Abstract
Necroptosis is a form of regulated cell death that critically depends on receptor-interacting serine-threonine kinase 3 (RIPK3) and mixed lineage kinase domain-like (MLKL) and generally manifests with morphological features of necrosis. The molecular mechanisms that underlie distinct instances of necroptosis have just begun to emerge. Nonetheless, it has already been shown that necroptosis contributes to cellular demise in various pathophysiological conditions, including viral infection, acute kidney injury, and cardiac ischemia/reperfusion. Moreover, human tumors appear to obtain an advantage from the downregulation of key components of the molecular machinery for necroptosis. Although such an advantage may stem from an increased resistance to adverse microenvironmental conditions, accumulating evidence indicates that necroptosis-deficient cancer cells are poorly immunogenic and hence escape natural and therapy-elicited immunosurveillance. Here, we discuss the molecular mechanisms and relevance to disease of necroptosis.
Keywords: caspases, damage-associated molecular patterns, immunogenic cell death, inflammation, mitochondrial permeability transition, necrostatin-1
INTRODUCTION
Extreme microenvironmental conditions (e.g., elevated pressures, shear forces, high temperatures) provoke the physical disassembly of cellular constituents, hence causing a virtually instantaneous and uncontrollable form of cellular demise that is commonly known as accidental cell death (ACD) (1). By contrast, cells succumb to relatively mild perturbations of homeostasis or developmental signals when a dedicated molecular machinery is activated (1). This latter instance of cellular demise can be modulated (i.e., accelerated or retarded) by pharmacological agents or genetic interventions and is generally referred to as regulated cell death (RCD) (2, 3). The term programmed cell death is employed to define instances of RCD that occur in purely physiological settings (e.g., embryonic and postnatal development, the maintenance of adult tissue homeostasis) (4) as opposed to RCD triggered by exogenous perturbations of the intracellular or extracellular microenvironment, which occurs once adaptive processes such as autophagy or the endoplasmic reticulum stress response fail (5, 6).
Because ACD invariably manifests with morphological features of necrosis (i.e., plasma membrane breakdown, generalized swelling of the cytoplasm and organelles, spillage of cellular constituents into the microenvironment), researchers commonly assumed that necrosis always occurs in accidental settings (7, 8). Data accumulating over the past 15 years, however, has conclusively demonstrated that several forms of RCD manifest with a partial or complete necrotic morphology, including necroptosis, as well as mitochondrial permeability transition (MPT)-driven regulated necrosis, parthanatos, ferroptosis, and pyroptosis (7, 8) (see sidebar, Other Forms of Regulated Necrosis). This conceptual transition has initiated a completely new and active field of investigation. Here, we discuss the signal transduction cascades that control necroptosis and analyze the relevance of necroptotic RCD for human physiopathology.
MOLECULAR MECHANISMS OF NECROPTOSIS
Pharmacological and genetic evidence collected over the past decade has provided profound insights into the molecular machinery that precipitates necroptosis (Table 1).
Table 1.
Major traits of mice with genetic knockouts or knockins in core and upstream regulators of necroptosis
| Genotype | Viable | Observations | Reference |
|---|---|---|---|
| Camk2g−/− | Yes | Are protected from doxorubicin-driven cardiomyopathy Are protected from cardiac ischemia/reperfusion |
114 |
| Casp8−/− | No | Die in utero on E10.5 | 60 |
| Casp8−/−Ripk3−/− | Yes | Exhibit lymphoproliferative disorders Are protected against systemic FAS ligation Fail to control Yersinia pestis infection |
56, 61, 132 |
| Casp8−/−Ripk3D161N | Yes | Exhibit lymphoproliferative disorders | 31 |
| Casp8−/−Ripk3K51A | Yes | Are viable, but not better characterized | 49 |
| Casp8−/−Tnfrsf1a −/− | No | Fail to survive to birth, but progress well beyond E10.5 | 41 |
| Cflar−/− | No | Die in utero on E10.5 | 65 |
| Cflar−/−Ripk3−/− | No | Die in utero on E12–13.5, owing to massive Casp3 activation in various tissues | 57 |
| Cflar−/−Ripk3−/−Fadd−/− | Yes | Exhibit lymphoproliferative disorders | 57 |
| Cflar−/−Ripk3−/−Tnfrsf1a−/− | No | Fail to survive to birth, but progress well beyond E10.5 | 41 |
| Cflar−/−Tnfrsf1a −/− | No | Fail to survive to birth, but progress well beyond E10.5 | 41 |
| Fadd−/− | No | Die in utero on E10.5 | 63, 64 |
| Fadd−/−Ripk3−/− | Yes | Exhibit lymphoproliferative disorders Are protected against systemic FAS ligation |
57 |
| Fadd−/−Tnfrsf1a −/− | No | Fail to survive to birth, but progress well beyond E10.5 | 41 |
| Mlkl−/− | Yes | Are protected from cerulein-driven pancreatitis Are protected from AKI driven by oxalate crystals or cisplatin Are not protected from acetaminophen-driven hepatitis Are not protected from a rheumatoid arthritis–like syndrome |
12, 52, 163, 166, 176 |
| Ripk1−/− | No | Die before 3 days of age, owing to widespread Casp3 activation and consequent multiorgan pathology | 43, 124 |
| Ripk1−/−Casp8−/− | No | Die in a few hours after birth | 41 |
| Ripk1−/−Fadd−/− | No | Most (>90%) die before 3 days of age | 45 |
| Ripk1−/−Faslg−/−Tnfrsf1a−/− | No | Die in a few hours after birth | 124 |
| Ripk1−/−Ifnar1−/− | No | Most (>90%) die before 5 days of age | 41 |
| Ripk1−/−Mlkl−/− | No | Most (>90%) die before 4 days of age | 124 |
| Ripk1−/−Myd88−/− | No | Most (>90%) die before 4 days of age | 124 |
| Ripk1−/−Ripk3−/− | No | Most (>90%) die before 10 days of age | 41, 124 |
| Ripk1−/−Ripk3−/−Casp8−/− | Yes | Exhibit lymphoproliferative disorders Display normal response to viral challenge |
41, 44, 124 |
| Ripk1−/−Ripk3−/−Fadd−/− | Yes | Exhibit lymphoproliferative disorders | 41 |
| Ripk1−/−Ripk3−/−Tnfrsf1a−/− | Yes | Most (>90%) die before 150 days of age, owing to the inability to maintain intestinal barrier functions | 41 |
| Ripk1−/−Ripk3+/−Casp8−/− | Yes | Are significantly underweight Most (>80%) die before 180 days of age |
44 |
| Ripk1−/−Ticam1−/− | No | Most (>90%) die before 3 days of age | 41 |
| Ripk1−/−Tnfrsf1a −/− | No | Die in a few hours after birth | 41, 124 |
| Ripk1−/−Tnfrsf1a−/−Ifnar1−/− | No | Most (>90%) die before 25 days of age | 41 |
| Ripk1−/−Tnfrsf1a−/−Ticam1−/− | No | Most (>90%) die before 25 days of age | 41 |
| Ripk3−/− | Yes | Are protected from TNF-driven SIRS Are protected from cerulein-driven pancreatitis Are protected from doxorubicin-driven cardiomyopathy Are protected from cardiac I/R Are protected from elastase-induced abdominal aortic aneurysm Are protected from atherogenesis driven by the Apoe−/− or Ldlr−/− genotype Are protected from RD driven by hyaluronate or poyI:C injection Are protected from spinal cord injury Are protected from AKI driven by I/R, oxalate crystals, or cisplatin Are protected from acetaminophen-driven hepatitis (?) Are protected from concanavalin A-driven hepatitis Are protected from ethanol and dietary intoxication Are protected from nonalcoholic steatohepatitis Are protected from a rheumatoid arthritis-like syndrome Fail to control HSV-1 and vaccinia virus infections |
32, 33, 34, 52, 114, 115, 127, 134, 139, 143, 150, 152, 159, 163, 166, 170, 172, 173, 174, 175, 179 |
| Ripk1D138N | Yes | Are protected against TNF-driven SIRS Fail to control vaccinia virus infections |
31, 48 |
| Ripk1K45A | Yes | Are protected against TNF-driven SIRS Are protected against SIRS driven by the Sharpin−/− genotype |
44, 47 |
| Ripk3D161N | No | Die in utero on E10.5, owing to uncontrolled Ripk1- and Casp8-driven apoptosis | 31 |
| Ripk3K51A | Yes | Are fertile but not better characterized | 49 |
Abbreviations: AKI, acute kidney injury; Apoe, apolipoprotein E; Camk2g, calcium/calmodulin-dependent protein kinase II gamma; Casp, caspase; Cflar, CASP8 and FADD like apoptosis regulator; Fadd, Fas associated via death domain; HSV-1, herpes simplex virus type 1; Ifnar1, interferon alpha and beta receptor 1; I/R, ischemia reperfusion; Ldlr, low density lipoprotein receptor; Mlkl, mixed lineage kinase domain-like; polyI:C, polyinosinic:polycytidylic acid; RD, retinal detachment; Ripk, receptor-interacting serine-threonine kinase; Sharpin, SHANK-associated RH domain interacting protein; SIRS; systemic inflammatory response syndrome; Ticam1, toll-like receptor adaptor molecule 1; TNF, tumor necrosis factor; Tnfrsf1a, tumor necrosis factor receptor superfamily member 1A.
Core Components
Necroptosis is a variant of RCD that critically relies on the phosphorylation of the pseudokinase MLKL (mixed lineage kinase domain-like) on S345 (in mice) or T357 and S358 (in humans) by the functional kinase RIPK3 (receptor-interacting serine-threonine kinase 3) (9–13). MLKL contains several other serine and threonine residues that can be phosphorylated by RIPK3, including S124, S158, S228, and S248 (11, 14). The phosphorylation of these residues seems to be dispensable for full-blown MLKL activation (11), yet it may mediate an important role in the fine-tuning of necroptotic signaling (14). Irrespective of this unknown, phosphorylated MLKL exposes an N-terminal 4-helix bundle (NB) domain and a central brace region, hence forming homo-oligomers and acquiring the ability to bind phosphatidylinositol phosphate (PIP) species of the inner leaflet of the plasma membrane (15–19). Such an initial binding is mediated by the brace domain and is characterized by a relatively low affinity but is critical for MLKL translocation to the plasma membrane (20). At the membrane, the NB domain of MLKL undergoes a rolling-over process to expose high-affinity binding sites for PIP species (phosphatidylinositol 4,5-bisphosphate being the preferred interaction partner), which results in the displacement of the brace domain and binding stabilization (20, 21). The exact stoichiometry of MLKL oligomers has not been clarified yet (16, 17), but it seems that MLKL oligomerization and/or its translocation to the plasma membrane relies on the cytosolic chaperone heat shock protein 90kDa alpha family class A member 1 (HSP90AA1; best known as HSP90) (22–24). The precise mechanisms whereby oligomerized MLKL provokes necroptotic plasma membrane permeabilization also remain obscure. One study proposed that transient receptor potential cation channel subfamily M member 7 (TRPM7) may act downstream of oligomerized MLKL to cause a lethal influx of Ca2+ ions (16), but these findings have not been confirmed by independent investigators so far. Initial beliefs that necroptosis would proceed upon mitochondrial fragmentation mediated by PGAM family member 5, serine/threonine protein phosphatase, mitochondrial (PGAM5) and dynamin 1-like (DNM1L) (25) have been conclusively rejected (26–29). Thus, at odds with several (but not all) other RCD variants (30), necroptosis may not depend on mitochondria.
As mentioned above, RIPK3 operates immediately upstream of MLKL in the signal transduction cascade that precipitates necroptosis (9), in a manner that depends strictly on RIPK3 kinase activity (31). In line with this notion, Ripk3−/− mice (which are viable and develop into fertile adults) and cells thereof are markedly more resistant to tumor necrosis factor (TNF) receptor superfamily member 1A (TNFRSF1A; best known as TNFR1) ligation in caspase-deficient conditions, as compared with their wild-type (WT) counterparts (32–34). In this setting, RIPK3 is activated by RIPK1, which results in the assembly of a RIPK1- and RIPK3-containing amyloid-like signaling complex assisted by HSP90 and the cochaperone cell division cycle 37 (CDC37) (35, 36), which has been dubbed necrosome (37). RIPK1 and RIPK3 mutually interact via their RIP homotypic interaction motif (RHIM) (38) and undergo trans- or autophosphorylation, endowing the necrosome with the ability to activate MLKL (9, 33). According to two recent reports, protein phosphatase, Mg2+/Mn2+ dependent 1B (PPM1B) and the E3 ubiquitin ligase STIP1 homology and U-Box containing protein 1 (Stub1; also known as Chip) exert prominent necroptosis-inhibitory functions by catalyzing the dephosphorylation of RIPK3 (39) or its ubiquitination and proteasomal degradation of Ripk3 (40), respectively. These findings, however, have not been confirmed by independent investigators yet.
RIPK1 not only activates RIPK3 upon TNFR1 ligation (in the presence of caspase inhibitors and/or SMAC mimetics), but also operates (when chemically inhibited or catalytically inactive) as a potent inhibitor of spontaneous and Toll-like receptor 3 (TLR3)-driven RIPK3 activation (41, 42). Ripk1−/− mice die at 1–3 days of age (43), a perinatal lethality that can be fully reverted by the concomitant deletion of Ripk3 and caspase 8 (Casp8), or Ripk3 and Fas associated via death domain (Fadd) but not by the knockout of Ripk3, Casp8, or Fadd (41, 44, 45). The deletion of Tnfsf1a delays, but does not rescue, the accelerated mortality of Ripk1−/−Ripk3−/− mice, owing to the inability of these animals to maintain intestinal barrier function (41). Along similar lines, the intestinal epithelial cell (IEC)-specific codeletion of Ripk1 and Fadd causes a lethal inflammatory syndrome that depends on Ripk3 (46). Mice expressing kinase-dead variants of Ripk1 (i.e., Ripk1K45A or Ripk1D138N) and Ripk3 (Ripk3K51A) are viable and mature into fertile adults (31, 47–49). These animals are protected from TNF-driven systemic shock, and their cells exhibit an accrued resistance to TNFR1 ligation in the presence of Z-VAD-fmk, as well as to TLR3 ligation (31, 47, 48). However, Ripk1D138N is not sufficient to block necroptosis in Fadd−/− IECs (46), but the lack of RIPK1 kinase activity is sufficient to abolish the severe multiorgan inflammatory disorders provoked by the absence of one component of the linear ubiquitin chain assembly complex (LUBAC), namely, SHANK-associated RH domain interacting protein (Sharpin) (47).
The observations above indicate that RIPK1 mediates complex effects in the core signal transduction cascades that precipitate necroptosis, only some of which depend on its kinase activity and RIPK3 (Figure 1). Importantly, RIPK1, RIPK3, and MLKL have also been implicated in the activation of supramolecular complexes commonly known as inflammasomes, which are responsible for the CASP1-dependent maturation and secretion of interleukin 1 beta (IL1B) and IL18 (50–54). The precise mechanisms underlying inflammasome activation by core components of the necroptotic machinery remain a matter of debate. However, the functional link between the necrosome and the inflammasome is a key aspect of necroptosis, because it endows this lethal subroutine with a prominent proinflammatory potential.
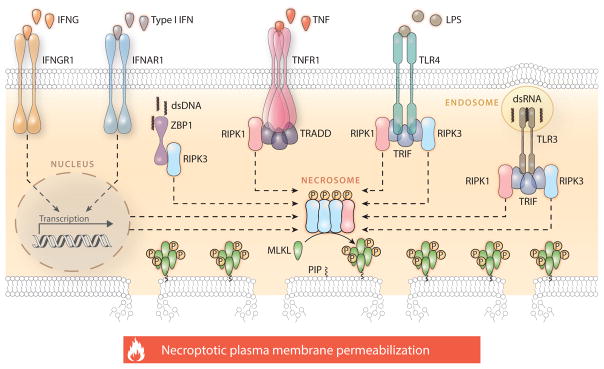
Figure 1.
Molecular mechanisms of necroptosis. Necroptosis critically depends on the receptor-interacting serine-threonine protein kinase 3 (RIPK3)–mediated phosphorylation of mixed lineage kinase domain-like (MLKL), resulting in MLKL oligomerization, translocation to the inner leaflet of the plasma membrane, and cell death. The formation of the RIPK3- and MLKL-containing complex that precipitates necroptosis, the so-called necrosome, can be elicited by extracellular signals (such as the ligation of death receptors) as well as by intracellular cues (such as the presence of viral nucleic acids) and is regulated by a complex network of physical and functional protein-to-protein interactions. The best characterized signal transduction cascade resulting in necroptotic cell death is initiated by TNFR1 (official name: TNFRSF1A, tumor necrosis factor receptor superfamily member 1A) ligation in the presence of caspase inhibitors and/or SMAC (official name: DIABLO, diablo IAP-binding mitochondrial protein) mimetics and critically depends on the phosphorylation of RIPK3 by RIPK1. In several other circumstances, however, RIPK1 is dispensable for necroptotic responses or even inhibits them in an active manner. This applies to various other TNFR1 interactors that participate in necroptotic signaling, most of which also regulate caspase 8 (CASP8)-dependent apoptosis and proinflammatory NF-κB activation. Please note that several physical or functional interactions have been omitted for the sake of simplicity. Abbreviations: dsDNA, double-stranded DNA; dsRNA, double-stranded RNA; IFN, interferon; IFNAR1, interferon (alpha and beta) receptor 1; IFNG, interferon gamma; IFNGR1, interferon gamma receptor 1; LPS, lipopolysaccharide; P, phosphate; PIP, phosphatidylinositol phosphate; TLR, Toll-like receptor; TNF, tumor necrosis factor; TRADD, TNFRSF1A associated via death domain; TRIF (official name TICAM1), toll-like receptor adaptor molecule 1; ZBP1, Z-DNA binding protein 1.
Upstream Regulators
Perhaps the best-characterized signal transduction pathway promoting necroptosis stems from the ligation of death receptors, notably TNFR1 (7). The ultimate outcome of TNF signaling, however, exhibits an elevated degree of context dependency, ranging from the cytoprotective and proinflammatory activation of NF-κB-dependent transcriptional programs to RCD via CASP8-and CASP3-mediated apoptosis or RIPK3- and MLKL-dependent necroptosis (55). Thus, several physical and functional interactors of TNFR1 contribute to the regulation of necroptosis. In particular, necroptosis is under tonic inhibition by an enzymatically active trimeric complex formed by FADD, CASP8, and the long isoform of CASP8 and FADD like apoptosis regulator (CFLAR, best known as cFLIP) (56–59). Indeed, whereas Casp8−/− mice die in utero on E10.5 (60), such an embryonic lethal phenotype is rescued by the concomitant absence of Ripk3 (56, 61). Casp8−/−Ripk3−/− mice develop a lymphoproliferative disease resembling that imposed by the Fas−/− or Faslg−/− genotype (62), which highlights the cooperative nature of apoptosis and necroptosis in the preservation of lymphocyte homeostasis. The Fadd−/− and Cflar−/− genotypes are also associated with early embryonic lethality (63–65). However, whereas Fadd−/−Ripk3−/− mice develop into adulthood, Cflar−/−Ripk3−/− embryos do not survive past E12–13.5, owing to hyper-activation of Casp3 in the endothelium and other regions (57). The concomitant absence of Fadd allows Cflar−/−Ripk3−/− embryos to develop into adulthood, although Fadd−/−Cflar−/−Ripk3−/−mice also exhibit lymphoproliferative disorders (57). These observations underscore the complexity of the system that controls Casp8 and Ripk3 activation during development.
Similar data have been obtained with the tissue-specific and/or acute Casp8, Cflar, and Fadd loss in murine embryonic or adult epithelia (58, 66–68). Moreover, the deletion of Fadd from the myeloid cell compartment provokes systemic inflammation that can be abolished by the concomitant absence of Ripk3 or myeloid differentiation primary response 88 (Myd88) (69), whereas Casp8−/− or Fadd−/− T cells exhibit considerable activation and proliferation defects, which can be partially rescued by the RIPK1 inhibitor necrostatin-1 (Nec-1) or by the concomitant absence of Ripk3 (45, 70–72). Thus, the regulation of lethal signal transduction cascades emanating from TNFR1 plays a critical role not only in embryonic development but also in the maintenance of epithelial and immunological homeostasis.
The initiation of RIPK1-dependent necroptosis (over apoptosis) by TNFR1 agonists is supported by two conditions: (a) CASP8 is inactivated pharmacologically (for instance, with the broad-spectrum protease inhibitor Z-VAD-fmk) (73) or genetically (61); and (b) RIPK1 deubiquitination is favored [for instance, with so-called SMAC mimetics, which inhibit several E3 ligases of the inhibitor of apoptosis (IAP) protein family] (32). Various members of this family, including baculoviral IAP repeat containing 2 (BIRC2; best known as cIAP1), BIRC3 (best known as cIAP2), and X-linked inhibitor of apoptosis (XIAP), robustly inhibit TNFR1-driven necroptosis (while favoring the activation of the proinflammatory and prosurvival transcription factor NF-κB) as they promote RIPK1 ubiquitination (74–77). Accordingly, the RIPK1-deubiquitinating enzyme cylindromatosis (CYLD) is required for optimal necroptotic responses following TNFR1 ligation (78–80), whereas the ubiquitin-modifying TNF alpha induced protein 3 (TNFAIP3; best known as A20) and LUBAC limit TNFR1-driven necroptosis by favoring RIPK1 ubiquitination (81–85). A20 also suppresses necroptosis by deubiquitinating RIPK3 at K5 (86), whereas CYLD mediates necroptotic effects by deubiquitinating TNF receptor associated factor 2 (TRAF2) (87).
Aside from being involved in the regulation of extrinsic apoptosis, TRAF2 inhibits necroptosis driven by TNFR1 and other death receptors by directly interacting with MLKL (87, 88). Accordingly, the whole-body deletion of Traf2 in adult mice is lethal, owing to an acute hepatotoxic response that can be prevented by deleting Ripk3 (87). Mice lacking Traf2 and Ripk3, however, succumb in a delayed manner upon the hyperactivation of Casp8 in the intestinal epithelium, and this process can be completely blocked with a cocktail of monoclonal antibodies that antagonize TNFR1 and other death receptors (87). Of note, whereas some authors propose that mitogen-activated protein kinase kinase kinase 7 (MAP3K7; best known as TAK1) mediates antinecroptotic effects by preventing RIPK1 signaling (77, 89, 90), others suggest that prolonged TAK1 activation (which is usually a transient consequence of TNFR1 ligation) may result in RIPK3 phosphorylation and the consequent initiation of a feedforward amplification circuitry with potent pronecroptotic activity (91). Another member of the TNFR1 interactome that is responsible for canonical NF-κB activation, the so-called inhibitor of kappa B kinase complex (IKK), has recently been shown to exert necroptosis- (and apoptosis-) inhibitory effects by directly phosphorylating RIPK1 (92). Taken together, these observations indicate that lethal TNFR1 signaling is regulated by a highly intertwined protein network, many components of which remain to be elucidated.
RIPK1 is not the sole activator of RIPK3 in murine and human cells. Rather, at least two other proteins can physically interact with RIPK3 through a bona fide RHIM and hence drive necroptosis, namely Z-DNA binding protein 1 (ZBP1; also known as DAI) (93, 94) and toll-like receptor adaptor molecule 1 (TICAM1; best known as TRIF) (95). Both these proteins are involved in innate immunity to invading pathogens. ZBP1 responds to exogenous DNA in the cytosol by promoting the synthesis of type I interferon (IFN) and downstream components of type I IFN responses (96, 97), as well as by activating NF-κB signaling (93, 98). TRIF is an adaptor in the signal transduction cascades elicited by TLR3 and TLR4, which are major players in the first line of defense against bacterial, viral, and endogenous threats (99).
Thus, at least theoretically, a wide panel of microbe-associated molecular patterns (MAMPs) or damage-associated molecular patterns (DAMPs) can trigger necroptosis through TLRs. So far, this hypothesis has been demonstrated for polyinosinic:polycytidylic acid (polyI:C, a TLR3 agonist) (95, 100) and lipopolysaccharide (LPS, which binds TLR2 and TLR4) (101). Pam3CSK4 (a mixed TLR1/TLR2 agonist) and resiquimod (a TLR7 agonist) also promote necroptosis in microglial cells (but not astrocytes, oligodendrocytes, and neurons) concomitantly exposed to Z-VAD-fmk (101). Because TLR1, TLR2, and TLR7 do not usually signal via TRIF, these data are compatible with the existence of another, hitherto uncharacterized, mechanism through which TLRs trigger necroptosis, perhaps depending on the common TLR adaptor MYD88 or resulting from the autocrine production of TNF. As an alternative possibility, cell type–related parameters underlie these observations. Of note, TLR3 may also initiate a pronecroptotic signaling pathway that involves the translocation of lysosomal cathepsin D (CTSD) to the cytoplasm, at least in dendritic cells (DCs) (100). In this setting, cytoplasmic CTSD would interact with mitochondrial antiviral signaling protein (MAVS), a transducer of innate immune responses against viral RNA, followed by the CTSD-dependent cleavage of CASP8, the recruitment of RIPK1 to the CTSD/MAVS complex, and final activation of necroptosis (100). These findings, however, have not been reproduced by independent investigators so far.
Interferon (alpha and beta) receptor 1 (IFNAR1) and interferon gamma receptor 1 (IFNGR1) can also initiate necroptosis, at least in some cell types including macrophages (102–104). The pronecroptotic activity of type I IFN partially relies on TRIF and stems from the persistent stimulation of signal transducer and activator of transcription 1 (STAT1), STAT2, and interferon regulatory factor 9 (IRF9) (104), perhaps promoting the upregulation of eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2; best known as PKR) (103). A similar STAT1- and PKR-dependent mechanism accounts for necroptosis initiation by interferon gamma (IFNG, best known as IFN-γ) (103). Type I IFN signaling may be required for several instances of necroptosis, including those elicited by TNFR1 ligation (104).
Similar to TNFR1-driven necroptosis, the necroptotic response to type I IFN and IFN-γ is suppressed by caspases (most likely CASP8) and FADD, an effect that (in the mouse system) is relieved by FADD phosphorylation on S191 (103). It will be interesting to test the ability of kinases that phosphorylate FADD on S191 (in humans, S194), such as casein kinase 1 alpha 1 (CSNK1A1), on the sensitivity of various cell types to necroptosis. In summary, the necrosome receives activatory and inhibitory signals not only from TNFR1 and its interactors, but also from other receptors that respond to perturbations of extracellular and intracellular homeostasis, such as ZBP1, TLR3, IFNAR1, and IFNGR1 (Figure 1).
Cross Talk with Other Regulated Cell Death Variants
The molecular machinery that regulates necroptosis is intimately connected with the signal transduction cascades that control several other forms of RCD, including apoptosis, MPT-driven necrosis, parthanatos, and ferroptosis. Such mutual interactions delineate a complex signaling network that integrates various inputs (e.g., developmental cues, indicators of metabolic fitness, growth factor availability, infectious threats) to initiate either an adaptive response focused on the preservation of cellular homeostasis or degenerative processes leading to RCD (which can also be seen as a homeostatic mechanism for the organism).
Apoptosis
As we have discussed above, various components of the signal transduction pathways that promote or retard apoptotic RCD in response to death receptor ligation also impact on necroptotic signaling. This applies not only to the TNFR1 interactors FADD, cFLIP, CASP8, CYLD, and various IAP family members (see above), but also to proteins that propagate or execute apoptotic signals, such as CASP6 (105), which is generally activated by CASP8 or CASP3 as one of the final events of apoptosis, and cathepsins (106), which promote mitochondrial outer membrane permeabilization (MOMP) in response to lysosomal damage (2). Similar to CASP8, which cleaves (and hence inhibits) RIPK1 (72, 107), RIPK3 (72, 108), and CYLD (80), CASP6 and cathepsins have been attributed necroptosis-inhibitory effects by virtue of their ability to proteolitically inactivate RIPK1 (105, 106). This explains, at least in part, the ability of Z-VAD-fmk (which inhibits several proteases including CASP8, CASP6, and cathepsins) (109) and the genetic inhibition of CASP8 (see above) to favor necroptosis over apoptosis upon ligation of death receptors or TLR3.
The depletion of RIPK3 or MLKL in mouse L929-N fibrosarcoma cells (which mount a necroptotic response to TNF alone) shifts the RCD modality triggered by TNFR1 ligation from rapid necroptosis to Nec-1-sensitive, delayed apoptosis (13). However, the small-interfering RNA (siRNA)-mediated downregulation of RIPK1 mediates similar switching effects (81), and so does the transgene-driven expression of a RIPK1 variant lacking the intermediate domain (RIPK1ΔID) (110). In this latter setting, RIPK1ΔID favors apoptosis over necroptosis by promoting the recruitment of FADD and CASP8 to ligand-bound TNFR1, an effect that can be limited by Nec-1 (110). These observations reinforce the notion that different RIPK1 domains have a differential impact on the signal transduction cascades elicited by TNFR1 ligation. In addition to this complexity, mice expressing a specific kinase-dead variant of Ripk3 (Ripk3D161N), but not mice expressing Ripk3K51A (which is also catalytically inactive), die around E10.5, owing to uncontrolled Ripk1-and Casp8-dependent apoptosis (31, 49). Moreover, three distinct RIPK3 kinase inhibitors activate a FADD-, CASP8-, and RIPK1-dependent apoptotic response (49), and RIPK3 was found to contribute to TNFR1-driven CASP8-dependent apoptosis in cells either depleted of cIAP1 and cIAP2 or exposed to a pharmacological inhibitor of TAK1 (90). Thus, RIPK1, RIPK3, and CASP8 appear to exist in a complex equilibrium that not only has major relevance for normal embryonic development and adult tissue homeostasis, but also dictates the modality (apoptosis versus necroptosis) through which cells succumb to lethal TNFR1 signaling.
According to two recent reports, upstream necroptotic signaling may drive (at least to some extent) MOMP-related RCD (111, 112). Investigators have proposed that Ripk1 may promote the Casp8-mediated cleavage of BH3 interacting domain death agonist (Bid), resulting in the activation of BCL2-associated X protein (Bax) and BCL2-antagonist/killer 1 (Bak1)-dependent (but caspase-independent) RCD, at least in L929-A cells (a variant of L929 cells that undergo TNF-driven necroptosis in the presence of Z-VAD-fmk) (111). In addition, Bax and Bak1 oligomerization into the outer mitochondrial membrane accompanies the necroptotic response of mouse embryonic fibroblasts (MEFs) to TNF plus Z-VAD-fmk, and Bax−/−Bak1−/− MEFs are considerably more resistant to this treatment than are their WT counterparts (112). Although these data are rather preliminary and have not been reproduced by other laboratories, they suggest the existence of a heretofore unsuspected link between necroptosis and MOMP-driven apoptosis (Figure 2).
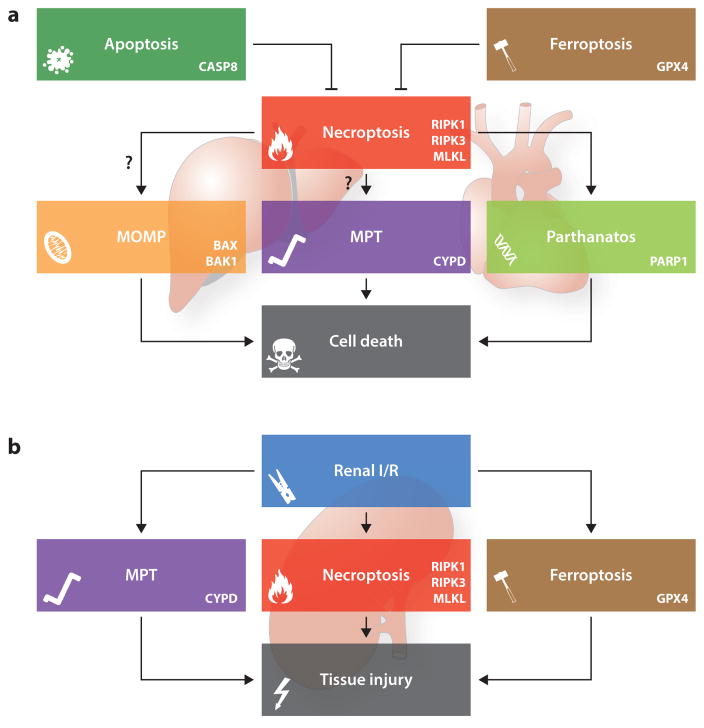
Figure 2.
Cross talk between necroptosis and other forms of regulated cell death. The signal transduction cascades that precipitate necroptosis and other forms of regulated cell death are highly intertwined. A heterotrimeric complex containing caspase 8 (CASP8), Fas associated via death domain (FADD), and the long isoform of cFLIP (official name CFLAR, CASP8 and FADD like apoptosis regulator), in which CASP8 retains partial proteolytic activity, robustly inhibits necroptosis, most likely owing to the CASP8-dependent cleavage of receptor-interacting serine-threonine kinase 1 (RIPK1), RIPK3, and cylindromatosis (CYLD). According to some reports that await confirmation, RIPK1 and/or RIPK3 may also mediate lethal effects by precipitating mitochondrial outer membrane permeabilization (MOMP), mitochondrial permeability transition (MPT)-driven regulated necrosis, or parthanatos. Along similar lines, the endogenous inhibitor of ferroptosis glutathione peroxidase 4 (GPX4) may suppress necroptosis by preserving optimal CASP8 functions (a). In specific situations, however, it seems that the signal transduction cascades precipitating necroptosis, MPT-driven regulated necrosis, ferroptosis, and parthanatos operate in a manner that is completely independent from each other. One of these scenarios is represented by renal ischemia/reperfusion (I/R) (b). Abbreviations: BAK1, BCL2-antagonist/killer 1; BAX, BCL2-associated X protein; CYPD (official name, PPIF), peptidylprolyl isomerase F; MLKL, mixed lineage kinase domain-like; PARP1, poly(ADP-ribose) polymerase 1.
Nonnecroptotic forms of regulated necrosis
Necroptosis is clearly not the only form of necrotic RCD (see sidebar, Other Forms of Regulated Necrosis). However, the interactions between the molecular machinery for necroptosis and the signal transducers that control MPT-driven regulated necrosis, parthanatos, and ferroptosis remain largely elusive. Ppif−/− MEFs lack the sole key regulator of MPT discovered so far and appear to be less sensitive to TNF plus Z-VAD-fmk than WT MEFs (112). Accordingly, Ppif−/− mice display a reduced sensitivity to cerulein-induced pancreatitis (which involves necroptosis as an etiological determinant) (32, 112) and are not sensitive to the cardioprotective effects of Nec-1 (113). Moreover, calcium/calmodulin-dependent protein kinase II gamma (Camk2g, best known as CaMKII) appears to respond to Ripk3-dependent phosphorylation, oxidation, or both by favoring MPT in a Ripk1- and Mlkl-independent manner, which is relevant for myocardial injury triggered by ischemia/reperfusion (I/R) (114). However, neither the Ripk3−/− and Ppif−/− genotypes nor the coadministration of Nec-1 and the MPT inhibitor sanglifehrin A (SfA) interact epistatically in models of renal I/R, cisplatin-triggered acute kidney injury, and TNF shock (115). Some authors have suggested that PARP1 also operates downstream of RIPK1 and RIPK3 in the lethal signal transduction cascades elicited by death receptor ligation in human HT29 colon and HepG2 hepatocellular carcinoma cells or by concanavalin A administration in the mouse liver (116). However, pharmacological and genetic manipulations suggest that necroptosis (as triggered by TNF) and parthanatos (as elicited by DNA-alkylating agents) constitute two distinct routes to necrotic RCD, at least in L929 cells (117).
Along similar lines, the main endogenous inhibitor of ferroptosis, i.e., glutathione peroxidase 4 (GPX4), reportedly mediates robust necroptosis-inhibitory effects in erythroid precursor cells, reflecting its capacity to preserve normal CASP8 functions (118). Nonetheless, the administration of a potent inhibitor of ferroptosis (i.e., 16–86) further exacerbates the protection afforded by Nec-1 plus SfA to mice experiencing renal I/R (119), which suggests that necroptosis is functionally distinct from ferroptosis in this setting. It remains to be determined whether these findings reflect (at least in part) some peculiar features of kidneys (which are not sensitized to necroptosis upon the tissue-specific deletion of Fadd or Casp8) (119). Additional work is required to clarify the precise links between necroptosis and MPT-driven regulated necrosis, parthanatos, and ferroptosis (Figure 2).
PATHOPHYSIOLOGICAL RELEVANCE OF NECROPTOSIS
Similar to other forms of RCD, necroptosis mediates major physiological functions but is also involved in the etiology of multiple human disorders.
Physiological Functions
During the past decade, researchers have devoted considerable efforts to generating mice that lack one or several components of the necroptotic machinery at the whole-body level or in specific tissues, as well as to developing ever-more potent inhibitors of RIPK1, RIPK3, and MLKL (8). Thanks to these models, the physiological functions of proteins with pro- or antinecroptotic activity, as well as of necroptosis as a lethal signaling pathway, have begun to emerge. Moreover, some of these models have allowed us to shed new light on observations that had remained partially unexplained for years, including the embryonic or perinatal lethality caused by the Casp8−/−(60), Fadd−/− (63), Cflar−/− (65), and Ripk1−/− (43) genotypes. Indeed, as amply discussed above, proper embryonic and perinatal development depends on a delicate equilibrium between various components of the core necroptotic machinery and their upstream regulators but not on necroptosis per se, as demonstrated by the viability of both Ripk3−/− and Mlkl−/− mice and their lack of developmental defects (12, 120). Along similar lines, the conditional deletion of Ripk1 in intestinal epithelial cells or keratinocytes demonstrated that Ripk1 mediates a key, nonredundant role in maintaining epithelial homeostasis in the adult (46, 121), reflecting the ability of Ripk1 to inhibit Fadd- and Casp8-dependent apoptosis (in the intestine) and Ripk3-dependent necroptosis (in the skin) independent of its kinase activity (46, 121). Of note, Casp8 resembles Ripk1 in its fundamental role in maintaining the intestinal barrier (68). Moreover, mice with a codeletion of Birc2, Birc3, and Xiap in the myeloid cell lineage exhibit (a) increased levels of proinflammatory cytokines in the blood, (b) granulocytosis, and (c) sterile inflammation, which can be corrected in part by the absence of Ripk1 or Ripk3 (but not Mlkl) (122). Taken together, these observations suggest that a delicate balance between various RCD modalities exerts major homeostatic functions in the adult.
Further corroborating this notion, the tamoxifen-inducible systemic knockout of Ripk1 in adult mice is lethal, owing to an accrued wave of RCD in the intestine and to bone marrow failure, cumulatively resulting in lethal systemic inflammation (123, 124). Confirming the prominent role of Ripkl in the survival of hematopoietic stem and progenitor cells (because of its antiapoptotic and antinecroptotic effects), fetal liver cells subjected to the tamoxifen-inducible deletion of Ripk1 as well as Ripk1−/− progenitors fail to repopulate irradiated recipients, a defect that can be partially corrected by the concomitant absence of Ripk3 (123, 124). Moreover, the systemic inflammation caused by the Ripk1−/− genotype is limited in Ripk3−/−, Mlkl−/−, and Myd88−/−hosts, yet only Ripk1−/−Ripk3−/−Casp8−/− mice survive past weaning (41, 124). These latter animals display an age-associated lymphoproliferative disorder that resembles, but may be less severe than, that developed by Ripk3−/−Casp8−/− mice (56, 61, 124). However, the mechanisms underlying such a difference remain to be clarified.
According to one recent report, Ripk1+/− mice, mice receiving a Ripk1-targeting siRNA intravenously, and mice treated with Nec-1 also exhibit reduced rates of physiological enterocyte turnover (which manifests with nonapoptotic morphological features) in the small intestine as compared with control animals (125). Moreover, the negative effects of Nec-1 on enterocyte turnover persist in Ripk3−/− mice, which do not exhibit any intestinal phenotype per se (125). These observations suggest the existence of a nonapoptotic (but RIPK3-independent) variant of RCD that would contribute to the preservation of intestinal homeostasis. However, the authors of this work did not conclusively demonstrate the nonapoptotic nature of such a physiologically relevant instance of RCD, which may hence constitute a bona fide (CASP8- and CASP3-dependent) variant of apoptosis manifesting with limited apoptotic morphology (1).
Gathering evidence indicates that necroptosis is particularly important for the activation of innate and adaptive immunity against malignant and infectious threats, hence contributing to physiological immunosurveillance. Thus, the RIPK3-driven, RIPK1-dependent activation of NF-κB is critical to allow cancer cells succumbing to necroptosis to release DAMPs that promote the capacity of DCs to cross-present tumor-associated antigens (TAAs) to CD8+ cytotoxic T lymphocytes (CTLs), hence initiating a tumor-targeting immune response (126). As compared with WT mice, Ripk3−/− mice and mice expressing Ripk1D138N exhibit severely impaired necroptotic responses to the vaccinia virus, which renders them unable to control viral infection (33, 48). Similar data have been obtained with Ripk3−/− mice inoculated with herpes simplex virus type 1 (HSV-1) as well as with Ripk1−/− and Mlkl−/− L929 cells exposed to HSV-1 plus Z-VAD-fmk (127). The necroptosis-inducing activity of HSV-1 in mice does not depend on TNFR1 but stems from the interaction between the HSV-1 protein ICP6 (which contains a bona fide RHIM domain) and Ripk1 and/or Ripk3, which directly activates the necrosome (127, 128). The interaction of ICP6 with RIPK1 and/or RIPK3 robustly suppress necrosome formation in human cells (127, 129), exemplifying the advantage that some viruses have obtained from the evolution of proteins that inhibit necroptosis. In addition to ICP6, these proteins include ICP10 from HSV-2 (129) and vIRA from murine cytomegalovirus (both of which contain a bone fide RHIM domain) (38, 94, 130, 131). Finally, Casp8−/−Ripk3−/− (but not Ripk3−/−) mice are highly susceptible to an otherwise sublethal infection with Yersinia pestis as they exhibit reduced levels of various proinflammatory cytokines involved in bacterial control coupled with lower-than-normal death rates of Cd11b+ myeloid cells (132). In vitro, such an inhibitory effect was extended to Ripk1 (with Ripk1−/− fetal liver macrophages and Nec-1 treatment) and linked to inflammasome activation, delineating a Ripk1-, Ripk3-, and Casp8-dependent proinflammatory pathway impinging on core components of the necroptotic machinery (132), which has been confirmed by others (133, 134). The administration of Nec-1 and Nec-1s (a Nec-1 derivative) also limits the demise of Kupffer cells in mice inoculated with Listeria monocytogenes, which restrains both microbicidal inflammation and tissue repair (135). In this setting, however, the deletion of Ripk3 has a paradoxical cytoprotective effect (135), meaning that it is difficult to identify the implications of necroptosis in the physiological control of L. monocytogenes infection. Despite this unknown, prominent physiological roles in pre-natal development and in the maintenance of adult organismal homeostasis have been attributed not only to various components of the necroptotic machinery, but also to necroptosis as process. Because this wave of investigation is still in its infancy, we surmise that many other physiological functions of necroptosis will be revealed in the next few years.
Nonmalignant Disorders
In addition to their involvement in multiple physiological processes, necroptosis and necroptosis-relevant proteins contribute to the etiology of a variety of nonmalignant disorders characterized by unwarranted cell loss and/or a prominent inflammatory component (Table 2).
Table 2.
Pharmacological inhibitors of necroptosis
| Molecule | Target | Observations |
|---|---|---|
| Dabrafenib | RIPK3 | Initially developed as a BRAF inhibitor, approved for use in patients with BRAFV600E-driven melanoma |
| GW806742X | MLKL | Experimental KDR inhibitor, also binds to MLKL pseudokinase domain and inhibits its translocation to the plasma membrane |
| Necrostatin-1 | RIPK1 | Experimental agent, mediates various off-target effects, including the inhibition of IDO1 |
| Necrostatin-1s (7-Cl-O-Nec-1) | RIPK1 | Experimental agent, exhibits increased stability and specificity as compared with necrostatin-1 |
| Necrostatin-3 | RIPK1 | Experimental agent, operates in a mechanistically distinct manner as compared with necrostatin-1 |
| Necrostatin-5 | RIPK1 | Experimental agent, inhibits RIPK1 via an indirect mechanism |
| Necrostatin-7 | ? | Experimental agent structurally and biologically different from other necrostatins, does not target RIPK1 |
| Necrosulfonamide | MLKL | Experimental agent, selective for human over murine MLKL |
| Pazopanib | RIPK1 | Multitarget tyrosine kinase inhibitor, approved for use in patients affected by renal cell carcinoma and advanced soft tissue sarcoma |
| Ponatinib | RIPK1 RIPK3 |
Multitarget tyrosine kinase inhibitor, approved for use in patients affected by various forms of leukemia |
Abbreviations: BRAF, B-Raf proto-oncogene, serine-threonine kinase; IDO1, indoleamine 2,3-dioxygenase 1; KDR, kinase insert domain receptor; MLKL, mixed lineage kinase domain-like; RIPK, receptor-interacting serine-threonine kinase.
Infectious diseases
Ifnar1−/− mice are remarkably more resistant to Salmonella enterica serovar Typhimurium than are their WT littermates, which has been linked to the ability of type I IFN to trigger the Ripk1- and Ripk3-dependent demise of macrophages, resulting in impaired bacterial control (102). Along similar lines, diverse bacterial pathogens including Serratia marcescens, Staphylococcus aureus, Streptococcus pneumoniae, L. monocytogenes, and uropathogenic Escherichia coli produce pore-forming toxins that trigger Ripk1-, Ripk3-, and Mlkl-dependent necroptosis in macrophages, which mechanistically contributes to bacterial hemorrhagic pneumonia (136). Engagement of the T cell receptor in HIV-1-specific CD8+ CTLs from patients with chronic HIV-1 infection (but not from patients who spontaneously control viremia) fails to promote normal proliferation, which has been attributed to the activation of RIPK3 and consequent necroptosis (137). Moreover, Nec-1 limits the formation of syncytia between HIV-1+ CD4+ T cells (138), which suggests that RIPK1 plays a pathogenic role in this setting. Additional experiments evaluating the impact of necroptosis on these and other infectious diseases are urgently needed.
Cardiovascular pathologies
As compared with WT mice, Ripk3−/− mice are less sensitive to heart failure triggered by I/R or doxorubicin (a cardiotoxic chemotherapeutic agent), correlating with a reduced necroptotic response of the myocardium (114, 139). Experiments with isolated cardiomyocytes indicate that the cardiotoxic effects of Ripk3 activation do not depend on Ripk1 or Mlkl (114). Rather, Ripk3 activation in the myocardium appears to elicit a CaMKII-dependent signaling pathway, resulting in MPT and consequent necrosis. Accordingly, both Camk2g−/− and Ppif−/− mice are remarkably protected from cardiac I/R and/or cardiotoxic chemotherapy (113, 114, 140). Nec-1 also mediates cardioprotective activity in mouse and pig models of cardiac I/R (113, 141, 142), but it remains unclear whether such an effect truly stems from the inhibition of Ripk3-dependent necroptosis (113). Ripk3−/− and Ripk3+/− mice are more resistant to elastase-induced abdominal aortic aneurysm than are their WT littermates, a protective effect that can also be observed upon transplantation of Ripk3+/− aortae into WT recipients and that involves a reduced loss of smooth muscle cells as well as a limited expression of proinflammatory genes (143). Along similar lines, Ripk3 activation and consequent necroptosis of macrophages in atheromas may contribute to the local and systemic inflammatory responses that accompany atherogenesis in mice lacking apolipoprotein E (Apoe) or low-density lipoprotein receptor (Ldlr) (144, 145). Thus, Ripk3−/−Apoe−/− mice and Ripk3−/−Ldlr−/− mice exhibit considerably delayed disease onset, mitigated inflammatory responses in atherosclerotic plaques and in circulation, limited lymphocyte infiltration in adipocyte tissue and in skin lesions, and dramatically delayed mortality as compared with Apoe−/− or Ldlr−/− mice, respectively (144, 145). Taken together, these observations suggest that necroptosis contributes to the etiology of various diseases of the cardiovascular system.
Neurological disorders
Nec-1 mediates neuroprotective effects in rodent models of acute and chronic neurological conditions, including adult stroke (146), neonatal hypoxia/ischemia (H/I) (147, 148), retinal ischemia (149), retinal detachment (150–152), subarachnoid hemorrhage (153, 154), traumatic brain injury (155), spinal cord injury (156–159), and amyotrophic lateral sclerosis (160). The ability of Nec-1 to counteract manifestations of amyotrophic lateral sclerosis in mice was backed up by the knockdown of RIPK1 as well as by data obtained using human cells treated with necrosulfonamide (NSA, a chemical that inhibits human MLKL) (160). However, the authors of most of these studies did not evaluate their hypotheses in Ripk3−/− or Mlkl−/−systems, which implies that the neuroprotective effects of Nec-1 remain to be formally linked to the inhibition of necroptosis. As notable exceptions, Ripk3−/− mice exhibit an increased resistance to retinal detachment triggered by subretinal injections of sodium hyaluronate (150) or polyI:C (152), as compared with their WT littermates. Similarly, Ripk3−/− mice exhibit a sizeable reduction in astrocytic demise upon spinal cord injury as compared with WT mice, which correlates with an improved preservation of neurotrophic function (159). Moreover, the administration of a Ripk3-targeting siRNA limits the demise of outer hair cells exposed to noise that causes a permanent threshold shift in hearing, an otoprotective effect that can be increased by the concomitant administration of Z-VAD-fmk (161). Taken together, these observations suggest that necroptosis may play an etiological role in both acute and chronic neurological conditions. However, further experiments with Ripk3−/− or Mlkl−/− animals are needed to confirm this conjecture.
Renal conditions
Signs of an ongoing necroptotic response (e.g., increased RIPK1, RIPK3, and/or MLKL expression and/or phosphorylation levels) are common to various renal disorders (at least in mice), including acute kidney injury (AKI) caused by I/R (115, 119, 162), urolithiasis (163), cisplatin-based chemotherapy or radiocontrast (115, 164–167), and chronic kidney disease after unilateral nephrectomy (168). Nec-1 mediates nephroprotective effects in virtually all these settings, which supports the contention that necroptosis contributes to the etiology of various renal disorders, at least in mice. Corroborating this hypothesis, Ripk3−/− and Mlkl−/− mice are less sensitive to oxalate crystal–induced and cisplatin-driven AKI than are their WT counterparts, which correlates with reduced levels of plasma creatinine, limited tubular injury, and neutrophil infiltration (163, 166). Along similar lines, the Ripk3−/− genotype confers considerable protection against mild I/R, and such a protection can be extended to severe I/R by the concomitant deletion of Ppif (115). Similar data were obtained with Nec-1, SfA, and 16–86, employed alone or in combination (115, 165), which suggests that various forms of necrotic RCD contribute to the etiology of AKI. Some authors report that Z-VAD-fmk can enhance the nephroprotective effects of Nec-1 in models of cisplatin-induced AKI (164, 167), whereas others demonstrate that Z-VAD-fmk has no beneficial effects in mice experiencing radiocontrast- or I/R-driven AKI (162, 165). This apparent discrepancy may stem from the capacity of cisplatin to trigger apoptotic RCD in some tubule cells and necroptosis in others. Such a possibility, however, has not been formally addressed yet.
Hepatic disorders
Several lines of evidence suggest that necroptosis is intimately involved in the etiology of various hepatic conditions with a prominent inflammatory component but may not contribute to hepatic I/R (169). Thus, the Ripk3−/− genotype, the intraperitoneal delivery of Ripk3-targeting antisense oligonucleotides, or the administration of dabrafenib (an FDA-approved inhibitor of oncogenic BRAF that also suppresses RIPK3 kinase activity) provides considerable hepatoprotection in mouse models of acetaminophen toxicity (134, 170, 171), concanavalin A–driven hepatitis (134), ethanol and dietary intoxication (172, 173), and nonalcoholic steatohepatitis (NASH) (174, 175). Along similar lines, mice treated with Nec-1 exhibit an increased resistance to the hepatotoxic effects of acetaminophen as compared with control animals (134). In a recent study on acetaminophen toxicity in mice, however, the beneficial effects of the Ripk3−/− genotype were not confirmed, no hepatoprotection by the Mlkl−/− genotype could be detected, and the delivery of a Ripk1-targeting antisense oligonucleotide equally protected WT and Ripk3−/− mice (176). These data contradict previous findings and argue against the implication of necroptosis as a whole in the etiology of hepatic acetaminophen intoxication. Rather, it seems that RIPK1 supports the hepatotoxicity of acetaminophen independent of necroptosis. The authors of the latter report suggest that the RIPK1-dependent activation of the hepatotoxic kinase mitogen-activated protein kinase 8 (MAPK8; best known as JNK1) may underlie their observations (176). The NASH-aggravating effects of Ripk3 have also been linked to the activation of JNK1 (174), perhaps pointing to the existence of a pathophysiologically relevant but poorly characterized (and possibly indirect) cross talk between RIPKs and JNK1. Additional studies are required to elucidate this aspect of the biology of RIPKs.
Inflammatory diseases
Necroptosis has a profound proinflammatory effect, as it potentially initiates a vicious circle of RCD-driven inflammation and inflammation-driven RCD (177). In line with this notion, necroptosis or core components of the necroptotic machinery contribute to various inflammatory disorders, including Crohn disease (68); rheumatoid arthritis (52); multiple sclerosis (178); TNF-driven autoinflammatory disorders, including the so-called systemic inflammatory response syndrome (SIRS) (47, 179) and the toxicity of systemic irradiation (180); severe cutaneous reactions to drugs (181); allograft rejection (182, 183); remote lung injury postengraftment (184); cigarette smoke–driven chronic obstructive pulmonary disease (COPD) (185, 186); acute pancreatitis (12, 32, 112); and some manifestations of systemic lupus erythematosus (187). Some of these findings, however, have been obtained in mice receiving Nec-1, NSA, or Ripk1-targeting siRNAs as sole inhibitors of necroptosis and, hence, should be taken with caution. As notable exceptions, Ripk3−/− mice appear to be more resistant to TNF-driven SIRS than are control mice (179). Similarly, the severe SIRS-like disease caused by the Sharpin−/− genotype is abolished when these mice are crossed with mice engineered to express catalytically inactive Ripk1K45A (47). Moreover, Ripk3−/− (but not Mlkl−/−) mice are less prone to develop a rheumatoid arthritis–like syndrome in response to serum from K/BxN transgenic mice as compared with their WT littermates (52), whereas Ripk3−/− hearts are less likely to be rejected from WT hosts than are Ripk3+/− organs, especially in the presence of immunosuppressive agents (182). Finally, Ripk3−/−and Mlkl−/− mice are more resistant to cerulein-induced hepatitis than are their WT counterparts (12, 32). Of note, biomarkers of necroptosis, including increased expression or activation of RIPK3 or MLKL, have been detected in pathological samples from patients with multiple sclerosis (178), Crohn disease (68), and COPD (185), which further underscores the probable pathophysiological relevance of necroptosis in human inflammatory disorders.
Neoplastic Conditions
Preclinical and clinical evidence suggests that the pathophysiological relevance of necroptosis is not limited to human pathologies that are characterized by an excess demise of postmitotic cells (see above) but extends to oncological conditions, mainly by virtue of its prominent proinflammatory profile.
NIH-3T3 mouse fibroblasts driven into necroptosis through a dimerizable variant of Ripk3, as well as TC-1 mouse lung carcinoma and EL4 mouse lymphoma cells responding to TNF plus SMAC mimetics and Z-VAD-fmk (TSZ), expose or secrete several DAMPs that are involved in anticancer immunosurveillance, including, but perhaps not limited to, calreticulin (CALR), ATP, and high mobility group box 1 (HMGB1) (126, 188). Accordingly, the coincubation of necroptotic NIH-3T3 cells with bone marrow–derived DCs promotes the maturation of the latter, as assessed by Cd86 and MHC class II surface staining (126). Moreover, the subcutaneous inoculation of ovalbumin (OVA)-expressing NIH-3T3 cells succumbing to Ripk3 dimerization into syngeneic mice confers long-term protection against a subsequent tumor challenge to more than 30% of animals, a process that is associated with the expansion of OVA-specific, multifunctional CD8+ CTLs (126). In this model, the Ripk3-driven Ripk1-mediated activation of NF-κB is crucial for the elicitation of optimal antitumor immunity (126). The subcutaneous delivery of TC-1 cells treated with TSZ to syngeneic mice also results in virtually complete protection against the subsequent inoculation of living cancer cells of the same type, as does this administration of TC-1 cells succumbing to the canonical immunogenic cell death (ICD) inducer mitoxantrone (MTX) (188). Only WT TC-11 cells treated with MTX induce such a protective anticancer immune response, whereas Ripk3−/− or Mlkl−/− TC-1 cells fail to do so (188). Moreover, although WT TC-1 and EL4 cells growing in syngeneic mice respond to MTX-based chemotherapy, which is accompanied by intense tumor infiltration by Cd11c+Cd86+ DCs and Cd3+Cd8+ CTLs, their Ripk3−/− or Mlkl−/− counterparts fail to do so, a defect that can be partially complemented by the concomitant administration of a TLR4 agonist and an inhibitor of extracellular ATPases (which increase ATP concentrations in the tumor microenvironment) (188). Taken together, these observations suggest that necroptosis is a central determinant of the immunogenicity of RCD.
Further supporting this contention, B16 mouse melanoma cells growing in syngeneic mice exhibit an improved sensitivity to radiation therapy in the presence of Z-VAD-fmk, promoting necroptosis over apoptotic RCD and hence favoring the release of immunostimulatory DAMPs and consequent DC maturation (189). Moreover, the antineoplastic effects of Newcastle disease virus (NDV) in an orthotopic GL261 mouse glioblastoma model are associated with CALR exposure on the surface of dying cells and HMGB1 release, depend on a functional immune system, and can be abolished by Nec-1 (190). The coadministration of polyI:C and Z-VAD-fmk may also trigger a necroptotic response mediated by the TLR3→TRIF→RIPK3 signaling axis in cultured CT26 mouse colorectal carcinoma cells (191). However, such a response is blunted when CT26 cells are grown in immunocompetent mice (191), casting doubts on its actual therapeutic relevance. As an additional line of evidence supporting the inflammatory potential of necroptosis and its importance in oncological settings, Nec-1 reportedly limits the development of colitis-driven colorectal tumors in mice (192), which is a well-known model of inflammation-dependent carcinogenesis.
Recent results of some clinical studies reinforce the notion that necroptosis promotes natural or therapy-driven anticancer immunosurveillance. In a cohort of 112 patients with colorectal carcinoma, malignant cells exhibited reduced RIPK3 levels as compared with normal adjacent cells, and low RIPK3 expression constituted an independent prognostic factor for overall survival and disease-free survival (193, 194). Along similar lines, CD34+ blasts from 31 acute myeloid leukemia patients exhibited reduced levels of the RIPK3-coding mRNA as compared with CD34+ cells from healthy volunteers (195). Moreover, RIPK3 was downregulated in malignant tissues from 75 breast carcinoma patients, regardless of tumor subtype, and this was associated with increased RIPK3 methylation (196). The authors of this latter study also gathered RIPK3 expression data from 6 previous studies that enrolled 1,166 women with breast carcinoma, and they demonstrated that patients with greater than median RIPK3 mRNA expression exhibited improved metastatic relapse-free survival over a 10-year period (196). Unfortunately, the software employed in this analysis did not allow for a refined patient stratification, which may have resulted in a considerable underestimation of the prognostic value of RIPK3 expression levels in breast carcinoma. MLKL was upregulated in neoplastic tissues from 54 cervical squamous carcinoma patients, as compared with 16 normal cervical samples, yet elevated MLKL expression was associated with a low histological grade, limited metastatic dissemination, and improved overall survival (197). Similarly, low MLKL levels correlated with decreased overall survival and decreased disease-free survival in 80 patients with pancreatic adenocarcinoma who were subjected to surgery and optional adjuvant chemotherapy (198), and in 153 individuals with resectable primary ovarian cancer (198), on both univariate and multivariate Cox regression analyses. Although this line of investigation is still in its infancy, it seems that the expression levels of core components of the necroptotic machinery, such as RIPK3 and MLKL, have prognostic value in patients with various neoplasms. Additional studies on large clinical cohorts are warranted to confirm and extend these findings.
CONCLUDING REMARKS
Necroptosis, as a lethal signaling pathway, and various components of the core necroptotic machinery not only provide a fundamental contribution to embryonic and postnatal development, but also participate in the maintenance of adult organismal homeostasis and constitute etiological determinants of multiple human pathologies (8). Thus, necroptosis-relevant proteins constitute attractive targets for the development of novel drugs, and at least three therapeutic paradigms can be envisioned in this setting: (a) the inhibition of necroptosis, as a strategy to limit the loss of postmitotic cells in ischemic, neurodegenerative, inflammatory, and toxic syndromes; (b) the activation of necroptosis, as a means to bypass the accrued resistance of most tumors to apoptosis; and (c) the biochemical conversion of apoptotic RCD into its necroptotic counterpart, as a means to exacerbate the immunostimulatory potential of cell death. As discussed above, preclinical evidence supporting the validity of all these approaches has already been provided. However, the pharmacological agents that are currently available to target necroptosis are still far from entering the clinics, with the notable exceptions of dabrafenib (which is approved by the FDA for the treatment of BRAFV600E-driven melanoma) (171), pazopanib (a multitarget tyrosine kinase inhibitor currently used to treat renal cell carcinoma and advanced soft tissue sarcoma patients, which inhibits RIPK1), and ponatinib (another multitarget tyrosine kinase inhibitor employed against various forms of leukemia, which limits the activity of both RIPK1 and RIPK3) (199). It will be interesting to see whether these agents efficiently prevent necroptotic RCD and its detrimental effects in the clinic.
Chemical inhibitors of necroptosis employed as standalone therapeutic agents often exhibit suboptimal protective effects in preclinical models of disease, reflecting two different but highly intertwined situations: (a) the concurrent activation of various signal transduction cascades that lead to RCD, in the same cell or the same tissue (115, 119); and (b) the involvement of the immune system (177). Combinatorial strategies that simultaneously target different forms of RCD, intercept immunostimulatory DAMPs, and/or limit immune activation may therefore mediate superior therapeutic effects in various pathologies characterized by excess cell death coupled with inflammation. It is perhaps not a coincidence that cyclosporin A, the most successful agent for preventing graft rejection, not only mediates robust immunosuppressive effects but also blocks MPT-driven necrosis (7).
Finally, investigators must keep in mind that all necroptosis-relevant proteins most likely mediate several RCD-unrelated functions, which have just begun to be characterized. Carefully dissecting the necroptotic versus nonnecroptotic functions of proteins such as RIPK1, RIPK3, and MLKL will be instrumental for developing novel therapeutic approaches that specifically target this regulated variant of necrosis.
OTHER FORMS OF REGULATED NECROSIS.
Mitochondrial permeability transition (MPT)-driven regulated necrosis critically relies on peptidylprolyl isomerase F (PPIF, best known as CYPD) and hence can be retarded with pharmacological CYPD inhibitors such as cyclosporin A and sanglifehrin A. Parthanatos ensues the hyperactivation of poly(ADP-ribose) polymerase 1 (PARP1), which causes a potentially lethal depletion of NAD+ coupled with the translocation of apoptosis-inducing factor, mitochondria associated 1 (AIFM1) from the mitochondrial intermembrane space to the nucleus, where it mediates large-scale chromatin condensation. Parthanatos can be delayed with pharmacological inhibitors of PARP1, such as olaparib and PJ34. Ferroptosis is an iron-dependent form of RCD that involves lethal lipid peroxidation and is under negative regulation by glutathione peroxidase 4 (GPX4). Ferroptosis is sensitive to Ferrostatin-1, an inhibitor of lipid peroxidation. Pyroptosis depends on the cleavage of gasdermin D (GSDMD) by inflammatory caspases including caspase-1 (CASP1), CASP4, and Casp11 and hence can be delayed by broad-spectrum caspase inhibitors such as Z-VAD-fmk. In addition, all these necrotic instances of RCD can be deferred by the downregulation of key signal transducers (e.g., CYPD for MTP-driven regulated necrosis, PARP1 for parthanatos, GSDMD for pyroptosis), or the overexpression of endogenous inhibitors of the process (e.g., GPX4 for ferroptosis).
SUMMARY POINTS.
Necroptosis is a regulated variant of necrosis that critically relies on the RIPK3-dependent phosphorylation of MLKL, which endows MLKL with the ability to translocate to the inner leaflet of the plasma membrane to compromise its integrity.
In some instances, necroptosis is precipitated by the formation of an amyloid-like RIPK1-, RIPK3-, and MLKL-containing complex known as necrosome, which is assisted by several chaperones including HSP90 and CDC27.
RIPK1 plays a complex role in the regulation of necroptosis: it promotes TNFR1-driven necroptosis by virtue of its kinase activity but also inhibits necroptosis (and apoptosis) by a mechanism that depends on its scaffold but not on kinase functions.
Necroptosis is tonically suppressed by an enzymatically active supramolecular complex containing FADD, CASP8, and the long isoform of cFLIP, presumably owing to the ability of this complex to proteolytically inactivate RIPK1, RIPK3, and CYLD.
A delicate equilibrium between the signal transduction cascades that precipitate apoptosis and necroptosis is required for normal embryonic and postnatal development, as well as for the maintenance of tissue homeostasis in the adult.
Necroptosis as a process or core components of the necroptotic machinery provide an etiological contribution to the development of various nonmalignant pathologies characterized by unwarranted cell loss and/or a prominent inflammatory component. In some cases, the proinflammatory effects of necroptosis are aggravated by the ability of some components of the necroptotic machinery to drive cytokine secretion.
The molecular machinery for necroptosis is required for dying cells to be perceived as immunogenic and hence elicits an adaptive immune response, perhaps explaining why high RIPK3 or MLKL levels correlate with improved disease outcomes in various cohorts of cancer patients.
Although targeting core components of the necroptotic machinery stands out as an attractive approach for the therapy of various human conditions, additional work is required for the development of necroptosis-modulatory agents with clinical applications.
FUTURE ISSUES.
It will be important to clarify the molecular mechanisms through which oligomeric MLKL bound to the inner leaflet of the plasma membrane compromises its structural integrity.
Efforts will have to be dedicated to obtaining further insights into the molecular cross talk between necroptosis and other forms of regulated necrosis in various pathophysiologically relevant settings.
Confirming the implication of necroptosis as a lethal signal transduction cascade in the etiology of several nonmalignant conditions with Ripk3−/− and Mlkl−/− models may provide additional targets for pharmacological interventions.
It will be important to discriminate the nonnecroptotic functions of core components of the molecular machinery for necroptosis from their lethal activities.
A major objective for future efforts is developing clinically implementable combinatorial strategies for the therapeutic modulation of necroptotic RCD and necroptosis-driven inflammation.
Glossary
- RCD
regulated cell death
- MPT
mitochondrial permeability transition
- MLKL
mixed lineage kinase domain-like
- RIPK
receptor-interacting serine-threonine kinase
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- CASP
caspase
- FADD
Fas associated via death domain
- Nec-1
necrostatin-1
- IFN
interferon
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- I/R
ischemia/reperfusion
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol. 2014;16:728–36. doi: 10.1038/ncb3005. [DOI] [PubMed] [Google Scholar]
- 3.Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sica V, Galluzzi L, Bravo-San Pedro JM, Izzo V, Maiuri MC, Kroemer G. Organelle-specific initiation of autophagy. Mol Cell. 2015;59:522–39. doi: 10.1016/j.molcel.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159:1263–76. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–47. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 8.Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–66. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Wang H, Wang Z, He S, Chen S, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. PNAS. 2012;109:5322–27. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23:76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Huang Z, Ren J, Zhang Z, He P, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remijsen Q, Goossens V, Grootjans S, Van den Haute C, Vanlangenakker N, et al. Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis. 2014;5:e1004. doi: 10.1038/cddis.2013.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanzer MC, Tripaydonis A, Webb AI, Young SN, Varghese LN, et al. Necroptosis signalling is tuned by phosphorylation of MLKL residues outside the pseudokinase domain activation loop. Biochem J. 2015;471:255–65. doi: 10.1042/BJ20150678. [DOI] [PubMed] [Google Scholar]
- 15.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–53. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Li W, Ren J, Huang D, He WT, et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–21. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Sun L, Su L, Rizo J, Liu L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. PNAS. 2014;111:15072–77. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quarato G, Guy CS, Grace CR, Llambi F, Nourse A, et al. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol Cell. 2016;61:589–601. doi: 10.1016/j.molcel.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–81. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7:e2051. doi: 10.1038/cddis.2015.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao XM, Chen Z, Zhao JB, Zhang PP, Pu YF, et al. Hsp90 modulates the stability of MLKL and is required for TNF-induced necroptosis. Cell Death Dis. 2016;7:e2089. doi: 10.1038/cddis.2015.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigenzahn JW, Fauster A, Rebsamen M, Kandasamy RK, Scorzoni S, et al. An inducible retroviral expression system for tandem affinity purification mass-spectrometry-based proteomics identifies mixed lineage kinase domain-like protein (MLKL) as an heat shock protein 90 (HSP90) client. Mol Cell Proteom. 2016;15:1139–50. doi: 10.1074/mcp.O115.055350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–43. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Moujalled DM, Cook WD, Murphy JM, Vaux DL. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis. 2014;5:e1086. doi: 10.1038/cddis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tait SW, Oberst A, Quarato G, Milasta S, Haller M, et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 2013;5:878–85. doi: 10.1016/j.celrep.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriwaki K, Farias Luz N, Balaji S, De Rosa MJ, O’Donnell CL, et al. The mitochondrial phosphatase PGAM5 is dispensable for necroptosis but promotes inflammasome activation in macrophages. J Immunol. 2016;196:407–15. doi: 10.4049/jimmunol.1501662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu W, Sun J, Yoon JS, Zhang Y, Zheng L, Murphy E, Mattson MP, Lenardo MJ. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLOS ONE. 2016;11:e0147792. doi: 10.1371/journal.pone.0147792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–88. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 31.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–60. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 32.He S, Wang L, Miao L, Wang T, Du F, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Cho YS, Challa S, Moquin D, Genga R, Ray TD, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–36. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 35.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–50. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D, Xu T, Cao Y, Wang H, Li L, et al. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. PNAS. 2015;112:5017–22. doi: 10.1073/pnas.1505244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 38.Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283:16966–70. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Wu J, Li L, Zhang Z, Ren J, et al. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat Cell Biol. 2015;17:434–44. doi: 10.1038/ncb3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seo J, Lee EW, Sung H, Seong D, Dondelinger Y, et al. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat Cell Biol. 2016;18:291–302. doi: 10.1038/ncb3314. [DOI] [PubMed] [Google Scholar]
- 41.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, et al. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014;21:1511–21. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 44.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. PNAS. 2014;111:7753–58. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–76. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–80. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 2014;193:1539–43. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–95. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, et al. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature. 2013;498:224–27. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cullen SP, Kearney CJ, Clancy DM, Martin SJ. Diverse activators of the NLRP3 inflammasome promote IL-1β secretion by triggering necrosis. Cell Rep. 2015;11:1535–48. doi: 10.1016/j.celrep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Moriwaki K, Bertin J, Gough PJ, Chan FK. A RIPK3-caspase 8 complex mediates atypical pro-IL-1 β processing. J Immunol. 2015;194:1938–44. doi: 10.4049/jimmunol.1402167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362–74. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 56.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–67. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–7. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinlich R, Oberst A, Dillon CP, Janke LJ, Milasta S, et al. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 2013;5:340–48. doi: 10.1016/j.celrep.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ram DR, Ilyukha V, Volkova T, Buzdin A, Tai A, et al. Balance between short and long isoforms of cFLIP regulates Fas-mediated apoptosis in vivo. PNAS. 2016;113:1606–11. doi: 10.1073/pnas.1517562113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 61.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, et al. Enhanced and accelerated lymphoproliferation in Fas-null mice. PNAS. 1996;93:2131–36. doi: 10.1073/pnas.93.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 64.Yeh WC, de la Pompa JL, McCurrach ME, Shu HB, Elia AJ, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–58. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 65.Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–42. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 66.Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–82. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–34. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 68.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–39. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schock SN, Young JA, He TH, Sun Y, Winoto A. Deletion of FADD in macrophages and granulocytes results in RIP3- and MyD88-dependent systemic inflammation. PLOS ONE. 2015;10:e0124391. doi: 10.1371/journal.pone.0124391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, et al. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. PNAS. 2010;107:13034–39. doi: 10.1073/pnas.1005997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ch’en IL, Beisner DR, Degterev A, Lynch C, Yuan J, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. PNAS. 2008;105:17463–68. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. PNAS. 2011;108:15312–17. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–30. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McComb S, Cheung HH, Korneluk RG, Wang S, Krishnan L, Sad S. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. 2012;19:1791–801. doi: 10.1038/cdd.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yabal M, Muller N, Adler H, Knies N, Gross CJ, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18:656–65. doi: 10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLOS ONE. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–23. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–42. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vanlangenakker N, Bertrand MJ, Bogaert P, Vandenabeele P, Vanden Berghe T. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2011;2:e230. doi: 10.1038/cddis.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rickard JA, Anderton H, Etemadi N, Nachbur U, Darding M, et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. eLife. 2014;3:e03464. doi: 10.7554/eLife.03464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Almagro MC, Goncharov T, Newton K, Vucic D. Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination. Cell Death Dis. 2015;6:e1800. doi: 10.1038/cddis.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bellail AC, Olson JJ, Yang X, Chen ZJ, Hao C. A20 ubiquitin ligase-mediated polyubiquitination of RIP1 inhibits caspase-8 cleavage and TRAIL-induced apoptosis in glioblastoma. Cancer Discov. 2012;2:140–55. doi: 10.1158/2159-8290.CD-11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peltzer N, Rieser E, Taraborrelli L, Draber P, Darding M, et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Rep. 2014;9:153–65. doi: 10.1016/j.celrep.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 86.Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16:618–27. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Petersen SL, Chen TT, Lawrence DA, Marsters SA, Gonzalvez F, Ashkenazi A. TRAF2 is a biologically important necroptosis suppressor. Cell Death Differ. 2015;22:1846–57. doi: 10.1038/cdd.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Karl I, Jossberger-Werner M, Schmidt N, Horn S, Goebeler M, et al. TRAF2 inhibits TRAIL-and CD95L-induced apoptosis and necroptosis. Cell Death Dis. 2014;5:e1444. doi: 10.1038/cddis.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG. TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol. 2013;33:582–95. doi: 10.1128/MCB.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20:1381–92. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morioka S, Broglie P, Omori E, Ikeda Y, Takaesu G, et al. TAK1 kinase switches cell fate from apoptosis to necrosis following TNF stimulation. J Cell Biol. 2014;204:607–23. doi: 10.1083/jcb.201305070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 93.Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, et al. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-κB. EMBO Rep. 2009;10:916–22. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–97. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–79. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 97.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–14. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 98.Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-κB activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181:6427–34. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14:546–58. doi: 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- 100.Zou J, Kawai T, Tsuchida T, Kozaki T, Tanaka H, et al. Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity. 2013;38:717–28. doi: 10.1016/j.immuni.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 101.Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 2013;4:e716. doi: 10.1038/cddis.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–62. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. PNAS. 2013;110:E3109–18. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, et al. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. PNAS. 2014;111:E3206–13. doi: 10.1073/pnas.1407068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Raam BJ, Ehrnhoefer DE, Hayden MR, Salvesen GS. Intrinsic cleavage of receptor-interacting protein kinase-1 by caspase-6. Cell Death Differ. 2013;20:86–96. doi: 10.1038/cdd.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McComb S, Shutinoski B, Thurston S, Cessford E, Kumar K, Sad S. Cathepsins limit macrophage necroptosis through cleavage of Rip1 kinase. J Immunol. 2014;192:5671–78. doi: 10.4049/jimmunol.1303380. [DOI] [PubMed] [Google Scholar]
- 107.Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–51. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 108.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–67. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 109.Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999;442:117–21. doi: 10.1016/s0014-5793(98)01640-8. [DOI] [PubMed] [Google Scholar]
- 110.Duprez L, Bertrand MJ, Vanden Berghe T, Dondelinger Y, Festjens N, Vandenabeele P. Intermediate domain of receptor-interacting protein kinase 1 (RIPK1) determines switch between necroptosis and RIPK1 kinase-dependent apoptosis. J Biol Chem. 2012;287:14863–72. doi: 10.1074/jbc.M111.288670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen G, Cheng X, Zhao M, Lin S, Lu J, et al. RIP1-dependent Bid cleavage mediates TNFα-induced but Caspase-3-independent cell death in L929 fibroblastoma cells. Apoptosis. 2015;20:92–109. doi: 10.1007/s10495-014-1058-0. [DOI] [PubMed] [Google Scholar]
- 112.Karch J, Kanisicak O, Brody MJ, Sargent MA, Michael DM, Molkentin JD. Necroptosis interfaces with MOMP and the MPTP in mediating cell death. PLOS ONE. 2015;10:e0130520. doi: 10.1371/journal.pone.0130520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther. 2007;21:467–69. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, et al. CaMKII is a RIP3 substrate mediating ischemia-and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–82. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 115.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. PNAS. 2013;110:12024–29. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–14. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sosna J, Voigt S, Mathieu S, Lange A, Thon L, et al. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci. 2014;71:331–48. doi: 10.1007/s00018-013-1381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Canli O, Alankus YB, Grootjans S, Vegi N, Hultner L, et al. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127:139–48. doi: 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, et al. Synchronized renal tubular cell death involves ferroptosis. PNAS. 2014;111:16836–41. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–69. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 122.Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–72. doi: 10.1182/blood-2013-06-510743. [DOI] [PubMed] [Google Scholar]
- 123.Roderick JE, Hermance N, Zelic M, Simmons MJ, Polykratis A, et al. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. PNAS. 2014;111:14436–41. doi: 10.1073/pnas.1409389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–88. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 125.Matsuoka Y, Tsujimoto Y. Role of RIP1 in physiological enterocyte turnover in mouse small intestine via nonapoptotic death. Genes Cells. 2015;20:11–28. doi: 10.1111/gtc.12193. [DOI] [PubMed] [Google Scholar]
- 126.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, et al. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science. 2015;350:328–34. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17:229–42. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Wang X, Li Y, Liu S, Yu X, Li L, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. PNAS. 2014;111:15438–43. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, et al. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–51. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem. 2015;290:11635–48. doi: 10.1074/jbc.M115.646042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. PNAS. 2014;111:7391–96. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, Jiang W, Yan Y, Gong T, Han J, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15:1126–33. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 134.Deutsch M, Graffeo CS, Rokosh R, Pansari M, Ochi A, et al. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis. 2015;6:e1759. doi: 10.1038/cddis.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–58. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLOS Pathog. 2015;11:e1005337. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gaiha GD, McKim KJ, Woods M, Pertel T, Rohrbach J, et al. Dysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosis. Immunity. 2014;41:1001–12. doi: 10.1016/j.immuni.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pan T, Wu S, He X, Luo H, Zhang Y, et al. Necroptosis takes place in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T lymphocytes. PLOS ONE. 2014;9:e93944. doi: 10.1371/journal.pone.0093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–16. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 140.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 141.Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 142.Koudstaal S, Oerlemans MI, Van der Spoel TI, Janssen AW, Hoefer IE, et al. Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur J Clin Investig. 2015;45:150–59. doi: 10.1111/eci.12391. [DOI] [PubMed] [Google Scholar]
- 143.Wang Q, Liu Z, Ren J, Morgan S, Assa C, Liu B. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res. 2015;116:600–11. doi: 10.1161/CIRCRESAHA.116.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meng L, Jin W, Wang X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. PNAS. 2015;112:11007–12. doi: 10.1073/pnas.1514730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin J, Li H, Yang M, Ren J, Huang Z, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–10. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 146.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–19. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 147.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–89. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chavez-Valdez R, Martin LJ, Flock DL, Northington FJ. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience. 2012;219:192–203. doi: 10.1016/j.neuroscience.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569–76. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. PNAS. 2010;107:21695–700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dong K, Zhu H, Song Z, Gong Y, Wang F, et al. Necrostatin-1 protects photoreceptors from cell death and improves functional outcome after experimental retinal detachment. Am J Pathol. 2012;181:1634–41. doi: 10.1016/j.ajpath.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 152.Murakami Y, Matsumoto H, Roh M, Giani A, Kataoka K, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21:270–77. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.King MD, Whitaker-Lea WA, Campbell JM, Alleyne CH, Jr, Dhandapani KM. Necrostatin-1 reduces neurovascular injury after intracerebral hemorrhage. Int J Cell Biol. 2014;2014:495817. doi: 10.1155/2014/495817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Su X, Wang H, Kang D, Zhu J, Sun Q, Li T, Ding K. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochem Res. 2015;40:643–50. doi: 10.1007/s11064-014-1510-0. [DOI] [PubMed] [Google Scholar]
- 155.You Z, Savitz SI, Yang J, Degterev A, Yuan J, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–73. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang Y, Wang H, Tao Y, Zhang S, Wang J, Feng X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91–101. doi: 10.1016/j.neuroscience.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 157.Liu M, Wu W, Li H, Li S, Huang LT, et al. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J Spinal Cord Med. 2015;38:745–53. doi: 10.1179/2045772314Y.0000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fan H, Tang HB, Kang J, Shan L, Song H, et al. Involvement of endoplasmic reticulum stress in the necroptosis of microglia/macrophages after spinal cord injury. Neuroscience. 2015;311:362–73. doi: 10.1016/j.neuroscience.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 159.Fan H, Zhang K, Shan L, Kuang F, Chen K, et al. Reactive astrocytes undergo M1 microglia/macrophages-induced necroptosis in spinal cord injury. Mol Neurodegener. 2016;11:14. doi: 10.1186/s13024-016-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Re DB, Le Verche V, Yu C, Amoroso MW, Politi KA, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–8. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zheng HW, Chen J, Sha SH. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014;5:e1262. doi: 10.1038/cddis.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–61. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 163.Mulay SR, Desai J, Kumar SV, Eberhard JN, Thomasova D, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun. 2016;7:10274. doi: 10.1038/ncomms10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tristao VR, Goncalves PF, Dalboni MA, Batista MC, de Durao MS, Jr, Monte JC. Nec-1 protects against nonapoptotic cell death in cisplatin-induced kidney injury. Ren Fail. 2012;34:373–77. doi: 10.3109/0886022X.2011.647343. [DOI] [PubMed] [Google Scholar]
- 165.Linkermann A, Heller JO, Prokai A, Weinberg JM, De Zen F, et al. The RIP1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced AKI in mice. J Am Soc Nephrol. 2013;24:1545–57. doi: 10.1681/ASN.2012121169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu Y, Ma H, Shao J, Wu J, Zhou L, et al. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol. 2015;26:2647–58. doi: 10.1681/ASN.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tristao VR, Pessoa EA, Nakamichi R, Reis LA, Batista MC, et al. Synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity. Apoptosis. 2016;21:51–59. doi: 10.1007/s10495-015-1190-5. [DOI] [PubMed] [Google Scholar]
- 168.Zhu Y, Cui H, Gan H, Xia Y, Wang L, et al. Necroptosis mediated by receptor interaction protein kinase 1 and 3 aggravates chronic kidney injury of subtotal nephrectomised rats. Biochem Biophys Res Commun. 2015;461:575–81. doi: 10.1016/j.bbrc.2015.03.164. [DOI] [PubMed] [Google Scholar]
- 169.Rosentreter D, Funken D, Reifart J, Mende K, Rentsch M, Khandoga A. RIP1-dependent programmed necrosis is negatively regulated by caspases during hepatic ischemia-reperfusion. Shock. 2015;44:72–76. doi: 10.1097/SHK.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 170.Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li JX, Feng JM, Wang Y, Li XH, Chen XX, et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 2014;5:e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–83. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang S, Ni HM, Dorko K, Kumer SC, Schmitt TM, et al. Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget. 2016;7:17681–98. doi: 10.18632/oncotarget.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062–74. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin Sci. 2015;129:721–39. doi: 10.1042/CS20140732. [DOI] [PubMed] [Google Scholar]
- 176.Dara L, Johnson H, Suda J, Win S, Gaarde W, et al. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 2015;62:1847–57. doi: 10.1002/hep.27939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14:759–67. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 178.Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–49. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–18. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 180.Huang Z, Epperly M, Watkins SC, Greenberger JS, Kagan VE, Bayir H. Necrostatin-1 rescues mice from lethal irradiation. Biochim Biophys Acta. 2016;1862:850–56. doi: 10.1016/j.bbadis.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saito N, Qiao H, Yanagi T, Shinkuma S, Nishimura K, et al. An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med. 2014;6:245ra95. doi: 10.1126/scitranslmed.3008227. [DOI] [PubMed] [Google Scholar]
- 182.Pavlosky A, Lau A, Su Y, Lian D, Huang X, et al. RIPK3-mediated necroptosis regulates cardiac allograft rejection. Am J Transplant. 2014;14:1778–90. doi: 10.1111/ajt.12779. [DOI] [PubMed] [Google Scholar]
- 183.Lau A, Wang S, Jiang J, Haig A, Pavlosky A, et al. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant. 2013;13:2805–18. doi: 10.1111/ajt.12447. [DOI] [PubMed] [Google Scholar]
- 184.Zhao H, Ning J, Lemaire A, Koumpa FS, Sun JJ, et al. Necroptosis and parthanatos are involved in remote lung injury after receiving ischemic renal allografts in rats. Kidney Int. 2015;87:738–48. doi: 10.1038/ki.2014.388. [DOI] [PubMed] [Google Scholar]
- 185.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Investig. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pouwels SD, Zijlstra GJ, van der Toorn M, Hesse L, Gras R, et al. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2016;310:L377–86. doi: 10.1152/ajplung.00174.2015. [DOI] [PubMed] [Google Scholar]
- 187.Fan H, Liu F, Dong G, Ren D, Xu Y, et al. Activation-induced necroptosis contributes to B-cell lymphopenia in active systemic lupus erythematosus. Cell Death Dis. 2014;5:e1416. doi: 10.1038/cddis.2014.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang H, Ma Y, Chen G, Zhou H, Yamazaki T, et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology. 2016;5:e1149673. doi: 10.1080/2162402X.2016.1149673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Werthmoller N, Frey B, Wunderlich R, Fietkau R, Gaipl US. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 2015;6:e1761. doi: 10.1038/cddis.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Koks CA, Garg AD, Ehrhardt M, Riva M, Vandenberk L, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer. 2015;136:E313–25. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- 191.Takemura R, Takaki H, Okada S, Shime H, Akazawa T, et al. PolyI:C-induced, TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo. Cancer Immunol Res. 2015;3:902–14. doi: 10.1158/2326-6066.CIR-14-0219. [DOI] [PubMed] [Google Scholar]
- 192.Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am J Cancer Res. 2015;5:3174–85. [PMC free article] [PubMed] [Google Scholar]
- 193.Feng X, Song Q, Yu A, Tang H, Peng Z, Wang X. Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma. 2015;62:592–601. doi: 10.4149/neo_2015_071. [DOI] [PubMed] [Google Scholar]
- 194.Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015;6:e1636. doi: 10.1038/cddis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nugues AL, El Bouazzati H, Hetuin D, Berthon C, Loyens A, et al. RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis. 2014;5:e1384. doi: 10.1038/cddis.2014.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25:707–25. doi: 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ruan J, Mei L, Zhu Q, Shi G, Wang H. Mixed lineage kinase domain-like protein is a prognostic biomarker for cervical squamous cell cancer. Int J Clin Exp Pathol. 2015;8:15035–38. [PMC free article] [PubMed] [Google Scholar]
- 198.He L, Peng K, Liu Y, Xiong J, Zhu FF. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. OncoTargets Ther. 2013;6:1539–43. doi: 10.2147/OTT.S52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fauster A, Rebsamen M, Huber KV, Bigenzahn JW, Stukalov A, et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 2015;6:e1767. doi: 10.1038/cddis.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]