Abstract
Background and Aims
Comparative floral ontogeny represents a valuable tool to understand angiosperm evolution. Such an approach may elucidate subtle changes in development that discretely modify floral architecture and underlie reproductive lability in groups with superficial homogeneous morphology. This study presents a comparative survey of floral development in Eugenia (Myrtaceae), one of the largest genera of angiosperms, and shows how previously undocumented ontogenetic trends help to explain the evolution of its megadiversity in contrast to its apparent flower uniformity.
Methods
Using scanning electron microscopy, selected steps of the floral ontogeny of a model species (Eugenia punicifolia) are described and compared with 20 further species representing all ten major clades in the Eugenia phylogenetic tree. Additional floral trait data are contrasted for correlation analysis and character reconstructions performed against the Myrtaceae phylogenetic tree.
Key results
Eugenia flowers show similar organ arrangement patterns: radially symmetrical, (most commonly) tetramerous flowers with variable numbers of stamens and ovules. Despite a similar general organization, heterochrony is evident from size differences between tissues and structures at similar developmental stages. These differences underlie variable levels of investment in protection, subtle modifications to symmetry, herkogamic effects and independent androecium and gynoecium variation, producing a wide spectrum of floral display and contributing to fluctuations in fitness. During Eugenia’s bud development, the hypanthium (as defined here) is completely covered by stamen primordia, unusual in other Myrtaceae. This is the likely plesiomorphic state for Myrteae and may have represented a key evolutionary novelty in the tribe.
Conclusions
Floral evolution in Eugenia depends on heterochronic patterns rather than changes in complexity to promote flexibility in floral strategies. The successful early establishment of Myrteae, previously mainly linked to the key innovation of fleshy fruit, may also have benefitted from changes in flower structure.
Keywords: Androecium, floral ontogeny, gynoecium, hypanthium, Myrteae, perianth
INTRODUCTION
Flower organs (i.e. calyx, corolla, androecium and gynoecium) and associated tissues are responsible for two main functions in the angiosperm life cycle. The primary function of these organs is forming male and female gametes and their connection for sexual reproduction. The secondary function is to enhance and protect this process, as well as balancing in- and out-breeding (Endress, 1994). In this way, evolutionary changes in floral traits affect reproductive success and promote fitness fluctuations in individual lineages (e.g. de Jager and Ellis, 2014; Antiqueira and Romero, 2016) and comparative floral developmental studies are a useful tool to comprehend evolution in angiosperms (e.g. Endress, 2002, 2006; Rudall and Bateman, 2004). By comparing floral ontogeny in distinct but closely related taxa, changes in rates of organ initiation and development (i.e. heterochronies) are documented, explaining differences in flower architecture (Endress, 1994; Tucker, 2003; Prenner, 2004; Prenner et al., 2008). These alterations in developmental rhythms promote differential investments in organs implicated in adaptive features for plant reproduction (e.g. changes in breeding system; see review in Li and Johnston, 2000).
Such comparative surveys of floral ontogeny are often hampered by a lack of systematic understanding and the difficulty of finding suitable material for analysis of the group of interest (i.e. spirit collections of floral buds in different developmental stages). For this reason, studies on large, tropical and/or taxonomically complicated taxa are rare in comparison with relatively species-poor (e.g. Endress, 2003) and/or temperate plant groups (e.g. Webster and Gilmartin, 2003). Systematic complexity in large genera is often a result of morphological homogeneity (e.g. Briggs and Johnson, 1979). The absence of comparative ontogenetic surveys in these groups means that remarkable but discrete patterns that are key to the explanation of evolutionary trends and diversification patterns are overlooked.
The tropical Myrtaceae genus Eugenia is an example of this deficit. Eugenia, with around 1000 species (WCSP, 2017), is one of the largest angiosperm genera, the second most diverse tree genus (Beech et al., 2017) and listed among the genera with highest diversity of species in threatened Neotropical biomes (Mori et al., 1983; Oliveira-Filho and Fontes, 2000). Being so huge and ecologically important, it is surprising that there is so little information available on the evolution of its floral structure. Flowers of Eugenia are known to display a series of Myrtaceae features: they are epigynous, radially symmetrical and polyandrous (Fig. 1; Ronse De Craene and Smets, 1991; Belsham and Orlovich, 2002, 2003; Vasconcelos et al., 2017a). However, they differ from other Myrtaceae flowers in presenting straight stamens in the bud, a character shared by only a few other related genera within tribe Myrteae (Vasconcelos et al., 2015). Certain histogenetic aspects have been described for a few species of Eugenia in isolated studies (Schmid, 1972; Pimentel et al., 2014; Martos et al., 2017); these focus on the highly similar vascular structure and a lack of infrageneric variation, reinforcing the homogeneous aspects of the genus’s floral morphology. The absence of information regarding floral evolution in Eugenia is aggravated by sample inaccessibility (due to its usual tree habit and tropical distribution) and, until recently, the absence of a phylogenetic framework (available in Mazine et al., 2014) with which to study an evolutionarily coherent sample.
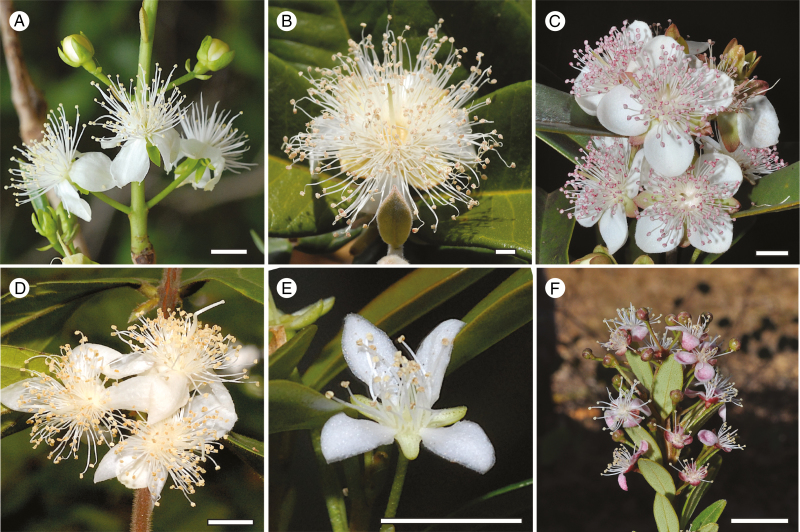
Fig. 1.
Field pictures of flowers in distinct Eugenia clades. (A) E. dichroma (Sect. Speciosae); (B) E. azurensis (Sect. Pseudeugenia); (C) E. involucrata (Sect. Phyllocalyx); (D) E. stipitata (Sect. Pilothecium); (E) E. ligustrina (Sect. Eugenia); (F) E. angustissima (Sect. Racemosae). Scale bars = 5 mm.
In this study, it is hypothesized that large groups with morphologically homogeneous flowers, such as Eugenia, rely on heterochronies to promote lability of reproductive strategies. This hypothesis is tested here by documenting floral ontogeny in a phylogenetically representative sample of Eugenia. Dimensions of organs and tissues at selected stages are compared with observed changes in developmental rates. Developmental differences are discussed in the context of flexibility of functional traits in the flower (e.g. protection and breeding system). Eugenia floral development data also provide understanding of changes in floral structure and their influence on stamen posture in Myrteae, the most species-rich tribe of Myrtaceae.
MATERIALS AND METHODS
Sampling
A complete ontogenetic sequence for Eugenia punicifolia (Eugenia Sect. Umbellatae) is described and used as a base for comparing variation in selected developmental stages between samples from 20 species representing ten consistent clades [i.e. clades that re-occur in independent phylogenetic analysis (Mazine et al., 2014, 2016; Bünger et al., 2016; Vasconcelos et al., 2017b)]. A list of all analysed species and the clades to which they belong is given in Table 1. Eugenia punicifolia represents the most common floral phenotype for the genus (tetramery, bilocular ovaries and multiple ovules) and is a common, widespread shrub in South America. Additional samples for stage-specific comparison of development and correlation between ovule number/stamen number and size were selected to represent both phylogenetic variation and geographical distribution of Eugenia.
Table 1.
Analysed species, vouchers, collection location and selected traits averaged for three flowers per collection
| Section1 | Species | Analysed voucher | Collection locality | Diameter [mm] | Stamen number | Ovule number | Ovary locules |
|---|---|---|---|---|---|---|---|
| Umbellatae | Eugenia punicifolia (Kunth) DC. | J.E.Q. Faria 4051 | Brazil (Distrito Federal) | 2.1 ± 0.1 | 88 ± 3.3 | 23.3 ± 0.5 | 2 |
| Umbellatae | Eugenia citrifolia Poir. | A. Giaretta 1441 | Brazil (Roraima) | 4.36 ± 0.5 | 101 ± 3.4 | 50 ± 2.2 | 2 |
| Umbellatae | Eugenia flavescens DC. | J.E.Q. Faria 4168 | Brazil (Bahia) | 2.47 ± 0.1 | 92 ± 4.5 | 16 ± 1.5 | 2 |
| Umbellatae | Eugenia protenta McVaugh | T. Vasconcelos 350 | Brazil (Amazonas) | 1.88 ± 0.2 | 69 ± 3.3 | 17 ± 0.8 | 2 |
| Clade Jossinia | Eugenia gacognei Montrouz. | T. Vasconcelos 595 | New Caledonia | ||||
| Clade Jossinia | Eugenia paludosa Pancher ex Brongn. & Gris | T. Vasconcelos 646 | New Caledonia | 3.66 ± 0.03 | 267 ± 5.2 | 107.3 ± 14.1 | 2 or 3 |
| Racemosae | Eugenia inversa Sobral | J.E.Q. Faria 4230 | Brazil (Espirito Santo) | 1.19 ± 0.1 | 60 ± 2.1 | 6.4 ± 1.4 | 2 |
| Racemosae | Eugenia angustissima O.Berg | D.F.Lima 490 | Brazil (Goiás) | 1.65 ± 0.1 | 33 ± 3.4 | 7.7 ± 2.1 | 2 |
| Racemosae | Eugenia longiracemosa Kiaersk. | T. Vasconcelos 310 | Brazil (Amazonas) | 2.02 ± 0.1 | 67 ± 4.9 | 21 ± 2.1 | 2 |
| Eugenia | Eugenia uniflora L. | T. Vasconcelos s.n. | RBG Kew (cultivated - originally from Brazil) | 2.06 ± 0.2 | 43 ± 4.2 | 19 ± 4.9 | 2 |
| Eugenia | Eugenia ligustrina (Sw.) Willd. | T. Vasconcelos 570 | Dominican Republic | 1.5 ± 0.5 | 44 ± 6.2 | 7 ± 2.3 | 2 |
| Pilothecium | Eugenia stipitata McVaugh | T. Vasconcelos 677 | Singapore (cultivated - originally from Brazil) | 3.1 ± 0.4 | 149 ± 13.9 | 21 ± 8.5 | 3 or 4 |
| Pilothecium | Eugenia itajurensis Cambess. | J.E.Q. Faria 4250 | Brazil (Espirito Santo) | 6.2 ± 0.3 | 180 ± 8.7 | 18 ± 3.2 | 2 |
| Pilothecium | Eugenia pohliana DC. | J.E.Q. Faria 4184 | Brazil (Bahia) | 3.75 ± 0.05 | 121 ± 9.4 | 5 ± 1.4 | 2 |
| Pseudeugenia | Eugenia azurensis O.Berg | J.E.Q. Faria 4186 | Brazil (Bahia) | 10.44 ± 0.7 | 354 ± 33.2 | 35 ± 6.9 | 2 or 3 |
| Pseudeugenia | Eugenia splendens O.Berg | J.E.Q. Faria 4196 | Brazil (Bahia) | 4.05 ± 0.3 | 149 ± 2.6 | 34.3 ± 4.5 | 2 |
| Hexachlamys | Eugenia myrcianthes Nied. | J.E.Q. Faria 6547 |
Brazil (Brasilia) | 5.4 ± 0.3 | 150 ± 8.3 | 4 ± 0 | 2 |
| Calycorectes | Eugenia acutata Miq. | T. Vasconcelos 506 | Brazil (Distrito Federal) | 5.2 ± 0.2 | 168 ± 14 | 32 ± 8.3 | 2 |
| Phyllocalyx | Eugenia involucrata DC. | T. Vasconcelos 256 | Brazil (Distrito Federal) | 6.02 ± 0.3 | 218 ± 20.1 | 66 ± 3.5 | 2 |
| Speciosae | Eugenia dichroma O.Berg | T. Vasconcelos 466 | Brazil (Espirito Santo) | 3.86 ± 0.1 | 130 ± 5.7 | 34.7 ± 4.5 | 2 |
1Nomenclature follows Mazine et al. (2016), except for clade Jossinia.
Flower samples of Eugenia were collected mainly from their natural environments during field expeditions to South America, the Caribbean and New Caledonia. In a few cases, samples were taken from cultivated collections in botanic gardens. Samples of young inflorescence shoots, flower buds and open flowers were collected and fixed in FAA (formalin, acetic acid and ethanol) or 70 % ethanol in Falcon tubes; field pictures at anthesis were also registered. Herbarium vouchers for all collections are deposited at the Royal Botanic Garden Kew (K) with duplicates in local herbaria from where the collections originate.
Ontogenetic examination
Flower buds in different developmental stages were selected and dissected in 50 or 70 % ethanol to expose structures of interest, then dehydrated through an ethanol series to 100 % ethanol. Critical-point drying was performed using an Autosamdri-815B critical-point dryer (Tousimis Research, Rockville, MD, USA). Dried material was mounted on metal specimen stubs using a carbon stick disc and coated with platinum using a Quorum Q-150-T sputter coater (Quorum Technologies, East Grinstead, UK). Stubs were examined and distinct floral developmental stages were documented using a cold-field emission scanning electron microscope (S-4700-II; Hitachi High Technologies, Tokyo, Japan).
Glossary of terms.
Floral ontogeny interpretation uses very specific terminology and different authors favour similar but not quite identical terms when describing structures, processes and configurations. To avoid confusion and to standardize the terminology used in this study, we provide a brief glossary of frequently used terms and their interpretation in Table 2.
Table 2.
Floral ontogeny terminology used in this study
| Term | Meaning |
|---|---|
| Structures | |
| Floral receptacle | The central part of the flower on which floral parts are inserted (Ronse De Craene, 2010). |
| Hypanthium | Cup-shaped floral base (Endress, 1996). Here used to indicate the tissue between corolla and gynoecium in the centre of the flower in the broad sense (see also Belsham and Orlovich, 2002, 2003) |
| Meristem | Undifferentiated tissue with capacity for morphogenesis (Endress, 1994) |
| Primordium | Early differentiated meristem that will develop into an organ; ‘The first visible stage of an organ’ (Endress, 1994) |
| Processes | |
| Heterochrony | ‘The alteration in developmental timing’ (Endress, 1994); ‘The changes in the duration of events during ontogeny’ (Lord, 1991); ‘Differences in rate of organ development’ (Raff and Wray, 1989). Here used to indicate any variation in developmental rate of organs and tissues between species |
| Morphogenesis | Process of organ formation during development (Wagner, 2009) or origin of form during development (Endress, 1994) |
| Anthesis | ‘The open flower phase’, or, more precisely, ‘the phase of a flower when pollen is presented and/or the stigma is receptive’ (Endress, 1994) |
| Aestivation | The mutual position of perianth organs in a floral bud (or vegetative leaves in a vegetative bud) (Endress, 1994) |
| Herkogamy | Spatial separation of male and female organs of a flower preventing self-pollination (Endress, 1996) |
| Configurations | |
| Polyandry | General term to refer to a flower with a large number of stamens (Endress, 1994) |
| Oligandry | General term to refer to flowers with few stamens (e.g. Ronse De Craene and Smets, 1993) |
| Decussate | Adjective describing the condition in which alternate pairs of organs are at right angles to each other (Beentje, 2012) |
| Haplostemonous | Adjective describing a flower with one whorl of stamens opposite the sepals (Endress, 1994, Ronse De Craene and Smets, 1995) |
| Diplostemonous | Adjective describing a flower with two whorls of stamens, the outer one opposite the sepals (Endress, 1994) |
| Obhaplostemonous | Adjective describing a flower with one whorl of stamens which are situated opposite the petals (Ronse De Craene, 2010) |
Flower measurements and correlation analysis
Additional measurements were taken to analyse correlation and disparity in the number of floral parts as a consequence of changes in developmental patterns. These were: floral receptacle diameter (the base of the flower); total number of stamens; and total number of ovules. All measurements were taken in mature, pre-anthetic buds or recently opened flowers and annotated as an average of observations from at least three buds per sample. Missing data correspond to samples that only presented buds in inadequate stages for reliable measurements (e.g. receptacle diameter was not recorded for samples that did not present open flowers, because the staminal ring appears to continuously expand in later stages of development and anthesis). All resulting measurements are presented in Table 1.
Linear regressions between flower receptacle diameter and total number of stamens and ovules were performed using the lm function in the stats package in R (R Core Team, 2017). This analysis was executed to test correlation between investment in receptacle diameter and formation of male (stamens) and female (ovules) reproductive structures.
Supporting analysis of character reconstruction
Ancestral state reconstruction analysis was conducted to interpret stamen posture and evolution of the combined androecium–hypanthium development in Myrtaceae. The Myrtaceae cladogram presented is based on the phylogenetic hypothesis published by Thornhill et al. (2015); it was used to reconstruct the characters as: (1) stamen primordia forming along the whole hypanthial surface; and (2) stamen primordia forming only on the edges of the hypanthial surface. The tree was trimmed to include only the Myrtoideae subfamily (all Myrtaceae except the monotypic and non-polyandrous Psyloxylon and Heteropyxis). Reconstruction was performed using the function ace in the R package ape (R Core Team, 2017). The character matrix and associated references are available in Supplementary Data S1.
RESULTS
Floral structure in Eugenia
Flowers of Eugenia are variable in size, reaching between 5 and >30 mm in diameter when open. Most analysed flowers of Eugenia share the same general floral ground-plan and formula (Fig. 2). The Eugenia calyx and corolla are tetramerous, with decussate aestivation. Eugenia myrcianthes is exceptional in its pentamery and imbricate quincuncial aestivation (i.e. two external sepals, two internal and one intermediate; Supplementary Data S2, Plate 1). Symmetry is radial to slightly asymmetrical. The androecium is polyandrous, with stamen number varying from ~30 to ~350 (Table 1). Stamens are free throughout flower development. The ovary is inferior, with two (most common phenotype) to three or four locules (Supplementary Data S2, Plate 2). Ovules are attached radially to an axillary placenta positioned at a single point on each locule wall of the ovary septum. Number of ovules per locule varied between 2 and 50 in analysed species. The complete ontogenetic sequence of E. punicifolia is described below.
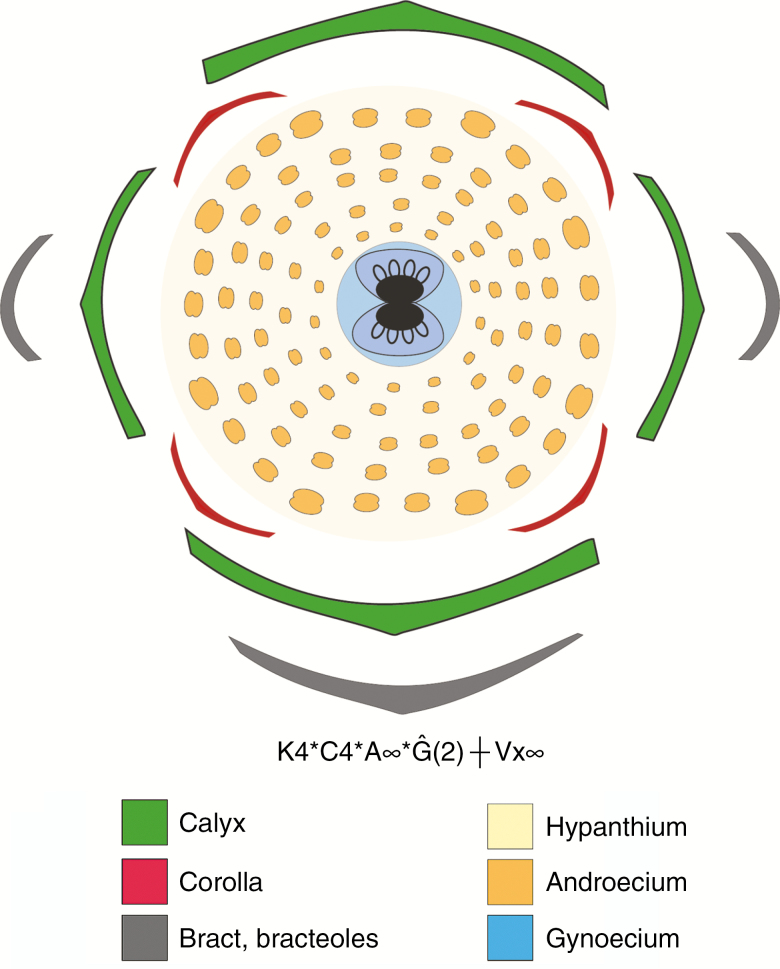
Fig. 2.
Floral diagram of Eugenia punicifolia, showing the most common floral ground-plan and floral formula for the genus (bilocular ovaries). For floral formulae interpretation see Prenner et al. (2010).
Flower development in Eugenia punicifolia
The complete floral ontogenetic sequence of E. punicifolia is divided into five main stages (Stages 1–5) and seven substages (Stages 1a, 1b, 2a, 2b, 3, 4, 5), according to meristematic differentiation, from sepal initiation to anthesis. Stages can be summarized as follows: Stage 1a, calyx initiation; Stage 1b, corolla initiation; Stage 2a, androecium and gynoecium initiation; Stage 2b, hypanthium elongation/expansion; Stage 3, differentiation of ovules and anthers; Stage 4, pre-anthetic bud enlargement and final maturation of sexual organs; Stage 5, anthesis.
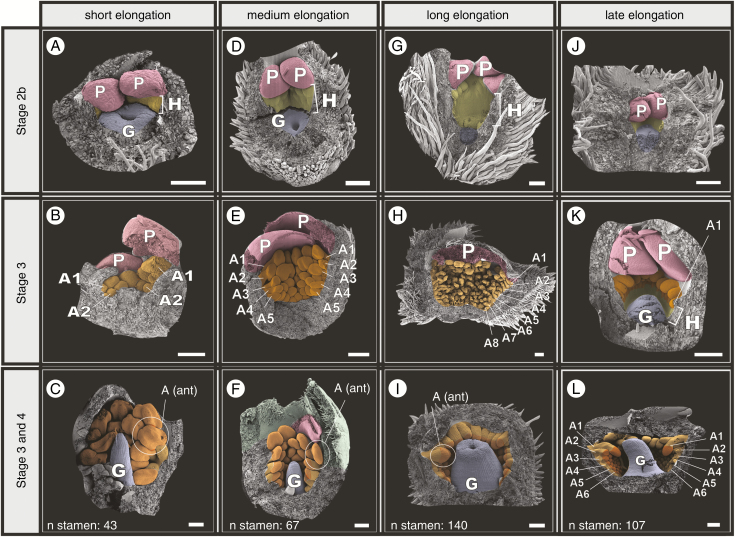
At Stage 1a, the first two sepals initiate almost simultaneously in a median position (labelled S1 and S1* in Fig. 3A) with the abaxial (lower) sepal appearing slightly older than the adaxial one. Shortly afterwards, two sepals form simultaneously in transverse positions, decussate to the first two sepals (S2 in Fig. 3A, B). During early bud elongation, the first pair of sepals overlaps the second (Fig. 3B, C). At this point, the difference in initiation timing between the two sepals from the first pair (S1 and S1* in Fig. 3A) is almost indistinguishable (S1 in Fig. 3B). Single-celled hairs appear on the tips of the sepals at this very early stage. These keep the edges of each pair of sepals tightly closed against each other and act like eyelashes, protecting the young bud during early floral development (arrow in Fig. 3C). Sepals are free throughout flower development.
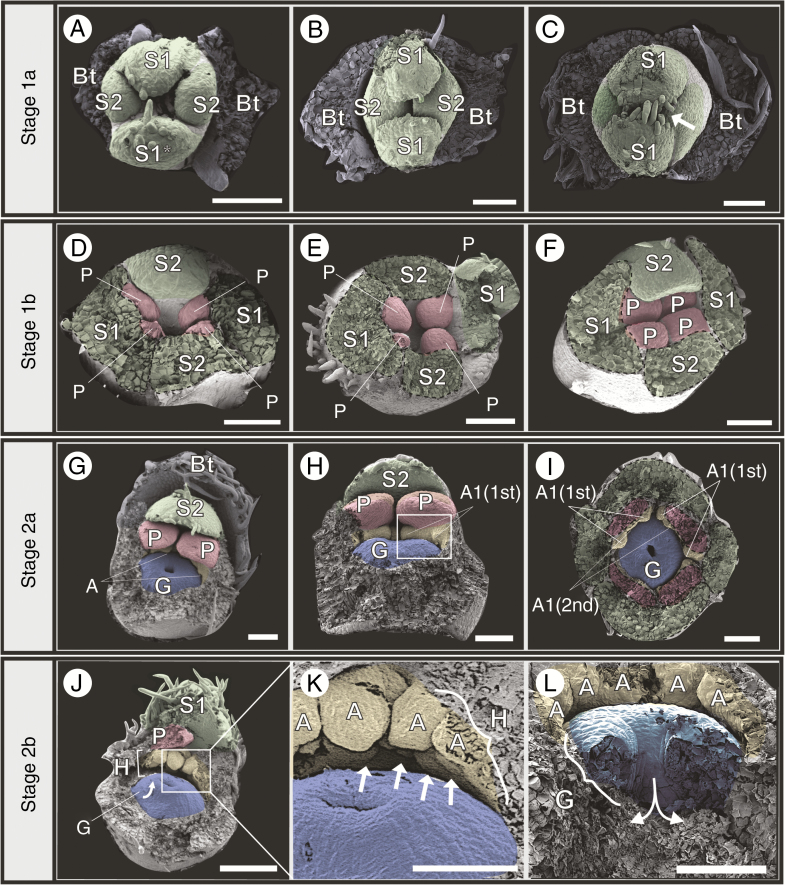
Fig. 3.
Early stages of floral ontogeny in Eugenia punicifolia. Stage 1a: (A) Early sepal development showing transverse bracteoles (removed), and first and second pair of sepals. S1* appears to be slightly older, a discrepancy only noticeable at this stage. (B, C) Early sepal development. Sepals enclose the bud; single-celled hairs develop at the tips of the sepals (arrow in C). Stage 1b: (D) Petal initiation; four petals arising almost simultaneously as bulges alternate with sepals. (E, F) Continuously growing petals eventually meeting in the middle of the bud. Stage 2a: (G, H) Petals overlap; one sepal and one bracteole left to highlight proportion between organs. (H) Lateral view, showing first stamen initiation [A1(1st)] flanking the petals. (I) As (G) and (H), but in frontal view; calyx and corolla removed; first group of stamen primordia prominent. Stage 2b: (J) Proto-style developing upwards (arrow) and initiation of second staminal whorl; stamens of the first whorl similar in size. (K) Detail of (J), showing stamen primordia covering the hypanthium below the first staminal girdle. (L) Same stage as (J), but further dissected and in lateral view; gynoecium depression (ovary) expands downwards while proto-style grows upwards. Bt, bracteole; S, sepals; P, petals; A, androecium; G, gynoecium; H, hypanthium. Bracteoles removed in all. Scale bars: (K) = 5 µm; (A–E, G–I, L) = 100 µm; (F, J) = 250 µm. Colour coding in online version: sepals, green; corolla, red; androecium, yellow; gynoecium, blue.
The corolla is the second whorl to develop, during early floral ontogenetic stages. In Stage 1b, four petals initiate almost simultaneously as bulges in alternate positions to the sepals on the inner slopes of the developing hypanthium (labelled P in Fig. 3D). The four petals enlarge, eventually touch each other in the centre of the bud (Fig. 3E, F) and overlap in the next stages (Fig. 3G–H), providing a cover of four layers of tissue below the calyx on the top of the bud. Because of the nearly simultaneous initiation of the four petals there is no clear pattern of aestivation, even within the same species (Supplementary Data S2, Plate 3). Petals are free throughout flower development.
Stage 2a starts with the initiation of the androecium and gynoecium. The development of the first staminal ring occurs on the hypanthial tissue just underneath each petal (labelled A in Fig. 3G), where two stamen primordia are formed flanking each petal [labelled A1(1st) in Fig. 3H, I]. The first ring continues to develop laterally and after a longer time gap (plastochron), second groups of stamen primordia appear between the first ones [labelled A1(2nd) in Fig. 3I], resulting in a complete first staminal ring. The plastochron between the appearance of the first group of stamen primordia and the appearance of the second group of stamen primordia in the first whorl is noticeable at this stage (Supplementary Data S2, Plate 4); as the flower continues to develop this size distinction almost disappears, so that the dissimilarity in age between stamens is barely visible in later stages (e.g. Fig. 3J, K). The gynoecium originates as a depression that appears on the apical surface of the flower base simultaneously with the appearance of the first androecial primordia (labelled G in Fig. 3G, H).
In Stage 2b, the hypanthium tissue expands (labelled H in Fig. 3J, K). Simultaneously, the androecium continues to develop as centripetal and concentric loosely distributed stamen primordia originating along the inner surface of the hypanthium, covering the whole area below the first staminal ring to the gynoecium (arrows in Fig. 3K; for other species see Figs 6 and 7). During this process, the gynoecium starts to form a proto-style (labelled G in Fig. 3J), whereas the initial depression, now a pore, represents the area that will form the proto-stigma. As the proto-style elongates, two ovary locules are formed below (Fig. 3L).
Fig. 6.
Comparative style development in Eugenia Sect. Umbellatae and other clades. (A) Swollen proto-style in E. punicifolia, contrasted with (B) flat proto-style in same stage of E. angustissima. (C, E) Continuous development of style in E. punicifolia and (E) E. protenta, showing style bending on top of the anthers. (D, F) Continuous development of style of E. angustissima, showing comparatively shorter style than in panels (C) and (E). (G) Pre-anthetic bud in E. protenta, showing long style that folds on top of the anthers, in contrast with (H) E. angustissima, where the style is always shorter than the stamens. (I) Open flowers of E. citrifolia, with highlighted folding mark in the middle of the style, in contrast with (J) open flower of E. angustissima, showing style at roughly the same height as stamens. S, sepal; P, petal; A, androecium; G, gynoecium. Scale bars: (A, B) = 150 µm; (C–F) = 250 µm; (G, H) = 500 µm; (I, J) = 5 mm. Colour coding in online version: sepals, green; corolla, red; androecium, yellow; gynoecium, blue.
Fig. 7.
Comparative hypanthium and androecium development in Eugenia and differential rate of pollen sac maturation (in orange) according to number of stamens per flower. (A, B) Androecium initiation and hypanthium development of E. angustissima, showing two or three loose stamen whorls forming on the expanded hypanthium. (C) Early maturation of pollen sacs in E. uniflora. (D, E) Androecium initiation and hypanthium development of E. dichroma, showing five loose stamen whorls forming on the hypanthium. (F) Pollen sacs in maturation process in E. longiracemosa. (G, H) Androecium initiation and hypanthium development of E. azurensis, showing eight loose stamen whorls forming on the hypanthium. (I) Late pollen sac maturation in E. stipitata. (J, K) Androecium initiation and hypanthium development of (J) E. gacognei and (K) E. paludosa, showing a gap between the development of the first and five following stamen whorls. (L) Pollen sac maturation in E. paludosa, showing anthers from the first staminal whorl in a more advanced state of development. A(ant), anther; G, gynoecium; P, petal; H, hypanthium. Scale bars: (A–D, F, G, I–L) = 100 µm; (E, H) = 200 µm. Colour coding in online version: sepals, green; corolla, red; androecium, yellow; gynoecium, blue.
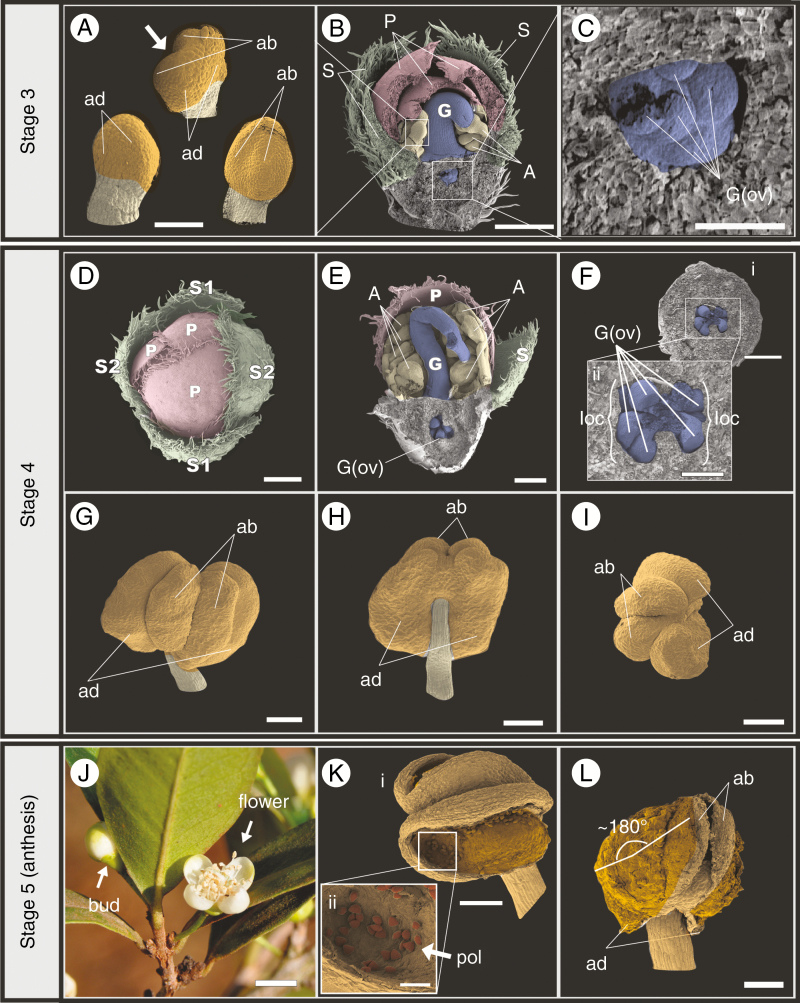
After all organs have been formed, the floral bud continually enlarges towards anthesis (Stages 3 and 4). In Stage 3, each stamen differentiates into a proximal filament and a distal anther. The tetrasporangiate anthers start to differentiate as sagittate structures (Fig. 4A) and a longitudinal depression appears in the middle of the abaxial side when the pollen sacs start to form (arrow in Fig. 4A). During this process, the style reaches the inner surface of the corolla and bends sidewards on top of the developing anthers (labelled G in Fig. 4B, E). Ovules start to develop at this stage, as protuberances on the axial placentas in both locules [labelled G(ov) in Fig. 4C].
Fig. 4.
Stages 3–5 of floral ontogeny in Eugenia punicifolia. (A–C) Initiation of anthers and ovules. Note style bending in panel (B). (D) Exposure of the corolla prior to anthesis. (E) Longitudinal section of pre-anthetic bud showing maturation of anthers and ovules. Note that the style is sharply bent downwards. (F) Detail of ovule maturation in both ovary locules. (G–I) Mature pollen sacs in pre-anthetic anther. (J) Bud with exposed corolla (arrow) and recently opened flower of E. punicifolia (field image). (K, L) Thecae opening during anthesis of Eugenia dichroma; thecae are reflexed 180° to expose pollen grains. S, sepals; P, petals; A, androecium; G, gynoecium; G(ov), ovules; A(ant), anther; loc, locule; ad, adaxial pollen sac; ab, abaxial pollen sac; pol, pollen grain. Scale bars: (Kii) = 50 µm; (A, C, G, H, I, Ki, L) = 100 µm; (Fii) = 200 µm; (B, D, E, Fi) = 500 µm; (J) = 5 mm. Colour coding in online version: sepals, green; corolla, red; androecium, yellow; gynoecium, blue.
In Stage 4, the sexual organs (androecium and gynoecium) finish pre-anthetic development, producing mature ovules and dorsifixed pollen sacs. Mature ovules are organized in loose series on the placenta [labelled G(ov) in Fig. 4E, F]. Counts in mature flowers show distinct ovule numbers per locule, apparently reflecting a short plastochron between each locule. The abaxial pollen sacs of each anther (labelled ab in Fig. 4G–I) are slightly smaller than the adaxial pollen sacs (labelled ad in Fig. 4G–I). The stigma is thin and simple, with single-celled papillae. During Stage 4, sexual organs mature faster than the perianth elongates (calyx and corolla). As a consequence, the corolla, which until this point has remained covered by the calyx lobes, is pushed upwards and exposed (Fig. 4D). This exposure of the corolla is the last step before anthesis. Also at this point, the sepal pairs (labelled S1 and S2) approach each other in size, producing four sepals of similar proportions. This process occurs either by developmental acceleration of S2, slowing of S1 or both (S1 and S2 in Fig. 4D).
Stage 5 represents anthesis. During this process (Fig. 4J) the perianth opens, the style straightens and the anthers are exposed. Tissue between each pollen sac (anther locules) opens longitudinally and laterorsely (Fig. 4K, L) until the thecae are held at nearly 180° to expose the pollen [Fig. 4K, L; labelled pol in Fig. 4K]. The flower is then ready for pollination.
Heterochronical pattern 1: perianth growth rate
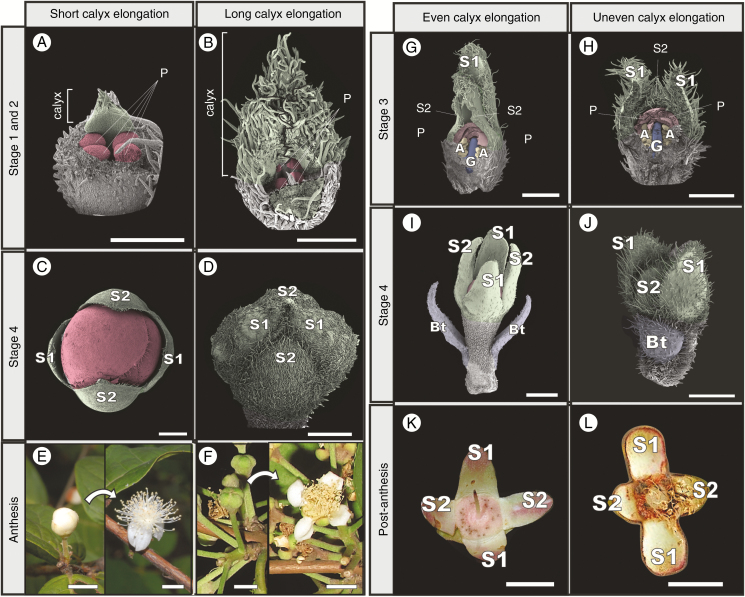
Using E. punicifolia as a reference, it is possible to compare organ size proportions at similar stages of development between species to infer changes in development rate (heterochronies). The first clear heterochronical pattern is observed when comparing perianth development between species. Most analysed samples showed a rate of perianth development similar to that of E. punicifolia. However, in at least three species (Eugenia involucrata, E. acutata and E. dichroma) sepal enlargement occurs at a noticeably faster rate (Fig. 5A) in early developmental stages. In these taxa the sepals elongate at least twice as fast as in similar stages in other Eugenia (see contrast between Fig. 5A and B). As a consequence, in these species the calyx covers the whole bud until developmental Stage 4, with no corolla exposure prior to anthesis (see contrasts between Fig. 5C and D and between Fig. 5E and F).
Fig. 5.
Variation of perianth developmental rate in Eugenia. (A) Early development of E. stipitata, showing short calyx contrasting with (B) the extremely elongated calyx of E. acutata. (C) Pre-anthetic stages in E. protenta, showing corolla exposition prior to anthesis. (D) The same stage in E. acutata, showing sepals that cover the whole bud prior to anthesis. (E) Anthesis in E. stipitata, highlighting how exposed the corolla is in the pre-anthetic stage. (F) Anthesis in E. acutata, showing sepals that cover the whole bud prior to anthesis. (G) Stage 3 bud in E. involucrata and (H) E. inversa, showing S1 more developed than S2. (I) Pre-anthetic buds of E. dichroma, with S1 and S2 equally developed, in contrast to the same stage in (J) E. inversa, where S1 is still more developed than S2. (K) Calyx from post-anthetic flower of E. involucrata, showing all sepals the same size in contrast with (L) post-anthetic flower of E. splendens, showing disymmetrical calyx with distinctly larger S1 than S2 sepal pairs. Bt, bracteole; S, sepal; P, petal; A, androecium; G, gynoecium. Scale bars: (A, B) = 250 µm; (C, D, H) = 500 µm; (G, I, J) = 1 mm; (E, F, K, L) = 5 mm. Colour coding in online version: sepals, green; corolla, red; androecium, yellow; gynoecium, blue. Picture in (F) by Augusto Giaretta.
Another distinct pattern of sepal development was observed in Eugenia inversa and E. splendens (Fig. 5G, I, K). In all other species, the ultimate size of the second pair of sepals (S2) is similar to the first one (S1) in developmental Stage 4, producing a radially symmetrical calyx at anthesis (Fig. 5G, I, K). In contrast, in E. inversa and E. splendens the size difference between sepals S1 and S2 is constant during and after anthesis, resulting in unequal sepals and a disymmetrical calyx, still evident in post-anthetic stages (highlighted in Fig. 5L).
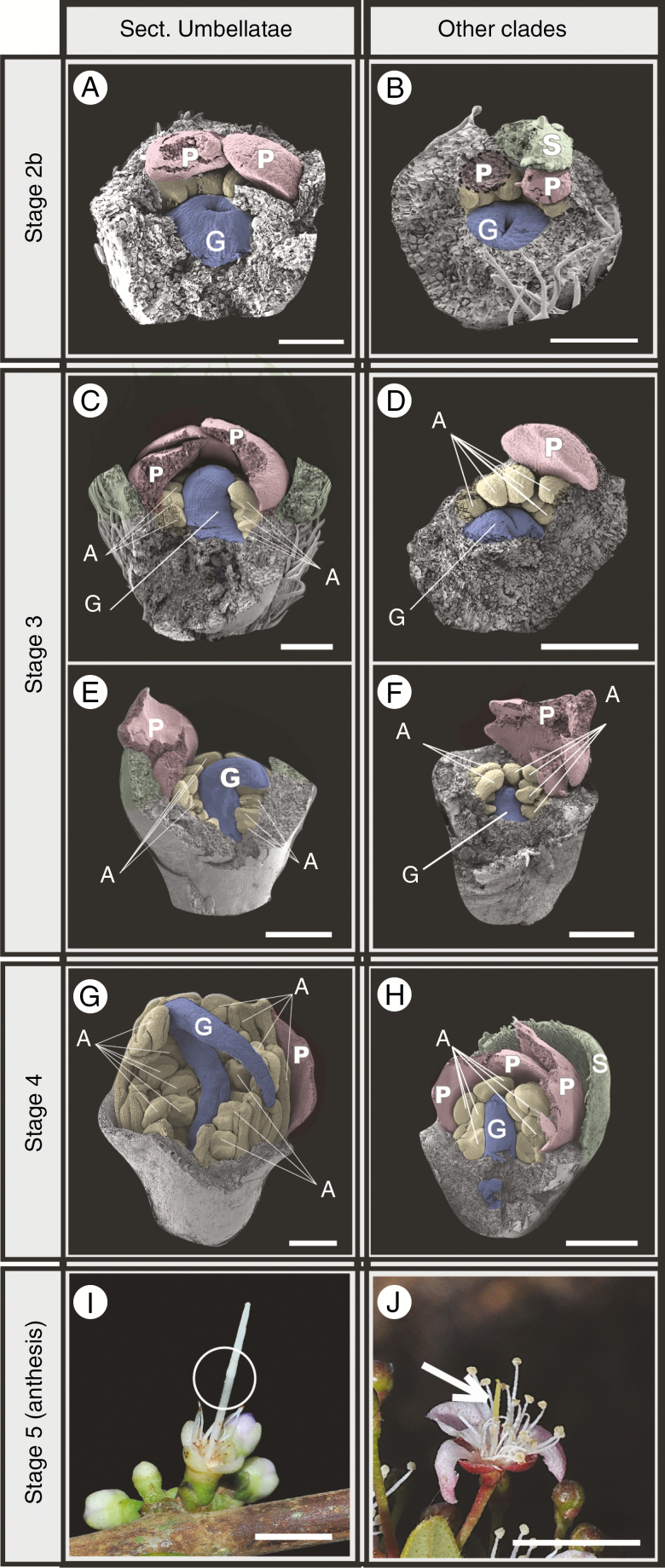
Heterochronical pattern 2: style gigantism in Eugenia Sect. Umbellatae
A second heterochronical pattern is found in the rate of stylar growth. Two main patterns of style elongation are observed across the sampled species. In species within Sect. Umbellatae (here represented by E. punicifolia, E. citrifolia, E. flavescens and E. protenta), the style develops faster, reaching the inner surface of the closed corolla early in Stage 3, bending to one side and resting upon the anthers (Fig. 6A, C, E, G, I, column labelled ‘Sect. Umbellatae’). In these species, the long style is twice the length of the androecium in anthetic flowers, with a visible mark in the middle where it was folded (highlighted in Fig. 6I). In all other analysed species, the rate of style development is slower than in E. punicifolia (Fig. 6B, D, F, H, J, column labelled ‘Other clades’) and the style never bends over the androecium in the pre-anthetic bud (Fig. 6J). After anthesis, the style in these species has the same length as the stamens (arrow in Fig. 6J). This variation was observed to be particular to each species, with no infraspecific distinction that would characterize heterostyly detected.
Heterochronical pattern 3: hypanthium elongation and androecium development
A third heterochronical pattern concerns early hypanthium elongation and its effects on the initiation and morphogenesis of the androecium. Androecium development is similar in all analysed species: initially, two stamen primordia appear below each petal followed by a continuous sequence of newly appearing stamen primordia in between, forming the first rings of stamens in Stage 2a (Fig. 7; see additional images in Supplementary Data S2, Plate 4). Sequentially, the hypanthium broadens and stamen primordia cover the entire surface of the hypanthium tissue, from corolla to the stylar base, in Stage 2b. The degree of early hypanthial elongation varies between species and thus the number of stamens also varies from species to species (Table 1). Stamens can be distributed in two to eight or nine rings (Fig. 7A–I) depending on the width of the available surface as a result of hypanthium expansion. An additional pattern was observed in the closely related New Caledonian species Eugenia paludosa and E. gacognei (clade Jossinia). In these species, hypanthial expansion occurs later in development, after the first staminal whorl has become prominent (Fig. 7J, K). This results in a clearer plastochron between the first and following staminal whorls; the first is already well developed when the later primordia appear. In this case, stamens in the first whorl end up folding slightly towards the centre of the bud in the available cavity (Fig. 7L).
The observed variation in androecium development is also responsible for a clear difference in the rate of anther maturation among the analysed species. In flowers with fewer stamens, the whole androecium matures faster in comparison with flowers with a higher number of stamens. As a result, flowers at apparently similar stages of development present anthers in different maturation stages according to stamen number (Fig. 7C, F, I, L).
Hypanthial heterochrony effects on androecium/gynoecium proportion
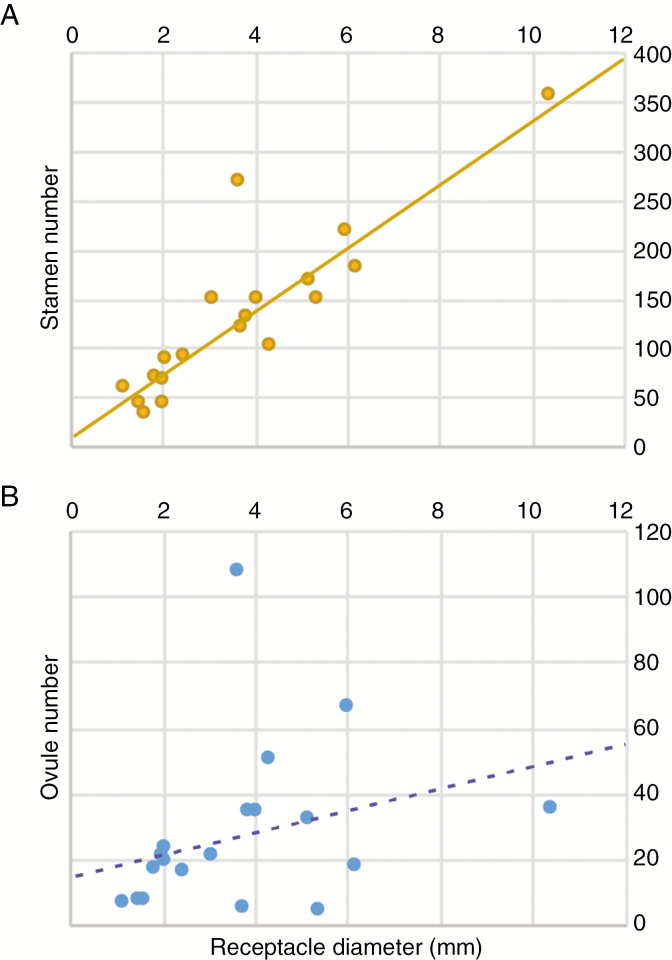
The relative hypanthial expansion in Eugenia flowers is also responsible for the final size of the floral receptacle. Therefore, species with longer initial hypanthial expansion (e.g. E. azurensis, E. itajurensis, E. paludosa) have larger floral receptacles in comparison with those with short hypanthial expansion (e.g. E. angustissima, E. ligustrina, E. punicifolia) (Table 1). Since the production of stamen primordia is continuous throughout hypanthium expansion, stamen number is directly linked to the growth of the hypanthium. This relationship of dependence strongly correlates stamen number with floral receptacle size (P < 0.001, R2 > 0.7; Fig. 8A). Thus, larger flowers bear more stamens and consequently more pollen sacs (reproductive male parts). Curiously, however, the same is not true for the relationship between floral receptacle and number of ovules (reproductive female parts). Because the hypanthium expands above the ovary, changes in hypanthium expansion rate have little influence on the number of ovules. Therefore, the size of the floral receptacle is not significantly correlated with total ovule number per flower (P = 0.214, R2 < 0.1; Fig. 8B), meaning that larger flowers do not necessarily bear more ovules than smaller ones. There is a slight difference in ovule size (Supplementary Data S1, Plate 5), but most variation appears to result from differential investment in receptacle tissue. These results suggest that shifts in the rate of hypanthial development, responsible for the total number of stamens formed, affect the final size of the flower and the production of male structures (androecium) but not female floral parts (gynoecium) in Eugenia.
Fig. 8.
Correlation between total diameter of the floral receptacle and (A) total stamen number per flower (linear regression P < 0.001, R2 = 0.769) and (B) total ovule number per flower (linear regression P = 0.2140, R2 = 0.089).
DISCUSSION
Eugenia flower development in the context of Myrtaceae
Flower morphology in Eugenia is similar to that in other Myrtaceae and Myrtales. The tetramerous–decussate phenotype is very frequent in other Myrtaceae (e.g. Eucalyptus, Syzygium) and even the variation between tetramerous–decussate and pentamerous–quincuncial aestivation can be found in other closely related genera (e.g. Myrcia; Vasconcelos et al., 2017a).
Polyandry is the most frequent androecium arrangement in Myrtaceae (Ronse De Craene and Smets, 1991) and is common in other core eudicot families (e.g. Prenner et al., 2008; Prenner, 2011; Paulino et al., 2014) and in Magnoliales (e.g. Ronse De Craene and Smets, 1998). Eudicots differ from the latter, however, in presenting whorled rather than spiral stamen formation (Ronse De Craene and Smets, 1992, 1998), having evolved from ancestral oligandrous arrangements, i.e. secondary polyandry (Endress, 1996).
The acquisition of secondary polyandry is not as evident in Eugenia as in other Myrtaceae (e.g. Melaleuca; Orlovich et al., 1999), but some heterogeneity in the appearance of the first and second group of stamen primordia suggests this pathway in the genus. During androecium initiation, a first group of staminal primordia is formed in an antepetalous position, so that the flower is initially obhaplostemonous. This pattern may represent a relic from a plesiomorphic stage, where these areas would have shown a more apparent primary primordium that would further divide into secondary primordia and sequential rings (Ronse De Craene and Smets, 1992; Endress, 1996). Diplostemony is hypothesized to be the plesiomorphic state for Myrtales (Dahlgren and Thorne, 1984) but there is no evidence for this state in Eugenia or Myrtaceae (Ronse De Craene and Smets, 1995) because even though two primary primordia are flanking each petal, these are arranged in a single whorl.
Heterochronic trends and adaptive features
When very few changes in complexity are observed within the morphologically homogeneous flowers of Eugenia (Supplementary Data S2), lability of reproductive strategies must rely on an alternative strategy. In this sense, heterochronies are an important component of secondary flower function (see definition in the first paragraph of the Introduction section). Examples of how heterochronies may affect fitness (i.e. the efficiency of the flower as a reproductive organ) in Eugenia are observed in all floral organs. In perianth development for instance, early calyx elongation in E. acutata, E. dichroma and E. involucrata may protect the bud in late development stages, hiding the reproductive organs until anthesis [as reported by Vasconcelos et al. (2017a) in Calyptranthes and Marlierea]. Likewise, the constant disparity between the first and second pair of sepals in E. inversa and E. splendens causes the open flower to be slightly disymmetrical instead of actinomorphic (the most common arrangement in the genus), which in turn may affect pollinator behaviour (Endress, 1999).
Regarding the gynoecium, the hyper-style elongation present in all four observed species of Eugenia Sect. Umbellatae creates a spatial gap between the stigma and the anthers after anthesis, i.e. herkogamy, a trait not observed in the other section (Fig. 9). Herkogamy is traditionally thought to increase the rate of cross-pollination, by avoiding accidental self-pollination (Webb and Lloyd, 1986). Although flowers of Eugenia present a certain degree of self-compatibility (Proença and Gibbs, 1994; Silva and Pinheiro, 2007), higher levels of cross-pollination are related to higher diversification rates (with abundant examples in flowering plants; e.g. Ferrer and Good, 2012; de Vos et al., 2014). The systematic consistency of this character and its relationship to the most diverse section of Eugenia may implicate this innovation in the accelerated diversification rates found in Eugenia Sect. Umbellatae (one of the highest in tribe Myrteae; Vasconcelos et al., 2017b).
Fig. 9.
Flower development and systematics in Eugenia. (A, B) Style gigantism and resulting herkogamy of Eugenia Sect. Umbellatae. (A) Flower of E. dichroma (Sect. Speciosae), in which the style is at the same height as stamens in the open flower (arrow). (B) Flower of E. adenocalyx (Sect. Umbellatae), showing a style almost twice as long as the stamens (arrow).
Hypanthium versus androecium: space matters
The most prominent effects of heterochrony in Eugenia flowers are seen in the development of the androecium. Changes in the rate of early hypanthial development are shown to affect the final diameter of the floral receptacle and consequently the number of stamens formed. Variation in stamen number is especially likely to affect aspects of reproductive strategies in Eugenia. It has been shown, for example, that the bottle-brush appearance that results from the large number of stamens in Myrtaceae flowers is the main agent of floral display and pollinator attraction (Proença and Gibbs, 1994; Willmer, 2011), so changes in stamen number could be related to variations within this syndrome. It is also clear that smaller flowers with fewer stamens undergo faster anther maturation, suggesting that the whole flower might have a faster rate of development. This could relate to a trade-off between investment in receptacle size and number of stamens (floral display) and faster maturation, with consequences for flowering phenology (Primack, 1985, 1987).
An alternative (or additional) hypothesis for variation in stamen number is that these changes affect the proportions of male and female parts in the flower and consequently relate to changes in breeding systems (Cruden, 1977; Charlesworth, 2006). An indication of this is that stamen and ovule numbers respond independently to variations in the size of the floral receptacle (resulting from hypanthium expansion) in different species (Fig. 8). While stamens and anther numbers are highly dependent on the space available after hypanthial expansion (similar development of corona size in Passifloraceae; Claßen-Bockhoff and Meyer, 2016), gynoecium configuration is more clade-specific, with lower number of ovules characteristic of certain clades (e.g. Faria, 2014). If hypanthium extension rate disparity affects the number of male but not of female parts, this heterochronic pattern might drive, or be implicated in, a flexible reproductive system and increased adaptive value of the genus throughout evolution. Pollen counts and ovule viability tests are required to fully test the importance of this character (Harder and Barret, 1993).
Relevance of hypanthium/androecium dependency for early Myrteae evolution
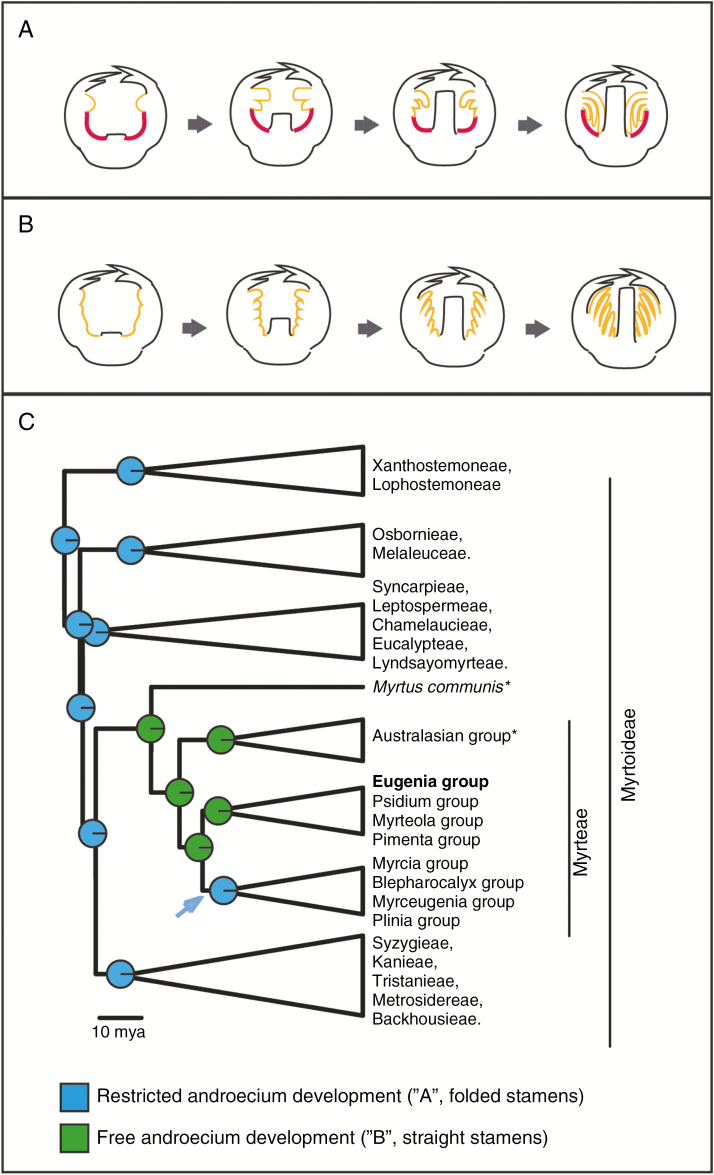
Even though polyandry is a configuration shared by most members of the Myrtoideae subfamily, the trait varies between lineages. A recent systematic survey showed that Eugenia, alongside other related genera within tribe Myrteae, are exceptions within Myrtales in presenting straight (as opposed to folded) stamens in the bud (Vasconcelos et al., 2015). Comparison of hypanthium and androecium development in Eugenia with that of other Myrtoideae genera (e.g. Drinnan and Ladiges, 1991; Orlovich et al., 1999; Bohte and Drinnan, 2005; Vasconcelos et al., 2017b; see list in Supplementary Data S1) suggests the distinction between straight and folded stamens in the bud is related to the area occupied by stamen primordia over the expanded hypanthium.
Eugenia (and related genera) produces an indeterminate number of staminal primordia that cover the whole hypanthial tissue up to the stylar base during androecium development (Fig. 4C–J). Conversely, Myrtaceae genera with folded stamens in the bud (including some Eucalyptus species with slightly straight stamens in the bud; e.g. McDonald et al., 2009) present staminal primordia development only on a restricted area of the hypanthial rim, below the corolla (Drinnan and Ladiges, 1991; Orlovich et al., 1999; Bohte and Drinnan, 2005; Vasconcelos et al., 2017b). The restricted development of stamen primordia on the hypanthial rim during bud development creates an open space below the youngest staminal ring when the hypanthium expands (shown in red in Fig. 10A), forming a hypanthial cup. This explains the position of the stamens in Myrtaceae buds as a physical matter: gravitropy folds the stamens down when adequate space is available (Fig. 10A). Meanwhile, Eugenia species [and related genera, e.g. Acca, Ugni (Belsham and Orlovich, 2003; Vasconcelos et al., 2015)] do not present any open space during early bud development to allow stamens to fold, as the whole hypanthial tissue is covered by stamen primordia (Fig. 10B). This leaves no space for folding and causes stamens to develop in a straight posture.
Fig. 10.
Evolution of androecium development in Eugenia and related taxa. (A) Restricted androecium development, where stamen primordia appear just in the apical part of the hypanthium, leading to folded stamens in the bud. (B) Free androecium development, where stamen primordia cover the whole hypanthium tissue, leading to straight stamens in the bud. Stamens are shown in yellow and ‘empty’ hypanthial tissue in red. Other floral organs are kept the same size to help interpretation. (C) Reconstruction of stamen position in the bud on the Myrtaceae phylogeny. Arrow shows reversal to the plesiomorphic state in Myrcia, Plinia, Blepharocalyx and Myrceugenia groups. *The position of the Myrtus and Australasian groups are swapped in more recent phylogenies (Vasconcelos et al., 2017b)
Therefore, the ‘folded stamen in the bud’ trait indicates that androecium development is restricted to the hypanthial rim while the ‘straight stamens in the bud’ trait indicates unrestricted androecium development over the hypanthium. By plotting these traits on the Myrtaceae phylogenetic hypothesis, a shift from restricted to unrestricted androecium development is estimated to have occurred at the crown node of tribe Myrteae (Fig. 10C). This shift may also be related to the loss of nectar production: while nectaries are present in many Myrtaceae (e.g. Beardsell et al., 1993; see also Supplementary Data S2, Plate 6), favoured also by the hypanthium cup, where the nectar can accumulate [as in other Myrtales (Davis, 1997; Varassin et al., 2008)], they are absent in most Myrteae (Nic Lughadha and Proença, 1996).
The unrestricted development state appears then to have reversed to a plesiomorphic restricted development state in Myrcia and related genera (arrow in Fig. 10C), the clade in Myrteae with folded stamens (Vasconcelos et al., 2015). This shift and the consequent lability of reproductive strategies provided by the association between unrestricted stamen formation and hypanthium expansion (Fig. 8) may have been important in the early evolution of tribe Myrteae. The high acceleration in diversification rates associated with the early evolution of the tribe (Biffin et al., 2010; Berger et al., 2016) has been traditionally linked to the key innovation of the fleshy fruit (Biffin et al., 2010), but this study provides evidence that not only fruit but also adaptations of the flower may have contributed to early establishment of tribe Myrteae.
Conclusions and future directions
The present study demonstrates that Eugenia presents diversity in reproductive strategies associated with the flowers, despite their superficially morphological similarities. These are mainly driven by subtle changes in developmental rates that have altered proportions between floral organs throughout the evolutionary history of the group. Heterochronies observed between Eugenia species are shown to be implicated in subtle breeding system changes (affecting differential production of male and female parts), phenology (floral development rate changes) and unbalanced clade diversity (in the case of the style in Eugenia Sect. Umbellatae). This study also provides insights into the evolution of characteristic Myrtaceae polyandry by indicating unrestricted primordia initiation throughout the hypanthium to have been an evolutionary novelty in Myrteae. Recognition that superficially homogeneous flowers may present an array of possible reproductive strategies by fine tuning developmental rhythms is a step forward from traditional deterministic concepts in plant reproductive biology. Future directions include field hypothesis testing, including environmental factors that play a role in the heterochronic patterns discussed, and trait-dependent diversification rate analyses, particularly regarding longer styles in the mega-diverse Eugenia Sect. Umbellatae.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Data S1: character matrix for reconstruction of androecium evolution in Myrtoideae. Data S2: floral ontogenetic aspects that are not directly linked to heterochrony in Eugenia.
Supplementary Material
ACKNOWLEDGEMENTS
For assistance with field collections, permits and species identification we thank J. Soewarto, L. Barrabe (New Caledonia), D.F. Lima (Brazil) and B. Peguero (Dominican Republic). For crucial assistance in the laboratory we thank M. Conejero, A. Giaretta and C. Phychid. We also thank A. Wingler and L. Ronse de Craene for useful comments on the manuscript. This work was supported by Reflora, Capes (SwB grant 7512-13-9) and Emily Holmes Memorial Scholarships (2015, 2016).
LITERATURE CITED
- Antiqueira PAP, Romero GQ. 2016. Floral asymmetry and predation risk modify pollinator behavior, but only predation risk decreases plant fitness. Oecologia 181: 475–485. [DOI] [PubMed] [Google Scholar]
- Beech E, Rivers M, Oldfield S, Smith PP. 2017. GlobalTreeSearch: the first complete global database of tree species and country distributions. Journal of Sustainable Forestry 1–36. http://dx.doi.org/10.1080/10549811.2017.1310049
- Beentje H. 2012. The Kew plant glossary: An illustrated of plant terms.Royal Botanic Gardens Kew; London, UK. [Google Scholar]
- Beardsell DV, Obrien SP, Williams EG, Knox RB, Calder DM. 1993. Reproductive biology of australian Myrtaceae. Australian Journal of Botany 41: 511–526. [Google Scholar]
- Belsham SR, Orlovich DA. 2002. Development of the hypanthium and androecium in New Zealand Myrtoideae (Myrtaceae). New Zealand Journal of Botany 40: 687–695. [Google Scholar]
- Belsham SR, Orlovich DA. 2003. Development of the hypanthium and androecium in South American Myrtoideae (Myrtaceae). New Zealand Journal of Botany 41: 161–169. [Google Scholar]
- Biffin E, Lucas EJ, Craven LA, Costa IR, Harrington MG, Crisp MD. 2010. Evolution of exceptional species richness among lineages of fleshy-fruited Myrtaceae. Annals of Botany 106: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger BA, Kriebel R, Spalink D, Sytsma KJ. 2016. Divergence times, historical biogeography, and shifts in speciation rates of Myrtales. Molecular Phylogenetics and Evolution 95: 116–136. [DOI] [PubMed] [Google Scholar]
- Bohte A, Drinnan A. 2005. Floral development and systematic position of Arillastrum, Allosyncarpia, Stockwellia and Eucalyptopsis (Myrtaceae). Plant Systematics and Evolution 251: 53–70. [Google Scholar]
- Briggs BG, Johnson LAS. 1979. Evolution in the Myrtaceae – evidence from inflorescence structure. Proceedings of the Linnean Society of New South Wales 102: 157–256. [Google Scholar]
- Bünger M, Mazine FF, Lucas EJ, Stehmann JR. 2016. Circumscription and synopsis of Eugenia section Speciosae Bünger & Mazine (Myrtaceae). PhytoKeys 61: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth D. 2006. Evolution of plant breeding systems. Current Biology 16: R726–R735. [DOI] [PubMed] [Google Scholar]
- Claßen-Bockhoff R, Meyer C. 2015. Space matters: meristem expansion triggers corona formation in Passiflora. Annals of Botany 117: 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden RW. 1977. Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46. [DOI] [PubMed] [Google Scholar]
- Dahlgren R, Thorne RF. 1984. The order Myrtales: circumscription, variation, and relationships. Annals of the Missouri Botanical Garden 71: 633–699. [Google Scholar]
- Davis AR. 1997. Influence of floral visitation on nectar-sugar composition and nectary surface changes in Eucalyptus. Apidologie 28: 27–42. [Google Scholar]
- Drinnan AN, Ladiges PY. 1991. Floral development and systematic position of Eucalyptus curtisii (Myrtaceae). Australian Systematic Botany 4: 539–551. [Google Scholar]
- Endress PK. 1994. Floral structure and evolution of primitive angiosperms: recent advances. Plant Systematics and Evolution 192: 79–97. [Google Scholar]
- Endress PK. 1996. Diversity and evolutionary biology of tropical flowers. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Endress PK. 1999. Symmetry in flowers: diversity and evolution. International Journal of Plant Sciences 160: S3–S23. [DOI] [PubMed] [Google Scholar]
- Endress PK. 2002. Morphology and angiosperm systematics in the molecular era. Botanical Review 68: 545–570. [Google Scholar]
- Endress PK. 2003. Early floral development and nature of the calyptra in Eupomatiaceae (Magnoliales). International Journal of Plant Sciences 164: 489–503. [Google Scholar]
- Endress PK. 2006. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research 44: 1–61. [Google Scholar]
- Faria JEQ. 2014. Revisão taxonômica e filogenia de Eugenia sect. Pilothecium (Kiaersk.) D. Legrand (Myrtaceae). PhD Thesis, Universidade de Brasilia, Brazil. [Google Scholar]
- Ferrer MM, Good SV. 2012. Self-sterility in flowering plants: preventing self-fertilization increases family diversification rates. Annals of Botany 110: 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Barrett SC. 1993. Pollen removal from tristylous Pontederia cordata: effects of anther position and pollinator specialization. Ecology 74: 1059–1072. [Google Scholar]
- de Jager ML, Ellis AG. 2014. Floral polymorphism and the fitness implications of attracting pollinating and florivorous insects. Annals of Botany 113: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Johnston MO. 2000. Heterochrony in plant evolutionary studies through the twentieth century. Botanical Review 66: 57–88. [Google Scholar]
- Lord EM. 1991. The concepts of heterochrony and homeosis in the study of floral morphogenesis. Flowering Newsletter 11: 4–13. [Google Scholar]
- Martos L, Galan ATOF, Souza LAD, Mourão KSM. 2017. The flower anatomy of five species of Myrteae and its contribution to the taxonomy of Myrtaceae. Acta Botanica Brasilica 31: 42–50. [Google Scholar]
- Mazine FF, Souza VC, Sobral M, Forest F, Lucas E. 2014. A preliminary phylogenetic analysis of Eugenia (Myrtaceae: Myrteae), with a focus on Neotropical species. Kew Bulletin 69: 1–14. [Google Scholar]
- Mazine FF, Bünger MO, Faria JEQ, Lucas E, Souza VC. 2016. Sections in Eugenia (Myrteae, Myrtaceae): nomenclatural notes and a key. Phytotaxa 289: 225–236. [Google Scholar]
- McDonald MW, Brooker MIH, Butcher PA. 2009. A taxonomic revision of Eucalyptus camaldulensis (Myrtaceae). Australian Systematic Botany 22: 257–285. [Google Scholar]
- Mori SA, Boom BM, Carvalino AM. 1983. Ecological importance of Myrtaceae in an eastern Brazilian wet forest. Biotropica 15: 68–70. [Google Scholar]
- Nic Lughadha E, Proença C. 1996. A survey of the reproductive biology of the Myrtoideae (Myrtaceae). Annals of the Missouri Botanical Garden 83: 480–503. [Google Scholar]
- Oliveira-Filho AT, Fontes MAL. 2000. Patterns of floristic differentiation among Atlantic forests in southeastern Brazil and the influence of climate. Biotropica 32: 793–810. [Google Scholar]
- Orlovich DA, Drinnan AN, Ladiges PY. 1999. Floral development in Melaleuca and Callistemon (Myrtaceae). Australian Systematic Botany 11: 689–710. [Google Scholar]
- Paulino JV, Prenner G, Mansano VF, Teixeira SP. 2014. Comparative development of rare cases of a polycarpellate gynoecium in an otherwise monocarpellate family, Leguminosae. American Journal of Botany 101: 572–586. [DOI] [PubMed] [Google Scholar]
- Pimentel RR, Natália AD, Barreira P et al. . 2014. Development and evolution of the gynoecium in Myrteae (Myrtaceae). Australian Journal of Botany 62: 335–346. [Google Scholar]
- Prenner G. 2004. New aspects in floral development of Papilionoideae: initiated but suppressed bracteoles and variable initiation of sepals. Annals of Botany 93: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner G. 2011. Floral ontogeny of Acacia celastrifolia: an enigmatic mimosoid legume with pronounced polyandry and multiple carpels. In: Wanntorp L, Ronse De Craene LP, eds. Flowers on the tree of life. Cambridge: Cambridge University Press, 256–278. [Google Scholar]
- Prenner G, Box MS, Cunniff J, Rudall PJ. 2008. The branching stamens of Ricinus and the homologies of the angiosperm stamen fascicle. International Journal of Plant Sciences 169: 735–744. [Google Scholar]
- Prenner G, Bateman RM, Rudall PJ. 2010. Floral formulae updated for routine inclusion in formal taxonomic descriptions. Taxon 59: 241–250. [Google Scholar]
- Primack RB. 1985. Longevity of individual flowers. Annual Review of Ecology and Systematics 16: 15–37. [Google Scholar]
- Primack RB. 1987. Relationships among flowers, fruits, and seeds. Annual Review of Ecology and Systematics 18: 409–430. [Google Scholar]
- Proença CE, Gibbs PE. 1994. Reproductive biology of eight sympatric Myrtaceae from Central Brazil. New Phytologist 126: 343–354. [Google Scholar]
- R Core Team , 2017. A Language and Environment for Statistical Computing. Vienna: Foundation for Statistical Computing. [Google Scholar]
- Raff RA, Wray GA. 1989. Heterochrony: developmental mechanisms and evolutionary results. Journal of Evolutionary Biology 2: 409–434. [Google Scholar]
- Ronse De Craene LP. 2010. Floral diagrams: an aid to understanding flower morphology and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1991. The impact of receptacular growth on polyandry in the Myrtales. Botanical Journal of the Linnean Society 105: 257–269. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1992. Complex polyandry in the Magnoliatae: definition, distribution and systematic value. Nordic Journal of Botany 12: 621–649. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1993. Dédoublement revisited: towards a renewed interpretation of the androecium of the Magnoliophytina. Botanical Journal of the Linnean Society 113: 103–124. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1995. The distribution and systematic relevance of the androecial character oligomery. Botanical Journal of the Linnean Society 118: 193–247. [Google Scholar]
- Ronse De Craene LP, Smets EF. 1998. Notes on the evolution of androecial organisation in the Magnoliophytina (angiosperms). Plant Biology 111: 77–86. [Google Scholar]
- Rudall PJ, Bateman RM. 2004. Evolution of zygomorphy in monocot flowers: iterative patterns and developmental constraints. New Phytologist 162: 25–44. [Google Scholar]
- Schmid R. 1972. A resolution of the Eugenia-Syzygium controversy (Myrtaceae). American Journal of Botany 59: 423–436. http://dx.doi.org/10.2307/2441553 [Google Scholar]
- Silva ALG, Pinheiro MCB. 2007. Biologia floral e da polinização de quatro espécies de Eugenia L. (Myrtaceae). Acta Botanica Brasilica 21: 235–247. [Google Scholar]
- Thornhill AH, Ho SY, Külheim C, Crisp MD. 2015. Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Molecular Phylogenetics and Evolution 93: 29–43. [DOI] [PubMed] [Google Scholar]
- Tucker SC. 2003. Floral development in legumes. Plant Physiology 131: 911–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varassin IG, Penneys DS, Michelangeli FA. 2008. Comparative anatomy and morphology of nectar-producing Melastomataceae. Annals of Botany 102: 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos TNC, Proença CEB. 2015. Floral cost vs. floral display: insights from the megadiverse Myrtales suggest that energetically expensive floral parts are less phylogenetically constrained. American Journal of Botany 102: 900–909. [DOI] [PubMed] [Google Scholar]
- Vasconcelos TNC, Prenner G, Bünger MO, De-Carvalho PS, Wingler A, Lucas EJ. 2015. Systematic and evolutionary implications of stamen position in Myrteae (Myrtaceae). Botanical Journal of the Linnean Society 179: 388–402. [Google Scholar]
- Vasconcelos TNC, Prenner G, Santos MF, Wingler A, Lucas EJ. 2017a. Links between parallel evolution and systematic complexity in angiosperms – a case study of floral development in Myrcia s.l. (Myrtaceae). Perspectives in Plant Ecology, Evolution and Systematics 24: 11–24. [Google Scholar]
- Vasconcelos TNC, Proença CEB, Ahmad B et al. . 2017b. Myrteae phylogeny, calibration, biogeography and diversification patterns: increased understanding in the most species rich tribe of Myrtaceae. Molecular Phylogenetics and Evolution 109: 113–137. [DOI] [PubMed] [Google Scholar]
- De Vos JM, Hughes CE, Schneeweiss GM, Moore BR, Conti E. 2014. Heterostyly accelerates diversification via reduced extinction in primroses. Proceedings of the Royal Society B 281: 20140075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, 2009. Flower morphogenesis: timing is key. Developmental Cell 16: 621–622. [DOI] [PubMed] [Google Scholar]
- WCSP 2017. World checklist of selected plant families. apps.kew.org/wcsp/. [Google Scholar]
- Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms I. Dichogamy. New Zealand Journal of Botany 24: 135–162. [Google Scholar]
- Webster MA, Gilmartin PM. 2003. A comparison of early floral ontogeny in wild-type and floral homeotic mutant phenotypes of Primula. Planta 216: 903–917. [DOI] [PubMed] [Google Scholar]
- Willmer P. 2011. Pollination and floral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.