Abstract
Purpose of the review
Recent studies in the kidney have revealed that the well-characterized tumor antigen mucin 1 (MUC1/Muc1) also has numerous functions in the normal and injured kidney.
Recent findings
Mucin 1 is a transmembrane mucin with a robust glycan-dependent apical targeting signal and efficient recycling from endosomes. It was recently reported that the TRPV5 calcium channel is stabilized on the cell surface by galectin-dependent cross-linking to mucin 1, providing a novel mechanism for regulation of ion channels and normal electrolyte balance.
Our recent studies in mice show that mucin 1 is induced after ischemia, stabilizing HIF-1α and β-catenin levels, and transactivating the HIF-1 and β-catenin protective pathways. However, prolonged induction of either pathway in the injured kidney can proceed from apparent full recovery to chronic kidney disease. A very recent report indicates that aberrant activation of mucin1 signaling after ischemic injury in mice and humans is associated with development of chronic kidney disease and fibrosis. A frame-shift mutation in MUC1 was recently identified as the genetic lesion causing Medullary Cystic Kidney Disease type 1, now appropriately renamed MUC1 Kidney Disease (MKD).
Summary
Studies of mucin 1 in the kidney now reveal significant functions for the extracellular mucin-like domain and signaling through the cytoplasmic tail.
Keywords: Mucin 1, MUC1, Muc1, MUC1 Kidney Disease, acute kidney injury, chronic kidney disease, TRPV5, inflammation
Introduction
Mucin 1, known as MUC1 in humans and Muc1 in other mammals, is a multi-functional protein expressed on the apical surface of most epithelial cells (for review, see [1-4]). The numerous biological activities attributed to MUC1/Muc1 in promoting cell growth and survival, are sometimes overshadowed by its name and classification as a tethered mucin. MUC1/Muc1 does undergo autocatalytic cleavage early in its biogenesis yielding a large mucin-like subunit (>300 kDa) composed of a variable number of tandem repeats (VNTR, range 40-140) that provides cell surface protection from bacteria, viruses and other environmental insults [5-9]. MUC1 glycans on this subunit also bind to galectins which are associated with crosslinking cell surface proteins and thereby preventing endocytosis [10-12] (Fig. 1). The smaller transmembrane subunit (25 kDa) includes the 72-residue cytoplasmic tail with sites for (i) binding adaptors for endocytosis and recycling, (ii) phosphorylation by kinases, and (iii) docking proteins involved in either signaling or transcriptional transactivation by trafficking to the nucleus (Fig. 2) [1-3,13-15]. The cytoplasmic juxtamembrane sequence (CQCRRK) is implicated in nuclear targeting of mucin 1 by disulfide crosslinking of MUC1 dimers, thereby forming a non-conventional nuclear targeting sequence despite the reported dual Cys-palmitoylation of this CQC motif [13,16].
Figure 1. Mucin 1 structure and functional domains.
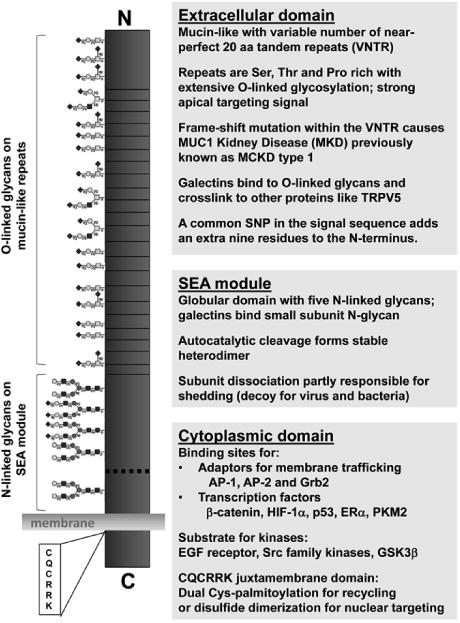
Mucin 1 is a type 1 transmembrane protein with a cleaved signal sequence and cytoplasmic C-terminus. The extracellular domain includes a SEA (sea urchin sperm protein, enterokinase and agrin) module with five N-linked glycans that exhibits autocatalytic cleavage yielding a heterodimeric structure (dashed line). The large mucin-like subunit also includes a variable number of tandem repeats (VNTR) that are rich in Pro and O-glycosylated Ser and Thr residues. The smaller subunit includes the transmembrane domain and cytoplasmic tail detailed in Figure 2. Functions attributed to each domain are noted with details in the text.
Figure 2. Functional activities of the mucin 1 cytoplasmic tail.
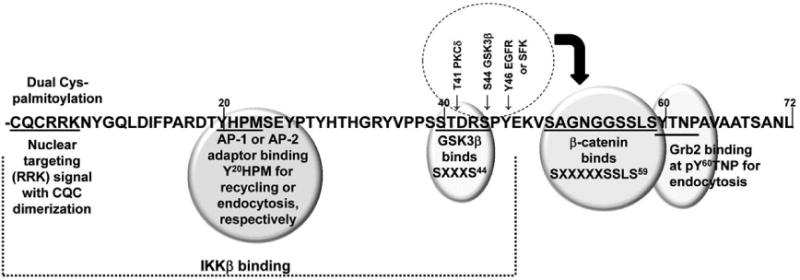
The 72-residue cytoplasmic domain is numbered from the transmembrane domain due to the extracellular VNTR. MUC1 binding of Grb2 at pY60TNP and the clathrin adaptor AP-2 at Y20HPM is required for endocytosis, while binding of clathrin adaptor AP-1 at Y20HPM and dual palmitoylation of the CQC3 motif is required for recycling from endosomes back to the cell surface [13,14]. Mucin dimerization through the CQC3 motif yields a nuclear targeting signal based on the adjacent RRK [16]. Peptides based on the CQCRRK motif can block MUC1 activities in the nucleus [67]. The site of IKKβ interaction is indicated which initiates NF-kB activation [68]. β-catenin binding to a canonical SXXXXXSSLS59 site is(i) enhanced by PKC5 phosphorylatin of Thr41 and Tyr46 phosphorylation by either the EGF receptor or Src family kinases, and (ii) blocked by Ser44 phosphorylation by GSK3p that binds at SXXXS44 (for review, see [1-3]).
The highest expression of MUC1 is reported in the stomach>lung>kidney>esophagus>colon> pancreas>breast tissues, etc. based on gene expression (RPKM, reads per kilobase per million reads from gtexportal.org). Interestingly, MUC1 is also expressed in immune cells including B cells, T cells, monocytes, macrophages and dendritic cells [17-20]. However, MUC1 activities have been primarily characterized in tumor cells where high expression levels in tumors correlate with a poor prognosis for the patient due to its ability to promote cell growth and survival [21-23]. In fact, the National Cancer Institute has priority-ranked MUC1 at #2 on a list of cancer vaccine target antigens where major criteria were immunogenicity, oncogenicity and therapeutic function [24]. Epithelial tumors in Muc1 global knockout (KO) mice exhibit reduced growth when compared to congenic controls consistent with a role for mucin 1 in promoting tumor growth and survival [25].
Under normal conditions the Muc1 KO mice have no obvious phenotype except that they are more sensitive to bacterial infections [26,27]. For this reason, the studies of MUC1/Muc1 function in stomach and lung have focused primarily on infection of either cell lines or Muc1 KO and congenic control mice with Helicobacter pylori and Pseudomonas aeruginosa, respectively (discussed below). The function of Muc1/MUC1 in kidney has been more difficult to address as direct infection by bacteria and virus is much less common. While sepsis does stress the kidney primarily by indirect means, the most well characterized model of acute kidney injury is produced by clamping both the renal artery and vein, or the artery alone, to produce ischemia, and studying recovery of kidney function and morphology after return of blood flow for hours to days [28-30]. Using this approach, we found that Muc1 is protective in the kidney in a mouse model of ischemia-reperfusion injury by transactivation of the HIF-1 and β-catenin protective pathways [31,32].
Lessons learned from MUC1 Kidney Disease (MKD)
A frame-shift mutation in MUC1 (MUC1-fs) was recently identified as the genetic lesion causing Medullary Cystic Kidney Disease type 1 (MCKD1 variant of autosomal dominant tubule- interstitial kidney disease) [33]. As these patients rarely have cysts, the disease has been appropriately renamed autosomal dominant tubule-interstitial kidney disease due to MUC1 mutations, with an abbreviated name of Mucin 1 Kidney Disease (MKD) [34,35]. The most common causative mutation is a cytosine duplication in a string of seven cytosines within any one of the G-C-rich tandem repeats. This cytosine insertion results in a frameshift mutation, producing a chimeric protein with the N-terminus of normal MUC1 and a C-terminus with a unique highly basic repeating sequence rich in Cys and His [33]. The frame-shift in mutant MUC1 (MUC1-fs) also places a stop codon after the repeats. Although the chimera should be secreted, it is found as intracellular staining by imunohistochemistry in patient kidney tissue and as diffuse and/or fine granular intracellular staining by immunofluorescence microscopy with puncta co-localizing with wild-type MUC1 staining at the apical surface [33]. Patients with MKD present with asymptomatic elevation of serum creatinine, exhibit bland urinary sediment with minimal blood or protein, non-specific tubulointerstitial fibrosis, a gradual decline in glomerular filtration rate, and a need for dialysis between the third and eighth decade of life [35,36]. Most notably, the patients usually have a family history of chronic kidney disease and exhibit only renal disease despite the presence of MUC1-fs in multiple organs. The basis for the highly variable age of disease onset is an active area of research and could be influenced by both the reduced levels of normal MUC1 and the accumulation of mutant MUC1-fs within the cell.
A role for MUC1 in surface expression of transporters
Uromodulin (UMOD, also known as Tamm-Horsfall protein) is the most abundant protein in human urine and mutations in uromodulin are the most common genetic cause of autosomal dominant tubule-interstitial kidney disease. These patients exhibit reduced urinary UMOD due to its intracellular accumulation in the thick ascending limb (TAL), as well as hypouricosuric hyperuricemia, hypertension, renal fibrosis and progressive renal failure. Mice expressing UMOD with the corresponding human mutations also have reduced urinary UMOD but exhibit hypercalciura, renal calcium crystals and reduced immunofluorescence staining of the renal calcium channel TRPV5, which is localized to the distal convoluted tubule (DCT) and collecting ducts (CD) [37]. Studies in transiently transfected HEK293 cells revealed that current density of TRPV5 was enhanced by either co-expression with UMOD or by addition of exogenous UMOD, through a mechanism that reduced TRPV5 endocytosis and increased its cell surface expression. The data are consistent with a role for UMOD after shedding from the TAL in directly stabilizing TRPV5 in the distal segments of the tubule.
As MUC1 is also expressed in the TAL and found in the urine while the mutant MUC1 accumulates within cells as described for UMOD mutations, similar studies were carried out in HEK293 cells to determine if MUC1 can also enhance activity of the renal TRPV5 channel. Interestingly, urinary MUC1 was also reduced in patients with calcium nephrolithiasis, a common type of kidney stone, supporting the possibility that shed MUC1 could have a role in enhancing calcium reabsorption [37]. Transient expression of MUC1 in HEK293 cells revealed a MUC1 dose-dependent increase in TRPV5 surface currents associated with reduced TRPV5 endocytosis and stabilization at the cell surface [37]. Earlier studies revealed that TRPV5 surface expression is also enhanced by binding galectin-1 after Klotho-dependent removal of sialic acid from TRPV5, and by galectin-3 binding to the N-glycan of TRPV5 [38,39]. These more recent studies in HEK293 cells revealed that MUC1 enhancement of TRPV5 surface expression proceeds by galectin-3-dependent crosslinking of O-glycans on MUC1 with the N- glycan on TRPV5, whereas galectin-1 had no role [37].
MUC1 does have an exceptionally strong glycan-dependent apical targeting signal that can redirect a basolaterally expressed protein to the apical cell surface in polarized epithelia; and the rate of MUC1 recycling from endosome to the cell surface is quite efficient and significantly higher than its rate of endocytosis (4-fold) [13,40]. It is therefore possible that the MUC1 mucinlike subunit directly enhances surface expression of additional channels by a similar mechanism of crosslinking and maintenance at the cell surface. For example, a large genome-wide association study focused on serum concentrations of cations revealed that the highest association with low serum magnesium levels (hypomagnesemia) was a very common genetic variant of MUC1 that adds nine amino acids to the extracellular N-terminus of the protein (MUC1 SNP: rs4072037 with coded allele frequency of 0.46) [41]. A Single nucleotide polymorphism (SNP) in the magnesium transporter TRPM6 was also associated with low serum magnesium but to a lesser extent than the MUC1 variant [41]. Interestingly, the MUC1 SNP was associated with higher bone mineral density and lower fasting glucose levels which could proceed by a direct interaction of either the transmembrane MUC1 or shed MUC1 with transporters within the kidney tubule [41].
MUC1 has been previously implicated in enhancing glucose uptake and metabolism. Transfection of pancreatic tumor cells with MUC1 enhanced uptake of [3H]2-deoxyglucose, while knockdown of MUC1 in liver tumor cells reduced uptake [42]. Injection of the pancreatic tumor cells into mice produced tumors with significantly higher uptake of dye-coupled 2- deoxyglucose than tumors lacking MUC1. The MUC1-expressing tumor cells had increased levels of the glucose transporter GLUT-1, as well as increased levels of HIF-1α and LDHA, and increased staining with Ki67, a marker of cell prolilferation. Studies using the same pancreatic tumor cells in culture were the first to reveal that MUC1 (small subunit) stabilizes HIF-1α by direct binding and enhances expression of downstream targets of HIF-1 by enhancing promoter occupancy of genes that shift metabolism in response to hypoxia [42]. Clearly, MUC1 can also modulate metabolism and solute transport by transcriptional means that alter the expression of enzymes and transporters. While Wang et al. [43] showed that intestinal uptake and absorption of cholesterol was significantly reduced in Muc1 KO mice compared with the wild-type mice by an undefined mechanism, Nath et al. [44] showed that MUC1 up-regulates multidrug resistance genes including the ABCC1 gene that encodes a cholesterol efflux pump, by direct binding to the promoter region of the ABCC1 gene in pancreatic cancer cells.
Lessons learned from Muc1 activities during acute kidney injury
The mouse model of ischemia-reperfusion injury (IRI) results in significant damage to primarily the proximal tubule (PT) that is normally well-oxygenated by the extensive renal vasculature in the cortex. Deep sequencing of microdissected adult rat renal tubule segments revealed that MUC1 was present in collecting duct (CD)> medullary loop of Henle> thick ascending limb (TAL)> distal convoluted tubule (DCT)≫≫ PT (based on RPKM) [45]. We also found the highest levels of Muc1 in the DCT, CD and TAL in the kidneys of sham treated mice using immunohistochemistry (IHC), but we also found Muc1 staining at very low levels in the PT that was absent in the Muc1 KO mouse kidney [31]. Muc1 appeared in the cytoplasm of all tubule epithelia immediately after 19 min ischemia, and was found in the nuclei after 4 h recovery [31]. Total Muc1 levels as assessed by immunblotting kidney homogenates was increased 4.2-fold after 3 d recovery when Muc1 staining by IHC was observed on the apical surface of flattened cells in the recovering proximal tubule [31]. The appearance of Muc1 on the apical surface of the recovering PT at 3 d recovery is even more convincing using immunofluorescence microscopy and co-staining with antibodies against the organic ion transporter 1 (OAT1) localized on the basolateral surface (Fig. 3). Published studies indicate that mucin 1 is expressed on the apical surface of polarized epithelial cells in the proximal tubule during development and potentially after injury, such that our findings in this mouse model is consistent with previous reports [46,47].
Figure 3. Mucin 1 is expressed in the proximal tubule during ischemia-reperfusion injury.
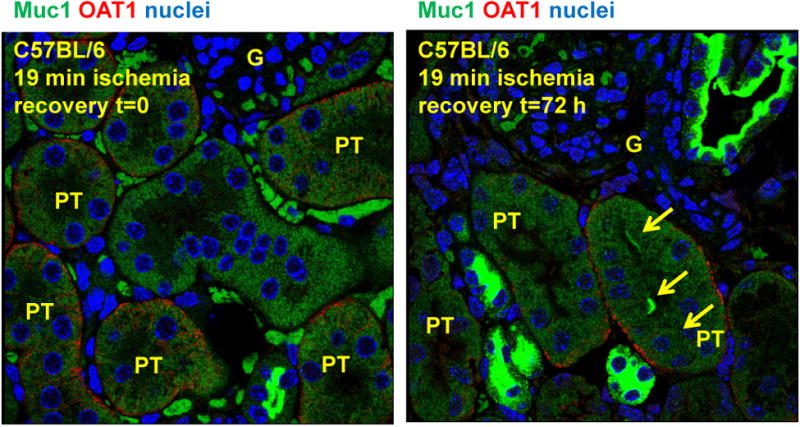
Mice were subjected to 19 min ischemia and recovery for t=0 or 3 days. Kidneys were fixed in PFA, embedded in paraffin, subjected to antigen retrieval, and incubated with rabbit anti-OAT1 (organic anion transporter 1) antibodies (red) to label the basolateral surface of the proximal tubule cells (PT), and with Armenian hamster CT-2 anti-MUC1 cytoplasmic tail antibodies (green). Nuclei were stained blue. Muc1 appears on the apical surface of flatten cells in the recovering proximal tubule at 3 d recovery (yellow arrows). Glomeruli (G) are also indicated.
A role for Muc1 in effective recovery from ischemic injury in the proximal tubule is supported by the fact that mice do not efficiently recover kidney function when Muc1 is absent [31]. When we followed sCr levels in mice for 3 d after 19 min ischemia, we found that sCr peaked after 24 h in both Muc1 knockout (KO) and congenic control mice and returned to normal in control mice, but not Muc1 KO mice, at 3 d recovery. The appearance of regenerating flatten cells in the proximal tubule after 3 d was also absent in Muc1 KO mice [31]. As MUC1 is known to co- immunoprecipitate with the transcription factors HIF-1α and β-catenin in tumor cell lines and prevent their degradation [42,48], we assessed levels of these two proteins in whole kidney homogenates and discovered that Muc1 stabilizes HIF-1α and β-catenin in the injured kidney [31,32].
HIF-1α was previously observed in the TAL and CD of sham-treated rats and appeared in the PT during IRI [49], tubule segments where we observed Muc1 expression during IRI [31]. Staining for HIF-1α was noticeably reduced in kidneys of Muc1 KO mice after 4 h recovery when compared to control mice, consistent with reduced HIF-1α levels (32%) measured by immunoblotting kidney tissue [31]. Induction of downstream targets of the HIF-1 protective pathway involved in a shift of metabolism from oxidative phosphorylation to glycolysis, were also aberrant in Muc1 KO kidneys during IRI [31]. MUC1 stabilization of HIF-1α and transactivation of HIF-1 target genes was previously reported in studies of pancreatic tumor cells [42]. In line with this, we found that levels of AMP-activated protein kinase (AMPK) were higher in Muc1 KO kidneys when compared to controls, and AMPK activation by phosphorylation (phosphor-Thr172 AMPK-a) was prolonged, indicating metabolic stress in the absence of Muc1 [31].
MUC1 is also reported to stabilize β-catenin in tumor cells by blocking its phosphorylation by glycogen synthase kinase (GSK) 3p [48]. Consistent with this, we found that induction of β- catenin during IRI was blocked in Muc1 KO mice while GSK3p activity was increased [32]. Targeting of β-catenin to the nucleus during IRI was also completely blocked in Muc1 KO mice, as was induction of the β-catenin protective pathway including stimulation of prosurvival factors (activated Akt, survivin, transcription factor T cell factor 4 (TCF4) and downstream target cyclin D1), and repression of proapoptotoc factors (p53, active Bax and cleaved caspase-3) [32].
Both HIF-1α and β-catenin are induced during ischemic injury in the kidney tubule epithelial cells and transactivate complex protective pathways [50,51]. This was established in part by finding that kidney injury and recovery is worse in mice with tubule knockout of either HIF-1α or β-catenin [51-53]. However, prolonged induction of either HIF-1α or β-catenin in response to kidney injury can progress from apparent full recovery to chronic kidney injury with fibrosis. While studies in mice have shown that stabilization of HIF-1α expression improves the kidney's response to ischemia, prolonged HIF activation through knockout of the von Hippel-Lindau E3 ligase, promotes interstitial fibrosis [50,54]. In turn, genetic ablation of HIF-1α blocked development of fibrosis in a unilateral ureteral obstruction (UUO) model of injury [55]. Haase has also identified a significant correlation of percent of tubular cells expressing HIF-1α and the stage of kidney nephropathy in diabetic patients [56].
Xiao et al. [57] have now established that moderate ischemia (20 min) in a mouse model of IRI causes a transient induction of β-catenin and apparent full recovery of kidney morphology and function, while a more severe ischemia (30 min) produced a prolonged elevation of β-catenin and progression to fibrosis after just ten days. Using mouse models, cultured kidney cells, and human biopsy specimens, Gibier et al. [58] now report that prolonged aberrant activation of mucin 1 signaling is associated with the development and progression of chronic kidney disease (CKD) including fibrosis. In their mouse model, sustained activation of Muc1 is associated with induction of epithelial-to-mesenchymal transition (EMT) features such as fibronectin, type I collagen, and Snail 1, which have been previously reported to cause renal fibrosis in alternative mouse models of kidney injury [59-61]. Furthermore, there was a positive correlation between MUC1 expression and expression of EMT markers in human biopsies which also exhibit more interstitial fibrosis levels [58]. This correlation could be explained by the role of mucin 1 in prolonged transactivation of both the HIF-1 and β-catenin protective pathways. Altogether, the data indicate that an early and transient activation of mucin 1 signaling appears to be renoprotective by promoting repair and recovery of kidney function, while sustained activation of mucin 1 appears to promote renal fibrosis and accelerate AKI to CKD progression [58].
The promoter of the mucin 1 gene contains HIF-responsive elements and is induced by hypoxia in a HIF-1-dependent manner [62]. HIF-1 also induces β-catenin expression [63]. As mucin-1 stabilizes both HIF-1α and β-catenin [1,48], it is clear that mucin 1 is a key figure in the kidney's normal recovery from transient ischemia and aberrant response to chronic ischemia, progressing to EMT and fibrosis.
Lessons learned from Muc1 activities in other tissues
Mucin 1 is highly expressed on the lung and gastric mucosal surfaces, and there is considerable evidence that mucin 1 provides both a physical barrier to potential pathogens, and modulates inflammatory and immune responses to bacterial infection. For example, Muc1 binds directly to the flaggelins of Pseudomonas aeruginosa (PA) and to adhesins on H. pylori, common pathogens in lung and stomach, respectively [8,64]. However, mucin 1 protection of these organs is primarily through its anti-inflammatory actions. PA stimulates alveolar macrophages to release TNF-a, and TNF-a induces mucin 1 levels in airway epithelial cells (AEC). PA also stimulates AEC to secrete TGF-a that activates the EGF receptor (EGFR), the EGFR phosphorylates mucin 1, and mucin 1 then associates with toll like receptor (TLR) 5 [65]. Mucin 1 expressed on macrophages also regulates the host immune system during H. pylori infection and limits gastritis by negatively regulating NLRP3 inflammasome activity that promotes IL-1p production [66]. This protective effect of mucin 1 is mediated by its interaction with TLRs, effecting NF-kB signaling and inhibition of IRAK4 activation [66]. As mucin 1 is known to limit inflammation by regulating the NLRP3 inflammasome and TLR signaling, future studies will address this role during kidney injury.
Conclusion
Mucin 1 is essential for both normal renal function and recovery from injury. Galectin crosslinking of mucin 1 with transporters such as TRPV5 provides a novel mechanism for regulation of ion balance. In response to ischemia, mucin 1 levels increase and stabilize both HIF-1α and β-catenin to potentiate downstream protective pathways, although prolonged increases lead to chronic kidney disease with fibrosis. As mucin 1 is reported to regulate inflammation in other tissues and the immune system by suppressing the NLRP3 inflammasome and TLR signaling, these topics should be the focus of future studies of renal injury.
Key points.
Mucin1 membrane trafficking insures its apical localization, and galectin binding to mucin 1 glycans enhances crosslinking and surface stability of channels such as TRPV5.
A global frame-shift mutation in mucin 1 causes only kidney disease, while mice lacking mucin 1 are seemingly normal but more susceptible to stressors.
Mucin1 induced during ischemia-reperfusion injury of the kidney stabilizes HIF-1α and β-catenin, thereby enhancing the associated protective pathways.
Transient increases in mucin 1 enhance recovery from acute kidney injury, but prolonged increases lead to chronic kidney disease and fibrosis.
Mucin 1 is a cell surface barrier to bacteria and viruses but also limits inflammation by regulating the NLRP3 inflammasome and TLR signaling.
Acknowledgments
We would like to thank Drs. Sandra J. Gendler and Anthony J. Bleyer for careful reading of this manuscript. We want to thank Henry E. Mang for his technical expertise,
Financial support and sponsorship: This work was supported by the National Institutes of Health (K01 DK109038 to MMB, and P30-DK-079307). T.A.S. received grant from NIH (grant no. R01 DK099345)
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- 1.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 3.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehla K, Singh PK. MUC1: a novel metabolic master regulator. Biochim Biophys Acta. 1845:126–135. doi: 10.1016/j.bbcan.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilkens J, Buijs F. Biosynthesis of MAM-6, an epithelial sialomucin. Evidence for involvement of a rare proteolytic cleavage step in the endoplasmic reticulum. J Biol Chem. 1988;263:4215–4222. [PubMed] [Google Scholar]
- 6.Arcasoy SM, Latoche J, Gondor M, Watkins SC, Henderson RA, Hughey R, Finn OJ, Pilewski JM. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am J Respir Cell Mol Biol. 1997;17:422–435. doi: 10.1165/ajrcmb.17.4.2714. [DOI] [PubMed] [Google Scholar]
- 7.McGuckin MA, Every AL, Skene CD, Linden SK, Chionh YT, Swierczak A, McAuley J, Harbour S, Kaparakis M, Ferrero R, et al. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology. 2007;133:1210–1218. doi: 10.1053/j.gastro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L809–815. doi: 10.1152/ajplung.00385.2003. [DOI] [PubMed] [Google Scholar]
- 9*.McAuley JL, Corcilius L, Tan HX, Payne RJ, McGuckin MA, Brown LE. The cell surface mucin MUC1 limits the severity of influenza A virus infection. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.16. The authors report that MUC1, but not MUC13 or MUC16, protects cells from influenza binding, and increased MUC1 levels or synthetic MUC1 glycopeptides blocks infection. Muc 1 knockout mice exhibit higher morbity and a greater inflammatory response than control mice which is similar to earlier studies by the McGuckin lab with H. pylori infection of stomach (see Ref 66) [DOI] [PubMed] [Google Scholar]
- 10.Poland PA, Rondanino C, Kinlough CL, Heimburg-Molinaro J, Arthur CM, Stowell SR, Smith DF, Hughey RP. Identification and characterization of endogenous galectins expressed in Madin Darby canine kidney cells. J Biol Chem. 2011;286:6780–6790. doi: 10.1074/jbc.M110.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Poland PA, Kinlough CL, Hughey RP. Cloning, expression, and purification of galectins for in vitro studies. Methods Mol Biol. 2015;1207:37–49. doi: 10.1007/978-1-4939-1396-1_2. An earlier publication (Ref 10) reported that MUC1 large subunit interacted best with Gal-3 and Gal-9 while the small subunit interacted best with Gal-3. In this study, a chimera carrying MUC1 repeats with the apical targeting signal interacted best with Gal-9, which is essential in MDCK cells forming polarized monolayers. The functional significance of MUC1 binding Gal-3 is highlighted in Ref 37. [DOI] [PubMed] [Google Scholar]
- 12.Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. J Cell Sci. 2015;128:2213–2219. doi: 10.1242/jcs.151159. [DOI] [PubMed] [Google Scholar]
- 13.Kinlough CL, McMahan RJ, Poland PA, Bruns JB, Harkleroad KL, Stremple RJ, Kashlan OB, Weixel KM, Weisz OA, Hughey RP. Recycling of MUC1 is dependent on its palmitoylation. J Biol Chem. 2006;281:12112–12122. doi: 10.1074/jbc.M512996200. [DOI] [PubMed] [Google Scholar]
- 14.Kinlough CL, Poland PA, Bruns JB, Harkleroad KL, Hughey RP. MUC1 membrane trafficking is modulated by multiple interactions. J Biol Chem. 2004;279:53071–53077. doi: 10.1074/jbc.M409360200. [DOI] [PubMed] [Google Scholar]
- 15.Bouillez A, Gnemmi V, Gaudelot K, Hemon B, Ringot B, Pottier N, Glowacki F, Butruille C, Cauffiez C, Hamdane M, et al. MUC1-C nuclear localization drives invasiveness of renal cancer cells through a sheddase/gamma secretase dependent pathway. Oncotarget. 2014;5:754–763. doi: 10.18632/oncotarget.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, Kufe D. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 17.Brugger W, Buhring HJ, Grunebach F, Vogel W, Kaul S, Muller R, Brummendorf TH, Ziegler BL, Rappold I, Brossart P, et al. Expression of MUC-1 epitopes on normal bone marrow: implications for the detection of micrometastatic tumor cells. J Clin Oncol. 1999;17:1535–1544. doi: 10.1200/JCO.1999.17.5.1535. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal B, Krantz MJ, Parker J, Longenecker BM. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res. 1998;58:4079–4081. [PubMed] [Google Scholar]
- 19.Leong CF, Raudhawati O, Cheong SK, Sivagengei K, Noor Hamidah H. Epithelial membrane antigen (EMA) or MUC1 expression in monocytes and monoblasts. Pathology. 2003;35:422–427. doi: 10.1080/00313020310001602576. [DOI] [PubMed] [Google Scholar]
- 20.Wykes M, MacDonald KP, Tran M, Quin RJ, Xing PX, Gendler SJ, Hart DN, McGuckin MA. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. J Leukoc Biol. 2002;72:692–701. [PubMed] [Google Scholar]
- 21.Ceriani RL, Chan CM, Baratta FS, Ozzello L, DeRosa CM, Habif DV. Levels of expression of breast epithelial mucin detected by monoclonal antibody BrE-3 in breast-cancer prognosis. Int J Cancer. 1992;51:343–354. doi: 10.1002/ijc.2910510303. [DOI] [PubMed] [Google Scholar]
- 22*.Liu XuF, Zhao F, An H, Feng G. Prognostic Significance of Mucin Antigen MUC1 in Various Human Epithelial Cancers: A Meta-Analysis. Medicine (Baltimore) 2015;94:e2286. doi: 10.1097/MD.0000000000002286. MUC1 expression has been associated with poor survival in numerous studies including Refs. 21 and 23. This current analysis of 3425 patients covering 23 studies continues to confirm that positive MUC1 staining is a negative predictor of overall survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270:30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 24.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, Hwang SI, Lee YY, Gendler SJ, Mukherjee P. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 2011;71:4432–4442. doi: 10.1158/0008-5472.CAN-10-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kardon R, Price RE, Julian J, Lagow E, Tseng SC, Gendler SJ, Carson DD. Bacterial conjunctivitis in Muc1 null mice. Invest Ophthalmol Vis Sci. 1999;40:1328–1335. [PubMed] [Google Scholar]
- 27.DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, Gendler S, Hilkens J, Carson DD. MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol. 1999;45:127–158. doi: 10.1016/s0165-0378(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 28*.Gomez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsisinduced AKI. Nat Rev Nephrol. 2017;13:143–151. doi: 10.1038/nrneph.2016.186. A novel discussion on the role of resistance and tolerance including metabolic adaptations, in the host defense to infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol. 2012;303:F1487–1494. doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Jackson EK, Menshikova EV, Mi Z, Verrier JD, Bansal R, Janesko-Feldman K, Jackson TC, Kochanek PM. Renal 2′,3′-Cyclic Nucleotide 3′-Phosphodiesterase Is an Important Determinant of AKI Severity after Ischemia-Reperfusion. J Am Soc Nephrol. 2016;27:2069–2081. doi: 10.1681/ASN.2015040397. The authors describe a unique two-kidney, hanging-weight model of renal bilateral ischemia that provides highly reproducible outcomes by occluding only the artery and avoids stasis of the blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Pastor-Soler NM, Sutton TA, Mang HE, Kinlough CL, Gendler SJ, Madsen CS, Bastacky SI, Ho J, Al-Bataineh MM, Hallows KR, et al. Muc1 is protective during kidney ischemia- reperfusion injury. Am J Physiol Renal Physiol. 2015;308:F1452–1462. doi: 10.1152/ajprenal.00066.2015. This study was the first to show that Muc1 is protective in a mouse model of acute kidney injury. Using Muc1 knockout and control mice, the authors reported that induction of Muc1 after ischemic injury stabilizes HIF-1α, enhances the HIF-1 protective pathway and prevents metabolic stress. This was also the first report that Muc1 is induced in the proximal tubule after injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Al-Bataineh MM, Kinlough CL, Poland PA, Pastor-Soler NM, Sutton TA, Mang HE, Bastacky SI, Gendler SJ, Madsen CS, Singh S, et al. Muc1 enhances the beta-catenin protective pathway during ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2016;310:F569–579. doi: 10.1152/ajprenal.00520.2015. This study was a follow-up to Ref. 31 and reported that Muc1 stabilizes β-catenin after ischemic injury and enhances the β-catenin protective pathway. Using Muc1 knockout and control mice, the authors found that β-catenin does not enter the nucleus in the absence of Muc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Kirby A, Gnirke A, Jaffe DB, Baresova V, Pochet N, Blumenstiel B, Ye C, Aird D, Stevens C, Robinson JT, et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet. 2013;45:299–303. doi: 10.1038/ng.2543. This manuscript presents the initial identification of the genetic cause of Medullary Cystic Kidney Disease type 1 as a frameshift mutation in the MUC1 gene. The mutated protein has a high pI and is not secreted but rather deposited intracellularly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int. 88:676–683. doi: 10.1038/ki.2015.28. Clinicians and scientists with expertise in ADTKD met and developed consensus guidelines on the nomenclature, diagnosis and management of ADTKD, including ADTKD due to MUC1 mutations. [DOI] [PubMed] [Google Scholar]
- 35*.Bleyer AJ, Kidd K, Zivna M, Kmoch S. Autosomal Dominant Tubulointerstitial Kidney Disease. Adv Chronic Kidney Dis. 2017;24:86–93. doi: 10.1053/j.ackd.2016.11.012. The authors provide a comparison between MUC1 Kidney Disease (MKD) and Uromodulin Kidney Disease (UKD), previously known as Medullary Cystic Kidney Disease type 1 and 2, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Bleyer AJ, Kmoch S, Antignac C, Robins V, Kidd K, Kelsoe JR, Hladik G, Klemmer P, Knohl SJ, Scheinman SJ, et al. Variable clinical presentation of an MUC1 mutation causing medullary cystic kidney disease type 1. Clin J Am Soc Nephrol. 2014;9:527–535. doi: 10.2215/CJN.06380613. The authors provide a detailed description of Medullary Cystic Kidney Disease type 1 now known as MUC1 Kidney Disease. The variable age of development of end-stage kidney disease (between 30 and 70) is emphasized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Nie M, Bal MS, Yang Z, Liu J, Rivera C, Wenzel A, Beck BB, Sakhaee K, Marciano DK, Wolf MT. Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients. J Am Soc Nephrol. 2016;27:3447–3458. doi: 10.1681/ASN.2015101100. This is the first report that patients with hypercalciuric nephrolithiasis have reduced levels of urinary MuC1. Further studies in cultured HEK cells revealed that MUC1 is cross-linked to the renal calcium channel TRPV5 in a lattice with Gal-3, and that MUC1 expression reduces TRPV5 endocytosis and thereby increases TRPV5 activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 39.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinlough CL, Poland PA, Gendler SJ, Mattila PE, Mo D, Weisz OA, Hughey RP. Coreglycosylated mucin-like repeats from MUC1 are an apical targeting signal. J Biol Chem. 2011;286:39072–39081. doi: 10.1074/jbc.M111.289504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, Zhang B, Mehla K, Brown RB, Caffrey T, et al. MUC1 mucin stabilizes and activates hypoxia- inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci U S A. 2012;109:13787–13792. doi: 10.1073/pnas.1203339109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HH, Afdhal NH, Gendler SJ, Wang DQ. Lack of the intestinal Muc1 mucin impairs cholesterol uptake and absorption but not fatty acid uptake in Muc1-/- mice. Am J Physiol Gastrointest Liver Physiol. 2004;287:G547–554. doi: 10.1152/ajpgi.00097.2004. [DOI] [PubMed] [Google Scholar]
- 44.Nath S, Daneshvar K, Roy LD, Grover P, Kidiyoor A, Mosley L, Sahraei M, Mukherjee P. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis. 2013;2:e51. doi: 10.1038/oncsis.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol. 2015;26:2669–2677. doi: 10.1681/ASN.2014111067. The authors carried out RNA-seq on 14 dissected rat renal tubule segments and glomeruli, and provide an incredibly extensive and highly useful data base of gene expression (transcriptome) across the nephron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braga VM, Pemberton LF, Duhig T, Gendler SJ. Spatial and temporal expression of an epithelial mucin, Muc-1, during mouse development. Development. 1992;115:427–437. doi: 10.1242/dev.115.2.427. [DOI] [PubMed] [Google Scholar]
- 47.Howie AJ. Epithelial membrane antigen in normal and proteinuric glomeruli and in damaged proximal tubules. J Pathol. 1986;148:55–60. doi: 10.1002/path.1711480109. [DOI] [PubMed] [Google Scholar]
- 48.Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 49.Sutton TA, Wilkinson J, Mang HE, Knipe NL, Plotkin Z, Hosein M, Zak K, Wittenborn J, Dagher PC. p53 regulates renal expression of HIF-1{alpha} and pVHL under physiological conditions and after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2008;295:F1666–1677. doi: 10.1152/ajprenal.90304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haase VH. Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol. 2013;24:537–541. doi: 10.1681/ASN.2012080855. [DOI] [PubMed] [Google Scholar]
- 51.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fahling M, Mathia S, Paliege A, Koesters R, Mrowka R, Peters H, Persson PB, Neumayer HH, Bachmann S, Rosenberger C. Tubular von Hippel-Lindau knockout protects against rhabdomyolysis-induced AKI. J Am Soc Nephrol. 2013;24:1806–1819. doi: 10.1681/ASN.2013030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schley G, Klanke B, Schodel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt KU, et al. Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol. 2011;22:2004–2015. doi: 10.1681/ASN.2010121249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haase VH. Hypoxia-inducible factor signaling in the development of kidney fibrosis. Fibrogenesis Tissue Repair. 2012;5:S16. doi: 10.1186/1755-1536-5-S1-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y. Sustained Activation of Wnt/beta-Catenin Signaling Drives AKI to CKD Progression. J Am Soc Nephrol. 2016;27:1727–1740. doi: 10.1681/ASN.2015040449. The authors were the first to show that β-catenin is protective during acute kidney injury in mice (Ref. 53). In this current paper they show that while transient induction of β-catenin is protective (after 20 min ischemia), more severe injury (30 min) that prolongs β-catenin induction drives the progression to chronic kidney injury with fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58**.Gibier JB, Hemon B, Fanchon M, Gaudelot K, Pottier N, Ringot B, Van Seuningen I, Glowacki F, Cauffiez C, Blum D, et al. Dual role of MUC1 mucin in kidney ischemia- reperfusion injury: Nephroprotector in early phase, but pro-fibrotic in late phase. Biochim Biophys Acta. 2017 doi: 10.1016/j.bbadis.2017.03.023. Using Muc1 KO and control mice, the authors report that Muc1 was protective after unilateral ischemic injury (25 min) for the first week of recovery, but Muc1 induction was then associated with progression to fibrosis at 28 d recovery. MUC1 was also found in injured proximal tubules from patient kidney biopsies associated with markers of epithelial-to- mesenchymal transition. [DOI] [PubMed] [Google Scholar]
- 59.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grande MT, Sanchez-Laorden B, Lopez-Blau C, De Frutos CA, Boutet A, Arevalo M, Rowe RG, Weiss SJ, Lopez-Novoa JM, Nieto MA. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 61.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT. The role of HIF-1 alpha in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem. 2003;278:40296–40304. doi: 10.1074/jbc.M302560200. [DOI] [PubMed] [Google Scholar]
- 63.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 64.Linden SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin TH, Sutton P, McGuckin MA. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009;5:e1000617. doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Kato K, Lillehoj EP, Kim KC. Pseudomonas aeruginosa stimulates tyrosine phosphorylation of and TLR5 association with the MUC1 cytoplasmic tail through EGFR activation. Inflamm Res. 2016;65:225–233. doi: 10.1007/s00011-015-0908-8. Earlier publications by this group showed that MUC1 limits inflammation in the lung during infection by Pseudomonas aeruginosa (PA) by suppression of Toll-like receptor signaling. The current paper elucidates the mechanism involving both macrophages and lung epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Ng GZ, Menheniott TR, Every AL, Stent A, Judd LM, Chionh YT, Dhar P, Komen JC, Giraud AS, Wang TC, et al. The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome. Gut. 2016;65:1087–1099. doi: 10.1136/gutjnl-2014-307175. The authors had previously published that mucin 1 is protective against H. pylori infection of the stomach by acting as a barrier and releasable decoy. In long term infections, they now show that Muc1 is critical for preventing severe gastritis, and for survival, because Muc1 expression in immune cells suppresses inflammation by regulating the NF-KB pathway and reducing the NLRP3 inflammasome activity. [DOI] [PubMed] [Google Scholar]
- 67.Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T, Vasir B, Avigan D, Kharbanda S, Kufe D. Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res. 2009;69:5133–5141. doi: 10.1158/0008-5472.CAN-09-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S, Kufe D. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat Cell Biol. 2007;9:1419–1427. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]


