Abstract
Integrated multi-organ microphysiological systems are an evolving tool for preclinical evaluation of the potential toxicity and efficacy of drug candidates. Such systems, also known as Body-on-a-Chip devices, have a great potential to increase the successful conversion of drug candidates entering clinical trials into approved drugs. Systems, to be attractive for commercial adoption, need to be inexpensive, easy to operate, and give reproducible results. Further, the ability to measure functional responses, such as electrical activity, force generation, and barrier integrity of organ surrogates, enhances the ability to monitor response to drugs. The ability to operate a system for significant periods of time (up to 28 d) will provide potential to estimate chronic as well as acute responses of the human body. Here we review progress towards a self-contained low-cost microphysiological system with functional measurements of physiological responses.
Impact statement
Multi-organ microphysiological systems are promising devices to improve the drug development process. The development of a pumpless system represents the ability to build multi-organ systems that are of low cost, high reliability, and self-contained. These features, coupled with the ability to measure electrical and mechanical response in addition to chemical or metabolic changes, provides an attractive system for incorporation into the drug development process. This will be the most complete review of the pumpless platform with recirculation yet written.
Keywords: Pumpless, serum free, organ on a chip, organ–organ interactions, functional measurement, microphysiological systems
Introduction
Under the current paradigm of pharmaceutical innovation, only around 10% on average of drug candidates (5% for oncology) entering clinical trials make their way to regulatory approval.1 The major issue lies in the disease models used for drug discovery and preclinical development. Most drug screening relies on in vitro cell culture models and uses animal models to predict drug toxicity and efficacy in humans. While traditional cell culture models utilizing multiwell plates enable fast and inexpensive high-throughput screening, the accuracy of the prediction is often compromised due to oversimplified cell microenvironment and tissue structure, and their inability to reproduce the complex interactions among organs and tissues that occur in a living animal.2 Animal models, on the other hand, are costly, raise ethical issues, and have shown poor predictive power for human response to drugs due to cross-species discrepancies.3,4 Alternative models that can improve the accuracy of preclinical evaluation of drug candidates would reduce drug attrition rates during clinical stages and have a large economic impact.
The development of human cell-based multi-organ microphysiological systems has gained momentum in recent years. These systems, also known as Body-on-a-Chip (BOC) devices, integrate multiple microscale organ models and connect them with microfluidic channels to mimic multi-organ interactions within the body.5 Advanced in vitro cell culture technology and microfluidics are combined to create a “human surrogate” that could be used to emulate drug absorption, distribution, metabolism, and action in the body.2 Such human-based systems hold a great potential to better identify drug candidates for clinical trials with humans.
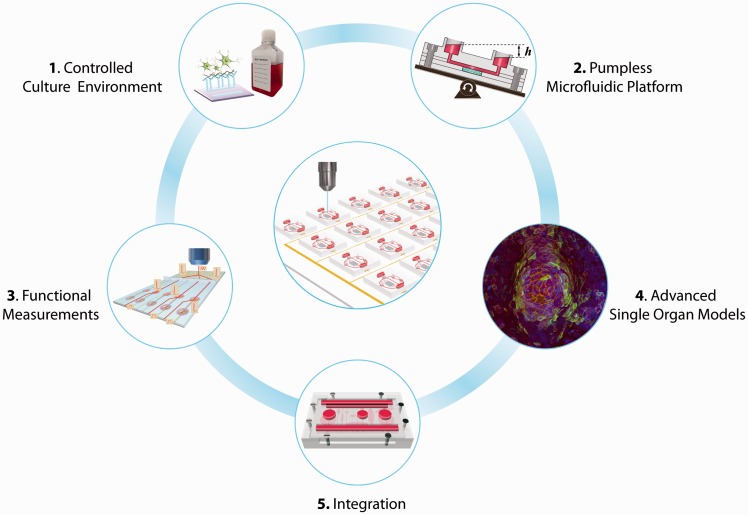
For effective adoption by the pharmaceutical industry, BOC systems need to be relatively inexpensive, easy to operate, and produce reliable results. This review summarizes our recent progress towards self-contained, low-cost BOC systems with functional measurements of physiological responses. A BOC system is considered “self-contained” if the operation of the system does not require any fluid or gas loops outside the device and functional measurements can be performed through non-invasive, non-destructive approaches, such as optical interrogation and in situ electrical recording. The only time that outside intervention is needed is when the system needs medium replenishment for long-term operation, as required for the human body. The review discusses five core aspects of such systems (Figure 1), including:
Controlled culture environment: The development of a blood surrogate with defined chemical formulation as well as the preparation of cell culture substrates with customized surface properties have given BOC systems better control over the cell microenvironment and thus helped generate reproducible results.
Pumpless microfluidic platform: Gravity-driven flow and passive fluidic controls have been combined to create fluid recirculation within the BOC model, allowing dynamic organ–organ interactions without the need for external pumps and tubing.6–10 Such pumpless microfluidic platforms form the basis of a self-contained BOC system, making low-cost operation of a large number of BOC units possible.
Functional measurements: On-chip integration of biosensors through adoption of microfabrication technologies has enabled non-invasive in situ functional measurements of electrical activity, contractile force, and barrier integrity.7,9–12 Along with metabolic analysis, these functional measurements have demonstrated high sensitivity in evaluating responses to drugs compared to morphology and viability analysis commonly used in drug toxicity screening.7
Advanced single organ models: Advanced single organ models, utilizing novel tissue engineering methods, human stem cell technology and microfabrication,12–17 provide sophisticated organ mimics as functional units for BOC systems to predict human responses to drugs.
System integration: Integration of multiple single organ units and functional measurement tools into one pumpless system has been demonstrated in several BOC systems. The integration, guided by residence time-based scaling and physiologically based pharmacokinetic (PBPK) models, enables physiologically relevant organ–organ interactions on chip, enhancing the predictive power of BOC systems for human responses.
Figure 1.
Self-contained low-cost Body-on-a-Chip (BOC) systems hold great potential for high-content, high-throughput drug screening. There are five core aspects of such systems: (1) controlled culture environment with defined culture medium formulations and surface chemistry helps with generating reproducible conditions and extending the depth of functional analysis. (2) Pumpless microfluidic platforms are the basis of a self-contained BOC system; (3) non-invasive functional measurements are enabled with integrated biosensors; (4) advanced single organ models provide improved organ mimics for BOC systems. Image shows a three-dimensional model of gastrointestinal (GI) macrovilli,74 reproduced from Esch et al.74 with permission from Springer. (5) Integration of multiple organ units based on proper scaling rules and integration strategies recreates physiologically relevant organ–organ interactions. (A color version of this figure is available in the online journal.)
Development in all five areas provides essential elements for building self-contained, low-cost BOC systems for drug development.
Controlled culture environment
A drastic change in the cell microenvironment occurs when cells are isolated from the body and placed into a petri dish.2,18 Mimicking the chemical composition of the cellular interspace and the cellular anchorage in vitro helps to offset some of the effects (i.e., phenotypic changes) triggered by the environmental shift.19 Standardized serum-free culture medium formulations enable precise control over the chemical environment surrounding the cells.2,18 Customized surface composition of cell culture substrates that favors the anchorage of specific cell adhesion molecules prevents phenotype loss and improves specific cellular functions.7,20–23 These strategies allow one to experiment with BOC systems under more reproducible conditions.
Defined cell culture medium formulation
To fully control the chemical microenvironment of cell cultures in vitro, well-defined culture medium is essential.2 Human or animal sera, typically supplemented in culture medium as a source for a variety of biologically important components, can vary considerably in composition from batch to batch. The elimination of serum from cell culture medium is critical to achieving full chemical definition. These undefined supplements can be replaced with a chemically well-defined formula that provides similar effects in supporting cell growth and maintenance. In 1995, the Hickman group implemented such an approach in experiments with hippocampal neurons.21 Further research has led to the successful adoption of defined and serum-free medium formulations for (i) single cultures of skeletal myotubes,22 cardiomyocytes,23 and motoneurons20,24; (ii) dual cultures of motoneurons with skeletal muscle (to form neuromuscular junctions),17 sensory neurons (to recreate the stretch reflex arc),16,25 or oligodendrocyte progenitor cells (to model myelination)26; and (iii) multi-organ cultures with four different organ types.7
Surface modifications
The cell microenvironment is also influenced by the functional groups present on the surface of culture substrates, especially for planar cultures, such as those grown on microelectrode arrays and microcantilevers for functional measurements. The surface chemistry controls the adsorption and activity of cell adhesive proteins,27 influencing cell–surface interactions such as adhesion and migration.28–30 Silane chemistry, an effective and widely used surface modification technique, has been used to produce self-assembled monolayers (SAMs) of cytophilic and cytophobic surfaces on glass coverslips and BioMicroelectromechanical Systems (bioMEMS) devices.7,22,23,25,30–43 With deep ultraviolet excimer laser lithography, SAMs can be patterned on cell culture substrates to control cell adhesion and alignment.44 These patterned silanes can produce defined tissue structures that enable high-content functional analysis.23,33,43,45,46 For example, chemical patterning of SAMs was used to engineer neuronal networks.46 The directionality of the network architecture was controlled with carefully designed discontinuities in chemical patterns on which hippocampal axonal growth was directed and “feed-forward” structures were created. In this way, the engineered chemical pattern pathways led to a layered architecture of neuronal networks where communication could travel within each layer and with directed communication pathways between layers. These defined neuronal networks allowed comprehensive network analysis when combined with extracellular electrical activity recording techniques.46
Pumpless microfluidic culture platforms
BOC systems utilize microfluidic technology to interconnect individual organ units and facilitate nutrient and metabolite exchange between chambers. Several multi-organ systems have been developed on microfluidic platforms.47–51 To achieve reliable and precise fluid transport, BOC microsystems often require extensive support from external pumps and connecting accessories, which consume significant laboratory space and complicate system assembly and operation. Developing a pumpless microfluidic platform is a key step towards building self-contained BOC systems.
In 2010, Sung et al. proposed a novel design of microfluidic devices that utilized gravity-induced flow and a rocking motion to recirculate cell culture medium.6 Microfluidic components, including three organ chambers and connecting channels were built on a single 4 cm x 4 cm chip. The integrated chip was placed on a tilting rocker plate to allow gravity to drive medium through the cell culture chambers of the device. Medium recirculation between two reservoirs was achieved by reversing the tilt direction periodically (Figure 1 (2)). This pumpless design using gravity as the driving force offers several key advantages: (i) it is space saving. Elimination of external pumps and tubing allows one to arrange self-contained BOC devices side by side closely to accommodate more units on a rocker plate. With additional stacking trays, tens to hundreds of units in parallel operation can be easily achieved on a single rocker. This pumpless solution holds great potential for relatively high-throughput application of microphysiological systems in pharmaceutical industries. (ii) It reduces unwanted drug adsorption on surfaces. Removal of connecting tubing also minimizes unwanted drug adsorption on the device by significantly reducing the exposed surface area. By decreasing the amount of drug adsorbed on device surfaces, more of the drug is available to interact with the organs on the chip. (iii) It is bubble-free. Air bubble formation is a notorious issue that afflicts most microfluidic cell culture systems. Air bubbles are detrimental to cells and generally lead to failure of a culture system. Yet they can be prevented in a gravity-driven system due to their buoyancy. This clears a major obstacle towards long-term, reliable operation of BOC systems. (iv) It is user-friendly. With all fluidic components integrated in a single platform, the assembly and operation of the devices are greatly simplified, and sterility is more easily sustained than when external tubing is utilized. (v) It provides easy access for liquid handling. The pumpless platform uses open access reservoirs that are similar to culture plate wells. This feature can facilitate automated liquid handling to remove samples and replenish medium. Overall, the gravity-driven pumpless platform provides a simple solution to achieve long-term reliable recirculating perfusion in BOC systems. By designing the channel dimensions and the tilt angle, the pumpless culture platform can achieve a wide range of flow rates that are suitable for BOC systems, from submicroliters to milliliters per minute. It should be noted that with highly integrated fluidic components on chip, the ratios of flow rates among organs are fixed once the chips are fabricated, although the overall flow rates are still adjustable through modifying the tilt angle. Such integration may reduce some flexibility for prototyping, but will benefit the long-term reliability and throughput of BOC operation.
The pumpless platform has evolved into several versions, and has been applied to various single-organ12,13,15 and multi-organ systems.7–10 Details will be discussed later in this review. It should be noted that the original pumpless platform using gravity-driven flow and a rocking motion creates a reciprocating mode of recirculation rather than a continuous unidirectional perfusion. Such reciprocating recirculation causes little deviation in the pharmacokinetic profiles of drugs compared to closed-loop recirculation.6 Similarly, the resulting bidirectional flow has not had adverse effects on the metabolism of liver tissue constructed from primary cells.15
The reciprocating flow induces bi-directional shear stress (SS), which could potentially affect SS-sensitive cells, such as endothelial cells and epithelial cells. Several modified versions have thus been developed to better accommodate SS-sensitive cells and tissues. Wang et al. introduced a “step chamber” in a microfluidic blood–brain barrier (BBB) model,12 in which the barrier cell culture plane was offset by a distance from the channel plane to minimize the magnitudes of bi-directional SS on the cell surface. Such modification allows brain microvascular endothelial cells (BMECs) to survive and maintain their unique BBB phenotype under a reciprocating circulation. Esch et al. designed a set of passive valves and a backflow channel to create a self-contained unidirectional pumpless system.9 The system supported gastrointestinal (GI) tract tissue in coculture with liver tissue for 14 d. In contrast to culture in a bidirectional system, the GI tract epithelium retained its barrier function in the unidirectional flow system, as evidenced through transepithelial electrical resistance (TEER) measurements.9
Overall, the development of the “pumpless” platform is a contribution toward effective adoption of BOC systems in drug discovery research. The “pumpless” platform is a low-cost, relatively high-throughput solution to achieve reliable long-term operation of BOC systems. It can be applied to both barrier and non-barrier tissues, easily adaptable for single organ and multi-organ microsystems, and versatile in integrating on-line analysis tools.
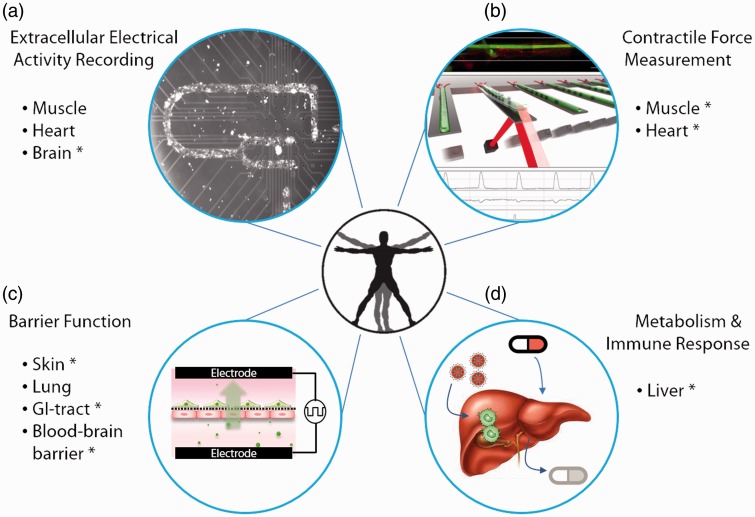
Functional measurements
Compared to morphology, viability, and biochemical analysis that are traditionally used in drug screening studies, measurements of organ-specific functions, such as cardiac electrical activity, muscle contraction, and intestinal barrier permeability, could provide valuable functional information and produce more sensitive evaluation of drug responses.7 Several in situ biosensors, such as microfabricated microelectrode arrays (MEAs) and microcantilevers, have been developed and incorporated into cell culture platforms to provide non-invasive functional readouts.7,9,11,12 These technologies can be readily integrated into microfluidic BOC systems and combined with optical interrogation and metabolic analysis to provide comprehensive functional assessment of drug responses in a self-contained BOC system without interrupting the cultures.
Extracellular electrical activity recording
Electrical propagation in the human body is exclusive to electrically conductive tissues such as nerves, muscles, and cardiac tissues. Functional analysis of those tissues has been extended beyond mere viability analysis and optical measurements (such as for cardiomyocyte beat frequency, BF) with extracellular electrical recording techniques, which have greatly advanced our knowledge of electrophysiology of populations of neurons. Among them, MEA-based multichannel recording systems are a non-invasive and high-throughput approach that provides comprehensive electrical signal recordings from conductive tissues (Figure 2(a)). The microfabricated nature of the MEA’s also makes it easier to be integrated into microfluidic BOC systems for electrical activity analysis.
Figure 2.
Functional measurements for Body-on-a-Chip systems. (a) Extracellular electrical activity recording with microelectrode arrays (MEAs) for conductive tissues, such as myotubes,77 cardiac microtissues,7,23 and nerves.7,20 (b) Measurement of force generation by muscles7,22 using a microfabricated cantilever-based system. Illustration reproduced from Smith et al.84 with permission from World Scientific Publishing. (c) Barrier function analysis through transepithelial electrical resistance (TEER) monitoring and permeability studies for barrier tissue, such as skin,13 gastrointestinal tract,71,72 and blood–brain barrier.12 (d) Analysis of hepatic enzymatic activity and immune response.8,9,15 *Organ-specific functional measurements that have been incorporated in a pumpless microfluidic system. (A color version of this figure is available in the online journal.)
The utility and ease of use of cell-based MEA recordings have enabled researchers to replace the time-consuming and cumbersome patch clamp technique for extracellular electrical activity recording. The sensitivity of toxin detection based on the electrical response of cardiac cells recorded using MEA’s systems has been found comparable to those using standard patch clamp techniques.11 Combining the MEA recording ability with surface patterning techniques that facilitate tissue alignment further expands the complexity and depth of electrical properties that can be measured (Figure 2(b)). This strategy has been applied to human stem cell derived cardiomyocytes to form aligned cardiac microtissues and enabled the measurement of a variety of critical parameters in assessing cardiac electrical response to drugs, including conduction velocity, rhythm generation (frequency and amplitude), action potential (AP) length (QT interval), and the length of the refractory period after an AP.23 The differential side effects of sotalol, norepinephrine, and verapamil on cardiac electrical functions presented in that combined system were in line with published clinical data,23 indicating great potential for the systems to evaluate cardiac electrical response to drugs.
Extracellular electrical activity measurements via MEAs have also been used to study the network architecture and electrophysiological behavior of hippocampal networks engineered with surface chemistry patterning. The extracellular recordings via MEAs have been used to measure the communication pathways both within layers and among layers. For example, long-term potentiation-like activity was induced in hippocampal networks to facilitate network analysis.46
Contractile force measurement
A primary functional output of muscle tissues is their contractile force, which can be measured in a number of ways in vitro.52 An approach developed by the Hickman group that combines microfabricated cantilevers and optical interrogation to measure cardiac or skeletal muscle force output (Figure 2(b)) is of particular interest due to its compatibility with microfluidic cell culture platforms, high precision, and ability to directly calculate force.
The force measurement is based on a microfabricated cantilever beam made of single-crystal silicon, whose dimensions and mechanical properties are well defined.34,38 Myocytes are cultured on top of the cantilevers, such that mechanical forces generated within the tissue is transferred to the cantilever beam causing a bending of the silicon cantilever. To measure the force, a laser is directed at the tip of the cantilever at an angle, and the reflected beam is directed onto a photodetector. As the cantilever bends, the reflected beam shifts on the photodetector, and this shift is measured.32 The geometry of the system, the sensitivity of the detector, and the mechanical properties of the cantilever all go into the calculation of the force from these measurements.31,39
This cantilever-based force measurement system was first developed to assess the physiological characteristics of skeletal muscle, including contractile force, time to peak force and time to half relaxation (the time needed for the muscle to generate peak force and to relax to half of the peak stress, respectively).31 An array of 32 cantilevers was fabricated on a single 1.5 cm x 1.5 cm chip to increase the throughput of measurements.31,32,34,38 The geometry of the cantilever was designed such that myoblasts on a cantilever fuse into a single functional skeletal muscle unit, a myotube. Each myotube can be investigated individually, allowing for measurement of myotube-level changes as well as changes to the population of myotubes. For example, data from this system can distinguish whether an effect occurs on the entire population of myotubes or only a subset. The effects of chronic drug treatments and long-term exercise protocols have been measured with respect to force and fatigue using this system.40 While initial experiments were carried out with primary embryonic rat myotubes,31,34,38,39 C2C12 mouse-based cells,32 and primary adult rat myotubes,40 this system has successfully performed force measurements on primary human myotubes.22
A similar system optimized for cardiomyocytes has also been developed.23 Cantilevers were customized for cardiomyocytes such that the cardiomyocytes aligned along the length of the cantilever to match in vivo syncytium structure. In this model, several drugs with cardiac effects on various aspects of the mechanical and electrical function were tested in multiple dosages, and these drugs demonstrated effects as expected from in vivo data. This system has been incorporated into a pumpless microfluidic device and monitored cardiomyocyte force over a 14-d period in a four-organ coculture system.7
Transepithelial and transendothelial electrical resistance (TEER) monitoring
Barrier tissues such as the gut, BBB, and pulmonary tissues are lined with monolayers of epithelial cells or endothelial cells, separating either the body from the external environment or the blood from tissues.53 Neighboring cells are bound together mainly through intercellular junctions, such as tight junctions, forming barrier layers that regulate mass transport across the interface. A variety of approaches can be used to evaluate barrier functions, including immunostaining of tight junction proteins as well as permeability studies. Among them, the transepithelial/ TEER measurement is the simplest. It can be performed non-invasively on live cells with quantitative and real-time readouts.54
The TEER value reflects the paracellular ionic conductance of cell monolayers and is a sensitive and reliable indicator of the barrier integrity.54 The values are determined by measuring the electrical resistance or impedance over a spectrum of frequencies, normalized to the effective monolayer area. A four-point probe method54 is commonly used in custom-designed and commercial TEER measuring systems, such as Endohm chambers and the “chopstick” electrode systems developed for transwells. Those systems measure ohmic resistance by utilizing a pair of electrodes on the different sides of the cell monolayer to apply square-wave current of microamps, and then use another pair of electrodes to record the voltage drop across the monolayer.
A uniform current density throughout the cellular monolayer is critical to ensure a valid TEER reading. In the “chopstick” system, the eccentric and suspended positioning of the electrodes tends to give uneven distribution of current flow across the cell layer that can result in 20% to 40% higher readings for large transwells (e.g., six-well transwells).55 The Endohm chambers adopt a fixed electrode geometry to minimize variation and a pair of concentrically centered circular disk current electrodes to achieve uniform current field.
A similar design to the Endohm chambers has been employed for Organ-on-a-Chip (OOC) systems with customized microelectrodes or thin-film microelectrodes56 (Figure 2(c)). Voltage-sensing Ag/AgCl pellets and annular current electrodes have been integrated into the pumpless platform to monitor the growth and barrier tightness of BBB12 and GI tissue9 constructs. Resistance measurements using these on-chip electrode systems have been compared to measurements from two commercial systems. The electrodes were also wired to give a real-time readout, without disrupting the culture. On-chip TEER monitoring can provide a non-invasive quality control measure of barrier integrity prior to the use of barrier tissues for drug permeability testing.
Metabolic and immunogenic measurements
Synthesis of plasma proteins, ammonia detoxification, drug metabolism, and immune response represent important hepatic functions. Previous in vitro models have demonstrated the possibility to quantify these functions (individually) from human primary hepatocytes in coculture with non-parenchymal cells (NPCs).57–61 However, the maintenance of stable function through time in a microfluidic pumpless system has been a challenge. A multicellular three dimensional liver model on the pumpless platform was developed and maintained for up to 14 d under flow and functional measurements were carried out through the experimental time non-invasively.15 The daily synthesis of albumin and urea were quantified from the medium exchange samples through the biochemical reactions of immunodetection (enzyme-linked immunosorbent assay, ELISA) and nucleophilic addition, both with final colorimetric determinations. The metabolism of drug compounds or xenobiotics by the hepatocytes is a tool that aims to improve the molecule physicochemical properties to facilitate elimination from the body. The liver is the major metabolizer in the body. Stable phase I metabolic enzyme activities were confirmed in the microfluidic platform throughout the 14 d period by evaluating the enzyme activities of 1A1 and 3A3 isoforms with a luminescence assay. A fluorescent-based reaction can also be used in these microfluidic devices avoiding the sampling step from the reservoir.62 Immune response studies of the hepatic platform were also possible with the coculture of human primary hepatocytes and hepatic NPCs upon the induction of Kupffer cells with lipopolysaccharide. An induced secretion of the cytokine IL-8 was quantified through a biochemical reaction using immunodetection (ELISA) and maintained for 14 d under flow demonstrating stable immune capacity in the system.9 All the parameters measured above reflect important hepatic functions among all others.
The platform consisted of human primary hepatocytes cocultured with human hepatic NPCs. The applied flow improved stabilization of the production of albumin and urea as well as cytochrome p450 1A1 and 3A4 enzymatic activities.15 The combination of a functional hepatic module together with other organs is of great importance for drug screening, not only for prodrug effects, but for the effect of the produced metabolites after the drug has been metabolized. Neither the addition of a GI tract module to the liver9 nor the addition of the other three organs into a liver module in a 4-Organ system7 affected the hepatic metabolic function.
Drug studies in such microfluidic models can be used to create PBPK models to predict the molecular fate of other compounds in the human body.63 It should be noted that to obtain accurate metabolic parameters from these studies, it is important to minimize or carefully characterize the absorption and adsorption of drugs to the microfluidic devices. Drug compounds with hydrophobic characteristics are known to have high affinity for absorption into polydimethylsiloxane (PDMS), a commonly used material for microfluidic devices, or adsorbed on the surface.64,65 The pumpless BOC system inherently benefits from the elimination of external tubing which reduces non-specific adsorption. Other strategies to reduce drug absorption and adsorption include PDMS surface modification,66 alternative materials for device fabrication,7,8 and pinhole-free parylene coating.12,15 Quantification of the predrug and metabolites can be carried out through mass spectrometry to further characterize the absorption and adsorption in the systems.
Advanced single OOC models
Human cell-based miniaturized organ models are the functional units of a BOC microsystem used in simulating human responses to drugs. Advances in human stem cell technology and tissue engineering have driven rapid development of OOC models for both barrier and non-barrier tissues.53,67 Those technologies have enabled efficient generation of high-fidelity human cells and in vivo-like cellular microenvironments to produce authentic metabolic and functional activities and simulate drug responses in the organs.12,13,15 Rapid adoption of microfabrication technology in biological contexts has also allowed integration of various biosensors for in situ functional analysis for OOC models.
Barrier tissues
BBB-on-a-Chip
The BBB, mainly composed of BMECs and overlying astrocytic foot processes, constitutes a dynamic physical and metabolic barrier between the blood and the brain. Most central nervous system (CNS) drug candidates have failed due to poor brain penetration across the BBB.68 High-fidelity in vitro models of the BBB would boost the development of neurotherapeutics by increasing the efficiency of brain drug permeability screening.
Wang et al. have developed a microfluidic BBB model with in vivo-like barrier properties based on the pumpless platform.12 The BBB-on-a-Chip (BBBoC) microsystem consists of a cell insert carrying BBB constructs and a pumpless culture platform providing luminal perfusion. The BBB constructs were prepared by coculturing human-induced pluripotent stem cell (hiPSC)-derived BMECs with primary astrocytes on two sides of a porous membrane of the cell insert. The constructs were then transferred onto the pumpless device for long-term maintenance. The adoption of the stand-alone cell insert simplifies dual-side cell seeding, ensures uniform membrane cell coverage critical to establishing barrier integrity, and separates the preparation of premature BBB constructs from long-term maintenance of mature BBB on chip.
The barrier properties of the BBBoC model were validated by TEER measurement, immunofluorescence staining and permeability studies. The BBBoC integrated electrodes for in situ monitoring of TEER values and is the first microfluidic BBB model that has demonstrated sustained TEER levels within the range of reported in vivo values (1500 to 8000 Ωcm2).69 It formed continuous networks of tight junction proteins (zonula occludens-1 and claudin-5). The permeability toward large molecules (fluorescein isothiocyanate [FITC]-dextran tracers) and small molecule model drugs (caffeine, cimetidine and doxorubicin) was demonstrated to be comparable to in vivo values. Such a microfluidic BBB model providing in vivo-like barrier properties could be a useful single-organ model for CNS drug permeability screening as well as a functional BBB unit to be integrated in the BOC systems.
In addition to modeling the BBB itself, Brown et al. has also included pericytes and neurons in a three-dimensional culture setting to recreate the neurovascular unit for studies where the mutual interaction between the BBB and CNS is of interest.70 This model and other 3D BBB–CNS are also reviewed in detail in the current issue.
Human skin equivalent (HSE)-on-a-Chip
Biomimetic in vitro skin models can be useful tools to predict skin responses to new drugs and evaluate the efficacy of transdermal drug delivery. Abaci et al., in collaboration with the Christiano group at Columbia University, have developed a microfluidic skin model based on the pumpless platform.13 Full thickness HSEs with both epidermal and dermal components were grown on porous support membranes with air on one side and with microfluidic perfusion on the other side. Establishment of a stable air–liquid interface that allows maturation and terminal differentiation of HSEs is usually challenging in a gravity-driven flow system. HSE-on-a-Chip has overcome this challenge by designing and carefully leveling the microfluidic housing to keep the medium levels from both reservoirs lower than the bottom layer of the HSE at all times. The system was able to maintain skin constructs on chip for three weeks with sustained barrier function validated by transdermal transport analysis. Immunohistochemistry stains revealed differentiation and localization of keratinocytes and establishment of all epidermis sub-layers after a week of on-chip culture. The keratinocytes at the epidermal–dermal interface remain proliferative up to three weeks. HSE-on-a-Chip also reproduced the toxic effects of doxorubicin on skin cells and structure, suggesting it could be a useful model for skin drug testing. Notably, the model used 36 fold less amount of culture medium and cells compared to conventional transwell models.
GI tract model
Replicating the barrier function of the GI tract epithelium accurately is important when using the tissue for evaluating drug candidates. In order to achieve physiologically relevant drug bioavailability profiles, the cellular barrier must be intact with similar permeability as in vivo.
The GI tract epithelium is a multi-cell type barrier tissue that regulates the uptake of orally administered drugs. Mahler et al. have developed a multi-cell type GI tract model that consists of epithelial cells, mucous-secreting goblet cells and M-cells.71 This model was used to simulate the oral uptake of nanoparticles of different sizes.72 The authors showed that differently sized nanoparticles preferred different routes of transport across the GI tract epithelium. Those routes were provided by the different cell types, demonstrating the importance of building a GI tract model that contains the major cell types found in vivo.
GI tract epithelial cells are also SS sensitive cells, and align under the fluidic flow. It is critical to create in vivo-like fluid flow conditions to establish and maintain GI tract epithelial barrier integrity as measured by TEER values. GI tract epithelial cells did not develop high TEER on a pumpless platform with reciprocating flow, but achieved and retained a high TEER level when the system was outfitted with a set of valves that created unidirectional flow.9
Another aspect of the GI tract model that has been recreated on-chip is its three dimensional architecture, consists of macrovilli that greatly increase the absorptive surface area of the gut. The gut macrovilli was replicated in a hydrogel73 and on microfabricated porous polymer membranes74 using microfabrication techniques.
Chen et al. have recently developed an ex vivo colon model by recellularizing the acellular human colon matrix with primary colon cells, including epithelial cells, endothelial cells, and myofibroblasts.75 In that study, they established a primary culture of colonic epithelial cells (hCECs) that were originally harvested from patient samples and immortalized with human telomerase reverse transcriptase (hTERT). These cells were able to form organoids with microcypt-like structure when cultured in 3D hydrogel. They can be a long-term stable source for hCECs for GI tract models for BOC systems.
Non-barrier tissues
Heart-on-a-Chip
Cardiac adverse effects are one of the main reasons for drug failure. The current preclinical and clinical models fail to predict the toxicity in advance, reflected in the number of withdrawn drugs due to cardiotoxicity observations after market release.76 Trying to overcome this problem, a human cardiac system was engineered to study basic physiology and toxicology of the two key cardiac functions: electrical conduction and contractile force. In Stancescu et al.23 a stable function of the human embryonic stem cell derived cardiomyocytes was achieved by combining surface modifications and a serum-free medium formulation. Cardiomyocytes were cultured on BioMEMS components to enable the electrical activity measurements from microelectrodes and the contractile force from cantilever chips. This system was challenged with three cardiotoxic drugs affecting different mechanism of action (chronotropic and inotropic effects): sotalol, norepinephrine, and verapamil. Functional responses were dose-dependent and were as expected from human clinical observations.
Muscle-on-a-Chip
The mechanical properties of the muscle have been of great interest for a broad field in science (i.e., bioengineering, neuromuscular studies and pharmacology). A human in vitro muscle platform was developed for studying the contractile forces of single myotubes. Human myotube formation has been successfully reproduced in vitro from human skeletal muscle stem cell/progenitors.77 Myotubes showed stable electrical properties similar to differentiated myotubes after two weeks under controlled culture conditions (serum-free and chemically modified surfaces).77 Myotube contractile force has been studied in this culture on cantilever chips upon electrical stimulation. The myotube culture was characterized for increasing its contractile force with maturity and showed a physiologic response of fatigue under a continuous electrical stimulation.22
CNS and Peripheral Nervous System (PNS)-on-a-Chip
The underlying mechanisms of the nervous system (NS) are incompletely understood. Solutions to NS-related diseases are affected by the incomplete puzzle.78 In vitro cultures for studies of the nervous system are a challenge due to the delicate culturing conditions required. Hickman and others have shown over a number of years the controlled culturing of several neuronal types under serum-free conditions and for long-term periods (weeks to months). Hippocampal neurons,79 motoneurons,24 sensory neurons,80 and HiPSc-derived cortical-like neurons20 have been fully characterized with stable electrical function. The integration of this neuronal culture with other cells types and with bioMEMS chips are in progress towards building a CNS and PNS-on-a-chip. While these systems have not been incorporated into microfluidic devices yet, the progress outlined below shows great promise.
The communication between neurons in the CNS occurs within complex network architectures. The behavior of neurons within this natural environment, particularly the neuronal plasticity, can be studied by controlling the cellular network architecture and the conduction path (communication) using chemical surface patterning and MEA chips.45,46 Participation of other cell types in the neuronal communication is also of interest. Supporting cells such as myelinating cells are involved in both the development of cell–cell connections as well as play a critical role in protecting and maintaining neuronal communication. Models for both CNS and PNS myelination have been established to promote neuron–neuron and neuron–muscle communication and to study diseases involving demyelination and injury repair.26,81,82 A higher degree of neuronal communication is the reflex arc circuit (CNS–PNS link). The sensory and the effector portions have been successfully reproduced in vitro under controlled conditions. The use of bioMEMS chips in different sections of this circuit helped validate the functional communication under electrical or chemical challenges.16,17,25,37,83–85
Liver-on-a-Chip
The liver is of great importance to the drug development industry due to its metabolic capacity and being a recurrent target for drug-induced toxicity.86,87 Esch et al. constructed multi-cellular 3D liver tissues that consisted of primary human NPCs (Kupffer cells, stellate cells, and fibroblasts) and primary human hepatocytes. When placed into a microfluidic cell culture device and subjected to gravity-driven flow that changes direction periodically, this tissue responded with an increase in metabolic activity.15 The observed result is similar to what others have seen with pumped systems,88 confirming that pumpless systems can replicate tissue function well.
BOC system integration
Multiple OOC models and on-chip functional analysis tools can be ultimately integrated onto a microfluidic platform to build a “human surrogate” that can be used to improve predictions of human response to drug candidates. The biggest advantage of such BOC systems over traditional multiwell plate cell culture models is the ability to mimic the complexity of organ–organ interactions in vivo. In addition to diffusion-mediated interactions that can be easily achieved by submerging all organ units in a common medium, such as in the “wells-within-a-well” coculture system,89 the microfluidics-based BOC systems allow for precise control over fluid transport between organs and thus are able to provide time-dependent dynamics of organ interaction. To recapitulate physiologically relevant drug dynamics and actions, and effectively interpret on-chip data into human clinical parameters, proper scaling rules and integration strategies are need.63 Shuler has suggested scaling rules based on blood residence time in each organ, which controls overall chemical conversion in a scale-independent manner. Others have proposed alternative approaches, such as allometric scaling and functional scaling.90–92 Residence time-based scaling is advantageous in replicating organ–organ interactions by taking into account the rate of chemical conversion in the tissue. PBPK models have also been proposed and applied to guide the design and assist the data interpretation of BOC systems.2,6,9,10,18 One might purposely make a BOC system non-physiological by removing or changing the size of certain organs to investigate mechanisms or disease conditions.
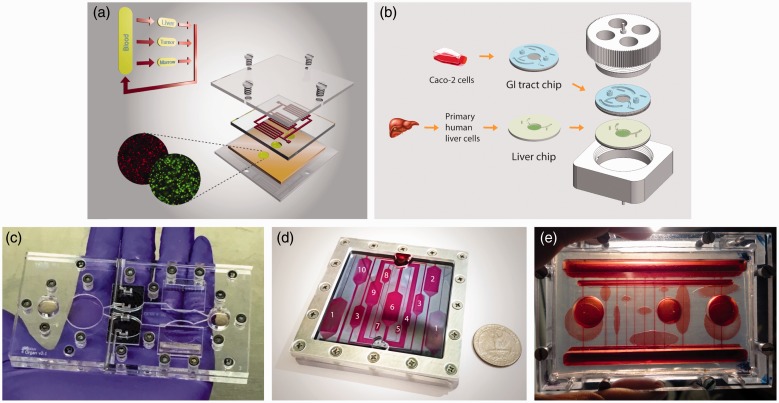
A full realization of a perfect “human surrogate” is not yet achievable, and may not be necessary for the purpose of drug screening. The time and money it could cost to build such a model may not yield sufficient advantages over simpler systems. Multi-organ systems with two to 13 organs have been demonstrated on pumpless microfluidic platforms (Figure 3).6–10 In 2010, Sung et al. published a pumpless system that contained three tissues: liver, bone marrow, and colon cancer tissue (Figure 3(a)).6 The actions of the cancer drugs 5-fluorouracil (5-FU) and uracil were recreated within that system, showing that metabolites generated in the liver tissue can recirculate and achieve the expected outcomes in the tumor tissue compartment. Lee et al. developed a liver-tumor model using a pumpless format.10 With that two-organ model, they reproduced the simultaneous metabolism and tumor cytotoxicity action of a flavonoid luteolin, and demonstrated the importance of using a proper scaling approach for multi-organ system design, as well as the power of combining a pharmacokinetic/pharmacodynamics (PK/PD) model with on-chip experiments in interpreting on-chip data and gaining mechanistic insights.
Figure 3.
Currently developed self-contained multi-organ systems. Integrated Body-on-a-Chip (BOC) systems that support coculture of two to 13 organs have been developed on pumpless microfluidic platforms. (a) A liver–tumor–marrow three-organ system. Reproduced from Sung et al.6 with permission from the Royal Society of Chemistry; (b) a modular system for the coculture of GI tract epithelium and 3D primary liver tissue with in situ transepithelial electrical resistance (TEER) measurement capacity.9 (c) A palm-size system for 4–5-organs with integrated electrical activity and contractile force sensors for non-invasive functional read-outs.7 (d, e) BOC systems for 10 or more organs.8 (A color version of this figure is available in the online journal.)
Two-organ systems of GI tract and liver have been developed previously on a platform that was operated with pumps to estimate the bioavailability of acetaminophen,48 and to evaluate the effects of 50 nm polystyrene nanoparticles on the GI tract and on liver tissue.93 The latest version of the GI tract–liver system developed on a pumpless platform features a modular design.9 The GI tract and liver tissues were cultured on separate microfluidic chips, and after maturation those chips were combined with each other to yield the two organ system (Figure 3(b)). Modular designs offer advantages because they allow the preparation of different tissues with special maturation requirements, such as tissues constructed from primary or stem cell sources, at their own pace before assembly into a single BOC system.
In 2016, Oleaga et al. reported a multi-organ model with four organs: liver, heart, muscle, and brain.7 The different cell types were located in communicating compartments inside a pumpless microfluidic system sharing the same culture medium with defined composition. Cellular morphology, viability, and function were measured for each cell type. The relevance of the multi-organ system as an in vitro platform for the study of systemic toxicity was achieved after the validation with five known drugs that exert side effects at high concentrations. The system responded in concordance with previous toxicity reported in in vivo and in vitro studies.7 Miller and Shuler presented a whole-body model on a pumpless microfluidic platform, which consider explicitly thirteen organs (14 chambers) (Figure 3(c)).8 Their work demonstrated the feasibility of constructing, operating, and maintaining a simple, self-contained, multi-organ microphysiological system with a large number of cell compartments for a sustained period with the capability of measuring cellular functions. They used a layered design to separate barrier and non-barrier types of cell cultures. The barrier chamber layer allowed for direct access that could be used to force chemical or biological reagents to pass through barrier cells before exposing the non-barrier tissues. These platforms represent the next generation of in vitro systems towards the BOC.
Concluding remarks
There have been many OOC and BOC models developed over the last decade, and we believe more of such microfluidics-based organ and whole-body models will be developed. We anticipate such systems will grow more sophisticated and will be a paradigm-shifting technology that will be adopted by pharmaceutical and chemical companies in reducing and replacing animal models. We also believe that self-contained, low-cost BOC systems, because of their simplicity and multiplex features, will become the workhorse of drug development. Under the new paradigm of pharmaceutical innovation, the time and cost to bring a new medicine to patients will be significantly reduced as the clinical approval success rate improves by adopting these simple but powerful BOC models.
Acknowledgment
The preparation of this mini-review has been supported by the National Institutes of Health (R44 TR001326-0A1), and is based on work accomplished under a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health (UH2TR000516).
Author Contributions
YIW, CO, JJH, and MLS devised the manuscript. YIW wrote the Introduction and the sections on pumpless microfluidic platforms, TEER monitoring, single organ models for the BBB and the skin, and the introduction for the Functional measurements and the Advanced single OOC models sections. CO, CL, and YIW wrote the section on controlled culture environment. CL, CM, and YIW wrote the section on extracellular electrical activity recording. CL and YIW wrote the section on contractile force measurement. CO wrote the sections on metabolism and immune response analysis and single cell models for the heart and muscles. MBE wrote the sections on single organ models of the liver and GI tracts and contributed to review on pumpless platforms. CO and CL wrote the CNS and PNS-on-a-Chip section. YIW, CO, MBE, and PM wrote the Body-on-a-Chip system integration section. MLS and YIW wrote the concluding remarks. YIW, MBE, CO, CL, PGM, JJH, and MLS contributed to editing the manuscript.
Declaration of Conflicting Interests
C Long, C McAleer, JJ Hickman (chief scientist), and ML Shuler (CEO/president) are also associated with Hesperos, Inc. (Orlando, FL) which is commercializing this technology. JJ Hickman has larger than a 5% equity in Hesperos, Inc., and C Long, C McAleer, and ML Shuler have less than a 5% equity in Hesperos, Inc. YI Wang, C Oleaga, MB Esch, and PG Miller have no conflicts of interest or financial ties to disclose.
References
- 1.Mullard A. Parsing clinical success rates. Nat Rev Drug Discov 2016; 15: 447–447. [DOI] [PubMed] [Google Scholar]
- 2.Esch MB, Smith AS, Prot JM, Oleaga C, Hickman JJ, Shuler ML. How multi-organ microdevices can help foster drug development. Adv Drug Deliv Rev 2014; 69–70: 158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, Burns C, Thorpe R, Stebbings R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol 2010; 161: 512–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U, Investigators SIT. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med 2007; 357: 562–71. [DOI] [PubMed] [Google Scholar]
- 5.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng 2011; 13: 55–72. [DOI] [PubMed] [Google Scholar]
- 6.Sung JH, Kam C, Shuler ML. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 2010; 10: 446–55. [DOI] [PubMed] [Google Scholar]
- 7.Oleaga C, Bernabini C, Smith AS, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B, Najjar S, Akanda N, Guo X, Martin C, Ekman G, Esch MB, Langer J, Ouedraogo G, Cotovio J, Breton L, Shuler ML, Hickman JJ. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep 2016; 6: 20030–20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller PG, Shuler ML. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol Bioeng 2016; 113: 2213–27. [DOI] [PubMed] [Google Scholar]
- 9.Esch MB, Ueno H, Applegate DR, Shuler ML. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 2016; 16: 2719–29. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Kim DS, Ha SG, Choi I, Lee JM, Sung JH. A pumpless multi-organ-on-a-chip (MOC) combined with a pharmacokinetic-pharmacodynamic (PK-PD) model. Biotechnol Bioeng 2017;114:432–43. [DOI] [PubMed]
- 11.Natarajan A, Molnar P, Sieverdes K, Jamshidi A, Hickman JJ. Microelectrode array recordings of cardiac action potentials as a high throughput method to evaluate pesticide toxicity. Toxicol In Vitro 2006; 20: 375–81. [DOI] [PubMed] [Google Scholar]
- 12.Wang YI, Abaci HE, Shuler ML. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng 2017;114:184–94. [DOI] [PMC free article] [PubMed]
- 13.Abaci HE, Gledhill K, Guo Z, Christiano AM, Shuler ML. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab Chip 2015; 15: 882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HJ, Sun J, Huang Z, Hou H, Arcilla M, Rakhilin N, Joe DJ, Choi J, Gadamsetty P, Milsom J, Nandakumar G, Longman R, Zhou XK, Edwards R, Chen J, Chen KY, Bu P, Wang L, Xu Y, Munroe R, Abratte C, Miller AD, Gümüş ZH, Shuler M, Nishimura N, Edelmann W, Shen X, Lipkin SM. Comprehensive models of human primary and metastatic colorectal tumors in immunodeficient and immunocompetent mice by chemokine targeting. Nat Biotechnol 2015; 33: 656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esch MB, Prot J-M, Wang YI, Miller P, Llamas-Vidales JR, Naughton BA, Applegate DR, Shuler ML. Multi-cellular 3D human primary liver cell culture elevates metabolic activity under fluidic flow. Lab Chip 2015; 15: 2269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Ayala JE, Gonzalez M, Stancescu M, Lambert S, Hickman JJ. Tissue engineering the monosynaptic circuit of the stretch reflex arc with co-culture of embryonic motoneurons and proprioceptive sensory neurons. Biomaterials 2012; 33: 5723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials 2011; 32: 9602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung JH, Srinivasan B, Esch MB, McLamb WT, Bernabini C, Shuler ML, Hickman JJ. Using physiologically-based pharmacokinetic-guided “body-on-a-chip” systems to predict mammalian response to drug and chemical exposure. Exp Biol Med 2014; 239: 1225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009; 326: 1216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry BJ, Akanda N, Smith AS, Long CJ, Schnepper MT, Guo X, Hickman JJ. Morphological and functional characterization of human induced pluripotent stem cell-derived neurons (iCell Neurons) in defined culture systems. Biotechnol Prog 2015; 31: 1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J Neurosci Methods 1995; 62: 111–9. [DOI] [PubMed] [Google Scholar]
- 22.Smith AS, Long CJ, Pirozzi K, Najjar S, McAleer C, Vandenburgh HH, Hickman JJ. A multiplexed chip-based assay system for investigating the functional development of human skeletal myotubes in vitro. J Biotechnol 2014; 185: 15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stancescu M, Molnar P, McAleer CW, McLamb W, Long CJ, Oleaga C, Prot JM, Hickman JJ. A phenotypic in vitro model for the main determinants of human whole heart function. Biomaterials 2015; 60: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X, Johe K, Molnar P, Davis H, Hickman J. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J Tissue Eng Regen Med 2010; 4: 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rumsey JW, Das M, Bhalkikar A, Stancescu M, Hickman JJ. Tissue engineering the mechanosensory circuit of the stretch reflex arc: sensory neuron innervation of intrafusal muscle fibers. Biomaterials 2010; 31: 8218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis H, Gonzalez M, Stancescu M, Love R, Hickman JJ, Lambert S. A phenotypic culture system for the molecular analysis of CNS myelination in the spinal cord. Biomaterials 2014; 35: 8840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson KA, Finch CA, Anderson P, Vollmer F, Hickman JJ. Whispering gallery mode biosensor quantification of fibronectin adsorption kinetics onto alkylsilane monolayers and interpretation of resultant cellular response. Biomaterials 2012; 33: 225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spargo BJ, Testoff MA, Nielsen TB, Stenger DA, Hickman JJ, Rudolph AS. Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc Nat Acad Sci 1994; 91: 11070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res 1993; 630: 136–47. [DOI] [PubMed] [Google Scholar]
- 30.Hickman JJ, Bhatia SK, Quong JN, Shoen P, Stenger DA, Pike CJ, Cotman CW. Rational pattern design for in vitro cellular networks using surface photochemistry. J Vacuum Sci Technol A 1994; 12: 607–607. [Google Scholar]
- 31.Wilson K, Das M, Wahl KJ, Colton RJ, Hickman J. Measurement of contractile stress generated by cultured rat muscle on silicon cantilevers for toxin detection and muscle performance enhancement. PLoS One 2010; 5: e11042–e11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson K, Molnar P, Hickman J. Integration of functional myotubes with a Bio-MEMS device for non-invasive interrogation. Lab Chip 2007; 7: 920–2. [DOI] [PubMed] [Google Scholar]
- 33.Molnar P, Wang WS, Natarajan A, Rumsey JW, Hickman JJ. Photolithographic patterning of C2C12 myotubes using vitronectin as growth substrate in serum-free medium. Biotechnol Prog 2007; 23: 265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das M, Gregory CA, Molnar P, Riedel LM, Wilson K, Hickman JJ. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials 2006; 27: 4374–80. [DOI] [PubMed] [Google Scholar]
- 35.Das M, Rumsey JW, Bhargava N, Gregory C, Riedel L, Kang JF, Hickman JJ. Developing a novel serum-free cell culture model of skeletal muscle differentiation by systematically studying the role of different growth factors in myotube formation. In Vitro Cell Dev Biol Anim 2009; 45: 378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. Skeletal muscle tissue engineering: a maturation model promoting long-term survival of myotubes, structural development of the excitation-contraction coupling apparatus and neonatal myosin heavy chain expression. Biomaterials 2009; 30: 5392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials 2010; 31: 4880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das M, Wilson K, Molnar P, Hickman JJ. Differentiation of skeletal muscle and integration of myotubes with silicon microstructures using serum-free medium and a synthetic silane substrate. Nat Protoc 2007; 2: 1795–801. [DOI] [PubMed] [Google Scholar]
- 39.Pirozzi KL, Long CJ, McAleer CW, Smith AS, Hickman JJ. Correlation of embryonic skeletal muscle myotube physical characteristics with contractile force generation on an atomic force microscope-based bio-microelectromechanical systems device. Appl Phys Lett 2013; 103: 83108–83108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAleer CW, Smith AS, Najjar S, Pirozzi K, Long CJ, Hickman JJ. Mechanistic investigation of adult myotube response to exercise and drug treatment in vitro using a multiplexed functional assay system. J Appl Physiol (1985) 2014; 117: 1398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natarajan A, Chun C, Hickman JJ, Molnar P. Growth and electrophysiological properties of rat embryonic cardiomyocytes on hydroxyl- and carboxyl-modified surfaces. J Biomater Sci Polym Ed 2008; 19: 1319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith AS, Long CJ, Pirozzi K, Hickman JJ. A functional system for high-content screening of neuromuscular junctions. Technology 2013; 1: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natarajan A, Stancescu M, Dhir V, Armstrong C, Sommerhage F, Hickman JJ, Molnar P. Patterned cardiomyocytes on microelectrode arrays as a functional, high information content drug screening platform. Biomaterials 2011; 32: 4267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson K, Stancescu M, Das M, Rumsey J, Hickman J. Direct patterning of coplanar polyethylene glycol alkylsilane monolayers by deep-ultraviolet photolithography as a general method for high fidelity, long-term cell patterning and culture. J Vacuum Sci Technol B 2011; 29: 0210201–10–0210201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards D, Stancescu M, Molnar P, Hickman JJ. Two cell circuits of oriented adult hippocampal neurons on self-assembled monolayers for use in the study of neuronal communication in a defined system. ACS Chem Neurosci 2013; 4: 1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natarajan A, DeMarse TB, Molnar P, Hickman JJ. Engineered in vitro feed-forward networks. J Biotechnol Biomater 2013; 3: 10001531–7–10001531–7. [Google Scholar]
- 47.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol Prog 2004; 20: 338–45. [DOI] [PubMed] [Google Scholar]
- 48.Mahler GJ, Esch MB, Glahn RP, Shuler ML. Characterization of a gastrointestinal tract microscale cell culture analog used to predict drug toxicity. Biotechnol Bioeng 2009; 104: 193–205. [DOI] [PubMed] [Google Scholar]
- 49.Wagner I, Materne EM, Brincker S, Sussbier U, Fradrich C, Busek M, Sonntag F, Sakharov DA, Trushkin EV, Tonevitsky AG, Lauster R, Marx U. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013; 13: 3538–47. [DOI] [PubMed] [Google Scholar]
- 50.Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hubner J, Lindner M, Drewell C, Bauer S, Thomas A, Sambo NS, Sonntag F, Lauster R, Marx U. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015; 15: 2688–99. [DOI] [PubMed] [Google Scholar]
- 51.An F, Qu Y, Luo Y, Fang N, Liu Y, Gao Z, Zhao W, Lin B. A laminated microfluidic device for comprehensive preclinical testing in the drug ADME process. Sci Rep 2016; 6: 25022–25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sung JH, Esch MB, Prot JM, Long CJ, Smith A, Hickman JJ, Shuler ML. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip 2013; 13: 1201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakolish CM, Esch MB, Hickman JJ, Shuler ML, Mahler GJ. Modeling barrier tissues in vitro: methods, achievements, and challenges. EBioMedicine 2016; 5: 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 2015; 20: 107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.CaliCell instruction manual: World Precision Instruments, 2014.
- 56.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood–brain barrier (muBBB). Lab Chip 2012; 12: 1784–92. [DOI] [PubMed] [Google Scholar]
- 57.Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell–cell interactions in vitro. Hepatology 2015; 61: 1370–81. [DOI] [PubMed] [Google Scholar]
- 58.Cameron K, Tan R, Schmidt-Heck W, Campos G, Lyall MJ, Wang Y, Lucendo-Villarin B, Szkolnicka D, Bates N, Kimber SJ, Hengstler JG, Godoy P, Forbes SJ, Hay DC. Recombinant laminins drive the differentiation and self-organization of hESC-derived hepatocytes. Stem Cell Rep 2015; 5: 1250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi K, Pfund WP, Andersen ME, Thomas RS, Clewell HJ, LeCluyse EL. Development of 3D dynamic flow model of human liver and its application to prediction of metabolic clearance of 7-ethoxycoumarin. Tissue Eng Part C Methods 2014; 20: 641–51. [DOI] [PubMed] [Google Scholar]
- 60.Ferrini JB, Pichard L, Domergue J, Maurel P. Long-term primary cultures of adult human hepatocytes. Chem Biol Interact 1997; 107: 31–45. [DOI] [PubMed] [Google Scholar]
- 61.Ware BR, Berger DR, Khetani SR. Prediction of drug-induced liver injury in micropatterned co-cultures containing iPSC-derived human hepatocytes. Toxicol Sci 2015; 145: 252–62. [DOI] [PubMed] [Google Scholar]
- 62.Sung JH, Choi JR, Kim D, Shuler ML. Fluorescence optical detection in situ for real-time monitoring of cytochrome P450 enzymatic activity of liver cells in multiple microfluidic devices. Biotechnol Bioeng 2009; 104: 516–25. [DOI] [PubMed] [Google Scholar]
- 63.Abaci H, Shuler M. Human-on-a-chip design strategies and principles for physiologically based pharmocokinetics/pharmacodynamics modeling. Integr Biol 2015; 7: 383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006; 6: 1484–6. [DOI] [PubMed] [Google Scholar]
- 65.Wang JD, Douville NJ, Takayama S, ElSayed M. Quantitative analysis of molecular absorption into PDMS microfluidic channels. Ann Biomed Eng 2012; 40: 1862–73. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Sjoberg R, Leyrat AA, Houseman BT, Shokat K, Quake SR. Biocompatibility and reduced drug absorption of sol-gel-treated poly(dimethyl siloxane) for microfluidic cell culture applications. Anal Chem 2010; 82: 8954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng F, Fu F, Cheng Y, Wang C, Zhao Y, Gu Z. Organ-on-a-chip systems: microengineering to biomimic living systems. Small 2016; 12: 2253–82. [DOI] [PubMed] [Google Scholar]
- 68.Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx 2005; 2: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolff A, Antfolk M, Brodin B, Tenje M. In vitro blood-brain barrier models – an overview of established models and new microfluidic approaches. J Pharm Sci 2015; 104: 2727–46. [DOI] [PubMed] [Google Scholar]
- 70.Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM, Samson PC, McCawley LJ, May JM, Webb DJ, Li D, Bowman AB, Reiserer RS, Wikswo JP. Recreating blood–brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015; 9: 054124–054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahler GJ, Shuler ML, Glahn RP. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J Nutr Biochem 2009; 20: 494–502. [DOI] [PubMed] [Google Scholar]
- 72.Mahler GJ, Esch MB, Tako E, Southard TL, Archer SD, Glahn RP, Shuler ML. Oral exposure to polystyrene nanoparticles affects iron absorption. Nat Nanotechnol 2012; 7: 264–71. [DOI] [PubMed] [Google Scholar]
- 73.Sung JH, Yu J, Luo D, Shuler ML, March JC. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 2011; 11: 389–92. [DOI] [PubMed] [Google Scholar]
- 74.Esch MB, Sung JH, Yang J, Yu C, Yu J, March JC, Shuler ML. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip' devices. Biomed Microdevices 2012; 14: 895–906. [DOI] [PubMed] [Google Scholar]
- 75.Chen HJ, Wei Z, Sun J, Bhattacharya A, Savage DJ, Serda R, Mackeyev Y, Curley SA, Bu P, Wang L, Chen S, Cohen-Gould L, Huang E, Shen X, Lipkin SM, Copeland NG, Jenkins NA, Shuler ML. A recellularized human colon model identifies cancer driver genes. Nat Biotechnol 2016; 34: 845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siramshetty VB, Nickel J, Omieczynski C, Gohlke BO, Drwal MN, Preissner R. WITHDRAWN – a resource for withdrawn and discontinued drugs. Nucleic Acids Res 2016; 44: D1080–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo X, Greene K, Akanda N, Smith A, Stancescu M, Lambert S, Vandenburgh H, Hickman J. In vitro differentiation of functional human skeletal myotubes in a defined system. Biomater Sci 2014; 2: 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang ZJ, Luo L. NEUROSCIENCE. It takes the world to understand the brain. Science 2015; 350: 42–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edwards D, Das M, Molnar P, Hickman JJ. Addition of glutamate to serum-free culture promotes recovery of electrical activity in adult hippocampal neurons in vitro. J Neurosci Methods 2010; 190: 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo X, Spradling S, Stancescu M, Lambert S, Hickman JJ. Derivation of sensory neurons and neural crest stem cells from human neural progenitor hNP1. Biomaterials 2013; 34: 4418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis H, Gonzalez M, Bhargava N, Stancescu M, Hickman JJ, Lambert S. Rat cortical oligodendrocyte-embryonic motoneuron co-culture: an in vitro axon-oligodendrocyte interaction model. J Biomater Tissue Eng 2012; 2: 206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rumsey JW, McAleer C, Das M, Bhalkikar A, Wilson K, Stancescu M, Lambert S, Hickman JJ. Myelination and node of Ranvier formation on sensory neurons in a defined in vitro system. In Vitro Cell Dev Biol Anim 2013; 49: 608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo X, Das M, Rumsey J, Gonzalez M, Stancescu M, Hickman J. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng Part C Methods 2010; 16: 1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith AS, Long CJ, Pirozzi K, Hickman JJ. A functional system for high-content screening of neuromuscular junctions in vitro. Technology 2013; 1: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo X, Sommerhage F, McAleer C, Martin C, Long C, Wang Y, Santhanam N, Colon A, Sancho CO, Hickman J. In vitro modeling of nervous system: engineering of the reflex arc. In: Zhang LG, Kaplan DL (eds) Neural engineering: from advanced biomaterials to 3D fabrication techniques. Cham: Springer International Publishing, 2016, pp.261–98.
- 86.Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, Giacomini KM, Krauss RM. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov 2007; 6: 904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Bottger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho CS, Choi YJ, Craig Rowlands J, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CE, Gomez-Lechon MJ, Groothuis GM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Haussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhutter HG, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Kevin Park B, Kordes C, Kullak-Ublick GA, LeCluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi J, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EH, Stieger B, Stober R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM, Hengstler JG. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 2013; 87: 1315–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ebrahimkhani MR, Neiman JA, Raredon MS, Hughes DJ, Griffith LG. Bioreactor technologies to support liver function in vitro. Adv Drug Deliv Rev 2014; 69–70: 132–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li AP, Bode C, Sakai Y. A novel in vitro system, the integrated discrete multiple organ cell culture (IdMOC) system, for the evaluation of human drug toxicity: comparative cytotoxicity of tamoxifen towards normal human cells from five major organs and MCF-7 adenocarcinoma breast cancer cells. Chem Biol Interact 2004; 150: 129–36. [DOI] [PubMed] [Google Scholar]
- 90.Wikswo JP, Curtis EL, Eagleton ZE, Evans BC, Kole A, Hofmeister LH, Matloff WJ. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 2013; 13: 3496–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moraes C, Labuz JM, Leung BM, Inoue M, Chun TH, Takayama S. On being the right size: scaling effects in designing a human-on-a-chip. Integr Biol 2013; 5: 1149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vozzi F, Mazzei D, Vinci B, Vozzi G, Sbrana T, Ricotti L, Forgione N, Ahluwalia A. A flexible bioreactor system for constructing in vitro tissue and organ models. Biotechnol Bioeng 2011; 108: 2129–40. [DOI] [PubMed] [Google Scholar]
- 93.Esch MB, Mahler GJ, Stokol T, Shuler ML. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip 2014; 14: 3081–92. [DOI] [PMC free article] [PubMed] [Google Scholar]