Abstract
Despite the fact that they are not typically life-threatening, low-grade gliomas (LGGs) remain a significant clinical challenge in pediatric neuro-oncology due to comorbidities associated with these tumors and/or their treatments, and their propensity to multiply recurs. LGGs, in total the most common brain tumors arising in childhood, can often become a chronic problem requiring decades of management. The Second International Consensus Conference on Pediatric Low-Grade Gliomas held in Padua, Italy in 2016 was convened in an attempt to advance the pace of translating biological discoveries on LGGs into meaningful clinical benefit. Topics discussed included: the implications of our growing biological understanding of the genomics underlying these tumors; the assessment of the model systems available; the implications of the molecular and histopathologic differences between adult and pediatric diffuse gliomas; and steps needed to expedite targeted therapy into late-stage clinical trials for newly diagnosed cases. Methods for the diagnostic assessment of alterations in the Ras/mitogen-activated protein kinase pathway, typical for these tumors, were also considered. While the overall tone was positive, with a consensus that progress is being and will continue to be made, the scale of the challenge presented by this complex group of tumors was also acknowledged. The conclusions and recommendations of the meeting panel are provided here as an outline of current thinking and a basis for further discussion.
Keywords: low-grade glioma, MAPK pathway, molecular diagnostics, neurooncology, pediatric brain tumor, targeted therapy
Pediatric low-grade gliomas (LGGs) and glioneuronal tumors, defined as World Health Organization (WHO) grade I or II lesions of the central nervous system (CNS), are an extremely diverse group of tumors.1 Together they comprise approximately one-third of all brain tumor diagnoses in children, making them the most common CNS neoplasia arising in the pediatric setting.2 While a great deal has been learned over the past decade about some of the more common entities such as pilocytic astrocytoma, the complex spectrum of pediatric LGGs is just beginning to be truly understood. There is no doubt that new knowledge is opening up significant therapeutic opportunities. Despite this clear potential, however, many challenges in how best to convert this growing biological understanding into improved clinical care remain unsolved. In the summer of 2016, a second international consensus meeting was convened in Padua, Italy to develop a path forward for the study of LGGs, so as to address these issues and challenges and suggest a blueprint that scientific and clinical investigators could pursue to make meaningful progress. Clinical and translational data, some unpublished at the time of the meeting, was presented in an open forum and was the basis of mutually agreed upon recommendations, designed to expedite the translation of molecular advances into clinical care.
Implications of Tumor Biology for Diagnostics and Therapy
Genetic Alterations in Pediatric LGG
A full description of all genetic alterations known to be present in pediatric LGG was covered, in detail, in a recently published report of the first consensus conference.3 It is now increasingly clear that the vast majority of tumors falling in the pediatric LGG spectrum are caused by one of a variety of alterations in the signaling pathway of mitogen-activated protein kinase (MAPK), including BRAF mutation or fusion, FGFR1 mutation or structural rearrangement, NF1 mutation, NTRK-family fusions, and other rarer events.4–6 Notable exceptions include subependymal giant cell astrocytoma (typically associated with germline TSC1/2 mutations) and a histologically mixed group of tumors including a substantial fraction of angiocentric and diffuse gliomas that harbor activating alterations in MYB or MYBL1.6–8 It is currently unclear whether the latter alterations, resulting in altered transcriptional activity, also function partly via the MAPK pathway, but the consequences are certainly more complex than that alone.
The discovery of these alterations over the last 5–10 years as key drivers in pediatric LGG has led to excitement and optimism both from a biological standpoint and from a clinical perspective—the latter because of the parallel development of drugs specifically targeting several of these alterations (eg, BRAF V600E, fibroblast growth factor receptor 1 [FGFR1], neurotrophic tyrosine receptor kinase [NTRK] inhibitors) or the downstream mediators of the pathway (eg, mitogen-activated protein kinase kinase [MEK] inhibitors). Early-phase trials with some of these compounds are currently in progress or nearing completion. In order for these new treatments to provide the greatest possible benefit, however, it is crucial to properly stratify patients entered into future trials to optimize the matching of drugs to patients in a rational way. Such stratification is complicated by the fact that while some degree of specificity for certain alterations in certain histologies has been observed (eg, KIAA1549:BRAF fusion is much more common to pilocytic astrocytoma; BRAF V600E is enriched in ganglioglioma and pleomorphic xanthoastrocytoma [PXA]), there are no 100% concrete associations between LGG morphology and genetics. Teasing out the details of this interplay will be a major focus of ongoing efforts and a key aspect of translating the wealth of genomics data into clinical care and, in time, benefit. Another major caveat is the fact that these genetic events are currently not routinely tested for in a majority of diagnostic laboratories. A critical step for the widespread molecular evaluation of pediatric LGGs is the development of more easily available assays to reliably diagnose the molecular abnormalities believed to be critical. Until then, referral to centralized laboratories focusing on the molecular abnormalities of LGGs may be required.
Implications for Diagnostic Stratification, Prognostication, and Therapy
The recent 2016 update of the WHO classification of nervous system tumors recognized in some tumor types, such as in medulloblastoma, that both morphological and molecular features are important to tumor behavior, introducing the concept of a layered, integrated diagnosis.1,9 As yet, however, it does not cover all of the pediatric-specific features of low-grade glial/glioneuronal neoplasms, especially diffuse gliomas. Not just molecularly (eg, IDH1 mutations are extremely rare in childhood gliomas, but partially defining in adult LGGs), but also clinically, pediatric LGGs are distinct from their adult counterparts. Although fully comprehensive data are still lacking, it is believed that pediatric-type diffuse gliomas currently classed as WHO grade II do not have the same propensity for malignant progression, and thus have a better prognosis, compared with their adult counterparts.10 In a similar fashion, pediatric tumors displaying oligodendroglial morphology usually do not harbor the IDH1/2 mutations and combined 1p/19q loss seen in adults, and may represent rarer, pediatric-type entities.11,12 Gliomas in infants are yet another special case, whereby apparently undifferentiated morphology and an elevated proliferation rate can represent the unique environment of the developing brain and do not always indicate malignancy.10
There are some clear enrichments of certain genetic alterations occurring more frequently in conjunction with specific histologies, as well as in certain locations. For example, the classical KIAA1549:BRAF fusion is much more common in pilocytic astrocytoma than other LGGs, and is particularly common in the cerebellum.4,6FGFR1 alterations, including point mutations and kinase domain duplications, are more frequent in dysembryoplastic neuroepithelial tumors,6,13 while alterations of MYB/MYBL1 seem to define a class of pediatric-type diffuse gliomas, often with features of angiocentric glioma.6,8 DNA methylation profiles have been suggested to distinguish biological subgroups with enrichment for particular aberrations,5 in a similar way to what has been shown for other pediatric brain tumors, and hold great promise to further subgroup LGGs.14–16
Conversely, there are also some genetic alterations which seem to be distributed across different histologies and which may have a varying prognostic impact. The BRAF V600E mutation is a good example of one such change, which is enriched in ganglioglioma and PXA, but can also be found in dysembryoplastic neuroepithelial tumors, pilocytic astrocytoma, and high-grade gliomas (HGGs), among others. It is clear that not all of these groups have a similar clinical course, with PXA having a worse outcome than WHO grade I tumors. Notably, many PXAs additionally show a focal genetic loss of the CDKN2A/B locus at 9p21, and this combination (V600E + 9p21 loss) has recently been proposed to mark a subset of LGGs that show a propensity toward malignant progression,17 as well as a group of histologically high-grade tumors that show a slightly more favorable prognosis than classical glioblastoma.18 Within histologically defined ganglioglioma series, V600E mutation has also been suggested as a negative prognostic marker.19 Further work is therefore required to precisely define the role of histology or epigenetic changes in determining the outcome of V600E-positive tumors—a task of particular urgency to ensure appropriate patient stratification in BRAF-based clinical trials.
Even the role of the histone 3 K27M mutation, common in pediatric HGGs and initially thought to perhaps be exclusive to them,20,21 is not yet completely clear. There are now multiple case reports of histologically low-grade tumors harboring this mutation (often in conjunction with a MAPK alteration such as BRAF V600E or FGFR1 mutation) and showing longer than expected survival.4,6,20 Thus, although true in the vast majority of cases, the presence of the K27M mutation is not automatically an indicator of aggressive behavior. This highlights the need for some degree of caution in using molecular findings to connote different LGG subsets and associated prognosis, and the need to continuously reevaluate the significance of molecular findings.21–23
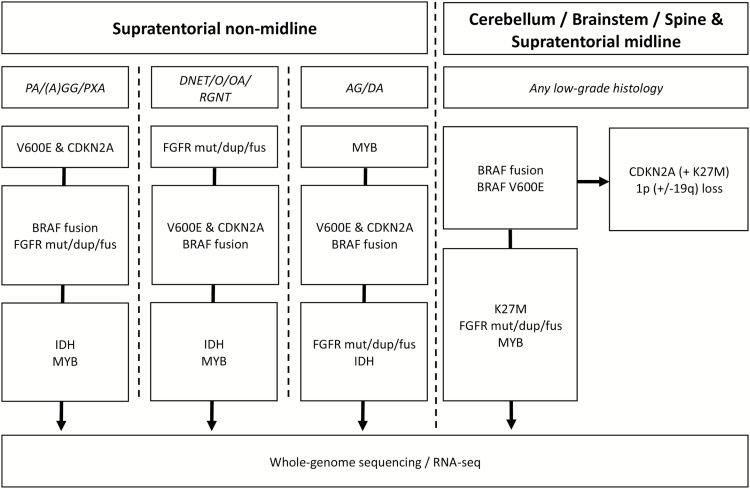
Further elucidating these complex relationships is important not just as an academic exercise, but also because of the expanding role that personalized therapy for these tumors is already playing and will continue to play in the future.24,25 Since many targeted therapy trials are now recruiting patients based on presence of a particular target across multiple histologies, rather than solely within one entity, one could postulate a framework for pediatric LGG which builds on the WHO 2016 concept of an integrated diagnosis, incorporating molecular aberrations. Low-grade neuroepithelial tumors would then be categorized first by their particular genetic change, with a secondary histologic subclassification (see Fig. 1).
Fig. 1.
Possible order of investigation for rationally targeted molecular testing based on tumor location and histology. Upfront next-generation sequencing (DNA + RNA) can also be used to supersede this order and give the most comprehensive overview. PA, pilocytic astrocytoma; (A)GG, (anaplastic) ganglioglioma; PXA, pleomorphic xanthoastrocytoma; DNET, dysembryoplastic neuroepithelial tumor; O, oligodendroglioma; OA, oligoastrocytoma; RGNT, rosette-forming glioneuronal tumor; AG, angiocentric glioma; DA (pediatric-type) diffuse glioma; mut, mutation; dup, tyrosine kinase internal tandem duplication; fus, fusion.
The primary goal of any such framework should be to facilitate the routine assessment of these important characterizing features in a clinical diagnostic setting and the stratification of patients by prognosis (and possibly treatment response). It would also facilitate the optimization of matching patients to targeted therapy trials. In order to achieve this, considerably more data are required on the natural course and prognosis of certain histologies in combination with certain molecular aberrations, as well as on the response of these different groups to current therapies. This is something which can only be achieved in collaboration and with the support of clinical trial groups for acquisition of sample material and outcome data. Once these data can be made available, however, it is hoped that this will rapidly translate into a robust classification scheme and will be one of the first priorities for the recently announced cIMPACT-NOW consortium for advancing nervous system tumor classification.26,27
Molecular Diagnostic Methods
To answer the outstanding questions regarding this histology-biology interplay, and for the expansion of knowledge on diverse genetic alterations to be of utility in clinical practice, standardized methods of accurately and reliably detecting them are required. In terms of molecular subgrouping, most systems proposed to date make use of either gene expression or DNA methylation changes.
For genetic tests, there is currently no consensus on how to optimally assess the multitude of changes that can potentially be found in pediatric LGGs. Some laboratories are already implementing a comprehensive approach based on next-generation sequencing, applying whole exome/whole genome and transcriptome sequencing to newly diagnosed LGGs. While this clearly offers the best opportunity to cover the whole spectrum of possible changes, and is to be encouraged where possible, it is currently not feasible from a cost and logistic point of view to perform such an analysis in every pathology lab worldwide. A rationally planned series of more targeted tests possible through a variety of methods (Sanger sequencing, fluorescence in situ hybridization, real-time PCR, single nucleotide polymorphism array, etc) is also able to identify the majority of the most common changes (see Fig. 2). Based on the observed enrichment of certain alterations in particular locations and histologies, the order of these tests can potentially be optimized to minimize time and costs for the molecular diagnostic process. A suggested structure for this testing is outlined in Fig. 2. Prospective clinical trials should mandate the collection of sufficient tumor material to perform a panel of tests for every recruited patient (with the optimum being fresh frozen tumor material and a matched germline control). Other than for children with neurofibromatosis type 1 (NF1), the optimal treatment of children with LGGs requires the obtainment of tissue for molecular characterization, especially when treatment is considered imminent. Even in children with NF1, with “atypical” lesions or those with aggressive clinical courses, biopsy may be needed.
Fig. 2.
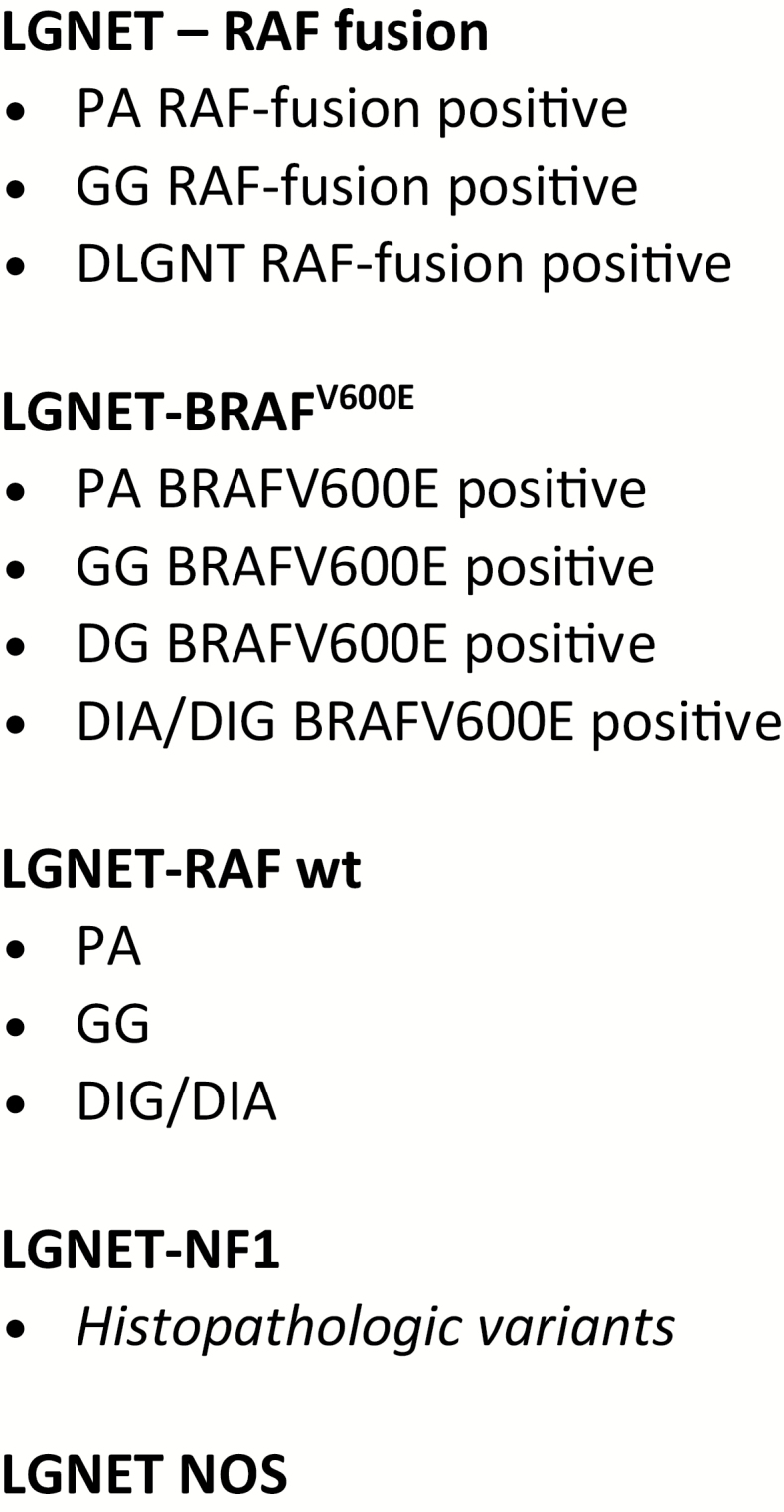
Example of a possible framework for an integrated molecular-histological stratification of pediatric LGG. LGNET, low-grade neuroepithelial tumor; PA, pilocytic astrocytoma; GG, ganglioglioma; DLGNT, diffuse leptomeningeal glioneuronal tumor; DG, diffuse glioma (pediatric type); DIA/DIG, desmoplastic infantile astrocytoma/ganglioglioma; NOS, not otherwise specified.
Conclusions and Recommendations
• Expanding knowledge of tumor genetic alterations is already impacting on patient management in terms of diagnostics and targeted therapeutic options.
• The current WHO classification, particularly for diffuse gliomas, does not satisfactorily address the spectrum of LGG seen in children.
• More data are required, particularly on survival and functional outcomes, in order to further examine the complex interplay between genetics and histology in LGG.
• The classification of pediatric LGG on an integrated histomolecular basis is the mandatory backbone for future clinical trial stratification.
Models of Pediatric Low-Grade Glioma
The other major topic of discussion from a tumor biology perspective was the pressing need for more and better model systems of pediatric LGGs. Both cell-based and in vivo models are essential for learning more about the biology and mechanisms of transformation of this disease, as well as for preclinical screening and drug testing. The particular importance of the latter element is highlighted by the study of sorafenib, a drug which resulted in an unexpected acceleration of tumor growth when treating these typically BRAF-altered tumors with a RAF inhibitor.28 This effect was subsequently explained as resulting from paradoxical MAPK pathway activation due to interactions between the drug and dimerization between mutant and wild-type B/CRAF.29 The models used to investigate this effect, cells transduced with relevant oncogenic constructs, are a convenient tool for mechanistic and inhibitor studies. They have also been used in various other settings, from the initial demonstration of the transforming potential of KIAA1549:BRAF30 and the abilities of the fusion to regulate neuroglial cell growth31 to more recent functional investigations of MYB alterations.7,8 While they certainly have a role to play for interrogation of signaling pathway dynamics and initial screening, an expanded repertoire of such systems (as is being explored in various labs) would be of major importance, as the model systems presently available incompletely reflect the genetic/epigenetic background of the primary tumor. Many efforts to derive primary cultures of LGGs that can be grown for more than a few passages have failed due to the intrinsic slow growth and benign behavior of these tumors, hampering the development of more accurate in vitro models. One method by which cells can be maintained long enough for preclinical drug screening is to overlay them onto mouse brain slices, whereby the cells are supported by this feeder-like layer of cellular and microenvironmental support.32 An additional novel approach was recently published using an inducible Simian virus 40 T-antigen system in primary cells.33 This method allowed the cells to overcome oncogene-induced senescence, the main intrinsic growth barrier, for long enough to allow cell expansion and subsequent drug testing. Although the method is still an artificial system, the advantages of being able to work with patient-derived cells make it a promising addition to the toolbox, especially if the same approach can now be used to generate a broader panel of lines with different MAPK alterations.
Finally, other more sophisticated in vitro approaches are also currently being investigated for their application in modeling LGG. For example, labs are exploring the potential for induced pluripotent stem cells derived from patients with NF1 as a system for generating tractable MAPK-activated lines (W. Weiss, unpublished). Still in development at present, this represents a further promising method for expanding the repertoire available to the field.
A similar dearth of animal models is unfortunately also a feature of pediatric LGG research. One of the first models to be developed was an NF1 model, which elegantly demonstrated the need for an Nf1 heterozygous microenvironment, as well as complete loss in astrocytes, in order to develop full-blown optic glioma in mice.34 Although this model, simulating hereditary optic pathway LGG, was first developed in 2003 (and subsequently used in several related follow-up studies), work to establish a model of the sporadic disease took substantially longer. The first such model was published in 2011, and used the RCAS (replication-competent avian sarcoma) somatic gene transfer system to introduce BRAF V600E into nestin-expressing cells.35 The resulting tumors histologically resemble human pilocytic astrocytoma and show strong MAPK pathway activation as well as a very slow growth rate (to the extent that the animals typically die from old age before they die of their tumor, despite an age of onset of approximately 4 wk; J. Gronych, personal communication). Further investigations into the kinetics of these tumors and their response to targeted therapies are ongoing.
A second attempt to model the sporadic disease used the KIAA1549:BRAF fusion.36 By introducing the fusion into different cell types, Kaul and colleagues were able to demonstrate that only neural stem cells (NSCs), and not mature astrocytes or neural glial antigen 2–positive progenitors, showed an increased proliferation in response to the oncogenic stimulus. The fusion-expressing NSCs also formed small glial lesions in vivo but did not recapitulate a full tumor.
There are no other representative genetic models available, although several are currently under development. An additional feature of these models, which may be of substantial interest in the current era of excitement about immunotherapy, is their fully immunocompetent background. This makes them well suited for investigation into, for example, immune checkpoint inhibitors or macrophage/microglia-modulating agents.
In addition to the genetic models, transplant models have been used as a way to rapidly investigate the tumorigenic potential of different oncogenes. For example, p53-null mouse astrocytes transduced with tyrosine kinase–duplicated FGFR1 generate tumors when implanted orthotopically into mouse brains.6 Murine NSCs transduced with QKI:MYB or QKI:NTRK also form tumors when injected orthotopically,7,37 while 3T3 cells transduced with activated myeloblastosis (MYB) or myeloblastosis oncogene-like 1 (MYBL1) are able to generate tumors in the mouse flank.7,8 These models are valuable tools for preclinical proof-of-concept studies, but the artificial background (including cell cycle deregulation as a result of additional genetic alterations) makes their broader utility as a faithful recapitulation of LGG somewhat limited. As long as these caveats are acknowledged, however, an expanded catalogue of similar models would certainly be of use.
Thus, there was a very clear consensus among meeting attendees that a lack of LGG models is currently a major hurdle and bottleneck for further advancing research into these tumors. Without such models, the advances that are possible in further interrogating the consequences of MAPK activation and how to inhibit it are limited. Both in vitro and in vivo models are also required for addressing the question of how drug resistance can emerge against targeted therapies including inhibition of BRAF and of MEK, and how we might overcome this in the clinic (eg, by combination strategies).
Conclusions and Recommendations
• A lack of suitable in vitro and in vivo models of pediatric LGG is a bottleneck hampering functional and preclinical investigation.
• Several efforts are ongoing with, importantly, a variety of different methods in an attempt to expand the catalogue of suitable model systems.
• Such efforts should be pursued collaboratively where possible in order to reduce duplication and maximize efficiency.
Low-Grade Gliomas: Clinical Trial Results and Design Implications
Critical issues for the development of future studies for children with LGGs remain, including whether there is enough information to justify the use of molecular-targeted agents in newly diagnosed gliomas and, if so, how clinical trials should be structured to best assess the efficacy and safety of such agents. As noted in Tables 1 and 2, since 1998 there have been multiple prospective clinical trials performed by well-established consortia and working groups.38–46 Nearly 2000 patients have been treated on these studies and results have been relatively consistent between studies.
Table 1.
Prospective clinical trials for patients with LGGs without NF1
| STUDY TYPE |
Single-Arm46 (multicentered) |
Single- Arm44 (POG) |
Single- Arm43 (SFOP) |
Single- Arm41 (HIT-LGG-1996) |
Randomized39 (COG) |
Single- Arm40 (SIOP) |
Single- Arm45 (COG) |
Randomized40 (SIOP) |
|---|---|---|---|---|---|---|---|---|
| AGENT | CARBO/VCR | CARBO | PCV/CARBO; VP16/CPDD VCR/CYTOX |
CARBO/VCR | CARBO/VCR vs TPCV |
CARBO/VCR | CARBO/VCR/ TEMO |
CARBO/VCR vs CARBO/VCR/VP16 |
| NUMBER PATIENTS |
63 | 29 | 62 | 161 | 274 | 166 | 66 | 497 |
| YEARS UNDERTAKEN |
1989–1993 | 1989–1994 | 1990–1998 | 1996–2004 | 1997–2005 | 1993–2000 | 2004–2007 | 2004–2012 |
| AGE RANGE |
0–180 mo | 0–71 mo | 0–180 mo | 0–192 mo | 0–120 mo | 0–170 mo | 0–120 mo | 0–180 mo |
| *NON- DIENCEPHALIC INVOLVEMENT |
*20% | 0% | 0% | *20% | *50% | *25% | *50% | *50% |
| EFS/PFS | 2-y 79 ± 11% |
3-y 51 ± 9% |
3-y PFS 42 ± 12% |
5-y PFS 47% |
5-y EFS 45 ± 3.2% |
5-y 40 ± 11% |
5-y 46 ± 13% |
5-y PFS 46% |
| OS 5–10 y | 97% | 83% | 89% | 90% | 86 ± 2% | 88 ± 1% | 87 ± 12% | 90 ± 2% |
*Includes both NF1 and non-NF1 patients when reporting sites of origin.
CARBO, carboplatin; VCR, vincristine; POG, Pediatric Oncology Group; SFOP, French Society of Pediatric Oncology; PCV, procarbazine; VP16, etoposide; CPDD, cisplatin; cytox, cyclophosfamide; TPCV, thioguanine, procarbazine, CCNU and vincristine; TEMO, temozolomide; EFS, event-free survival.
Table 2.
Prospective clinical trials for patients with LGGs with NF1
| STUDY TYPE |
Single-Arm46 (multicentered) |
Single-Arm44 (POG) |
Single-Arm43 (SFOP) |
Single-Arm43 (HIT-LGG-1996) |
Single-Arm38 (COG) |
Single-Arm41 (SIOP I) |
Single-Arm45 (SIOP II) |
|---|---|---|---|---|---|---|---|
| AGENT | CARBO/VCR | CARBO | PCV/CARBO; VP16/CPDD VCR/CYTOX |
CARBO/VCR | CARBO/VCR | CARBO/VCR | CARBO/VCR |
| NUMBER PATIENTS |
15 | 21 | 23 | 55 | 127 | 44 | 284 |
| YEARS UNDERTAKEN |
1989–1993 | 1998-94 | 1990–1998 | 1996–2004 | 1997–2005 | 1993–2000 | 2004–2012 |
| AGE RANGE |
0–180 mo | 0–120 mo | 0–72 mo | 0–180 mo | 0–120 mo | 0–180 mo | 0–180 mo |
| EFS/PFS | 2-y 79 ± 11% |
5-y 61 ± 12% |
3-y 62 ± 93% |
5-y 68% |
5-y 69 ± 4% |
5-y 60 ± 6% |
NA |
| OS 5–10 y | 100% | 100% | NA | NG | 98% | 100% | NA |
CARBO, carboplatin; VCR, vincristine; POG, Pediatric Oncology Group; SFOP, French Society of Pediatric Oncology; PCV, procarbazine; VP16, etoposide; CPDD, cisplatin; cytox, cyclophosfamide; TPCV, thioguanine, procarbazine, CCNU and vincristine; EFS, event, free survival; OS, overall survival. NA, not available; NG, not given.
Prospective Chemotherapy Trials
Over 1400 children without NF1 have been treated.38–46 Although the majority of children treated had tumors of the optic nerves/chiasm/hypothalamus/optic tracks/optic radiations, many recent studies have included up to 50% of children with LGGs outside these regions, especially those of the brainstem. The most common regimen tested has been the combination of carboplatin and vincristine, utilized in nearly 1000 children both in single-arm studies and in randomized trials.38–42,45,46 Drug combinations directly compared with carboplatin and vincristine in randomized studies included the 4-drug combination of thioguanine, procarbazine, lomustine, and vincristine and carboplatin and vincristine combined with either etoposide or temozolomide.39,40,45 Only one relatively small single-arm study utilized carboplatin alone.44 These prospective trials varied widely in the number of patients entered on study, with the largest being the International Society of Paediatric Oncology (SIOP) 2004 study.45 Most studies entered children over a wide age range, spanning as long as birth to 18 years. Response to the regimens tested was difficult to compare across studies, as some studies utilized only enhanced images, while others evaluated both enhanced and non-enhanced images.38–46 Overall responses as assessed by either complete or partial responses (>50% reduction in tumor greatest bidirectional area) usually were in the 30%–35% range; some investigations also utilized a minor response criterion connoting reduction between 25% and 49%. After adding minor responses, the carboplatin and vincristine regimens resulted in tumor shrinkage in up to 60% of patients. Making assessment across trials even more difficult was that there was no consistency in whether central neuroradiographic or local institution review was utilized or reported. Furthermore, the relationship between radiographic response and progression-free survival (PFS) was variable, with most studies showing no clear-cut association. The greatest consistency among studies was PFS, with 5-year rates in patients without neurofibromatosis being in the 35%–45% range. Overall survival estimates ranged between 85% and 100%.
For patients with LGG and NF1, study designs were more uniform, the majority being single-arm studies utilizing carboplatin and vincristine. The 2 randomized LGG trials from the Center for Cancer Genomics and SIOP had a single stratum for patients with NF1, as there was reluctance to expose patients with NF1 to alkylating agents.38,41 In over 550 patients with NF1 who have been treated on these prospective trials, the complete and partial response rates seemed somewhat higher than in patients without NF1—when reported, being closer to 50%. Similarly, 5-year event-free survival or PFS rates were better than those found in non-NF1 patients and were remarkably consistent in the 60%–70% range in almost all studies. Overall survivals also were consistent across studies and in most were between 90% and 100% at 5 years.
Smaller studies have tested other agents with overall similar results. The combination of cisplatin and etoposide, utilized at different dose intensities of cisplatin, has been prospectively evaluated in 50 children and had a high overall response rate (70%); the PFS also seemed somewhat higher than seen in the carboplatin and vincristine prospective studies.47,48 Single-agent vinblastine has been utilized in 54 patients, including 13 with NF1.49 The response rate to this regimen seems lower than what was reported after treatment with the carboplatin and vincristine regime, but the 5-year PFS rate was quite similar. The combination of vinblastine and carboplatin has been tested but not expanded to a phase II trial because of excess toxicity.50 Single-agent temozolomide has been used with variable efficacy.51,52
What can be gleaned from these prospective studies includes that, almost independently of the type of regimen utilized, approximately 35%–45% of children without NF1 and a higher proportion with NF1 will be event free or progression free 5 years following initiation of treatment. Disease control in those without NF1 progressively falls over the first 5–10 years. Most trials demonstrate a higher rate of progression for children under 1 year of age at diagnosis.42,43,46 There is a seeming plateau in the trajectory of loss of disease control between years 3 and 5 in children with NF1, most likely due to the natural history of LGGs to grow primarily during the first few years of life. Age less than 1 year has been a consistent risk factor for a higher rate of progression.38,42,46 The majority of studies have utilized relatively lax entry criteria, allowing patients to be entered on the basis of either clinical or radiographic progression, with the criteria being utilized for clinical progression being nonspecific and at the discretion of the treating physician. In some studies, patients could be entered without progressive clinical or radiographic disease if the LGG was believed to have the potential to cause significant morbidity, such as threat of loss to vision. Slightly more consistency was perhaps found in the European HIT/Children’s Cancer & Leukaemia Group studies, with specific criteria given for observation versus treatment.53 In those trials evaluating children without NF1, the majority did not require tissue confirmation for tumors isolated to the optic nerve or the optic nerve and chiasm for entry. Even in those studies requiring tissue, molecular characterization was not done, which was not surprising given the era in which these prospective studies were initiated. The randomized trials took, on average, between 8 and 10 years to accrue and another 3 to 5 before results were reported. Response across studies is essentially impossible to compare and although some of these studies attempted to address functional outcomes, the vast majority did this only in an anecdotal fashion.
Molecular-Targeted Trials: Early Results
As regards the status of molecular-targeted approaches for patients with LGG, although there have been studies assessing receptor kinase inhibitors and mammalian target of rapamycin inhibitors, most efforts over the past 5 years have centered on evaluation of the efficacy of drugs aimed directly at interrupting Ras-MAPK hyperactivation. There are presently 4 MEK inhibitors in active study, including 3 evaluating tumor control and response in children with progressive LGG, with and without NF1.54 The most mature results have been reported in a Pediatric Brain Tumor Consortium trial with the AstraZeneca drug selumetinib.54 Responses were seen in the phase I study in patients both with and without NF1. In the phase I study, some non-NF patients did not have molecular testing performed for BRAF mutation. The preliminary results of the phase II study in children with refractory or recurrent LGGs have been released in abstract form.55 Approximately one-third of patients (8 of 25) with LGGs harboring either a V600E mutation or the KIAA1549:BRAF fusion experienced a partial response to treatment, and many others had some degree of tumor shrinkage, although less than 50%. Two-year PFS in these patients, all of whom had failed at least one form of previous therapy, was 66 ± 11%. Responses seem to be somewhat higher in the children with NF1-related LGGs, as 10 of 25 achieved a partial response (40%) and almost all patients with NF1 have had some degree of tumor shrinkage. The 2-year PFS for patients with NF1 was 96 ± 4% and only one patient progressed while on therapy.
The duration of response off-treatment is being closely followed in the Pediatric Brain Tumor Consortium trial. In the selumetinib trial, patients with responding or stable disease could elect to stop treatment between years 1 and 2 after initiation, and if the tumor then progressed off-treatment, they could be placed back on. To date, only the minority of patients had to go back on treatment, which is evidence either of the erratic nature of growth of LGGs (which can spontaneously cease growth as the child ages) or of a somewhat unexpected prolonged effect of the drug after cessation.
Adding to the enthusiasm for the use of selumetinib, or for that matter other MEK inhibitors in patients with NF1, has been the recently published experience of the results of the phase I study performed in patients with NF1 and plexiform neurofibromas.56 In this single-arm study, partial responses were noted in 17 of 24 children, with anecdotal evidence of decreases in tumor-related pain, in disfigurement, and in functional impairment. The higher response rate should not be taken as clear evidence that plexiform neurofibromas are more responsive than LGGs; in the plexiform trial a partial response was greater than or equal to a 20% reduction in volume, compared with the 50% criteria utilizing greatest bidimensional area used for the LGG studies.
Results with the use of other MEK inhibitors in children with LGGs should soon be forthcoming. The Novartis agent trametinib has recently been studied in a completed phase II trial which included a stratum for children with BRAF-mutated tumors (either fusion or point mutation). The Array drug (MEK 162) has been assessed in a nearly completed pediatric phase I trial and is soon to be expanded into a phase II trial for children with LGGs with or without NF1. The Genentech/Roche drug cobimetinib is presently in phase I trials.
Experience with V600E mutation inhibitors is likewise growing. Ongoing pediatric phase II clinical trials include the 2 commercially available type I BRAF V600E inhibitors vemurafenib (Roche/Genentech) and dabrafenib (Novartis). The phase I results for dabrafenib in pediatric BRAF V600E–positive tumors was reported at ASCO 2015, and the final phase II result for pediatric LGGs treated with dabrafenib has recently been reported in abstract and oral presentations at the European Society of Medical Oncology 2016 (ESMO).57 The clinical trial population included children 2–17 years of age with progressive pediatric LGGs who had failed at least one chemotherapy or radiation regimen. Overall, the drug was well tolerated, with the most common side effects related to skin rash, similar to those observed in adults treated with this drug. Of the 32 patients enrolled on the trial with LGGs (15 in the phase I and 17 in the phase II), all but 3 had stoppage of tumor growth; 6 had minor responses, 11 had partial responses, and 1 patient demonstrated a complete response.57 Case reports of responses to BRAF inhibitors have also been published.58 While the response rate of BRAF V600E mutant LGGs is promising, the majority of pediatric low-grade tumors harbor the KIAA1549:BRAF truncated fusion and this variant is paradoxically activated by BRAF V600E inhibitors, including sorafenib.23 As such, it is critical that patients be properly profiled and that only those with sequence confirmed BRAF V600E mutations be exposed to these type I inhibitors.
To overcome the paradoxical activation of KIAA1549: BRAF truncated fusion variants of pediatric LGGs, newer types of BRAF inhibitors have recently been developed. Called type II inhibitors, these targeted agents shut down both BRAF and MEK signaling and thus can be considered for all BRAF pathway mediated tumors.59 A clinical trial of the first type II inhibitor in pediatric LGGs (TAK580, which has excellent CNS penetration) is just now opening and clinical results are therefore unavailable at the present time.59
Experience from the use of BRAF V600E inhibitors in malignant melanoma has demonstrated rapid development of resistance to type I inhibitors. To address this, dual BRAF V600E and MEK inhibition is now approved and results in adults have demonstrated improved response rates, improved duration of response, and in some cases reduction in toxicity, especially related to the development of squamous cell carcinoma (M. Kieran, unpublished). Through an international consortium and funded by Novartis, the combination of dabrafenib (targeting BRAF V600E) and trametinib (targeting MEK) is now also being tested in pediatric patients with tumors harboring the BRAF V600E mutation, including those with gliomas. The likelihood of resistance development in pediatric LGGs is probably different than that of melanomas (and for that matter HGGs), and this difference requires further study. The phase I component of both of these inhibitors has been completed and both single agent phase II trametinib for KIAA1549:BRAF fusion tumors as well as the phase II combination of dabrafenib and trametinib for BRAF V600E tumors are currently accruing patients. The safety and optimal duration of treatment remain unknown.
Conclusions and Recommendations
• Over the past quarter-century, multiple prospective clinical trials, some randomized and some not, have been performed in children with progressive LGGs and demonstrate very similar 5-year PFS rates and better 5-year disease control in children with NF1 compared with those without.
• Studies have varied in the inclusion criteria used, and the prospective trials have by and large been very long in duration; because of the structure of the trials, results are not available for up to 5 years following completion of accrual.
• Radiographic response has been used as a surrogate for efficacy; however, the relationship between response and 5-year disease control has been variable among studies, as have image sequences utilized to assess response.
• There is an increasing experience demonstrating that molecular-targeted therapies such as BRAF inhibitors and MEK inhibitors are effective in patients who have failed first-line chemotherapy; these studies have incomplete but somewhat reassuring short-term safety data.
• The rationale for utilizing molecular-targeted therapy in clinical trials for children with newly diagnosed LGGs is strong. It is unclear, however, whether this needs to be done as part of large, prospective randomized trials; there are a wealth of historical data available from chemotherapy-based trials, and conventional randomized trials will likely require over 8 years to accrue and another 3–5 years for evaluation.
• A major flaw in all prospective studies that have been done to date is the lack of consistent evaluation of functional outcome and biology. Future studies utilizing biologic-targeted therapy should focus on functional outcome; functional outcome measures may supply a quicker read-out of efficacy of the drug utilized and a more accurate test of its “true” benefit—important also from a health-economic perspective with expensive new drugs.
Evaluation of Therapies: Functional Outcome
Tumor measurements by MRI are increasingly being recognized as an insufficient assessment of the clinical response to therapy for children with LGGs. Neurologic, cognitive, endocrine, behavioral, emotional, visual, and adaptive functions are critical endpoints, if not the most critical, for pediatric LGGs.60 Therapies may exacerbate patients’ tumor-associated symptoms and thus may impair their overall function and quality of survival. The clinical impact of both the tumor and treatment effect may be measured by objective testing, clinician’s rating of symptoms and functional status, and patient-reported outcome (PRO) scales. PROs are often used to evaluate the impact of the disease and treatment in terms of symptoms, impact on activities of daily living, and quality of survival. The functional evaluations are summarized in Supplementary Table S1. These measures may take on even more importance in the future, as a major criterion for federal approval of the drug will likely include demonstration that the novel biologic agent shows greater functional improvement than “conventional” therapy, such as the chemotherapeutic agents presently in use.
Neurologic Function
Assessments of neurologic function, including both neurologic examination and cognitive testing, are critical in assessing therapy response. Tumor location, presence of hydrocephalus, and prior treatments may, however, have substantial impact upon the neurologic examination. There are currently no standardized neurologic assessment scales for children with LGGs. The Lansky performance scale attempts to reflect global neurologic function comparable to the Karnofsky performance status scale, but has been shown to be subjective and lacking reproducibility.60–63 Its use in response assessment is limited given that it is a global measure and may not address individual symptoms. The lack of a measurement scale of neurologic function specifically for brain tumor patients has been addressed by the Response Assessment in Neuro-Oncology (RANO) group with development of the Neurologic Assessment in Neuro-Oncology (NANO) scale.64 Assessment tools for specific functional motor skills, such as the 6-minute walk test, are available for a variety of other neurologic diseases but have yet to be evaluated for children with LGG.65
Visual Outcomes Among Children with Optic Pathway Gliomas
Outcome measures for optic pathway glioma (OPG) clinical trials should include visual endpoints, and treatment success should be based on these endpoints. Several studies have identified limitations of imaging outcome measures for OPGs, including discordance with visual outcomes following treatment.66,67 Consensus-based, evidence-driven recommendations for visual endpoints for OPG clinical trials, including Teller acuity cards, HOTV charts, optic disc pallor, and visual quality of life (utilizing the Children’s Visual Function Questionnaire), have been defined by the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) Visual Outcomes Committee68 and are applicable to sporadic OPG as well. Visual field assessments have not been included, due to concerns of difficulty in reproducibility in younger children, although visual field loss can be a major functional deficit in those with visual pathway LGGs; this gap needs to be rectified. Potential markers currently under evaluation for future testing include retinal nerve fiber layer measured by optical coherence tomography and MRI fractional anisotropy of the optic radiations. Both have been shown to correlate with vision in OPG patients69–71 but require further validation prior to routine inclusion in OPG clinical trials.
Cognitive, Adaptive Functioning and Quality of Survival
Risk factors for poor cognitive outcomes include age at diagnosis, male gender, tumor size and location, hydrocephalus, surgical resection, and dose and treatment volume of cranial radiation therapy.72–76 Although children with LGGs often demonstrate normal cognitive and adaptive functioning, they have increased risks for cognitive and adaptive functioning impairments. Due to costs and patients’ access to testing, embedded studies of cognitive functioning within clinical trials have often been hindered by poor compliance with cognitive evaluations, thereby limiting progress in understanding the cognitive functioning of survivors.
The selection of evaluations for cognitive functioning for clinical trials for children with LGGs must meet several criteria: They must be clinically meaningful, validated in multiple languages, simple, brief, and inexpensive to administer. The CogState is a relatively brief, validated, patient-completed, computer-based questionnaire of those neurocognitive processes which are known to be most affected among brain tumor survivors (ie, attention, processing speed, and memory) for children 5 years and older.77,78 Adaptive behavior scales like the Vineland Adaptive Behavior Scale (VABS) address everyday performance in the following domains: communication (expressive and receptive), daily living skills (personal, domestic, and community), socialization (interpersonal relationships, play and leisure, coping skills), motor skills (gross and fine with a ceiling of abilities at 7 y), and problem behaviors.79 The questionnaire is a widely available, multilanguage assessment of adaptive functioning that has been used in pediatric brain tumor populations, including LGG cohorts.80–86 It is applicable to all ages and can be completed from responses to telephone interviews or to a parent- or caregiver-rating form.87 The study design of the upcoming LOGGIC study in Europe will use the VABS as a primary endpoint. For this study, a trained research nurse will help to interview parents/guardians in an attempt to enhance participation and consistency of data. The 24-item Pediatric Quality of Life Inventory (PedsQL) Brain Tumor Module, validated for children age 2 to 18 years, encompasses the following 6 scales: cognitive problems, pain, movement and balance, procedural anxiety, nausea, and worry; it has been incorporated into a recently published phase II study of weekly vinblastine for children with LGG.49,88 Long-term assessments with the European Organisation for Research and Treatment of Cancer (EORTC) 30-item quality of life core questionnaire (QLQ-C30) and the 20-item QLQ for brain neoplasm (BN20) among adults surviving childhood LGG showed impaired quality of life (QoL) among those exposed to cranial radiotherapy.89 Other studies using PedsQL, the German Children’s Quality of Life questionnaire (KINDL), and the Netherlands Organization for Applied Scientific Research Academic Medical Centre Children’s Quality of Life Questionnaire (TACQOL-P) revealed relatively high QoL concerning psychosocial, physical, emotional, social, and school-functioning scales among pediatric LGG patients.90–93 Finally, the Patient-Reported Outcome Measurement Information System (PROMIS) QoL battery has been used for serial measurements of patient mobility, fatigue, pain interference, peer relationships, anxiety, and depressive symptoms among children with newly diagnosed cancer and neurofibromatosis.94,95
Endocrine Outcome Measures
Supratentorial midline LGGs potentially disturb the pituitary and/or the hypothalamus. Diencephalic syndrome is a rare disorder presenting with severe emaciation often associated with preservation or acceleration of linear growth, euphoria, hyperactivity, vomiting, irritability, nystagmus, and increasing head circumference occurring especially in children less than 2 years of age with diencephalic LGGs.96–98 In response to therapy, severe emaciation may be conversely replaced by inappropriate and rapid weight gain and central precocious puberty.99,100 Outcome measures for suprasellar tumors presenting with or without hormone dysfunction or failure require comprehensive auxological assessment (height, weight, body mass index), pubertal assessment (Tanner staging), and full evaluation of the hypothalamic pituitary axis (insulin-like growth factor 1, growth hormone dynamic testing, luteinizing hormone, follicle stimulating hormone, testosterone/estradiol, am cortisol, thyroid stimulating hormone, free thyroxine-4, prolactin, paired morning urine/plasma osmolality measurements).101,102
Conclusions and Recommendations
• Outcome for children with LGGs cannot be assessed by neuro-imaging alone, and neurologic, cognitive, visual, endocrine, behavioral, emotional, visual, and adaptive function are critical endpoints.
• Objective testing techniques are available to assess functional outcome but have not been widely used in prospective studies to date.
• Although visual outcomes, neurocognitive assessments, endocrinologic measures, and to some extent adaptive and QoL measures have been employed in studies of children with brain tumors and are ready to utilize as endpoints in prospective clinical trials, other important assessments, such as that of neurologic function, need to be better refined.
• The potential cumulative toxicities of these approaches, especially given the chronic nature of LGGs and the need for repeated treatment, will require ongoing assessments of benefits and sequelae; distinguishing the toxicities of these different therapies and their potential synergistic effects may be difficult.
Summary
Progress continues to be made both in our biological understanding of the complexity of pediatric LGG but also in our thinking of how this and other knowledge from past clinical studies can be used to more intelligently design the next generation of trials. Preliminary signals from early-phase trials of more targeted compounds are certainly promising, but more work is required to understand which patients will benefit most from such therapies; and if/how tumors will develop resistance that requires further targeting or preemptive blocking. A broader repertoire of model systems that recapitulates the diverse catalogue of LGG is therefore urgently needed. The addition of novel agents also does not mean simply adding a targeted inhibitor into old protocols and hoping for a “magic bullet” effect—much more detailed and rigorous ways of measuring the quality, not just the duration, of PFS and overall survival are required to truly understand the impact that these new treatments may have on patients with LGG. The overall spirit of this consensus meeting was positive and collaborative, with clear grounds for optimism, but also with an acknowledgment that the job remains far from over.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the A Kids’ Brain Tumor Cure‒PLGA Foundation and the Gilbert Family Neurofibromatosis Institute.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1. Louis DN, et al. WHO Classification of Tumours of the Central Nervous System, Revised. 4th ed IARC; 2016. [Google Scholar]
- 2. Ostrom QT, de Blank PM, Kruchko C et al. . Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;16(Suppl 10):x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Packer RJ, Pfister S, Bouffet E et al. . Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6):750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones DT, Hutter B, Jäger N et al. ; International Cancer Genome Consortium PedBrain Tumor Project Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qaddoumi I, Orisme W, Wen J et al. . Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131(6):833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Wu G, Miller CP et al. ; St Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bandopadhayay P, Ramkissoon LA, Jain P et al. . MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48(3):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramkissoon LA, Horowitz PM, Craig JM et al. . Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 2013;110(20):8188–8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louis DN, Perry A, Reifenberger G et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 10. Ryall S, Tabori U, Hawkins C. A comprehensive review of paediatric low-grade diffuse glioma: pathology, molecular genetics and treatment. Brain Tumor Pathol. 2017;34(2):51–61. [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez FJ, Perry A, Rosenblum MK et al. . Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol. 2012;124(5):627–641. [DOI] [PubMed] [Google Scholar]
- 12. Huse JT, Snuderl M, Jones DT et al. . Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133(3):417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rivera B, Gayden T, Carrot-Zhang J et al. . Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol. 2016;131(6):847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hovestadt V, Remke M, Kool M et al. . Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pajtler KW, Witt H, Sill M et al. . Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sturm D, Witt H, Hovestadt V et al. . Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 17. Mistry M, Zhukova N, Merico D et al. . BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol. 2015;33(9):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korshunov A, Ryzhova M, Hovestadt V et al. . Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129(5):669–678. [DOI] [PubMed] [Google Scholar]
- 19. Dahiya S, Haydon DH, Alvarado D, Gurnett CA, Gutmann DH, Leonard JR. BRAF(V600E) mutation is a negative prognosticator in pediatric ganglioglioma. Acta Neuropathol. 2013;125(6):901–910. [DOI] [PubMed] [Google Scholar]
- 20. Schwartzentruber J, Korshunov A, Liu XY et al. . Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 21. Ryall S, Krishnatry R, Arnoldo A et al. . Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun. 2016;4(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu G, Broniscer A, McEachron TA et al. ; St Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pages M, Beccaria K, Boddaert N et al. . Co-occurrence of histone H3 K27M and BRAF V600E mutations in paediatric midline grade I ganglioglioma. Brain Pathol. 2016;doi: 10.1111/bpa.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia MA, Solomon DA, Haas-Kogan DA. Exploiting molecular biology for diagnosis and targeted management of pediatric low-grade gliomas. Future Oncol. 2016;12(12):1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olow A, Mueller S, Yang X et al. . BRAF status in personalizing treatment approaches for pediatric gliomas. Clin Cancer Res. 2016;22(21):5312–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louis DN, Aldape K, Brat DJ et al. . cIMPACT-NOW (the Consortium To Inform Molecular and Practical Approaches to CNS Tumor Taxonomy): a new initiative in advancing nervous system tumor classification. Brain Pathol. 2016;doi:10.1111/bpa.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Louis DN, Aldape K, Brat DJ et al. . Announcing cIMPACT-NOW: the consortium to inform molecular and practical approaches to CNS tumor taxonomy. Acta Neuropathol. 2017;133(1):1–3. [DOI] [PubMed] [Google Scholar]
- 28. Karajannis MA, Legault G, Fisher MJ et al. . Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16(10):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sievert AJ, Lang SS, Boucher KL et al. . Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A. 2013;110(15):5957–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones DT, Kocialkowski S, Liu L et al. . Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaul A, Chen YH, Emnett RJ, Dahiya S, Gutmann DH. Pediatric glioma-associated KIAA1549:BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner. Genes Dev. 2012;26(23):2561–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Y, Alberta JA, Pilarz C et al. . A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro Oncol 2017;19(6):774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selt F, Hohloch J, Hielscher T et al. . Establishment and application of a novel patient-derived KIAA1549:BRAF-driven pediatric pilocytic astrocytoma model for preclinical drug testing. Oncotarget. 2016;8(7):11460–11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bajenaru ML, Hernandez MR, Perry A et al. . Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63(24):8573–8577. [PubMed] [Google Scholar]
- 35. Gronych J, Korshunov A, Bageritz J et al. . An activated mutant BRAF kinase domain is sufficient to induce pilocytic astrocytoma in mice. J Clin Invest. 2011;121(4):1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaul A, Chen YH, Emnett RJ, Gianino SM, Gutmann DH. Conditional KIAA1549:BRAF mice reveal brain region- and cell type-specific effects. Genesis. 2013;51(10):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ni J, Xie S, Ramkissoon SH et al. . Tyrosine receptor kinase B is a drug target in astrocytomas. Neuro Oncol. 2017;19(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ater JL, Xia C, Mazewski CM et al. . Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ater JL, Zhou T, Holmes E et al. . Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chintagumpala M, Eckel SP, Krailo M et al. . A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: a Children’s Oncology Group study. Neuro Oncol. 2015;17(8):1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gnekow AK, Falkenstein F, von Hornstein S et al. . Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy—report from the multicenter treatment study for children and adolescents with a low grade glioma—HIT-LGG 1996—of the Society of Pediatric Oncology and Hematology (GPOH). Klin Padiatr. 2004;216(6):331–342. [DOI] [PubMed] [Google Scholar]
- 43. Laithier V, Grill J, Le Deley MC et al. ; French Society of Pediatric Oncology Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy—results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21(24):4572–4578. [DOI] [PubMed] [Google Scholar]
- 44. Mahoney DH Jr, Cohen ME, Friedman HS et al. . Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro Oncol. 2000;2(4):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gnekow, et al. A European controlled trial of the addiction of etoposide to standard vincristine and carboplatin as part of an 18 month treatment program for childhood (<16 years) low-grade gliomas—a final report. Eur J Cancer. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Packer RJ, Ater J, Allen J et al. . Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. [DOI] [PubMed] [Google Scholar]
- 47. Massimino M, Spreafico F, Cefalo G et al. . High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20(20):4209–4216. [DOI] [PubMed] [Google Scholar]
- 48. Massimino M, Spreafico F, Riva D et al. . A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol. 2010;100(1):65–71. [DOI] [PubMed] [Google Scholar]
- 49. Lassaletta A, Scheinemann K, Zelcer SM et al. . Phase II weekly vinblastine for chemotherapy-naive children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium study. J Clin Oncol. 2016;doi:10.1200/JCO.2016.68.1585. [DOI] [PubMed] [Google Scholar]
- 50. Jakacki RI, Bouffet E, Adamson PC et al. . A phase 1 study of vinblastine in combination with carboplatin for children with low-grade gliomas: a Children’s Oncology Group phase 1 consortium study. Neuro Oncol. 2011;13(8):910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gururangan S, Fisher MJ, Allen JC et al. . Temozolomide in children with progressive low-grade glioma. Neuro Oncol. 2007;9(2):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nicholson HS, Kretschmar CS, Krailo M et al. . Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110(7):1542–1550. [DOI] [PubMed] [Google Scholar]
- 53. Stokland T, Liu JF, Ironside JW et al. . A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro Oncol. 2010;12(12):1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Banerjee A, Jakacki RI, Onar-Thomas A et al. . A phase I trial of the MEK inhibitor Selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor (PBTC) study. Neuro Oncol. 2017. doi: 10.1093/neuonc/now282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fangusaro JR, Onar-Thomas A, Young-Poussaint T et al. . A phase II prospective study of selumetinib in children with recurrent or refractory low-grade glioma (LGG): a Pediatric Brain Tumor (PBTC) study. Proceedings ASCO. 2017: 10504–10504. [Google Scholar]
- 56. Dombi E, Baldwin A, Marcus LJ et al. . Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kieran M, et al. in ESMO LBA19_PR. Copenhagen, Denmark, 2016. [Google Scholar]
- 58. Lassaletta A, Guerreiro Stucklin A, Ramaswamy V et al. . Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer. 2016;63(11):2038–2041. [DOI] [PubMed] [Google Scholar]
- 59. Limond JA, Bull KS, Calaminus G, Kennedy CR, Spoudeas HA, Chevignard MP; Brain Tumour Quality of Survival Group, International Society of Paediatric Oncology (Europe) (SIOP-E) Quality of survival assessment in European childhood brain tumour trials, for children aged 5 years and over. Eur J Paediatr Neurol. 2015;19(2):202–210. [DOI] [PubMed] [Google Scholar]
- 60. Hutchinson TA, Boyd NF, Feinstein AR, Gonda A, Hollomby D, Rowat B. Scientific problems in clinical scales, as demonstrated in the Karnofsky index of performance status. J Chronic Dis. 1979;32(9-10):661–666. [DOI] [PubMed] [Google Scholar]
- 61. Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987;60(7):1651–1656. [DOI] [PubMed] [Google Scholar]
- 62. Orr ST, Aisner J. Performance status assessment among oncology patients: a review. Cancer Treat Rep. 1986;70(12):1423–1429. [PubMed] [Google Scholar]
- 63. Roila F, Lupattelli M, Sassi M et al. . Intra and interobserver variability in cancer patients’ performance status assessed according to Karnofsky and ECOG scales. Ann Oncol. 1991;2(6):437–439. [DOI] [PubMed] [Google Scholar]
- 64. Nayak L, DeAngelis L, Wen P,. et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration in the Radiologic Assessment in Neuro-Oncology (RANO) Criteria (S22. 005). Neurology. 2014;82(10):S22–005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ulrich S, Hildenbrand FF, Treder U et al. . Reference values for the 6-minute walk test in healthy children and adolescents in Switzerland. BMC Pulm Med. 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fisher MJ, Loguidice M, Gutmann DH et al. . Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shofty B, Ben-Sira L, Freedman S et al. . Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer. 2011;57(3):481–485. [DOI] [PubMed] [Google Scholar]
- 68. Fisher MJ, Avery RA, Allen JC et al. ; REiNS International Collaboration Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81(21 Suppl 1):S15–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Avery RA, Hwang EI, Ishikawa H et al. . Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol. 2014;132(3):265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Avery RA, Liu GT, Fisher MJ et al. . Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151(3):542–549.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Blank PM, Berman JI, Liu GT, Roberts TP, Fisher MJ. Fractional anisotropy of the optic radiations is associated with visual acuity loss in optic pathway gliomas of neurofibromatosis type 1. Neuro Oncol. 2013;15(8):1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Beebe DW, Ris MD, Armstrong FD et al. . Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). J Clin Oncol. 2005;23(22):5198–5204. [DOI] [PubMed] [Google Scholar]
- 73. Ris MD, Beebe DW, Armstrong FD et al. ; Children’s Oncology Group Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(29):4765–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tonning Olsson I, Perrin S, Lundgren J, Hjorth L, Johanson A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 2014;51(4):515–521. [DOI] [PubMed] [Google Scholar]
- 75. Turner CD, Chordas CA, Liptak CC et al. . Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53(3):417–423. [DOI] [PubMed] [Google Scholar]
- 76. Ullrich NJ, Embry L. Neurocognitive dysfunction in survivors of childhood brain tumors. Semin Pediatr Neurol. 2012;19(1):35–42. [DOI] [PubMed] [Google Scholar]
- 77. Maruff P, Thomas E, Cysique L et al. . Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24(2):165–178. [DOI] [PubMed] [Google Scholar]
- 78. Roddy E, Sear K, Felton E et al. . Presence of cerebral microbleeds is associated with worse executive function in pediatric brain tumor survivors. Neuro Oncol. 2016;18(11):1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Organization, W. H. International Classification of Functioning, Disability and Health: ICF. 2001. [Google Scholar]
- 80. Ashford JM, Netson KL, Clark KN et al. . Adaptive functioning of childhood brain tumor survivors following conformal radiation therapy. J Neurooncol. 2014;118(1):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Msall ME. Developing preschool surveillance tools for adaptive functioning: lessons for neuro-oncology. Eur J Paediatr Neurol. 2010;14(5):368–379. [DOI] [PubMed] [Google Scholar]
- 82. Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children’s adaptive functioning following conformal radiation therapy for localized ependymoma. Int J Radiat Oncol Biol Phys. 2012;84(1):217–223.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Papazoglou A, King TZ, Morris RD, Morris MK, Krawiecki NS. Attention mediates radiation’s impact on daily living skills in children treated for brain tumors. Pediatr Blood Cancer. 2008;50(6):1253–1257. [DOI] [PubMed] [Google Scholar]
- 84. Pastore V, Colombo K, Villa F et al. . Psychological and adjustment problems due to acquired brain lesions in pre-school-aged patients. Brain Inj. 2013;27(6):677–684. [DOI] [PubMed] [Google Scholar]
- 85. Poggi G, Liscio M, Pastore V et al. . Psychological intervention in young brain tumor survivors: the efficacy of the cognitive behavioural approach. Disabil Rehabil. 2009;31(13):1066–1073. [DOI] [PubMed] [Google Scholar]
- 86. Vago C, Bulgheroni S, Usilla A et al. . Adaptive functioning in children in the first six months after surgery for brain tumours. Disabil Rehabil. 2011;33(11):953–960. [DOI] [PubMed] [Google Scholar]
- 87. Limperopoulos C, Majnemer A, Steinbach CL, Shevell MI. Equivalence reliability of the Vineland adaptive behavior scale between in-person and telephone administration. Phys Occup Ther Pediatr. 2006;26(1-2):115–127. [PubMed] [Google Scholar]
- 88. Palmer SN, Meeske KA, Katz ER, Burwinkle TM, Varni JW. The PedsQL brain tumor module: initial reliability and validity. Pediatr Blood Cancer. 2007;49(3):287–293. [DOI] [PubMed] [Google Scholar]
- 89. Nwachukwu CR, Youland RS, Chioreso C et al. . Health related quality of life (HRQOL) in long-term survivors of pediatric low grade gliomas (LGGs). J Neurooncol. 2015;121(3):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aarsen FK, Paquier PF, Reddingius RE et al. . Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106(2):396–402. [DOI] [PubMed] [Google Scholar]
- 91. Bhat SR, Goodwin TL, Burwinkle TM et al. . Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol. 2005;23(24):5493–5500. [DOI] [PubMed] [Google Scholar]
- 92. Bull KS, Liossi C, Peacock JL, Yuen HM, Kennedy CR; Children’s Cancer and Leukaemia Group (CCLG) Screening for cognitive deficits in 8 to 14-year old children with cerebellar tumors using self-report measures of executive and behavioral functioning and health-related quality of life. Neuro Oncol. 2015;17(12):1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Musial-Bright L, Panteli L, Hernáiz Driever P. Pediatric low-grade glioma survivors experience high quality of life. Childs Nerv Syst. 2011;27(11):1895–1902. [DOI] [PubMed] [Google Scholar]
- 94. Dobrozsi S, Yan K, Hoffmann R, Panepinto J. Patient-reported health status during pediatric cancer treatment. Pediatr Blood Cancer. 2017;64:doi:10.1002/pbc.26295. [DOI] [PubMed] [Google Scholar]
- 95. Wolters PL, Martin S, Merker VL et al. ; REiNS International Collaboration Patient-reported outcomes of pain and physical functioning in neurofibromatosis clinical trials. Neurology. 2016;87(7 Suppl 1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cappelli C, Grill J, Raquin M et al. . Long-term follow up of 69 patients treated for optic pathway tumours before the chemotherapy era. Arch Dis Child. 1998;79(4):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gan HW, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine morbidity after pediatric optic gliomas: a longitudinal analysis of 166 children over 30 years. J Clin Endocrinol Metab. 2015;100(10):3787–3799. [DOI] [PubMed] [Google Scholar]
- 98. Gnekow AK, Falkenstein F, von Hornstein S et al. . Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brauner R, Trivin C, Zerah M et al. . Diencephalic syndrome due to hypothalamic tumor: a model of the relationship between weight and puberty onset. J Clin Endocrinol Metab. 2006;91(7):2467–2473. [DOI] [PubMed] [Google Scholar]
- 100. Kilday JP, Bartels U, Huang A et al. . Favorable survival and metabolic outcome for children with diencephalic syndrome using a radiation-sparing approach. J Neurooncol. 2014;116(1):195–204. [DOI] [PubMed] [Google Scholar]
- 101. Clement SC, Schouten-van Meeteren AY, Boot AM et al. . Prevalence and risk factors of early endocrine disorders in childhood brain tumor survivors: A Nationwide, Multicenter Study. J Clin Oncol. 2016;34(36):4362–4370. [DOI] [PubMed] [Google Scholar]
- 102. Gan HW, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine morbidity after pediatric optic gliomas: a longitudinal analysis of 166 children over 30 years. J Clin Endocrinol Metab. 2015;100(10):3787–3799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.