Abstract
The blood–brain barrier is a dynamic and highly organized structure that strictly regulates the molecules allowed to cross the brain vasculature into the central nervous system. The blood–brain barrier pathology has been associated with a number of central nervous system diseases, including vascular malformations, stroke/vascular dementia, Alzheimer’s disease, multiple sclerosis, and various neurological tumors including glioblastoma multiforme. There is a compelling need for representative models of this critical interface. Current research relies heavily on animal models (mostly mice) or on two-dimensional (2D) in vitro models, neither of which fully capture the complexities of the human blood–brain barrier. Physiological differences between humans and mice make translation to the clinic problematic, while monolayer cultures cannot capture the inherently three-dimensional (3D) nature of the blood–brain barrier, which includes close association of the abluminal side of the endothelium with astrocyte foot-processes and pericytes. Here we discuss the central nervous system diseases associated with blood–brain barrier pathology, recent advances in the development of novel 3D blood–brain barrier -on-a-chip systems that better mimic the physiological complexity and structure of human blood–brain barrier, and provide an outlook on how these blood–brain barrier-on-a-chip systems can be used for central nervous system disease modeling.
Impact statement
The field of microphysiological systems is rapidly evolving as new technologies are introduced and our understanding of organ physiology develops. In this review, we focus on Blood–Brain Barrier (BBB) models, with a particular emphasis on how they relate to neurological disorders such as Alzheimer’s disease, multiple sclerosis, stroke, cancer, and vascular malformations. We emphasize the importance of capturing the three-dimensional nature of the brain and the unique architecture of the BBB – something that until recently had not been well modeled by in vitro systems. Our hope is that this review will provide a launch pad for new ideas and methodologies that can provide us with truly physiological BBB models capable of yielding new insights into the function of this critical interface.
Keywords: Vascular, models, engineering, diseases, brain, endothelium
Introduction
The blood–brain barrier (BBB) is a dynamic and highly organized structure that strictly regulates the passage of molecules from the brain vasculature into the central nervous system (CNS) and thereby functions as a critical defense system that protects the brain from toxins and infection. The barrier results from a combination of extensive tight and adherens junctions between cerebral endothelial cells (CECs), which dramatically reduce the rate of transcytosis relative to vessels outside of the CNS. Additionally, both pericytes (PCs) and astrocytes, located on the abluminal side of the endothelium, provide critical support for this barrier. Dysfunction of the BBB is associated with a number of CNS diseases, including vascular malformations, stroke/vascular dementia, Alzheimer’s disease, multiple sclerosis, and various neurological tumors including glioblastoma multiforme, and thus understanding this critical interface will be crucial in combating these pathologies. While current research relies heavily on rodent models and two-dimensional (2D) in vitro systems, neither can fully recapitulate the complexities of the human BBB. The three-dimensional (3D) nature of microvasculature and its association with PCs and astrocytes precludes the use of 2D model systems, and physiological differences between humans and mice, such as species differences in the P-glycoprotein (P-gp) transport of drug molecules across the BBB, make translation of mouse studies to the clinic problematic.1 In this review, we discuss CNS diseases associated with BBB pathology, recent advances in the development of novel 3D BBB-on-a-chip systems that better mimic the physiological complexity and structure of human BBB, and provide an outlook on how these BBB-on-a-chip systems can be used for CNS disease modeling.
Structure and function of the BBB
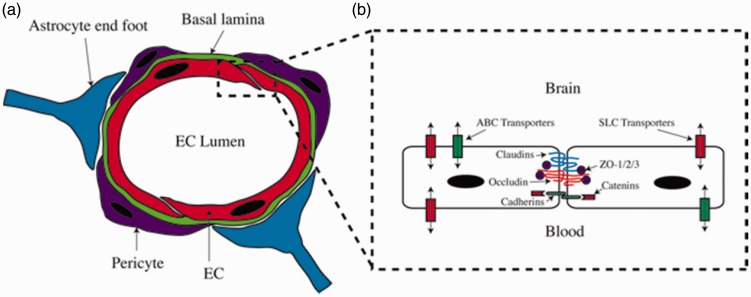
The BBB is a complex, dynamic, and highly organized structure consisting of four specialized components that together form the neurovascular unit (NVU). These components include: (1) specialized CECs that form the microvascular network; (2) a basal lamina that supports the abluminal surface of the endothelium; (3) PCs that wrap around these vessels; and (4) astrocyte end feet that extend to the CECs (Figure 1(a)).2 The anatomical structure and functions of this neurovascular unit have been reviewed extensively elsewhere3; therefore, for the purpose of this review, we only highlight the key BBB features that are relevant for the specific pathologies we discuss below.
Figure 1.
The human blood–brain barrier (BBB). (a) The human NVU consists of CECs forming the blood vessel, the basal lamina, pericyte, and astrocyte end foot. (b) Tight junctions, adherens junctions, SLC transporters, and ABC transporters of CECs
Of the BBB components, the endothelium is the primary gatekeeper for ion exchange, transportation of neurotransmitters, essential water-soluble nutrients and metabolites, and the efflux of neurotoxins. This regulatory function relies on a combination of specific ion channels and active transporters expressed on the endothelial surface.3,4 At the cellular level, CECs express several proteins that contribute to adherens junctions (AJ) and tight junctions (TJ) at the interface between neighboring CECs (Figure 1(b)). These junctions function to significantly reduce paracellular diffusion of macromolecules from the blood plasma to the brain parenchymal region. AJs, consisting of cadherins that are associated with cytoplasmic scaffolding proteins such as alpha, beta, and gamma catenin, function to hold neighboring cells together while also providing structural support.5 TJs on the other hand consist of occludin and claudin family proteins that are linked to numerous cytoplasmic scaffolding proteins, including ZO-1, ZO-2, and ZO-3, and act to tighten the contact surface between neighboring cells.6 While occludin is considered a major component of TJs, particularly those found on CECs, evidence has shown that a functional BBB can still form in occludin-deficient mice.7 This suggests that other proteins enriched in BBB TJs, such as marveld2, cingulin-like-1, and pard3, perform redundantly to maintain BBB barrier function even in the absence of occluding.8
While some lipid-soluble molecules are able to cross the BBB by passive diffusion, non-lipid-soluble molecules require active transport by transporter proteins on both the luminal and abluminal surfaces of the endothelium. These transporters are classified into two main families: soluble carriers (SLC), which transport a variety of organic molecules and inorganic ions according to their affinities with a specific transporter, and ATP-binding cassette (ABC) transporters, which actively transport a variety of substrates such as biosynthetic precursors, vitamins, and metabolites using an ATP-dependent process (Figure 1(b)).3 The specificity of these transporter families has important implications for both BBB pathology and the development of pharmacologic agents capable of crossing the BBB. For example, the SLC transporter GLUT1 normally functions as the primary BBB glucose transporter, yet mutations in this transporter are known to contribute to pathophysiological disorders such as Alzheimer's disease (AD), epilepsy, ischemia, and traumatic brain injury.9 On the other hand, ABC transporters such as ABCB1 (P-gp), ABCG2 (BCRP), and the ABCC subfamily (ABCC1, ABCC2, and ABCC4), which normally serve as efflux pumps to remove toxins from the brain, pose challenges to drug delivery to the CNS. Many useful, lipid-soluble drugs that theoretically can cross the BBB according to their partition coefficient are unintentional substrates for these efflux transporters, thus preventing their accumulation in brain tissue.10
In addition to the SLC and ABC transporters, molecules can be transported across the BBB through endothelial transcytosis. A unique feature of CECs compared to other types of endothelial cells (EC) in the body is that they have a one to two log lower level of transcytosis,11 thus further increasing the selectivity of the BBB.12 Endothelial transcytosis can be receptor-mediated for ligands such as transferrin and insulin, or caveolae-mediated for low-density proteins (LDL), all of which are important for CNS activities.13
The basal lamina surrounding the abluminal side of CECs consists of proteoglycans and basement membrane proteins such as fibronectin, laminin, and collagen IV, all of which contribute to selective filtering of microparticles.14,15 Disruption of the basal lamina, and subsequent BBB disruption, is implicated in several pathological conditions, such as Alzheimer’s disease (AD), multiple sclerosis (MS) and stroke, and is a major step in facilitating tissue inflammation.16 Sharing the basal lamina with the CECs are the PCs. These cells wrap around the microvessels and play various roles at the BBB. They have been shown to regulate both BBB-specific gene expression patterns in EC, and endothelial transcytosis, and also induce polarization of astrocyte end-feet surrounding the CNS blood vessels.17 Astrocytes are involved in various processes in the brain, such as regulating ion and water concentration, facilitating neurotransmitter clearance, and matching oxygen and glucose transportation to neuronal activity.18 Although the role of astrocytes in regulating the BBB is still not fully understood, several in vitro studies have shown that they can induce barrier properties in cerebral and other types of EC.19 In addition, recent findings have shown that astrocytes release nitric oxide, arachidonic acid, and prostaglandins, all of which can regulate vessel diameter to control blood flow.20
Together, CECs, the basal lamina, PCs, and astrocytes cooperate to form a selectively permeable barrier that actively regulates the type and quantity of molecules that traffic from the blood into the CNS. While each component is critical to normal BBB function, defects in individual components can lead to BBB dysfunction, enhanced permeability, and subsequent CNS disease.
The role of BBB dysfunction in CNS diseases
Vascular malformations
Disruption in the normal flow of blood to the brain, either by malfunctioning capillaries or hemorrhaging vessels, can have devastating effects, including mental deficits, epileptic fits, headaches, balance deficits, and psychiatric disorders.21 There are a number of vascular malformations that can cause such disruptions, including cerebral cavernous malformations (CCMs)22 and arteriovenous malformations (AVMs). CCMs form as the result of mutations in the CCM1, 2 or 3 genes, and are composed of tight clusters of small, thin-walled capillaries that often support only minimal blood flow, and in the case of CCM2, have a disrupted BBB.23 AVMs are lesions in which blood flows directly from arteries/arterioles to veins/venules, thereby bypassing downstream capillary beds. While these lesions can occur in other tissues, AVMs in the brain cause particularly severe symptoms, likely as a result of the reduced oxygen that results from the lack of a capillary bed between the oxygenated arterial blood and the venous return. AVMs are often congenital and are a major component of several hereditary disorders, including hereditary hemorrhagic telangiectasia (HHT, also known as Osler–Weber–Rendu disease), Wyburn–Mason syndrome, and Sturge–Weber syndrome.24 While mutations in several genes have been identified to cause these diseases, specific pathological mechanisms are still unknown. In HHT, for example, which is an autosomal dominant disease, mutations of endoglin (ENG) or Alk1 (ACVRL1) are responsible for the majority of cases, but the location of developing lesions remains unpredictable.25–27 Several contributing factors may determine where these lesions form, including: local trauma, such as a wound, that triggers angiogenesis,26,28 alterations of blood flow,29 or altered mural cell recruitment and deficient interactions with the endothelium.30 This final hypothesis is of particular interest in the context of the BBB, as disruption of pericyte function may also lead to breakdown of the BBB. Accurately evaluating the role of BBB dysfunction in AVM formation will require a platform that faithfully recreates the intricate structure of brain microvasculature and surrounding BBB components. Such a platform must recapitulate the endogenous branching of native vasculature as this is an essential component of AVM lesions. BBB-on-a-chip technologies that allow for physiologic vascular network formation will provide a novel approach for studying the pathogenesis of cerebral vascular malformations.
Alzheimer’s disease
Growing evidence suggests that BBB dysfunction is a major contributor in disorders of cognitive decline in the CNS. In one such disorder, Alzheimer’s disease (AD), a major driver of pathology, is the accumulation of amyloid-β (Aβ) peptide in the form of amyloid plaques in the brain.31 Transportation of Aβ across the BBB plays a crucial role in determining peptide concentration in the CNS, and its pathological consequence in AD.32 In individuals affected by AD, BBB dysfunction has been correlated with pathological progression, as measured by decreased tight junction protein expression, thickening basal lamina, and increased vascular permeability.33 With the failure of several large drug trials designed to lower the Aβ load in the brain of AD patients, efforts have focused on developing therapies around other AD-associated defects, such as vascular dysfunction. In recent years, a “two-hit” vascular hypothesis has been proposed, in which a pre-existing, non-AD related disease causes vascular dysfunction and subsequently accelerates the development of AD.34 Metabolic disorders such as type 2 diabetes (T2D) and the related hyperglycemic condition are linked to vascular abnormality and hyperpermeability.35 T2D is now considered an additional risk factor for AD progression in addition to aging.36 Longitudinal studies have shown that T2D patients have an increased risk of Alzheimer's disease by 50–100%.37 Thus, it is hypothesized that T2D is the first hit that causes vascular malfunctions, leading to an imbalance in amyloid transportation across the BBB and an acceleration of AD development. A BBB-on-a-chip model would be an ideal platform for investigating BBB transport of Aβ and the potential role of hyperglycemia in driving disease progression.
Stroke & vascular dementia
Microvessel BBB dysfunction is also considered a contributing factor in ischemic stroke and non-Alzheimer’s cognitive decline (vascular dementia). Nevertheless, how BBB dysfunction leads to the onset of these diseases has yet to be fully elucidated. While ischemic stroke is most commonly attributed to decreased blood flow due to vessel blockage in the brain, recent theories suggest that BBB permeability and microbleeds in the brain may significantly contribute to disease pathology.38 Magnetic resonance imaging (MRI) of individuals who have suffered lacunar stroke (25% of all ischemic stroke individuals) reveals accumulation of the contrast agent gadolinium in brain white matter, indicating BBB permeability at these sites.39 A detailed evaluation across 31 clinical studies indicates that BBB permeability increases not only with age, but is also higher in individuals with dementia.40 BBB permeability appears especially important in vascular dementia, as these individuals have higher vascular leak compared to individuals with AD. While these studies demonstrate a strong correlation between BBB damage and disease onset, they cannot determine whether BBB dysfunction is a primary cause or a consequence of stroke and vascular dementia. This question has been partially addressed using a spontaneous hypertensive stroke-prone rat (SHRSP) model where sequential MRIs have revealed that BBB permeability precedes immune infiltration at specific foci in the brain.41 While the SHRSP rat model recapitulates human small vessel pathology quite well, there is still a critical need in the stroke field to develop better tools to model changes in microvasculature relevant to stroke pathology. BBB-on-a-chip models offer tremendous potential for recreating microvasculature in the laboratory that will allow controlled study of the mechanics of BBB permeability and immune infiltration as they relate to the process of stroke.
Multiple sclerosis
In healthy individuals, oligodendrocytes generate a myelin sheath that insulates CNS neuronal axons and facilitates signal propagation to neighboring neurons. However, in the neuroinflammatory disease multiple sclerosis (MS), these myelin sheaths are degraded by activated immune cells, leading to damage and death of the underlying neurons. While the exact mechanisms of MS remain unclear, one leading hypothesis suggests that CEC dysfunction and breakdown of the BBB are critical steps to the pathogenesis of this disease. Indeed, inflammation and blood vessel damage are evident at both active and chronic MS lesion sites in brain white matter when compared to healthy individuals.42,43 More specifically, recent evidence suggests that localized BBB breakdown is the result of several processes, including: (1) increased inflammation and activation of CECs; (2) decreased expression of CEC junction proteins; and (3) increased adhesion and extravasation of T cells. Elevated levels of the proinflammatory cytokines interferon-γ and TNF-α have been observed in individuals with MS and are thought to function in activating CECs.44 Subsequent in vitro studies demonstrated that the presence of these cytokines, or whole serum from MS patients, can trigger breakdown of the BBB by downregulating the key junctional proteins occludin, VE-cadherin, and ZO-1.42,45 The loss of these junction proteins compromises the integrity of the BBB and allows T cells to cross the endothelium. Despite evidence supporting this model of MS pathogenesis, controversy remains regarding the role of proinflammatory cytokines46,47 and the requirement of selectins for T cell extravasation.48,49 Significant clarity on MS pathogenesis is likely to be gained through human-specific, tissue-on-a-chip platforms that more accurately model MS when compared to existing experimental autoimmune encephalomyelitis (EAE) mouse models, which are the current standard for MS studies.50
Glioblastoma
Glioblastoma multiforme (GBM) is a high-grade brain tumor arising from astrocytes. The high growth rate and relative insensitivity of these tumors to pharmacologic agents contribute to an especially poor prognosis for individuals with GBM.51 The inability of these agents to significantly affect GBM growth is largely due to their limited transport across the BBB. Paradoxically, high-grade GBM are characterized by major alterations to normal vascular function, including down-regulation of key TJ proteins and increased BBB permeability.51–53 Nevertheless, local disruption within the tumor vasculature is not sufficient to allow drug penetration in relevant quantities to effectively target GBM growth.54 Moreover, cell migration may be increased across a newly permeable BBB, thereby facilitating the development of brain metastases.55 The mechanisms underlying the inability of pharmacologic agents to cross the BBB have yet to be fully elucidated, but one possibility is altered expression of junction proteins. Recent work shows that the CEC receptor molecule Roundabout 4 (Robo4) is upregulated in an in vitro BBB model of GBM, resulting in a less permeable BBB.56 In this same model, genetic deletion of Robo4 increased BBB permeability by down-regulation of the key TJ proteins ZO-1, occludin-1 and claudin-5. These results suggest that despite overall increased BBB permeability in GBM, Robo4 may maintain relative impermeability of the BBB against anti-tumor therapeutic agents.57 Additionally, the affinity of some anti-tumor drugs for the ABC efflux transporter proteins likely prevents their accumulation in GBM tumors.57 The importance of tumor vasculature in providing nutrients to growing tumor cells has spurred efforts to develop anti-angiogenic therapies targeting GBM.58 However, new data suggest that these therapies may not prolong overall patient survival when added to the standard of care.59 Indeed, an anti-angiogenic regimen may impair the efficacy of chemotherapy in the GBM by compromising intratumoral delivery of these agents.60 Although existing organ-on-a-chip platforms have sought to combine vasculature and GBM tumor components,61 these platforms will be further enhanced by the addition of a functioning BBB.
The current state of BBB organ-on-a-chip models
Currently, in vivo models (most frequently, mice) are considered the standard for studies on the BBB and related CNS diseases.62 These models are especially conducive to multilevel evaluation of complex tissues while allowing for pharmacodynamic and pharmacokinetic evaluation of potential pharmacologic agents. While these models have contributed significantly to new discoveries of BBB mechanics, their utility is inherently limited by physiological differences between humans and rodents, which often impede reliable translation to the clinic.
Microphysiological systems (MPS) combine the advantages of in vivo and in vitro models of tissue and organs by using microfluidics technology to incorporate dynamic fluid flow within a 3D environment that better mimics native tissues.63,64 One unique feature of these organs-on-a-chip that extends far beyond 2D and animal models is the capability to recapitulate patient-specific pathology.64 While animal models provide a platform for studying complex diseases beyond 2D systems, these same models fail to capture human inter-individual genetic differences that may contribute to disease. Therefore, future studies will require the use of advanced MPS approaches to better evaluate patient-specific disease etiologies, with the ultimate goal being the development of truly personalized therapies. To this end, several BBB-on-a-chip models have been developed within the past five years (Table 1).65–74
Table 1.
Summary of current BBB organ-on-chips and their key features
| Year Published | Reference | Geometry/ dimension of blood vessel channel (width × height) | Endothelial cell source | Co-cultured cells | TJ qualitative assessment | Transendothelial electrical resistance (TEER) measurement | Permeability Test | Physiological test | Shear Stress* |
|---|---|---|---|---|---|---|---|---|---|
| 2016 | Wang et al.65 | Polycarbonate insert and micro-channel (300 µm × 160 µm) | Human iPSC-derived brain EC | Primary rat astrocytes (Life Technologies) | Claudin-5, ZO-1 | 2000–4000 Ω.cm2 | 4, 20, and 70 kDa dextran (0.5 mg/mL) = 10−7 to 10−8 cm2/s | Exposure to small molecule drugs (caffeine, doxorubicin) | 2–3 MPa |
| 2016 | Herland et al.66 | Square PDMS channel (1 mm × 20 mm) filled with collagen I gel to pattern a circular channel. | Primary human brain microvascular EC (Cell Systems) | Primary human brain pericytes (Cell Systems) + astrocytes (Sciencell) | VE-Cadherin, ZO-1 | N/A | 3 kDa dextran (5 µg/mL) = 2–3 × 10−6 cm2/s | Exposure to T NF-α | ∼100 MPa (5 min once a day) |
| 2015 | Brownet al.67 | Rectangular PDMS channel (6.2 mm × 100 µm) | Primary human brain microvascular EC (Applied Cell Biology) | Primary human pericytes (Sciencell), astrocytes (ATCC), and human iPSC-derived glutamatergic neurons | ZO-1 | 1950–2210 Ω.cm2 | 10 and 70 kDa dextran (relative comparison) | Exposure to glutamate, ascorbate, cold shock | 2 MPa |
| 2015 | Deosarkar et al.68 | Rectangular PDMS channel (200 µm × 100 µm) | Primary rat brain capillary EC | Primary rat astrocytes | ZO-1 | N/A | 40 kDa dextran = 1.1–2.9 × 10−6 cm2/s | N/A | 0.38–7.6 MPa |
| 2015 | Sellgren et al.69 | Rectangular PDMS channel (1 mm × 150 µm) | Immortalized mouse brain EC – bEnd.3 (ATCC) | Immortalized mouse astrocytes – C8D1A (ATCC) | Claudin-5 | N/A | 70 kDa dextran = ∼9 × 10−7 cm2/s | N/A | 500 MPa |
| 2015 | Kim et al.70 | Circular collagen gel channel (235–360 µm diameter) | Immortalized mouse brain EC – bEnd.3 (ATCC) | N/A | ZO-1 | N/A | 40 kDa dextran = 2.7 × 10−7 to 6.03 × 10−10 cm2/s | Hyperosmotic exposure with mannitol | N/A |
| 2013 | Prabhakarpandian et al.71 | Rectangular PDMS channel (200 µm × 100 µm) | Rat brain EC (RBE4) | N/A (+Astrocyte conditioned media) | ZO-1, Claudin-1 | N/A | 3–5 kDa dextran (relative comparison) | Pgp efflux activity of Rhodamine 123 under Verapamil | 3 MPa |
| 2013 | Achyuta et al.72 | Rectangular PDMS channel (10 mm × 100 µm) | Rat brain EC (RBE4) | E18 neural cells | ZO-1 | N/A | 3 kDa dextran (relative comparison) | Exposure to TNF-α | N/A |
| 2013 | Griep et al.73 | Rectangular PDMS channel (500 µm × 100 µm) | Immortalized human brain EC (hCMEC/D3) | N/A | ZO-1 | 37–120 Ω.cm2 | N/A | Exposure to TNF-α | 600 MPa |
| 2012 | Yeon et al.72 | Rectangular PDMS channel (25 µm in height) | Primary HUVEC (Sciencell) | N/A (+Astrocyte conditioned media) | ZO-1 | N/A | 4, 40, 70 kDa dextran (relative comparison) | N/A | N/A |
Note: “N/A” indicates no reported value or no measurement.
*Shear stress was reported in MPa, Pa, or dyn/cm2. For simplicity, all reported values were converted to MPa (1 dyn/cm2= 100 MPa).
Overall, these BBB organ-on-a-chip models better recapitulate the physical structure and physiological complexity of the human BBB by incorporating a 3D environment and in some cases exposing the endothelium to physiological fluid flow. In particular, fluid flow and shear stress are critical contributors to EC structure and function related to vascular network formation.75 The barrier function of these models is broadly improved over traditional 2D Transwell assays, as assessed by dextran diffusion across the endothelium. While the utility of these models has been examined elsewhere,63,76 there are several challenges with these systems that must be considered in the development of future platforms and for the adoption of organ-on-a-chip systems for BBB disease modeling.
First, the geometry and dimensions of the blood vessel compartment within these systems do not truly represent cerebral blood vessels in vivo. The blood vessel compartment in all but two models (Herland et al. and Kim et al.) has a square or rectangular cross section, in contrast to the circular cross section found in living blood vessels. Additionally, the dimensions of these vessels vary in diameter, from 100 µm up to millimeter sizes. For comparison, in the human brain, cerebral capillaries are 7–10 µm in diameter,77 while arterioles and venules are 50–100 µm in diameter.78 This difference in geometry is important as it can alter the distribution of shear stress on the endothelial surface. While shear stress positively influences BBB functions, square or rectangular cross-section channels do not have uniform shear stress distribution compared to circular cross-section channels under steady laminar flow. This shear stress is particularly important in regulating two transcription factors Krueppel-like factor (KLF)2 and KLF4 that suppress endothelial responses to inflammatory stimuli such as TNF-α to maintain a quiescent phenotype.79 A non-uniform shear stress distribution could potentially affect how EC respond to inflammatory signals inside the channel.
Second, the models listed in Table 1 all consist of a single channel and more appropriately represent a single blood vessel rather than a network of interacting vasculature. As such, the BBB-on-a-chip models have been unable to recapitulate the hierarchical branching of in vivo vasculature, which is a defining feature of microvascular networks. Further, branch points are often the foci where many defects originate, as these regions are exposed to the most turbulent flow. Thus, it is clear that while a single channel approach may prove useful in understanding basic blood vessel biology, these models cannot adequately reproduce and interrogate disease phenotypes inherent to more complex, hierarchical vascular networks.
Third, the anatomical structure of the human NVU is not truly represented in these models. The physical contacts and interactions between CECs, the basal lamina, PC, and astrocytes require a 3D environment and the absence of an intervening membrane such as that present in Transwells. Of all the models listed in Table 1, the closest to the anatomical structure of a human NVU in vivo is reported by Herland et al. However, even in this model, which does have direct contact between CEC and astrocytes unimpeded by an artificial membrane, all four components of the human NVU were not present together, there was no capillary network (see above), and the single vessel was considerably wider than normal brain capillaries.
Lastly, the most recent platforms demonstrate a trend away from rodent CECs towards more ambitious approaches that employ human CECs. While it remains a challenge to obtain human CECs and maintain their specific phenotypes in vitro, it is crucial to utilize human cell sources when possible. Transcriptional profiling of human CECs versus mouse CECs reveals differences in immune response genes, tight junction proteins, transporters, and cell surface receptors, all of which are important features of the BBB.80 It is also notable that commercially available, immortalized human brain CEC lines perform inconsistently in these modeling systems and there are no specific lines which appear optimal for in vitro modeling.81 In terms of a co-culture system, it has been demonstrated that the presence of PC and/or astrocytes enhances EC barrier functions in vitro,82 and so it will be essential to incorporate these cells into BBB-on-a-chip models. Indeed, the majority of models discussed in this review incorporate PC and/or astrocytes in co-culture. However, not all models utilize a human cell source, nor do they capture the 3D structure of the neurovascular unit. Despite the anatomical deficiencies in many of these models, some useful outputs can be obtained. For example, transport characteristics of the barrier can be assessed quite easily with the use of fluorescent or radioactive tracers, although in most of these systems only blood-to-brain transport is readily measurable. Inflammatory responses of the endothelium can also be assessed, along with leukocyte adhesion, although as discussed above, the flow characteristics may be disrupted to the point where the endothelium is more sensitive to inflammatory stimuli than would be the case in vivo. Finally, in only a limited number of these models can the interactions between the EC and the pericytes and astrocytes be investigated, as in many cases an artificial membrane separates the cell types. There is clearly much still to be done in creating fully representational BBB models in vitro.
Outlook
While efforts to develop more complex, sophisticated in vitro BBB-on-a-chip platforms have advanced significantly in recent years, challenges remain in utilizing these models to study CNS diseases.
As discussed above, animal cells are still widely used in BBB-on-a-chip platforms due to the limited availability of human tissues. While these models are useful for comparison to in vivo studies, there must also exist a ‘gold standard’ to benchmark for clinical translation.83,84 Although challenges remain in differentiation protocols, recent advances in deriving brain endothelium from human induced pluripotent stem cells (iPSCs)83,85 adds another to the list of brain cell types that can be used to create clinically relevant models for CNS disease. Particularly for complex CNS syndromes, it is crucial to investigate whether the pathology involves cell types other than those primarily thought to be involved, and these could include CEC. As previously discussed, perturbation of vascular integrity in the BBB has been implicated in diverse neurological disorders. To understand the role of the BBB in neurological diseases, it will be essential to develop a fully functional in vitro model that integrates multiple human cell types within a perfused vascular network and appropriate ECM. This prototypical BBB-on-a-chip will allow real-time study of events leading to neurovascular compromise, such as aberrant angiogenesis – often in the presence of a tumor, deregulated transport across the BBB, inflammation, and arterial dysfunction. It should also allow for meaningful screening of CNS-targeting drugs that must penetrate the BBB to enable effectiveness.84 BBB-on-a-chip platforms that can incorporate patient-derived iPSC to model specific diseases, or patient-derived tumor tissue to model brain cancer, have the potential to lower many barriers to drug discovery. Furthermore, BBB-on-a-chip platforms generated from iPSCs (or cancer cells) that are isolated from unique populations of patients can be studied to reveal patient-specific, gene–drug interactions based on differential treatment response. These adaptations may facilitate drug discovery targeted to specific genetic subgroups and inform patient stratification in clinical trial design.
In addition to cell sourcing, incorporation of other key components of the BBB such as PC, astrocytes, and a brain-like ECM environment should be considered for future in vitro model development. These components are often neglected, yet they are potentially as important as the EC in driving CNS pathological conditions in vivo. As such it will be critical to incorporate them into BBB-on-a-chip models.
Aside from platform development, new standardized criteria for quantitative evaluation of BBB functions are required. Traditionally, Transendothelial Electrical Resistance (TEER) and vascular permeability of molecules with different molecular weights have been used to assess the barrier properties in 2D Transwell assays. However, in new BBB-on-a-chip models, this may no longer prove to be a viable metric. As shown in Table 1, the majority of these models do not assess TEER due to challenges in probing electrical resistance inside complex microfluidic configurations. Other factors, such as the presence of co-cultured cells and ECM, the materials used for device fabrication, and ionic composition in cell culture medium could affect TEER measurements, hindering comparison between different BBBs-on-a-chip. Thus for future model development, TEER should not be considered as a major benchmark for barrier functions. Vascular permeability, on the other hand, is more useful and relevant to evaluate vascular integrity relevant to BBB function. In general, if the permeability of reference molecules measured in the BBB-on-a-chip model is close to physiological levels, the model can be considered to mimic the BBB in vivo, at least for transport studies. Fluorescently tagged 70 kDa dextran is routinely used as a reference to determine vascular permeability, partially due to its close molecular weight to albumin (MW = 68 kDa), a major protein present in the bloodstream. Under physiologic conditions, little to no albumin can cross the BBB in vivo, thus vascular permeability of 70 kDa dextran in BBB-on-a-chip models should be minimal, and can be considered a benchmark to evaluate BBB properties within the platform. Nevertheless, several factors need to be considered when comparing vascular permeability in these models relative to in vivo results. For instance, the dextran concentration, the flow rate, and the pressure difference between compartments (or inside and outside of the vascular channel) could affect permeability measurements. While there is no perfect in vitro model that can reflect all aspects of the BBB in vivo, it is critical to understand the limitations and caveats of each modeling platform. Thus, standardization for measuring vascular permeability is required, and the permeability coefficient should be reported with specific parameters related to that measurement such as flow rate, dextran concentrations, and exposure duration.
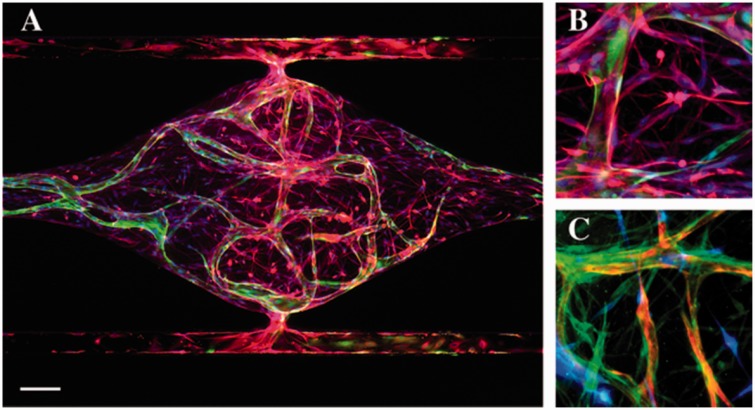
Over the past five years, we have significantly improved the methodology to generate perfused human microvascular networks in our organ-on-a-chip system.86,87 In addition, we have demonstrated that this system can be adapted to model the tumor microenvironment in vitro for therapeutic screening.88 These results are important milestones in our platform development effort, providing an opportunity to adapt the system for different organ-specific applications. With a vision to create an in vitro BBB model to study CNS diseases, we have been developing a BBB-on-a-chip that incorporates perfused human blood vessels, a brain-like ECM environment, PC and astrocytes. As a proof-of-concept, we have cultured human endothelial colony forming cell-endothelial cells (ECFC-EC), PC enriched from human stroma, and astrocytes derived from human neural stem cells in an ECM consisting of several basement membrane and interstitial structural proteins (Figure 2). We observed multicellular interactions between PC, astrocytes and the microvasculature. Furthermore, we found that the presence of astrocytes increases expression of several key adherens and tight junction genes, such as VE-Cadherin and Claudin-5. We are currently exploring the use of iPSC-derived EC, along with strategies to induce a brain-type phenotype, with the goal of enhancing barrier functions in our system. Current limitations of the system include: problems sourcing low passage human brain EC, which likely will prove essential for generating the correct pericyte-EC and astrocyte-EC interactions; maintaining the correct extracellular environment, which will likely need to be bathed in a CSF-like medium; and modeling the BBB in different parts of the brain, as there is a growing awareness that astrocytes differ in different regions and the BBB may have differential selectivity in some (possibly congruent) regions. In summary, by combining the permeability assays, non-invasive optical imaging of metabolism,89 and incorporation of multiple cell types (including glioma cells), we believe this system will provide unprecedented insights into the functions of the BBB in health and disease.
Figure 2.
BBB-on-a-chip supported by living microvascular network. (a) Global view of astrocytes (GFAP – red) and the microvascular network formed by ECFC-EC (green) inside a tissue chamber. Nuclei were stained with DAPI (blue). Scale bar = 100 µm. (b) Astrocytes (GFAP – red) lay down end feet and interact with the vessel (green). (c) Pericytes (PDGFR-β – green) wrap around the vessel (red) and interact with astrocytes (blue)
Acknowledgement
This work was supported by the following grants: UH3 TR000481 (SCG) and R01 PQD5-CA180122 (CCWH).
Authors’ contribution
DTTP and RHFB wrote and edited the manuscript. DTTP prepared the table and figures. DTTP, RHFB, JWA, AG, and SJH contributed knowledge and wrote the disease section. DTTP contributed knowledge and wrote the current state of BBB organ-on-a-chip model and outlook section. SJH contributed knowledge and wrote the outlook section. SCG and CCWH conceived the idea, wrote, and edited the manuscript. All authors read and approved the manuscript.
Declaration of Conflicting Interests
Christopher CW Hughes and Steven C George are founders of 4Design Biosciences, LLC, which has been established to commercialize the microfluidic platform mentioned in the text.
References
- 1.Suzuyama N, Katoh M, Takeuchi T, Yoshitomi S, Higuchi T, Asashi S, Yokoi T. Species differences of inhibitory effects on P-glycoprotein-mediated drug transport. J Pharm Sci 2007; 96: 1609–18. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins B, Davis T. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173–85. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Brites D, Brito M. Looking at the blood–brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 2010; 64: 328–63. [DOI] [PubMed] [Google Scholar]
- 4.Abbott N, Patabendige A, Dolman D, Yusof S, Begley D. Structure and function of the blood–brain barrier. Neurobiol Dis 2010; 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 5.Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res 2009; 335: 75–96. [DOI] [PubMed] [Google Scholar]
- 6.Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vascul Pharmacol 2002; 38: 323–37. [DOI] [PubMed] [Google Scholar]
- 7.Saitou M, Furuse M, Sasaki H, Schulzke J, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11: 4131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daneman R, Zhou L, Agalliu D, Cahoy J, Kaushal A, Barres B. The mouse blood–brain barrier transcriptome: a new resource for understanding thedevelopment and function of brain endothelial cells. PLoS One 2010; 5: e13741–e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X, Geng M, Du G. Glucose transporter 1, distribution in the brain and in neural disorders: its relationship with transport of neuroactive drugs through the blood–brain barrier. Biochem Genet 2005; 43: 175–87. [DOI] [PubMed] [Google Scholar]
- 10.Löscher W, Potschka H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005; 2: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bock M, Van Haver V, Vandenbroucke R, Decrock E, Wang N, Leybaert L. Into rather unexplored terrain-transcellular transport across the blood–brain barrier. Glia 2016; 64: 1097–123. [DOI] [PubMed] [Google Scholar]
- 12.Stamatovic S, Keep R, Andjelkovic A. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol 2008; 6: 179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgieva J, Hoekstra D, Zuhorn I. Smuggling drugs into the brain: an overview of ligands targeting transcytosis for drug delivery across the blood–brain barrier. Pharmaceutics 2014; 6: 557–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanwar Y, Linker A, Farquhar M. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol 1980; 86: 688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieleg O, Baumgärtel R, Bausch A. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys J 2009; 97: 1569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zlokovic B. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57: 178–201. [DOI] [PubMed] [Google Scholar]
- 17.Armulik A, Genové G, Mäe M, Nisancioglu M, Wallgard E, Niaudet C, He L, Norlin J, Linblom P, Strittmatter K, Johansson B, Betscholtz C. Pericytes regulate the blood–brain barrier. Nature 2010; 468: 557–61. [DOI] [PubMed] [Google Scholar]
- 18.Sofroniew M, Vinters H. Astrocytes: biology and pathology. Acta Neuropathol 2010; 119: 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott N, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 20.Gordon G, Mulligan S, MacVicar B. Astrocyte control of the cerebrovasculature. Glia 2007; 55: 1214–21. [DOI] [PubMed] [Google Scholar]
- 21.Ajiboye N, Chalouhi N, Starke R, Zanaty M, Bell R. Cerebral arteriovenous malformations: evaluation and management. Sci World J 2014; 2014: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross B, Du R. Cerebral cavernous malformations: natural history and clinical management. Expert Rev Neurother 2015; 15: 771–7. [DOI] [PubMed] [Google Scholar]
- 23.Choquet H, Pawlikowska L, Lawton M, Kim H. Genetics of cerebral cavernous malformations: current status and future prospects. J Neurosurg Sci 2015; 59: 211–20. [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas J, Surendran S, Abraham M, Rajavelu A, Kartha C. Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin Epigenet 2016; 8: 78–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peacock H, Caolo V, Jones E. Arterovenous malformations in hereditary haemorrhagic telangiectasia: looking beyond ALK1-NOTCH interactions. Cardiovasc Res 2016; 109: 196–203. [DOI] [PubMed] [Google Scholar]
- 26.Arthur H, Geisthoff U, Gossage J, Hughes C, Lacombe P, Meek M, Oh P, Roman R, Trerotola S, Velthuis S, Woodercak-Donahue W. Executive summary of the 11th HHT international scientific conference. Angiogenesis 2015; 18: 511–24. [DOI] [PubMed] [Google Scholar]
- 27.Sam C, Li F, Liu S. Inherited neurovascular diseases affecting cerebral blood vessels and smooth muscle. Metab Brain Dis 2015; 30: 1105–16. [DOI] [PubMed] [Google Scholar]
- 28.Choi E, Kim Y, Choe S, Tak Y, Garrido-Martin E, Chang M, Lee Y, Oh P. Enhanced responses to angiogenic cues underlie the pathogenesis of hereditary hemorrhagic telangiectasia 2. PLoS One 2013; 8: e63138–e63138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corti P, Young S, Chen C, Patrick M, Rochon E, Pekkan K, Roman B. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 2011; 238: 1573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shovlin C. Hereditary haemorrhagic telangiectasia: pathology, diagnosis and treatment. Blood Rev 2010; 24: 203–19. [DOI] [PubMed] [Google Scholar]
- 31.Wisniewski T, Ghiso J, Frangione B. Biology of A beta amyloid in Alzheimer's disease. Neurobiol Dis 1997; 4: 313–28. [DOI] [PubMed] [Google Scholar]
- 32.Zlokovic B, Ghiso J, Mackic J, McComb J, Weiss M, Frangione B. Blood–brain barrier transport of circulating Alzheimer's amyloid beta. Biochem Biophys Res Commun 1993; 197: 1034–40. [DOI] [PubMed] [Google Scholar]
- 33.Kelleher R, Soiza R. Evidence of endothelial dysfunction in the development of Alzheimer's disease: Is Alzheimer's a vascular disorder? Am J Cardiovasc Dis 2013; 3: 197–226. [PMC free article] [PubMed] [Google Scholar]
- 34.Zlokovic B. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scalia R, Gong Y, Berzins B, Zhao L, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: the role of mu-calpain. Diabetes 2007; 56: 1842–9. [DOI] [PubMed] [Google Scholar]
- 36.Carlsson C. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J Alzheimers Dis 2010; 20: 711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biessels G, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet 2006; 5: 64–74. [DOI] [PubMed] [Google Scholar]
- 38.Wardlaw J, Sandercock P, Dennis M, Starr J. Is breakdown of the blood–brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003; 34: 806–12. [DOI] [PubMed] [Google Scholar]
- 39.Wardlaw J, Doubal F, Armitage P, Chappell F, Carpenter T, Muñoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B. Lacunar stroke is associated with diffuse blood–brain barrier dysfunction. Ann Neurol 2009; 65: 194–202. [DOI] [PubMed] [Google Scholar]
- 40.Farrall A, Wardlaw J. Blood–brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging 2009; 30: 337–52. [DOI] [PubMed] [Google Scholar]
- 41.Sironi L, Guerrini U, Tremoli E, Miller I, Gelosa P, Lascialfari A, Zucca I, Eberini I, Gemeiner M, Paoletti R, Gianazza E. Analysis of pathological events at the onset of brain damage in stroke-prone rats: a proteomics and magnetic resonance imaging approach. J Neurosci Res 2004; 78: 115–22. [DOI] [PubMed] [Google Scholar]
- 42.Kirk J, Plumb J, Mirakhur M, McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J Pathol 2003; 201: 319–27. [DOI] [PubMed] [Google Scholar]
- 43.Plumb J, McQuaid S, Mirakhur M, Kirk J. Abnormal endothelial tight junctions in active lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol 2002; 12: 154–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hohnoki K, Inoue A, Koh CS. Elevated serum levels of IFN-gamma, IL-4 and TNF-alpha/unelevated serum levels of IL-10 in patients with demyelinating diseases during the acute stage. J Neuroimmunol 1998; 87: 27–32. [DOI] [PubMed] [Google Scholar]
- 45.Minagar A, Long A, Ma T, Jackson TH, Kelley RE, Ostanin DV, Sasaki M, Warren A, Jawahar A, Cappell B, Alexander J. Interferon (IFN)-beta 1a and IFN-beta 1b block IFN-gamma-induced disintegration of endothelial junction integrity and barrier. Endothelium 2003; 10: 299–307. [DOI] [PubMed] [Google Scholar]
- 46.Bahbouhi B, Berthelot L, Pettre S, Michel L, Wiertlewski S, Weksler B. Peripheral blood CD4+ T lymphocytes from multiple sclerosis patients are characterized by higher PSGL-1 expression and transmigration capacity across a human blood–brain barrier-derived endothelial cell line. J Leukoc Biol 2009; 86: 1049–63. [DOI] [PubMed] [Google Scholar]
- 47.Ottum PA, Arellano G, Reyes LI, Iruretagoyena M, Naves R. Opposing roles of interferon-gamma on cells of the central nervous system in autoimmune neuroinflammation. Front Immunol 2015; 6: 539–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doring A, Wild M, Vestweber D, Deutsch U, Engelhardt B. E- and P-selectin are not required for the development of experimental autoimmune encephalomyelitis in C57BL/6 and SJL mice. J Immunol 2007; 179: 8470–9. [DOI] [PubMed] [Google Scholar]
- 49.Engelhardt B. Immune cell entry into the central nervous system: involvement of adhesion molecules and chemokines. J Neurol Sci 2008; 274: 23–6. [DOI] [PubMed] [Google Scholar]
- 50.Croxford AL, Kurschus FC, Waisman A. Mouse models for multiple sclerosis: historical facts and future implications. Biochim Biophys Acta 2011; 1812: 177–83. [DOI] [PubMed] [Google Scholar]
- 51.Omuro A, DeAngelis L. Glioblastoma and other malignant gliomas: a clinical review. JAMA 2013; 310: 1842–50. [DOI] [PubMed] [Google Scholar]
- 52.Papadopoulos M, Saadoun S, Woodrow C, Davies D, Costa-Martins P, Moss R, Krishna S, Bell B. Occludin expression in microvessels of neoplastic and non-neoplastic human brain. Neuropathol Appl Neurobiol 2001; 27: 384–95. [DOI] [PubMed] [Google Scholar]
- 53.Liebner S, Fischmann A, Rascher G, Duffner F, Grote E, Kalbacher H, Wolburg H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol 2000; 100: 323–31. [DOI] [PubMed] [Google Scholar]
- 54.Dhermain F, Hau P, Lanfermann H, Jacobs A, van den Bent M. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol 2010; 9: 906–20. [DOI] [PubMed] [Google Scholar]
- 55.Jia W, Lu R, Martin T, Jiang W. The role of claudin-5 in blood–brain barrier (BBB) and brain metastases. Mol Med Rep 2014; 9: 779–85. [DOI] [PubMed] [Google Scholar]
- 56.Cai H, Liu W, Xue Y, Shang X, Liu J, Li Z, Wang P, Liu L, Hu Y, Liu Y. Roundabout 4 regulates blood-tumor barrier permeability through the modulation of ZO-1, Occludin, and Claudin-5 expression. J Neuropathol Exp Neurol 2015; 74: 25–37. [DOI] [PubMed] [Google Scholar]
- 57.Régina A, Demeule M, Laplante A, Jodoin J, Dagenais C, Berthelet F, Moghrabi A, Béliveau R. Multidrug resistance in brain tumors: roles of the blood–brain barrier. Cancer Metastasis Rev 2001; 20: 13–25. [DOI] [PubMed] [Google Scholar]
- 58.Popescu A, Purcaru S, Alexandru O, Dricu A. New perspectives in glioblastoma antiangiogenic therapy. Contemp Oncol (Pozn) 2016; 20: 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scribner E, Saut O, Province P, Bag A, Colin T, Fathallah-Shaykh H. Effects of anti-angiogenesis on glioblastoma growth and migration: model to clinical predictions. PLoS One 2014; 9: e115018–e115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Groot J, Reardon D, Batchelor T. Antiangiogenic therapy for glioblastoma: the challenge of translating response rate into efficacy. Am Soc Clin Oncol Educ Book 2013, pp. e71–e78. doi: 10.1200/EdBook_AM.2013.33.e71–e71–e78. doi: 10.1200/EdBook_AM.2013.33.e71. [DOI] [PubMed] [Google Scholar]
- 61.Rape A, Ananthanarayanan B, Kumar S. Engineering strategies to mimic the glioblastoma microenvironment. Adv Drug Deliv Rev 2014; 79–80: 172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbott N. Prediction of blood–brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today Technol 2004; 1: 407–16. [DOI] [PubMed] [Google Scholar]
- 63.van der Helm M, van der Meer A, Eijkel J, van den Berg A, Segerink L. Microfluidic organ-on-chip technology for blood–brain barrier research. Tissue Barriers 2016; 4: e1142493–e1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatia S, Ingber D. Microfluidic organs-on-chips. Nat Biotechnol 2014; 32: 760–72. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Abaci H, Shuler M. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng 2016; 114: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herland A, van der Meer A, FitzGerald E, Park T, Sleeboom J, Ingber D. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood–brain barrier on a chip. PLoS One 2016; 11: e0150360–e0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown J, Pensabene V, Markov D, Allwardt V, Neely M, Shi M, Britt C, Hoilett O, Yang Q, Brewer B, Samson P, McCawley L, May J, Webb D, Li D, Bowman A, Reiserer R, Wikswo J. Recreating blood–brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015; 9: 054124–054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deosarkar S, Prabhakarpandian B, Wang B, Sheffield J, Krynska B, Kiani M. A novel dynamic neonatal blood–brain barrier on a chip. PLoS One 2015; 10: e0142725–e0142725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellgren K, Hawkins B, Grego S. An optically transparent membrane supports shear stress studies in a three-dimensional microfluidic neurovascular unit model. Biomicrofluidics 2015; 9: 061102–061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Kim H, Im S, Chung S, Kang J, Choi N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015; 9: 024115–024115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prabhakarpandian B, Shen M, Nichols J, Mills I, Sidoryk-Wegrzynowicz M, Aschner M, Pant K. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip 2013; 13: 1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achyuta A, Conway A, Crouse R, Bannister E, Lee R, Katnik C, Behensky A, Cuevas J, Sundaram S. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip 2013; 13: 542–53. [DOI] [PubMed] [Google Scholar]
- 73.Griep L, Wolbers F, de Wagenaar B, ter Braak P, Weksler B, Romero I, Couraud P, Vermes I, van der Meer A, van den Berg A. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood–brain barrier function. Biomed Microdevice 2013; 15: 145–50. [DOI] [PubMed] [Google Scholar]
- 74.Yeon J, Na D, Choi K, Ryu S, Choi C, Park J. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevice 2012; 14: 1141–8. [DOI] [PubMed] [Google Scholar]
- 75.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in blood–brain barrier endothelial physiology. BMC Neurosci 2011; 12: 40–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolff A, Antfolk M, Brodin B, Tenje M. In vitro blood–brain barrier models – an overview of established models and new microfluidic approaches. J Pharm Sci 2015; 104: 2727–46. [DOI] [PubMed] [Google Scholar]
- 77.Duvernoy H, Delon S, Vannson J. The vascularization of the human cerebellar cortex. Brain Res Bull 1983; 11: 419–80. [DOI] [PubMed] [Google Scholar]
- 78.Wiedeman M. Dimensions of blood vessels from distributing artery to collecting vein. Circ Res 1963; 12: 375–8. [DOI] [PubMed] [Google Scholar]
- 79.Atkins G, Jain M. Role of Krüppel-Like Transcription Factors in Endothelial Biology. Circ Res 2007; 100: 1686–95–1686–95. [DOI] [PubMed] [Google Scholar]
- 80.Urich E, Lazic S, Molnos J, Wells I, Freskgård P. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood–brain barrier models. PLoS One 2012; 7: 6:31589–6:31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahman N, Rasil A, Meyding-Lamade U, Craemer E, Diah S, Tuah A, Muharram S. Immortalized endothelial cell lines for in vitro blood–brain barrier models: a systematic review. Brain Res 2016; 1642: 532–45. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Wang N, Cai B, Wang G, Li J, Piao X. In vitro model of the blood–brain barrier established by co-culture of primary cerebral microvascular endothelial and astrocyte cells. Neural Regen Res 2015; 10: 2011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bellin M, Marchetto M, Gage F, Mummery C. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol 2012; 13: 713–26. [DOI] [PubMed] [Google Scholar]
- 84.Pamies D, Hartung T, Hogberg H. Biological and medical applications of a brain-on-a-chip. Exp Biol Med (Maywood) 2014; 239: 1096–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lippmann E, Azarin S, Kay J, Nessler R, Wilson H, Al-Ahmad A, Palecek S, Shusta E. Derivation of blood–brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 2012; 30: 783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Phan D, Sobrino A, George S, Hughes C, Lee A. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 2016; 16: 282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Phan D, Zhao D, George S, Hughes C, Lee A. An on-chip microfluidic pressure regulator that facilitates reproducible loading of cells and hydrogels into microphysiological system platforms. Lab Chip 2016; 16: 868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sobrino A, Phan D, Datta R, Wang X, Hachey S, Romero-López M, Gratton E, Lee A, George S, Hughes C. 3D microtumors in vitro supported by perfused vascular networks. Sci Rep 2016; 6: 31589–31589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stringari C, Cinquin A, Cinquin O, Digman M, Donovan P, Gratton E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc Natl Acad Sci U S A 2011; 108: 13582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]