Abstract
Background and Aims
Aridification is considered a selective pressure that might have influenced plant diversification. It is suggested that plants adapted to aridity diversified during the Miocene, an epoch of global aridification (≈15 million years ago). However, evidence supporting diversification being a direct response to aridity is scarce, and multidisciplinary evidence, besides just phylogenetic estimations, is necessary to support the idea that aridification has driven diversification. The cycad genus Dioon (Zamiaceae), a tropical group including species occurring from humid forests to arid zones, was investigated as a promising study system to understand the associations among habitat shifts, diversification times, the evolution of leaf epidermal adaptations, and aridification of Mexico.
Methods
A phylogenetic tree was constructed from seven chloroplast DNA sequences and the ITS2 spacer to reveal the relationships among 14 Dioon species from habitats ranging from humid forests to deserts. Divergence times were estimated and the habitat shifts throughout Dioon phylogeny were detected. The epidermal anatomy among Dioon species was compared and correlation tests were performed to associate the epidermal variations with habitat parameters.
Key Results
Events of habitat shifts towards arid zones happened exclusively in one of the two main clades of Dioon. Such habitat shifts happened during the species diversification of Dioon, mainly during the Miocene. Comparative anatomy showed epidermal differences between species from arid and mesic habitats. The variation of epidermal structures was found to be correlated with habitat parameters. Also, most of the analysed epidermal traits showed significant phylogenetic signals.
Conclusions
The diversification of Dioon has been driven by the aridification of Mexico. The Miocene timing corresponds to the expansion of arid zones that embedded the ancestral Dioon populations. As response, species in arid zones evolved epidermal traits to counteract aridity stress. This case study provides a robust body of evidence supporting the idea that aridification is an important driver of biodiversity.
Keywords: Aridification, climate change, cycads, Dioon, diversification, epidermal anatomy, habitat shift, Mexico, Zamiaceae
INTRODUCTION
Climate change strongly affects the distribution, diversity and abundance of biological groups (Crowley and North, 1988; Parmesan, 2006; Hoffmann and Sgrò, 2011). This effect is particularly drastic when environmental change is rapid, and species or populations can be at risk of extinction if unable to migrate (Petit et al., 2008). This possible scenario has gained considerable attention in conservation biology due to the great loss of diversity being inflicted during the current stage of climate change (Wake and Vredenburg, 2008; Mawdsley et al., 2009). Nevertheless, the appearance of new abiotic conditions can give rise to natural selection pressures that may promote adaptations and phenotypic plasticity (Chevin et al., 2010; Hoffmann and Sgrò, 2011). Adaptations corresponding to habitat variations tend to lead to the divergence of lineages (Arnegard et al., 2014), as has been observed from population genetics (Andrew et al., 2013), phylogenetics (Becerra, 2005; Good-Avila et al., 2006; Arakaki et al., 2011) and comparative micromorphology (Raven, 2002; Hetherington and Woodward, 2003) studies. Thus, climate change can be not only a threat to species, but also a driver of biodiversity (Bell and Collins, 2008).
Aridification, which is an acute manifestation of climate change, is an environmental pressure that can undermine the fitness of perennial tropical plants due to water stress (Kertész and Mika, 1999; Osakabe et al., 2014). Arid conditions have had a long-term influence on the distribution and composition of tropical forests in all continents (Briones, 1994; Becerra, 2005; Pennington et al., 2009). In response, diverse adaptations and strategies to aridity have originated across many plant groups (Chaves et al., 2002). For example, drought-adapted plant groups such as succulents diversified in synchrony with the expansion of arid zones during the Miocene (Good-Avila et al., 2006; Arakaki et al., 2011; Hernández-Hernández et al., 2014). This timing is consistent for ancient gymnosperm lineages that partially occur in arid areas, such as Cupressaceae (Pittermann et al., 2012) or Cycadales (Nagalingum et al., 2011; Salas-Leiva et al., 2013; Condamine et al., 2015). The phylogenetic studies cited above showed that the association between aridification and species diversification seems reasonable. However, evidence supporting the idea that diversification happened in direct response to aridity is scarce, and multidisciplinary evidence, besides just phylogenetic estimations, is necessary to support the idea that aridification has driven diversification.
Among the cycads, the Neotropical genus Dioon (Zamiaceae) is a promising group to trace the process of species diversification during the process of aridification. Whereas three species occur exclusively in humid forests in Mexico and Honduras, 12 species inhabit a mosaic of habitats with considerable variation in elevation, temperature and annual precipitation, ranging from humid forests to arid zones throughout the Sierra Madre mountain chains (Sabato and De Luca, 1985; Moretti et al., 1993; González et al., 2008). Such habitat variability occurs even between neighbouring populations and sister species (González et al., 2008), which suggests that the expansion of arid conditions produced a habitat continuum effect throughout the Mexican landscapes that restrained the ancestral humid habitats of Dioon in refugia areas (Hewitt, 2004; González et al., 2008). Empirical (Langin et al., 2015) and theoretical (Mallet et al., 2009) studies have shown that habitat continuums usually provide heterogeneous environmental pressures that facilitate divergence. Divergence in these varied environments can be favoured by spatially autocorrelated natural selection, which may be stronger in species with discrete distribution and localized dispersal (Richardson et al., 2014), as seems to be the case for Dioon (Vovides, 1990).
The comparison of phenotypic variation among species and its correlation to habitats can aid in understanding the evolutionary response to aridity. Previous studies have shown that micromorphology, rather than macromorphology, can provide promising insights into cycad evolution (Griffith et al., 2014; Barone Lumaga et al., 2015). In particular, epidermal structures that protect leaves from radiation and prevent water loss can be useful to explore the relationship between phenotypes and aridity (Lange et al., 1971; Yeats and Rose, 2013). Thus, we expect that the phylogenetic reconstruction of Dioon will depict the long-term effect of aridification by providing evidence that (1) cladogenesis and speciation events are associated with habitat shifts towards arid zones, and (2) phylogenetically independent epidermal variation reflects habitat variations among species. In this study, we show the timing of habitat shifts towards arid zones during the diversification of Dioon. Also, we found that the current habitat differentiation is consistent with variation of epidermal traits, suggesting that xeric Dioon species evolved phenotypic traits to counteract arid environmental pressures. With this evidence, we conclude that aridification has been a major driver in the diversification of Dioon.
MATERIALS AND METHODS
Phylogenetic analyses
Since the history of expansion and diversification of Dioon remained unsolved in previous studies (Sabato and De Luca, 1985; Moretti et al., 1993; González et al., 2008), we first needed to reconstruct an improved phylogeny to include species across all distribution ranges and habitats reached by the genus. We sampled 35 specimens representing all the distribution ranges of 14 Dioon species (Supplementary Data Fig. S1). The species D. planifolium was excluded because it was described after we completed our sampling (Salas-Morales et al., 2016). Our samples were obtained from three different sources: fresh leaflets of 32 living plants deposited in the F.J. Clavijero Botanic Garden (FCBG) of the Instituto de Ecología, A.C, Xalapa, Mexico; one dry leaflet of a herbarium specimen deposited in the National Herbarium of Universidad Nacional Autónoma de México (MEXU); and fresh leaflets of two living plants of D. sonorense collected in the field (Table 1).
Table 1.
List of Dioon accessions used in this study. Localities are indicated by a number (1–35) in the column headed Accession labels, which correspond to the distributions illustrated in Supplementary Data Fig. S1. Asterisks in the Accession labels column indicate accessions also included in epidermal anatomy analyses. Phylogenetic groups are coded as in Fig. 1: W, western; S, southern; E, eastern; Spi, Spinulosum clade. Habitat categories are based on Köppen climate classification. Climate variables refer to the nearest climate station as listed in the CONABIO database (García, 2004). Vouchers and sources (MEXU and FCBG) are annotated
| Accession label | Locality | Group | Köppen climate category | Precipitation (mm) | Altitude (m above sea level) | Temperature (°C) | Station name | Source | Voucher |
|---|---|---|---|---|---|---|---|---|---|
| (1) D. mejiae* | Honduras | Spi | Humid: Am | 1128 | 666 | 26.2 | Olanchito | FCBG | 2005-008B |
| (2) D. spinulosum* | Oaxaca, Tuxtepec | Spi | Humid: Am | 2167.7 | 19 | 24.4 | Tuxtepec | FCBG | 1980-208D |
| (3) D. rzedowskii | Oaxaca, San Bartolomé Ayautla | Spi | Humid: A(C)f(m) | 3463.8 | 733 | 21.8 | San Bartolomé Ayautla | FCBG | 1987-113 |
| (4) D. rzedowskii* | Oaxaca, Santo Domingo | Spi | Humid: A(C)f(m) | 3463.8 | 733 | 21.8 | San Bartolomé Ayautla | FCBG | 1993-129.01 |
| (5) D. edule* | Veracruz, Monte Obscuro | E | Subhumid: Aw0(w) | 843.6 | 160 | 24.4 | Actopan | FCBG | 2002-026H |
| (6) D. edule | Veracruz, Chavarrillo | E | Subhumid: Aw0(w) | 843.6 | 160 | 24.4 | Actopan | FCBG | 1982-327.01 |
| (7) D. edule* | Veracruz, Tuzamapan | E | Subhumid: Aw0(w) | 843.6 | 160 | 24.4 | Actopan | FCBG | 1975-002.03 |
| (8) D. edule | Querétaro, Landa de Matamoros | E | Humid: (A)C(m)(w) | 2024.7 | 800 | 19.6 | Abritas, El | FCBG | 2001-153.01 |
| (9) D. edule | San Luis Potosí, Tamasopo | E | Humid: (A)C(m)(w) | 1891.8 | 372 | 23.0 | Agua Buena | FCBG | 2001-071.01 |
| (10) D. angustifolium | Tamaulipas, Casas | E | Semidry: BS1(h’)hw | 755.9 | 25 | 23.9 | Soto la Marina | FCBG | 2001-151A |
| (11) D. angustifolium* | Tamaulipas, San Carlos | E | Subhumid: (A)C(w0) | 769.8 | 30 | 22.2 | San Carlos | FCBG | 1999-056.03 |
| (12) D. angustifolium* | Nuevo León, Linares | E | Subhumid: (A)C(x’)(w0) | 753.2 | 350 | 22.2 | Linares | FCBG | 2001-147A |
| (13) D. merolae* | Chiapas, Jiquipilas | S | Subhumid: Aw0 | 1041.6 | 480 | 25.2 | Las Flores | FCBG | 1992-180.03 |
| (14) D. merolae | Chiapas, La Sepultura | S | Subhumid: Aw0 | 1041.6 | 480 | 25.2 | Las Flores | FCBG | 2008-006C |
| (15) D. merolae | Oaxaca, Cerro El Gavilán | S | Semidry: BS0(h’)w(w) | 511.9 | 620 | 25.1 | Boquilla de Nejapa # 1 | FCBG | 2000-008-04 |
| (16) D. merolae | Oaxaca, La Yerbabuena | S | Semidry: BS1(h’)w(w) | 671.8 | 1003 | 23.5 | San Carlos Yautepec | MEXU | 1204147 |
| (17) D. holmgrenii | Oaxaca, Loxicha | S | Subhumid: Aw0(w) | 934.7 | 160 | 26.5 | Pochutla | FCBG | 2005-061D |
| (18) D. holmgrenii* | Oaxaca, San Gabriel Mixtepec | S | Subhumid: Aw0(w) | 1774.4 | 130 | 26.1 | Limón, El | FCBG | 1981-698.03 |
| (19) D. holmgrenii | Oaxaca, San Pedro Juchatengo | S | Subhumid: Aw0(w) | 908.6 | 875 | 24.7 | San Pedro Juchatengo | FCBG | 1987-382.01 |
| (20) D. argenteum* | Oaxaca, San Pedro Yolox | S | Semidry: BS0(h’)w(w) | 489.3 | 545 | 25.1 | Quiotepec | FCBG | 2000-007A |
| (21) D. purpusii* | Oaxaca, Cuicatlán | S | Semidry: BS0(h’)w(w) | 510.7 | 595 | 26.1 | Cuicatlán | FCBG | 1979-128A |
| (22) D. purpusii | Oaxaca, Pápalo | S | Subhumid: Cb(w1)(w) | 837.7 | 1850 | 16.0 | Concepción Pápalo | FCBG | 2000-001M |
| (23) D. caputoi* | Puebla, Caltepec | S | Dry: BWhw(w) | 319.1 | 1800 | 18.1 | Caltepec | FCBG | 2004-200.1 |
| (24) D. califanoi* | Oaxaca, Teotitlán | S | Semidry: BS1(h’)w(w) | 733.7 | 1050 | 21.9 | Teotitlán del Camino | FCBG | 1986-193.01 |
| (25) D. stevensonii | Guerrero, Achotla | W | Subhumid: Aw2(w) | 1277.5 | 1460 | 22.5 | Campo Morado | FCBG | 2004-046J |
| (26) D. stevensonii* | Guerrero, Coyuca de Catalán | W | Subhumid: Aw0(w) | 1035.1 | 340 | 27.5 | Placeres del Oro | FCBG | 2001-120A |
| (27) D. stevensonii | Michoacán, Arteaga | W | Subhumid: Aw0(w) | 917.3 | 860 | 22.7 | Arteaga | FCBG | 1982-122A |
| (28) D. tomasellii | Jalisco, El Tuito | W | Subhumid: Aw2(w) | 1958.0 | 600 | 22.6 | Tuito, El | FCBG | 1993-007.01 |
| (29) D. tomasellii | Jalisco, Huaxtla | W | Subhumid: (A)C(w0)(w) | 820.0 | 1366 | 20.5 | Acatlán de Juárez | FCBG | 2004-043B |
| (30) D. tomasellii | Nayarit, Las Varas | W | Subhumid: Aw0(w) | 976.3 | 872 | 22.7 | Compostela | FCBG | 1986-171.01 |
| (31) D. tomasellii* | Nayarit, Compostela | W | Subhumid: Aw0(w) | 976.3 | 872 | 22.7 | Compostela | FCBG | 2004-028A |
| (32) D. tomasellii | Sinaloa, Concordia | W | Subhumid: Aw0(w) | 830.9 | 178 | 24.5 | Concordia, La | FCBG | 2004-033A |
| (33) D. sonorense* | Sonora, Sierra de Álamos | W | Semidry: BS1(h’)hw | 641.4 | 389 | 23.6 | Álamos | FCBG | 2000-066A |
| (34) D. sonorense | Sonora, Nuri | W | Semidry: BS1(h’)hw | 677.2 | 450 | 23.5 | Nuri | Field | - |
| (35) D. sonorense | Sonora, Sierra de Mazatán | W | Semidry: BS1hw(x’) | 504.5 | 530 | 21.5 | Mazatán | Field | - |
For each individual, we isolated genomic DNA according to Doyle and Doyle (1987). Amplification of DNA sequences, by the polymerase chain reaction (PCR), was performed in a Takara PCR Thermal Cycler Dice (Takara Shuzo, Shiga, Japan) using 10 µL volumes containing 10× Ex Taq buffer (Takara), 2.5 mm MgCl2, 250 µm of each dNTP, 0.2 U rTaq DNA Polymerase (Takara), 1 µL of template DNA and 0.4 µm of each primer. Seven chloroplast DNA (cpDNA) regions and the nuclear ribosomal DNA (nrDNA) spacer ITS2 were amplified using the primers listed in Supplementary Data Table S1. For all the PCR runs, thermocycling conditions were 95 ºC for 1 min; 35 cycles of 95 ºC for 45 s, annealing temperature for 45 s and 72 ºC for 1 min; and a final extension at 72 ºC for 5 min. The amplified products were confirmed by electrophoresis in 1 % agarose gel with ethidium bromide (1 µg/ml). Purification of PCR products was done using ExoSAP-IT (USB, Cleveland, OH, USA) and cycle-sequencing reactions were performed using BigDye Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) following the instructions of the manufacturer. We purified the sequencing reaction products by ethanol precipitation, and Sanger sequencing was carried with an ABI 3500 DNA sequencer (Applied Biosystems). All 280 of the sequences produced can be found in the DNA Data Bank of Japan under the accession numbers LC183483–LC183762.
Editing of sequences was done with Bioedit v7.1.11 software (Hall, 1999). The alignment of sequences was performed with the Clustal X program (Thompson et al., 1997) with minimal manual adjustments. Indels were not considered, and thus the program TrimAl v.1.3 of Phylemon 2.0 (Sánchez et al., 2011) was used to remove sites with gaps and trim to uniform length for each partition. The cpDNA sequences for each accession were manually concatenated and a partition homogeneity test was run, as implemented in PAUP* 4.0 (Swofford, 2003), to test the significance of tree incongruence between concatenated cpDNA and nrDNA loci. Our results showed weak significance for tree incongruence (P = 0.02), which allowed us to concatenate nrDNA with cpDNA data.
To construct the phylogenetic relationships of the 35 accessions, Bayesian inference (BI) analyses were run in MrBayes 3.2.5 (Huelsenbeck and Ronquist, 2001). The most likely substitution model for each partition estimated by separate in jModelTest (Posada, 2009) was assigned for each partition. Monte Carlo Markov chains consisted of four iterations with 100 million generations, discarding 25 % of the generations as burn-in. To determine the root position, the homologous sequences of Cycas revoluta were retrieved from GenBank (EU497698.1 for nrDNA and JN867588.1 for cpDNA) and aligned into our matrix data for a parallel analysis. The lengths of partitions were trimmed to fit the length of the outgroup, but sites with gaps were not discarded in this run to avoid a large data loss. The software MEGA 6 (Tamura et al., 2013) was used to construct a parsimony tree with 100 000 bootstraps, considering gaps as a fifth state. The root position for the clade topology was found to be similar to Moretti et al. (1993), Nagalingum et al. (2011), Salas-Leiva et al. (2013) and Condamine et al. (2015), and was also used for our BI tree.
Estimation of divergence times
We constructed an ultrametric tree using Bayesian Evolutionary Analysis Sampling Trees (BEAST) v.1.8 software, preparing the input file using BEAUti (Drummond et al., 2012). The stem of the genus Dioon was constrained by a height of 56 Ma, as defined by the fossil showing the greatest macro-morphological resemblance to Dioon, Dioonopsis praespinulosa (Hollick, 1932; Erdei et al., 2012), which was also used for calibration in the studies of Condamine et al. (2015), Salas-Leiva et al. (2013) and Nagalingum et al. (2011). This stem calibration is a relaxed constriction that assumes that synapomorphies that link Dioonopsis with Dioon might have evolved at any time along the stem branch (Nagalingum et al., 2011), and it is a more conservative approach than attaching the fossils at the crown node. In view of the lack of fossil records within the genus Dioon, we used two biogeographical events to calibrate inner nodes. Using a lognormal distribution with standard deviation of 0.75, the Pacific Seaboard group and Southern group nodes were calibrated to 34 and 23 Ma, respectively. These times correspond to the start of the formation of the Sierra Madre Occidental (Van Devender, 2002) and Transversal Volcanic Belt (Morán-Zenteno, 1994), two events that promoted the flourishing and maintenance of the Neotropical flora in Mexico (Becerra, 2005). The distribution of Dioon is clearly associated with the orography of Mexico (González et al., 2008), and thus it is reasonable to associate the clade divergence of Dioon with the history of local orography. Also, the advantage of using these biogeographical events is that they are well dated, and can give more robust and accurate estimations than using only one fossil calibration (Landis, 2016). Priors for birth–death mean growth rate, birth–death relative death rate, lognormal relaxed clock and underlying lognormal distribution model were adjusted as in Condamine et al. (2015). The BI tree obtained from the combined data of the 35 accessions obtained in MrBayes with fixed root position was used as a starting tree after resolving its polytomies (as 0 branch length) with Mesquite 3.10 (Maddison and Maddison, 2016). The Monte Carlo Markov chain analyses were run for 100 million generations and sampled every 1000 generations. The software Tracer v1.6 (URL http://beast.community/tracer) was used to ensure that estimated sample sizes (ESSs) for all the priors were >200 to assess their convergence. The Tree Annotator program of BEAST was used to build a maximum clade credibility tree, discarding 25 % of trees as burn-in.
Anatomical descriptions
We described the epidermal variation of Dioon to test whether the anatomy is consistent with the current habitat differentiation between mesic zones (humid and subhumid) and arid zones (semidry and dry). There is a general tendency for plants in arid zones to show epidermal features that can potentially reduce water loss (Ehleringer, 1981). These features can be intrinsic to the species, but seasonal polymorphisms can also exist due to an effective phenotypic response to the environment (Aronne and De Micco, 2001). For this reason, we examined the epidermal anatomy of 14 Dioon species by using samples obtained from living plants deposited in the FCBG. These living plants seem to have conserved their intrinsic phenotype over at least one decade, regardless of the common humid environmental conditions of the botanic garden location where they are planted (A. P. Vovides, pers. obs.). We used leaflet material from 16 populations representing 14 species of Dioon (Table 2).
Table 2.
Variation of epidermal anatomical traits within the genus Dioon. Values (µm) are mean ± s.d. Accession labels correspond to those indicated in Table 1
| Accession label | Trait | Ring type | Furrows | Papillae | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||||
| (1) D. mejiae | 2.86 ± 0.1 | 24.2 ± 1.3 | 39.2 ± 1.49 | 420 ± 100 | 230 ± 40 | 15 ± 0 | 4 ± 2 | Edule | − | − |
| (2) D. spinulosum | 4.16 ± 0.2 | 29.38 ± 1.78 | 30.5 ± 1.57 | 490 ± 110 | 240 ± 60 | 21 ± 0 | 7.5 ± 1.5 | Edule | − | − |
| (4) D. rzedowskii | 3.76 ± 0.3 | 32.8 ± 1.56 | 38.3 ± 2.77 | 450 ± 150 | 225 ± 75 | 16 ± 0 | 8 ± 2 | Edule | − | − |
| (5) D. edule | 4.36 ± 0.3 | 23 ± 0.77 | 42 ± 2.89 | 270 ± 130 | 150 ± 50 | 10 ± 0 | 8.5 ± 3.5 | Edule | − | − |
| (7) D. edule | 4.36 ± 0.3 | 23 ± 0.77 | 42 ± 2.89 | 270 ± 130 | 150 ± 50 | 10 ± 0 | 8.5 ± 3.5 | Edule | − | − |
| (11) D. angustifolium | 4.52 ± 0.2 | 17.5 ± 1.6 | 42.5 ± 2.89 | 375 ± 125 | 185 ± 65 | 5 ± 0 | 2.5 ± 1.5 | Edule | + | − |
| (12) D. angustifolium | 4.52 ± 0.2 | 17.5 ± 1.6 | 42.5 ± 2.89 | 375 ± 125 | 185 ± 65 | 5 ± 0 | 2.5 ± 1.5 | Edule | + | − |
| (13) D. merolae | 6.06 ± 0.4 | 23.6 ± 1.5 | 42.1 ± 1.2 | 320 ± 30 | 205 ± 25 | 13 ± 0 | 7.5 ± 2.5 | Edule | + | − |
| (18) D. holmgrenii | 4.54 ± 0.2 | 24.5 ± 1.07 | 36.3 ± 1.07 | 300 ± 100 | 165 ± 15 | 12.5 ± 1.5 | 9 ± 3 | Edule | + | − |
| (20) D. argenteum | 7.81 ± 0.8 | 22.9 ± 0.9 | 56.3 ± 1.07 | 225 ± 75 | 200 ± 100 | 11 ± 0 | 8 ± 2 | Purpusii | + | + |
| (21) D. purpusii | 7.59 ± 0.5 | 19 ± 1.45 | 49.8 ± 0.87 | 325 ± 105 | 165 ± 85 | 11 ± 0 | 13.5 ± 3.5 | Purpusii | + | + |
| (23) D. caputoi | 9.26 ± 0.8 | 18.4 ± 1.6 | 64.8 ± 2.89 | 235 ± 85 | 450 ± 200 | 8 ± 0 | 8 ± 3 | Purpusii | + | + |
| (24) D. califanoi | 5.64 ± 0.3 | 23.6 ± 0.8 | 59.3 ± 1 | 275 ± 75 | 215 ± 35 | 13 ± 0 | 12 ± 5 | Purpusii | + | + |
| (26) D. stevensonii | 5.22 ± 0.4 | 13.1 ± 0.8 | 57 ± 1.2 | 300 ± 50 | 200 ± 50 | 13 ± 0 | 8.5 ± 3.5 | Purpusii | + | + |
| (31) D. tomasellii | 4.68 ± 0.3 | 17.8 ± 1.92 | 49.8 ± 0.87 | 350 ± 100 | 375 ± 75 | 15 ± 1 | 10 ± 3 | Purpusii | + | + |
| (33) D. sonorense | 4.36 ± 0.3 | 21.3 ± 0.98 | 38.5 ± 2.11 | 275 ± 75 | 225 ± 75 | 11 ± 0 | 8 ± 2 | Purpusii | + | + |
Traits: A, adaxial cuticle thickness; B, epistomatal chamber pore width; C, epistomatal chamber depth; D, stomatal band width; E, interstomatal band width; F, number of mucilaginous canals; G, number of circumvascular fibres.
Furrows and papillae: +, presence; −, absence
For transverse sectioning (TS), 2 cm pieces of freshly collected leaflets were sectioned with a sliding microtome. The sections were suspended in distilled water; the best were selected and subjected to histochemical staining with phloroglucinol–HCl for lignin (Chamberlain, 1932) and a mixture of Sudan III and IV for cuticles. Additional sections were fixed in formaldehyde/acetic acid/ethanol (FAA) for several hours before obtaining permanent microscopic preparations by double staining in safranin and fast green (Purvis et al., 1966) and mounting them in Histoclad®.
The following TS measurements were taken on ten randomly chosen replicates of each cell or tissue type for each of the leaflets sampled per taxon: thickness of adaxial epidermis, abaxial epidermis and cuticle at leaflet margin (measurement taken from the top anticlinal limit of the epidermal cell where cuticle is thinnest); TS dimensions of adaxial and abaxial epidermal cells; number of layers of girder sclerenchyma associated with the vascular bundles and sclerenchyma layers at leaflet margin; depth and width of epistomatal chamber (depth measurement taken from the top surface of the guard cells to the top of the uppermost encircling cell (Florin ring sensuVovides and Galicia, 2016) width measurement taken at the cuticle level of the epistomatal pore); and number of perivascular and intervascular G-fibres and number of mucilaginous canals. Observations and measurements were done with a calibrated microscope; digital photomicrographs were taken using a Zeiss Fomi III photomicroscope (Zeiss, Oberkochen, Germany) fitted with a Canon® digital camera.
For observation with a scanning electron microscope (SEM) of the outer cuticular abaxial surfaces, a piece of fresh leaflet (~1 cm) per species was dehydrated in a desiccator for 24 h. The samples were sputter-coated with gold–palladium (1.5 kV, 5 mA for 8 min) with a JEOL Fine Coat JFC 1100 (JEOL, Tokyo, Japan) sputter coater. Observations were made with a JEOL JSM-5600LV (JEOL, Tokyo, Japan) SEM.
Reconstruction of ancestral traits
Köppen climate classification, mean annual precipitation, annual mean temperature and altitude for each of the populations were assigned by referencing the nearest Mexican climate station published in the geoinformation database of Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (García, 2004). For D. mejiae, climate data were taken from World Weather Online (http://www.worldweatheronline.com) for the nearest station (Olanchito, Honduras). For further analyses, similar Köppen climate classifications were merged into four categories: humid (Am, A(C) or (A)C); subhumid (Aw or Cbw); semidry (BS); and dry (BW).
The reconstruction of ancestral habitats was made by tracing the four climate categories on the BI tree constructed from 35 accessions using the maximum likelihood (Markov k-state one-parameter model) method (Lewis, 2001) implemented in Mesquite (Maddison and Maddison, 2016). In addition, the anatomical categorical data annotated in Table 2 (Florin ring and absence/presence of papillae and furrows) were each traced using the same maximum likelihood method in a Bayesian tree constructed in MrBayes, running four iterations of 100 million MCMC, sampling every 1000 generations and discarding 25% of generations as burn-in, but using only the 16 accessions for which both DNA sequences and anatomical data were available.
Association between anatomical traits and habitat variables
Anatomical measurement data (characters A–G in Table 2) were subjected to estimations of linear regressions with altitude, annual precipitation and mean annual temperature variables, by using two methods: considering and not considering the phylogenetic relationships among accessions. We estimated both the cross-species correlations with the square of the Pearson correlation coefficient (R2) and P-values in Excel®, and the correlations with the square of the standardized phylogenetically independent contrast coefficients (PICC2) (Felsenstein, 1985) determined with the R package ape (Paradis et al., 2004).
The assumption of phylogenetic independence was tested to verify whether the variation of anatomical traits among species is affected by phylogenetic signal (Abouheif, 1999). Anatomical data were linked to the tips of the phylogenetic tree of 16 accessions using the R package phylobase (Hackathon et al., 2015). We estimated Abouheif’s C (Cmean) and P-values for each trait by using Abouheif’s test (abouheif.moran with 100 000 repetitions) as implemented in the R package adephylo (Jombart et al., 2010). In addition, Pagel’s λ was estimated with corPagel correlation structure using the R package ape (Paradis et al., 2004) in a generalized least squares model.
RESULTS
Phylogenetic reconstruction of Dioon
We produced a data matrix (6048 bp) from seven concatenated sequences of the maternally inherited cpDNA (Cafasso et al., 2001), and the nrDNA ITS2 spacer. Both of the Bayesian trees we obtained from cpDNA and ITS2 data resolved the previously well-recognized Spinulosum clade (Moretti et al., 1993; González et al., 2008) as sister to the clade consisting of the 11 remaining Dioon species (hereinafter called ‘Dioon major clade’) (Supplementary Data Fig. S2). Furthermore, in combination, cpDNA and ITS2 data showed that the Dioon major clade comprises three phylogenetic groups that are consistent with the geographical distributions: eastern, southern and western (Fig. 1, Table 1). At the inner nodes, we found that some species appear to be paraphyletic, especially in the eastern and the southern groups (Fig. 1). However, since we represented all habitat variants and distribution ranges for all the analysed species, we suggest that these apparent paraphyletic patterns are caused by incomplete taxonomic descriptions, in which certain populations that we included might represent new species. Nonetheless, our results represent a clearer and better supported infrageneric phylogeny of Dioon, improved from previous studies (Moretti et al., 1993; González et al., 2008).
Fig. 1.
Definition of infrageneric clades and groups in Dioon. A Bayesian tree was constructed from seven cpDNA regions and one nrDNA sequence region of 35 accessions representing 14 species of the cycad genus Dioon. Accession labels correspond to those shown in Table 1. Posterior probability values are indicated above branches. Phylogenetic groups are annotated on the right and their geographical distributions are illustrated in the upper-left inset. Asterisks indicate accessions that were also analysed on epidermal anatomy.
Dioon diversified towards arid zones since the Miocene
Our Bayesian tree topology from combined data was useful to construct an ultrametric tree, which estimated that Dioon lineages date back to the early Cenozoic (~56 Ma) (Fig. 2, Table 3, Supplementary Data Fig. S3). This estimation was congruent with a previous robust estimation on the global diversification of the gymnosperms (Crisp and Cook, 2011). In turn, the ultrametric tree was useful to reconstruct the ancestral states of habitats during the evolution of Dioon. We found that arid lineages repeatedly originated from mesic habitats during the diversification of Dioon, especially during the Miocene. The lineage that gave rise to the Spinulosum clade diversified exclusively in humid habitats at lower altitudes and latitudes of south-east Mexico and Central America. In addition, the Dioon major clade expanded along eastern Mexico, where one shift to dry habitat occurred. Also, two lineages (southern and western groups) expanded towards the Pacific Seaboard during the early orogenic events of the Miocene that promoted diversification driven by aridification in southern Mexico (Morán-Zenteno, 1994; Nieto-Samaniego et al., 1999). Moreover, the consolidation of the Sierra Madre Occidental along western Mexico resulted in a corridor towards the Nearctic zone (Halffter, 1987) that allowed the establishment of Dioon in central Sonora, the northernmost distribution for Dioon in western Mexico. There, the habitat became drier due to the Pleistocene aridification (Briones, 1994), ~2 Ma, leading to the most recent speciation with a habitat shift in D. sonorense.
Fig. 2.
The history of diversification of Dioon is associated with the aridification of Mexico. This ultrametric tree was constructed with BEAST (Drummond et al., 2012) using a birth–death model. Bold node labels (i, ix and xii) indicate calibration nodes. Left inset indicates the climate-based habitat categories, and the pie charts at nodes represent probabilities for the ancestral climate categories estimated with the maximum likelihood method in Mesquite (Maddison and Maddison, 2016). Ages of relevant nodes are annotated in Table 3. The distribution of groups is illustrated in the upper left inset (W, western; S, southern; E, eastern; Spi, Spinulosum clade). Accession labels correspond to those used in Table 1. Asterisks indicate accessions that were also analysed on epidermal anatomy.
Table 3.
Divergence times of relevant nodes within the genus Dioon. Node labels correspond to those used in Fig. 2. Mean age was estimated from the highest posterior density 95 %
| Node | Label | Age (Ma) | Highest posterior density 95 % |
|---|---|---|---|
| Dioon crown | i | 55.92 | 53.99–57.89 |
| Spinulosum clade | ii | 30.15 | 15.47–46.57 |
| D. spinulosum–D. rzedowskii | iii | 14.58 | 5.69–25.8 |
| Dioon major clade crown | iv | 43.33 | 30.49–55.39 |
| Eastern group A | v | 28.61 | 12.62–45.36 |
| Dioon major clade A | vi | 37.43 | 25.19–49.38 |
| Eastern group B | vii | 24.11 | 9.5–39.12 |
| Dioon major clade B | viii | 25.7 | 17.21–35.7 |
| Southern group | ix | 19.08 | 12.11–26.77 |
| Southern – east | x | 14.49 | 8.23–21.4 |
| Southern – west | xi | 14.07 | 7.73–20.93 |
| Western group | xii | 18.02 | 10.51–26.26 |
| D. tomaselii–D. sonorense | xiii | 10.07 | 4.89–16.18 |
| D. sonorense | xiv | 1.82 | 0.17–4.4 |
Association between habitat variations and epidermal traits
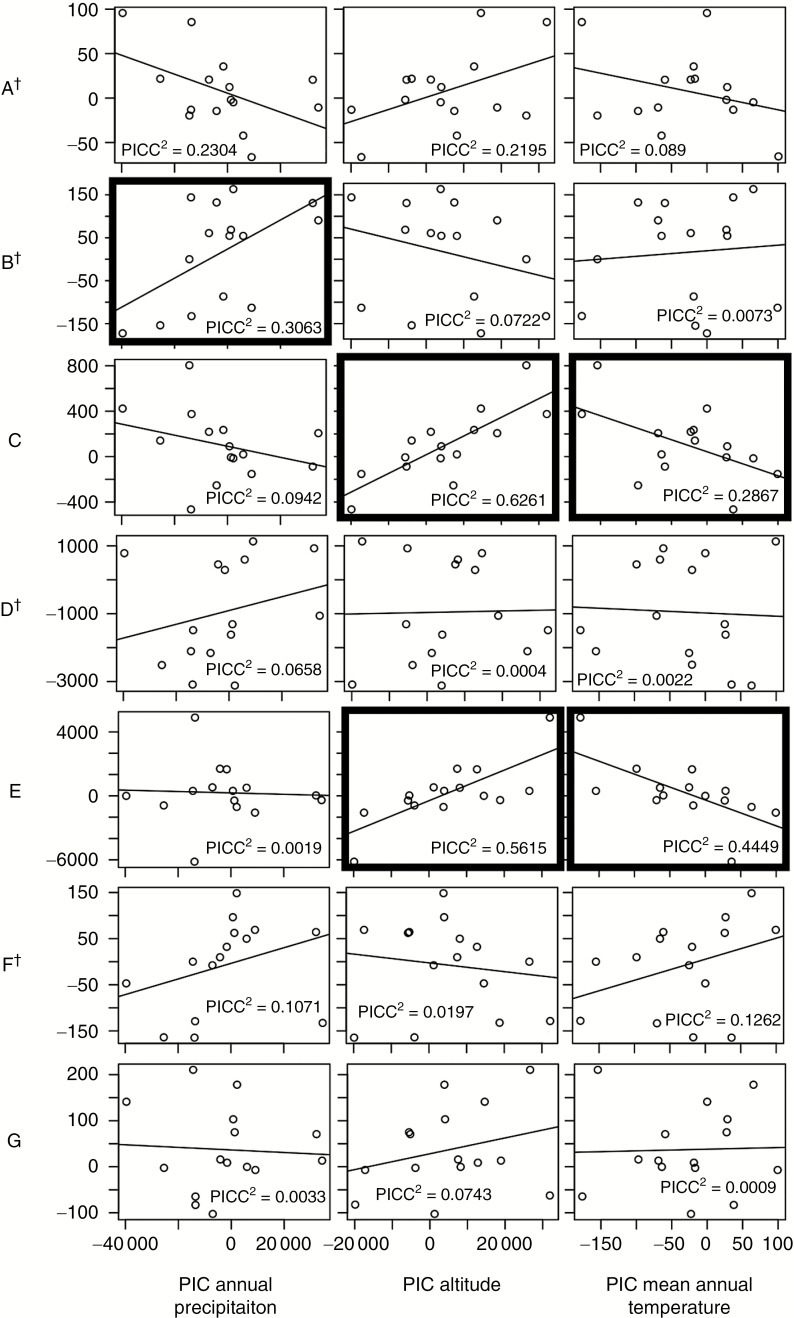
We found correlations suggesting that arid environments influenced the formation of xeromorphic epidermal traits in Dioon (Table 4, Figs 3 and 4). Cuticle thickness, stomatal chamber width and depth, stomatal band width, and number of mucilaginous canals are significantly correlated with habitat variables, especially with annual mean precipitation, in the cross-species correlations. Furthermore, traits corresponding to the architecture of the stomatal chambers significantly correlate with the habitat variations in both the cross-species and the phylogenetically independent contrast (PIC) correlations: deep and narrow chambers are associated with arid habitats or high altitudes, whereas shallow and wide chambers are associated with mesic habitats or low altitudes. Yet in many cases cross-species correlations are weakened in the PIC analyses. Four out of seven traits with measurement data showed phylogenetic signal, as estimated with Abouheif’s Cmean (Table 4). Cuticle thickness, epistomatal chamber pore width, band width and number of mucilaginous canals showed significant Cmean and their significances were confirmed by finding relatively high Pagel’s λ values, >0.5 in all significant cases).
Table 4.
Relationships among epidermal traits, habitat variables and phylogeny of Dioon. Values obtained from phylogenetic signal tests, cross-species correlations and PIC correlations between habitat variables and measurements of epidermal traits of Dioon are indicated. Values supported by significant P-values are indicated in bold text
| Trait | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |||
| Phylogenetic signal tests | Abouheif’s test | Cmean | 0.333* | 0.4146** | 0.1813 | 0.5379** | 0.0436 | 0.4632** | 0.2381 |
| P | 0.0266 | 0.0055 | 0.1304 | 0.0012 | 0.2944 | 0.0015 | 0.0686 | ||
| Pagel’s test | λ | 0.5826 | 0.8603 | 0.3887 | 0.7983 | -0.3985 | 2.6585 | 0.5012 | |
| Cross-species correlations | Precipitation | R 2 | 0.2592* | 0.5574*** | 0.3145* | 0.4796** | 0.0104 | 0.3788* | 0.0018 |
| P | 0.044 | 0.0008 | 0.024 | 0.0029 | 0.7072 | 0.0111 | 0.8761 | ||
| Altitude | R 2 | 0.3473* | 0.012 | 0.5106** | 0.0827 | 0.6248*** | 0.0015 | 0.0818 | |
| P | 0.0162 | 0.6864 | 0.0018 | 0.2802 | 0.0002 | 0.8868 | 0.2828 | ||
| Temperature | R2 | 0.0824 | 0.0008 | 0.0976 | 0.0032 | 0.4111* | 0.1038 | 0.0208 | |
| P | 0.2811 | 0.9174 | 0.2387 | 0.8353 | 0.0074 | 0.2237 | 0.5941 | ||
| PIC correlations | Precipitation | PICC2 | 0.2304 | 0.3063* | 0.0942 | 0.0658 | 0.0019 | 0.1071 | 0.0033 |
| P | 0.0702 | 0.0323 | 0.266 | 0.3557 | 0.875 | 0.234 | 0.838 | ||
| Altitude | PICC2 | 0.2195 | 0.0722 | 0.6261*** | 0.0004 | 0.5615** | 0.0197 | 0.0743 | |
| P | 0.0782 | 0.3328 | 0.0004 | 0.94 | 0.0013 | 0.6175 | 0.3254 | ||
| Temperature | PICC2 | 0.089 | 0.0073 | 0.2867* | 0.0022 | 0.4449** | 0.1262 | 0.0009 | |
| P | 0.28 | 0.7611 | 0.0397 | 0.8653 | 0.0066 | 0.1938 | 0.915 | ||
Traits: A, adaxial cuticle thickness; B, epistomatal chamber pore width; C, epistomatal chamber depth; D, stomatal band width; E, interstomatal band width; F, number of mucilaginous canals; G, number of circumvascular fibres.
*P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 3.
Pearson product-moment correlation plots between trait measurements (vertical axes, in μm) and habitat variables (horizontal axes) in Dioon. Squared correlation coefficient (R2) values are indicated in each plot. Colour of plotted values corresponds to habitat categories as indicated in Fig. 1. Significant correlations (P < 0.05) are indicated with bold borders. Traits A–G correspond to those indicated in Table 2, and daggers (†) indicate traits that show significant phylogenetic signal. Values are detailed in Table 4.
Fig. 4.
Phylogenetically independent contrast (PIC) correlation plots between trait measurements (vertical axes) and habitat variables (horizontal axes) in Dioon. Values of the squared PIC coefficient (PICC2) (Paradis et al., 2004) are indicated in each plot. Significant correlations (P < 0.05) are indicated by bold borders. Traits A–G correspond to those indicated in Table 2, and daggers (†) indicate traits that show significant phylogenetic signal. Values are detailed in Table 4.
We confirm the presence of Florin rings associated with stomata in all the Dioon species. Florin rings are thick cutin layers overlying the uppermost cells surrounding the stomata (Vovides and Galicia, 2016). These Florin rings can be categorized into two types (Fig. 5A, Supplementary Data Fig. S4). The Edule ring type (with papillae absent) is the basal ring type of the phylogeny. It is present in the Spinulosum clade and mesic species of the eastern and southern groups. The Purpusii ring type (with papillae present) is associated with the diversification of species from arid habitats, especially in the western group. We suggest that rings of the Purpusii ring type, with their varying number of papillae, have an opening–closure function that regulates water loss through evaporation during the gas exchange process or following dust exposure. In addition, both ring types were often found to contain wax plugs, which are usually involved in resistance to water loss, and prevention of stomatal closure under conditions of stress (Feild et al., 1998; Mohammadian et al., 2007). This seems to be advantageous during crassulacean acid metabolism (CAM) cycling (Haworth and McElwain, 2008), as this process has been reported for D. edule (Vovides et al., 2002; Yáñez-Espinosa et al., 2014) and is probably present in other Dioon species.
Fig. 5.
Categorical anatomical traits traced in the phylogeny of Dioon using the maximum likelihood method in Mesquite (Maddison and Maddison, 2016). (A) Ancestral reconstruction of absence/presence of papillae and its associated ring type, defined in the photographs at the right. Bars = 20 μm. (B) Ancestral reconstruction of furrows (photograph at the right; bar = 100 μm). Pie charts at nodes of trees represent the likelihood of the trait’s ancestral state.
In Dioon, the stomata are arranged in parallel bands (Vovides and Galicia; 2016). The widths of these bands positively correlate with annual precipitation (Fig. 3D), while separation lengths (interstomatal band width) positively correlate with altitude (Fig. 3E). Stomatal bands in most of the Dioon major clade species occur along furrows, which are structures associated with various layers of girder sclerenchyma that are associated with veins. If traced in the phylogeny of Dioon (Fig. 5B), we find that furrows are absent in the more mesic basal species, and they only originated during the divergence of the Dioon major clade.
DISCUSSION
Aridification as a driver of diversification in Dioon
This study suggests that aridification drove the diversification of species in the genus Dioon. Habitat shifts towards arid zones mostly occurred during the Miocene epoch, when the arid zones started to expand in Mexico due to orogenic events and climate change (Fig. 2). The current patchy distribution and the disparity of habitats suggest that the expansion of dry tracts might have caused the divergence of ancient mesic populations. This process might have been followed by climatic oscillations that initially promoted non-adaptive isolation and followed by speciation under different ecological conditions (Pérez-Farrera et al., 2014). Such ecological variation might have promoted the divergence of xeromorphic lineages in response to aridity.
Ecological and evolutionary trends in the diversification of Dioon
A previous study proposed the hypothesis that volcanism might have promoted the xeromorphic variation of Dioon (Barone-Lumaga et al., 2015). However, in our study we demonstrate that xeromorphic variations, such as thick cuticles, deep and narrow stomatal chambers, and the presence of furrows and papillae, might have originated as adaptations against water loss (Supplementary Data Text S1). We show for example that the Spinulosum clade, which comprises species that inhabit mesic habitats, show the thinnest cuticles in the genus. In contrast, the thickest cuticles are found in species from arid habitats of the Dioon major clade, where thick cuticles can be advantageous against water stress (Caldwell et al., 2007) or mechanical stress (Vovides and Galicia, 2016), and as protection from ultraviolet radiation in deserts and at high altitudes (Körner, 2007; Barone-Lumaga et al., 2015). In the same manner, stomatal chamber architecture shows significant correlations in both cross-species and PIC analyses, suggesting direct functional associations between stomata and habitat differentiations. Thus, whereas xeromorphic stomata are phylogenetically predisposed in the Dioon major clade, environmental pressure is also a factor that defines the presence of xeromorphic stomata. Furthermore, the presence of papillae, wax plugs and wax induments in species from arid areas and high altitudes may have multiple functions, ranging from solar radiation resistance or reduction of transpiration to repelling water or dust (Haworth and McElwain, 2008; Barone Lumaga et al., 2015; Vovides and Galicia, 2016). These patterns are consistent with other studies that also found deep and narrow chambers to be correlated to arid zones (Aronne and De Micco, 2001; Roth-Nebelsick, 2007).
Several traits we analysed show significant phylogenetic signals that indicate a unidirectional evolutionary trend towards aridity tolerance (Table 4, Figs 3 and 4), which might have been advantageous for dispersal to drier or elevated areas. This suggests that the acquisition of xeromorphic traits tends to be conserved during the evolution of Dioon, and xeromorphic variations that had arisen favoured the diversification trend towards arid habitats. This is backed by the PIC correlations, which better support the influence of phylogenetic dependence rather than local adaptation for most traits. However, significant correlations that were not supported by the PIC analysis do not necessarily preclude the possibility that traits associated with aridity were maintained under repeated stabilizing selection.
A phylogenetic predisposition of Dioon was favourable for adaptation to aridity in local landscapes, especially in the Dioon major clade, where in many cases populations co-habit with drought-avoiding (deciduous) and drought-resistant (succulent) plants. The evolution of xeromorphic cycads would result from the plasticity of the sustained ancestral traits that limited them to occupying underexploited niches, such as poor soils and extreme conditions with scarce competition from angiosperms (Vovides, 1990). Many populations inhabit sunlit slopes with rocky or poor shallow soils having high drainage. This niche occupancy is concordant with the local dispersal of Dioon species, which occurs through rodents that deposit the seeds in rock fissures (Vovides, 1990). Here, only a low proportion of seedlings survive if they are able to avoid high radiation and desiccation, and rapidly develop xeromorphic physiology (Yáñez-Espinosa et al., 2014; Yáñez-Espinosa and Flores, 2016). Such seedling survival might be related to natural selection under dry conditions.
The diversification of Dioon might also be favoured by other intrinsic factors, such as CAM-cycling photosynthesis (Vovides et al., 2002; Yáñez-Espinosa et al., 2014), or extrinsic factors, such as specificity of pollinators or dependence on association with mycorrhiza (Vovides, 1991; Vessey et al., 2005). Future studies that evaluate seed dispersal on a micro-scale and the in situ response of seedlings under different environmental conditions, considering natural selection during this stage, would help to better elucidate the success of cycads under these habitats, which are stressing and unusual for tropical forest trees (Webb and Peart, 2000) but common in the Dioon major clade.
Aridification as a driver of global biodiversity
The Miocene global aridification coincides with the timing of diversification of Dioon in Mexico, as well as for most of the other cycad genera in the world (Nagalingum et al., 2011; Salas-Leiva et al., 2013; Condamine et al., 2015). During the Miocene, cycads were able to diversify in a global scenario of climate change, and a predisposition to adapt into novel habitats would explain their long lineage persistence even through drastic mass extinction events (McElwain and Punyasena, 2007). Indeed, the Miocene was also a period of diversification for succulents, driven by the expansion of deserts, especially in America, Africa and Australia (Arakaki et al., 2011). Not surprisingly, these regions are the main centres of cycad diversity (Osborne et al., 2012). We suspect, because of habitat affinities, that other cycad genera in Australia (Cycas and Macrozamia) and Africa (Encephalartos) may have followed a similar diversification mechanism. Also, C4 grasses, succulent genera or agamosporic ferns with aridity tolerance and wide distributions (Anthelme et al., 2011) might have evolved with a similar pattern throughout tropical regions.
Our study helps expand our understanding of the long-term influence of climate change by showing the opposing facet of the effects of this phenomenon on biodiversity, not only as a threat to species, but also as a driver of the formation of new lineages. Since cycads represent an old lineage of seed plants, new evidence on diversification mechanisms obtained by using multiple approaches would provide a solid framework for the comprehension of plant evolution and speciation pathways, and also contribute to the conservation of this charismatic but threatened plant group.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: map of distribution of the 35 localities represented in this study. Figure S2: comparison of Bayesian tree topologies from cpDNA and ITS2 data. Figure S3: ultrametric tree of Dioon with highest posterior density 95 % bars at tree nodes. Figure S4: definition of stomatal structures in Dioon. Table S1: list of primers used in PCR. Text S1: discussion about the hypothesis of Barone-Lumaga et al. (2015).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Japan Society for the Promotion of Science (Japan) (grant 16J11945 to J.S.G.O) and the Consejo Nacional de Ciencia y Tecnología (Mexico) (grant CB-2011-01-169468 to A.V.). Thanks to Sonia Galicia for technical support, to FCBG for providing leaflet material from the Living National Cycad Collection, and to David Gernandt for facilitating access to the vouchers deposited in MEXU. Thanks to Takashi Tsuchimatsu, Alan Meerow, Nathalie Nagalingum and two anonymous reviewers for critical comments that helped improve the manuscript. Field living plants of D. sonorense were collected with permission of SEMARNAT, Mexico (No. SGPA/DGVS/12198).
LITERATURE CITED
- Abouheif E. 1999. A method for testing the assumption of phylogenetic independence in comparative data. Evolutionary Ecology Research 1: 895–909. [Google Scholar]
- Andrew RL, Kane NC, Baute GJ, Grassa CJ, Rieseberg LH. 2013. Recent nonhybrid origin of sunflower ecotypes in a novel habitat. Molecular Ecology 22: 799–813. [DOI] [PubMed] [Google Scholar]
- Anthelme F, Abdoulkader A, Viane R. 2011. Are ferns in arid environments underestimated? Contribution from the Saharan Mountains. Journal of Arid Environments 75: 516–523. [Google Scholar]
- Arakaki M, Christin PA, Nyffeler R et al. 2011. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proceedings of the National Academy of Sciences of the USA 108: 8379–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnegard ME, McGee MD, Matthews B et al. 2014. Genetics of ecological divergence during speciation. Nature 511: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronne G, De Micco V. 2001. Seasonal dimorphism in the Mediterranean Cistus incanus L. subsp. incanus. Annals of Botany 87: 789–794. [Google Scholar]
- Barone Lumaga MR, Coiro M, Truernit E, Erdei B, De Luca P. 2015. Epidermal micromorphology in Dioon: did volcanism constrain Dioon evolution?Botanical Journal of the Linnean Society 179: 236–254. [Google Scholar]
- Becerra JX. 2005. Timing the origin and expansion of the Mexican tropical dry forest. Proceedings of the National Academy of Sciences of the USA 102: 10919–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Collins S. 2008. Adaptation, extinction and global change. Evolutionary Applications 1: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones O. 1994. Origen de los desiertos mexicanos. Ciencia 45: 263–279. [Google Scholar]
- Cafasso D, Cozzolino S, Caputo P, Luca PD. 2001. Maternal inheritance of plastids in Encephalartos Lehm. (Zamiaceae, Cycadales). Genome 44: 239–241. [PubMed] [Google Scholar]
- Caldwell MM, Bornman JF, Ballaré CL, Flint SD, Kulandaivelu G. 2007. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochemical and Photobiological Sciences 6: 252–266. [DOI] [PubMed] [Google Scholar]
- Chamberlain CJ. 1932. Methods in plant histology. Chicago: University of Chicago Press. [Google Scholar]
- Chaves MM, Pereira JS, Maroco J. et al. 2002. How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany 89: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevin LM, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8: e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine FL, Nagalingum NS, Marshall CR, Morlon H. 2015. Origin and diversification of living cycads: a cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evolutionary Biology 15: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp MD, Cook LG. 2011. Cenozoic extinctions account for the low diversity of extant gymnosperms compared with angiosperms. New Phytologist 192: 997–1009. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, North GR. 1988. Abrupt climate change and extinction events in earth history. Science 240: 996–1002. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer J. 1981. Leaf absorptances of Mohave and Sonoran desert plants. Oecologia 49: 366–370. [DOI] [PubMed] [Google Scholar]
- Erdei B, Manchester SR, Kvaček Z. 2012. Dioonopsis Horiuchi et Kimura leaves from the Eocene of western North America: a cycad shared with the Paleogene of Japan. International Journal of Plant Sciences 173: 81–95. [Google Scholar]
- Feild TS, Zweiniecki MA, Donoghue MJ, Holbrook NM. 1998. Stomatal plugs of Drimys winteri (Winteraceae) protect leaves from mist but not drought. Proceedings of the National Academy of Sciences of the USA 95: 14256–14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- García E. 2004. Modificaciones al sistema de clasificación climática de Koppen. Mexico City: Universidad Nacional Autónoma de México. [Google Scholar]
- González D, Vovides AP, Bárcenas C. 2008. Phylogenetic relationships of the Neotropical genus Dioon (Cycadales, Zamiaceae) based on nuclear and chloroplast DNA sequence data. Systematic Botany 33: 229–236. [Google Scholar]
- Good-Avila SV, Souza V, Gaut BS, Eguiarte LE. 2006. Timing and rate of speciation in Agave (Agavaceae). Proceedings of the National Academy of Sciences of the USA 103: 9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith MP, Magellan TM, Tomlinson PB. 2014. Variation in leaflet structure in Cycas (Cycadales: Cycadaceae): does anatomy follow phylogeny and geography?International Journal of Plant Sciences 175: 241–255. [Google Scholar]
- Hackathon R, Bolker B, Butler M et al. 2015. phylobase: Base Package for Phylogenetic Structures and Comparative Data, version 0.8.0 https://github.com/fmichonneau/phylobase.
- Halffter G. 1987. Biogeography of the montane entomofauna of Mexico and Central America. Annual Review of Entomology 32: 95–114. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Haworth M, McElwain J. 2008. Hot, dry, wet, cold or toxic? Revisiting the ecological significance of leaf and cuticular micromorphology. Palaeogeography, Palaeoclimatology, Palaeoecology 262: 79–90. [Google Scholar]
- Hernández‐Hernández T, Brown JW, Schlumpberger BO, Eguiarte LE, Magallón S. 2014. Beyond aridification: multiple explanations for the elevated diversification of cacti in the New World Succulent Biome. New Phytologist 202: 1382–1397. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424: 901–908. [DOI] [PubMed] [Google Scholar]
- Hewitt GM. 2004. Genetic consequences of climatic oscillations in the Quaternary. Philosophical Transactions of the Royal Society of London B: Biological Sciences 359: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470: 479–485. [DOI] [PubMed] [Google Scholar]
- Hollick A. 1932. Descriptions of new species of Tertiary cycads, with a review of those previously recorded. Bulletin of the Torrey Botanical Club 59: 69–189. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jombart T, Balloux F, Dray S. 2010. Adephylo: new tools for investigating the phylogenetic signal in biological traits. Bioinformatics 26: 1907–1909. [DOI] [PubMed] [Google Scholar]
- Kertész Á, Mika J. 1999. Aridification – climate change in South-Eastern Europe. Physics and Chemistry of the Earth, Part A: Solid Earth and Geodesy 24: 913–920. [Google Scholar]
- Körner C. 2007. The use of ‘altitude’ in ecological research. Trends in Ecology and Evolution 22: 569–574. [DOI] [PubMed] [Google Scholar]
- Landis MJ. 2016. Biogeographic dating of speciation times using paleogeographically informed processes. Systematic Biology. doi:10.5061/dryad.dq666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange OL, Lösch R, Schulze ED, Kappen L. 1971. Responses of stomata to changes in humidity. Planta 100: 76–86. [DOI] [PubMed] [Google Scholar]
- Langin KM, Sillett TS, Funk WC, Morrison SA, Desrosiers MA, Ghalambor CK. 2015. Islands within an island: repeated adaptive divergence in a single population. Evolution 69: 653–665. [DOI] [PubMed] [Google Scholar]
- Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology 50: 913–925. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. 2016. Mesquite: a modular system for evolutionary analysis. Version 3.10 http://mesquiteproject.org.
- Mallet J, Meyer A, Nosil P, Feder JL. 2009. Space, sympatry and speciation. Journal of Evolutionary Biology 22: 2332–2341. [DOI] [PubMed] [Google Scholar]
- Mawdsley JR, O’Malley R, Ojima DS. 2009. A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conservation Biology 23: 1080–1089. [DOI] [PubMed] [Google Scholar]
- McElwain JC, Punyasena SW. 2007. Mass extinction events and the plant fossil record. Trends in Ecology and Evolution 22: 548–557. [DOI] [PubMed] [Google Scholar]
- Mohammadian MA, Watling JR, Hill RS. 2007. Do waxy stomatal plugs impact leaf gas exchange in a rain forest gymnosperm Agathis robusta?General and Applied Plant Physiology 33: 203–220. [Google Scholar]
- Morán-Zenteno DJ. 1994. The geology of the Mexican Republic. AAPG studies in geology, Vol. 39 Tulsa, OK: American Association of Petroleum Geologists. [Google Scholar]
- Moretti A, Caputo P, Cozzolino S et al. 1993. A phylogenetic analysis of Dioon (Zamiaceae). American Journal of Botany 80: 204–214. [Google Scholar]
- Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S. 2011. Recent synchronous radiation of a living fossil. Science 334: 796–799. [DOI] [PubMed] [Google Scholar]
- Nieto-Samaniego ÁF, Ferrari L, Alaniz-Alvarez SA, Labarthe-Hernández G, Rosas-Elguera J. 1999. Variation of Cenozoic extension and volcanism across the southern Sierra Madre Occidental volcanic province, Mexico. GSA Bulletin 111: 347–363. [Google Scholar]
- Osakabe Y, Osakabe K, Shinozaki K, Tran LSP. 2014. Response of plants to water stress. Frontiers in Plant Science 5 doi:10.3389/fpls 2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R, Calonje MA, Hill KD, Stanberg L, Stevenson DW. 2012. The world list of cycads. In: Proceedings of Cycad 2008. The 8th International Conference on Cycad Biology (Memoirs of the New York Botanical Garden Volume 106), 480–508. [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37: 637–669. [Google Scholar]
- Pennington RT, Lavin M, Oliveira-Filho A. 2009. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annual Review of Ecology, Evolution, and Systematics 40: 437–457. [Google Scholar]
- Pérez-Farrera MA, Vovides AP, Avendaño S. 2014. Morphology and leaflet anatomy of the Ceratozamia norstogii (Zamiaceae, Cycadales) species complex in Mexico with comments on relationships and speciation. International Journal of Plant Sciences 175: 110–121. [Google Scholar]
- Petit RJ, Hu FS, Dick CW. 2008. Forests of the past: a window to future changes. Science 320: 1450–1452. [DOI] [PubMed] [Google Scholar]
- Pittermann J, Stuart SA, Dawson TE, Moreau A. 2012. Cenozoic climate change shaped the evolutionary ecophysiology of the Cupressaceae conifers. Proceedings of the National Academy of Sciences of the USA 109: 9647–9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. 2009. Selection of models of DNA evolution with jModelTest. In: Posada D. ed. Bioinformatics for DNA sequence analysis. New York: Humana Press, 93–112. [DOI] [PubMed] [Google Scholar]
- Purvis MJ, Collier DC, Walls D. 1966. Laboratory techniques in botany. London: Butterworths. [Google Scholar]
- Raven JA. 2002. Selection pressures on stomatal evolution. New Phytologist 153: 371–386. [DOI] [PubMed] [Google Scholar]
- Richardson JL, Urban MC, Bolnick DI, Skelly DK. 2014. Microgeographic adaptation and the spatial scale of evolution. Trends in Ecology and Evolution 29: 165–176. [DOI] [PubMed] [Google Scholar]
- Roth-Nebelsick A. 2007. Computer-based studies of diffusion through stomata of different architecture. Annals of Botany 100: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabato S, De Luca P. 1985. Evolutionary trends in Dion (Zamiaceae). American Journal of Botany 72: 1353–1363. [Google Scholar]
- Salas-Leiva DE, Meerow AW, Calonje M et al. 2013. Phylogeny of the cycads based on multiple single-copy nuclear genes: congruence of concatenated parsimony, likelihood and species tree inference methods. Annals of Botany 112: 1263–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Morales SH, Chemnick J, Gregory TJ. 2016. A new cycad species in the genus Dioon (Zamiaceae) from the Mixteca region of Oaxaca, Mexico. Cactus and Succulent Journal 88: 35–42. [Google Scholar]
- Sánchez R, Serra F, Tárraga J et al. 2011. Phylemon 2.0: a suite of web-tools for molecular evolution, phylogenetics, phylogenomics and hypotheses testing. Nucleic Acids Research 39: W470–W474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP*: Phylogenetic analysis using parsimony (* and other methods). Version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Devender TR. 2002. Cenozoic environments and the evolution of the gopher tortoises (genus Gopherus). In: Van Devender TR. ed. The Sonoran desert tortoise. Tucson, AZ: University of Arizona Press and Arizona-Sonora Desert Museum, 29–51. [Google Scholar]
- Vessey JK, Pawlowski K, Bergman B. 2005. Root-based N2-fixing symbioses: legumes, actinorhizal plants, Parasponia sp. and cycads. In: Lambers H, Colmer TD eds. Root physiology: from gene to function. Netherlands: Springer, 51–78. [Google Scholar]
- Vovides AP. 1990. Spatial distribution, survival and fecundity of Dioon edule (Zamiaceae) in a tropical deciduous forest in Veracruz, Mexico, with notes on its habitat. American Journal of Botany 77: 1532–1543. [Google Scholar]
- Vovides AP. 1991. Vesicular-arbuscular mycorrhiza in Dioon edule Lindl. (Zamiaceae, Cycadales) in its natural habitat in central Veracruz, Mexico. Brenesia 35: 97–103. [Google Scholar]
- Vovides AP, Galicia S. 2016. G-fibers and Florin ring-like structures in Dioon (Zamiaceae). Botanical Sciences 94: 263–268. [Google Scholar]
- Vovides AP, Etherington JR, Dresser PQ, Groenhof A, Iglesias C, Ramirez JF. 2002. CAM‐cycling in the cycad Dioon edule Lindl. in its natural tropical deciduous forest habitat in central Veracruz, Mexico. Botanical Journal of the Linnean Society 138: 155–162. [Google Scholar]
- Wake DB, Vredenburg VT. 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences of the USA 105: 11466–11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CO, Peart DR. 2000. Habitat associations of trees and seedlings in a Bornean rain forest. Journal of Ecology 88: 464–478. [Google Scholar]
- Yáñez-Espinosa L, Flores J. 2016. Effects of shade on germination traits of the endangered cycad Dioon edule (Zamiaceae). Botanical Sciences 94: 1–6. [Google Scholar]
- Yáñez-Espinosa L, Flores J, Millán PSR, Méndez GR. 2014. Influence of germination date on Dioon edule (Zamiaceae) seedling tolerance to water stress. Journal of Plant Research 127: 413–422. [DOI] [PubMed] [Google Scholar]
- Yeats TH, Rose JK. 2013. The formation and function of plant cuticles. Plant Physiology 163: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.