Abstract
Objectives
To estimate mortality directly attributable to HIV in HIV-infected adults in low and middle income countries and discuss appropriate methodology.
Design
Illustrative analysis of pooled data from six studies across sub-Saharan Africa and Thailand with data on individuals with known dates of seroconversion to HIV.
Methods
Five of the studies also had data from HIV-negative subjects and one had verbal autopsies. Data for HIV-negative cohorts were weighted by the initial age and sex distribution of the seroconverters. Using the survival of the HIV-negative group to represent the background mortality, net survival from HIV was calculated for the seroconverters using competing risk methods. Mortality from all causes and ‘net’ mortality were modelled using piecewise exponential regression. Alternative approaches are explored in the dataset without information on mortality of uninfected individuals.
Results
The overall effect of the net mortality adjustment was to increase survivorship proportionately by 2 to 5% at 6 years post-infection. The increase ranged from 2% at ages 15–24 to 22% in those 55 and over. Mortality rate ratios between sites were similar to corresponding ratios for all-cause mortality.
Conclusion
Differences between HIV mortality in different populations and age groups are not explained by differences in background mortality, although this does appear to contribute to the excess at older ages. In the absence of data from uninfected individuals in the same population, model life tables can be used to calculate background rates.
Keywords: HIV/AIDS, life tables, mortality, relative survival, survival
Introduction
Differences in the natural history of HIV across study sites and by age at infection have been reported [1,2]. In both high income and low and middle income countries median survival after HIV infection decreases with increasing age at seroconversion [1,2]. A comparison across different study sites showed large differences between survival post-infection in studies from Thailand compared with studies in east and South Africa [2].
The variation in the natural history of HIV infection by study site and by age at infection could be caused, at least partly, by different levels of background mortality. Adult mortality invariably increases with age [3] and varied considerably from country to country even before the HIV epidemic [4].
In this paper we use data from six of the studies described in the paper by Todd et al. [2], and investigate the effect of removing mortality from causes other than HIV in order to compare the ‘net’ effect of HIV on survival post-infection across study sites and in comparisons by age at infection and sex. We also explore the use of alternative methods for eliminating background mortality, depending on data availability.
Methods
The study used data on individuals with known dates of HIV seroconversion from three east African community studies with repeat serosurveys, and from cohorts of South African mineworkers, Thai soldiers, and Thai blood donors and their partners. These studies are described in detail elsewhere [2,5–10].
The methods used to estimate net HIV-related mortality varied by site. Five of the sites also had data on survival in HIV-negative individuals. The other study had verbal autopsy information on causes of death for all deaths occurring during the follow-up [10].
The three east African community-based studies all collected data on uninfected individuals and follow-up procedures were identical for infected and uninfected people [5–7].
The South Africa miners cohort also followed up miners who had a negative HIV test; but these observations were censored at 2 years to ensure that if there were any undetected seroconversions the observation period would cover only the very early stages after infection in which the impact on mortality would be minimal [8]. In order to obtain a survival curve for uninfected individuals in the miners cohort over the whole period, the age-specific mortality rates were calculated from the first 2 years of follow-up and were used to estimate mortality rates up to 11 years post-infection, allowing for the ageing of the cohort over the period using one-year age bands. As there were few miners over the age of 50 years, directly measured mortality rates were unreliable. Therefore, for these older age groups, age-specific death rates were taken from the South African population in 1990 [11] (and I. Timaeus, personal communication), which were assumed to be little influenced by HIV.
The Thai military cohort study followed up a comparison group of HIV-negative men matched by time of induction into the Royal Thai Army and district of residence [9]. Uninfected individuals were not followed up in the Thai blood donor and partner cohort [10], but verbal autopsy data were collected, which recorded whether HIV / AIDS was the likely cause of death.
In order to allow comparison of HIV-positive and negative cohorts, allowing for any differences in age and sex composition, the data in the HIV-negative cohorts were directly standardized to the sex and age distribution at the time of seroconversion in the HIV-positive cohorts.
Net survival was calculated from the gross survival and survival of the (weighted) population of uninfected individuals using the relationship:
| (1) |
where SN(x) represents net survival up to time x, SG(x) is the gross survival (i.e. the observed survival) among seroconverters, and SU(x) is the corresponding survival among uninfected individuals. In some subgroups of the data, the survival of uninfected individuals was less favourable than the survival of infected individuals. This can occur because of the small numbers at risk, or in the period shortly after infection when events may be unobservable as a result of left truncation. These are minor effects and in these cases the net survival was taken to be equal to the survival during the previous interval, thereby satisfying the logical requirement that a survival curve cannot increase over time.
For the Thailand blood donor and partner cohort, for which there was no HIV-negative comparison group, net survival was calculated by two methods. The first method used verbal autopsy data, censoring those who were reported to have died from causes other than HIV. The second method makes use of general population life tables. A ‘without AIDS’ model life table for Thailand by sex was taken from the U N Population Division estimates and projections [12]. The tabulated 5-year life table was expanded to obtain yearly estimates, the death rates within 5-year age groups are assumed to be constant and the annual mortality hazard, l, in the interval between age x and age x + 5 is computed from the tabulated survival function lx at these ages:
| (2) |
The annual mortality hazards were applied to a population with a sex and age structure equivalent to the seroconverter cohort at the time of seroconversion to estimate the survival that would have been experienced by the cohort from the time of seroconversion if they had not been infected. Net survival was then calculated using Eq. (1). To assess the reliability of this method, it was also applied to the Tanzanian and Ugandan data using the appropriate country and sex-specific life tables in order to compare results with those directly measured in the cohorts.
Kaplan–Meier analysis was used to describe unadjusted survival functions. A piecewise exponential regression based on single-year intervals of time since infection was used to investigate adjusted rate ratios. Stata version 9.0 (Stata Corp., College Station, Texas, USA) was used for all analyses.
Excess mortality was modeled using piecewise exponential regression [13]. This was implemented in Stata in the framework of a generalized linear model using a customized link function that scales the results in terms of excess mortality rather than total deaths [14]. The probabilities of dying by study site, age at infection and sex were generated for the HIV-negative individuals. These were then applied to each year of follow-up for the seroconverter cohort to obtain the expected number of deaths if the cohort of seroconverters had experienced the same mortality as the uninfected cohort.
Results
Table 1 shows the numbers of uninfected individuals for whom survival data were available. Person-years are much larger in the community sites than in the occupational cohorts from South Africa and Thailand.
Table 1.
Description of HIV-negative individuals in each cohort.
| Age at first test (years) | Negative follow-up time | |||||
|---|---|---|---|---|---|---|
| Study | No. negative at first test | Median | Range | Deaths | Total person-years | Maximum person-years |
| Kisesa, Tanzania | 14 451 | 25 | 15–95 | 326 | 60 262 | 10.6 |
| Masaka, Uganda | 9067 | 27 | 15–95 | 644 | 52 299 | 14.1 |
| Rakai, Uganda | 27 581 | 23 | 15–89 | 608 | 108 584 | 12.8 |
| South African miners | 6164 | 32 | 17–78 | 84 | 11 777 | 2.0 |
| Thailand military | 251 | 22 | 20–30 | 8 | 1295 | 6.6 |
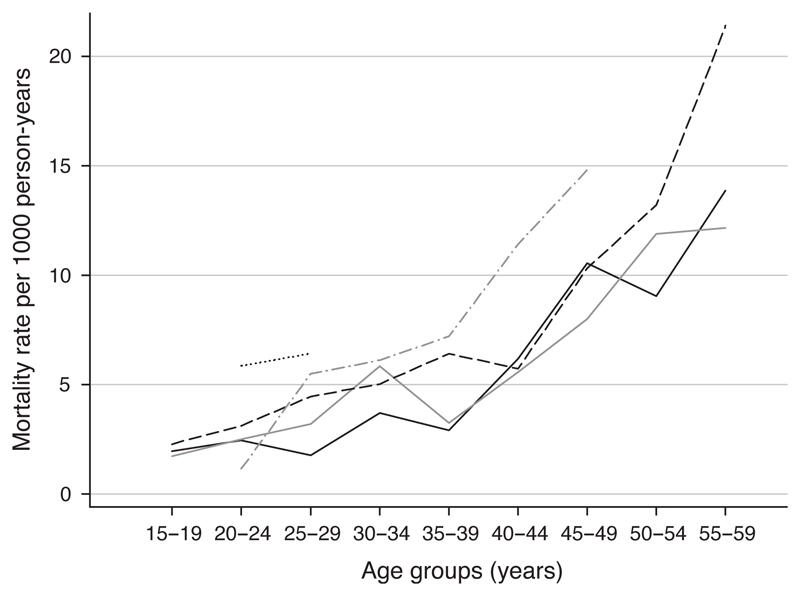
Less than 12% of uninfected individuals died within 10 years of their first test. Figure 1 shows the age-specific mortality rates of the HIV-negative individuals by site. The Thai military cohort shows a much higher mortality in the 20–29 year age range than the other sites, with a mortality rate of 6.4 [95% confidence interval (CI) 2.7–15.4] per 1000 person-years in the 25–29 year age group compared with 2.7 (95% CI 2.1–3.4) in the combined east African sites. Mortality in Kisesa was generally lower at younger ages than in the other sites. At older ages the South African miners cohort had higher mortality rates, but the confidence intervals are wide as a result of the small numbers of person-years of follow up. Overall mortality rates among the miners were slightly higher than in the combined east African sites (age-adjusted mortality rate ratio 1.4, 95% CI 1.1–1.8).
Fig. 1.
Age-specific mortality rates for HIV-negative individuals, by study site.  Kisesa;
Kisesa;  Masaka;
Masaka;  Rakai;
Rakai;  South African miners; Thai military.
South African miners; Thai military.
Within each of the African studies the HIV-negative cohorts were older than the HIV-positive cohorts at the time of origin of follow-up. Estimates of net mortality from HIV within each study were adjusted for age differences by using the weighted rates for the HIV-negative groups. Table 2 shows the measured gross survival patterns in each site, and the estimated net survival after removing background mortality. Differences were small: less than 5% even in sites with long follow-ups. The proportionate increase in survival at 8 years ranged from 3 to 7%. In terms of years added, 25% net mortality was reached 0.2–0.6 years later than the 25% gross mortality in each cohort.
Table 2.
Gross (observed) and net (adjusted for non-HIV mortality) survival of seroconverters.
| Kisesa |
Masaka |
Rakai |
SA miners |
Thai military |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Years after HIV seroconversion |
Gross | Net | Gross | Net | Gross | Net | Gross | Net | Gross | Net |
| 0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 2 | 94.1 | 94.6 | 95.5 | 96.2 | 96.9 | 97.5 | 97.3 | 98.6 | 96.9 | 98.0 |
| 4 | 85.4 | 86.6 | 86.1 | 87.6 | 87.7 | 89.1 | 91.2 | 93.8 | 90.9 | 93.1 |
| 6 | 79.2 | 80.8 | 71.8 | 73.9 | 72.3 | 74.3 | 78.2 | 81.8 | 70.7 | 73.1 |
| 8 | 77.0 | 78.6 | 61.3 | 63.9 | 54.1 | 56.3 | 65.5 | 69.8 | ||
| 10 | 50.4 | 53.0 | 53.9 | 58.8 | ||||||
| 12 | 38.7 | 41.2 | ||||||||
SA, South African.
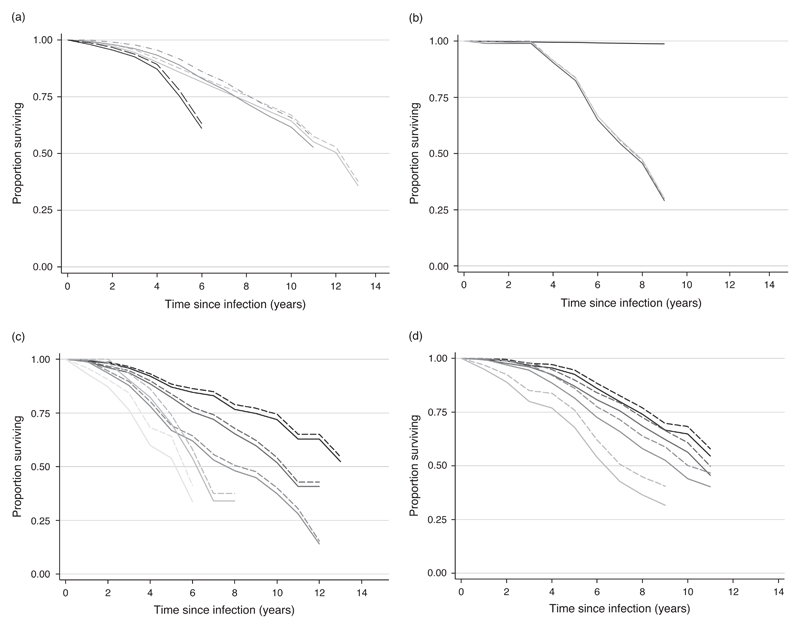
To allow comparison between sites, net and gross survival curves were adjusted to age 25–29 years at infection. Figure 2a shows the results for grouped studies. Table 3 shows rate ratios by study population and sex, comparing the gross and net survival adjusted by age and site. It is clear that the differences between study populations seen when the gross survival is compared are just as large using net survival. Differences between the sexes remain non-significant.
Fig. 2.
Survival curves. (a) Net and grosssurvival curves adjusted to age 25–29 years at infection (seroconversion), grouped for studies from east Africa (population cohorts), South Africa (miners) and Thailand (military).  East Africa population (gross);
East Africa population (gross);  east Africa population (net);
east Africa population (net);  South Africa miners (gross);
South Africa miners (gross);  South Africa miners (net);
South Africa miners (net);  Thai military (gross);
Thai military (gross);  Thai military (net). (b) Survival curves from time since infection for Thai blood donor and partner cohort using a general population lifetable method and verbal autopsy data to calculate net survival.
Thai military (net). (b) Survival curves from time since infection for Thai blood donor and partner cohort using a general population lifetable method and verbal autopsy data to calculate net survival.  Survival of HIV-negative individuals using weighted U N Population Division ‘without AIDS’ life table;
Survival of HIV-negative individuals using weighted U N Population Division ‘without AIDS’ life table;  gross survival of seroconverters;
gross survival of seroconverters;  net survival using verbal autopsy data;
net survival using verbal autopsy data;  net survival using weighted U N Population Division ‘without AIDS’ life table. (c) Net and gross survival curves by age at infection (seroconversion) for east African studies.
net survival using weighted U N Population Division ‘without AIDS’ life table. (c) Net and gross survival curves by age at infection (seroconversion) for east African studies.  15–24 years (gross);
15–24 years (gross);  15–24 years (net);
15–24 years (net);  25–34 years(gross);
25–34 years(gross);  25–34 years(net);
25–34 years(net);  35–44 years(gross);
35–44 years(gross);  35–44 years(net);
35–44 years(net);  45–54 years (gross);
45–54 years (gross);  45–54 years (net);
45–54 years (net);  55+ years (gross);
55+ years (gross);  55+ years (net). (d) Net and gross survival curves by age at infection (seroconversion) for South African miners.
55+ years (net). (d) Net and gross survival curves by age at infection (seroconversion) for South African miners.  15–24 years (gross);
15–24 years (gross);  15–24 years (net);
15–24 years (net);  25–34 years (gross);
25–34 years (gross);  25–34 years (net);
25–34 years (net);  35–44 years (gross);
35–44 years (gross);  35–44 years (net);
35–44 years (net);  45+ years (gross);
45+ years (gross);  45+ years (net).
45+ years (net).
Table 3.
Rate ratios of ‘gross’ and ‘net’ survival of seroconverters in five studies with follow-up of uninfected individuals.a
| Gross |
Net |
|||||
|---|---|---|---|---|---|---|
| Adjusted for age, site and duration of follow-up | Adjusted for duration of follow-up | Adjusted for age, site and duration of follow-up | ||||
| Characteristic | (95% CI) | (95% CI) | (95% CI) | |||
| Study populations | ||||||
| East Africa | 1 | 1 | 1 | |||
| South Africa | 0.80 | (0.68–0.94) | 0.75 | (0.62–0.91) | 0.84 | (0.69–1.05) |
| Thailand | 1.70 | (1.25–2.32) | 1.26 | (0.94–1.68) | 1.69 | (1.19–2.39) |
| Sex (east Africa) | ||||||
| Male | 1 | 1 | 1 | |||
| Female | 0.90 | (0.69–1.18) | 0.88 | (0.65–1.20) | 0.93 | (0.69–1.28) |
Note that the gross figures differ slightly to those of Todd et al. [2]. A piecewise exponential regression was used here rather than Weibull regression to enable direct comparison with adjusted net survival values.
The Thailand blood donor and partners cohort verbal autopsy data suggested that only three of the seroconverter deaths were not caused by HIV. Figure 2b shows the estimated survival of an uninfected population with the same sex and age structure as the seroconverters using the ‘without AIDS’ life table for men and women. This gave a survival at 6 years post-infection of 99%, predicting 2.1 non-HIV deaths in the seroconverters. Net survival calculated using verbal autopsy data, in which non-HIV deaths were treated as censored, gave a very similar survival to that calculated using the without AIDS life table, with survival at 6 years post-infection at 67 and 66%, respectively.
After weighting by the sex and age distribution of seroconverters at the time of infection, the Uganda national ‘without AIDS’ model life table implied that 95% would still be alive after 6 years of follow-up in Masaka and 96% in Rakai compared with 95 and 97%, respectively, directly observed among the uninfected individuals in those two studies. Similarly, the Tanzanian ‘without AIDS’ life table implies a 97% 6-year survival compared with 98% directly observed. For the Thai military, the national life table would have predicted 99.9% 6-year survival compared with 97% estimated using cohort data. For the east African studies this translates into a one percentage point increase in net survival at 6 years. For the Thai military the difference is slightly larger, with net survival at 6 years decreasing from 73% using the directly measured data to 71% using the weighted national without AIDS life table.
Net and gross survival curves by age group for the pooled east African sites and the South African miners are shown in Fig. 2c and d, respectively; the net and gross curves diverge more for the older age groups. The proportionate increase in survivorship for the east African populations at 6 years (the maximum follow-up available for all age groups) is 22% in the 55+ year age group, followed by 8, 4, 3 and 2% for age groups 45–54, 35–44, 25–34 and 15–24 years, respectively. The South African miners show a similar pattern. The striking differences in survival by age at infection seen in the gross survival data, however, persist after the net survival adjustment for background mortality.
Rate ratios comparing mortality in older age groups with mortality in 15–24 year olds for the uninfected population increase steadily with age in both pooled east African data and the South African data (Table 4). In the east African data, the gradient of the increase in rate ratio with age is very similar in the uninfected individuals and in seroconverters, apart from the age group 55+ years, in which the ratio for the HIV-negative individuals (13.5) is much higher than the corresponding ratio for seroconverters (4.9). In the South African dataset, there is a suggestion that the gradient of the increase in rate ratio with age is steeper for the uninfected individuals than for seroconverters. As a result, the net mortality rate ratios for the pooled east African data are almost the same as the gross mortality rate ratios except in the age group 55+ years, in which there is a slight narrowing in the net ratio (4.0) compared with the gross ratio (4.9). This difference is, however, well within the 95% confidence intervals for the ratio estimates. In South Africa the net ratios are somewhat smaller than the gross ratios throughout the age range, but again, none of the differences fall outside the confidence range.
Table 4.
Rate ratios for age at infection (or age at first negative test) for gross and net survival of seroconverters, and survival of HIV-negative individuals.
| Gross |
Net |
HIV-negative individuals |
||||
|---|---|---|---|---|---|---|
| Adjusted for age, site and duration of follow-up |
Adjusted for age, site and duration of follow-up |
Adjusted for age, site and duration of follow-up |
||||
| Characteristic | (95% CI) | (95% CI) | (95% CI) | |||
| Age at infection (years) | ||||||
| (East Africa) | ||||||
| 15–24 | 1 | 1 | 1 | |||
| 25–34 | 1.65 | (1.16–2.35) | 1.64 | (1.11–2.45) | 1.41 | (1.18–1.67) |
| 35–44 | 3.00 | (2.03–4.44) | 2.96 | (1.90–4.61) | 2.49 | (2.10–2.95) |
| 45–54 | 3.37 | (2.06–5.51) | 3.38 | (1.94–5.90) | 4.57 | (3.83–5.46) |
| 55+ | 4.88 | (2.72–8.73) | 4.01 | (1.89–8.51) | 13.51 | (11.63–15.69) |
| (South Africa) | ||||||
| 15–24a | 1 | 1 | 1 | |||
| 25–34 | 1.26 | (0.99–1.60) | 1.18 | (0.88–1.58) | 2.45 | (0.75–7.98) |
| 35–44 | 1.76 | (1.35–2.29) | 1.59 | (1.16–2.20) | 3.66 | (1.12–12.01) |
| 45+ | 3.24 | (2.28–4.59) | 2.71 | (1.71–4.30) | 4.64 | (1.35–15.93) |
For seroconverters this group has 963 people, of whom only 37 are below the age of 20 years.
Discussion
This paper shows that differences in survival post-infection comparing the Thai studies with the African studies are not caused by differences in background mortality. The small differences between the African studies persist after accounting for background mortality; but these differences are not statistically significant. The possible reasons for these differences are discussed elsewhere [2].
We also showed that background mortality does not explain the differences between survival post-infection by age group at the time of seroconversion, although there was some evidence that in older age groups (45+ years in South Africa and 55+ years in east Africa), the difference between net and gross survival is larger than in the younger age groups.
The ALPHA network collaboration [15] has allowed these issues to be explored in much more detail than has previously been possible within single-site analyses [8,16,17]. Common analytical methods were used as far as possible, but there were some differences between the sites.
In order to avoid the inclusion of unknown seroconverters, the South African miners’ HIV-negative cohort was censored at 2 years. Although the miners were followed up after leaving the mines, by definition all would have been employed within the previous 2 years, and a ‘healthy worker’ effect is possible. Mortality rates may be low because of recruitment strategies in the industry; but there could be a bias in the opposite direction from occupational mortality. In practice, the measured mortality rates in the HIV-negative miners were similar to those in South Africa before HIV was widespread, suggesting that the data are reasonably representative of the wider population [8].
In the community cohorts in east Africa, the survival of uninfected individuals is based on follow-up after the first negative test in order to achieve as long a period of observation as possible. As individuals were re-tested at each survey round, the number of unidentified seroconverters is likely to be small. The incidence of HIV is low in these countries, at less than 2% per annum [16,18,19] so it is not necessary to censor at 2 years after the last negative test. Only 23% of deaths of uninfected individuals in the pooled dataset occurred more than 2 years after the last negative test. To estimate the maximum bias introduced by undetected infections, a 2% incidence rate was assumed along with the fitted model mortality rates of seroconverters in the pooled east African studies. These rates were applied to the length of time the HIV-negative group was followed up after their last negative test. With these assumptions, after 5 years of follow-up, 1% of the deaths in the HIV-negative east African cohorts could have been caused by HIV/AIDS, rising to 3% at 14 years.
The two methods for calculating net survival in the Thai blood donor and partners cohort gave similar results, with a slightly more favourable net survival obtained by using the ‘without AIDS’ model life table. The cohort was situated in northern Thailand so we assume that the mortality of the whole of Thailand is the same as in northern Thailand, and that blood donors and their partners have the same mortality as the general population. In this case this assumption appears to have been reasonable and either method could be used. The use of verbal autopsy data to measure non-AIDS mortality among infected persons may, however, in other circumstances, result in under or overestimates. When the HIV status of the patient is known, it is possible that an interviewer or reviewer would be more inclined to put HIV down as the cause of death, even when it could have been caused by another factor. Underestimation could occur if the HIV status of the deceased is unknown or is not recorded as a result of the stigma associated with HIV/AIDS [20]. Current work on the sensitivity and specificity of verbal autopsy data questions designed to identify AIDS deaths, could improve the reliability of verbal autopsy data for calculating net survival from causes other than HIV/AIDS [21,22].
The use of model life tables purporting to represent ‘without AIDS’ mortality in east African countries gave very similar survival rates to those directly measured from the cohorts, suggesting that mortality among adults uninfected with HIV in these sites is close to the models used to represent national mortality of uninfected individuals. On the time scale of interest for estimating net mortality (6–12 years after infection) the small differences have little impact on the net survival adjustments. The national ‘without AIDS’ life tables for Thailand underestimate the observed mortality of uninfected individuals in the Thai military cohort. This gives a slightly less favourable net survival rate than that calculated from the directly measured survival of those uninfected in the cohort. In the absence of other data, the use of model life tables is a useful alternative, although care must be taken in choosing a model that is representative of the population.
Overall, adjustments for background mortality had a minor influence on patterns of mortality seen in HIV seroconverter cohorts. Except for the oldest age groups, the background rates are dwarfed by HIV-related mortality, so variations in the background have little influence on overall mortality. Larger differences might be expected with longer follow-up, as cohorts age, and background mortality should be considered when making comparisons across populations in which it varies more widely, such as between high income and low and middle income countries.
Acknowledgements
The authors would like to thank all study sites for contributing their data and participating in the discussions leading to this analysis. They wish to acknowledge the hard work of their staff, and the generosity of their funders in making the data available. Key individuals from each site include: Kisesa (Mark Urassa, Basia Zaba,Wambura Mwita, Milly Marston, Raphael Isingo, Milalu Ndege); Masaka (Sam Biraro, Heiner Grosskurth, Agnes Kasirye, Jessica Nakiyingi-Miiro, Lieve van der Paal, Leigh Anne Shafer, Duncan Ssematimba, Jim Todd); Rakai (Anthony Ndyanabo, JohnBosco Bwanika, Tom Lutalo); South African miners (Jill Murray, Gill Nelson, Andre Bester, Stuart Shearer, Pam Sonnenberg, Judith Glynn); Thai military cohort (Ram Rangsin, Phunlerd Piyaraj,Thira Sirisanthana, Narongrid Sirisopana, Onsri Short, Kenrad E. Nelson, Lauri E. Markowitz, Joseph Chiu, Chirasak Khamboonruang, Sakol Eiumtrakul, Arthur E. Brown, Merlin Robb, Chris Beyrer, Surinda Kawichai); Thai blood donors and partners (Ann Duerr, Kenrad E. Nelson, Caroline Costello, Vinai Suriyanon).
Footnotes
Conflicts of interest: None.
References
- 1.Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- 2.Todd J, Glynn JR, Marston M, Lutalo T, Biraro S, Mwita W, et al. Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy. AIDS. 2007;21(Suppl. 6):S55–S63. doi: 10.1097/01.aids.0000299411.75269.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations. Model life tables for developing countries. New York: United Nations; 1982. [Google Scholar]
- 4.Population Division of Department of Economic and Social Affairs of the United Nations Secretariat. World Population Prospects: The 2002 Revision, Volume 1: Comprehensive tables. New York: United Nations; 2003. [Google Scholar]
- 5.Mulder DW, Nunn AJ, Wagner HU, Kamali A, Kengeya-Kayondo JF. HIV-1 incidence and HIV-1-associated mortality in a rural Ugandan population cohort. AIDS. 1994;8:87–92. doi: 10.1097/00002030-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Urassa M, Boerma JT, Isingo R, Ngalula J, Ng’weshemi J, Mwaluko G, et al. The impact of HIV/AIDS on mortality and household mobility in rural Tanzania. AIDS. 2001;15:2017–2023. doi: 10.1097/00002030-200110190-00015. [DOI] [PubMed] [Google Scholar]
- 7.Wawer MJ, Serwadda D, Gray RH, Sewankambo NK, Li C, Nalugoda F, et al. Trends in HIV-1 prevalence may not reflect trends in incidence in mature epidemics: data from the Rakai population-based cohort, Uganda. AIDS. 1997;11:1023–1030. doi: 10.1097/00002030-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Glynn JR, Sonnenberg P, Nelson G, Bester A, Shearer S, Murray J. Survival from HIV-1 seroconversion in Southern Africa: a retrospective cohort study in nearly 2000 gold-miners over 10 years of follow-up. AIDS. 2007;21:625–632. doi: 10.1097/QAD.0b013e328017f857. [DOI] [PubMed] [Google Scholar]
- 9.Rangsin R, Chiu J, Khamboonruang C, Sirisopana N, Eiumtrakul S, Brown AE, et al. The natural history of HIV-1 infection in young Thai men after seroconversion. J Acquir Immune Defic Syndr. 2004;36:622–629. doi: 10.1097/00126334-200405010-00011. [DOI] [PubMed] [Google Scholar]
- 10.Costello C, Nelson KE, Suriyanon V, Sennun S, Tovanabutra S, Heilig CM, et al. HIV-1 subtype E progression among northern Thai couples: traditional and non-traditional predictors of survival. Int J Epidemiol. 2005;34:577–584. doi: 10.1093/ije/dyi023. [DOI] [PubMed] [Google Scholar]
- 11.Dorrington RB, Bradshaw D, Laubscher R, Timaeus IM. MRC technical report. Cape Town: Medical Research Council; 2001. The impact of HIV/AIDS on adult mortality in South Africa. [Google Scholar]
- 12.Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. World Population Prospects: The 2004 Revision, CD-ROM edition. New York: United Nations; 2005. [Google Scholar]
- 13.Dickman PW, Sloggott A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 14.Dickman PW, Coviello E, Hills M. Estimating and modelling relative survival. The Stata J. in press. [Google Scholar]
- 15.London School of Hygiene and Tropical Medicine. ALPHA Network. [Accessed: 27 July 2007]; Available at: http://www.lshtm.ac.uk/cps/alpha/
- 16.Isingo R, Zaba B, Marston M, Ndege M, Mngara J, Mwita W, et al. Survival following HIV infection in the pre-ART era in a rural Tanzanian cohort. AIDS. 2007;21(Suppl. 6):S5–S13. doi: 10.1097/01.aids.0000299405.06658.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Paal L, Shafer LA, Todd J, Mayanja BN, Whitworth JAG, Grosskurth H. HIV-1 disease progression and mortality before the introduction of highly active antiretroviral therapy in rural Uganda. AIDS. 2007;21(Suppl. 6):S21–S29. doi: 10.1097/01.aids.0000299407.52399.05. [DOI] [PubMed] [Google Scholar]
- 18.Arroyo MA, Sateren WB, Serwadda D, Gray RH, Wawer MJ, Sewankambo NK, et al. Higher HIV-1 incidence and genetic complexity along main roads in Rakai District, Uganda. J Acquir Immune Defic Syndr. 2006;43:440–445. doi: 10.1097/01.qai.0000243053.80945.f0. [DOI] [PubMed] [Google Scholar]
- 19.Whitworth JA, Mahe S, Mbulaiteye SM, Nakiyingi J, Ruberantwari A, Oiwiya A, Kamali A. HIV-1 epidemic trends in rural south-west Uganda over a 10-year period. Trop Med Int Health. 2002;7:1047–1052. doi: 10.1046/j.1365-3156.2002.00973.x. [DOI] [PubMed] [Google Scholar]
- 20.Hosegood V, Vanneste AM, Timaeus IM. Levels and causes of adult mortality in rural South Africa: the impact of AIDS. AIDS. 2004;18:663–671. doi: 10.1097/00002030-200403050-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lopman BA, Barnabas RV, Boerma JT, Chawira G, Gaitskell K, Harrop T, et al. Creating and validating an algorithm to measure AIDS mortality in the adult population using verbal autopsy. PLoS Med. 2006;3:e312. doi: 10.1371/journal.pmed.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopman BA, Cook A, Urassa M, Isingo R, Żaba B, Charwira G, et al. Finding the best questions for measuring AIDS mortality using verbal autopsy: a validation study in Kisesa, Tanzania and Manicaland, Zimbabwe. 7th IN DEPTH AGM; Nairobi, Kenya. 3–7 September 2007. [Google Scholar]