Abstract
Background and Aims
Defective hybrid seed development in angiosperms might mediate the rapid establishment of intrinsic post-zygotic isolation between closely related species. Extensive crosses within and among three lineages of wild tomatoes (Solanum section Lycopersicon) were performed to address the incidence, developmental timing and histological manifestations of hybrid seed failure. These lineages encompass different, yet fairly recent, divergence times and both allopatric and partially sympatric pairs.
Methods
Mature seeds were scored visually 2 months after hand pollinations, and viable-looking seeds were assessed for germination success. Using histological sections from early-developing seeds from a sub-set of crosses, the growth of three major seed compartments (endosperm, embryo and seed coat) was measured at critical developmental stages up to 21 d after pollination, with a focus on the timing and histological manifestations of endosperm misdevelopment in abortive hybrid seeds.
Key Results
For two of three interspecific combinations including the most closely related pair that was also studied histologically, almost all mature seeds appeared ‘flat’ and proved inviable; histological analyses revealed impaired endosperm proliferation at early globular embryo stages, concomitant with embryo arrest and seed abortion in both cross directions. The third interspecific combination yielded a mixture of flat, inviable and plump, viable seeds; many of the latter germinated and exhibited near-normal juvenile phenotypes or, in some instances, hybrid necrosis and impaired growth.
Conclusions
The overall results suggest that near-complete hybrid seed failure can evolve fairly rapidly and without apparent divergence in reproductive phenology/biology. While the evidence accrued here is largely circumstantial, early-acting disruptions of normal endosperm development are most probably the common cause of seed failure regardless of the type of endosperm (nuclear or cellular).
Keywords: Endosperm, hybrid necrosis, hybrid seed failure, post-zygotic isolation, seed development, Solanum peruvianum (wild tomato), Solanum chilense, Solanum arcanum ‘marañón’
INTRODUCTION
Understanding barriers to reproduction is fundamental for plant biologists, contributing to a wide range of fields such as evolutionary biology, developmental biology and plant breeding. Hybridization between lineages can be a crossroads in speciation: while natural hybrids can serve as a substrate for nascent species or facilitate the exchange of adaptive alleles, hybridization between species is in most cases prevented by barriers that preserve their genetic integrity (Mayr, 1942; Seehausen et al., 2014). As such, the establishment of reproductive isolation between diverging lineages is inherent to the speciation process (Dobzhansky, 1937; Coyne and Orr, 2004). However, the mechanisms limiting the formation of viable and/or fertile hybrids between closely related plant species are not fully understood, as they can be complex and have multiple origins (Baack et al., 2015).
Reproductive barriers have been defined according to their developmental chronology and origin. Intrinsic post-zygotic barriers manifest after successful fertilization and do not rely on environmental conditions or other extrinsic factors. In contrast to pre-zygotic barriers that operate before fertilization, these barriers can hardly be overcome, making the speciation process irreversible (Seehausen et al., 2014). The broad definition of intrinsic post-zygotic barriers encompasses a variety of developmental failures; such barriers may appear early in seed development, potentially leading to hybrid seed failure (HSF), but they can also arise via sterility or inviability of subsequent hybrid generations. HSF has been documented in several angiosperm families, both in crosses between closely related species at the same ploidy level (Cooper and Brink, 1940; Rick and Lamm, 1955; Walker, 1955; Rick, 1963; Burkart-Waco et al., 2015; Rebernig et al., 2015; Oneal et al., 2016) and in interploidy, nominally intraspecific crosses (Cooper and Brink, 1945; Beamish, 1955; Ortiz and Ehlenfeldt, 1992; Dilkes et al., 2008; Jullien and Berger, 2010; Lu et al., 2012).
In previous efforts to understand how seeds abort, seed morphology and histology were utilized to compare the relative growth of the three main seed compartments: the seed coat (maternal origin), the endosperm (biparental, triploid) and the embryo (biparental, diploid). Typically, some form of endosperm misdevelopment was observed and interpreted as the main cause of seed abortion. In flowering plants, this tissue co-ordinates nutrient and hormone provisioning from maternal tissues to the embryo (Olsen, 2007). Current human nutrition is highly dependent on so-called endospermic seeds, where the endosperm stores large quantities of carbohydrates, proteins and/or lipids that remain available after seed maturity (e.g. starch in wheat, maize and rice). This explains why current knowledge on endosperm biology is dominated by cereals, together with the model plant Arabidopsis thaliana (reviewed in Olsen, 2007). These species are united by all having a nuclear endosperm, characterized by an initial syncytial phase where the triploid nucleus divides via karyokinesis, followed by a later cellular phase where cell walls are formed (via cytokinesis) after every nuclear division.
The best-characterized cases of HSF with this type of endosperm are in the genus Arabidopsis, where impaired cellularization causes seed abortion (Scott et al., 1998; Hehenberger et al., 2012; Wolff et al., 2015). However, two other modes of endosperm development, cellular and helobial, are known in flowering plants (Lopes and Larkins, 1993). In the cellular type, karyo- and cytokinesis occur simultaneously from the very first division after fertilization. HSF has also been described in taxa with cellular endosperm, such as members of the Solanaceae (Cooper and Brink, 1940; Rick and Lamm, 1955; Walker, 1955; Rick, 1963, 1986; Lester and Kang, 1998; Baek et al., 2016) and more recently in Mimulus (Garner et al., 2016; Oneal et al., 2016). The histological manifestations of HSF via endosperm failure are necessarily different between cellular- and nuclear-type endosperm because the cellular type does not undergo a cellularization phase. Indeed, two recent studies in Mimulus (Oneal et al., 2016) and Solanum (Baek et al., 2016) reported impaired endosperm proliferation in abortive hybrid seeds. Clearly, more observational data are needed to assess whether HSF exhibits common, shared features in species that do not have nuclear-type endosperm development.
Given their ecological, morphological and genetic diversity, wild tomatoes (Solanum section Lycopersicon) have recently come to the fore as a model system in evolutionary biology (Städler et al., 2005, 2008; Moyle, 2008; Tellier et al., 2011; Pease et al., 2016). Some of their crossing relationships have long been studied in the context of cultivar improvement, for instance to introgress traits of agronomic interest from wild species to the domesticated tomato (reviewed by Grandillo et al., 2011), often necessitating the use of embryo rescue (Segeren et al., 1993; Chen and Adachi, 1996). Our study describes and quantifies post-zygotic barriers among three lineages of wild tomatoes, with dual emphases on quantifying levels/patterns of HSF and comparisons of endosperm (and seed) growth in intra- and interspecific crosses. Briefly, S. arcanum ‘marañón’ (Am) grows in northern Peru from the Pacific drainages to the western and eastern slopes of the Andes (100–2900 m altitude; Peralta et al., 2008). Solanum peruvianum (P) is the most polymorphic wild tomato species with an elongated north–south distribution from central Peru to northern Chile, ranging from sea level to 3300 m altitude. Finally, S. chilense (C) is native to southern Peru and northern Chile (where it is regionally sympatric with P for parts of its geographic range) from sea level to 3500 m altitude. It was also sampled in the driest place in the world, the Atacama desert (Chetelat et al., 2009), and is well known for its resistance to diverse kinds of abiotic stress (Nosenko et al., 2016).
The crossing relationships between these three wild tomato lineages have been partially described in the last century by C. M. Rick’s seminal work (Rick and Lamm, 1955; Rick, 1963, 1986). He found high proportions of HSF in several cross combinations and hypothesized a causal role for the endosperm in seed failure, yet without providing quantitative data beyond the rates of inviable seed formation in hybrid crosses. The observed variability in the incidence of HSF between pairs of species appears to make wild tomatoes a promising system to study this type of reproductive barrier. We have built on Rick’s early work by performing more hybrid crosses with Am in both interspecific combinations than were possible due to limited sampling and recognition of Am as a separate evolutionary lineage at the time of Rick’s (1986) work. Importantly, we have additionally focused on the developmental time course of endosperm and embryo growth in compatible and abortive crosses. This part of our work found general agreement regarding the developmental phase of embryo arrest in abortive seeds between taxa with nuclear-type and those with cellular-type endosperm, despite the somewhat different endosperm pathologies involved.
MATERIALS AND METHODS
Plant material and crosses
We conducted a large crossing experiment to assess intrinsic pre- and post-zygotic barriers in our study system, consisting of three wild tomato lineages (Peralta et al., 2008): Solanum arcanum Peralta ‘marañón’ (Am), S. chilense (Dunal) Reiche (C) and S. peruvianum L. (P). Plants were grown from seeds obtained from the Tomato Genetics Resource Center (TGRC, University of California, Davis, USA; http://tgrc.ucdavis.edu;Supplementary Data Table S1). Individual plant identifiers consist of the TGRC accession number for each population to which an additional letter was added to distinguish between different plant genotypes within populations, which were grown to maximize the number of within- and among-species crosses. For our study species, the TGRC accessions are almost equivalent to samples from naturally outbred populations and can be expected to contain dissimilar genotypes (see Baek et al., 2016). All plants were maintained in an insect-free greenhouse at the ETH station at Lindau-Eschikon (canton Zurich, Switzerland). Plants were grown in 5 L pots filled with Ricoter Substrate 214 (Ricoter Erdaufbereitung AG, Switzerland) and watered 3–4 times per week. Given their fast and continuous growth, the plants were trimmed every 2 weeks. In spring and autumn, plants were repotted in fresh soil, and root areas were fertilized with granulates (Gartensegen, Hauert HBG SA, Switzerland). Additional fertilizer was applied once or twice per month with Wuxal® NPK solution (Aglukon Spezialdünger GmbH & Co. KG, Germany). Day and night phases were under controlled temperature and humidity conditions (day, 20 °C, 50 % relative humidity; night, 16 °C, 60 % relative humidity). During daytime, light was maintained above 25 kLux with artificial light if needed.
Pollinations were performed within and between species via manual pollen transfer. As all plant source populations in this study were described as self-incompatible (http://tgrc.ucdavis.edu/), we did not emasculate flowers before the collection and transfer of pollen. Hands were washed between each cross to avoid any contamination (ethanol 70 %, water); no fruit set was observed on non-pollinated inflorescences. A summary of all crosses performed is presented in Table 1 and detailed results for each cross can be found in Supplementary Data Table S2. We adopted a specific nomenclature to distinguish between unidirectional and reciprocal crosses. For example, the reciprocal Am–C cross refers to both Am × C and C × Am single crosses (mother × father).
Table 1.
Summary of controlled crosses, seed viability and germination success within and between the three wild tomato lineages Solanum arcanum ‘marañón’ (Am), S. chilense (C) and S. peruvianum (P)
| Cross type | No. of individual crosses | No. of fruits sampled | Mean no. of seeds per fruit | Total no. of seeds counted | No. of viable seeds | Median % viable seeds | Median % germination of viable seeds (success/all) | |
|---|---|---|---|---|---|---|---|---|
| Intraspecific | Am × Am | 55 | 374 | 44.6 | 16 675 | 15 502 | 94.5 | 64.1 (14/16) |
| C × C | 54 | 616 | 49.7 | 30 617 | 18 197 | 80.1 | 42.9 (9/9) | |
| P × P | 82 | 987 | 67.9 | 67 052 | 60 016 | 95.4 | 90.7 (11/11) | |
| Interspecific | Am × C | 39 | 312 | 34.5 | 10 778 | 7 493 | 68.2 | 30.0 (5/5) |
| C × Am | 47 | 636 | 45.8 | 29 146 | 15 244 | 53.9 | 58.4 (11/11) | |
| Am × P | 31 | 218 | 37.9 | 8 262 | 37 | 0 | – | |
| P × Am | 29 | 284 | 51.2 | 14 551 | 150 | 0 | 13.2 (1/2) | |
| C × P | 58 | 747 | 59.1 | 44 132 | 660 | 0 | 9.5 (1/1) | |
| P × C | 41 | 443 | 51.4 | 22 756 | 22 | 0 | – |
Cross types are identified as mother × father; individual crosses refer to combinations of two single genotypes in one cross direction.
Each individual cross yielded a single seed viability/germination value (summing results from independent pollination events = fruits); these values were used to compute medians for each cross type.
In the last column, the number of crosses with any germination success/the number of crosses tested is given in parentheses; –, not tested.
Visual seed phenotyping and germination tests
Hybrid seed failure arises after successful double fertilization and during seed development; it has the potential to establish an early, irreversible intrinsic post-zygotic barrier. To quantify the incidence and strength of HSF among the three wild tomato lineages, seed viability was first assessed visually for seeds generated by crosses within each species and by all interspecific combinations of Am, C and P. Fruits were sampled 2 months after pollination and dissected on paper sheets. Once seeds were dry, visual assessment of seed viability was performed: a seed was considered as (potentially) viable if it had a plump aspect with a visible coiled embryo. In contrast, inviable seeds were flatter and looked ‘shrivelled’. Although seeds considered to be inviable were also often – but not necessarily – very small compared with viable seeds, seed size per se was not taken into account for seed viability assessment.
Germination may appear as the ultimate confirmation of seed viability. However, this validation is partial: germinating seeds are necessarily viable, while intrinsic and extrinsic causes can inhibit germination of otherwise good seeds. To test and quantify germination success, we put mature seeds on autoclaved 1 % agar gel added with PPM™ diluted to 0.2 % in sterile water (Plant Cell Technology, Inc., USA). Seeds visually scored as viable vs. inviable were tested separately; those showing a radicle after a maximum duration of 14 d were considered to have germinated.
To describe HSF in more detail, we measured seed size in a sub-set of crosses. We selected three intraspecific and three reciprocal interspecific crosses involving Am, C and P (referred to later as Am1 × C1, C1 × Am1, C1 × P1, P1 × C1, Am1 × P1 and P1 × Am1 crosses); accessions and measurements are given in Supplementary Data Table S3. Seed size was measured for a minimum of 112 seeds per cross. All seeds were scanned on the paper used for dissection at 1200 dpi resolution (Epson Perfection® 3200 PHOTO).
Seed histology
Fruits were sampled at different stages between 4 and 21 days after pollination (DAP), halved, and fixed in a 9:1 ethanol:acetic acid solution. We subsequently embedded them either in plastic (Technovit® 7100 plastic) or in paraffin (Clark, 1981). Using an RM2145 Leica microtome (Leica Microsystems GmbH, Wetzlar, Germany), 10 µm thin slices were obtained, mounted on glass slides and stained with Toluidine Blue and Ruthenium Red (Sigma-Aldrich 198161 and R2751). In a last step, slides were covered with Roti®-Histol and sealed with Roti®-Histokit (Carl Roth, 6640 and 6638). For P × C crosses, it was not possible to recover slices of satisfactory quality with fruits harvested earlier than 10 DAP. All measurements were performed on individual sections of seeds. Through continuous micro-sectioning of entire embedded fruits, it was possible to identify the largest sagittal section of each developing seed; the most central (largest) section of each seed was thus selected for measurements. Observations were made with ×10 to ×40 objectives on an Olympus BX40 microscope (Olympus Corporation, Japan) and photographs were taken with a Canon EOS 600D digital camera connected to the microscope (Canon, Japan). Adobe© Photoshop® CS6 v.13.0 was used to format the images.
Hybrid plant phenotyping
We characterized a sub-set of germinating F1 plants by growing them in the greenhouse. Plant phenotyping was done on the progeny of 12 Am and C intra- and interspecific crosses. The starting seed material was obtained from crosses involving two plants of each species in a full diallele design (four intraspecific and eight hybrid crosses). The C parents were from populations LA4329 and LA2748, and the Am parents from populations LA1626 and LA2185 (Supplementary Data Table S1). Seeds were put on agar gel for germination (see above) and seedlings transferred to trays and fertilized every week. After 6 weeks, the occurrence of plant necrosis was assessed. We re-potted a sub-set of 151 plants in 5 L pots, including 24 necrotic plants. Plant height was measured after an additional 5 weeks on these progeny.
Data analyses
R via the RStudio interface was used for all numerical and graphical analyses (https://www.r-project.org/;RStudio Team, 2015). The following packages were needed: ggplot2, grid, gtable and agricolae. In order to measure total seed area on scans and the size of different seed compartments (embryo area, endosperm area, nucellus width and seed coat width) on sagittal cuts, image analysis was performed manually with ImageJ® version 1.48 (Schneider et al., 2012; Supplementary Data Table S4).
RESULTS
Lack of intrinsic pre-zygotic isolation
High fruit and seed set was obtained from our intra- and interspecific crosses (Table 1; Supplementary Data Table S2). In total, we performed 451 different crosses, sampled 4617 fruits and assessed 243 969 seeds. Fifteen crosses did not result in fruit set (three intraspecific and 12 hybrid combinations, including eight with the same maternal plant; Table S2), probably caused by shared S-alleles or weakness of particular maternal plants at the time of the cross. On average, 10.4 fruits per cross and 51.4 seeds per fruit were obtained in intraspecific crosses (191 crosses), while hybrid crosses yielded on average 10.8 fruits per cross and 45.9 seeds per fruit (245 crosses; Table 1). There is an overall pattern of somewhat reduced seed number per fruit in hybrid crosses on Am and P maternal plants, but no such signal for C maternal plants in hybrid crosses (Table 1). Together, these results suggest that intrinsic barriers to fertilization within and between the three lineages are negligible or (at most) modest, at least under the imposed non-competitive pollination conditions.
Patterns of hybrid seed failure among lineages
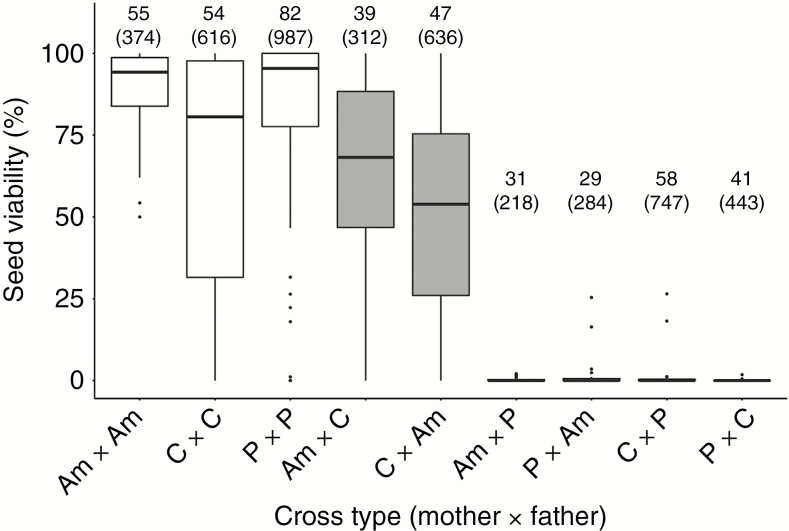
Seed viability was generally high in intraspecific crosses, with median proportions ranging between 0.806 and 0.954 in the cross categories C–C, Am–Am and P–P; the corresponding visual assessments of hybrid seeds revealed significantly lower proportions of viable seeds [Table 1; Fig. 1; Wilcoxon rank sum test (WRST), P < 2.2E-16]. However, we found disparate outcomes among different lineage combinations. All reciprocal crosses among Am–P and C–P consistently yielded very low seed viability, being distributed around a median of 0 % (Table 1; Fig. 1). Thus, hybrid seed failure is symmetric for reciprocal Am–P and C–P crosses and can be qualified as early and near-complete.
Fig. 1.
Box plots representing the distribution of seed viability in controlled crosses within and between the three wild tomato lineages Solanum arcanum ‘marañón’ (Am), S. chilense (C) and S. peruvianum (P). All crosses are identified as mother × father. White background, intraspecific crosses; grey background, hybrid crosses. On the top of each cross type, the number of individual crosses (combination of two single genotypes) and number of fruits sampled (in parentheses) are given.
In contrast, the reciprocal Am × C and C × Am crosses gave rise to substantial proportions of viable seeds, with median values of 68.2 and 53.9 %, respectively. These proportions were significantly lower than the within-species ranges from Am–Am and C–C crosses (Fig. 1; WRST, P < 2.9E-07). Moreover, for a given combination of Am and C genotypes, seed failure proportions appeared to be strongly influenced by the directionality of the cross (Supplementary Data Table S2). For example, we obtained 54.6 % mean seed viability in the LA4329A × LA2185B (C × Am) cross (n = 20 fruits) but 96.6 % seed viability in the reciprocal LA2185B × LA4329A cross (n = 13 fruits). Overall, Am × C crosses yielded higher proportions of viable seeds than C × Am crosses, but these differences were only marginally significant (Fig. 1; WRST, P = 0.083). To summarize, hybrid seed failure between C and Am appears to be partial and its manifestation variable, whereas the other species combinations failed to produce more than a few viable seeds.
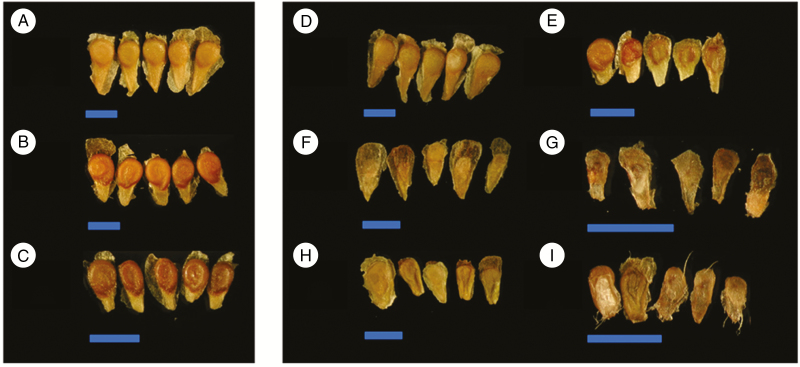
Representative examples of viable and inviable seeds are shown in Fig. 2. In intraspecific crosses and the hybrid Am × C cross, all depicted seeds were scored as viable, with well-developed coiled embryos visible (Fig. 2A–D). In the C × Am cross (Fig. 2E), only the seed shown on the far left appeared to be viable while the other four were much flatter with underdeveloped embryos, typical of inviable seeds. Finally, all seeds from the reciprocal crosses between Am–P (Fig. 2F, G) and C–P (Fig. 2H, I) appeared to be inviable, reflecting the near-complete post-zygotic barrier between these taxa.
Fig. 2.
Images of mature seeds from selected crosses within and between the three wild tomato lineages Solanum arcanum ‘marañón’ (Am), S. chilense (C) and S. peruvianum (P). In all images, blue scale bars represent 2 mm. (A–C) Intraspecific crosses within Am (A, LA2185A × LA1626B), C (B, LA4329B × LA2748B) and P (C, LA2744B × LA2964A); all seeds appear viable with plump aspect and coiled embryo. (D–I) Reciprocal hybrid crosses: Am × C (D, LA2185A × LA4329B) and C × Am (E, LA4329B × LA2185A), Am × P (F, LA2185A × LA2744B) and P × Am (G, LA2744B × LA2185A), C × P (H, LA4329B × LA2744B) and P × C (I, LA2744B × LA4329B). Note the variability in seed size and shape in hybrid crosses; seeds appear viable in (D), partially viable in (E) and completely inviable in (F–I). The same crosses were used in seed size measurements (see Fig. 3).
Assessment of seed germination rates
Germination rates were measured separately for seeds scored as viable vs. inviable, respectively. We tested 9190 viable-looking seeds of which 63.9 % germinated (germination in 94 of 101 crosses). Germination rates of intraspecific seeds ranged from 42.9 to 90.7 % and were overall higher than those of ‘viable’ hybrid seeds (from 9.5 to 58.4 %; Table 1). Substantial proportions of hybrid seeds from Am–C crosses germinated (30.0 and 58.4 %; Table 1) and appeared to represent viable F1 hybrids at this early developmental stage. Some seed germination was observed in two of eight selected hybrid crosses involving P, but at very low proportions (9.5 and 13.2 %; Table 1). Am × P and P × C crosses lacked sufficient numbers of viable-looking seeds to assess germination rates. We also evaluated seeds that were visually scored as inviable; of the 9073 seeds sown, only 0.4 % germinated (in 12 of 123 crosses). These results confirm that hybrid crosses involving P in both parental roles in combination with Am or C are strongly abortive, leading to near-complete and symmetric post-zygotic isolation between P and Am, and between P and C.
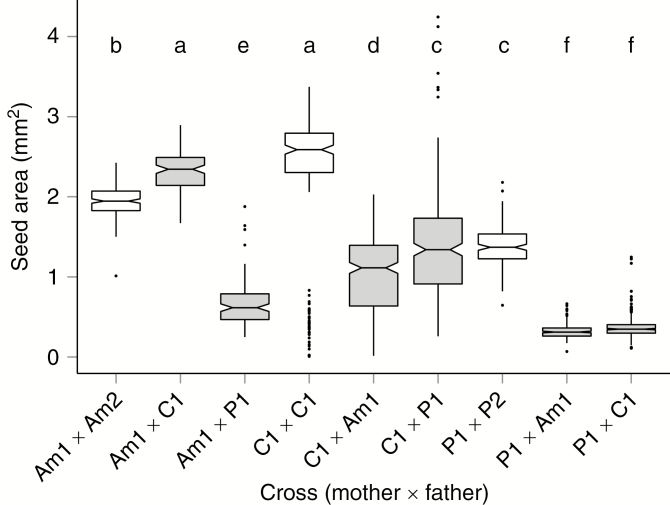
Patterns of seed size variation among cross types
We measured seed size in a total of nine crosses (three intraspecific and three reciprocal hybrid crosses). In total, 1909 seeds were measured, with a range of 112–328 seeds per cross. Seed size was significantly different between reciprocal hybrid crosses (Fig. 3; Supplementary Data Table S3; Tukey’s test). The largest difference between reciprocals was found in the C–P cross (Δ = 1.02 mm2, C seeds being larger). Overall, seed size was significantly reduced in hybrid seeds compared with seeds from intraspecific crosses (Welch-test, P < 1E-16). However, the largest seed size was found in the hybrid cross Am1 × C1 (round mean ± s.d., 2.31 ± 0.26 mm2, n = 112 seeds), significantly larger than seed size in the intraspecific cross with the same mother (Am1 × Am2, 1.95 ± 0.19 mm2, n = 204 seeds, Tukey’s test). Importantly, both hybrid crosses with the P1 mother uniformly produced extremely small seeds of overlapping size with very low size variability (P1 × Am1, 0.32 ± 0.09 mm2, n = 221 seeds; P1 × C1, 0.37 ± 0.13 mm2, n = 328 seeds). This corresponds to a homogeneous phenotype of early seed abortion. The Am1 × P1 cross yielded a similar seed phenotype but with larger variation in seed size (0.67 ± 0.28 mm2, n = 114 seeds). In contrast, hybrid seeds of the C1 mother were larger and of more variable size (C1 × Am1, 0.96 ± 0.57 mm2, n = 308 seeds; C1 × P1, 1.38 ± 0.63 mm2, n = 273 seeds). However, these hybrid seeds were smaller than those from the maternal cross C1 × C2 (Fig. 3; Tukey’s test).
Fig. 3.
Box plots representing the distribution of seed size in selected crosses within and between the three wild tomato lineages Solanum arcanum ‘marañón’ (Am), S. chilense (C) and S. peruvianum (P). All crosses are identified as mother × father, and numbers (1, 2) refer to different genotypes. White background, intraspecific crosses; grey background, hybrid crosses. Notches represent 95 % confidence intervals of the medians. Comparisons of means were evaluated with Tukey’s test (pairwise comparisons with P < 0.05 shown with different letters on top of the boxes); distributions with significantly overlapping means share the same letter. Representative images of seeds from the same crosses are shown in Fig. 2.
Quantification of seed developmental trajectories
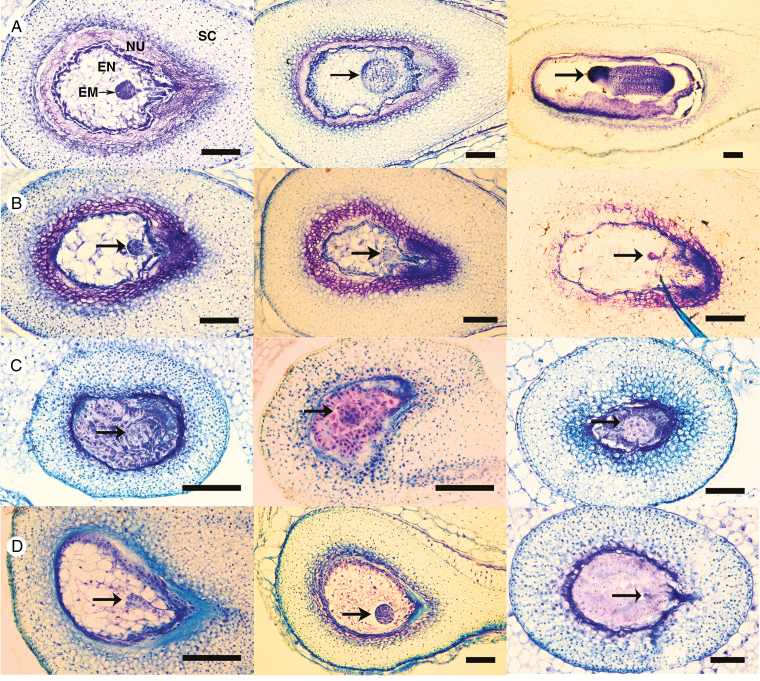
We assessed histological sections of different stages in seeds from intra- and interspecific crosses involving Am, C and P (Fig. 4). Normal seed development was exemplified by a C × C cross at early-globular, late-globular and torpedo stages, corresponding to 13, 16 and 21 DAP (Fig. 4A). In C × P and P × C hybrid crosses, endosperm abnormalities were visible at early stages (Fig. 4B, C). C × P endosperms featured large and vacuolated cells (Fig. 4B, left); cells from the suspensor and adjacent endosperm cells appeared to be collapsed (Fig. 4B, left and centre). At 21 DAP, embryos visibly started to degenerate (Fig. 4B, right). In P × C seeds, endosperm cells appeared small and dense, and the general aspect of both endosperm and embryo remained at the early-globular stage between 10 and 21 DAP, despite overall seed growth (Fig. 4C).
Fig. 4.
Histological sections of wild tomato developing seeds at different stages. EN, endosperm; EM, embryo; NU, nucellus; SC, seed coat. Black arrows point toward the embryo, and black bars represent 100 µm (magnification ×10 to ×40). (A) Normal seed development in a C × C cross (LA2750A × LA4329B): left, early-globular stage (13 DAP); centre, late-globular stage (16 DAP); right, torpedo stage (21 DAP). (B) Abnormal development in C × P hybrid seeds (LA2750A × LA1616A; left to right: 13, 16 and 21 DAP). (C) Abnormal development in P × C hybrid seeds [LA1616A × LA2750A left (10 DAP) and centre (16 DAP), LA0153A × LA2750A right (21 DAP)]. (D) Selected images from Am–C crosses: left, abnormal seed in C × Am (LA2750A × LA2185A) at 16 DAP; centre, normal Am × C seed at 18 DAP; right, abnormal Am × C seed at 21 DAP (both LA2185A × LA2750A).
Examples of normal and abnormal seed development in Am–C reciprocal crosses are presented in Fig. 4D. Developing seeds from a C × Am cross at 16 DAP should correspond to the late-globular stage (Fig. 4D, left). However, we observed pro-embryos with abnormal development, as shown by their asymmetric cellular organization (Fig. 4D, left). In the depicted (and typical) single Am × C cross, we observed both successfully developing and abortive seeds. In one case, the embryo was clearly round and connected to a well-developed suspensor at 18 DAP, matching the late-globular stage (Fig. 4D, centre). Other seeds showed degenerating embryos and small, optically dense endosperm cells at 21 DAP (Fig. 4D, right), similar to observations in the P × C cross at this late stage. The strongly diverging phenotypes of Am–C developing seeds matched our observations on mature Am–C seeds (Table 1; Figs 1 and 2).
Next, we detail seed compartment measurements to evaluate developmental trajectories between 4 and 21 DAP in strongly abortive crosses (Fig. 5; Supplementary Data Table S4). In particular, we compared the reciprocal C–P hybrids with their parental crosses P × P and C × C. Importantly, for these data, C × P and P × C crosses were performed among the same parents and were thus true reciprocals. Observations were made on 450 seed sagittal cuts with a range of 11–40 cuts per cross category and temporal stage (Supplementary Data Table S4).
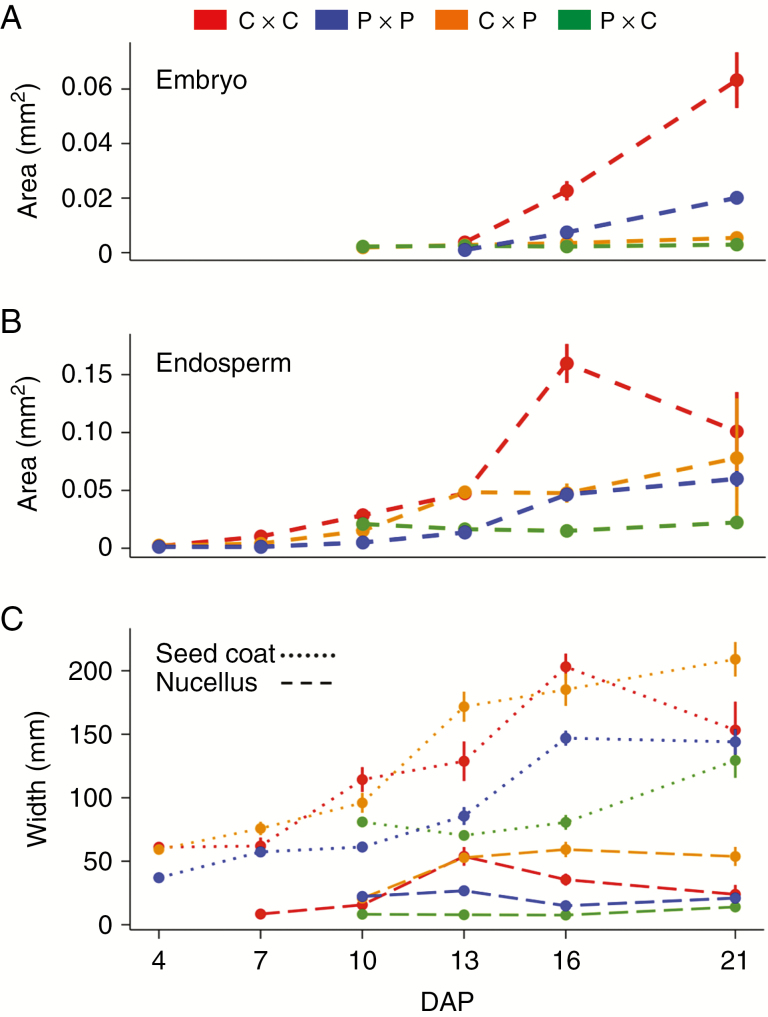
Fig. 5.
Measurements of seed compartment size over time (in DAP) in crosses within and between Solanum chilense (C) and S. peruvianum (P). For each data point, confidence intervals are represented by vertical bars. (A) Embryo development. (B) Endosperm development. (C) Seed coat (dotted line) and nucellus (dashed line) development. Details on the accessions/plants used are provided in Supplementary Data Table S4.
In intraspecific crosses, endosperm and maternal compartments (seed coat and nucellus) were steadily growing between 4 and 16 DAP (Fig. 5B, C). Growth of the C × C endosperm was exponential and its approximate area rose from 0.018 to 0.16 mm2 over 12 d. For both C × C and P × P, this growth was particularly rapid between 13 and 16 DAP for endosperm and seed coat: endosperm areas tripled while seed coat widths doubled, which was reflected in overall seed size (Supplementary Data Table S4). This critical phase corresponds to the globular stage where embryos expand from pro-embryos to heart-shape embryos. These measurements show that C × C and P × P, despite their different mature seed size (P × P seeds generally smaller than C × C seeds; Fig. 2), display synchronized development with very similar phenotypic features at 16 DAP, corresponding to the late-globular stage.
After 16 DAP, endosperm and maternal compartments exhibited reduced growth rate in P × P and shrank markedly in C × C (Fig. 5B, C), while embryo area tripled in both C × C and P × P seeds from 16 to 21 DAP (Fig. 5A). Embryos expanded at similar rates in both intraspecific crosses to reach the heart-to-torpedo stage, increasingly contributing to total seed size while ‘consuming’ the endosperm. This is expected given that mature tomato seeds contain very little residual endosperm and consist mainly of the seed coat surrounding the autonomous embryo. C × C embryos were larger than P × P embryos during seed development, consistent with seed size differences observed for mature seeds (Supplementary Data Table S4). Despite these quantitative differences, our measurements indicate that C × C and P × P seeds undergo parallel developmental sequences, both reaching the heart-to-torpedo stage at 21 DAP. In contrast, this timeline is strongly compromised in abortive hybrid seeds obtained from reciprocal C–P crosses.
Although C × C and C × P endosperms reached approximately the same size at 13 DAP (0.048 mm2), the latter did not grow appreciably until 16 DAP, whereas in C × C seeds, endosperm area tripled between 13 and 16 DAP (Fig. 5B). Between 16 and 21 DAP, C × P endosperm area enlarged by 64 % and embryos grew slowly, whereas C × C endosperms lost >50 % of their area while embryo size increased markedly. In the P × C hybrid cross, endosperm area shrank by 28 % between 10 and 16 DAP while it enlarged 9-fold in the P × P seeds. Between 16 and 21 DAP, both endosperm and embryos hardly expanded in P × C seeds.
Moreover, while embryos and endosperm exhibited reduced size in both hybrid classes compared with the corresponding intraspecific data, maternal tissues kept expanding after 16 DAP (Fig. 5). We observed an overgrowth of maternal layers in C × P compared with C × C seeds at 21 DAP. In P × C seeds, the seed coat remained the thinnest among all crosses but grew further after 16 DAP when it was expected to stabilize, based on our P × P observations. Jointly, these features suggest that the maternal layers of seeds can also be affected in their development by the hybrid state. To summarize, embryos were visible at 13 DAP in both C × P and P × C hybrid seeds; their growth, however, was markedly reduced in comparison with intraspecific crosses. Hybrid embryos did not reach the late-globular stage; they either remained at the early-globular stage or else degenerated (Figs 4B, C and 5). Hybrid endosperm suffered aberrant development in C × P and P × C as shown by slight shrinkage between 13 and 16 DAP followed by halting growth until 21 DAP.
Intrinsic post-zygotic isolation in later stages of Am–C F1 hybrids
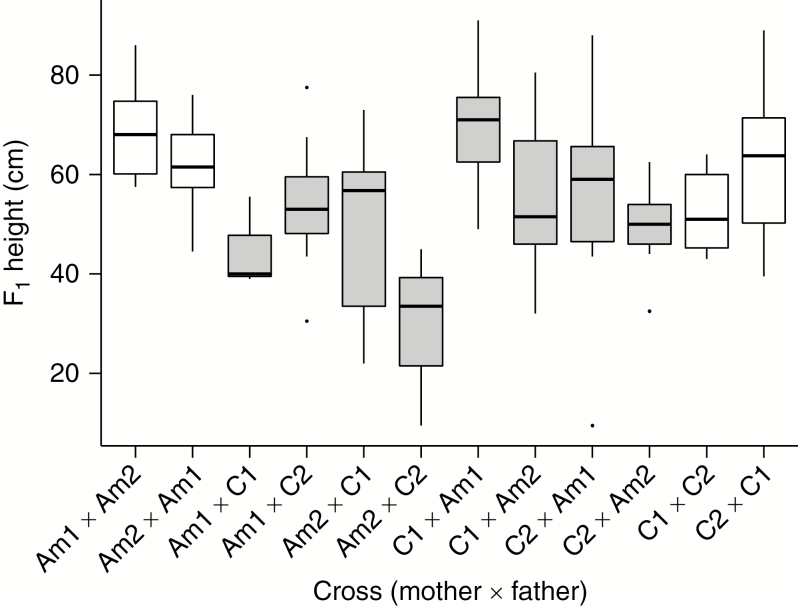
We have shown that reciprocal crosses between Am and C produce significant proportions of viable seeds, of which substantial numbers germinated (Table 1; Fig. 1). We thus conclude that early-acting intrinsic post-zygotic barriers are relatively weak between Am and C, at least in comparison with the two other species combinations tested here. To assess whether later intrinsic post-zygotic barriers exist, we compared plant growth after germination in intra- and interspecific progeny. During early growth, 44 of 531 seedlings exhibited leaf and/or stem necrosis (Supplementary Data Fig. S1). These necrotic plants were exclusively hybrids (C1 × Am1, 34 plants; Am1 × C1, two plants; C2 × Am2, eight plants). We planted a sub-set of 127 non-necrotic and 24 necrotic plants 6 weeks after germination. Five weeks after re-potting, non-necrotic plants were 9.5–91 cm tall, with large differences between reciprocals in both intra- and interspecific crosses (Fig. 6; Supplementary Data Table S5). The largest difference was between Am1–C1 reciprocals, where median heights differed by 31 cm (n = 3, n = 9). Some of these hybrids exhibited transgressive phenotypes compared with their parental F1s, as shown by hybrid weakness in Am1 × C1 and hybrid vigour in C1 × Am1; however, other parental combinations did not generate taller F1 hybrid progeny (Fig. 6). When merging observations on individual crosses into cross categories by species combinations, plant height differed significantly between Am × Am (n = 20) and C × C (n = 21; WRST, P = 0.03). Overall, C × Am hybrid progeny (n = 51) did not differ in height compared with C × C progeny (WRST, P = 0.901); however, Am × C hybrids (n = 34) were significantly smaller than Am × Am seedlings (WRST, P = 1.5E-4).
Fig. 6.
Box plots representing the distribution of plant height in F1s from controlled crosses between four plants representing the two wild tomato lineages Solanum arcanum ‘marañón’ (Am) and S. chilense (C). All crosses are identified as mother × father (Am1, LA2185A; Am2, LA1626B; C1, LA4329B; C2, LA2748B). In total, 126 non-necrotic plants were measured 3 months after germination. White background, intraspecific crosses; grey background, hybrid crosses. Note the lower height of hybrids with Am mothers compared with hybrids with C mothers (see text).
We kept 24 necrotic plants for several months of observation; three of them died and four were considered to have recovered, of which one flowered. The remaining 17 necrotic plants had a median height of 8 cm at 3 months. Unlike the vast majority of non-necrotic plants, they neither flowered nor recovered a normal size. Thus, hybrid necrosis appears to contribute to late post-zygotic isolation between Am and C.
DISCUSSION
Hybrid seed failure establishes strong reproductive barriers
Wild tomato species share yellow petals and anthers, a buzz pollination system, weak or absent floral scent and simultaneous flowering and fruit set; limited field observations suggest solitary bees as the main pollinators, with insufficient data to evaluate their degree of species specificity (Rick, 1950; Chetelat et al., 2009). Despite overlapping geographic ranges of several tomato species, no hybrid populations or individuals were reported based on field work spanning several decades (Taylor, 1986; Moyle, 2008; Baek et al., 2016). In our extensive crossing experiments, all species were found to be intercompatible, with high proportions of fruit and seed set in each cross combination. This suggests that extrinsic and/or intrinsic post-zygotic barriers may be crucial for species isolation. In particular, our study provides compelling evidence that post-zygotic barriers via HSF are common between three wild tomato lineages.
Visual assessment of large numbers of intraspecific and hybrid seeds revealed three categories of HSF: negligible, intermediate (‘weak’) and near-complete (‘strong’). Intraspecific crosses were characterized by generally high seed viability, with some exceptions, particularly in S. chilense. This latter finding could be related to the substantial sub-structure in this lineage, consisting of at least four genetic clusters reflecting range expansion and regional adaptation (Böndel et al., 2015). Although there were also several within-cluster C crosses yielding high proportions of inviable seeds, overall group comparisons revealed significantly lower proportions of viable seeds for among-cluster crosses (mean = 0.563, n = 31 crosses) than for within-cluster crosses (mean = 0.730, n = 23 crosses; WRST, P < 0.005; Supplementary Data Table S2).
Importantly, near-complete seed inviability was observed in all reciprocal hybrid crosses, with the exception of the Am–C crosses. At seed maturity, all failing hybrid seeds were small compared with those from intraspecific crosses and had a flat and empty aspect, suggesting improper endosperm development and eventual embryo arrest. Given that mature tomato seeds contain only a thin layer of endosperm (Baek et al., 2016), it is inherently difficult to evaluate the role of the endosperm in HSF by observing mature seeds. However, final seed size is generally expected to reflect earlier endosperm growth (Sabelli and Larkins, 2009). Of note, individual Am–C crosses yielded diverse proportions of viable seeds, and fairly high proportions of these did germinate in the majority of the crosses we tested. Based on 86 interspecific crosses using individuals from six Am and ten C accessions, our results are in agreement with earlier, much more limited observations (Rick, 1986) and suggest that they are typical of Am–C interspecific crosses. It is noteworthy that the geographic ranges as well as morphological features of Am and C represent opposite ends for the suite of lineages studied here (Rick, 1986; Peralta et al., 2008), while Am is equidistant to both P and C in terms of divergence time (Pease et al., 2016).
Histological analyses of perturbed hybrid seed development
To identify potential defects leading to seed abortion, we characterized the dynamics of early seed development using histological analyses. Not unexpectedly, this revealed strong differences in endosperm development between intraspecific and hybrid seeds. While the manifestation of endosperm failure in nuclear endosperm – typical for monocots and some dicots such as members of the Brassicaceae – is the result of impaired cellularization timing (Hehenberger et al., 2012; Rebernig et al., 2015), we inferred a different scenario in our study system. Solanum species are characterized by a cellular endosperm, i.e. cell divisions begin right after fertilization without any prior syncytial phase. Our observations in wild tomatoes confirm that endosperm cells are present in early-developing seeds regardless of whether these end up as viable or inviable at seed maturity. However, endosperm proliferation and details of typical cell size and optical density are demonstrably affected in strongly abortive crosses, at least from the early-globular stage onwards. This developmental stage has also been found to be crucial for seed abortion in other Solanum crosses (Lester and Kang, 1998; Baek et al., 2016), as well as in Arabidopsis (Bushell et al., 2003) and Mimulus (Oneal et al., 2016). Our finding that maternal seed compartments also exhibit disrupted development in hybrid crosses (in particular, relative overgrowth of the nucellus and seed coat in C × P hybrid seeds; Fig. 5) is consistent with the notion that the developing endosperm closely interacts with maternal tissues, mediated by hormonal and/or signalling pathways (Haughn and Chaudhury, 2005; Xu et al., 2016).
Successful embryo rescue at 13 DAP has recently been used to demonstrate the causative role of the endosperm in abortive Capsella hybrid seeds (Rebernig et al., 2015). We did not attempt embryo rescue in our study, primarily because earlier work using the cultivated tomato as pistillate parent and either C or P as staminate parents (like C–P, these crosses are characterized by strong HSF) have yielded fairly low success rates (Rick and Lamm, 1955; Segeren et al., 1993; Chen and Adachi, 1996). While the successfully excised embryos strongly implicate impaired endosperm function in the usual failure of these crosses, the many unsuccessful attempts imply that the overall evidence is still circumstantial. However, previous observations on HSF in Solanum and other angiosperm genera have been interpreted as primarily reflecting endosperm defects (Haig and Westoby, 1991; Lester and Kang, 1998). To summarize, despite the fact that nuclear- and cellular-type endosperms exhibit different developmental abnormalities in abortive seeds, endosperm disruption appears to affect seed development at largely equivalent stages. At such an early developmental stage, embryo development strongly relies on nutrient provisioning via the endosperm. If the latter is not fully functional, the embryo will most probably starve and abort, as inferred for many of the mature seeds we assessed.
The failing endosperm: evolutionary significance and potential mechanisms
From an evolutionary perspective, parental conflict is expected to occur in the developing seed with variable strength, depending on the prevailing mating system. Transgressive and complementary phenotypes between reciprocal crosses can reveal different levels of parental conflict between lineages (Haig and Westoby, 1991). In our study, hybrid phenotypes often deviated from intraspecific phenotypes during seed development, at seed maturity and during F1 seedling growth (Figs 2–6). In particular, our tested C × P and P × C combinations revealed different endosperm growth trajectories; C × P endosperm continued to grow until 21 DAP and was only somewhat smaller than C × C endosperm at that time point, but P × C endosperm showed no growth after 10 DAP and thus remained much smaller than P × P endosperm (Figs 4 and 5). Although our study system consisted of strictly outcrossing (self-incompatible) lineages, divergent endosperm/seed size between reciprocal crosses may result not only from major differences in mating system but more generally from different levels of parental conflict; this has recently been shown empirically with interpopulation crosses among outcrossing populations of Arabidopsis lyrata (Willi, 2013). Under the ‘Weak Inbreeder Strong Outcrosser’ (WISO) hypothesis (Brandvain and Haig, 2005), our observations may imply that the C plant, as pollen parent, was ‘weaker’ in terms of extracting maternal resources than the P plant, yielding smaller (transient) endosperms in the P × C than in the C × P cross. Generalizing from our initial findings, however, will require additional comparative data on reciprocal mature seed size and/or seed developmental trajectories.
Despite the relevance of HSF (probably via endosperm malfunction) in plant biology and speciation, its underlying mechanisms and level of conservation among taxa have not yet been satisfactorily elucidated (Lafon-Placette and Köhler, 2016). Decades of experimental work have evaluated the role of parental dosage in endosperm development; early work on interploidy crosses explored the possible phenotypic outcomes of parental imbalance (Beamish, 1955; Lin, 1984). The concept of endosperm balance number (EBN) came to be used as a predictive tool for successful seed development in interspecific and interploidy crosses among wild potatoes (Johnston et al., 1980). The EBN of a given lineage corresponds to its ‘effective’ endosperm ploidy, and only crosses among the same EBN were predicted to be viable. Our combined interspecific endosperm and seed failure phenotypes may thus reflect a higher EBN for P compared with both C and Am. Yet, the EBN conceptualizes a central role for parental dosage in interspecific crosses but does not entirely explain all empirical patterns of endosperm failure (Katsiotis et al., 1995; Masuelli and Camadro, 1997; Lafon-Placette et al., 2017).
Clearly, other levels of complexity, such as the determinants of gene expression, should be taken into account in attempts to explain HSF functionally. Special attention should be drawn to the potential involvement of perturbed genomic imprinting (Haig and Westoby, 1991; Gutierrez-Marcos et al., 2003; Josefsson et al., 2006; Walia et al., 2009; Jullien and Berger, 2010; Kradolfer et al., 2013; Burkart-Waco et al., 2015; Kirkbride et al., 2015; Wolff et al., 2015), de-repressed transposable elements (Castillo and Moyle, 2012; Fultz et al., 2015) and cellular processes mediated by small interfering RNAs (Bourc’his and Voinnet, 2010; Lu et al., 2012; Ng et al., 2012). All these factors might interact in the endosperm to determine the success or failure of particular cross combinations. Our recent study addressed some of these issues in C–P hybrid crosses, using transcriptome data obtained from laser-microdissected endosperm (Florez-Rueda et al., 2016). Abnormal endosperm development was characterized by a perturbation of paternally imprinted genes together with a genome-wide increase in maternal expression proportions in the P × C hybrid endosperm. It is tempting to interpret the more deviant endosperm development (and resulting smaller seed size in this cross direction; Figs 2, 4 and 5) as a consequence of greater perturbation of ‘normal’ expression proportions and/or misexpression of crucial, normally paternally expressed genes. Florez-Rueda et al. (2016) spearheaded the exploration of molecular correlates of HSF in plant species with cellular endosperm, and more detailed insights will probably be facilitated by current technological improvements in transcriptomics (Todd et al., 2016) and epigenomics (Zhang et al., 2014; Wang et al., 2015).
Partial escape from HSF and hybrid survival in Am–C crosses
Significant proportions of hybrid seeds from Am–C crosses escaped HSF and were able to germinate. We thus qualified the barrier caused by HSF between Am and C as ‘weak’ while recognizing that individual seeds meet with one of two quite distinct fates. Moreover, reproductive isolation can manifest after successful seed maturation by other mechanisms of hybrid failure. Therefore, we assessed intrinsic post-zygotic barriers that may arise during later phases of plant development in Am–C hybrids. In fact, F1 hybrid seedling performance was impaired by both hybrid necrosis and slower growth rate. Hybrid necrosis has been repeatedly observed in crosses between plant populations or distinct species, and has been interpreted as a manifestation of genetic incompatibilities resulting in autoimmune responses in hybrid progeny (reviewed in Bomblies and Weigel, 2007).
In summary, we obtained large numbers of healthy-looking F1 hybrid plants in reciprocal crosses between Am and C. Hybrid necrosis appears to contribute to ‘late’ post-zygotic isolation between Am and C, but occurred in only three of eight interspecific crosses, affecting 29–52 % of the F1 plants assessed in each of the three crosses. Necrotic phenotypes might be a manifestation of incompatibilities mediated by rapid evolution of pathogen response genes concurrent with species divergence (Bomblies and Weigel, 2007, and references therein). Regardless, we consider the apparent intrinsic post-zygotic barriers between Am and C at the vegetative post-germination stage to be rather modest, compared with those that manifest as reduced proportions of viable seeds.
Implications for modes of tomato speciation
Wild tomato divergence is recent and resulted in a rapid radiation across a wide range of ecological conditions from Ecuador to Chile and from low to high altitude (Moyle, 2008; Tellier et al., 2011; Nosenko et al., 2016). Ecological data suggest that speciation in wild tomatoes was mainly driven by divergent environmental variables such as temperature and precipitation regimes (Nakazato et al., 2010). On the other hand, the role of geographic isolation seems rather complex, and neither allopatric nor sympatric speciation appears to have been predominant across the history of the clade (Nakazato et al., 2010), estimated to have diverged from a common ancestor about 2.5 million years ago (Pease et al., 2016). Extensive crossing experiments revealed the diversity of mechanisms leading to pre- and post-zygotic barriers between many regionally sympatric pairs of wild tomato species; however, only one or a few crosses per species pair were tested (Baek et al., 2016).
In our largely complementary study that concentrated on fewer taxa but employed many interspecific crosses using range-wide sampling, P and C represent the only species pair occurring in regional sympatry. These taxa may have diverged as recently as 0.55 million years ago (Städler et al., 2008), and there is population-genetic evidence for post-divergence gene flow, possibly following a period of initial allopatric divergence (Städler et al., 2005, 2008). These lineages, like all other self-incompatible wild tomatoes, show no obvious signs of divergence in reproductive biology, sharing conspicuous yellow corollas with continuous flowering and fruit set, buzz pollination and overlapping pollen size (Chetelat et al., 2009). Consequently, past selection for enhanced reproductive isolation between C and P via reinforcement at the post-zygotic level may be plausible; such a scenario is feasible under conspicuous maternal investment, mixed pollinations and the potential for seed compensation (Coyne, 1974; Coyne and Orr, 2004). Moreover, the near-complete post-zygotic barrier we have documented regardless of current sympatry or allopatry of the crossed P and C accessions is consistent with post-speciation range expansion (Böndel et al., 2015). In contrast, the fact that the purely allopatric (and phylogenetically more distant; Pease et al., 2016) Am–C crosses typically yielded intermediate proportions of viable seeds may represent a case of gradual loss of ancestral compatibility, similar to several other wild tomato species retaining the ability to yield viable F1 progeny (as pollen donors) with the cultivated tomato (Grandillo et al., 2011). Interestingly, Am and P have evolved a very strong HSF phenotype similar to that between C and P, even though the former are fully allopatric and unlikely to have been in contact recently. Future molecular analyses may reveal whether similar genetic signals underlie this common seed failure phenotype.
Conclusions
Scientific interest in endosperm misdevelopment as a driver of potentially rapid establishment of reproductive isolation between nascent lineages has risen sharply, yet there is a paucity of studies attempting to bridge developmental and evolutionary biology. Our crossing experiments provide compelling evidence that hybrid seed failure is almost universal between two of the three studied pairs of lineages, while the third pair yields mixtures of inviable and viable hybrid seeds; the latter were shown to germinate at intermediate rates and show only weak signs of post-germination developmental problems. The high frequency of hybrid seed failure (even observed in among-population crosses within the nominal species S. chilense) implies that almost complete reproductive isolation can evolve fairly rapidly but is not an invariable outcome. In terms of timing and tissue malfunctions in early seed development, we identified endosperm proliferation defects at the early globular embryo stage as a plausible, circumstantially supported cause of seed abortion. Our observations highlight fundamental similarities in hybrid endosperm malfunction across developmental types, nuclear- and cellular-type endosperm.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: identity and geographic origin of TGRC accessions, and their utilization in our experiments. Table S2: complete data on seed viability per visual assessment in Solanum crosses. Table S3: individual seed size measurements via seed scanning in Solanum crosses. Table S4: measurements of seed compartment size in Solanum crosses, based on histological images. Table S5: plant height in hybrid and intraspecific F1 progeny 11 weeks after germination. Figure S1: representative examples of F1 seedlings from crosses within and between Solanum arcanum ‘marañón’ (Am) and S. chilense (C).
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Maja Frei and Esther Zürcher for taking expert care of the plants, to Markos Tsinganis for measuring seed size, to Franziska Berger and Benoit Villain for further technical help, and to Alex Widmer for general support of this project. We are also grateful to Ottmar Holdenrieder and Thomas Sieber for assistance with our histological work, and to the C. M. Rick Tomato Genetics Resource Center at U.C. Davis for generously supplying seed samples. We also thank two anonymous reviewers for their constructive comments on a previous version of our manuscript. This work was supported by the Swiss National Science Foundation [31003A_130702 to T.S.] and an ETH Research Grant [ETH-40 13-2 to T.S. and Alex Widmer].
LITERATURE CITED
- Baack E, Melo MC, Rieseberg LH, Ortiz-Barrientos D. 2015. The origins of reproductive isolation in plants. New Phytologist 207: 968–984. [DOI] [PubMed] [Google Scholar]
- Baek YS, Royer SM, Broz AK et al. 2016. Interspecific reproductive barriers between sympatric populations of wild tomato species (Solanum section Lycopersicon). American Journal of Botany 103: 1964–1978. [DOI] [PubMed] [Google Scholar]
- Beamish KI. 1955. Seed failure following hybridization between the hexaploid Solanum demissum and four diploid Solanum species. American Journal of Botany 42: 297–304. [Google Scholar]
- Bomblies K, Weigel D. 2007. Hybrid necrosis: autoimmunity as a potential gene-flow barrier in plant species. Nature Reviews Genetics 8: 382–393. [DOI] [PubMed] [Google Scholar]
- Böndel KB, Lainer H, Nosenko T, Mboup M, Tellier A, Stephan W. 2015. North–south colonization associated with local adaptation of the wild tomato species Solanum chilense. Molecular Biology and Evolution 32: 2932–2943. [DOI] [PubMed] [Google Scholar]
- Bourc’his D, Voinnet O. 2010. A small-RNA perspective on gametogenesis, fertilization, and early zygotic development. Science 330: 617–622. [DOI] [PubMed] [Google Scholar]
- Brandvain Y, Haig D. 2005. Divergent mating systems and parental conflict as a barrier to hybridization in flowering plants. American Naturalist 166: 330–338. [DOI] [PubMed] [Google Scholar]
- Burkart-Waco D, Ngo K, Lieberman M, Comai L. 2015. Perturbation of parentally biased gene expression during interspecific hybridization. PLoS One 10: e0117293. doi:10.1371/journal.pone.0117293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell C, Spielman M, Scott RJ. 2003. The basis of natural and artificial postzygotic hybridization barriers in Arabidopsis species. The Plant Cell 15: 1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo DM, Moyle LC. 2012. Evolutionary implications of mechanistic models of TE-mediated hybrid incompatibility. International Journal of Evolutionary Biology 2012: article ID 698198. http://dx.doi.org/10.1155/2012/698198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LZ, Adachi T. 1996. Efficient hybridization between Lycopersicon esculentum and L. peruvianum via ‘embryo rescue’ and in vitro propagation. Plant Breeding 115: 251–256. [Google Scholar]
- Chetelat RT, Pertuzé RA, Faúndez L, Graham EB, Jones CM. 2009. Distribution, ecology and reproductive biology of wild tomatoes and related nightshades from the Atacama Desert region of northern Chile. Euphytica 167: 77–93. [Google Scholar]
- Clark G. 1981. Staining procedures. London, UK: William & Wilkins. [Google Scholar]
- Cooper D, Brink R. 1940. Somatoplastic sterility as a cause of seed failure after interspecific hybridization. Genetics 25: 593–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DC, Brink RA. 1945. Seed collapse following matings between diploid and tetraploid races of Lycopersicon pimpinellifolium. Genetics 30: 376–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA. 1974. The evolutionary origin of hybrid inviability. Evolution 28: 505–506. [DOI] [PubMed] [Google Scholar]
- Coyne J, Orr H. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Dilkes BP, Spielman M, Weizbauer R et al. 2008. The maternally expressed WRKY transcription factor TTG2 controls lethality in interploidy crosses of Arabidopsis. PLoS Biology 6: 2707–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. 1937. Genetics and the origin of species. New York: Columbia University Press. [Google Scholar]
- Florez-Rueda AM, Paris M, Schmidt A, Widmer A, Grossniklaus U, Städler T. 2016. Genomic imprinting in the endosperm is systematically perturbed in abortive hybrid tomato seeds. Molecular Biology and Evolution 33: 2935–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz D, Choudury SG, Slotkin RK. 2015. Silencing of active transposable elements in plants. Current Opinion in Plant Biology 27: 67–76. [DOI] [PubMed] [Google Scholar]
- Garner AG, Kenney AM, Fishman L, Sweigart AL. 2016. Genetic loci with parent-of-origin effects cause hybrid seed lethality in crosses between Mimulus species. New Phytologist 211: 319–331. [DOI] [PubMed] [Google Scholar]
- Grandillo S, Chetelat R, Knapp S et al. 2011. Solanum sect. Lycopersicon. In: Kole C, ed. Wild crop relatives: genomic and breeding resources. Berlin–Heidelberg: Springer-Verlag, 129–215. [Google Scholar]
- Gutierrez-Marcos JF, Pennington PD, Costa LM, Dickinson HG. 2003. Imprinting in the endosperm: a possible role in preventing wide hybridization. Philosophical Transactions of the Royal Society B: Biological Sciences 358: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D, Westoby M. 1991. Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philosophical Transactions of the Royal Society B: Biological Sciences 333: 1–13. [Google Scholar]
- Haughn G, Chaudhury A. 2005. Genetic analysis of seed coat development in Arabidopsis. Trends in Plant Science 10: 472–477. [DOI] [PubMed] [Google Scholar]
- Hehenberger E, Kradolfer D, Köhler C. 2012. Endosperm cellularization defines an important developmental transition for embryo development. Development 139: 2031–2039. [DOI] [PubMed] [Google Scholar]
- Johnston SA, den Nijs TPM, Peloquin SJ, Hanneman RE. 1980. The significance of genic balance to endosperm development in interspecific crosses. Theoretical and Applied Genetics 57: 5–9. [DOI] [PubMed] [Google Scholar]
- Josefsson C, Dilkes B, Comai L. 2006. Parent-dependent loss of gene silencing during interspecies hybridization. Current Biology 16: 1322–1328. [DOI] [PubMed] [Google Scholar]
- Jullien PE, Berger F. 2010. Parental genome dosage imbalance deregulates imprinting in Arabidopsis. PLoS Genetics 6: e1000885. doi:10.1371/journal.pgen.1000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsiotis A, Hanneman RE, Forsberg RA. 1995. Endosperm balance number and the polar-nuclei activation hypotheses for endosperm development in interspecific crosses of Solanaceae and Gramineae, respectively. Theoretical and Applied Genetics 91: 848–855. [DOI] [PubMed] [Google Scholar]
- Kirkbride RC, Yu HH, Nah G, Zhang C, Shi X, Chen ZJ. 2015. An epigenetic role for disrupted paternal gene expression in postzygotic seed abortion in Arabidopsis interspecific hybrids. Molecular Plant 8: 1766–1775. [DOI] [PubMed] [Google Scholar]
- Kradolfer D, Wolff P, Jiang H, Siretskiy A, Köhler C. 2013. An imprinted gene underlies postzygotic reproductive isolation in Arabidopsis thaliana. Developmental Cell 26: 525–535. [DOI] [PubMed] [Google Scholar]
- Lafon-Placette C, Köhler C. 2016. Endosperm-based postzygotic hybridization barriers: developmental mechanisms and evolutionary drivers. Molecular Ecology 25: 2620–2629. [DOI] [PubMed] [Google Scholar]
- Lafon-Placette C, Johannessen IM, Hornslien KS et al. 2017. Endosperm-based hybridization barriers explain the pattern of gene flow between Arabidopsis lyrata and Arabidopsis arenosa in central Europe. Proceedings of the National Academy of Sciences, USA 114: E1027–E1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RN, Kang JH. 1998. Embryo and endosperm function and failure in Solanum species and hybrids. Annals of Botany 82: 445–453. [Google Scholar]
- Lin BY. 1984. Ploidy barrier to endosperm development in maize. Genetics 107: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MA, Larkins BA. 1993. Endosperm origin, development, and function. The Plant Cell 5: 1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang C, Baulcombe DC, Chen ZJ. 2012. Maternal siRNAs as regulators of parental genome imbalance and gene expression in endosperm of Arabidopsis seeds. Proceedings of the National Academy of Sciences, USA 109: 5529–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuelli RW, Camadro EL. 1997. Crossability relationships among wild potato species with different ploidies and Endosperm Balance Numbers (EBN). Euphytica 94: 227–235. [Google Scholar]
- Mayr E. 1942. Systematics and the origin of species. Cambridge, MA: Harvard University Press. [Google Scholar]
- Moyle LC. 2008. Ecological and evolutionary genomics in the wild tomatoes (Solanum Sect. Lycopersicon). Evolution 62: 2995–3013. [DOI] [PubMed] [Google Scholar]
- Nakazato T, Warren DL, Moyle LC. 2010. Ecological and geographic modes of species divergence in wild tomatoes. American Journal of Botany 97: 680–693. [DOI] [PubMed] [Google Scholar]
- Ng DWK, Lu J, Chen ZJ. 2012. Big roles for small RNAs in polyploidy, hybrid vigor, and hybrid incompatibility. Current Opinion in Plant Biology 15: 154–161. [DOI] [PubMed] [Google Scholar]
- Nosenko T, Böndel KB, Kumpfmüller G, Stephan W. 2016. Adaptation to low temperatures in the wild tomato species Solanum chilense. Molecular Ecology 25: 2853–2869. [DOI] [PubMed] [Google Scholar]
- Olsen O-A, ed 2007. Endosperm – developmental and molecular biology. Berlin–Heidelberg: Springer-Verlag. [Google Scholar]
- Oneal E, Willis JH, Franks RG. 2016. Disruption of endosperm development is a major cause of hybrid seed inviability between Mimulus guttatus and Mimulus nudatus. New Phytologist 210: 1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz R, Ehlenfeldt M. 1992. The importance of Endosperm Balance Number in potato breeding and the evolution of tuber-bearing Solanum species. Euphytica 60: 105–113. [Google Scholar]
- Pease JB, Haak DC, Hahn MW, Moyle LC. 2016. Phylogenomics reveals three sources of adaptive variation during a rapid radiation. PLoS Biology 14: e1002379. doi:10.1371/journal.pbio.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta IE, Spooner DM, Knapp S. 2008. Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Systematic Botany Monographs 84: 1–186. [Google Scholar]
- RStudio Team 2015. RStudio: integrated development for R URL http://www.rstudio.com (last accessed 12 November 2016).
- Rebernig CA, Lafon-Placette C, Hatorangan MR, Slotte T, Köhler C. 2015. Non-reciprocal interspecies hybridization barriers in the Capsella genus are established in the endosperm. PLoS Genetics 11: e1005295. doi:10.1371/journal/pgen.1005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick CM. 1950. Pollination relations of Lycopersicon esculentum in native and foreign regions. Evolution 4: 110–122. [Google Scholar]
- Rick CM. 1963. Barriers to interbreeding in Lycopersicon peruvianum. Evolution 17: 216–232. [Google Scholar]
- Rick CM. 1986. Reproductive isolation in the Lycopersicon peruvianum complex. In: D’Arcy WG, ed. Solanaceae – biology and systematics. New York: Columbia University Press, 477–495. [Google Scholar]
- Rick CM, Lamm R. 1955. Biosystematic studies on the status of Lycopersicon chilense. American Journal of Botany 42: 663–675. [Google Scholar]
- Sabelli PA, Larkins BA. 2009. The contribution of cell cycle regulation to endosperm development. Sexual Plant Reproduction 22: 207–219. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG. 1998. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125: 3329–3341. [DOI] [PubMed] [Google Scholar]
- Seehausen O, Butlin RK, Keller I et al. 2014. Genomics and the origin of species. Nature Reviews Genetics 15: 176–192. [DOI] [PubMed] [Google Scholar]
- Segeren MI, Sondahl MR, Siqueira WJ, Medina Filho HP, Nagai H, Lourenção AL. 1993. Tomato breeding: 1. Embryo rescue of interspecific hybrids between Lycopersicon esculentum Mill. and L. peruvianum (L.) Mill. Brazilian Journal of Genetics 16: 367–380. [Google Scholar]
- Städler T, Roselius K, Stephan W. 2005. Genealogical footprints of speciation processes in wild tomatoes: demography and evidence for historical gene flow. Evolution 59: 1268–1279. [PubMed] [Google Scholar]
- Städler T, Arunyawat U, Stephan W. 2008. Population genetics of speciation in two closely related wild tomatoes (Solanum section Lycopersicon). Genetics 178: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IB. 1986. Biosystematics of the tomato. In: Atherton JG, Rudich J, eds. The tomato crop: a scientific basis for improvement. London: Chapman and Hall Ltd, 1–34. [Google Scholar]
- Tellier A, Laurent SJY, Lainer H, Pavlidis P, Stephan W. 2011. Inference of seed bank parameters in two wild tomato species using ecological and genetic data. Proceedings of the National Academy of Sciences, USA 108: 17052–17057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd EV, Black MA, Gemmell NJ. 2016. The power and promise of RNA-seq in ecology and evolution. Molecular Ecology 25: 1224–1241. [DOI] [PubMed] [Google Scholar]
- Walia H, Josefsson C, Dilkes B, Kirkbride R, Harada J, Comai L. 2009. Dosage-dependent deregulation of an AGAMOUS-LIKE gene cluster contributes to interspecific incompatibility. Current Biology 19: 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RI. 1955. Cytological and embryological studies in Solanum, section Tuberarium. Bulletin of the Torrey Botanical Club 82: 87–101. [Google Scholar]
- Wang P, Xia H, Zhang Y et al. 2015. Genome-wide high-resolution mapping of DNA methylation identifies epigenetic variation across embryo and endosperm in maize (Zea mays). BMC Genomics 16: 21. doi:10.1186/s12864-014-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi Y. 2013. The battle of the sexes over seed size: support for both kinship genomic imprinting and interlocus contest evolution. American Naturalist 181: 787–798. [DOI] [PubMed] [Google Scholar]
- Wolff P, Jiang H, Wang G, Santos-González J, Köhler C. 2015. Paternally expressed imprinted genes establish postzygotic hybridization barriers in Arabidopsis thaliana. eLife 4: e10074. doi:10.7554/eLife.10074.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Fiume E, Coen O, Pechoux C, Lepiniec L, Magnani E. 2016. Endosperm and nucellus develop antagonistically in Arabidopsis seeds. The Plant Cell 28: 1343–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xie S, Dong X et al. 2014. Genome-wide high resolution parental-specific DNA and histone methylation maps uncover patterns of imprinting regulation in maize. Genome Research 24: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.