Abstract
Purpose
Pneumonia is common and more severe in human immunodeficiency virus (HIV)-infected patients. Alcohol consumption in pneumonia patients without HIV is associated with excess mortality and morbidity. However, studies are lacking on the impact of alcohol on pneumonia and HIV. Our goal was to determine if alcohol use was an independent risk factor for pneumonia severity in HIV-infected patients.
Methods
Secondary analysis of prospective cohort study data evaluating early bronchoscopy for pneumonia diagnosis in HIV patients between 2007 and 2011 was conducted. We defined AUDs using an alcohol use disorder identification test (AUDIT) score as follows: ≥8 indicates hazardous drinking and ≥14 indicates dependence. We quantified pneumonia severity using the pneumonia severity index (PSI). Multivariable linear regression was used to investigate the independent association between alcohol and pneumonia severity.
Results
A total of 196 HIV+ individuals comprised our cohort. Most cohort subjects were middle-aged African American men. Most subjects (70 %) reported not taking antiretroviral therapy. The overall prevalence of hazardous drinking was 24 % in our cohort (48/196) with 10 % (19/196) meeting the criteria for alcohol dependence. Alcohol consumption was significantly associated with pneumonia severity (r = 0.25, p < 0.001). Hazardous drinking (β-coefficient 10.12, 95 % CI 2.95–17.29, p = 0.006) and alcohol dependence (β-coefficient 12.89, 95 % CI 2.59–23.18, p = 0.014) were independent risk factors for pneumonia severity. Reported homelessness and men who have sex with men (MSM) status remained independent risk factors for more severe pneumonia after adjustment for the effects of alcohol.
Conclusions
In a cohort of HIV patients with pneumonia, presence of an AUD was an independent risk factor for pneumonia severity. Homelessness and MSM status were associated with greater pneumonia severity in AUD patients.
Keywords: Pneumonia severity, Alcohol use disorder, HIV/AIDS, Respiratory illness
Introduction
Approximately 1.2 million people in the United States are currently living with the human immunodeficiency virus (HIV) [1, 2]. HIV prevalence continues to rise with over 50,000 new HIV infections reported annually [1, 2]. Despite the widespread introduction of highly active antiretroviral therapy (HAART), people living with HIV (PLWH) remain susceptible to opportunistic infections (OIs), particularly due to respiratory pathogens [3]. HIV infection is associated with a 25-fold increase in lower respiratory tract infections with associated mortality rates ranging from 10 to 30 % [4].
Alcohol use disorders (AUD) are also highly prevalent in the United States, with 14 % of US adults meeting diagnostic criteria for alcohol abuse or dependence [5]. The frequency of harmful alcohol use is higher in the HIV+ population [6, 7]. Hazardous alcohol use negatively impacts HIV progression as evidenced by higher viral loads, lower CD4 counts, and greater comorbidity in HIV patients with AUDs [7, 8]. Alcohol use in the absence of HIV contributes to pneumonia incidence, severity, and mortality and prolongs hospital stays increasing costs [9, 10]. However, no studies specifically evaluate the impact of AUD on the severity or outcomes of pneumonia infections in PLWH. The goal of this study was to determine the relationship between AUD and HIV-associated pneumonia severity.
Materials and Methods
Study Design
HIV patients admitted to the Interim Louisiana State University Public Hospital in New Orleans between 2007 and 2011 with a presumptive diagnosis of pneumonia were approached to participate in a prospective, observational study of pneumonia diagnosis. Presumptive pneumonia diagnosis required (1) clinical suspicion of lower respiratory tract infection by the primary healthcare provider; and (2) new or changing radiographic infiltrate or unexplained hypoxia in the absence of an infiltrate (defined by partial pressure of oxygen <70 mm Hg or oxygen saturation <90 % or a drop in oxygen saturation >4 % with exertion). Clinical data were obtained by interview and medical record review. The Louisiana State University Health Sciences Center Institutional Review Board (IRB #5585) approved the study with informed consent obtained upon study enrollment.
Alcohol Use Assessment
The alcohol use disorder identification test (AUDIT) questionnaire [11], previously validated in PLWH [12], was administered by interview at the time of enrollment. All subjects with available alcohol use data were included in the current analysis. Based upon published thresholds, an AUDIT score ≥8 was defined as hazardous drinking [12] and a score ≥14 as alcohol dependence [13]. AUDIT-C scores, which estimate consumption (quantity–frequency), were also used to identify active hazardous drinking (score ≥4 for men; ≥3 for women) [14, 15]. Binge drinking was defined by an AUDIT-3 (question 3 of the AUDIT) score ≥2 [16].
Pneumonia Severity
The pneumonia severity index (PSI) has been extensively validated, including in HIV+ populations with risk groups predictive of pneumonia-related mortality [17–22]. All variables contributing to the PSI were obtained at the time of presentation. There were no missing data. The PSI was used as a continuous variable in models exploring the association between alcohol use and pneumonia severity. Subjects were categorized according to accepted PSI risk strata (category 1: PSI >51, category 2: PSI 51–70, category 3: PSI 71–90 and category 4: PSI >90) in order to determine the distribution of risk in our cohort.
Statistical Analysis
Descriptive statistics included means for continuous variables, medians for non-parametric data, and proportions for categorical variables. Bivariate analysis was performed using Student’s t tests for means, Wilcoxon rank-sum testing for non-parametric data, and Chi-square testing with Fischer’s exact tests for samples with <5 observations. Multivariable linear regression was used to determine whether AUDIT score was an independent predictor of PSI score. Two additional linear regression models were constructed using the presence of hazardous drinking (AUDIT score ≥8) and alcohol dependence (AUDIT score ≥14) as dichotomous variables. Models were adjusted for the following potential confounders chosen a priori based on clinical importance: homelessness, reported income of $0.00, current smoking status (yes/no), reported sexual preference as a man who has sex with men (MSM), most recent CD4 count, and self-report of HAART adherence. Given the clinical importance of the variables in potentially explaining the relationships between alcohol and pneumonia severity, all potential confounders were retained in the final model regardless of statistical significance. We did not adjust for age and sex, both significant variables in the univariate analysis as they are variables included in the PSI score. Sensitivity analyses were performed restricting the cohort to individuals meeting the criteria for dependence to determine whether similar relationships were observed with a more severe alcohol phenotype and to only variables with significant p values (<0.2) in the univariate analyses (smoking status, homelessness, MSM status, CD4 count, HAART, and/or prophylaxis use, race). All statistical analyses were performed using STATA software (STATACorp. Version 13.0, College Station, TX) and a p value <0.05 was considered statistically significant. R-squared values were used to assess model fit for each of the models.
Results
Cohort Characteristics
In total, 196 HIV+ subjects hospitalized with pneumonia between the years of 2007 and 2011 were included (Table 1). One subject was excluded from the analysis due to missing alcohol use data. The cohort was predominantly male (71 %) and self-identified as African American or black (87 %). Most subjects were middle-aged (mean age 44 years (SD 9). The majority of subjects reported a history of incarceration (71 %) and half reported having been homeless (51 %). Half of the cohort reported no annual income (50 %) with a median annual income of $8400 [Interquartile range (IQR) $7200–10,800] for subjects receiving an income. Smoking was common, as has been reported for PLWH [23]. Patient adherence to medications was low and only 30 % of subjects were receiving HAART. Twenty-eight percent of subjects were taking OI prophylactic medications. Subjects were severely immunosuppressed [median CD4 count of 64 cells/mm3 (IQR 16–140 cells/mm3)] with average viral loads of 31,300 copies [IQR 893–139,809 copies]. PSI scores were low with 70 % (n = 138) of subjects meeting PSI risk category 1 or 2 criteria (Table 2). Subjects with higher severity scores were significantly older, more often men and more likely to report a history of MSM. Three subjects died during hospitalization with an average PSI score of 52 (SD 20). HIV disease severity markers including CD4 count and use of ART were not significantly associated with PSI scores. Respiratory specimens identified bacterial pathogens in 121 subjects (61 %) and fungal pathogens in 89 subjects (45 %) including Pneumocystis Jiroveci in 53 (27 %) subjects.
Table 1.
Factors associated with presence of an alcohol use disorder (AUD) at hospital admission in HIV positive patients presenting with pneumonia
| Overall cohort (n = 196) | AUD (n = 48, 24 %) | No AUD (n = 148, 76 %) | p value* | |
|---|---|---|---|---|
| Male sex, % | 71 | 85 | 67 | 0.008 |
| Age (years), mean (SD**) | 44 (9) | 47 (7) | 44 (9) | 0.005 |
| Raceb, % | ||||
| Black | 87 | 83 | 89 | |
| White | 12 | 11 | 11 | 0.98 |
| No answer | 2 | 6 | 0 | |
| History of incarceration, % | 71 | 73 | 70 | 0.73 |
| History of homelessness, % | 51 | 52 | 50 | 0.80 |
| Man who has sex with men, % | 26 | 26 | 25 | 0.94 |
| No annual income, % | 50 | 44 | 53 | 0.28 |
| Median annual income [IQRa] | $8400 [$7200–10,800] | $9300 [$7500–12,000] | $8400 [$7200–9600] | 0.14 |
| Current smoker, % | 48 | 54 | 45 | 0.02 |
| Highly active antiretroviral use, % | 30 | 29 | 30 | 0.87 |
| Any prehospital infectious prophylaxis use, % | 28 | 36 | 25 | 0.14 |
| Median CD4 count [IQR] | 61 [16–140] | 67 [17–127] | 63 [16–149] | 0.84 |
Bold values indicate statistically significant differences
Comparison of alcohol use disorder (AUD) to no alcohol use disorder (AUD)
SD standard deviation
IQR interquartile range
Reported values may not add to 100 due to rounding
Table 2.
Pneumonia severity index class and subject characteristics
| Risk group I (n = 79, 40 %) |
Risk group II (n = 59, 30 %) |
Risk group III (n = 40, 21 %) |
Risk group IV (n = 18, 9 %) |
p valueb | |
|---|---|---|---|---|---|
| Age (mean, SD*) | 41 (7) | 42 (8) | 51 (6) | 50 (8) | <0.001 |
| Male sex, % | 61 | 61 | 93 | 89 | <0.001 |
| African American or black race, % | 92 | 90 | 87 | 76 | 0.09 |
| History of incarceration % | 68 | 71 | 68 | 76 | 0.77 |
| History of homelessness, % | 43 | 48 | 53 | 65 | 0.11 |
| Man who has sex with men, % | 22 | 22 | 33 | 41 | 0.04 |
| No annual income, % | 56 | 52 | 53 | 39 | 0.32 |
| Median CD4 count, [IQRa] | 63 [13–130] | 92 [41–165] | 46 [11–123] | 50 [14–140] | 0.24 |
| Highly active antiretroviral use, % | 28 | 27 | 33 | 50 | 0.13 |
Bold values indicate statistically significant differences
SD standard deviation
IQR interquartile range
ANOVA comparison across groups
Alcohol Use Disorder Profiles
One hundred and forty-nine (76 %) subjects reported any history of lifetime alcohol consumption with 107 (55 %) reporting alcohol use prior to hospital admission. Forty-three percent of those reporting alcohol use identified beer as their primary alcohol type with 25 % of subject reporting use of more than one alcohol type and 24 % primarily drinking wine.
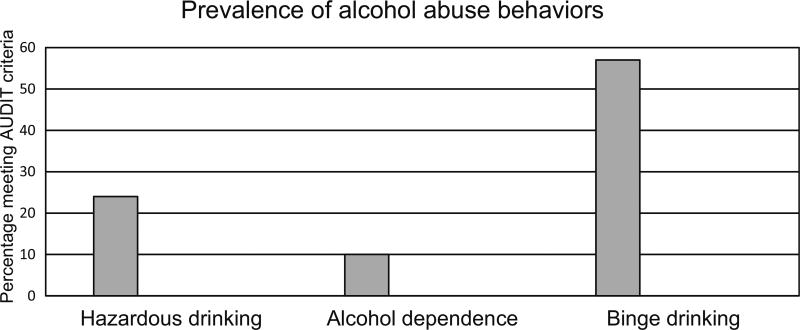
The varying approaches characterizing alcohol use yielded remarkably different results (Fig. 1). The overall prevalence of hazardous drinking (AUDIT score ≥8) was 24 % in our cohort (48/196 subjects) with 10 % (19/196 subjects) meeting criteria for alcohol dependence (AUDIT score ≥14). In contrast, AUDIT-C scores (quantifies recent consumption) indicated that 45 % of men (63/139) and 33 % of women (19/57) screened positive for potentially hazardous active drinking. Using question 3 of the AUDIT alone, 112 subjects (57 %) were identified as binge drinkers. Subjects with an AUD (hazardous drinking or dependence) identified on full AUDIT screening (Table 3) were more often men (85 vs. 67 %, p = 0.008) and older (47 vs. 44 years, p = 0.005). Current smoking was more common in subjects with AUDs (54 vs. 45 %, p = 0.02). Alcohol-dependent subjects (Supplementary Table 1) were mostly men (95 vs. 67 in non-AUD subjects, p = 0.02). Admission oxygen saturations (SpO2) were significantly lower for subjects with hazardous drinking (SpO2 94 vs. 96 %, p = 0.02) compared with non-AUD subjects. Mean admission serum sodium levels were lower for subjects with hazardous drinking (133 vs. 135 mEq/L non-AUD subjects, p = 0.001). Otherwise, there were no significant differences in admission physiologic values or markers of HIV disease management between subjects with and without AUDs.
Fig. 1.
Prevalence of alcohol abuse behaviors in a HIV positive cohort. Alcohol abuse behaviors were frequently reported with 24 % meeting criteria for hazardous drinking, 10 % alcohol dependence, and 57 % reporting binge drinking
Table 3.
Multivariate analysis of hazardous drinking or alcohol dependence and pneumonia severity index score
| β-coefficient | 95 % Confidence interval | p value | |
|---|---|---|---|
| Hazardous drinking | 10.12 | 2.95–17.29 | 0.006 |
| Homelessness | 12.06 | 5.52–18.61 | <0.001 |
| MSM* status | 10.28 | 3.46–17.10 | 0.003 |
| Smoking | −5.62 | −12.23–1.00 | 0.096 |
| No annual income | −0.63 | −6.59–5.32 | 0.834 |
| Recent CD4 count | −0.001 | −0.03–0.03 | 0.971 |
| HAARTa | 3.52 | −3.31–10.35 | 0.311 |
| Alcohol dependence | 12.89 | 2.59–23.18 | 0.014 |
| Homelessness | 11.06 | 4.54–17.59 | 0.001 |
| MSM | 10.27 | 3.45–17.10 | 0.003 |
| Smoking | −4.49 | −10.93–1.94 | 0.170 |
| No annual income | −1.45 | −7.46–4.55 | 0.633 |
| Recent CD4 count | 0.001 | −0.03–0.03 | 0.967 |
| HAART | 2.37 | −4.67–9.41 | 0.508 |
Bold values indicate statistically significant independent predictors of PSI score ≥ 8
MSM man who has sex with men
HAART highly active antiretroviral therapy
Alcohol Use Disorder and Pneumonia Severity
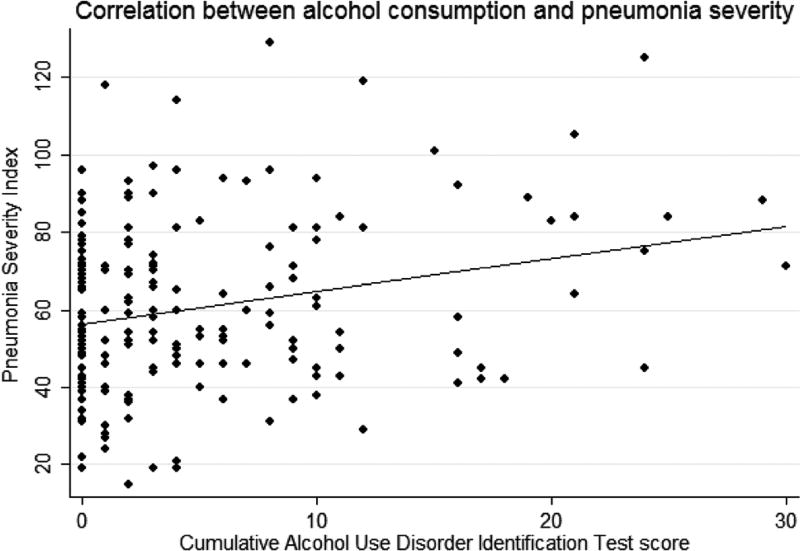
There was a significant relationship (r = 0.25, p < 0.001) between AUDIT and PSI scores in univariate analysis (Fig. 2). In multivariable analyses (Supplementary Table 1), presence of an AUD was independently associated with mean PSI scores with an average increase in mean PSI score of 10 points for subjects with hazardous drinking compared with non-hazardous drinking (β-coefficient 10.12, 95 % CI 2.95–17.29, p = 0.006, adjusted model R2 = 0.14). Similar results were observed restricting the cohort to patients with alcohol dependence with an average increase in PSI of 13 points for subjects with alcohol dependence compared with no dependence (adjusted model R2 = 0.14). PSI scores were significantly higher for subjects with homelessness and the presence of hazardous or dependent drinking. Reported homelessness and history of MSM status remained as independent risk factors for more severe pneumonia after adjustment for the effects of alcohol. However, there was no significant difference found between median PSI scores stratified by positive AUDIT-C scores (men: p = 0.14, women: p = 0.84), nor by binge drinking (p = 0.27). Restriction of the models to variables significant in univariate analyses changed the estimates minimally.
Fig. 2.
PSI scores as a function of AUDIT scores. Alcohol consumption was significantly correlated with pneumonia severity (r = 0.25, p < 0.001)
Discussion
In a cohort of PLWH, we found that one-quarter of subjects presenting to the hospital with pneumonia meet criteria for hazardous drinking, with up to 45 % of subjects meeting criteria for potentially harmful alcohol consumption, and 57 % meeting criteria for binge drinking. Presence of an AUD was significantly associated with other risky behaviors including tobacco abuse. Meeting criteria for an AUD at hospital admission was an independent risk factor for pneumonia severity with further increase in risk for subjects with concomitant AUD and homelessness. We failed to find significant associations between HIV disease markers and the presence of an AUD or greater pneumonia severity.
Prior studies report a high prevalence of AUDs in PLWH [12, 24, 25]. Reported at risk drinking ranges from 8 to 50 % of PLWH [12, 24–26], with our cohort falling around the midpoint. In addition to identifying a large proportion of patients with diagnostic criteria for an AUD using the full AUDIT, we identified an even larger proportion of individuals with potentially hazardous ongoing drinking beyond those with defined AUDs, as evidenced by excessive consumption on the AUDIT-C or AUDIT-3 binge drinking. These data highlight the fact that PLWH presenting with pneumonia represent a high-risk group for identification of alcohol misuse, and alcohol use screening may be beneficial in this patient demographic. We found a significant association between the presence of an AUD and ongoing smoking supporting prior studies suggesting that high-risk behaviors track together in PLWH [23, 27]. Studies are needed to understand how uniform screening strategies may identify AUDs or high-risk behaviors early in PLWH presenting to the hospital, and if early identification may lead to earlier treatment or more sustained remission from alcohol misuse.
Our study is the first, to our knowledge, to report an association between alcohol use and pneumonia severity in PLWH. Studies of HIV-undetermined cohorts identified greater pneumonia severity in patients with AUD [9]. Presence of an AUD increased pneumonia-related morbidity with excess mortality, hospitalizations, and healthcare utilizations attributed to prehospital alcohol use [28]. A single study of HIV-infected individuals admitted to the intensive care unit, however, found no association between ICU illness severity and history of alcohol abuse although the cohort was not restricted to pneumonia [29]. While our study suggests alcohol is a risk factor for more severe pneumonia, most subjects in our study represented lower pneumonia risk categories and there was little pneumonia-associated morbidity. Therefore, our sample was inadequately powered to investigate the impact of alcohol on pneumonia-related mortality and morbidity in PLWH. We failed to identify associations between HIV control and pneumonia severity or presence of AUD, but these effects were likely masked by the lack of adequate HIV care in our cohort. Further studies in a higher pneumonia risk severity cohort are needed to understand if alcohol is an independent risk factor for worse patient outcomes in PLWH.
Alcohol is a known contributor to immune dysregulation. Alcohol alters NF-κB and TNF-α signaling contributing to immunosuppression and host susceptibility early in the course of sepsis [10, 30–33]. Studies in murine models of chronic alcohol abuse demonstrate neutrophil impairments including decreased response to chemotactic signaling and reduced bactericidal activity with abnormal phagocytosis, superoxide generation, and degranulation [34, 35]. Bronchoalveolar lavage fluid sampling of chronic alcohol abusers in the absence of HIV demonstrates significantly different inflammatory profiles compared with non-alcoholic controls [36, 37]. Alcohol-related immunosuppression may have contributed to the increased pneumonia severity observed in our cohort; however, no data on the host inflammatory response were available to test this assumption.
Interestingly, although pneumonia severity scores were low on average in our cohort, falling into risk category 1 or 2, all patients were hospitalized for their pneumonia. Validation studies of the PSI in PLWH suggest it may be clinically appropriate to treat patients risk categories 1 and 2 with outpatient therapy [17]. However, our results show that, despite low clinical risk scores, healthcare providers opt for hospitalization of PLWH and presumed pneumonia. Studies of non-HIV positive patients suggest that sociodemographic factors play an important role in admission decisions for pneumonia patients with low-risk patients accounting or 35 % of excess pneumonia costs. Presence of an alcohol use disorder and/or homelessness was an important predictor of admission in this cohort [38]. Similarly, subjects in our cohort were frequently economically disadvantaged and experienced significant psychosocial stressors including high rates of incarceration and homelessness. This social instability likely contributed to the high hospitalization frequency in otherwise low-risk individuals observed in our study. Alternatively, concern over OIs may have driven hospitalization as the cohort was severely immunosuppressed. Studies are needed to better understand healthcare utilization patterns and provider biases toward inpatient care for PLWH presenting with respiratory illness.
Reinforcing the importance of psychosocial and behavioral factors in the health consequences of harmful alcohol use, we found disparate results depending on our alcohol use methodology. Neither the AUDIT-C nor AUDIT-3, which are reflective of recent consumption and binge drinking, were associated with pneumonia severity scores despite the observed association with pneumonia severity using the full AUDIT which accounts for the psychosocial consequences of harmful alcohol use. This suggests that the sequela of excessive alcohol consumption may be as, if not more, important than the direct toxicological effect of ethanol.
There are a number of important limitations to our study. First, as mentioned previously, individuals presented with less severe disease that may have limited our inferences and underestimated the magnitude of the association between alcohol use and pneumonia severity. Second, the majority of subjects in our cohort had poorly controlled HIV and were not on HIV therapy at the time of hospitalization. The lack of variance in HIV control in our cohort likely limited our ability to explore the associations between AUDs and HIV disease progression or HIV control on subsequent pneumonia severity. Third, while our results encompass a large cohort of PLWH collected over a 5-year time period, our results reflect a single center and may not generalize to the larger population of PLWH. Finally, given the observational nature of our study, there may be unmeasured confounders contributing to the observed relationship between alcohol use and pneumonia severity not accounted for in our study. In particular, although we attempted to obtain data regarding baseline lung disease by patient report, it is possible that patients were unaware or undiagnosed with existing lung disease such as chronic obstructive lung disease that may have contributed to their pneumonia risk. Future studies should attempt to obtain objective measures of lung function in addition to patient report.
Conclusions
AUDs were common in PLWH hospitalized for pneumonia with 24 % of subjects meeting criteria for an AUD, up to 45 % meeting criteria for potentially excessive active consumption, and 57 % meeting criteria for binge drinking. Presence of an AUD was an independent risk factor for more severe pneumonia with greater risk evident in subjects with concomitant AUD and homelessness. Further studies are needed to understand whether early identification of AUDs in PLWH presenting to the hospital results in greater AUD treatment and to determine whether AUDs are associated with pneumonia-related morbidity or mortality.
Supplementary Material
Acknowledgments
SJ, QA, and DW conceived of the study and generated the study design. SJ completed the data analysis. SJ, QA, DW, CH, and SJ contributed to drafting and revision of the manuscript.
Funding This work was funded by the following National Institutes of Health Grants: P60 AA009803, R24AA19661, PO1 HL076100, and U54 GM104940 (LA CaTS Center).
Abbreviations
- AUD
Alcohol use disorder
- AUDIT
Alcohol use disorders identification test
- HAART
Highly active antiretroviral therapy
- HIV
Human immunodeficiency virus
- IQR
Interquartile range
- OI
Opportunistic infection
- PLWH
People living with HIV
- PSI
Pneumonia severity index
- SpO2
Oxygen saturation
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00408-016-9920-1) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflicts of interest The authors have no significant conflicts of interest to report.
References
- 1.Sullivan PS, Jones JS, Baral SD. The global north: HIV epidemiology in high-income countries. Curr Opin HIV AIDS. 2014;9(2):199–205. doi: 10.1097/COH.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 2.CDC, editor. Prevention CfDCa: today’s HIV/AIDS Epidemic. 2015. [Google Scholar]
- 3.Buchacz K, Baker RK, Palella FJ, Jr, Chmiel JS, Lichtenstein KA, Novak RM, Wood KC, Brooks JT Investigators H. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24(10):1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 4.Benito N, Moreno A, Miro JM, Torres A. Pulmonary infections in HIV-infected patients: an update in the 21st century. Eur Respir J. 2012;39(3):730–745. doi: 10.1183/09031936.00200210. [DOI] [PubMed] [Google Scholar]
- 5.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant KJ, Nelson S, Braithwaite RS, Roach D. Integrating HIV/AIDS and alcohol research. Alcohol Res Health. 2010;33(3):167–178. [PMC free article] [PubMed] [Google Scholar]
- 7.Justice A, Sullivan L, Fiellin D. Veterans aging cohort study project T: HIV/AIDS, comorbidity, and alcohol: can we make a difference? Alcohol Res Health. 2010;33(3):258–266. [PMC free article] [PubMed] [Google Scholar]
- 8.Pandrea I, Happel KI, Amedee AM, Bagby GJ, Nelson S. Alcohol’s role in HIV transmission and disease progression. Alcohol Res Health. 2010;33(3):203–218. [PMC free article] [PubMed] [Google Scholar]
- 9.Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2010;138(12):1789–1795. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S. Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abuse Rev. 2008;1(1):56–67. doi: 10.2174/1874473710801010056. [DOI] [PubMed] [Google Scholar]
- 11.Babor T, Higgins-Biddle JC, Saunders JB, Monteiro MG. In: The alcohol use disorder identification test: guidelines for use in primary care. WHO Publication, vol 01.6a, editor. World Health Organization; Geneva: 2001. [Google Scholar]
- 12.McGinnis KA, Justice AC, Kraemer KL, Saitz R, Bryant KJ, Fiellin DA. Comparing alcohol screening measures among HIV-infected and -uninfected men. Alcohol Clin Exp Res. 2013;37(3):435–442. doi: 10.1111/j.1530-0277.2012.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2013;37(Suppl 1):E253–E259. doi: 10.1111/j.1530-0277.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- 14.Bradley KA, McDonell MB, Bush K, Kivlahan DR, Diehr P, Fihn SD. The AUDIT alcohol consumption questions: reliability, validity, and responsiveness to change in older male primary care patients. Alcohol Clin Exp Res. 1998;22(8):1842–1849. doi: 10.1111/j.1530-0277.1998.tb03991.x. [DOI] [PubMed] [Google Scholar]
- 15.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory care quality improvement project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 16.Tuunanen M, Aalto M, Seppa K. Binge drinking and its detection among middle-aged men using AUDIT, AUDIT-C and AUDIT-3. Drug Alcohol Rev. 2007;26(3):295–299. doi: 10.1080/09595230701247756. [DOI] [PubMed] [Google Scholar]
- 17.Chew KW, Yen IH, Li JZ, Winston LG. Predictors of pneumonia severity in HIV-infected adults admitted to an Urban public hospital. AIDS Patient Care STDS. 2011;25(5):273–277. doi: 10.1089/apc.2010.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curran A, Falco V, Crespo M, Martinez X, Ribera E, Villar del Saz S, Imaz A, Coma E, Ferrer A, Pahissa A. Bacterial pneumonia in HIV-infected patients: use of the pneumonia severity index and impact of current management on incidence, aetiology and outcome. HIV Med. 2008;9(8):609–615. doi: 10.1111/j.1468-1293.2008.00603.x. [DOI] [PubMed] [Google Scholar]
- 19.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 20.Pereira JM, Paiva JA, Rello J. Assessing severity of patients with community-acquired pneumonia. Semin Respir Crit Care Med. 2012;33(3):272–283. doi: 10.1055/s-0032-1315639. [DOI] [PubMed] [Google Scholar]
- 21.Sanders KM, Marras TK, Chan CK. Pneumonia severity index in the immunocompromised. Can Respir J. 2006;13(2):89–93. doi: 10.1155/2006/195464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52(1):9–17. [PubMed] [Google Scholar]
- 23.Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep. 2012;9(3):223–230. doi: 10.1007/s11904-012-0121-0. [DOI] [PubMed] [Google Scholar]
- 24.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63(2):179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 25.Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10(8):458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- 26.Surah S, Kieran J, O’Dea S, Shiel C, Raffee S, Mulcahy F, Keenan E, Lyons F. Use of the alcohol use disorders identification test (AUDIT) to determine the prevalence of alcohol misuse among HIV-infected individuals. Int J STD AIDS. 2013;24(7):517–521. doi: 10.1177/0956462412473885. [DOI] [PubMed] [Google Scholar]
- 27.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, Skarbinski J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 28.de Wit M, Zilberberg MD, Boehmler JM, Bearman GM, Edmond MB. Outcomes of patients with alcohol use disorders experiencing healthcare-associated infections. Alcohol Clin Exp Res. 2011;35(7):1368–1373. doi: 10.1111/j.1530-0277.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- 29.Palepu A, Khan NA, Norena M, Wong H, Chittock DR, Dodek PM. The role of HIV infection and drug and alcohol dependence in hospital mortality among critically ill patients. J Crit Care. 2008;23(3):275–280. doi: 10.1016/j.jcrc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza NB, Bagby GJ, Nelson S, Lang CH, Spitzer JJ. Acute alcohol infusion suppresses endotoxin-induced serum tumor necrosis factor. Alcohol Clin Exp Res. 1989;13(2):295–298. doi: 10.1111/j.1530-0277.1989.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 31.Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, Szabo G. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin Exp Res. 2002;26(11):1609–1614. doi: 10.1097/01.ALC.0000036926.46632.57. [DOI] [PubMed] [Google Scholar]
- 32.Nelson S, Bagby G, Summer WR. Alcohol suppresses lipopolysaccharide-induced tumor necrosis factor activity in serum and lung. Life Sci. 1989;44(10):673–676. doi: 10.1016/0024-3205(89)90472-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhang P, Bagby GJ, Boe DM, Zhong Q, Schwarzenberger P, Kolls JK, Summer WR, Nelson S. Acute alcohol intoxication suppresses the CXC chemokine response during endotoxemia. Alcohol Clin Exp Res. 2002;26(1):65–73. [PubMed] [Google Scholar]
- 34.Tamura DY, Moore EE, Partrick DA, Johnson JL, Offner PJ, Harbeck RJ, Silliman CC. Clinically relevant concentrations of ethanol attenuate primed neutrophil bactericidal activity. J Trauma. 1998;44(2):320–324. doi: 10.1097/00005373-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Bagby GJ, Stoltz DA, Summer WR, Nelson S. Granulocyte colony-stimulating factor modulates the pulmonary host response to endotoxin in the absence and presence of acute ethanol intoxication. J Infect Dis. 1999;179(6):1441–1448. doi: 10.1086/314763. [DOI] [PubMed] [Google Scholar]
- 36.Boe DM, Richens TR, Horstmann SA, Burnham EL, Janssen WJ, Henson PM, Moss M, Vandivier RW. Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcohol Clin Exp Res. 2010;34(10):1723–1732. doi: 10.1111/j.1530-0277.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burnham EL, Kovacs EJ, Davis CS. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. Am J Physiol Lung Cell Mol Physiol. 2013;304(12):L873–L882. doi: 10.1152/ajplung.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goss CH, Rubenfeld GD, Park DR, Sherbin VL, Goodman MS, Root RK. Cost and incidence of social comorbidities in low-risk patients with community-acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155. doi: 10.1378/chest.124.6.2148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.