Abstract
Surface-based cortical thickness (CT) analyses are increasingly being used to investigate variations in brain morphology across the spectrum of brain health, from neurotypical to neuropathological. An outstanding question is whether individual differences in cortical morphology, such as regionally increased or decreased CT, are associated with domain-specific performance deficits in healthy adults. Since CT studies are correlational, they cannot establish causality between brain morphology and cognitive performance. A direct comparison with classic lesion methods is needed to determine whether the regional specificity of CT-cognition correlations is similar to that observed in patients with brain lesions. We address this question by comparing the neuroanatomical overlap of effects when 1) whole brain vertex-wise CT is tested as a correlate of performance variability on a commonly used neuropsychological test of executive function, Trailmaking Test Part B (TMT-B), in healthy adults and 2) voxel-based lesion-symptom mapping (VBLSM) is used to map lesion location to performance decrements on the same task in patients with frontal lobe lesions. We found that reduced performance on the TMT-B was associated with increased CT in bilateral prefrontal regions in healthy adults and that results spatially overlapped in the left dorsomedial prefrontal cortex with findings from the VBLSM analysis in patients with frontal brain lesions. Findings indicate that variations in the structural integrity of the left dorsomedial prefrontal lobe, ranging from individual CT differences in healthy adults to structural lesions in patients with neurological disorders, are associated with poor performance on the TMT-B. These converging results suggest that the left dorsomedial prefrontal region houses a critical region for the complex processing demands of TMT-B, which include visuomotor tracking, sequencing, and cognitive flexibility.
Keywords: Cognitive flexibility, Trailmaking test, Cortical thickness, Voxel-based lesion symptom mapping, morphometry
Introduction
Quantitative MRI (qMRI) studies demonstrate that subtle variations in brain morphology are associated with individual differences in cognitive performance in healthy adults (reviewed in Kanai and Rees 2011). In patients with neurological disorders, gross abnormalities of cortical volume or thickness have long been appreciated as MRI markers of neuropathology, ranging from visually apparent atrophy in chronic epilepsy (Liu et al. 2003) to developmental hypertrophy in focal cortical dysplasia (Guerrini and Carrozzo 2001; Barkovich et al. 2005; Blümcke et al. 2011). The introduction of qMRI methods allows for precise measurement of cortical thickness (CT) and for statistical comparison between individual brains, which increases sensitivity to more subtle variations in brain morphology (Fischl and Dale 2000; Jones et al. 2000). This has launched several investigations of the relationship between morphological aberrations and cognition or behavior in healthy individuals with visually normal MRI scans (Bermudez et al. 2009; Blackmon et al. 2010, 2011). This work has also been extended to those with neurological disease, with evidence of a relationship between CT abnormalities and cognitive impairments in temporal lobe epilepsy (Dabbs et al. 2009).
Although qMRI metrics derived from voxel-based and surface-based morphometry can link individual differences in behavior and cognition to brain anatomy (Kanai and Rees 2011), these qMRI studies are correlational and therefore unable to establish causality between aberrations in brain morphology and reduced cognitive performance. Lesion studies are considered the gold standard for establishing causality between brain anatomy and function (Rorden and Karnath 2004). A direct comparison with classic lesion methods is needed to determine whether abnormalities in qMRI metrics, such as increased or decreased CT, confer a functional disadvantage that is comparable to that observed in patients with brain lesions. In other words, is the regional specificity of correlational effects between CT and cognition similar to that observed in patients who have suffered cognitive deficits from focal lesions?
The trailmaking test, part B (TMT-B) is an ideal behavioral measure for investigating multi-modal correspondence in brain-behavior effects across healthy controls and lesion patients due to its psychometric reliability (Sanchez-Cubillo et al. 2009) and sensitivity to executive dysfunction in healthy controls (Vasilopoulos et al. 2012; Lee et al. 2012) and patients with neurological disorders (Corrigan and Hinkeldey 1987; Crawford et al. 1992; Lezak 1995; Reitan 1958). Patients with frontal lobe lesions show the most consistent performance decrements on the TMT-B (Reitan 1958; Spreen and Benton 1965; Lezak 1995; Stuss et al. 2001; Yochim et al. 2007; McDonald et al. 2005); which makes them an optimal lesion group for establishing regionally specific correlates of impaired TMT-B performance.
To address the question of whether CTcorrelates of TMT-B performance in healthy controls correspond to regional effects observed in frontal lesion patients, we compared the spatial correspondence of results from a) correlational analysis of CT and TMT-B in healthy adults with no history of neurological or psychiatric illness (HA group); and b) voxel-based lesion-symptom analysis (VBLSM) of TMT-B task performance in adults with brain lesions in the frontal lobe (FL group). We hypothesized that there would be focal CT effects in the frontal lobe in the HA group that spatially overlap with VBLSM effects in the FL group. Such overlap would nominate the identified region(s) as critical for supporting executive functions such as cognitive flexibility.
In addition, given variability in lesion location in the FL group, we expected to find overlap in neuropsychological test score distributions with the HA group. In other words, we did not expect to find a significant difference in TMT-B scores between the HA group and the FL group, due to the variability in lesion location within the FL group. We did, however, expect that poor performance on TMT-B will be associated with cortical thickness variations (HA group) and lesion location (FL group) in spatially corresponding regions. Overlap in behavioral results (score distributions) and imaging results (spatial correspondence) between the HA and FL group would support the inference that individual differences in focal morphology detected with surface-based MRI analyses in healthy adults can impact performance efficiency in a manner similar to visually apparent focal brain lesions.
Materials and methods
Participants
This study was approved by the IRB of New York University. A sample of 30 HAs were recruited from the community through internet-based advertisement and gave consent to participate in the study. The absence of current or historical psychiatric and neurological disorders was established in HA participants by self-report during an initial semi-structured screening interview. All HA participants were administered the Wechsler Adult Intelligence Scale-Third Edition (Wechsler 1997) to rule out intellectual disability. A summary of descriptive information for the HA group and FL patient group is provided in Table 1.
Table 1.
Summary of demographic information for healthy adult and frontal lesion groups
| Lesion Patients (N=27) | Healthy Adults (N=30) | |||
|---|---|---|---|---|
| Sex | 16 males / 11 females | 14 males / 16 females | ||
| Handedness | 2 left / 2 ambidext / 23 right | 2 left / 28 right | ||
| Min-Max | Mean (SD) | Min-Max | Mean (SD) | |
| Age | 19–67 | 37.4 (10.9) | 21–65 | 42.7 (10.9) |
| Years of Education | 12–20 | 15.9 (3.6) | 11–20 | 16.1 (2.4) |
| Full–Scale IQ | 79–126 | 102.5 (13.59) | 84–143 | 107.9 (15.6) |
A sample of 27 (16 males/11 females) patients with lesions in the frontal lobe (FL group) was recruited from the New York University Patient Registry for the Study of Perception, Emotion, and Cognition (NYU PROSPEC). As part of the initial screening for this registry, FL patients completed a comprehensive battery of neuropsychological tests and a structural magnetic resonance imaging (MRI) scan (T1 MPRAGE). Patients were excluded if there was evidence of global cognitive dysfunction on the Full-Scale Intelligence Quotient (IQ) from the Wechsler Adult Intelligence Scale-Fourth Edition (i.e., IQ<70) (Wechsler 2008) or diffuse atrophy on the MRI scan. Participants were tested at least 6 months post surgery for the treatment of epilepsy, infiltrative low-grade tumors, or vascular lesions (mean=3.1 years, SD=3.1). Demographic and clinical information for each individual patient is shown in Table 2.
Table 2.
Demographic and clinical information for individual patients in the FL group
| Patient | Age | Sex | TMT-B | Years of Education | Onset Age | Years Since Surgery | Type of Lesion | Lesion Location | Handedness | Full-scale IQ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | F | 61″ | 16 | 4 | 1.54 | Epilepsy | L Frontal | Right | 94 |
| 2 | 30 | F | 61″ | 13 | 3 | 2.09 | Epilepsy | L Frontal | Right | 102 |
| 3 | 26 | M | 44″ | 16 | 14 | 4.39 | Oligodendroglioma | L Frontal | Right | 104 |
| 4 | 44 | F | 51″ | 18 | 42 | 1.54 | Oligodendroglioma | L Frontal | Right | 119 |
| 5 | 42 | M | 116″ | 23 | 15 | 3.28 | Low-grade Glioma | L Frontal | Right | 82 |
| 6 | 24 | F | 119″ | 15 | 10 | 4.84 | Epilepsy | L Frontal | Left | 82 |
| 7 | 45 | M | 51″ | 16 | 40 | 4.9 | Glial tumor | L Frontal | Right | 124 |
| 8 | 35 | F | 56″ | 17 | 12 | 3.01 | Epilepsy | R Frontal | Right | 82 |
| 9 | 41 | F | 50″ | 17 | 9 | 16.42 | Epilepsy | R Frontal | Right | 106 |
| 10 | 34 | M | 86″ | 12 | 5 | 12.54 | Epilepsy | R Frontal | Right | 98 |
| 11 | 29 | M | 41″ | 12 | 19 | 2.06 | Ganglioglioma | L/R Frontal | Ambidextrous | 110 |
| 12 | 39 | M | 54″ | 16 | 21 | 1.02 | Glioma | L Frontal | Right | 102 |
| 13 | 23 | F | 55″ | 16 | 22 | 0.73 | Glioma | L Frontal | Right | 100 |
| 14 | 31 | M | 53″ | 20 | 31 | 0.71 | Oligodendroglioma | R Frontal | Right | 120 |
| 15 | 37 | M | 69″ | 14 | 35 | 1.81 | Tumor | R Frontal | Right | 97 |
| 16 | 67 | M | 66″ | 3 | 65 | 1.65 | Meningioma | R Frontal | Right | 103 |
| 17 | 43 | F | 155″ | 19 | 29 | 0.21 | Astrocytoma | L Frontal | Left | 107 |
| 18 | 32 | M | 148″ | 12 | 0.5 | 0.56 | Epilepsy | R Frontal | Right | 80 |
| 19 | 42 | M | 57″ | 17 | 32 | 0.77 | Astrocytoma | L Frontal | Right | 93 |
| 20 | 45 | M | 67″ | 16 | 25 | 0.88 | Cavernoma | L Frontal | Right | 100 |
| 21 | 29 | M | 58″ | 16 | 1 | 6.97 | Hamartoma | L Frontal | Right | 126 |
| 22 | 19 | M | 49″ | 14 | 14 | 0.22 | Astrocytoma | R Frontal | Right | 118 |
| 23 | 29 | M | 63″ | 18 | 23 | 4.12 | Epilepsy | L Frontal | Left | 108 |
| 24 | 59 | F | 232″ | 16 | 32 | 1.28 | Meningioma and Hemorrhagic Stroke | L/R Frontal | Right | 79 |
| 25 | 43 | F | 63″ | 18 | 12 | 0.58 | Cavernoma | L Frontal | Right | 119 |
| 26 | 50 | F | 53″ | 20 | 49 | 1.02 | Astrocytoma | R Frontal | Ambidextrous | 108 |
| 27 | 44 | M | 57″ | 18 | 11 | 0.58 | Cavernoma | R Frontal | Right | 104 |
Cognitive assessment
All participants were administered the TMT-B in a distraction-free testing room using standardized procedures. This measure is one of the top five instruments most commonly used by licensed clinical neuropsychologists in the United States and Canada to assess attention and executive abilities (Rabin et al. 2005). The TMT-B has been validated as a measure of visuomotor tracking, sequencing, divided attention, and cognitive flexibility in healthy control (Vasilopoulos et al. 2012; Lee et al. 2012) and neurologically impaired adults (Corrigan and Hinkeldey 1987; Crawford et al. 1992; Lezak 1995; Reitan 1958). The TMT-B is frequently used in neuropsychological research due to its reliability (Sanchez-Cubillo et al. 2009) and sensitivity to frontal brain damage (Lezak 1995; Reitan 1958; Spreen and Benton 1965). Its sensitivity to neurological impairment may be related to the complex set of skills required for successful performance (Franzen 2000). It is primarily a visuomotor tracking task with different cognitive processing demands for each of its two parts (Lezak 1995). Part A requires the participant to draw a line as rapidly as possible joining consecutive numbers (1–25) pseudo-randomly arranged on an 8×11.5 in. page. In Part B, the participant draws a line alternating between consecutive numbers and letters that are also pseudo-randomly arranged on a page (1–A–2–B–3 …. L–13). Therefore, TMT-B demands more complex executive functions, such as divided attention, alpha-numeric sequencing, and cognitive flexibility (Sanchez-Cubillo et al. 2009).
TMT has a high coefficient of reliability [Part A; r=0.79; Part B: r=0.89 (Dikmen et al. 1999)]. The validity of TMT-B has been supported by its relationship to additional probes of cognitive flexibility such as the Wisconsin Card Sorting Task (Kortte et al. 2002; Ríos et al. 2004), set-shifting tasks (Arbuthnott and Frank 2000), and Go/No-Go tasks (Langenecker et al. 2007).
Participants are timed and raw time scores (in seconds) are the outcome measurements. For the current study, we used raw time scores from TMT-B as the dependent variable. Higher scores indicate poorer performance (i.e., more time needed to complete the task). TMT-A scores were used to index sufficient effort towards testing. There was no indication of insufficient effort, as TMT-A scores were within an acceptable range for the HA group [17″–49″; mean =32.6″; SD=8.9″] and the FL group [14″–67″; mean=34.5″; SD= 13.4″] and there was no significant difference in TMT-A performance between the two groups [t(55)=0.64, p=0.52].
MRI scanning and image processing
Imaging was performed at the NYU Center for Brain Imaging on a 3-Tesla Siemens Allegra head-only MR scanner. Image acquisitions included a conventional three-plane localizer and two T1-weighted gradient-echo sequence (MPRAGE) volumes (TE=3.25 ms, TR=2530 ms, TI=1.100 ms, flip angle = 7°, FOV = 256 mm, voxel size = 1 × 1 × 1.33 mm). Acquisition parameters were optimized for increased gray/white matter image contrast. The imaging protocol was identical for all subjects studied. The image files in DICOM format were transferred to a Linux workstation for morphometric analysis. Images were automatically corrected for spatial distortion due to gradient nonlinearity (Jovicich et al. 2006) and B1 field inhomogeneity (Sled et al. 1998), registered, and averaged to improve signal-to-noise ratio. Images were further processed with the FreeSurfer (5.1) software package (http://surfer.nmr.mgh.harvard.edu) and Matlab software (MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, Massachusetts, United States).
CT measurement and analysis
Freesurfer was used for whole brain CT analyses. Two surface models were generated using intensity and continuity information — the white matter (WM) surface representing the boundary between WM and gray matter (GM) and the pial surface representing the boundary between GM and cerebrospinal fluid (CSF). CT was calculated at every vertex as the shortest distance between the two surfaces (Fischl and Dale 2000). Major components of the applied procedure were described in detail in recent publications (Blackmon et al. 2010, 2011; Butler et al. 2012). In brief, MRI data were registered to Talairach space, intensity normalized and the skull was automatically removed (Dale et al. 1999; Ségonne et al. 2004). WM voxels were detected and labeled using intensity and neighborhood information to generate the WM surface. A connected components procedure assigned a solid body of WM voxels for each hemisphere. These WM bodies were tessellated into a polygon mesh, smoothed and refined by a deformable surface algorithm to follow intensity gradients between WM and GM. The pial surface between GM and CSF was defined similarly (Dale et al. 1999). Small topological inexactness was automatically detected and rectified (Ségonne et al. 2007). Each automated reconstruction was manually checked for errors and corrected, if necessary, by an experienced technician blind to the hypothesis of this study. Manual intervention was necessary only in<5 % of the scans and in every case only minimal.
Resulting surfaces were superposed to MR data. Finally, CT was individually assessed at each vertex (Fischl and Dale 2000). To enable intersubject averaging and statistical analysis individual brain surfaces were registered to a spherical atlas by aligning the folding patterns of any individual with the average of the atlas (Fischl et al. 1999). To specify localizations with anatomical differences the reconstructed cortical surface was automatically parcellated into gyrus-based regions (Desikan et al. 2006; Fischl et al. 2004).
For each hemisphere, vertexwise correlations were used to test for a relationship between CT at each vertex along the cortical surface and TMT-B scores, using age as a covariate. The spatial CT distribution was smoothed with a circularly symmetric Gaussian kernel of 15 mm full width half maximum to provide normal distribution of the results. We controlled the familywise error rate by means of Monte Carlo simulations as implemented in Freesurfer 5.1 (Hayasaka and Nichols 2003; Hagler et al. 2006). The data were tested against an empirical null distribution of maximum cluster size across 10,000 iterations synthesized with an initial cluster threshold of p<0.05, yielding significant clusters corrected for multiple comparisons across all vertices. Corrected significance clusters of CT correlations with TMT-B raw scores were mapped onto the pial surface of the average brain reconstruction for visual display.
Lesion analysis
High resolution T1 MPRAGE volumes from each patient were normalized to Montreal Neurological Institute (MNI) standard space using FSL FLIRT (FMRIB’s Linear Image Registration Tool; http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/flirt/) (Jenkinson and Smith 2001). To improve normalization, cost function weighting (weight=0) of the lesion area and any surrounding craniotomy defect was used during 12° of freedom affine transformation of the lesioned brain to the standard MNI 1 mm reference volume. Lesions were manually traced by a neuropsychologist (KB) on individual slices of the native brain, with crosschecking across all three planes. All patients had surgical lesions; therefore, margins were readily visible on T1-weighted MRI scans. The treating neurosurgeons were consulted if there was any ambiguity about lesion margins. Tracing produced a binary volume with B1^ indicating the presence of the lesion and B0^ the presence of normal tissue. Multi-slice images depicting lesion location and extent for each individual patient displayed on the MNI 152 standard brain template are provided in Supplementary Figure 1.
FSL BET toolbox was used for skull-stripping, and for calculation of whole-brain and lesion volumes. Lesion volumes were calculated with fslstats by counting non-zero voxels in the skull-stripped brain mask (Smith 2002). Lesion overlap maps were constructed by averaging the normalized lesion maps for each subject, giving a proportion overlap score at each voxel. The normalized lesion masks were used in subsequent VBLSM analyses, as implemented in MRIcro software (Rorden and Brett 2000) (www.mricro.com). A Brunner-Munzel analysis, with 4000 permutations and a false discovery rate of 10 %, was used to investigate which voxels, when lesioned, were associated with a performance decrement.
Results
CT analyses in the HC group
TMT-B scores in the HC group ranged from 27″–128″ (mean=65.93″, SD=24.07″), which is similar to what is reported in large adult normative samples (Drane et al. 2002; Tombaugh 2004). There was no association with TMT-B scores and age (r=0.10, p=0.59), which may be due to the age range of our HA group. In a large adult normative sample (N=911; age range=18–89) for TMT-B, age effects do not appear prominent until age 65, which is where our sample range ends (Tombaugh 2004). Consistent with other normative samples (Drane et al. 2002; Tombaugh 2004), there was no association between gender and TMT-B scores (t=0.96; p=0.35) and there was an inverse correlation between TMT-B scores and years of education (r=−0.36; p=0.001) as well as overall IQ (r=−0.55; p=0.001); therefore, education and IQ were entered as a nuisance variables in secondary CT analyses.
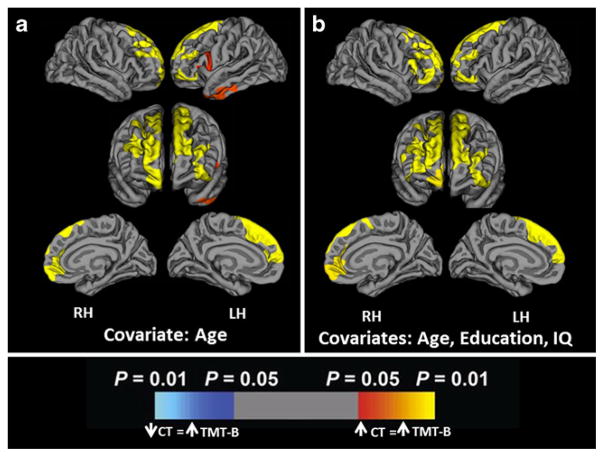
For each hemisphere, we assessed the vertex-wise correlational relationship between CT and TMT-B scores. CT was positively correlated with TMT-B scores across widespread frontal lobe regions bilaterally, independent of age, after correction for multiple comparison corrections (See Fig. 1a). Secondary analyses revealed that significant effects in bilateral dorsomedial prefrontal lobes were independent of education and IQ (see Fig. 1b). Areas of the LH where increased CT was associated with higher TMT-B scores include the left superior frontal gyrus (r=0.6384, p=0.0001) and anterior inferior temporal cortex (r=0.5791, p=0.0008). The left inferior temporal region did not remain significant after adding education and IQ as covariates. Areas of the RH where increased CT was associated with higher TMT-B scores were the superior and middle frontal gyri (r=0.6069, p=0.0002). There were no areas in either hemisphere where increased CT was associated with better TMT-B performance (i.e., lower scores).
Fig. 1.
Cortical areas where increased CT in healthy adult participants is associated with higher TMT-B scores, with a. age as a covariate; and b. age, education and IQ as covariates, following Monte-Carlo cluster-wise correction for multiple comparisons. Positive values (red, yellow) signify regions where increased CT is significantly associated with increased TMT-B scores (poorer performance). There were no instances of negative correlations between CT and TMT-B scores (dark blue, light blue)
Voxel-based Lesion-Symptom Mapping (VBLSM) in the Frontal Lobe (FL) group
Age, sex, education, and test score comparisons between the FL and HA groups
TMT-B scores for the FL group ranged from 41″ to 232″ (mean=75.37″, SD= 43.26″). There was no difference between the FL and HA groups in age (t(55) =1.82, p=0.08), education (t(55)=−0.31, p=0.76), Full-Scale IQ (t(55)=1.74, p=0.09), or gender distribution (χ2 =0.91; p=0.342).
Within the FL group, the distribution of TMT-B scores was non-normal due to the presence of several positive outliers; therefore, the non-parametric Brunner-Munzel rank order test was used for the VBLSM analyses. There was no difference in TMT-B scores between males and females (Mann–Whitney U Test: p = 0.65). There was no correlation between TMT-B scores and age (rho = −0.06, p = 0.79) or education (rho = 0.21; p=0.31), but there was an inverse relationship between TMT-B scores and IQ (rho=−0.57, p=0.002).
Comparison of TMT-B scores between the HA and FL groups revealed that the distribution of TMT-B scores was the same across groups (Mann–Whitney U Test: p=0.97). This remained the case after controlling for age (p=0.132), years of education (p=0.807), and IQ (p=0.73). Thus, there was no evidence of an overall performance decrement on the TMT-B in the FL group compared to the HA group.
Voxel-based lesion symptom mapping results
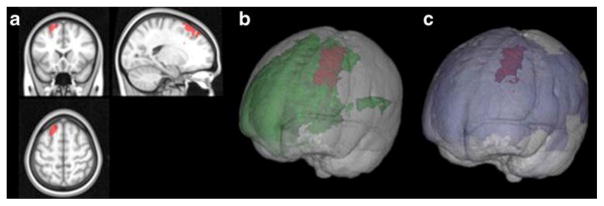
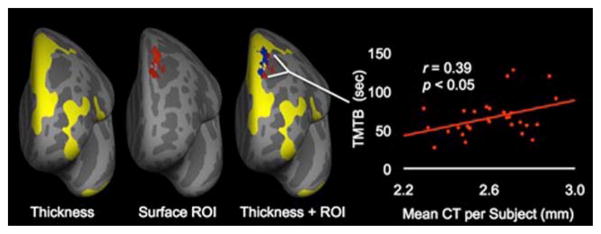
Results from VBLSM show a region in the left medial superior prefrontal region (see Fig. 2) that, when lesioned, is associated with higher TMT-B scores (indicating poorer performance), after correction for multiple comparisons (t-score> 2.2). This region of interest (ROI) was converted into a surface file via Freesurfer, using a depth of 0.5 mm, and overlaid on the results from CT analyses in the HA group to determine the degree of spatial overlap (see Fig. 3). There was substantial overlap in the left hemisphere dorsomedial prefrontal region. The proportion of the CT results in the HA group that overlapped with the significant cluster obtained from VBLSM results in the FL group was 66.5 and 15.2 % of the variance in TMT-B scores in the HA group was accounted for by CT in this overlapping cluster [r=0.39, R2 =0.152; see Fig. 3].
Fig. 2.
a Highlighted brain volume on MNI template in red signifies area of brain where patients’ lesions yielded poorer TMT-B performance. Area was calculated by Brunner-Munzel analysis with 4000 permutations and false discovery rate of 10 % (t-score>2.2). b Power map for volume obtained from VBLSM, green denotes regions with sufficient statistical power, red represents the significant region as shown in (a). c Lesion-overlap map for volume obtained from VBLSM, blue denotes any region where a patient lesion was identified, red represents the significant region as shown in (a) and (b)
Fig. 3.
Thickness cluster (yellow) along with surface reconstruction of the lesion volume (red) obtained from correlating lesion volume location with TMT-B performance, depth 0.5 mm. Also shown on third figure labeled ‘Thickness+ROI’ is region of overlap (blue) of thickness cluster (yellow) and surface reconstruction of lesion volume (red). Scatterplot demonstrates significant correlation of average CT per subject in surface ROI as it relates to TMT-B performance (r=0.39, p=0.033)
Overall GM volume and lesion volumes were calculated in the FL group. GM volume ranged from 1029613 to 1655245.5 mm3 (mean= 1386801.76; SD= 167310.96). There was no correlation between GM volume and TMT-B test performance (r=0.23; p=0.25). Individual lesion volumes ranged from 1127.28 mm3 to 155075.27 mm3 (mean= 36139.08; SD=35906.34). There was no relationship between total lesion volume and TMT-B scores (rho=0.132; p=0.51).
In order to determine whether clinical factors within the FL group had an impact on TMT-B scores, we tested the correlation between TMT-B scores and Bage of disease onset,^ and Byears since surgery.^ There was no correlation between TMT-B scores and age of disease onset (rho = −0.11; p = 0.58) or time since surgery (rho = −0.08; p = 0.7). Concerning lesion etiology there was no significant difference in TMT-B score distributions between patients with a chronic history of treatment resistant epilepsy versus those with a more recent discovery of a tumor or vascular lesion (Mann–Whitney U-test: p= 0.08); although it is important to note that many of the patients with tumors and vascular lesions also had seizures.
Taken together, these results demonstrate that the location of a lesion in the left dorsomedial prefrontal lobe had a greater impact on TMT-B performance than total brain volume, the extent of the lesion (i.e., lesion volume), or clinical factors such as disease duration and time since surgery.
Discussion
MRI morphometry tools are increasingly being used to investigate the relationship between cortical surface features and individual differences in cognition. Thus, it is important to determine whether regional effects overlap with those of more classic approaches to brain-behavior relationships, such as lesion methods. Overlapping effects provide support for the use of surface-based measures to probe the neuroanatomical basis for individual differences in cognitive abilities. In this study, we investigated the relationship between CT and TMT-B performance in healthy adults and directly compared the spatial overlap with results from voxelwise lesion-symptom mapping in patients with frontal lobe lesions. Our results demonstrate that stronger performance on the TMT-B task is associated with reduced CT in bilateral superior prefrontal lobes in healthy adults, independent of age, education, and IQ. Independence from general cognitive abilities (i.e., IQ) provides evidence that the regional effects in the superior prefrontal cortex are specific to the processing demands of the TMT-B task.
Critical to the hypothesis of the study, findings from the CT analysis in healthy adults overlapped with results from VBLSM analysis of TMT-B scores in patients with frontal lobe lesions. The area of overlap was limited to the left dorsomedial prefrontal cortex (PFC). Converging findings in this region suggest that it may serve as a critical region for the unique processing demands of the TMT-B test. Also in line with our hypothesis, average TMT-B performance in the HA group did not differ from the FL group, with evidence of overlapping distributions. In both groups, poorer performance was associated with reduced integrity of the left dorsomedial PFC. These findings support vertex-wise CT analysis as a valid method for probing neuroanatomical contributions to individual differences in TMT-B performance. They suggest that thicker cortex in the dorsomedial PFC confers a functional disadvantage in healthy adults similar to that observed in patients with surgical lesions in this region.
The left dorsomedial prefrontal cortex supports TMT-B performance
Impairment in TMT-B performance is robustly linked to frontal lobe pathology (Stuss et al. 2001; Yochim et al. 2007; McDonald et al. 2005). Two prior studies found that left frontal lobe lesions resulted in greater impairment (Stuss et al. 2001; Yochim et al. 2007); however, further sub-lobar specificity was difficult to determine due to variability in lesion location and extent. VBLSM overcomes this limitation by testing the voxel-wise relationship between task performance and the presence of a lesion (yes/no), allowing for greater spatial precision (Rorden and Karnath 2004). VBLSM has been previously used to investigate executive dysfunction in a large sample of lesion patients (Gläscher et al. 2012). Poor performance on the TMT was associated with damage to a small region of the left ventromedial PFC, inferior to the cluster we identified. This study utilized a different measure of TMT performance (TMT-B – TMT-A), which suggests that variable metrics derived from the TMT are sensitive to damage spanning the left medial PFC.
Functional MRI (fMRI) studies demonstrate activation in multiple areas of the frontal lobe during TMT-B performance in healthy controls, as opposed to the singular regions identified in lesion studies. There is asymmetric activation of the left hemisphere, most notable in the left dorsolateral PFC (Moll et al. 2002; Zakzanis et al. 2005). Additional areas implicated include the left middle temporal (Zakzanis et al. 2005; Jacobson et al. 2011) and superior temporal (Zakzanis et al. 2005) gyri, right supplementary motor area and cingulate sulcus (Zakzanis et al. 2005), and bilateral intraparietal sulcus (Moll et al. 2002). In sum, both lesion and functional imaging studies converge on the left PFC as a critical region for adept TMT-B performance; however, fMRI studies in healthy controls implicate a broader bilateral fronto-temporal-parietal network.
Increased CT in the prefrontal lobe can confer a functional disadvantage
Our findings show that poorer performance on TMT-B is associated with increased CT in bilateral PFC. If the assumption is Bmore-is-better,^ these results appear counterintuitive. Indeed, meta-analysis of PFC volumes and executive functions show that in healthy adults, larger volumes are often associated with better performance (Yuan and Raz 2014); however, this relationship is variable. In several studies that looked specifically at PFC gray matter volume and TMT-B performance, results varied from no effect (Hanninen et al. 1997; Koutsouleris et al. 2010; Gianaros et al. 2006), to a negative correlation (Paul et al. 2009) and a positive correlation (Zimmerman et al. 2006). The lack of significant effects in some studies could be explained by only including narrow regions outside of the dorsomedial PFC, such as the orbitofrontal cortex (Choi et al. 2004; Nakamura et al. 2008). In the only other study that investigated the relationship between CT and TMT-B scores in healthy adults, the relationship was negative: thicker cortex was associated with stronger performance (Dickerson et al. 2008). However, this study was performed in older adults (age 66–81), where age-related acceleration of performance decline (Tombaugh 2004) and brain atrophy (Storsve et al. 2014) might impact results.
In healthy young to middle age adults, decreased CT is associated with a performance advantage across a range of cognitive domains, including attention (Westlye et al. 2011), word-reading (Blackmon et al. 2011), and expert chess-playing (Hänggi et al. 2014). When cognitive domain-specific performance discrepancies are observed, a verbal over visuospatial advantage is associated with decreased CT in bilateral occipito-temporal and frontal regions; whereas, a visuospatial over verbal advantage is associated with increased CT in the same regions (Margolis et al. 2013). CT is negatively associated with general intellectual abilities until the 4th decade at which point, thicker cortex starts to confer a functional advantage (Schnack et al. 2015). In sum, evidence for both positive and negative relationships between PFC structure and TMT-B performance suggests that directionality may not be as important as regional specificity, particularly in healthy young to middle age adult samples where atrophy is not a primary factor driving performance correlations.
Bidirectional relationships between CT and performance in healthy adults may be due to multifactorial determinants of cortical gray matter morphology. Non-linear and dynamic lifespan CT trajectories (Storsve et al. 2014) suggest that both the region and the developmental period must be taken into consideration when assessing the relationship between CTand cognitive performance. Increased CT in older children and young adults could reflect insufficient pruning or myelination, implicating a pathological rather than adaptive process. During adolescence, reduction in CT might be associated with neuronal pruning (Kanai and Rees 2011; Huttenlocher and Dabholkar 1997), which results in more efficient cortical networks (Kharitonova et al. 2013). The frontal lobes are the last region to complete the myelination process; with frontal lobe myelination peaking in the 4th decade of life (Bartzokis et al. 2012). Therefore, thicker cortex in our healthy adult population may confer a functional disadvantage because it reflects either insufficient pruning or myelination. However, given the variable findings from volumetric studies of TMT-B correlates, other factors are likely contributory.
Spatial distribution of effects
The total area of significant results from the VBLSM analysis in the frontal lobe lesion group is considerably smaller than results from the surface-based analyses in the HA group. This might be partially explained by structural covariance in healthy adults (Evans 2013). Measures of gray matter density and CT show strong covariance between bilateral homologous regions (Chen et al. 2008; Mechelli et al. 2005), which has significant genetic influences (Schmitt et al. 2014), particularly in association cortices (Lenroot et al. 2009). If CT in homologous regions tends to covary as a result of genetic influence, rather than experience, practice, or expertise, then this suggests that bilateral CT results may not always represent true performance correlates but instead might reflect a network of general (i.e., non-performance related) structural covariance. Results from the VBLSM analyses support this by demonstrating that only a small region within the broader distribution of CT effects represents the critical region for TMT-B performance. These findings should be taken into account when interpreting results from studies that use CT to probe the neuroanatomical basis for specific cognitive abilities.
Limitations
Our study is limited by a relatively small number of patients in the VBLSM analysis. Although the sample included patients with lesions extending into the left dorsolateral PFC (Fig. 3c), they were few in number and our power to detect effects in this region was low (Fig. 3b). Therefore, we cannot exclude the possibility that more lateral left PFC regions subserve TMT-B performance, as is suggested by prior lesion studies (Stuss et al. 2001; Yochim et al. 2007) and by our CT data from the HA group. In the CT analysis, the strength of the linear relationship between CT and TMT-B was smaller than effects obtained in a prior sample of healthy controls (Dickerson et al. 2008). In this prior study, the relationship between CT and TMT-B scores was assessed in the same 15 subjects (ages 66 to 81) across 4 different time points and MRI scanners. Resulting effect sizes ranged from R2 =0.44 to R2 =0.83, which is more than twice that observed in our sample of 27 adults ranging in age from 21 to 65. The difference in effect sizes between our samples may be due to increased rates of change in both TMT-B performance (Tombaugh 2004) and CT (Lemaitre et al. 2012) in adults above the age of 65. Given the relatively smaller effect size we obtained, it is recommended that future studies of the relationship between CT and performance in healthy, middle-aged adults utilize larger samples.
In addition, TMT-B involves a myriad of cognitive processes, including visual attention, graphomotor speed, divided attention, set-shifting, and alphanumeric sequencing. Therefore, our results could be viewed as non-specific to these numerous aspects of executive function as probed by TMT-B. Many studies have employed methods to correct for these additional processes, such as subtracting TMT-A from TMT-B or dividing TMT-B by TMT-A. However, these metrics are not as commonly utilized in clinical practice (Rabin et al. 2005) and do not consistently show greater sensitivity to frontal lobe damage or concurrent validity with other measures of cognitive flexibility (Sanchez-Cubillo et al. 2009). Furthermore, in a separate VBLSM study that utilized the [TMT-B – TMT-A] metric, a similar left medial frontal result was obtained; suggesting that various metrics derived from the TMT-B may be comparably sensitive to left medial PFC damage.
Conclusion
In sum, our results demonstrate converging evidence from different imaging modalities for the role of the left dorsomedial PFC in supporting TMT-B performance. Spatial correspondence between CTand VBLSM results provide support for CT as a measure of regional brain integrity that can be used to probe the neuroanatomical correlates of individual differences in healthy adults. However, the broader distribution of CT effects suggests that additional factors, such as non-performance related structural covariance, should be taken into account when interpreting CT performance correlates in healthy adult populations.
Acknowledgments
This work was supported by Finding a Cure for Epilepsy and Seizures (FACES) and the Epilepsy Foundation (Targeted Research Initiative for Cognitive and Psychiatric Aspects of Epilepsy). The authors declare no competing financial interests.
References
- Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. Journal of Clinical and Experimental Neuropsychology. 2000;22(4):518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65(12):1873–1887. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Heydari P, Couvrette A, Lee GJ, Kalashyan G, Altshuler LL. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biological Psychiatry. 2012;72(12):1026–1034. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Bermudez P, Lerch JP, Evans AC, Zatorre RJ. Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cerebral Cortex. 2009;19(7):1583–1596. doi: 10.1093/cercor/bhn196. [DOI] [PubMed] [Google Scholar]
- Blackmon K, Barr WB, Kuzniecky R, DuBois J, Carlson C, Quinn BT, Thesen T. Phonetically irregular word pronunciation and cortical thickness in the adult brain. NeuroImage. 2010;51(4):1453–1458. doi: 10.1016/j.neuroimage.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmon K, Halgren E, Barr WB, Carlson C, Devinsky O, DuBois J, Thesen T. Individual differences in verbal abilities associated with regional blurring of the left gray and white matter boundary. The Journal of Neuroscience. 2011;31(43):15257–15263. doi: 10.1523/JNEUROSCI.3039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Spreafico R. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission1. Epilepsia. 2011;52(1):158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Blackmon K, McDonald CR, Carlson C, Barr WB, Devinsky O, Thesen T. Cortical thickness abnormalities associated with depressive symptoms in temporal lobe epilepsy. Epilepsy & Behavior. 2012;23(1):64–67. doi: 10.1016/j.yebeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cerebral Cortex. 2008;18(10):2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kang DH, Kim JJ, Ha TH, Lee JM, Youn T, Kwon JS. Left anterior subregion of orbitofrontal cortex volume reduction and impaired organizational strategies in obsessive-compulsive disorder. Journal of Psychiatric Research. 2004;38(2):193–199. doi: 10.1016/j.jpsychires.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the trail making test. Journal of Clinical Psychology. 1987;43(4):402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Parker DM, McKinlay WW, editors. A handbook of neuropsychological assessment. Hove: Psychology Press; 1992. [Google Scholar]
- Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy & Behavior. 2009;15(4):445–451. doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Fischl B. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. NeuroImage. 2008;39(1):10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen SS, Heaton RK, Grant I, Temkin NR. Test–retest reliability and practice effects of expanded Halstead–Reitan neuropsychological test battery. Journal of the International Neuropsychological Society. 1999;5(04):346–356. [PubMed] [Google Scholar]
- Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Cognitive and Behavioral Neurology. 2002;15(1):39–43. [PubMed] [Google Scholar]
- Evans AC. Networks of anatomical covariance. NeuroImage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Franzen MD. Reliability and validity in neuropsychological assessment. 2000. [Google Scholar]
- Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. NeuroImage. 2006;31(2):754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Tranel D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2012;109(36):14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Carrozzo R. Epilepsy and genetic malformations of the cerebral cortex. American Journal of Medical Genetics. 2001;106(2):160–173. doi: 10.1002/ajmg.1569. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage. 2006;33(4):1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi J, Brütsch K, Siegel AM, Jäncke L. The architecture of the chess player’s brain. Neuropsychologia. 2014;62:152–162. doi: 10.1016/j.neuropsychologia.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Hanninen T, Hallikainen M, Koivisto K, Partanen K, Laakso MP, Riekkinen PJ, Soininen H. Decline of frontal lobe functions in subjects with age-associated memory impairment. Neurology. 1997;48(1):148–153. doi: 10.1212/wnl.48.1.148. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. NeuroImage. 2003;20(4):2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobson SC, Blanchard M, Connolly CC, Cannon M, Garavan H. An fMRI investigation of a novel analogue to the trail-making test. Brain and Cognition. 2011;77(1):60–70. doi: 10.1016/j.bandc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones SE, Buchbinder BR, Aharon I. Three-imensional mapping of cortical thickness using Laplace’s equation. Human Brain Mapping. 2000;11(1):12–32. doi: 10.1002/1097-0193(200009)11:1<12::AID-HBM20>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12(4):231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JD, Sheridan MA. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Developmental Cognitive Neuroscience. 2013;6:61–71. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortte KB, Horner MD, Windham WK. The trail making test, part B: cognitive flexibility or ability to maintain set? Applied Neuropsychology. 2002;9(2):106–109. doi: 10.1207/S15324826AN0902_5. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Patschurek-Kliche K, Scheuerecker J, Decker P, Bottlender R, Schmitt G, Meisenzahl EM. Neuroanatomical correlates of executive dysfunction in the at-risk mental state for psychosis. Schizophrenia Research. 2010;123(2):160–174. doi: 10.1016/j.schres.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Zubieta JK, Young EA, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: convergent validity and test–retest reliability of the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology. 2007;29(8):842–853. doi: 10.1080/13803390601147611. [DOI] [PubMed] [Google Scholar]
- Lee T, Mosing MA, Henry JD, Trollor JN, Lammel A, Ames D, Sachdev PS. Genetic influences on five measures of processing speed and their covariation with general cognitive ability in the elderly: the older Australian twins study. Behavior Genetics. 2012;42(1):96–106. doi: 10.1007/s10519-011-9474-1. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiology of Aging. 2012;33(3):617. doi: 10.1016/j.neurobiolaging.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Giedd JN. Difference in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3 1995. [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Hammers A, Sisodiya SM, Bartlett PA, Duncan JS. Progressive neocortical damage in epilepsy. Annals of Neurology. 2003;53(3):312–324. doi: 10.1002/ana.10463. [DOI] [PubMed] [Google Scholar]
- Margolis A, Bansal R, Hao X, Algermissen M, Erickson C, Klahr KW, Naglieri JA, Peterson BS. Using IQ discrepancy scores to examine the neural correlates of specific cognitive abilities. The Journal of Neuroscience. 2013;33(35):14135–14145. doi: 10.1523/JNEUROSCI.0775-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui-Madoz VJ. Is impairment in set-shifting specific to frontal-lobe dysfunction? Evidence from patients with frontal-lobe or temporal-lobe epilepsy. Journal of the International Neuropsychological Society. 2005;11(04):477–481. [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. The Journal of Neuroscience. 2005;25(36):8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Oliveira-Souza RD, Moll FT, Bramati IE, Andreiuolo PA. The cerebral correlates of set-shifting: an fMRI study of the trail making test. Arquivos de Neuro-Psiquiatria. 2002;60(4):900–905. doi: 10.1590/s0004-282x2002000600002. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME, McCarley RW. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131(1):180–195. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, Gordon E. Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiology of Aging. 2009;30(3):457–465. doi: 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA Division 40 members. Archives of Clinical Neuropsychology. 2005;20(1):33–65. doi: 10.1016/j.acn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8(3):271–276. [Google Scholar]
- Ríos M, Periáñez JA, Muñoz-Céspedes JM. Attentional control and slowness of information processing after severe traumatic brain injury. Brain Injury. 2004;18(3):257–272. doi: 10.1080/02699050310001617442. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5(10):812–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the trail making test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society. 2009;15(03):438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Neale MC, Fassassi B, Perez J, Lenroot RK, Wells EM, Giedd JN. The dynamic role of genetics on cortical patterning during childhood and adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(18):6774–6779. doi: 10.1073/pnas.1311630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Pol HE. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cerebral Cortex. 2015;25(6):1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging. 2007;26(4):518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Comparative studies of some psychological tests for cerebral damage. The Journal of Nervous and Mental Disease. 1965;140(5):323–333. doi: 10.1097/00005053-196505000-00002. [DOI] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. Journal of Neuroscience. 2014;34(25):8488–8498. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The trail making test: a study in focal lesion patients. Psychological Assessment. 2001;13(2):230. [PubMed] [Google Scholar]
- Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Vasilopoulos T, Franz CE, Panizzon MS, Xian H, Grant MD, Lyons MJ, Toomey R, Jacobson KC, Kremen WS. Genetic architecture of the Delis-Kaplan executive function system trail making test: evidence for distinct genetic influences on executive function. Neuropsychology. 2012;26(2):238–250. doi: 10.1037/a0026768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III, Wechsler adult intelligence scale: Administration and scoring manual. Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. WAIS-IV, Wechsler adult intelligence scale: Administration and scoring manual. Psychological Corporation; 2008. [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cerebral Cortex. 2011;21(2):345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Yochim B, Baldo J, Nelson A, Delis DC. D-KEFS trail making test performance in patients with lateral prefrontal cortex lesions. Journal of the International Neuropsychological Society. 2007;13(04):704–709. doi: 10.1017/S1355617707070907. [DOI] [PubMed] [Google Scholar]
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2014;42:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the trail making test. Neuropsychologia. 2005;43(13):1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Gordon E. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. The American Journal of Geriatric Psychiatry. 2006;14(10):823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]