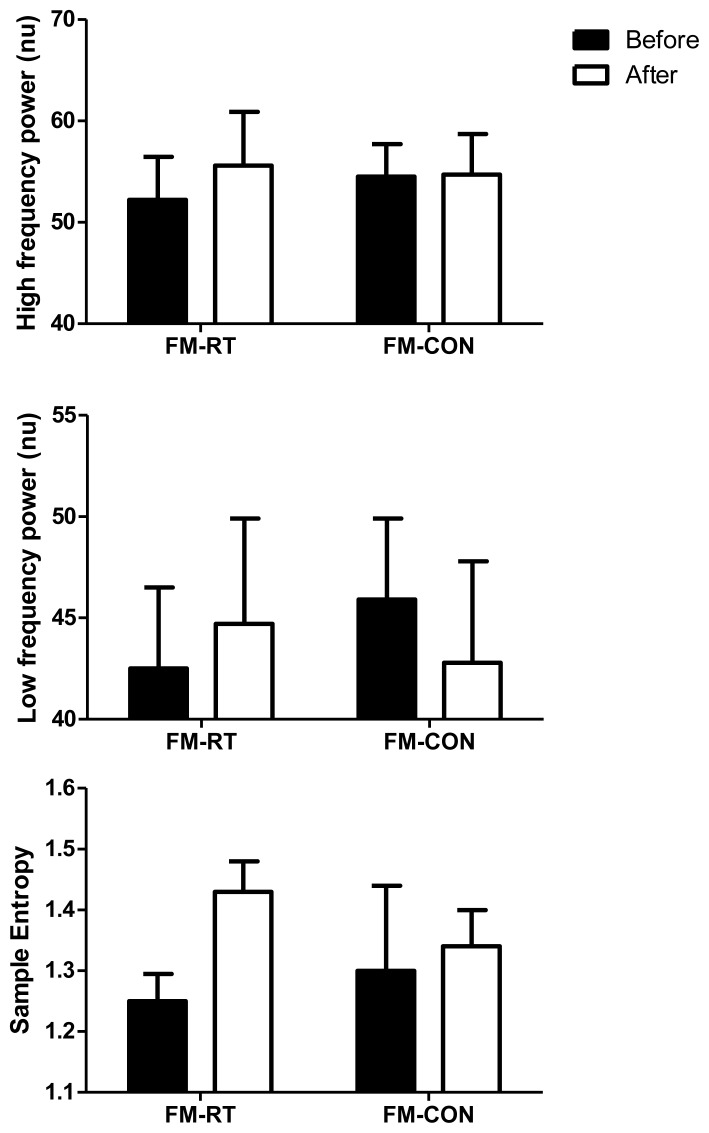
There were no differences between the women with FM at rest or across time on heart rate or any measures of autonomic modulation (Figure 2). In the FM-RT group, SampEn increased by 14% comparing before to after RET, however, it was not statistically significant (p=0.08).
Figure 2.
Changes in A) normalized high-frequency power, B) normalized low-frequency power, and C) sample entropy before and after 8 weeks of resistance exercise training in women with fibromyalgia (FM) that underwent resistance training (FM-RT; n=14) or a control (FM-CON; n=12). Values are expressed as mean ± SEM. ‡p=0.08 versus before training.
DISCUSSION
The primary finding of the present study is that 8 weeks of whole-body RET did not alter pain catastrophizing or autonomic modulation at rest in women with FM. However, 8 weeks of RET may be sufficient to significantly increase maximal strength, decrease disease impact, and improve quality of life in women with FM.
Our findings confirm previous work indicating women with FM have reduced HRV at rest compared to healthy controls (6, 7, 11, 12, 30, 31). Our results illustrate decreased levels of HF power, in both absolute and normalized units. This was also demonstrated by lower resting heart rates in the HC group compared to women with FM. Although previous researchers have not demonstrated changes in markers of sympathetic modulation at rest in women with FM (6, 7, 12), our data demonstrate significant increases in normalized LF power, which is a novel finding.
Our results for strength gains and FIQ scores reflect our previous work. After 16 weeks of RET, Figueroa et al., (2008) observed signifcant strength gains in women with FM, while (25) reported decreased FIQ scores utilizing a similar protocol but a different intervention period. Further work by Kingsley et al. (2010) reported both significant strength gains and decreased FIQ scores after only a 12-week intervention period. Our study is the first to successfully confirm the efficacy of an 8-week RET program on maximal strength gains and FIQ scores in women with FM.
Although FIQ and maximal strength were significantly impacted, it seems 8 weeks of RET did not significantly alter pain catastrophizing in women with FM. Smeets et al. (2006) found exercise training to attenuate pain catastrophizing in women with chronic low back pain. However, the participants performed both aerobic exercise and RET 3 days per week for 10 weeks (33). More data are needed in this area utilizing larger samples sizes to see if RET could be a useful modality to decrease pain catastrophizing in women with FM.
Heffernan et al. (2007) and Cooke et al. (2005) reported no change in HRV in healthy young men after 6 weeks, and 8 weeks of RET, respectively. Regardless of previous work utilizing healthy populations, some data suggest that individuals with autonomic dysfunction, such as those individuals with FM, may respond differently. Figueroa et al. (2008) reported significant increases in parasympathetic activity in women with FM, indicated by an increase in Ln RMSSD [the natural logarithm of the square root of the mean sum of the squared differences between R-R intervals], a measure of vagal modulation. Similar to our study, women in the study by Figueroa et al. (2008) had autonomic dysfunction at rest compared to healthy, age-matched controls.
Our results are comparable to our previous work in which we reported no change in HRV after 12 weeks of RET in women with FM (15). In the study by Kingsley et al. (15), participants had similar autonomic modulation at rest compared to healthy controls, which in turn may have lessened the likelihood of observing a noticeable change in autonomic modulation after RET. However, our results demonstrated some autonomic dysfunction at rest compared to health controls; yet, HRV was still not affected by the RET intervention. As such, it may be that autonomic modulation at rest may not be entirely indicative of subsequent physiological responses induced by RET. Despite inconsistencies in the literature exploring the effect of RET on HRV in women with FM, our results contribute to this body of research and provide evidence of the potential benefits an RET program may offer.
There is a paucity of research detailing the impact of RET on HRC. Heffernan et al. (2007) reported significant increases in SampEn after 6 weeks of RET in young, healthy men. In contrast, our results demonstrated no change in SampEn after 8 weeks of RET in women with FM. To our knowledge, there are no additional studies examining the effect of RET on HRC for any population.
Future studies should consider larger sample sizes to increase the likelihood of correctly identifying markers of autonomic modulation (28). Furthermore, 8-weeks of a RET program may not be enough to elicit significant changes in HRV in this population. We also recognize menstrual cycle in premenopausal women was not controlled, however, previous literature suggests menstrual cycle may not influence HRV (18). Finally, participants within the control group (FM-CON) were not required to keep a physical activity journal, as such, exercise behavior during the control period was not quantified.
In conclusion, results from this study suggest an 8-week RET intervention may be sufficient to increase maximal strength and reduce disease impact, thereby increasing quality of life in women with FM. However, this timeline may not be long enough to induce changes in pain catastrophizing, resting heart rate, or resting measures of autonomic modulation. These data demonstrate that while women with FM may still have autonomic dysfunction after undergoing a RET program, disease impact was significantly reduced. Researchers are encouraged to explore other methodological approaches, i.e., RET protocol and length of the intervention, to further understand the changes of autonomic modulation in women with FM.
REFERENCES
- 1.Billman GE. The LF/HF ratio does not accurately measure cardiac sympathovagal balance. Front Physiol. 2013;4:26. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borman P, Celiker R, Hascelik Z. Muscle performance in fibromyalgia syndrome. Rheumatol Int. 1999;19(1–2):27–30. doi: 10.1007/s002960050095. [DOI] [PubMed] [Google Scholar]
- 3.Boudreau P, Yeh WH, Dumont GA, Boivin DB. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol Int. 2012;29(6):757–768. doi: 10.3109/07420528.2012.674592. [DOI] [PubMed] [Google Scholar]
- 4.Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: development and validation. J Rheumatol. 1991;18:728–733. [PubMed] [Google Scholar]
- 5.Chervin RD, Teodorescu M, Kushwaha R, Deline AM, Brucksch CB, Ribbens-Grimm C, Ruzicka DL, Stein PK, Clauw DJ, Crofford LJ. Objective measures of disordered sleep in fibromyalgia. J Rheumatol. 2009;36(9):2009–2016. doi: 10.3899/jrheum.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen H, Neumann L, Kotler M, Buskila D. Autonomic nervous system derangement in fibromyalgia syndrome and related disorders. Isr Med Assoc J. 2001;3:755–760. [PubMed] [Google Scholar]
- 7.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum. 2000;29(4):217–227. doi: 10.1016/s0049-0172(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 8.Cooke WH, Carter JR. Strength training does not affect vagal-cardiac control or cardiovagal baroreflex sensitivity in young healthy subjects. Eur J Appl Physiol. 2005;93(5–6):719–725. doi: 10.1007/s00421-004-1243-x. [DOI] [PubMed] [Google Scholar]
- 9.de Bruijn ST, van Wijck AJ, Geenen R, Snijders TJ, van der Meulen WJ, Jacobs JW, Veldhuijzen DS. Relevance of physical fitness levels and exercise-related beliefs for self-reported and experimental pain in fibromyalgia: an explorative study. J Clin Rheumatol. 2011;17(6):295–301. doi: 10.1097/RHU.0b013e31822c5196. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RR, Bingham CO, 3rd, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55(2):325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa A, Kingsley JD, McMillan V, Panton LB. Resistance exercise training improves heart rate variability in women with fibromyalgia. Clin Physiol Funct Imaging. 2008;28(1):49–54. doi: 10.1111/j.1475-097X.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 12.Furlan R, Colombo S, Perego F, Atzeni F, Diana A, Barbic F, Porta A, Pace F, Malliani A, Sarzi-Puttini P. Abnormalities of cardiovascular neural control and reduced orthostatic tolerance in patients with primary fibromyalgia. J Rheumatol. 2005;32(9):1787–1793. [PubMed] [Google Scholar]
- 13.Hassett AL, Cone JD, Patella SJ, Sigal LH. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum. 2000;43(11):2493–2500. doi: 10.1002/1529-0131(200011)43:11<2493::AID-ANR17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Heffernan KS, Fahs CA, Shinsako KK, Jae SY, Fernhall B. Heart rate recovery and heart rate complexity following resistance exercise training and detraining in young men. Am J Physiol Heart Circ Physiol. 2007;293(5):H3180–3186. doi: 10.1152/ajpheart.00648.2007. [DOI] [PubMed] [Google Scholar]
- 15.Kingsley JD, McMillan V, Figueroa A. The effects of 12 weeks of resistance exercise training on disease severity and autonomic modulation at rest and after acute leg resistance exercise in women with fibromyalgia. Arch Phys Med Rehabil. 2010;91(10):1551–1557. doi: 10.1016/j.apmr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Kingsley JD, Panton LB, McMillan V, Figueroa A. Cardiovascular autonomic modulation after acute resistance exercise in women with fibromyalgia. Arch Phys Med Rehabil. 2009;90(9):1628–1634. doi: 10.1016/j.apmr.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34(2):364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 18.Leicht AS, Hirning DA, Allen GD. Heart rate variability and endogenous sex hormones during the menstrual cycle in young women. Exp Physiol. 2003;88(3):441–446. doi: 10.1113/eph8802535. [DOI] [PubMed] [Google Scholar]
- 19.Lombardi F. Clinical implications of present physiological understanding of HRV components. Card Electrophysiol Rev. 2002;6(3):245–249. doi: 10.1023/a:1016329008921. [DOI] [PubMed] [Google Scholar]
- 20.Marques AP, Ferreira EA, Matsutani LA, Pereira CA, Assumpcao A. Quantifying pain threshold and quality of life of fibromyalgia patients. Clinical Rheumatol. 2005;24:266–271. doi: 10.1007/s10067-004-1003-7. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Lavin M. Fibromyalgia as a sympathetically maintained pain syndrome. Curr Pain Headache Rep. 2004;8(5):385–389. doi: 10.1007/s11916-996-0012-4. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Lavin M, Hermosillo AG, Mendoza C, Ortiz R, Cajigas JC, Pineda C, Nava A, Vallejo M. Orthostatic sympathetic derangement in subjects with fibromyalgia. J Rheumatol. 1997;24(4):714–718. [PubMed] [Google Scholar]
- 23.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 24.Palazzolo JA, Estafanous FG, Murray PA. Entropy measures of heart rate variation in conscious dogs. Am J Physiol Heart Circ Physiol. 1998;274(4 Pt 2):H1099–1105. doi: 10.1152/ajpheart.1998.274.4.H1099. [DOI] [PubMed] [Google Scholar]
- 25.Panton LB, Figueroa A, Kingsley JD, Hornbuckle L, Wilson J, St John N, Abood D, Mathis R, VanTassel J, McMillan V. Effects of resistance training and chiropractic treatment in women with fibromyalgia. J Altern Complement Med. 2009;15(3):321–328. doi: 10.1089/acm.2008.0132. [DOI] [PubMed] [Google Scholar]
- 26.Panton LB, Kingsley JD, Toole T, Cress ME, Abboud G, Sirithienthad P, Mathis R, McMillan V. A comparison of physical functional performance and strength in women with fibromyalgia, age- and weight-matched controls, and older women who are healthy. Phys Ther. 2006;86(11):1479–1488. doi: 10.2522/ptj.20050320. [DOI] [PubMed] [Google Scholar]
- 27.Parati G, Di Rienzo M. Determinants of heart rate and heart rate variability. J Hypertens. 2003;21(3):477–80. doi: 10.1097/00004872-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Picard G, Tan CO, Zafonte R, Taylor JA. Incongruous changes in heart period and heart rate variability with vagotonic atropine: implications for rehabilitation medicine. PM R. 2009;1(9):820–826. doi: 10.1016/j.pmrj.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Raj SR, Brouillard D, Simpson CS, Hopman WM, Abdollah H. Dysautonomia among patients with fibromyalgia: a noninvasive assessment. J Rheumatol. 2000;27(11):2660–2665. [PubMed] [Google Scholar]
- 30.Reyes del Paso GA, Garrido S, Pulgar A, Duschek S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosomatic Res. 2011;70(2):125–134. doi: 10.1016/j.jpsychores.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Reyes Del Paso GA, Garrido S, Pulgar A, Martin-Vazquez M, Duschek S. Aberrances in autonomic cardiovascular regulation in fibromyalgia syndrome and their relevance for clinical pain reports. Psychosomatic Med. 2010;72(5):462–470. doi: 10.1097/PSY.0b013e3181da91f1. [DOI] [PubMed] [Google Scholar]
- 32.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 33.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7(4):261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan MJL, Bishop SC, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 35.Task-Force. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 36.Vaeroy H, Qiao ZG, Morkrid L, Forre O. Altered sympathetic nervous system response in patients with fibromyalgia (fibrositis syndrome) J Rheumatol. 1989;16(11):1460–1465. [PubMed] [Google Scholar]
- 37.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]