See the article by Olmez et al on pages 192–202.
Patients with glioblastoma (GBM) are uniformly treated with chemoradiation, but recurrence is inevitable in most cases. Advances in the molecular classification of GBMs have shown that 3 major subtypes, termed proneural (PN), classical (CL), and mesenchymal (MES), are consistently seen.1 The PN and MES subtypes show not only contrasting molecular signatures, but also differential biological and clinical characteristics. For example, in single-cell sequencing studies, survival rates in patients with tumors that had a higher proportion of cells with a mixed signature (PN/MES/CL) were poorer than those with tumors that consisted of predominantly PN cells.2 Recent clonal studies using primary GBM cells implicate a MES signature with multitherapy resistance.3 Notably, a switch to the MES subtype upon recurrence is associated with worse outcome in GBM.4 Therefore, therapies directed against or preventing the transition to a MES subtype could be beneficial to GBM patients. In a study published in this issue of Neuro-Oncology, Purow and colleagues identify a novel therapeutic target for the MES subtype of GBM.5 They show that the inhibition of diacylglycerol kinase alpha (DGKα) with a drug, ritanserin, a serotonin receptor inhibitor, or RNA interference specifically kills the MES subtype of glioma stemlike cells (GSCs) in vitro and in vivo. Moreover, they uncover a previously unknown molecular axis (DGKα–GGTase I–NF-κB) to be operational in MES GSCs.
DGKα is one of the 10 DGK family members that convert diacylglycerol (DAG) to phosphatidic acid (PA), and both DAG and PA are critical lipid second messengers in the plasma membrane.6 DGKα has been shown to play essential functions in the regulation of T-cell immunological response.7 In cancer, a host of molecular pathways are altered by DGKα, including hypoxia-inducible factor 1α, mammalian target of rapamycin, c-Met, and vascular endothelial growth factor.8 Additional known functions of DGKα include membrane ruffling, cell migration, proliferation, and angiogenesis.8 Previous studies by Purow and colleagues have shown that DGKα is a key oncogenic node in GBM and melanoma and is under regulation by miR-297.9,10 Furthermore, ritanserin was found to be a specific inhibitor of DGKα. In this study, the authors examined the expression of DGKα transcript in the database of The Cancer Genome Atlas and found higher expression of this gene in the MES subtype compared with all other subtypes. Next, they examined the effect of ritanserin on the proliferation and self-renewal of GSCs in vitro and found that this drug induces partial death via apoptosis only in MES GSCs. The inhibition was specific as confirmed by apoptosis induced using 2 independent small interfering RNAs against DGKα only in MES GSCs. Furthermore, another serotonin receptor inhibitor that lacks DGKα inhibition (ketanserin) was ineffective in inducing apoptosis in MES GSCs, and a serotonin agonist did not rescue the cytotoxicity induced by ritanserin. Importantly in an orthotopic model, oral ritanserin administration increased survival of mice bearing MES GSCs, but no effects were seen in a non-MES GSC line, pointing to the specific effects of ritanserin against the MES subtype of GBM. Given that MES cells are known to be radioresistant and that nuclear factor-kappaB (NF-κB) causes activation of PN to MES transition (PMT),11 the authors examined the effects of ritanserin on GSCs undergoing radiation-induced PMT as well as carcinoma cells undergoing an epithelial to MES transition and found strong reduction in viability with this agent.
To delineate the mechanisms underlying the specificity of ritanserin to the MES subtype, the authors hypothesized that DGKα affects NF-κB via RhoA, given a prior regulatory role of DGKα on RhoA, and NF-κB in the MES phenotype. Not only did silencing DGKα cause reduction of RhoA activation via geranylgeranylation of Rap1A, an upstream regulator of RhoA, the effects were dependent on geranylgeranyltransferase type 1 (GGTase I) enzyme. In a series of elegant experiments, it was demonstrated that DGKα regulation of GGTase I was directly upstream and that GGTase I inhibition affects not only geranylgeranylation of Rap1a, but also NF-κB transcriptional function.
Given the radioresistant functions of NF-κB in MES GBMs, combined treatment of ritanserin and radiation caused synergistic growth inhibition in MES GSCs. Mechanistically, DNA damage response pathway activation was attenuated by treatment with ritanserin. Interestingly, the authors found that a 5-day treatment with ritanserin caused not only growth inhibition of GSCs, but a switch from a MES to PN subtype as evidenced by induction of PN markers. The gene product of platelet derived growth factor receptor alpha amplification, a hallmark of PN GBMs, showed marked activation in ritanserin-treated cells, and treatment with a pan receptor tyrosine kinase inhibitor imatinib caused synergistic reduction of cell growth.
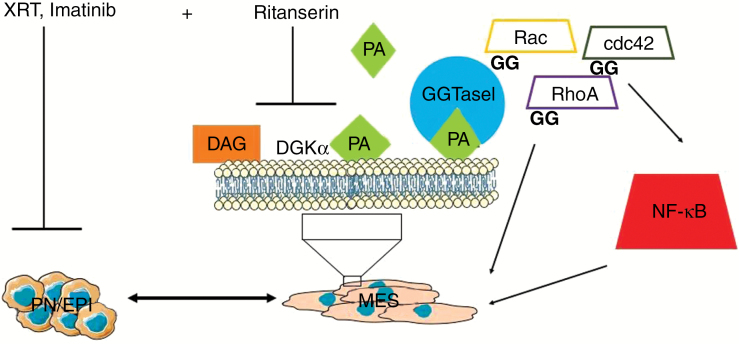
Although this is not the first study linking DGKα to oncogenesis, the authors have elucidated the mechanisms by which DGKα promotes survival in the MES subtype of GBMs (summarized in Fig. 1). The authors elegantly demonstrate that PA, the DGKα product, binds to GGTase I and cooperates in the geranylgeranylation of proteins after transcription of the Rho family of GTPases, which in turn activate NF-κB. Inhibition of DGKα or GGTase I specifically alters the growth and apoptosis in MES GSCs via activation of the NF-κB cascade. Given that ritanserin is an FDA-approved agent, the regulatory route to GBM clinical trials is less complex when repurposing drugs. However, some biological and clinical issues need to be addressed prior to planning such trials. First, the side effects of ritanserin on serotonin receptor need to be taken into consideration, as these can be long term and include mood disorders. Second, DGKα promotes T-cell maturation, and therefore inhibition of this molecule can affect immune functions. Examination of the effects of ritanserin in genetically engineered mouse models should clarify this. Finally, because GBMs are not clinically classified by transcriptional signatures, choosing the right patient populations (in this case MES) who are likely to benefit in a trial can be challenging. Nonetheless, with the high failure rates of newer drugs in GBM, ritanserin offers hope for GBM patients, and clinical trials with this agent are warranted.
Fig. 1.
Pathways altered by Ritansein. EPI, epithelial.
Funding
This research was supported by the University Cancer Foundation via the Institutional Research Grant program and Start up funds at the University of Texas MD Anderson Cancer Center (to KB).
References
- 1. Wang QH, Hu B, Hu X et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel AP, Tirosh I, Trombetta JJ et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Segerman A, Niklasson M, Haglund C et al. Clonal variation in drug and radiation response among glioma-initiating cells is linked to proneural-mesenchymal transition. Cell Rep. 2016;17(11):2994–3009. [DOI] [PubMed] [Google Scholar]
- 4. Wang JG, Cazzato E, Ladewig E et al. Clonal evolution of glioblastoma under therapy. Nature Genet. 2016;48(7):768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olmez I, Love S, Xiao A,. et al. Targeting the mesenchymal subtype in glioblastoma and other cancers via inhibition of diacylglycerol kinase alpha. NeuroOncol 2018;20(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mérida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409(1):1–18. [DOI] [PubMed] [Google Scholar]
- 7. Krishna S, Zhong X. Role of diacylglycerol kinases in T cell development and function. Crit Rev Immunol. 2013;33(2):97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Purow B. Molecular pathways: targeting diacylglycerol kinase alpha in cancer. Clin Cancer Res. 2015;21(22):5008–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kefas B, Floyd DH, Comeau L et al. A miR-297/hypoxia/DGK-α axis regulating glioblastoma survival. Neuro Oncol. 2013;15(12):1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dominguez CL, Floyd DH, Xiao A et al. Diacylglycerol kinase α is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov. 2013;3(7):782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhat KPL, Balasubramaniyan V, Vaillant B et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]