Abstract
Background and Aims
Plant species with fire-triggered germination are common in many fire-prone ecosystems. For such plants, fire timing in relation to the timing of reproduction may strongly influence post-fire population regeneration if: (a) flowering occurs infrequently (e.g. plants are mast seeders); and (b) seed survival rates are low and input from the current year’s flowering therefore contributes a large proportion of the viable dormant seedbank. The role of fire timing in relation to masting as a driver of post-fire recruitment has rarely been examined directly, so this study tested the hypothesis that fires shortly after masting trigger increased recruitment of the obligate-seeding arid zone spinifex, Triodia pungens R. Br., an iteroparous masting grass with smoke-cued germination.
Methods
Phenological monitoring of T. pungens was conducted over 5 years, while a longitudinal seedbank study assessed the influence of seeding events on soil-stored seedbank dynamics. Concurrently, a fire experiment with randomized blocking was undertaken to test whether T. pungens hummocks burnt shortly after masting have greater post-fire recruitment than hummocks burnt when there has not been recent input of seeds.
Key Results
Triodia pungens flowered in all years, though most flowerings were characterized by high rates of flower abortion. A mast flowering with high seed set in 2012 triggered approx. 200-fold increases in seedbank densities, and seedbank densities remained elevated for 24 months after this event. The fire experiment showed significantly higher recruitment around hummocks burnt 6 months after the 2012 mast event than around hummocks that were burnt but prevented from masting by having inflorescences clipped.
Conclusions
Fires shortly after masting trigger mass recruitment in T. pungens because such fires synchronize an appropriate germination cue (smoke) with periods when seedbank densities are elevated. Interactions between natural fire regimes, seedbank dynamics and fire management prescriptions must be considered carefully when managing fire-sensitive masting plants such as T. pungens.
Keywords: Synchronous flowering, arid vegetation, obligate seeder, seed predation, spinifex grasslands, seedling establishment, Triodia, pungens
INTRODUCTION
Mast seeding, the intermittent production of synchronized seed crops in polycarpic plant populations, is expected to have important consequences for seedling recruitment in systems where seedbank persistence is limited by rapidly declining seed viability or by granivores (Whelan, 1995; Wells and Bagchi, 2005; Barbera et al., 2006). Consistent with this, one of the most frequently cited ‘evolutionary’ explanations for masting is the predator satiation hypothesis (Salisbury, 1942; Janzen, 1971, 1976). Under this hypothesis, masting is an anti-predator adaptation that starves consumers during low output inter-mast years, and increases recruitment chances by ‘swamping’ granivores with an abundance of seeds during mast years. Other commonly cited explanations for masting include improved pollination efficiency for wind-pollinated species (Herrera et al., 1998; Kelly et al., 2001) and environmental prediction (the use of environmental cues to ‘anticipate’ future periods favourable for germination or seedling growth (Williamson and Ickes, 2002; Burns, 2012).
Mast seeding is prevalent in many fire-prone habitats (Keeley and Bond, 1999; Peters et al., 2005; Davies and Kenny, 2013). The timing of fires in relation to mast years is potentially of great importance to plant species for which recruitment is contingent on fire (i.e. that have smoke- or heat-cued germination), and for which input from the current year’s flowering contributes a large proportion of the viable dormant seedbank. For such species, fires shortly after a mast year may considerably enhance recruitment, by coinciding high seedbank densities with appropriate fire-related cues for germination and/or favourable post-fire establishment conditions (O’Dowd and Gill, 1984; Wright et al., 2016). Alternatively, if fires occur during the flowering or bud stage of a mast year, then recruitment chances may be minimized as seed crops will be destroyed and subsequent input to seedbanks might not occur for periods of up to a decade or more (Whelan, 1995). Similar fire-driven elimination of seed crops may also occur in non-masting plants, when ‘natural’ fire regimes are altered and the season of burn is discordant with the annual flowering phenology of a plant species (Whelan, 1995; Heelemann et al., 2008; Nield et al., 2016).
Despite the strong theoretical basis behind the hypothesis that fire timing in relation to reproduction should be an important determinant of post-fire recruitment success for masting plants, there have been few empirical studies that have examined this prediction. Experimental data are therefore needed, and may not be overly difficult to obtain if researchers can be opportunistic and take advantage of mast years when they occur.
The vastly distributed Triodia (common name spinifex) hummock grasslands of Australia offer an excellent opportunity to investigate plant population responses to interactions between fire timing and masting. These grasses occur primarily in arid and semi-arid inland regions, and, for all examined arid species, rainfall is the proximate trigger for flower initiation and seed production (Andrews, 1883; Jacobs, 1973, 1984; Wells et al., 2000). Interestingly, although arid Triodia species typically flower on an annual basis, rates of seed set during flowering are usually low (Westoby et al., 1988; Rice et al., 1994). It is only following protracted periods (e.g. ≥12 months) of exceptionally high rainfall that high seed set mast flowering occurs (Jacobs, 1984; Wright et al., 2014). As the rains that trigger mast flowerings also lead to grass growth and fuel accumulation (McArthur, 1972; Griffin et al., 1983), Triodia mast events are usually followed by wildfires (Jacobs, 1984; Wright et al., 2014).
Triodia seeds are long lived (i.e. ≥15 years) and subject to high levels of predation (Jacobs, 1973, 1984). Granivores that consume Triodia seeds include ants, bird species such as the night parrot (Pezoporus occidentalis), rodent genera including Notomys and Pseudomys, and rare and endangered small mammals such as the bilby (Macrotis lagotis) and the western hare wallaby (Lagorchestes hirsutus) (Andrews, 1883; Jacobs, 1973, 1982; Bolton and Latz, 1978; Murray and Dickman, 1994; Fig. 1A, B). Profuse seeding during Triodia mast years overwhelms seed predators, causing brief ‘satiation windows’ when seedbanks are dense and seed availability is high (Wright and Fensham, 2016). As a result, Triodia masts that are produced ‘in step’ with arid zone fire cycles may enhance post-fire recruitment, by synchronizing high densities of smoke-cued seeds with periods when populations are likely to burn [Triodia germination is enhanced by compounds such as karrikinolides which are present in smoke (Erickson et al., 2016)]. Moreover, burning during inter-mast periods may have negative demographic consequences for obligate-seeding Triodia, because seedbank persistence during such times is expected to be limited by predation pressure.
Fig. 1.
Context of the arid study area: (A) population of obligate-seeding T. pungens following the 2012 mast flowering event at Deep Well station, Northern Territory; (B) midden of empty T. pungens florets belonging to the seed-harvesting ant species Monomorium rothsteini.
The current study examined the influence of mast seeding on seedbank responses and post-fire recruitment in an arid zone population of the iteroparous masting grass Triodia pungens R.Br. (common name soft or gummy spinifex). The study was initiated in early 2012 at Deep Well pastoral station in Australia’s Northern Territory, when a T. pungens mast year was triggered following successive high rainfall years in 2010 (769.6 mm) and 2011 (366.4 mm) (Australian Government Bureau of Meteorology, 2017). The study sought explicitly to examine the dynamics of flowering to seed set, seed set to seedbank and seedbank to seedlings of the Deep Well T. pungens population following this rare masting event. The hypotheses tested by the study were: (1) that the 2012 mast event would lead to a short-lived seedbank pulse, because high levels of granivory rapidly deplete the seedbank, but mast events temporarily overwhelm seed predators; and (2) a fire occurring shortly after the mast event would trigger increased seedling regeneration compared with fires during an inter-mast period, because seedbank densities should be highest shortly after masting.
By addressing these key questions, this study (1) provided an empirical test of more general theories and models relating to the timing of fire disturbance in relation to mast seeding and seedbank dynamics; and (2) contributed toward an understanding of how fire management may need to be changed to avoid threatening populations of a keystone plant species in arid and semi-arid vegetation communities in Australia.
MATERIALS AND METHODS
Study species and study site
Spinifex (Triodia spp.) grasses are perennial, hummock-forming grasses that form the dominant component of grasslands across at least 25 % of Australia (Allan and Southgate, 2002). There are currently 81 described species of Triodia (Lazarides, 1997; Armstrong, 2008; Barrett and Barrett, 2015;Anderson et al., 2017), though a number of species complexes are currently under revision and the total species number is expected to increase. The study species, T. pungens, has highly resinous foliage and is widespread in arid and semi-arid regions across the northern half of the Australian continent (Burbidge, 1943; Nicholas et al., 2009). It occurs over a range of soil types, but is most commonly found on infertile sandy desert soils (Bowman et al., 2008; Nicholas et al., 2009).
Field observations indicate that T. pungens varies considerably in terms of its fire response and growth form between regions. For example, in semi-arid areas such as the Tanami Desert and Camooweal in western Queensland, T. pungens resprouts after fire, often forms rings as plants age and produces long adventitious runners that may form new clones (Latz, 2007; Gamage et al., 2014). Conversely, across the vast arid range of the species, such as in areas around Uluru-Kata Tjuta National Park and in the Finke Desert bioregion, it is normally killed by fire (i.e. is an obligate seeder), rarely forms rings and does not produce long adventitious runners (Bogusiak et al., 1990; Latz, 2007; Wright and Fensham, 2016). The T. pungens form that occurs at Deep Well pastoral station has only ever been observed to regenerate from seed after fires of a range of intensities and post-fire rainfall totals, and hereafter is referred to as the obligate-seeding form of T. pungens.
The study site was located approx. 30 km south of the Alice Springs airport, on Deep Well pastoral station in the Finke bioregion in the Northern Territory of Australia. The site was approx. 5 ha in size, and occurred on a mildly undulating sand plain with an understorey dominated by T. pungens and an overstorey of scattered shrubs of blue mallee (Eucalyptus gamophylla F. Muell.) and mulga (Acacia aptaneura Maslin & J.E. Reid). The site has a mean annual rainfall of 234 mm, and the majority of rains fall over summer months due to the influence of the northern Australian monsoon (Australian Government Bureau of Meteorology, 2017). Fire regimes that characterize the study area are similar to those of other arid Australian regions such as the Simpson-Strzelecki dunefields and the Macdonnell Ranges bioregion (Allan and Southgate, 2002; Bastin and Allan, 2012). Typically, long dry periods with little biomass accumulation and few wildfires are interspersed by occasional years of exceptionally high rainfall with high fuel loads and extensive wildfires (Fig. 2).
Fig. 2.
Relationship between area (km2) of the Finke bioregion burnt each calendar year and 3 and 24 month antecedent rainfall [fire data obtained from Parks and Wildlife Commission North Australia and Rangelands Fire Information (2017); rainfall data obtained from Alice Springs Airport weather station (Australian Government Bureau of Meteorology, 2017)].
Phenological and seedbank studies
A longitudinal phenological study was commenced in April 2012 to estimate rates of seed input by T. pungens populations to soil seedbanks. Specifically, the reproductive output of ten tagged T. pungens hummocks was monitored immediately prior to a mast event in May 2012, and then at each subsequent flowering until April 2016. Rainfall measurements for the study site were taken from the nearest reliable weather station at the Alice Springs Airport 30 km to the north (Australian Government Bureau of Meteorology, 2017). To check for spatial variability in flowering output, this phenological study was replicated at a second T. pungens population approx. 5 km to the south of the Deep Well population (Ooraminna site) (Supplementary Data Fig. S1). The site characteristics of the Ooraminna population were considered largely identical to those of the Deep Well site.
Estimates of the total seed production for each hummock during each flowering were assessed by multiplying the number of inflorescences produced in a flowering by the following three parameters (obtained from five inflorescences from five randomly selected individuals): (1) the proportion of seed-filled florets (estimated via visual inspection of n = 200 florets using a stereomicroscope); (2) the mean number of florets produced per spikelet; and (3) the mean number of spikelets produced per inflorescence. Estimates of the total viable seed production for each flowering were then made by multiplying the calculated total seeding output by the estimated percentage seed viability. Seed viability was calculated from a 1 × 100 sub-sample of seed using tetrazolium staining (2, 3, 5-triphenyl-tetrazolium chloride; AsureQuality Australia Pty. Ltd, Melbourne, Australia). All inflorescences at both sites were clipped following each flowering to avoid recounting during subsequent sampling.
Concurrent to the phenological study, a T. pungens seedbank study was initiated in April 2012 at Deep Well to quantify the contribution of the May 2012 mast seeding event to the overall seedbank density. For this study, soils from two (later pooled) 20 × 20 × 1 cm deep quadrats were collected from the edges of 15 randomly selected unburnt T. pungens hummocks (1–1.2 m diameter) over nine sampling rounds (with different randomly selected hummocks sampled in each round). The soil samples were gathered from hummock edges only, as previous studies had indicated that this is the microsite from which most post-fire seedling regeneration occurs (Westoby et al., 1988). Samples were gathered from the 0–1 cm soil depth because previous research had indicated this is the depth where the majority of the post-mast seedbank exists (Wright and Fensham, 2016). The first sampling round was conducted immediately prior to masting in April 2012, thereby providing an indication of inter-mast seedbank densities. The remaining eight sampling rounds occurred in May 2012 (immediately after seed fall) and then at 6, 12, 18, 24, 30, 42 and 48 months post-seed fall. The soil samples were initially passed through a 600 μm sieve, and then the remaining organic and inorganic matter was passed through a zigzag-style aspirator to remove unwanted chaff. The resulting material was then screened for Triodia seeds under a stereomicroscope. Viable seed densities for the seedbank study were estimated by multiplying the individual data points (observed seed densities) by 0.68 (the previously calculated percentage seed viability estimate).
Fire experiment
A fire experiment was commenced in November 2012 at Deep Well to examine the effect of the May 2012 T. pungens mast event on post-fire seedling regeneration. The experiment used a randomized block design, with 30 randomly selected experimental hummocks (1–1.2 m diameter) stratified across a northern and a southern block to account for any confounding spatial effects. The combined area of the two blocks was approx. 5 ha. The experiment aimed to compare seedling regeneration from hummocks that were burnt after masting with regeneration from hummocks that were burnt during a simulated inter-mast period. By simulating an inter-mast period (rather than waiting for a ‘natural’ inter-mast period), ambient post-fire rainfall levels (which would affect recruitment rates) would be consistent across both masted and inter-mast hummocks.
Hummocks allocated to the ‘inter-mast’ level of the masting treatment had flowers clipped in April 2012 just prior to masting (these hummocks are hereafter termed the ‘clipped’ hummocks). This limited the localized seedbank to residual seeds, as if no mast seeding had occurred for these plants. Additionally, the inflorescences of surrounding hummocks within a 5 m radius were clipped to minimize the possibility of seeds from the surrounding masting population being incorporated into the seedbank. Hummocks allocated to the ‘masted’ level of the masting treatment were allowed to go to seed during the May 2012 mast event, thereby providing fresh seed input to the soil seedbanks of these plants. Data from these ‘masted’ hummocks were previously published in an investigation on the effects of fire severity on post-fire recruitment (Wright and Fensham, 2016).
To examine differences in post-fire regeneration between ‘masted’ and ‘clipped’ hummocks, all hummocks were burnt approx. 6 months after the mast event on 3 November 2012. Burning occurred between 07.00 and 11.00 h, and 10 min prior to each burn the hummocks were watered with 10 L of rainwater. This approach reduced the intensity of the burns and permitted the greatest opportunity to observe any differences in regeneration that might occur between the ‘clipped’ and ‘masted’ plants. Moreover, conducting the burns under low-intensity conditions meant the results would be directly relevant to management burns of spinifex grasslands, which, in arid Australia, are usually conducted under mild low-temperature winter conditions, or after rain (Pitts and Matthews, 2000; Allan and Southgate, 2002; Duguid et al., 2008; Parks and Wildlife Service of the Northern Territory, 2011).
Soil temperatures during the experimental fires were monitored by inserting type K thermocouples (TC6-K, Onset Computer Corporation, USA) into soils during five of the burns at the surface, 1 cm and 4 cm soil depths (Wright and Fensham, 2016). Post-fire soil temperature conditions at a single burnt and unburnt hummock were also monitored at the soil surface and at 1 cm for 12 months after the burn experiment (Supplementary Data Fig. S2). All hummocks were watered daily to stimulate recruitment for 7 d after burning with 10 L of rainwater between 05.00 and 07.00 h and 10 L of rainwater between 17.00 and 19.00 h. Drying out of soils between watering periods was minimized by applying 1.5 × 1.5 m squares of 90 % light reduction shade cloth over each burnt hummock for the duration of the watering. This watering and shading regime mimicked a typical central Australian summer rainfall event, which occurs when monsoon-linked rainfall systems enter the arid central regions and provide rains and cloud cover that may last for up to a week or more (Suppiah, 1993). Given that these systems occasionally fail to penetrate deeply into arid Australia, watering was done in our experiment to ensure germination occurred after fire, and that the rare opportunity to test the effect of masting on post-fire recruitment could occur. Monitoring of seedling regeneration from all hummocks was conducted by counting Triodia seedlings within a 1.2 m radius from the centre of each hummock 10 d after the burns occurred, and then again at approx. 6 monthly intervals until 36 months after the burns.
Data analysis
Prior to conducting the seedbank and seedling recruitment analyses, detailed data explorations were applied following the protocol of Zuur et al. (2010). To model the response of seed counts in the seedbank to the categorical covariate ‘sampling time’, a negative binomial (NB) generalized linear model (GLM) was fitted using the statistical software R (R Core Team, 2016). This model was not overdispersed (overdispersion factor = 1.16). Post-hoc comparisons of means were conducted using Bonferroni corrections for multiple comparisons using the ‘multcomp’ package in R (Hothorn et al., 2008). A likelihood ratio test was applied to test the significance of terms in the fitted model. Model validations were carried out to verify the underlying assumptions of the model.
For the fire experiment analysis, the number of T. pungens seedlings that emerged after fire was modelled as a function of the fixed categorical covariates ‘masting treatment’, ‘sampling time’ and ‘block’, and all two-way interactions between these covariates. To account for dependencies due to sampling the same spinifex hummocks repeatedly over time, a generalized linear mixed modelling (GLMM) framework was used in which ‘hummock identity’ was employed as a random intercept. Due to the relatively large number of zeros (20.5 %), a zero inflated NB GLMM was fitted in the glmmADMD package in R (Fournier et al., 2012). This model was not overdispersed. To determine which covariates should be retained in this model, a backwards selection process was carried out using AICc (small sample size corrected Akaike information criterion) (Zuur et al., 2010). This process involved stepwise deletion of candidate variables, based on computed AICc values, until no further improvement to the model could be found. Following model selection, post-hoc tests with Bonferroni corrections were applied. Following analysis, graphical model validation procedures were undertaken.
RESULTS
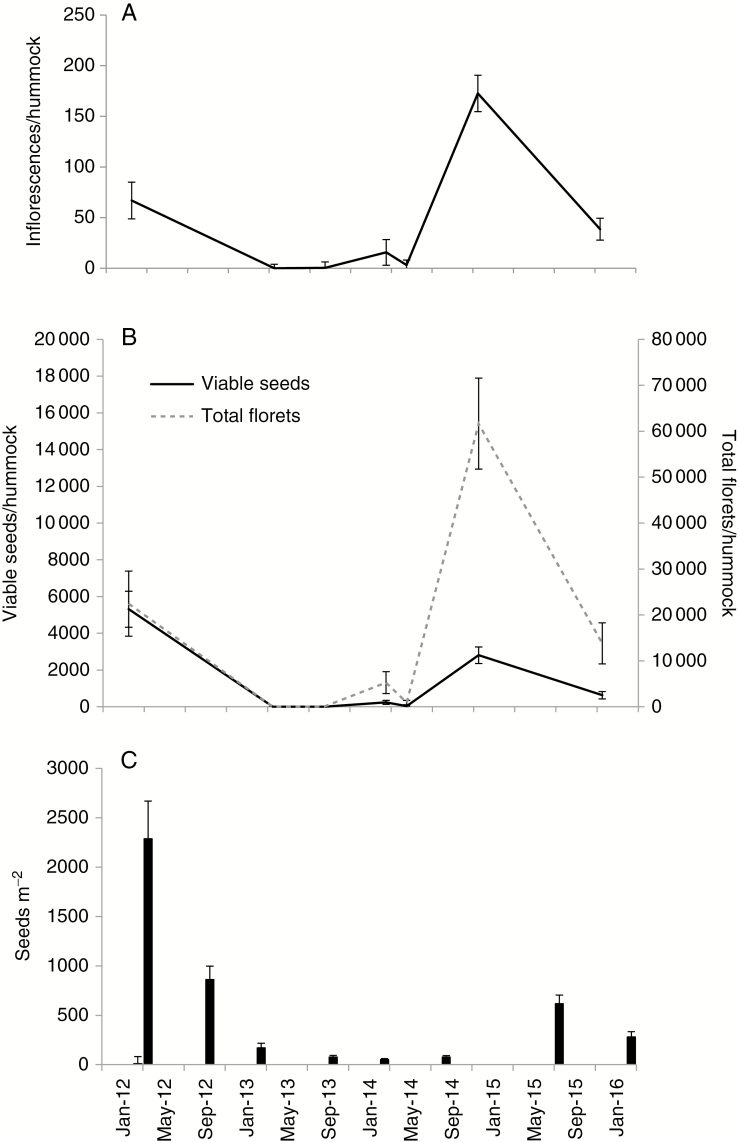
Triodia pungens flowered in all years, though only the 2012 mast flowering was characterized by high rates of seed setting (34.8 %) and high seed output (5311.4 viable seeds/hummock) (Fig. 3A, B). This event had a strong impact on the soil seedbank, with a massive but short-lived seedbank pulse occurring immediately after masting [likelihood ratio test (LRT) = 469.9, d.f. = 6, P ≥ 0.001] (Figs 3C and 5A). Post-hoc testing indicated that seed densities after May 2012 remained elevated over the pre-masting density for the next 30 months (Supplementary Data Table S1). Another smaller seedbank pulse was detected in September 2015, and this is presumed to have occurred in response to a seeding event in January 2015 (Fig. 3B, C). The 2015 event differed from the 2012 seeding event because although large quantities of viable seeds were produced during both events, the overall percentage of flowers that produced filled seeds was much lower in 2015 (6.7 %) than in 2012 (34.8 %).
Fig. 3.
(A) Mean T. pungens inflorescence production per hummock at the Deep Well study site between 2012 and 2016; (B) mean floret number and viable seed production per hummock for the T. pungens population at Deep Well between 2012 and 2016; (C) mean viable T. pungens seeds m–2 in 0–1 cm soil depth beneath hummock edges between 2012 and 2016.
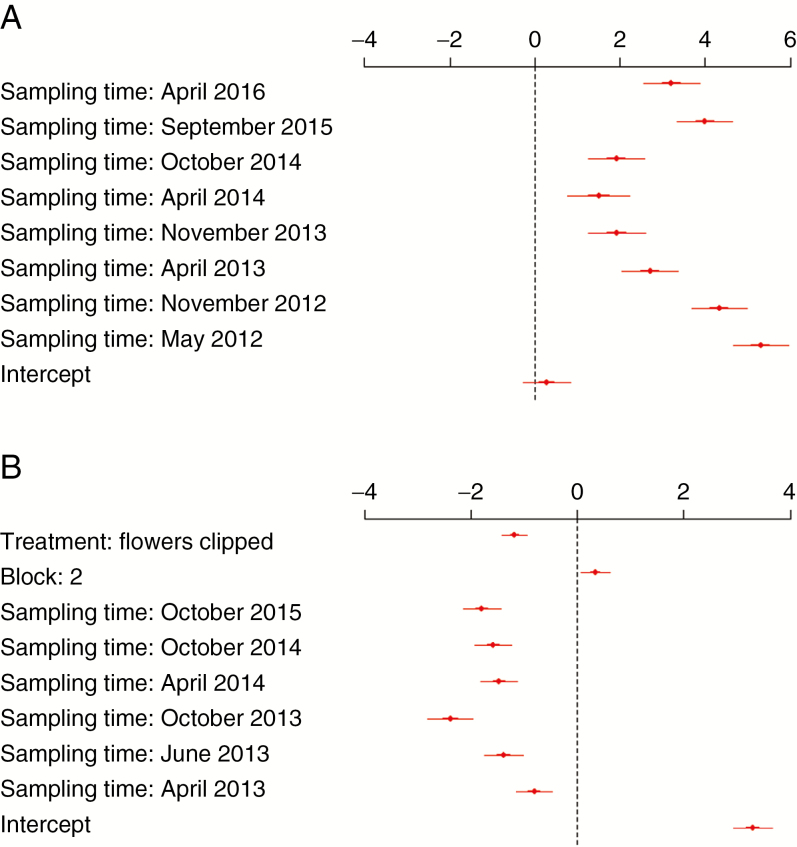
Fig. 5.
Parameter estimates (means and 95 % confidence intervals) from the (A) ‘seedbank’ GLM, and (B) ‘fire experiment’ GLMM. For the ‘seedbank’ GLM, ‘April 2012’ is the baseline ‘sampling time’ parameter. For the ‘fire experiment’ GLMM, ‘treatment: masted’ is the baseline ‘masting treatment’ parameter, block 1 the baseline ‘block’ parameter, and ‘November 2012’ the baseline ‘sampling time’ parameter.
From the ‘fire experiment’ analysis, AICc scores indicated that the preferred model (model 1) contained the predictors ‘masting treatment’, ‘sampling time’ and ‘block’ (Supplementary Data Table S2). The detection of a significant ‘masting treatment’ effect [LRT = 79.9, d.f. = 1, Pr (>Chi) ≥ 0.0001] indicated that fire timing in relation to masting was an important driver of post-fire T. pungens recruitment. Across all time periods and blocks, the mean post-fire recruitment of hummocks that were burnt after masting was significantly higher than at hummocks that were prevented from masting prior to fire by having inflorescences experimentally clipped (Figs 4 and 5B). The results of the post-hoc test on ‘sampling time’ indicated that across both masting treatments and blocks, seedling numbers in April 2013 were lower than in November 2012, and also that seedling numbers in October 2013 were lower than in June 2013 (Figs 4 and 5B; Supplementary Data Table S3). The protracted warm and dry conditions over these periods, with temperatures at the soil surface occasionally exceeding 70 °C (Supplementary Data Fig. S2), most probably caused high levels of seedling attrition during these periods, and therefore account for differences in seedling numbers between these sampling times. Additionally, seedling numbers at April 2014 were significantly higher than at October 2013 (Figs 4 and 5B; Supplementary Data Table S3), most probably indicating a flush of seedling recruitment that occurred after good rains over the summer of 2013/2014.
Fig. 4.
Mean (±s.e.) counts of Triodia pungens seedlings m–2 at masted and clipped hummocks, sampled at seven post-fire time periods between November 2012 and October 2015.
DISCUSSION
Although mast seeding is predicted to have strong impacts on seedbank dynamics in systems with high granivore densities (Janzen, 1971; Barbera et al., 2006), empirical studies that have quantified mast impacts on seed population densities in soils are few (Haase et al., 1995; Zhang et al., 2009). In our experimental studies at Deep Well, rainfall-stimulated T. pungens masting precipitated a large but transitory spike in soil seedbank size and burning populations 6 months after this event triggered mass recruitment. These findings suggest that T. pungens regeneration after most ‘natural’ wildfires should also be strong, as the high-rainfall conditions that promote grassy fuel growth and lead to states of high ecosystem combustibility also stimulate masting and attendant seedbank pulsing. Plants that were burnt but had inflorescences clipped prior to masting had significantly less recruitment, presumably because the seedbanks of these plants were sparse at the time of fire. This finding suggests that lightning-ignited or prescribed burns during inter-mast periods should likewise lead to reduced recruitment, even when post-fire rainfall provides sufficient soil moisture for germination.
The results of our experiment should be directly applicable to low fire severity management burns. Moreover, the positive effect of masting on post-fire recruitment we observed should also hold for ‘natural’ wildfire situations. However, for several reasons, the magnitude of effects of wildfires on masted and non-masted populations is likely to differ from those detected by our experiment. First, spinifex wildfires would normally have higher soil temperatures than our experimental burns (Bradstock et al., 1992), and this would increase mortality rates of shallowly buried seeds via direct exposure to lethal heat. Secondly, our experimentally burnt hummocks were given a very favourable watering regime after fire, which maximized chances for germinated seeds to establish. Such favourable rainfall conditions may not always be encountered following wildfires, and this could reduce recruitment levels because (1) extended intervals between initial post-fire rainfalls that trigger germination and subsequent rainfall events should lead to high rates of seedling attrition due to moisture stress (Thomas et al., 2010; Hewitt et al., 2015) and (2) long intervals between fire and germination-triggering rainfalls should allow additional time for granivores to deplete seedbanks (Noble, 1997). Given that post-fire recruitment rates of non-masted plants in our study were already marginal, additional research is therefore urgently needed to determine whether wildfires during inter-mast periods could reduce regeneration below levels sufficient for populations to recuperate.
Rapid seed transfer into the soil following masting is presumably important for obligate-seeding Triodia populations for ensuring that some seeds are buried and protected from lethal heats if wildfires occur. However, further research is required to determine whether movement of seed into the soil following masting occurs primarily via abiotic (e.g. rainfall-mediated) or biotic processes (e.g. dispersal by fauna or self-burial mechanisms in the seeds) (Benvenuti, 2007). A previous study on the depth distribution of Triodia seedbanks after masting indicated that although movement of Triodia seed into the soil after masting is fast, most seed is buried shallowly (Wright and Fensham, 2016). In this previous study, it was found that percentage seed densities 6 months after masting at the 0–0.5, 0.5–1 and 1–2 cm depths were 76.9, 20.6 and 1.6 %, respectively (as percentages of the total viable detected seedbank), with occasional seed clumps also occurring to 4 cm depth. Nevertheless, despite these shallow burial depths, a considerable fraction of the shallow post-mast seedbank presumably escapes lethal heat during wildfires. This is because Triodia seeds can withstand temperatures up to 80 °C for 60 min without loss of viability (Wright and Fensham, 2016), and maximum soil temperatures under simulated wildfire conditions rarely exceed 60 °C at 1 cm depth beneath hummocks (Bradstock et al., 1992), and approx. 50 °C at 1 cm depth beneath hummock edges (Wright and Clarke, 2008).
Consistent with the predator satiation hypothesis of masting (Janzen, 1971; Zwolak et al., 2016), it is likely that the seedbank pulses we observed in 2012 and 2015 occurred because masting in these years satiated granivores, and this allowed a fraction of the seed crop to ‘escape’ and become assimilated into the soil seedbank. Even so, following these initial seedbank pulses, steady declines in soil seedbank densities were observed over time. This suggests ongoing depredation of seeds by granivores, which could have been occurring if ants were consuming seeds that were initially cached in nests shortly after masting. Alternatively, surface-scavenging rodents could have been detecting buried seeds via olfaction, as occurs in North American and Siberian rodents (Downs and Vander Wall, 2009; Yi et al., 2016). Seed decay due to old age is unlikely to have accounted for the observed reductions in seed densities after initial post-mast seedbank pulsing, as Triodia seeds are known to be long lived (>15 years) (Jacobs, 1973, 1984).
It is possible that if populations remain unburnt for long periods following masting, then a small number of seeds could escape predators completely and go on to form a sparse persistent seedbank. If this is the case, then if populations remain unburnt and experience numerous mast events in their lifetime, densities of the persistent component of the seedbank could increase incrementally over time. However, such incremental increases over long periods of time are likely to be minor, as high levels of seed predation that are intrinsic to arid Triodia systems would still maintain seedbanks at low levels during inter-mast periods (Bolton and Latz, 1978; Morton, 1985; Andersen, 1991). Nevertheless, small increases in the persistent viable seedbank over time could be important for allowing populations to recover if severe droughts that kill adult plants occur during inter-mast periods.
Most conservation-based management prescriptions for arid spinifex grasslands advocate burning fire-breaks during average and below-average rainfall years, when fuel levels are low and ecosystem combustibility is reduced (Pitts and Matthews, 2000; Duguid et al., 2008; Parks and Wildlife Service of the Northern Territory, 2011). Burning fire-breaks in this manner is expected to minimize the likelihood of large-scale wildfires after high rainfall periods that trigger fuel accumulations. Managers of arid spinifex grasslands should be aware, however, that burns conducted during average and below-average rainfall times are likely to be taking place during inter-mast periods, when Triodia seedbank densities are low and recruitment potentials are greatly reduced. Hence, if the maintenance of Triodia populations is a management objective, care should be taken to minimize any negative impacts that burning might have on sparse inter-mast Triodia seedbanks. Ensuring that management burns are of low intensity, for example by burning at night, during winter seasons and/or immediately after rainfall, would be one way to achieve this outcome. Fire managers should also be aware, however, that reducing soil temperatures during burning may adversely affect recruitment dynamics of certain non-spinifex species, such as many members of the Fabaceae and Malvaceae, that often require heat to cue seeds to germinate (Paula and Pausas, 2008; Page, 2009).
Although future changes to atmospheric CO2 and precipitation regimes under anthropogenic climate change (ACC) are predicted strongly to affect all aspects of plant biology (Parmesan and Hanley, 2015), it is unclear how Triodia grasslands will respond to ACC. It has been forecast that inland Australia will experience increasing dryness, which in turn will diminish fire activity as fire occurrence in inland regions is dependent on fuel availability (Bradstock, 2010; Gibson et al., 2014). This could mean that Triodia mast events also become less frequent, which in turn would mean fewer opportunities for soil seedbanks to be ‘topped up’. Such disruptions to seedbank accrual processes could affect the ways populations regenerate after droughts and fires. Even so, before major generalizations are made, further experimental research is required to determine (1) how complex interactions between predicted changes in temperature, precipitation and CO2 could affect the reproductive phenology of Triodia, and (2) how such effects might interact with projected shifts in fire frequency and dryness to influence the regeneration dynamics of obligate-seeding populations.
In summary, rainfall-triggered masting in the obligate-seeding arid T. pungens buffers populations against the danger of fire-driven extirpation by naturally confining masting and concomitant seedbank pulsing to periods of high fuel loads and increased ecosystem flammability. Fire managers should therefore be cautious about conducting burns during inter-mast periods, as seedbank densities are likely to be low during such times, and post-fire regeneration potentials greatly reduced. In addition to seeding heavily after high rainfall periods, T. pungens populations do flower during lower rainfall periods, although such flowering events typically have low seed set and contribute less to viable seedbank densities. These inter-mast flowerings may help to maintain the persistence of seedbanks despite high ambient levels of granivory, and this could be important for the arid zone seeding form of T. pungens to tolerate droughts or fires during inter-mast periods. Overall, this study highlights the importance of marrying phenological, seedbank and recruitment-related field data for unravelling the intricacies of regeneration syndromes of obligate-seeding plants in fire-prone habitats.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: mean T. pungens inflorescence production/hummock at Deep Well compared with Ooraminna site between 2012 and 2016. Figure S2: mean monthly maximum and minimum ambient air temperatures and mean monthly maximum soil temperatures measured by thermocouples at the Deep Well site at the soil surface and at 1 cm depth from burnt and unburnt T. pungens hummocks from November 2012 to November 2013. Table S1: selected results of multiple comparison tests of mean seedbank densities between different sampling times from the ‘seedbank’ GLM. Table S2: small sample size corrected Akaike information criterion (AICc) scores for competing models from the ‘fire experiment’ analysis. Table S3: results of multiple comparison tests of mean seedling recruitment densities between successive sampling times from the ‘fire experiment’ GLMM.
Supplementary Material
ACKNOWLEDGEMENTS
Jan Hayes from Deep Well station is thanked for allowing fieldwork to occur at Deep Well station. Dorsey Debney is thanked for assisting with fire control and conducting field work at Deep Well. Arunrung Donkrathok, Minu Raj and Anstee Nichols are thanked for their assistance with field data collection. Alain Zuur from Highland Statistics analysed the data set from the fire experiment. Grant Allan from the Parks and Wildlife Commission of the Northern Territory provided fire data from the Finke bioregion. Scott Pulleybank provided access to facilities at the Alice Springs Desert Park for seedbank processing. Jeremy Bruhl and Wal Whalley are thanked for providing access to facilities to allow seedbank processing in the Botany department at the University of New England. Robert Whelan, Peter Clarke and Bob Wright provided important feedback on early drafts of the manuscript.
LITERATURE CITED
- Allan GE, Southgate R. 2002. Fire regimes in the spinifex grasslands of Australia. In: Bradstock RA, Williams JE, Gill AM, eds. Flammable Australia: fire regimes and biodiversity of a continent. Cambridge: Cambridge University Press, 145–176. [Google Scholar]
- Andersen AN. 1991. Seed harvesting by ants in Australia. In: Huxley CR, Cutler DF, eds. Ant–plant interactions. Oxford: Oxford University Press, 493–503. [Google Scholar]
- Anderson BM, Thiele KR, Barrett MD. 2017. A revision of the Triodia basedowii species complex and close relatives (Poaceae: Chloridoideae). Australian Systematic Botany30: 117–229. [Google Scholar]
- Andrews FW. 1883. Notes on the Night Parrot. Transactions and Proceedings of the Royal Society of South Australia 6: 29–30. [Google Scholar]
- Armstrong G. 2008. Triodia caelestialis (Triodieae: Chloridoideae: Poaceae), a new species from the central Kimberley, Western Australia. Journal of the Royal Society of Western Australia 91: 313–317. [Google Scholar]
- Australian Government Bureau of Meteorology 2017. Daily rainfall totals from Alice Springs Airport (2012–2017) and Deep Well station (1966–2016) Available at http://www.bom.gov.au/jsp/ncc/cdio/weather [accessed 5 January 2017].
- Barbera GG, Navarro-Cano JA, Castillo VM. 2006. Seedling recruitment in a semi-arid steppe: the role of microsite and post-dispersal predation. Journal of Arid Environments 67: 701–704. [Google Scholar]
- Barrett RL, Barrett MD. 2015. Twenty-seven new species of vascular plants from Western Australia. Nuytsia 26: 21–87. [Google Scholar]
- Bastin G, Allan G. 2012. After the smoke has cleared: 2011 fire in central Australia. Range Management Newsletter NO. 12/2 Australian Rangeland Society, Australia, 3–6. [Google Scholar]
- Benvenuti S. 2007. Natural weed seed burial: effect of soil texture, rain and seed characteristics. Seed Science Research 17: 211–219. [Google Scholar]
- Bogusiak A, Rice B, Westoby M. 1990. Seedling emergence of hummock grasses in relation to the effects of fire. Australian Rangeland Journal 12: 25–28. [Google Scholar]
- Bolton BL, Latz PK. 1978. The Western Hare-wallaby, Lagorchestes hirsutus (Gould) (Macropidae), in the Tanami Desert. Australian Wildlife Research 5: 285–93. [Google Scholar]
- Bowman D, Boggs GS, Prior LD. 2008. Fire maintains an Acacia aneura shrubland–Triodia grassland mosaic in central Australia. Journal of Arid Environments 72: 34–47. [Google Scholar]
- Bradstock RA, Auld TD, Ellis ME, Cohn JS. 1992. Soil temperatures during bushfires in semi-arid, mallee shrublands. Australian Journal of Ecology 17, 433–440. [Google Scholar]
- Bradstock RA. 2010. A biogeographic model of fire regimes in Australia: current and future implications. Global Ecology and Biogeography 19: 145–158. [Google Scholar]
- Burbidge NT. 1943. Ecological succession observed during regeneration of Triodia pungens R. Br. after burning. Journal of the Royal Society of Western Australia 28: 149–156. [Google Scholar]
- Burns KC. 2012. Masting in a temperate tree: evidence for environmental prediction?Austral Ecology 37: 175–182. [Google Scholar]
- Davies SJJF, Kenny SA. 2013. The ages and fecundity of some arid-zone plants in Western Australia. Rangeland Journal 35: 455–468. [Google Scholar]
- Downs CJ, Vander Wall SB. 2009. High relative humidity increases pilfering success of yellow pine chipmunks. Journal of Mammalogy 90: 796–802. [Google Scholar]
- Duguid A, Brock C, Gabrys K. 2008. A review of fire management on central Australian conservation reserves: towards best practice. In: Edwards GP, Allan GE, eds. Desert fire: fire and regional land management in the arid landscapes of Australia. Alice Springs: Desert Knowledge Cooperative Research Centre, 209–308. [Google Scholar]
- Erickson TE, Shackelford N, Dixon KW, Turner SR, Merritt DJ. 2016. Overcoming physiological dormancy in seeds of Triodia (Poaceae) to improve restoration in the arid zone. Restoration Ecology 24: 64–74. [Google Scholar]
- Fournier DA, Skaug HJ, Ancheta J et al. 2012. AD model builder: using automatic differenetiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods and Software 27: 233–249. [Google Scholar]
- Gamage HK, Memmott P, Firn J, Schmidt S. 2014. Harvesting as an alternative to burning for managing spinifex grasslands in Australia. Advances in Ecology 2014: 1–11. [Google Scholar]
- Gibson RK, Bradstock RA, Penman TD, Keith DA, Driscoll DA. 2014. Changing dominance of key plant species across a Mediterranean climate region: implications for fuel types and future fire regimes. Plant Ecology 215: 83–95. [Google Scholar]
- Griffin GF, Price NF, Portlock HF. 1983. Wildfires in the central Australian Rangelands, 1970–1980. Journal of Environmental Management 17: 311–323. [Google Scholar]
- Haase P, Francisco PI, Incoll LD. 1995. Seed production and dispersal in the semi-arid tussock grass Stipa tenacissima L. during masting. Journal of Arid Environments 31: 55–65. [Google Scholar]
- Heelemann S, Proches S, Rebelo AG, van Wilgen BW, Porembski S, Cowling RM. 2008. Fire season effects on the recruitment of non-sprouting serotinous Proteaceae in the eastern (bimodal rainfall) fynbos biome, South Africa. Austral Ecology 33: 119–127. [Google Scholar]
- Herrera CM, Jordano P, Guitian J, Traveset A. 1998. Annual variability in seed production by woody plants and the masting concept: reassessment of principles to pollination and seed dispersal. American Naturalist 152: 576–594. [DOI] [PubMed] [Google Scholar]
- Hewitt A, Holford P, Renshaw A, Stone G, Morris EC. 2015. Seed size and the regeneration niches of one rare (Melaleuca deanei) and three common (Melaleuca styphelioides, Melaleuca thymifolia and Melaleuca nodosa) Melaleuca (Myrtaceae) species of the Sydney region. Austral Ecology 40: 661–671. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometric Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Jacobs SWL. 1973. Ecological studies on the genera Triodia R. Br. and Plectrachne Henr. in Australia. PhD thesis, University of Sydney, Sydney. [Google Scholar]
- Jacobs SWL. 1982. Relationsips, distribution and evolution of Triodia and Plectrachne (Gramineae). In: Barker W, Greenslade J, eds. Evolution of the flora and fauna of arid Australia. Adelaide, South Australia: Peacock, 287–290. [Google Scholar]
- Jacobs SWL. 1984. Spinifex. In: Cogger HG, Cameron EE, eds. Arid Australia. Chipping Norton, NSW: Surrey Beatty & Sons Pty. Ltd, 131–142. [Google Scholar]
- Janzen DH. 1971. Seed predation by animals. Annual Review of Ecology and Systematics 2: 465–492. [Google Scholar]
- Janzen DH. 1976. Why bamboos wait so long to flower. Annual Review of Ecological Systematics 7: 347–391. [Google Scholar]
- Keeley JE, Bond W. 1999. Mast flowering and semelparity in bamboos: the bamboo fire cycle hypothesis. American Naturalist 154: 383–391. [DOI] [PubMed] [Google Scholar]
- Kelly D, Hart DE, Allen RB. 2001. Evaluating the wind pollination benefits of mast seeding. Ecology 82: 117–126. [Google Scholar]
- Latz PK. 2007. The flaming desert: arid Australia – a fire shaped landscape. Alice Springs: NT Print Management. [Google Scholar]
- Lazarides M. 1997. A revision of Triodia including Plectrachne (Poaceae, Eragrostideae, Triodiinae). Australian Systematic Botany 10: 381–489. [Google Scholar]
- McArthur AG. 1972. Fire control in the arid and semi-arid lands of Australia. In: Hall N, ed. The use of trees and shrubs in the dry counrty of Australia. Canberra: Australian Government Publishing Service, 488–516. [Google Scholar]
- Morton S. 1985. Granivory in arid regions: comparison of Australia with North and South America. Ecology 66: 1859–1866. [Google Scholar]
- Murray BR, Dickman CR. 1994. Food preferences and seed selection in two species of Australian desert rodents. Wildlife Research 21: 647–655. [Google Scholar]
- Nicholas AMM, Franklin DC, Bowman D. 2009. Coexistence of shrubs and grass in a semi-arid landscape: a case study of mulga (Acacia aneura, Mimosaceae) shrublands embedded in fire-prone spinifex (Triodia pungens, Poaceae) hummock grasslands. Australian Journal of Botany 57: 396–405. [Google Scholar]
- Nield AP, Enright NJ, Ladd PG. 2016. Fire-stimulated reproduction in the resprouting, non-serotinous conifer Podocarpus drouynianus (Podocarpaceae): the impact of a changing fire regime. Population Ecology 58: 179–187. [Google Scholar]
- Noble JC. 1997. The delicate and noxious scrub: CSIRO studies on native tree and shrub proliferation in the semi-arid woodlands of eastern Australia. Lyneham, Australian Capital Territory: CSIRO. [Google Scholar]
- North Australia and Rangelands Fire Information 2017. Fire history records for Finke Bioregion 1995–2017 Available at www.firenorth.org.au [accessed 1 April 2017].
- O’Dowd DJ, Gill AM. 1984. Predator satiation and site alteration following fire: mass reproduction of alpine ash (Eucalyptus delegatensis) in southeastern Australia. Ecology 65: 1052–1066. [Google Scholar]
- Page M. 2009. Using heat and smoke treatments to simulate the effects of fire on soil seed banks in four Australian vegetation communities. Proceedings of the Royal Society of Queensland 115: 1–9. [Google Scholar]
- Parks and Wildlife Service of the Northern Territory 2011. Finke Gorge National Park joint management plan. Alice Springs, Australia: Department of Natural Resources, Environment, the Arts, and Sport, Northern Territory Government. [Google Scholar]
- Parmesan C, Hanley ME. 2015. Plants and climate change: complexities and surprises. Annals of Botany 116: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S, Pausas JG. 2008. Burning seeds: germinative responses to heat treatments in relation to resprouting ability. Journal of Ecology 96: 543–552. [Google Scholar]
- Peters VS, Macdonald ES, Dale MRT. 2005. The interaction between masting and fire is key to white spruce regeneration. Ecology 86: 1744–1750. [Google Scholar]
- Pitts B, Matthews D. 2000. Biophysical mapping in parks: a park management manual for central Australia: using GIS and survey data for management support in National Parks. Alice Springs, Australia: Parks and Wildlife Commission of the Northern Territory. [Google Scholar]
- R Core Team 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rice BL, Westoby M, Griffin GF, Friedel MH. 1994. Effects of supplementary nutrients on hummock grasses. Australian Journal of Botany 42: 687–703. [Google Scholar]
- Salisbury E. 1942. The reproductive capacity of plants. London: Bell. [Google Scholar]
- Suppiah R. 1993. ENSO phenomenon and 30–50 day variability in the Australian summer monsoon rainfall. International Journal of Climatology 13: 837–851. [Google Scholar]
- Thomas PB, Morris EC, Auld TD, Haigh AM. 2010. The interaction of temperature, water availability and fire cues regulates seed germination in a fire-prone landscape. Oecologia 162: 293–302. [DOI] [PubMed] [Google Scholar]
- Wells GB, Davidson PJ, Dixon KW, Adkins SW. 2000. Defining seed quality of Australian arid zone hummock grasses (Triodia and Plectrachne spp.). In: Asher CJ, Bell LC, eds. Proceedings of the 3rd Australian Workshop on Native Seed Biology for Revegetation. Pinjarra Hills, Queensland: Australian Centre for Minesite Rehabilitation Research, 59–83. [Google Scholar]
- Wells K, Bagchi R. 2005. Eat in or take away – seed predation and removal by rats (Muridae) during a fruiting event in a Dipterocarp rainforest. Raffles Bulletin of Zoology 53: 281–286. [Google Scholar]
- Westoby M, Rice B, Griffin GF, Friedel MH. 1988. The soil seed bank of Triodia basedowii in relation to time since fire. Australian Journal of Ecology 13: 161–169. [Google Scholar]
- Whelan RJ. 1995. The ecology of fire. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Williamson GB, Ickes K. 2002. Mast fruiting and ENSO cycles – does the cue betray a cause?Oikos 97: 459–461. [Google Scholar]
- Wright BR, Clarke PJ. 2008. Relationships between soil temperatures and properties of fire in feathertop spinifex (Triodia schinzii (Henrard) Lazarides) sandridge desert in central Australia. Rangeland Journal 30: 317–325. [Google Scholar]
- Wright BR, Fensham RJ. 2016. Relationships between fire severity and recruitment in arid grassland dominated by the obligate-seeding soft spinifex (Triodia pungens). International Journal of Wildland Fire 25: 1264–1272. [Google Scholar]
- Wright BR, Zuur AF, Chan GCK. 2014. Proximate causes and possible adaptive functions of mast seeding and barren flower shows in spinifex grasses (Triodia spp.) in arid regions of Australia. Rangeland Journal 36: 297–308. [Google Scholar]
- Yi XF, Wang ZY, Zhang HM, Zhang ZB. 2016. Weak olfaction increases scatter-hoarding by Siberian chipmunks: implication in shaping plant–animal interactions. Oikos 125: 1712–1718. [Google Scholar]
- Zuur AF, Leno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14. [Google Scholar]
- Zhang H, Wang Y, Zhang Z. 2009. Domestic goat grazing disturbance enhances tree seed removal and caching by small rodents in a warm-temperate deciduous forest in China. Wildlife Research 36: 610–616. [Google Scholar]
- Zwolakl R, Bogdziewicz M, Wrobel A, Crone EE. 2016. Advantages of masting in European beech: timing of granivore satiation and benefits of seed caching support the predator dispersal hypothesis. Oecologia 180: 749–758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.