Abstract
Background
Antibody responses to the inactivated seasonal influenza vaccine in individuals with atopic dermatitis (AD) have not been carefully characterized.
Objective
The primary objective of this study was to compare antibody responses to intradermal vaccination in participants with moderate/severe AD to non-atopic participants. Secondary objectives were to evaluate the effect of route of administration, Staphylococcus aureus skin colonization, and disease severity on vaccine response.
Methods
This was an open-label study conducted in the 2012–2013 influenza season at 5 US clinical sites. A total of 360 participants with moderate/severe AD or non-atopic were assessed for eligibility, of which 347 received intradermal or intramuscular vaccination per label and followed for 28 days post-vaccination. The primary outcome was the difference in the proportion of participants achieving seroprotection (hemagglutination-inhibition (HAI) antibody titer ≥ 1:40 on day 28 post-vaccination).
Results
Seroprotection rates for influenza B, H1N1, and H3N2 were not different (a) between AD participants and non-atopic participants receiving intradermal vaccination and (b) between AD participants receiving intradermal and intramuscular vaccination. Following intradermal –but not intramuscular – vaccination, AD participants with S. aureus colonization experienced (a) lower seroprotection and seroconversion rates and lower HAI titer geometric mean fold-increase against influenza B and (b) lower seroconversion rates against influenza H1N1 than non-colonized AD participants.
Conclusion
AD participants colonized with S. aureus exhibited a reduced immune response to influenza vaccination compared to non-colonized participants after intradermal, but not intramuscular, vaccination. Since most patients with AD are colonized with S. aureus, intramuscular influenza vaccination should be given preference in these patients.
Keywords: Atopic Dermatitis, S. aureus, Eczema, Influenza, Vaccination, Skin, Antibody
INTRODUCTION
Atopic Dermatitis (AD) is the most common chronic skin disease, affecting more than 15% of children and persisting into adulthood in half of these patients 1,2. Patients with AD have a unique predisposition to infection by Staphylococcus aureus and herpes simplex virus3–6. The NIH/NIAID funded Atopic Dermatitis Research Network (ADRN) aims to elucidate mechanisms underlying cutaneous and systemic immunity in AD and to identify biomarkers that characterize groups of AD patients with and without a history of staphylococcal colonization and/or history of eczema herpeticum.
Intradermal (ID) vaccination in normal skin is more immunogenic than intramuscular (IM) vaccination7,8,9. The current knowledge of antibody responses to ID administration of antigens in individuals with AD is unknown, but over 6 million doses of ID seasonal influenza vaccine (personal communication, Dr. M. Decker, Sanofi Pasteur) have been administered since it was licensed in the U.S. in 20111.
In the current study, the primary analysis compared the antibody responses to ID vaccination against influenza strains B, H1N1 and H3N2 in AD as compared to non-atopic (NA) participants. We also compared, as secondary analyses, the antibody responses of participants with moderate/severe AD receiving ID versus IM vaccination,antibody responses in AD participants with and without S. aureus skin colonization (SASC), gender and race.
Methods and Statistics
Participants aged 18–64 years received open-label vaccination at five centers [National Jewish Health (NJH), University of Rochester, Oregon Health & Science University, Boston Children’s Hospital (BCH), and Northwestern University] upon approval from their institutional review boards. Participants with AD had active, moderate to severe skin lesions as per the Rajka-Langeland Severity Score11. Non-atopic participants had no personal or first-degree family history of AD, asthma, allergic rhinitis, or food allergy. See Online-Only Text for inclusion/exclusion criteria and classification method of race and ethnicity.
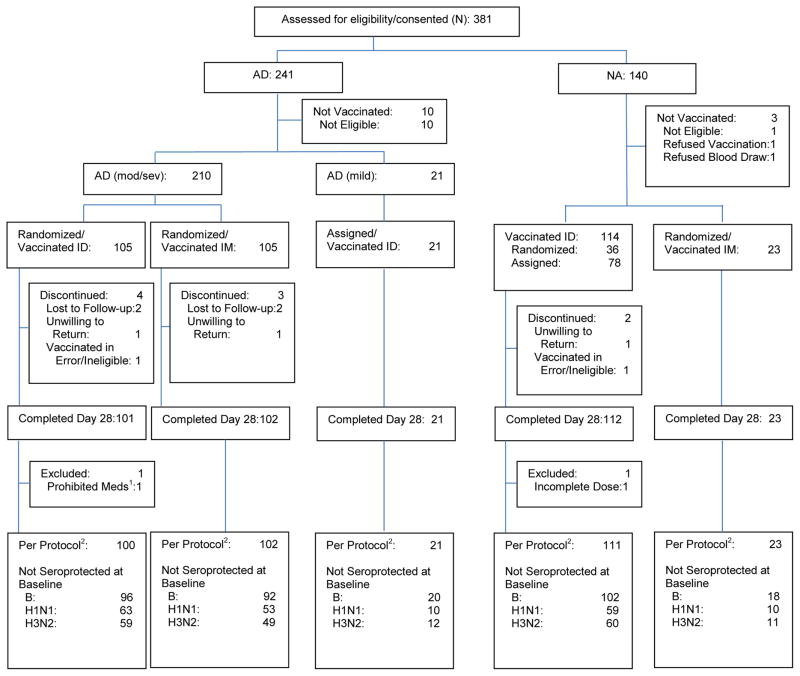
Participants with moderate/severe AD (hereafter referred to as AD) were randomized 1:1 to receive ID or IM administration of the 2012–2013 seasonal influenza vaccine12. At NJH, NA participants were randomized 3:2 to ID or IM vaccination until 23 participants received IM vaccination. Thereafter, remaining NA participants at NJH received ID vaccination. All NA participants at the remaining centers received ID vaccination. The 23 NA participants receiving IM vaccination served as a reference group for exploratory analyses (Figure 1). Stratified block randomization was used to balance gender and AD severity between vaccination routes by clinical site.
Figure 1. Consort Diagram of Study Participants.
Note: NA=Non-Atopic; AD=Atopic Dermatitis; ID=Intradermal; IM=Intramuscular; Mod/Sev=Moderate/Severe.
1Methotrexate (not allowed during the study)
2The per protocol population includes participants who 1) received a full dose of vaccine, 2) provided serum samples at baseline and day 28, 3) met eligibility criteria, 4) received no prohibited medications, and 5) had no major protocol deviations.
HAI antibody titers influenza B-specific IgG1, IgG2, IgG3 and IgA by enzyme-linked immunosorbent assay (ELISA) were measured pre-vaccination and 28 (+/−7) days post-vaccination. IgE and IgG antibodies specific for toxic shock staph toxin-1 (TSST-1) and Staphylococcal enterotoxin B (SEB), total IgE, and complete blood count (CBC) were measured pre-vaccination. Prior measurements of total IgE and CBC obtained within 30 days of vaccination were used if available.
S. aureus cultures of skin swabs had been previously obtained in NA and AD participants as part of the ADRN Registry. In AD participants, skin swabs were collected from the most severe AD lesion and also from adjacent non-lesional skin. Methodologies of S. aureus culture and laboratory assays are presented as Online-Only text. Sensitivity analyses involving SASC were also performed for two subgroups: (a) including only participants who had a S. aureus culture within 30 days of the vaccination date, or (b) including only participants with moderate disease.
For each of the three influenza strains, the primary outcome was the proportion of participants achieving seroprotection, (HAI antibody titer ≥ 1:40 on day 28 post-vaccination). Secondary outcomes included the geometric mean fold increase (GMFI) in HAI antibody titers from baseline to day 28 post-vaccination, and proportion of participants experiencing seroconversion (≥ 4-fold increase in baseline HAI antibody titers on day 28 post-vaccination). Participants having baseline HAI titers ≥1:40 for a particular strain were excluded from the analyses for that particular strain, and counts of those not seroprotected at baseline per strain are included in Figure 1.
Demographics and baseline characteristics were compared using Fisher’s exact test for categorical measures and Wilcoxon 2-sample test for continuous measures. Binary rates are presented as proportions and exact 95% confidence intervals (CI), and comparisons are summarized using odds ratios (OR) and Fisher’s exact test. Continuous variables were summarized with unadjusted geometric means and 95% CIs. Robust regression models using M-estimation were used to analyze continuous outcomes of log2 HAI titer fold-increase, log10 influenza B-specific IgG, IgG2, IgG3 and IgA. Geometric mean ratios (GMR) were defined as the ratio of geometric means of one group to the other. Multiple imputation methodology was used for influenza B-specific IgG1, IgG2, IgG3 and IgA values outside the limits of quantification. Baseline log10 IgE and IgG antibodies specific for TSST-1 and SEB were analyzed using left-censored Tobit regression models. All continuous models adjust for age and gender. The individual effects of SASC and disease severity were analyzed using an Rn2 test13 from a similar robust regression model as described above that included both SASC and disease severity as covariates.
Sample size calculations were based on H3N2 data from our previous ADRN Influenza Vaccine Pilot Study (NCT01518478)14 with the intradermal 2011–2012 seasonal influenza vaccine15 where 57% and 85% of AD and NA participants, respectively, achieved seroprotection post-vaccination. Because no adjustments were made for multiple comparisons among groups or endpoints, all p-values reported are descriptive/hypothesis generating except for the (inferential) p-value testing H3N2 seroprotection of AD vs NA among participants given ID vaccination.
Using Fisher’s exact test and assuming a two-sided significance level of 0·05, a sample size of at least 62 NA participants and 62 AD participants who were not seroprotected at baseline was necessary to detect a 28% difference in seroprotection rates between AD and NA participants receiving ID vaccination with at least 90% power. For secondary objective analyses, we similarly chose a sample size of at least 62 AD participants without seroprotection at baseline to receive IM vaccination.
Results
Demographic and Baseline Characteristics
Of 360 candidates screened, 347 were enrolled and vaccinated and 336 were evaluable in the per-protocol analysis (AD ID: 100, AD IM: 102, NA ID: 111, NA IM: 23) (Figure 1). A total of 136 of the 313 (43%) participants in the three main study groups (AD ID, AD IM and NA ID) were enrolled and vaccinated at NJH. The proportions of the three main study groups enrolled at each site were similar across all sites except BCH, where NA participants given ID vaccination comprised 65% of its enrollment. Among participants receiving ID vaccination, the age of the NA group was higher than the AD group (Table I). The AD group given ID vaccination was not different in gender, race and ethnicity to either the AD group given IM or the NA group given ID vaccination. Among recipients of ID vaccination, the NA group had lower total IgE levels, eosinophil counts and proportions of SASC than the AD group. These 3 characteristics were similar between AD participants receiving ID and IM vaccination.
Table I.
Demographic and Baseline Characteristics
| Characteristic | NA ID (N=111) | Moderate/Severe | Moderate/Severe | NA IM (N=23) |
|---|---|---|---|---|
| AD ID (N=100) | AD IM (N=102) | |||
| Gender, n (%) | ||||
| Female | 66 (59.5) | 56 (56.0) | 57 (55.9) | 12 (52.2) |
| Male | 45 (40.5) | 44 (44.0) | 45 (44.1) | 11 (47.8) |
| Race, n (%) | ||||
| Black or African American | 21 (18.9) | 30 (30.0) | 41 (40.2) | 3 (13.0) |
| Caucasian | 78 (70.3) | 55 (55.0) | 52 (51.0) | 16 (69.6) |
| Other | 12 (10.8) | 15 (15.0) | 9 (8.8) | 4 (17.4) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 10 (9.0) | 9 (9.0) | 10 (9.8) | 7 (30.4) |
| Not Hispanic or Latino | 101 (91.0) | 91 (91.0) | 92 (90.2) | 16 (69.6) |
| Age (yrs), Mean (SD) | 38.8 (11.9)* | 35.4 (11.3) | 36.6 (12.1) | 34.3 (9.8) |
| Total IgE (kU/L), Median (Q1, Q3) | 24.2 (10.1, 65.3)* | 196.5 (41.6, 1168.5) | 294.0 (85.9, 1017.0) | 24.9 (8.9, 79.8) |
| Eosinophils (cells/μL), Median (Q1, Q3) | 0.10 (0.05, 0.16)* | 0.20 (0.12, 0.35) | 0.19 (0.09, 0.38) | 0.10 (0.07, 0.13) |
| EASI Score, Median (Q1, Q3) | Not Applicable | 9.80 (4.1, 18.8) | 9.03 (4.2, 22.3) | Not Applicable |
| Rajka-Langeland Total Score, Median (Q1, Q3) | Not Applicable | 7 (6, 8) | 7 (6, 8) | Not Applicable |
| Rajka-Langeland Severity Categories, n (%) | ||||
| Moderate (4.5–7.5) | Not Applicable | 67 (67.0) | 69 (67.6) | Not Applicable |
| Severe (8–9) | Not Applicable | 33 (33.0) | 33 (32.4) | Not Applicable |
| S. aureus Skin Colonization, n (%) | ||||
| Positive | 1 (0.9)* | 38 (38.0) | 46 (45.1) | 3 (13.0) |
| Negative | 110 (99.1) | 60 (60.0) | 56 (54.9) | 20 (87.0) |
| Missing | 0 (0.0) | 2 (2.0) | 0 (0.0) | 0 (0.0) |
Notes: NA=Non-Atopic; AD=Atopic Dermatitis; ID=Intradermal; IM=Intramuscular. Percentages are based on column total (N). For total IgE, 1 kU/L = 2.4 μg/L.
Differences between the NA ID and moderate/severe AD ID groups (p<.05). Pairwise comparisons are based on Fisher’s exact test for proportions and Wilcoxon 2-sample test for continuous measures. There were no differences between the moderate/severe AD ID and moderate/severe AD IM groups for any measure. No other pairwise comparisons were assessed.
Baseline severity measures of AD, such as the eczema area and severity index (EASI) and the Rajka-Langeland Severity Score, were similar in participants given ID and IM vaccination. Baseline seroprotection rates were similar between the AD and NA groups for each influenza strain; however, baseline seroprotection rates were low for influenza B compared to H1N1 or H3N2 (Figure E1 in the Supplement).
Comparative Antibody Responses of NA and AD Participants Following ID Vaccination
There were no differences in seroprotection, seroconversion or HAI titer GMFI post-ID vaccination for either influenza B, H1N1 or H3N2 between the NA and AD participants overall (Table II).
Table II.
Baseline and Day 28 Post-vaccination Immune Response Summary
| NA ID | Moderate/Severe AD ID | Moderate/Severe AD IM | NA IM | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Moderate/Severe AD ID vs. NA ID | Moderate/Severe AD ID vs. AD IM | |||||
| B | ||||||
| N | 102 | 96 | 92 | 18 | ||
| Baseline GMT (95% CI) | 7.5 (6.8, 8.3) | 6.7 (6.1, 7.3) | 7.4 (6.7, 8.2) | 6.1 (4.8, 7.6) | ||
| Day 28 GMT (95% CI) | 16.4 (13.6, 19.8) | 20.4 (16.3, 25.6) | 21.4 (17.5, 26.2) | 20.0 (15.0, 26.7) | ||
| GM Fold Increase (95% CI) | 2.2 (1.8, 2.6) | 3.1 (2.4, 3.9) | 2.9 (2.4, 3.5) | 3.3 (2.2, 4.9) | ||
| GM Ratio (95% CI) | 1.25 (0.96, 1.61) | 0.93 (0.71, 1.22) | ||||
| p = .09 | p = .61 | |||||
| Seroprotection % (95% CI) | 23% (15, 32) | 34% (25, 45) | 34% (24, 44) | 22% (6.4, 48) | ||
| Odds Ratio (95% CI) | 1.80 (0.92, 3.55) | 1.03 (0.54, 1.97) | ||||
| p = .08 | p > .99 | |||||
| Seroconversion % (95% CI) | 30% (22, 40) | 41% (31, 51) | 48% (37, 58) | 61% (36, 83) | ||
| Odds Ratio (95% CI) | 1.57 (0.84, 2.94) | 0.75 (0.40, 1.38) | ||||
| p = .14 | p = .38 | |||||
| H1N1 | ||||||
| N | 59 | 63 | 53 | 10 | ||
| Baseline GMT (95% CI) | 7.9 (6.8, 9.1) | 7.8 (6.7, 9.0) | 8.8 (7.5, 10.3) | 12.3 (7.7, 19.7) | ||
| Day 28 GMT (95% CI) | 230 (151, 352) | 142 (95.6, 210) | 180 (125, 260) | 106 (43.7, 255) | ||
| GM Fold Increase (95% CI) | 29.1 (18.3, 46.3) | 18.3 (12.3, 27.1) | 20.5 (13.4, 31.3) | 8.6 (3.0, 24.7) | ||
| GM Ratio (95% CI) | 0.59 (0.32, 1.11) | 0.91 (0.50, 1.65) | ||||
| p = .10 | p = .75 | |||||
| Seroprotection % (95% CI) | 86% (75, 94) | 84% (73, 92) | 92% (82, 98) | 90% (55,100) | ||
| Odds Ratio (95% CI) | 0.83 (0.26, 2.56) | 0.43 (0.09, 1.63) | ||||
| p = .80 | p = .25 | |||||
| Seroconversion % (95% CI) | 88% (77, 95) | 86% (75, 93) | 89% (77, 96) | 60% (26, 88) | ||
| Odds Ratio (95% CI) | 0.81 (0.24 ,2.65) | 0.77 (0.21, 2.62) | ||||
| p = .79 | p = .78 | |||||
| H3N2 | ||||||
| N | 60 | 59 | 49 | 11 | ||
| Baseline GMT (95% CI) | 8.8 (7.6, 10.2) | 9.8 (8.3, 11.5) | 9.1 (7.7, 10.7) | 8.3 (5.7, 11.9) | ||
| Day 28 GMT (95% CI) | 79.1 (54.4, 115) | 93.2 (64.1, 135) | 108 (78.2, 148) | 75.1 (29.3, 193) | ||
| GM Fold Increase (95% CI) | 9.0 (6.2, 13.0) | 9.5 (6.6, 13.9) | 11.9 (8.2, 17.2) | 9.1 (3.2, 25.6) | ||
| GM Ratio (95% CI) | 1.04 (0.60, 1.80) | 0.76 (0.44, 1.31) | ||||
| p = .90 | p = .32 | |||||
| Seroprotection % (95% CI) | 73% (60, 84) | 85% (73, 93) | 94% (83, 99) | 91% (59,100) | ||
| Odds Ratio (95% CI) | 2.02 (0.75, 5.71) | 0.36 (0.06, 1.58) | ||||
| p = .18 | p = .22 | |||||
| Seroconversion % (95% CI) | 73% (60, 84) | 76% (63, 86) | 88% (75, 95) | 82% (48, 98) | ||
| Odds Ratio (95% CI) | 1.17 (0.47, 2.92) | 0.45 (0.13, 1.39) | ||||
| p = .83 | p = .14 | |||||
Notes: NA=Non-Atopic; AD=Atopic Dermatitis; ID=Intradermal; IM=Intramuscular, GMT=Geometric Mean Titer, GM=Geometric Mean, CI=Confidence Interval. Fold increase is defined as the day 28 hemagglutination-inhibition (HAI) antibody titer divided by the baseline HAI antibody titer. Seroprotection is defined as a day 28 HAI antibody titer ≥1:40. Seroconversion is defined as a ≥4-fold increase in HAI antibody titer from baseline to day 28. HAI antibody titers of <1:10 have been imputed as 1:5 and titers of ≥1:1280 have been imputed as 1:2560 for analyses. Participants with a baseline HAI antibody titer ≥1:40 for a particular strain are excluded from the analyses of that strain. GMT and GM fold increase statistics are raw estimates. GM ratios, 95% CIs and p-values are from pairwise robust regression models of log2 HAI titer fold-increase, adjusting for age and gender. Odds ratio 95% CIs are exact estimates and p-values are from pairwise Fisher’s exact tests. GM ratios and odds ratios compare the moderate/severe AD ID group to the adjacent groups.
Comparative Antibody Responses of AD Participants Following ID or IM Vaccination
Seroprotection and seroconversion rates and HAI titer GMFI at day 28 were similar in AD participants who received ID or IM vaccination for each of the 3 strains (Table II).
Comparative Antibody Responses of NA Participants Following ID and IM Vaccination
As an exploratory analysis, the seroprotection rates of NA participants were similar between those given ID vaccination compared to those given IM vaccination (Table II).
Impact of S. aureus Skin Colonization on Antibody Responses to Vaccination
Results of S. aureus cultures of skin swabs were available in 334 of 336 (99%) participants; cultures were collected up to 477 days pre-vaccination (mean 143 days) in 330 of 334 (99%) participants and post-vaccination (mean 37 days) in 4 of 334 (1%) participants. Cultures for 120 (36%) participants were collected within 30 days of vaccination, with cultures for 70 participants collected the same day as vaccination. Overall, 42% of participants with AD were colonized (Table I). Among all participants with AD who were not seroprotected at baseline separately for influenza B, H1N1 and H3N2, the rates of SASC were 41%, 44% and 41%, respectively. Participants with AD and SASC were divided evenly between ID and IM vaccines.
Baseline TSST-1-specific and SEB-specific IgE and IgG antibodies were higher in AD participants with SASC compared to AD participants without SASC (Figure E2 in the Supplement). Also, AD participants without SASC had higher baseline TSST-1 and SEB antibody levels than NA participants without SASC.
Comparative Antibody Responses to ID Vaccination
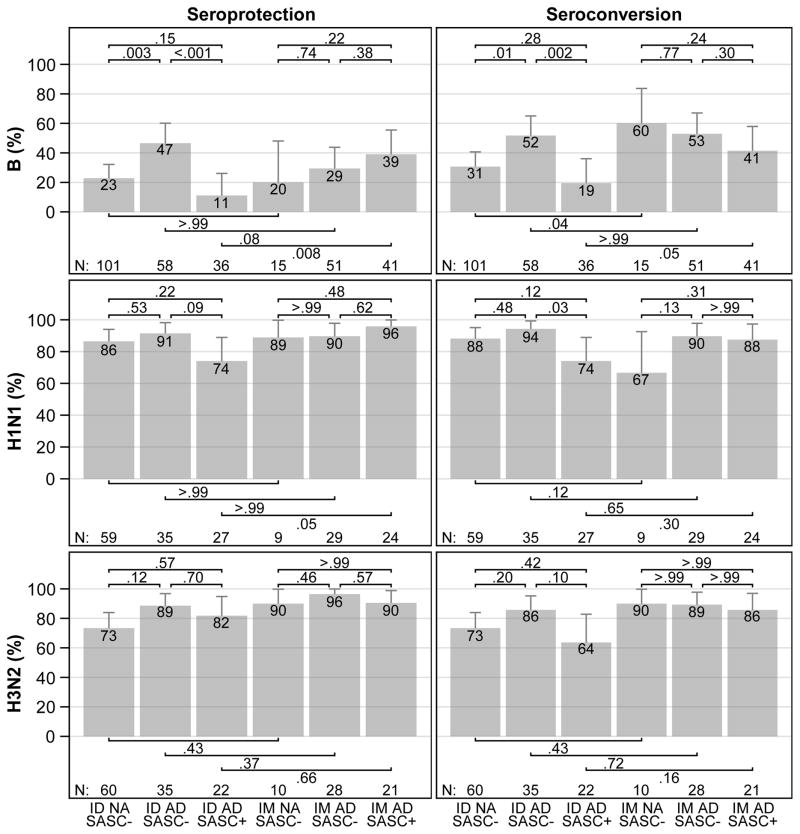
The rate of seroprotection to influenza B in AD participants with SASC was lower than in AD participants without SASC [11% vs 47%, OR 0.14 (95% CI, 0.03–0.49), p<.001] (Figure 2). The difference in rate of seroprotection to influenza B persisted when including only moderate AD participants [6% vs 51%, OR 0.06 (95% CI, 0.00–0.50), p=.002] or when including only participants with S. aureus cultures of skin swabs collected within 30 days of vaccination [0% vs 57%, OR 0.00 (95% CI, 0.00–0.40), p=.004].
Figure 2. Day 28 Post-vaccination Influenza B, H1N1 and H3N2 Seroprotection and Seroconversion, by Vaccination Route, Diagnostic Group and S. aureus Skin Colonization.
Seroprotection (HAI titers ≥ 1:40) and seroconversion (4-fold or greater increase in HAI titers over baseline titers) percentages and upper 95% confidence intervals are displayed. Pairwise comparisons are performed by the Fisher’s exact test.
Note: NA=Non-Atopic; AD=Atopic Dermatitis; ID=Intradermal; IM=Intramuscular; SASC= S. aureus Skin Colonization. Seroprotection is a day 28 hemagglutination-inhibition (HAI) antibody titer >=1:40. Seroconversion is a 4-fold or greater increase in HAI antibody titers from baseline to day 28. Participants with a baseline HAI antibody titer >=1:40 are excluded from the analyses. Pairwise comparisons are made using Fisher’s exact test. Error bars represent exact 95% upper confidence limits.
Additionally, there was a trend among AD participants toward a lower H1N1 strain seroprotection rate in those with SASC compared to those without [74% vs 91%, OR 0.27 (95% CI, 0.04–1.37), p=.09].
Among AD participants, the rate of seroconversion to influenza B and the rate of seroconversion to H1N1 in participants with SASC were also lower than those without SASC [19% vs 52%, OR 0.23 (95% CI, 0.07–0.64), p=.002 and 74% vs 94%, OR 0.17 (95% CI, 0.02–1.06), p=.03, respectively] (Figure 2), which persisted for influenza B when including only moderate AD participants [25% vs 55%, OR 0.27 (95% CI, 0.06–1.08), p=.05], or when including only participants with skin swabs collected within 30 days of vaccination [11% vs 65%, OR 0.07 (95% CI, 0.00–0.70), p=.02].
Participants with AD and SASC had lower HAI titer GMFI against influenza B compared with AD participants without SASC [GMR 0.50 (95% CI, 0.34–0.74), p<.001] (Figure E3 in the Supplement), which persisted when including only AD participants [GMR 0.52 (95% CI, 0.31–0.89), p=.02], or when including only participants with skin swabs collected within 30 days of vaccination [GMR: 0.39 (95% CI, 0.17–0.88), p=.02].
While the HAI titer GMFI against influenza B was influenced by the presence of SASC, it was not influenced by level of AD severity among ID vaccinees. When considering both SASC status and AD severity as covariates in a robust regression model including ID-vaccinated participants with AD, there were no pairwise differences in HAI titer GMFI against influenza B between severity levels, and only marginal evidence of an overall effect of severity (p=.07). However, there was evidence of an overall effect of SASC status on HAI titer GMFI against influenza B (p=.01).
In post hoc analyses, among participants without SASC receiving ID vaccination, the seroprotection rates were lower in NA participants than in AD participants for influenza B [23% vs 47%, OR 0.34 (95% CI, 0.16–0.72), p=.003], and the seroconversion rate for the B strain was also lower in NA than in AD participants [31% vs 52%, OR 0.41 (95% CI, 0.20–0.85), p=.01] (Figure 2).
Comparative Antibody Responses to IM Vaccination
Among AD participants receiving IM vaccination, SASC status did not impact either the seroprotection rate or the seroconversion rate to any of the three strains (Figure 2). There was a trend, among AD participants vaccinated IM, toward a lower HAI titer GMFI against influenza H3N2 in participants with SASC than in participants without SASC [GMR 0.49 (95% CI, 0.23–1.08), p=.08] (Figure E3 in the Supplement).
Comparison of Antibody Responses Between ID and IM Vaccination in Participants with AD and SASC
In a post hoc analysis, the proportion of AD participants with SASC achieving seroprotection to influenza B was lower among those receiving ID vaccination than among those receiving IM vaccination [11% vs 39%, OR 0.20 (95% CI, 0.04–0.72), p=.008] (Figure 2). There were similar trends in seroconversion to influenza B [19% vs 41%, OR 0.34 (95% CI, 0.10–1.06), p=.05] and in seroprotection to influenza H1N1 [74% vs 96%, OR 0.12 (95% CI, 0.00–1.14), p=.05] (Figure 2). Vaccination route did not influence immune responses to influenza H3N2 among AD participants with SASC.
Intradermal vaccination resulted in a lower HAI titer GMFI to the B strain than IM vaccination in AD participants with SASC [GMR 0.64 (95% CI, 0.45–0.90), p=.01] (Figure E3 in the Supplement).
Comparison of Antibody Responses Between ID and IM Vaccination in Participants with AD and without SASC
In a post hoc analysis, there were no differences between responses to ID and IM vaccinations among AD participants without SASC (Figure 2 and Figure E3 in the Supplement).
Impact of S. aureus Skin Colonization Status on Influenza B-specific IgG1, IgG2, IgG3 and IgA Responses to ID Vaccination among AD participants
There were no differences in baseline IgG1, IgG2, IgG3 or IgA titers to influenza B between AD participants with and without SASC (data not shown). Participants with AD and SASC had lower day 28 IgG1 responses to influenza B than AD participants without SASC [GMR 0.82 (95% CI, 0.69–0.97), p=.02], while there were no such differences in day 28 IgG2, IgG3 or IgA responses to influenza B (Figures E4 and E5 in the Supplement). There were no differences in day 28 IgG1, IgG2, IgG3 or IgA responses to influenza B between ID and IM vaccination among AD participants with SASC.
Comparison of Antibody Response by Gender and Race
There were no differences in seroconversion, seroprotection, or HAI titer GMFI to any vaccine strain between males and females or between Caucasian and Black or African Americans within the groups of AD or NA participants regardless of vaccination route (data not shown).
Safety Summary
A total of four AEs, two non-serious and two serious requiring hospitalization, were reported among three subjects. All adverse events were grade 3, resolved without sequelae, and were deemed not related to the vaccination. One AD IM subject experienced simultaneous vomiting and diarrhea 6 days after vaccination, one AD ID subject was hospitalized for an asthma exacerbation 6 days after vaccination, and one NA ID subject was hospitalized for a skin infection.
Discussion
The current study is the first immunological examination of ID vaccination against influenza in individuals with AD. Seroprotection and seroconversion rates were not different overall between AD participants and NA controls receiving ID vaccination for any of the three influenza strains (B, H1N1, and H3N2). In contrast, following ID vaccination in AD participants, compared to those without SASC, participants with SASC experienced (a) lower seroprotection and seroconversion rates and lower HAI titer GMFI against influenza B and (b) lower seroconversion rates against influenza H1N1. However, among participants who are AD with SASC, the response rate is higher among those receiving IM vaccination than those receiving ID vaccination.
Most differences were seen in response to influenza B. This result is probably due to a new B strain in the vaccine and low immunogenicity of B16,17. The low immunogenicity of B is an important handicap of inactivated influenza vaccines because recent studies show that B is not less pathogenic than A18.
The antibody response to influenza vaccines is mainly found within the IgG1 antibody subclass19. We therefore analyzed IgG1, IgG2, IgG3 and IgA antibody responses to influenza. Our finding that IgG1 antibody responses following ID vaccination were reduced in AD with SASC provided further support for a deficient cutaneous vaccination response in AD participants with SASC (Figure E4).
It is not known if SASC is simply a biomarker for reduced immune responses to ID vaccination or whether S. aureus directly inhibits immune responses to ID vaccination in AD. We considered the possibility that this association of diminished ID vaccine response due to S. aureus colonization was related to severity of AD. However, when we controlled for severity of skin disease, SASC remained strongly associated with reduced ID vaccine response to influenza vaccination. Previous studies have demonstrated that staphylococcal superantigenic toxins deplete dendritic cells from the skin by inducing migration of cutaneous antigen presenting cells to the draining lymph nodes20. Furthermore, it is known that S. aureus products such as staphylococcal protein A (SpA) have subversive effects on B cell and plasmablast antibody responses21. This provides biologic plausibility for the association of S. aureus colonization with reduced vaccine antibody responses.
Previous ADRN studies of transcutaneous vaccination to yellow fever virus in individuals with AD skin revealed an inverse association between total serum IgE levels and neutralizing anti-viral antibody titers22. In the current study of ID vaccination, however, reduced anti-influenza antibody responses were independent of baseline serum IgE. Our data suggest that the immunologic characteristics of the skin compartment and microbiome may dictate immune responses to influenza vaccines in AD. Considering that AD is a common health problem, these individuals, as well as those with other skin diseases, should be evaluated during early stage clinical trials that involve cutaneous delivery. New biomarkers, such as total serum IgE and SASC, may prove useful to identify population subsets that may not respond optimally to intradermal vaccination.
A limitation of our current study is that skin swabs for S. aureus were not collected on the day of vaccination for 79% of participants. However, microbiologic studies have demonstrated that S. aureus colonization may affect over 90% of severe AD patients23. Persistent S. aureus colonization in AD for up to one year has been demonstrated in other studies suggesting that skin swabs obtained at different time points will be relevant to future propensity to S. aureus colonization24,25,26. Another limitation of our study is that since ID vaccination is only approved for adults, the current study did not include children. However, it is immunologically plausible that S. aureus colonization subverts the skin immune response since eczema herpeticum in all age groups is associated with S. aureus colonization27.
In our current study, we conclude that NA and AD participants overall mount similar immune responses to ID vaccination. The subset of AD participants with SASC however, exhibited reduced immune responses after ID vaccination compared to AD participants without SASC. AD patients without S. aureus colonization had stronger seroprotection and seroconversion against influenza B than non-atopic controls (p=.003 and p=.01, respectively) and S. aureus-colonized AD patients (p<·001 and p=.002, respectively) when they were vaccinated ID suggesting the local environment of the S. aureus colonized skin subverts vaccine immune responses. Since SASC has been reported in the majority of AD23, the most prudent approach will be to avoid ID influenza vaccination in AD when a suitable vaccine with an alternative route of administration is available and not contraindicated.
Supplementary Material
CLINICAL IMPLICATIONS.
Atopic Dermatitis (AD) patients colonized with S. aureus exhibit reduced immune responses to influenza vaccination compared to non-colonized patients after intradermal, but not intramuscular, vaccination. Intramuscular influenza vaccination should be given preference in S. aureus colonized patients.
Acknowledgments
All sources of financial and material support and assistance were funded by NIH/NIAID Atopic Dermatitis Research Network contracts HHSN272201000020C and HHSN272201000017C and grants U19 AI117673-01 and UM2AI117870. This included design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review and approval of the manuscript. Dr. Donald Y.M. Leung and Brett Jepson had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Donald Leung, Gloria David, Margarita Gomez Lorenzo, Jon Hanifin, and Adriana Weinberg. Acquisition, analysis, and interpretation of data: Donald Leung, Brett Jepsen, Lisa Beck, Jennifer Canniff, Gloria David, Margarita Gomez Lorenzo, Jon Hanifin, Katherine Monti, Amy Paller, Lynda Schneider, and Adriana Weinberg. Drafting of the manuscript: Donald Leung, Brett Jepsen, Gloria David, Jennifer Canniff, Margarita Gomez Lorenzo, and AdrianaWeinberg. Critical revision of the manuscript for important intellectual content: Donald Leung, Lisa Beck, Gloria David, Margarita Gomez Lorenzo, Jon Hanifin, Katherine Monti, Amy Paller, Lynda Schneider, and Adriana Weinberg. Statistical analysis: Brett Jepsen, Katherine Monti, and Adriana Weinberg. Obtained funding: Donald Leung. Administrative, technical, or material support: Donald Leung, Jennifer Canniff, David, Margarita Gomez Lorenzo, Jon Hanifin, and Amy Paller. Study supervision: Donald Leung, Lisa Beck, Gloria David, Jon Hanifin, Amy Paller, and Adriana Weinberg. The authors acknowledge Joy Laurienzo Panza, RN, NIAID project manager to this study; Marshall Plaut, MD, NIAID project scientist and reviewer; Meghan McGinn at Rho, Inc. for study coordination; Barbara Jane Bate at University of Colorado Denver for laboratory analyses; and the following study coordinators for their hard work in recruiting human participants for this study: at National Jewish Health, Patricia Taylor, FNP-C, Gayle Spears, PA-C/CHA, Caroline Bronchick, RN, and Trudi Madigan, RN (supported in part by NIH/NCATS Colorado CTSI Grant number UL1 TR000154); at University of Rochester Medical Center, Jean Sauvain, Caitlyn Eberle and Kristopher Denby, MD; at Boston Children’s Hospital, Irene Borras-Coughlin; at Oregon Health & Science University, Emma Hill, and at Northwestern University Feinberg School of Medicine, Victoria Godinez-Puig; and the nurses at Clinical Trial Research Centers. Clinical Trial Research Centers are supported in part by the Colorado Clinical and Translational Science Award/Colorado Clinical & Translational Sciences Institute grant UL1 RR025780 from National Center for Research Resources/NIH and from NIH/National Center for Advancing Translational Sciences (grant UL1 TR000154). Additionally, the authors wish to acknowledge The Edelstein Family Foundation for their generous support of our work. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the NIH. Margarita Gomez Lorenzo, MD is a NIAID/NIH employee. Adriana Weinberg, MD has research support from MedImmune/Astra Zeneca, Sanofi Pasteur, Glaxo SmithKline, Merck, Roche Molecular, and Becton Dickinson. Dr. Weinberg’s spouse has intellectual property on Zostavax (Merck). The authors have no financial conflict of interest related to this manuscript. DYML, BJ and GD had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
ABBREVIATIONS
- AE
Adverse Event
- AD
Atopic Dermatitis
- ADRN
Atopic Dermatitis Research Network
- BCH
Boston Children’s Hospital
- CI
Confidence Interval
- CBC
Complete Blood Count
- EASI
Eczema Area and Severity Index
- ELISA
Enzyme-Linked Immunosorbent Assay
- GMFI
Geometric Mean Fold Increase
- GMT
Geometric Mean Titer
- GMR
Geometric Mean Ratio
- HAI
Hemagglutination-Inhibition
- ID
Intradermal
- IgA
Immunoglobulin A
- IgE
Immunoglobulin E
- IgG
Immunoglobulin G
- IM
Intramuscular
- NA
Non-Atopic
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institute of Health
- NJH
National Jewish Health
- OR
Odds Ratio
- Q1
1st Quartile
- Q3
3rd Quartile
- SASC
S. aureus Skin Colonization
- SEB
Staphylococcal Enterotoxin B
- SpA
Staphylococcal Protein A
- TSLP
Thymic Stromal Lymphopoietin
- TSST-1
Toxic Shock Staph Toxin-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150(6):593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769–79. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125(1):4–13. doi: 10.1016/j.jaci.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46(10):1555–61. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 6.Boguniewicz M, Leung DY. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunolog Rev. 2011;242(1):233–46. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roukens AH, Vossen AC, Bredenbeek PJ, van Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non-inferiority trial. PLoS One. 2008;3(4):e1993. doi: 10.1371/journal.pone.0001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ. 2011;89(3):221–6. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351(22):2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. [Accessed October 14, 2015];Sanofi Pasteur, 271/371 Fluzone®, 372 Fluzone®, 390 Fluzone® Intradermal. Retrieved from http://www.fda.gov/downloads/biologicsbloodvaccines/.../ucm195479.pdf.
- 11.Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–4. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. [Accessed October 14, 2015];Influenza Virus Vaccine for the 2012–2013 Season. Retrieved from http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Post-MarketActivities/LotReleases/ucm310644.htm.
- 13.Hampel FR, Ronchetti EM, Rousseeuw PJ, Stahel WA. Robust statistics: The approach based on influence functions. New York, NY: John Wiley & Sons; 1986. [Google Scholar]
- 14.ClinicalTrials.gov. [Accessed October 14, 2015];ADRN Influenza Vaccine Pilot. Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT01518478?term=adrn&rank=3.
- 15.U.S. Food and Drug Administration. [Accessed October 14, 2015];Influenza Virus Vaccine for the 2011–2012 Season. Retrieved from http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/post-marketactivities/lotreleases/ucm262681.htm.
- 16.Vinnemeier CD, Fischer-Herr J, Meyer S, et al. Immunogenicity and safety of an inactivated 2012/2013 trivalent influenza vaccine produced in mammalian cell culture (Optaflu®): An open label, uncontrolled study. Human Vaccines & Immunotherapeutics. 2014;10(2):441–448. doi: 10.4161/hv.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieninger D, Sheldon E, Lin W-Y, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infectious Diseases. 2013;13:343. doi: 10.1186/1471-2334-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su S, Chaves SS, Perez A, D’Mello T, Kirley PD, Yousey-Hindes K, et al. Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin Infect Dis. 2014;59(2):252–5. doi: 10.1093/cid/ciu269. [DOI] [PubMed] [Google Scholar]
- 19.Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13(9):981–90. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shankar G, Pickard-Elias S, Burnham K. Superantigen-induced Langerhans cell depletion is mediated by epidermal cell-derived IL-1α and TNFα. Cell Immunol. 1996;171(2):240–45. doi: 10.1006/cimm.1996.0199. [DOI] [PubMed] [Google Scholar]
- 21.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nature reviews Microbiology. 2015;13(9):529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slifka MK, Leung DY, Hammarlund E, Raué HP, Simpson EL, Tofte S, et al. Transcutaneous yellow fever vaccination of subjects with or without atopic dermatitis. J Allergy Clin Immunol. 2014;133(2):439–47. doi: 10.1016/j.jaci.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breuer K, Häussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147(1):55–61. doi: 10.1046/j.1365-2133.2002.04872.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoeger P, Niggemann B, Schroeder C. Enhanced basal and stimulated PMN chemiluminescence activity in children with atopic dermatitis: stimulatory role of colonizing staphylococci? Acta Paediatrica. 1992;81(6–7):542–546. doi: 10.1111/j.1651-2227.1992.tb12291.x. [DOI] [PubMed] [Google Scholar]
- 25.Kong H, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Abrams M, Tlougan B, Rademaker A, Paller A. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123(5):e808–e814. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 27.Beck LA, Boguniewicz M, Hata TR, Schneider LC, Hanifin J, Gallo R, et al. Phenotype of atopic dermatitis: Subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124(2):260–9. doi: 10.1016/j.jaci.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.