Abstract
Background and Purpose
Chymase is a unique, abundant secretory product of mast cells and a potent chemoattractant for eosinophils, monocytes and neutrophils, but little is known of its influence on mast cell accumulation.
Experimental Approach
A mouse peritoneal inflammation model, cell migration assay and flowcytometry analysis, were used to investigate the role of chymase in recruiting mast cells.
Key Results
Chymase increased, by up to 5.4‐fold, mast cell numbers in mouse peritoneum. Inhibitors of chymase, heat‐inactivation of the enzyme, sodium cromoglycate and terfenadine, and pretreatment of mice with anti‐intercellular adhesion molecule 1, anti‐L‐selectin, anti‐CD11a and anti‐CD18 antibodies dramatically diminished the chymase‐induced increase in mast cell accumulation. These findings indicate that this effect of chymase is dependent on its enzymatic activity and activation of adhesion molecules. In addition, chymase provoked a significant increase in 5‐HT and eotaxin release (up to 1.8‐ and 2.2‐fold, respectively) in mouse peritoneum. Since 5‐HT, eotaxin and RANTES can induce marked mast cell accumulation, these indirect mechanisms may also contribute to chymase‐induced mast cell accumulation. Moreover, chymase increased the trans‐endothelium migration of mast cells in vitro indicating it also acts as a chemoattractant.
Conclusion and Implications
The finding that mast cells accumulate in response to chymase implies further that chymase is a major pro‐inflammatory mediator of mast cells. This effect of chymase, a major product of mast cell granules, suggests a novel self‐amplification mechanism for mast cell accumulation in allergic inflammation. Mast cell stabilizers and inhibitors of chymase may have potential as a treatment of allergic disorders.
Abbreviations
- CI
calcium ionophore
- fMLP
N‐formyl‐methionyl‐leucyl‐phenylalanine
- HSA
human serum albumin
- ICAM‐1
intercellular adhesion molecule 1
- RANTES
regulated upon activation, normal T‐cell‐expressed and ‐secreted
- SAAPP
N‐succinyl‐L‐Ala‐L‐Ala‐L‐Pro‐L‐Phe‐p‐nitroanilide
- SBTI
soybean trypsin inhibitor
- SCF
stem cell factor
- SLPI
secretory leukocyte proteinase inhibitor
- TNBS
2,4,6‐trinitrobenzene sulfonic acid
- ZIGPFM
Z‐Ile‐Glu‐Pro‐Phe‐CO2 Me
Introduction
Chymase is a mast cell serine proteinase with a molecular weight of 30 kDa. It is exclusively located in the granules of mast cells together with tryptase, histamine, heparin, cytokines and other products (Schwartz, 1994). Since a large quantity of the active form of chymase is released by mast cells, into their adjacent environment, upon degranulation (He et al., 1999), it is likely to play a role in mast cell‐related diseases such as allergy. Indeed, it has been recently reported that airway higher responsiveness to mannitol in asthma is associated with chymase‐positive mast cells (Sverrild et al., 2016).
Chymase has been reported to be a potent chemoattractant for eosinophils (He and Walls, 1998a) via an ERK and p38 MAPK‐mediated mechanism (Terakawa et al., 2005). It is also a chemoattractant for monocytes and neutrophils (Tani et al., 2000). The finding that chymase inhibitors reduced the increase in the number of dermal mast cells in 2, 4‐dinitrofluorobenzene‐induced dermatitis and that intradermal injection of human chymase significantly increased the histamine content in skin suggests that chymase released by mast cells may participate in local mast cell accumulation (Tomimori et al., 2002a). However, little is known of the potential mechanisms of chymase‐induced mast cell accumulation.
Apart from its direct influence on mast cell accumulation, chymase may induce mast cell accumulation via indirect mechanisms. Since chymase can provoke mast cell degranulation (He et al., 1999) and induce the release of IL‐8 from human EoL‐1 cells (Terakawa et al., 2006), it may very likely induce the release of other mast cell products such as 5‐HT (serotonin) (Wong et al., 2002), eotaxin (CCL11) and regulated upon activation, normal T‐cell‐expressed and ‐secreted (RANTES; CCL5) (Shakoory et al., 2004) from mast cells upon activation. Moreover, it has been found that sumatriptan, a 5‐HT receptor agonist, enhanced thalamic mast cell population, especially those containing 5‐HT (Dubayle et al., 2005), and that RANTES can provoke a strong recruitment of mast cells selectively in rats (Conti et al., 1998), implicating the involvement of mast cell secretory products in mast cell accumulation. The aim of the current study was to investigate the ability of chymase to induce mast cell accumulation and the potential mechanisms involved in this effect.
Methods
Animals, cell line and culture
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). BALB/c mice (6–8 weeks) and C57BL/6J mice (6–8 weeks) were obtained from Guangdong Experimental Animal Centre, China, Grade II, Certificate No. 2001A049, and from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China), Certificate No. 11400700118760 and 11400700256540. They were housed in the Animal Experimental Centre of the First Affiliated Hospital of Jinzhou Medical University in a specific pathogen‐free environment with free access to standard rodent chow and water, at a constant temperature 23–28°C and relative humidity of 60–75%. Mast cell‐deficient (KitW‐4Bao) mice were kindly donated by Professor Shigang Li (China Three Gorges University, China). The animal experiment procedures were approved by the Animal Care Committee at Shantou University and Jinzhou Medical University. Human mast cell line, HMC‐1 cells, were a present from Dr Joseph H. Butterfield (Mayo Clinic, MN, USA). HMC‐1 cells were maintained in RPMI 1640 medium supplemented with 10% (v.v‐1) heat‐inactivated FBS, 100 U·mL−1 penicillin/streptomycin in 75 cm2 tissue culture flasks (Falcon) at 37°C in a humidified atmosphere of 5% (v.v‐1) CO2. HMEC‐1 (ATCC® CRL‐3243™, human dermal microvascular endothelium cells) were cultured in MCDB 131 medium containing 2.0 mM glutamine, 1.0 μg·mL−1 hydrocortisone and 10 ng·mL−1 epidermal growth factor supplemented with 10% (v.v‐1) heat‐inactivated FBS, 100 U·mL−1 penicillin/streptomycin. When HMEC‐1 reached 80% confluence in culture flasks, the recommended trypsin–EDTA solution was used to disperse the cells and the cells were used in experiments or reseeded in flasks.
Preparation of enzymes
Human chymase was purified from human skin tissue obtained at circumcision by high salt extraction, heparin agarose and S‐200 sephacryl gel filtration chromatography procedures as described previously (He and Walls, 1998a). The experimental procedures were approved by the Ethical Committee at Shenyang Medical College. Enzymatic activity was determined spectrophotometrically (410 nm) by the rate of hydrolysis of 0.7 mmol·L−1 SAAPP in 1.5 mol·L−1 NaCl, 0.3 mol·L−1 Tris, pH 8.0. The specific activity of chymase was 4.9 U·mg−1 protein, where 1 U of enzyme represents that required to hydrolyse 1 μM of SAAPP·min−1 at 25°C. Purity was evaluated using 10% SDS‐PAGE, and the identity of the protein band was confirmed by Western blotting with a monoclonal antibody CC1 against human chymase. Chymase prepared for this study had a single diffuse band on SDS‐PAGE. There was no elastolytic activity and negligible tryptase contamination in this preparation.
As chymase is enzymatically unstable in physiological solutions, considerable care was taken in its preparation. Purified chymase stored in high salt buffer was diluted immediately prior to its injection, first with sterile distilled water, adjusting the NaCl concentration to 0.15 mol·L−1, and then with normal saline (NS) to obtain the required chymase concentration. Human serum albumin (HSA) in saline was used as a foreign protein control. Where added, proteinase inhibitors or drugs were incubated with chymase for 30 min on ice before injection.
Tryptase was purified from human lung tissues by high salt extraction, heparin agarose and immunoaffinity chromatography procedures with monoclonal antibody AA5 against tryptase as described previously (He et al., 1997). The specific activity of the tryptase used in these studies was 1.96 U·mg−1. The preparation had no detectable chymotryptic or elastolytic activity, and endotoxin levels were very low, being less than 49 pg·mg−1 tryptase.
Mouse i.p. injection and cell count
The procedure was adapted from that described previously (He et al., 1997). Briefly, various concentrations of human chymase, calcium ionophore (CI), eotaxin, RANTES, chymotrypsin and HSA, 5‐HT at 1 μg·mL−1, soybean trypsin inhibitor (SBTI) at 20 μg·mL−1, α1‐antitrypsin at 20 μg·mL−1, α1‐antichymotrypsin at 20 μg·mL−1, secretory leukocyte proteinase inhibitor (SLPI) at 20 μg·mL−1, Z‐Ile‐Glu‐Pro‐Phe‐CO2 Me (ZIGPFM) at 400 ng·mL−1, chymostatin at 10 μg·mL−1, WAY100635 at 20 μg·mL−1, human tryptase at 1 μg·mL−1, heat inactivation of human chymase at 10 μg·mL−1, mouse neutrophil elastase at 1 μg·mL−1 or NS in 0.5 mL volume were injected i.p. into BALB/C mice, whose abdominal skin was swabbed with 70% ethanol. HSA, mouse neutrophil elastase and NS were used for foreign protein control, unrelated protease control and vehicle respectively. At 10 min, 3, 6 or 16 h following injection, animals were killed, and their peritoneal lavage fluids were collected into heparin‐treated tubes and centrifuged at 266× g for 10 min at 4°C. While supernatant was collected for elisa analysis, cells were resuspended in 2.0 mL MEM, stained with 0.1% trypan blue and counted using an Improved Neubauer haemocytometer (for total cell numbers). Cytocentrifuge preparations were made, air dried and stained with modified Wright's stain. Differential cell counts were performed for a minimum of 500 cells. The results are expressed as absolute numbers of mast cells per mouse peritoneum.
For certain experiments, various concentrations of human chymase and CI were injected in the peritoneum of mast cell‐deficient (KitW‐4Bao) mice and wild‐type C57BL/6J mice for 10 min, 3 and 6 h before their peritoneal lavage fluids were collected for mast cell count, 5‐HT and eotaxin measurement.
Pretreatment of mice with antibodies and drugs
For the experiments investigating mast cell migration mechanism, groups of mice were pretreated i.v. (tail vein injection) with monoclonal antibodies against the adhesion molecules L‐selectin, CD11a/CD18 and intercellular adhesion molecule 1 (ICAM‐1) (all at a dose of 1.0 mg·kg−1; Zamuner et al., 2005), mast cell stabilizer sodium cromoglycate (20 mg·kg−1; Leza et al., 1992), histamine H1 receptor antagonist terfenadine (2 mg·kg−1; Tryka et al., 1999) or 5‐HT antagonist WAY100635 (10 μg), respectively, for 30 min before intra‐peritoneal injection of 1.0 μg·mL−1 of chymase, 10 ng·mL−1 of eotaxin, 10 ng·mL−1 of RANTES or 1.0 μg·mL−1 of 5‐HT. Control animals received an equivalent dose of the corresponding normal rat or hamster IgG isotype control or drug alone. At 6 h following injection, the mice were killed and their peritoneal lavages were processed as described above.
Trans‐endothelial migration of mast cells in vitro
To further investigate the effect of chymase on mast cell migration, a co‐culture system with HMC‐1 and HMEC‐1 cells was established. The migration of HMC‐1 cells was assessed by using E‐16‐well plates and the xCELLigence technology (Acea Bioscience, San Diego, CA, USA) (Limame et al., 2012). Briefly, 165 μL medium solutions containing N‐formyl‐methionyl‐leucyl‐phenylalanine (fMLP) alone at 10 μM or chymase at 0, 0.1, 0.3 and 1.0 μg·mL−1 with or without chymostatin (1.0 μg·mL−1) or SBTI (1.0 μg·mL−1) were added into the lower chamber of E‐16‐well plates, respectively, and incubated for 1 h. For the upper chamber, 30 μL HMEC‐1 cells (6 × 104 cells per well) were seeded in, and cells grew for 1 h. Anti‐human ICAM‐1 antibody (50 μL) at a concentration of 10 μg·mL−1 was then added in specified wells and cultured for 15 min. This was followed by seeding 50 μL HMC‐1 cell suspension (6 × 104 cells per well). All control wells received only the equal volume of medium. The HMC‐1 migration number, expressed as a cell index value, was monitored for 48 h. Cells in the lower chamber were collected for flow cytometric analysis of HMC‐1. The experiments were conducted in duplicate and repeated four times.
Flow cytometry analysis of mast cells and endothelial cells
Cells in lower chambers were collected in 1 mL of 1% BSA/PBS and pelleted by centrifugation. This was followed by incubation of cells with PE‐conjugated anti‐human CD117 antibody at room temperature for 15 min in the dark. PE‐conjugated mouse IgG1 was used as an isotype control. After being washed, cells were resuspended in FACS‐Flow solution and analysed with a FACS Verse flow cytometer with FlowJo software version 7.0 (Treestar, Ashland, USA).
elisa
Levels of mouse mast cell chymase 1 (CAM‐1), 5‐HT, eotaxin and RANTES were measured by using elisa kits according to the manufacturer's instruction.
Statistics
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Statistical analyses were performed by using SPSS software (version 21.0, IBM Corporation). Data are displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values for the number of experiments indicated. Where Kruskal–Wallis analysis indicated significant differences between groups, a pairwise test was used for multiple comparisons between the groups. Data for trans‐endothelial migration of HMC‐1 cells are expressed as mean ± SEM. Where ANOVA indicated significant differences between groups with ANOVA, a Bonferroni method was applied for further comparison. For all analyses, P < 0.05 was considered statistically significant.
Materials
The following compounds were purchased from Sigma‐Aldrich (St Louis, MO, USA): human α1‐antitrypsin, human α1‐antichymotrypsin, human chymotrypsin, mouse SLPI, SBTI, chymostatin, CI A23187, fMLP, mouse eotaxin, mouse RANTES, sodium cromoglycate, terfenadine, SAAPP, N‐benzoyl‐D, L‐arginine‐p‐nitroanilide, HSA, 5‐HT and its receptor antagonist WAY100635. MCDB 131 medium, RPMI 1640 medium, FBS, penicillin/streptomycin, MEM containing 25 mM HEPES and Dulbecco's PBS (DPBS) were obtained from Invitrogen‐Gibco®/Life Technologies (Grand Island, USA). Heparin agarose and S‐200 Sephacryl agarose were purchased from Pharmacia (Uppsala, Sweden). Rat monoclonal antibodies including anti‐mouse CD11a lymphocyte function‐associated antigen 1 α chain, anti‐mouse CD18 (integrin β2 chain), anti‐mouse CD62L (L‐selectin), rat IgG2a isotype standard; hamster anti‐mouse CD54 (ICAM‐1) antibody and hamster IgG1 isotype standard, recombinant mouse neutrophil elastase and mouse eotaxin and RANTES elisa kits were purchased from R&D Systems (Minneapolis, USA). ZIGPFM was synthesized by CL Bio‐Scientific Inc (Xi'an, China) with a purity >98%. PE‐conjugated anti‐human CD117 antibody was obtained from Biolegend (San Diego, USA). The 5‐HT elisa kit was purchased from Abcam (Cambridge, UK). Mouse mast cell chymase 1 elisa kit was purchased from Aviva System Biology (San Diego, USA). Modified Wright's stain was from BaSo (Zhuhai, China). Most of the general chemicals, such as salts and buffer components, were of analytical grade.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Results
CI‐induced mast cell accumulation and chymase release in the peritoneum of mice
We recently reported that the mast cell secretagogue compound 48/80 is a potent stimulus of mast cell accumulation (Liu et al., 2016). Therefore, it is likely that CI, a mast cell activator, can also induce mast cell recruitment. To further confirm the concept that mast cell degranulation may provoke mast cell accumulation in vivo, we investigated the ability of CI to induce mast cell accumulation in a mouse peritoneal inflammation model. The result showed that CI induced a dose‐dependent mast cell accumulation in mouse peritoneum at 6 h (Figure 1A) and 16 h (Figure 1B) following injection. As little as 0.01 μM CI was capable of inducing up to approximately 86% increase in mast cell accumulation in mouse peritoneum at 16 h following injection. Up to 1.4‐fold increase in accumulation of mast cells was achieved when 1.0 μM of CI was injected for 16 h. It was observed that chymase inhibitors α1‐antitrypsin, SBTI and SLPI, inhibited CI‐induced mast cell accumulation by up to 100 and 88.5%, 81.3 and 100% and 94.5 and 98.6% at 6 and 16 h following injection, respectively, indicating that the unique mast cell granule product chymase may contribute to CI‐induced mast cell accumulation.
Figure 1.
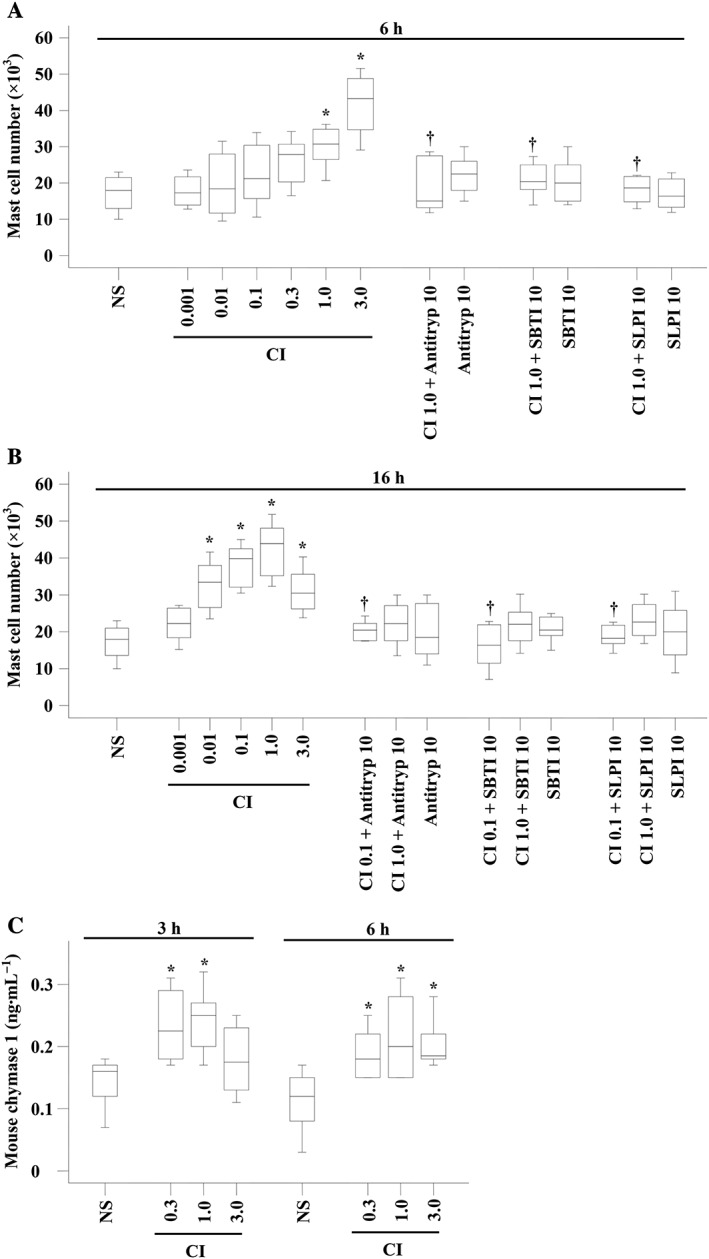
Mast cell accumulation and chymase production in the peritoneum of mice induced by CI A23187 (μM). Various concentrations of CI in the presence or absence of α1‐antitrypsin (Antitryp, μg), SBTI (μg) and SLPI (μg) were injected i.p. in mice for 6 h (A) and 16 h (B). Various concentrations of CI were injected for 3 and 6 h before their peritoneal lavage fluids were collected for chymase measurement (C). Normal saline (NS) was employed as a vehicle. Data are displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values. Each group of data represents results from six to seven animals. *P < 0.05 compared with the corresponding NS group. † P < 0.05 compared with the corresponding stimulus alone group.
In order to confirm that CI‐induced accumulation of mast cells is via the release of endogenous chymase from mast cells, we examined the levels of chymase in the peritoneum of mouse following CI administration. The results showed that CI at 0.3 and 1.0 μM provoked up to 44% increase in production of mouse mast cell chymase 1 in mouse peritoneum following 3 and 6 h injection (Figure 1C).
Induction of mast cell accumulation by chymase and α1‐chymotrypsin
We recently reported that tryptase is potent at inducing chemotaxis of mast cells (Liu et al., 2016), which suggests a self‐amplification mechanism of mast cell accumulation. Since chymase and tryptase are both located in the secretory granules of mast cells (Schwartz et al., 1987), we anticipated that chymase may also have the ability to elicit mast cell accumulation. It was found that chymase induced dose‐dependent mast cell accumulation in the peritoneum of mice following 10 min, 3, 6 and 16 h injection (Figure 2). Chymase provoked mast cell accumulation initiated at 10 min following injection (Figure 2D) and lasted at least to 16 h (Figure 2F). As little as 0.005 μg of chymase was capable of eliciting marked mast cell accumulation at 3 h following injection, and the maximum of 5.4‐fold increase in mast cells was achieved when 5.0 μg of chymase was injected for 3 h (Figure 2D).
Figure 2.
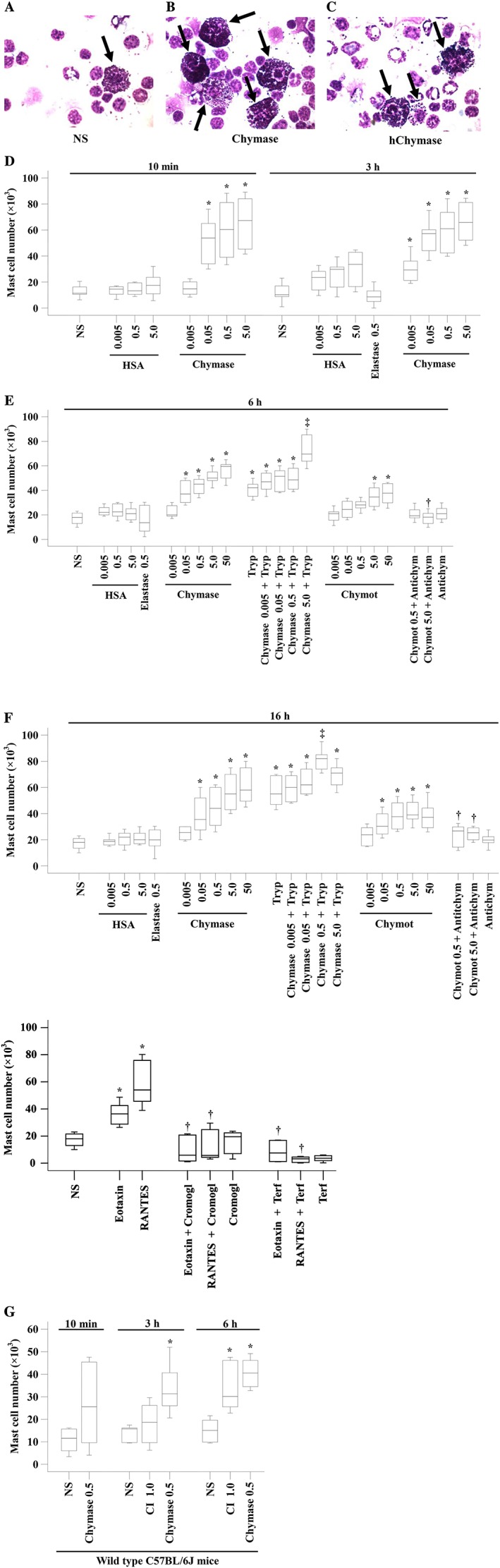
Mast cell accumulation in the peritoneum of mice induced by chymase and α1‐chymotrypsin. (A), (B) and (C) Representative graphs of mast cell accumulation following injection of normal saline (NS), chymase (5 μg) and heat‐inactived chymase (hChymase, 5 μg) respectively. (D) Various concentrations of chymase (μg), mouse neutrophil elastase (μg) and HSA (μg) were injected i.p. in mice for 10 min and 3 h. (E) Various concentrations of HSA, elastase and chymase in the presence or absence of tryptase (Tryp, 0.5 μg), or α1‐chymotrypsin (Chymot, μg) in the presence or absence of α1‐antichymotrypsin (Antichym, 10 μg) were injected i.p. for 6 h. (F) Various concentrations of HSA, elastase and chymase in the presence or absence of Tryp, or Chymot in the presence or absence of Antichym were injected i.p. for 16 h. (G) Chymase (μg) and CI (μM) were injected i.p. in wild‐type C57BL/6J mice. Data are displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values. Each data group represents results from six to seven animals. *P < 0.05 compared with the corresponding NS group. † P < 0.05 compared with the corresponding stimulus alone group. ‡ P < 0.05 compared with the corresponding chymase alone group.
Since chymase belongs to the chymotrypsin family of protease (Sukenaga et al., 1993) and chymase‐induced mast cell accumulation may depend on its enzymatic activity, we employed the common chymotryptic enzymes α1‐chymotrypsin (Niemann, 1964) and elastase as positive and negative controls, respectively, in the present study. The results showed that α1‐chymotrypsin also induced a dose‐dependent increase in mast cell accumulation at 6 h (Figure 2E) and 16 h (Figure 2F) following injection. As little as 0.05 μg of α1‐chymotrypsin was able to elicit marked mast cell accumulation at 16 h following injection, and the maximum of 1.2‐fold increase in mast cells was achieved when 5.0 μg of α1‐chymotrypsin was injected for 16 h (Figure 2F). These results suggest that chymase‐induced mast cell accumulation is likely to be as results of its enzymatic activity. In contrast, elastase failed to provoke mast cell accumulation at 3, 6 and 16 h following injection.
Inhibitors of chymase including α1‐antichymotrypsin, chymostatin, SBTI, SLPI and ZIGPFM suppressed chymase‐induced mast cell accumulation by up to 93.8, 95.9, 53.1, 59.9 and 90.5% respectively, whereas heat‐inactivating chymase (56°C for 120 min) abolished up to 89.1% of its ability to accumulate mast cells (Table 1), confirming that chymase‐induced mast cell accumulation is dependent on its enzymatic activity. Similarly, α1‐antichymotrypsin reduced α1‐chymotrypsin‐induced mast cell accumulation by up to 98.8% at 6 h following injection. These inhibitors by themselves did not alter the number of mast cells in the peritoneum of mice when injected alone (data not shown).
Table 1.
Inhibition of chymase‐induced mast cell infiltration by inhibitors of chymase and heat inactivation of the enzyme at 6 and 16 h following injection
| Percentage inhibition of mast cell infiltration | ||
|---|---|---|
| Treatment | 6 h Median (range) | 16 h Median (range) |
| α1‐antitrypsin (10 μg) | 93.8 (31.3–100)* | 86.5 (40.5–100)* |
| Chymostatin (5 μg) | 75 (56.3–100)* | 95.9 (59.5–100)* |
| SBTI (10 μg) | 53.1 (31.3–93.8)* | NA |
| SLPI (10 μg) | 56.6 (26.6–100)* | 59.9 (40.8–100)* |
| ZIGPFM (200 ng) | 68.8 (56.3–81.3)* | 90.5 (37.8–100)* |
| Heat inactivation (5 μg) | 89.1 (43.8–100)* | 74.3 (45.9–94.6)* |
The values shown are median (range) for six to eight individual mice. The inhibitors were incubated with 5 μg of chymase for 20 min before an i.p. injection.
P < 0.05 compared with the uninhibited control mice. NA, not available.
In order to further confirm the abilities of chymase and CI to induce mast cell accumulation, mast cell‐deficient (KitW‐4Bao) and wild‐type C57BL/6J mice were employed. The result showed that chymase at 0.5 μg and CI at 1.0 μM induced up to 1.69‐fold and 0.85‐fold increase in peritoneal mast cells in wild‐type C57BL/6J mice at 6 h following injection (Figure 2G). Mast cell‐deficient mice did not show any mast cells in their peritoneum.
Influence of tryptase on chymase‐induced mast cell accumulation
Tryptase and chymase are released from mast cells at the same time upon mast cell degranulation and possibly from the same granules (Schwartz, 1990). Therefore, they may have a mutual effect on their ability to accumulate mast cells. To investigate this possible interaction, we determined the influence of tryptase on chymase‐induced mast cell accumulation in the present study. The results show that tryptase at 0.5 μg had an additive effect on 5.0 μg chymase‐induced mast cell accumulation at 6 h (Figure 2E) and on 0.5 μg chymase‐induced mast cell accumulation at 16 h (Figure 2F) following injection.
Induction of trans‐endothelium migration of mast cells in vitro by chymase
It is reported that human tryptase can induce trans‐endothelium migration of mast cells in vitro (Liu et al., 2016), indicating that tryptase is a potent chemoattractant of mast cells. In order to understand the mechanism of chymase‐induced mast cell accumulation, we investigated the ability of chymase to induce the trans‐endothelium migration of mast cells in vitro. The results show that chymase induced up to 46.2% increase in migration of mast cells. An anti‐ICAM‐1 antibody, chymostatin and SBTI completely abolished chymase‐induced mast cell migration (Figure 3), suggesting that the action of chymase was mediated by ICAM‐1 and dependent on its enzymatic activity.
Figure 3.
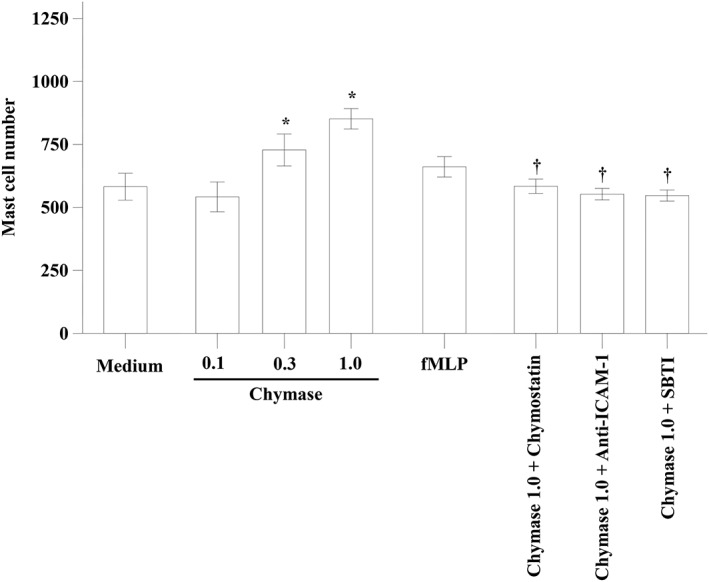
The trans‐endothelium migration of mast cells in vitro induced by chymase. Various concentrations of chymase (μg·mL−1) with or without chymostatin (1.0 μg·mL−1) or SBTI (1.0 μg·mL−1) were added to chambers of E‐16‐well plates, respectively, and incubated for 1 h. Anti‐human ICAM‐1 antibody (Anti‐ICAM‐1, 50 μL of 10 μg·mL−1 solution) was added to specified wells and cultured for 15 min. Cells in the lower chamber were collected for flowcytometric analysis of HMC‐1 mast cells. fMLP (10 μM) was used as a positive control. Values shown are mean ± SEM for four independent experiments. *P < 0.05 compared with the response to medium alone control group. † P < 0.05 compared with the response to the corresponding stimulus alone group.
Effect of anti‐ICAM‐1, anti‐L‐selectin, anti‐CD11a and anti‐CD18 antibodies and drugs on chymase‐induced mast cell accumulation
In order to further understand the mechanism of chymase‐induced mast cell accumulation, we investigated the effect of adhesion molecules on chymase‐induced mast cell accumulation in the peritoneum of mice. The results show that anti‐CD11a, anti‐CD18, anti‐L‐selectin, anti‐ICAM‐1 antibodies, sodium cromoglycate and terfenadine inhibited chymase provoked mast cell accumulation by 85.2, 81.5, 84.8, 74.1, 81.5 and 74.8%, respectively, when they were i.v. injected into mice, suggesting that chymase‐elicited mast cell accumulation was dependent upon the activities of the CD11a/CD18 complex, L‐selectin and ICAM‐1 (Figure 4).
Figure 4.
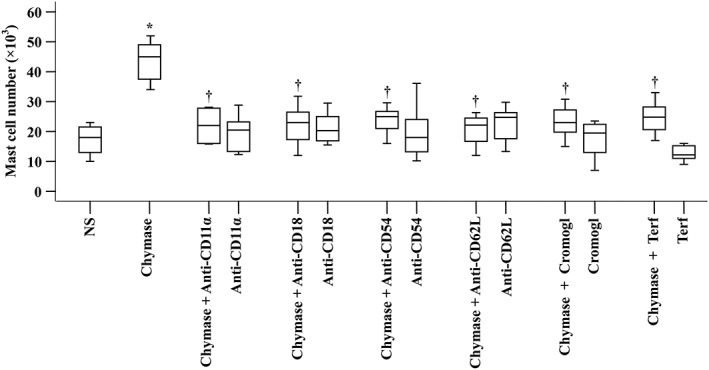
Inhibition of mast cell accumulation by antibodies against cell adhesion molecules and drugs. Mice were pretreated with a monoclonal antibody against either L‐selectin (anti‐CD62L), CD11a (anti‐CD11a), CD18 (anti‐CD18) or ICAM‐1 (anti‐CD54), respectively (all at a dose of 1 mg·kg−1), or with sodium cromoglycate (Cromogl, 20 mg·kg−1) or terfenadine (Terf, 2 mg·kg−1) for 30 min before i.p. injection of chymase (0.5 μg) for 6 h. NS was employed as a vehicle. Data were displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values. Each data group represents results from six and seven animals. *P < 0.05 compared with the NS group. † P < 0.05 compared with chymase alone group.
Effect of chymase on mast cell‐derived 5‐HT and eotaxin release in the peritoneum of mice
Since inhibitors of chymase did not completely abolish chymase‐induced mast cell accumulation and migration, it is possible that chymase may provoke mast cell accumulation through some indirect mechanisms. It has been reported previously that chymase is capable of inducing mast cell degranulation (He et al., 1998); we therefore investigated the ability of chymase to induce the release of mast cell‐derived 5‐HT, eotaxin and RANTES in the peritoneal lavage of mice. The results show that chymase at 0.5 μg increased, by up to 1.8‐fold, 5‐HT release in the peritoneum of BALB/C mice, which is comparable to that induced by CI at 3 h following injection (Figure 5A). Similarly, chymase provoked up to 3.2‐fold eotaxin increase (Figure 5B) but had little effect on level of RANTES in mouse peritoneum (data not shown). Chymostatin (5 μg) suppressed chymase‐induced 5‐HT release by 88.6% and completely abolished chymase‐induced eotaxin release (Figure 5B). 5‐HT at 0.5 μg appeared to be more potent than chymase at inducing the production of eotaxin. It increase the production of by more than 3.2‐fold in mouse peritoneum. It was observed that 5‐HT‐induced production of eotaxin was almost completely abolished by its inhibitor WAY100635 (Figure 5B). Chymase at 0.5 μg also increased the release of 5‐HT (Figure 5C) and eotaxin (Figure 5D) in wild‐type C57BL/6J mice. Compared with wild‐type C57BL/6J mice, mast cell‐deficient (KitW‐4Bao) mice had low 5‐HT (Figure 5C) and eotaxin (Figure 5D) levels in their peritoneum. Chymase had little effect on 5‐HT (Figure 5C) and eotaxin (Figure 5D) levels in mast cell deficient (KitW‐4Bao) mice.
Figure 5.
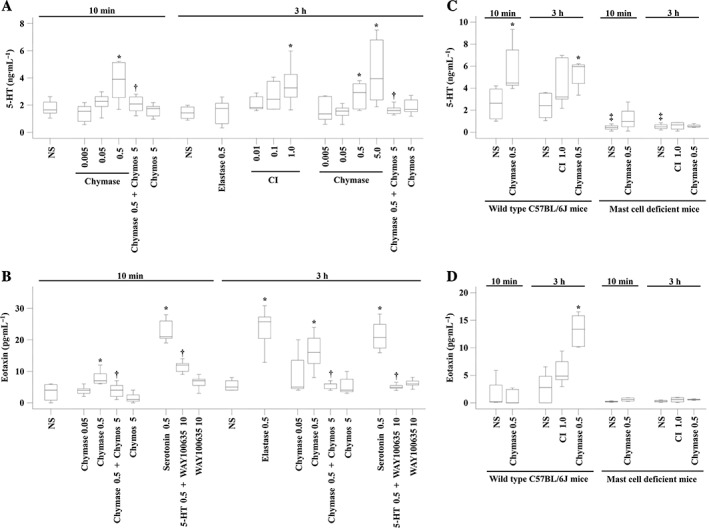
The release of 5‐HT and eotaxin induced by chymase in the peritoneum of mice. (A) Various concentrations of chymase in the presence or absence of chymostatin (Chymos, 5 μg), elastase (μg) or CI A23187 (μM) were injected i.p. 10 min and 3 h before the peritoneal lavage fluids were collected for 5‐HT measurement. (B) Various concentrations of chymase in the presence or absence of chymostatin (Chymos, 5 μg), 5‐HT (μg) in the presence or absence of WAY100635 (μg) or elastase (μg) were injected i.p. 10 min and 3 h before the peritoneal lavage fluids were collected for eotaxin measurement. Chymase (μg) and CI (μM) were injected i.p. in wild‐type C57BL/6J mice and mast cell‐deficient (KitW‐4Bao) mice before peritoneal levels of 5‐HT (C) or eotaxin (D) were determined. NS was employed as a vehicle. Data are displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values. Each data group represents the results from six to seven animals. *P < 0.05 compared with the corresponding NS group. † P < 0.05 compared with the corresponding stimulus alone group.
Induction of mast cell accumulation by chemokines
5‐HT is used as an indicator of mouse mast cell degranulation (Krop et al., 2010), but little is known of its ability to induce mast cell accumulation. After confirmation that chymase can provoke increased 5‐HT and eotaxin release in mouse peritoneum, the next issue that should be addressed is whether 5‐HT and these chemokines have the ability to provoke mast cell accumulation. We therefore investigated the effect of 5‐HT, eotaxin and RANTES on mast cell accumulation in mouse peritoneum. The results showed that 5‐HT at 0.5 μg induced dramatic mast cell accumulation in mouse peritoneum, which could be inhibited by WAY100635 at 10 μg (Figure 6A). Eotaxin induced marked mast cell accumulation in peritoneum of mice at 3, 6 and 16 h following injection (Figure 6B). As little as 0.05 ng eotaxin induced up to 1.4‐fold increase in mast cell number at 3 h following injection. The maximum increase in mast cell number, 3.2‐fold, was provoked by 5.0 ng eotaxin at 16 h following injection (Figure 6B). Similarly, 0.05 ng RANTES elicited up to 1.3‐fold increase in mast cell number at 3 h following injection. The maximum increase in mast cell number, 3.9‐fold, was provoked by 5.0 ng RANTES at 16 h following injection (Figure 6C).
Figure 6.
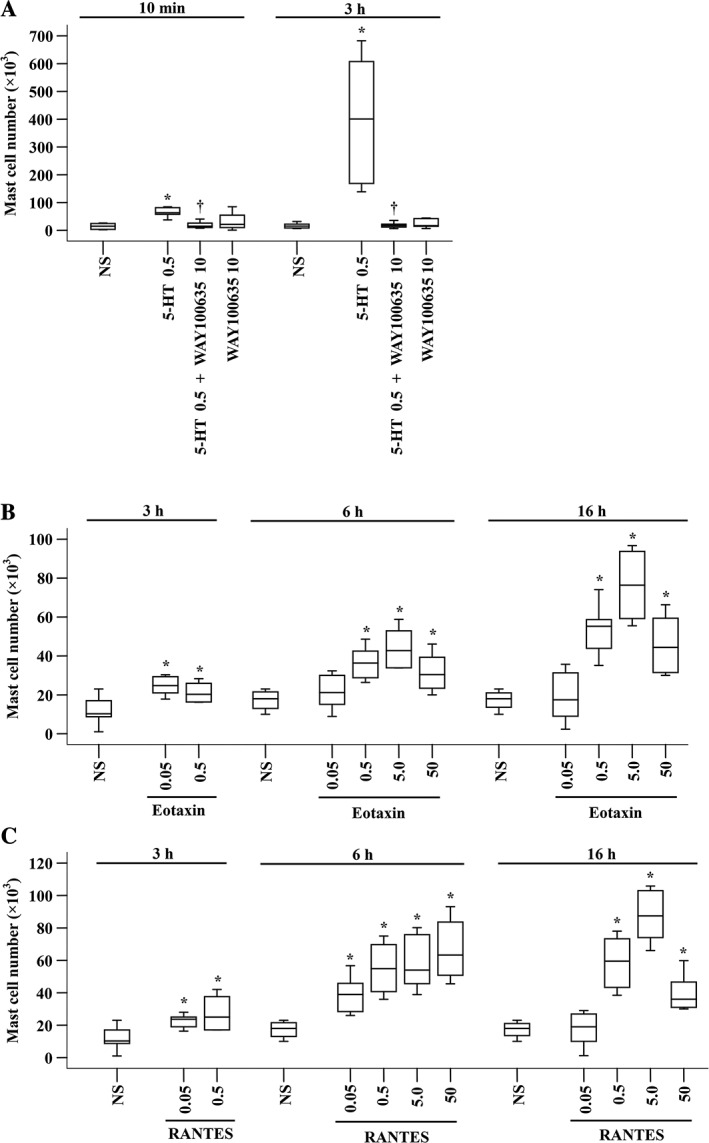
Mast cell accumulation in the peritoneum of mice induced by 5‐HT and chemokines. (A) 5‐HT (μg) in the presence or absence of WAY100635 (μg) was injected i.p. for 10 min or 3 h. (B) Various concentrations of eotaxin (ng) or (C) RANTES (ng) were injected i.p. for 3, 6 and 16 h. NS was employed as a vehicle. Data are displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values. Each group of data represents results from six to seven animals. *P < 0.05 compared with the corresponding NS group. † P < 0.05 compared with the corresponding stimulus alone group.
Effect of anti‐ICAM‐1, anti‐L‐selectin, anti‐CD11a and anti‐CD18 antibodies and drugs on chemokine‐induced mast cell accumulation
In order to understand the migration mechanism of the chemokine‐induced mast cell accumulation, we investigated the effect of adhesion molecules, sodium cromoglycate and terfenadine on eotaxin‐ and RANTES‐induced mast cell accumulation in the peritoneum of mice. The results show that eotaxin‐induced mast cell accumulation was inhibited by pretreatment with anti‐L‐selectin antibody (injected i.v.), and RANTES‐elicited mast cell infiltration was suppressed by pretreatment with anti‐CD11a, anti‐CD18 and anti‐CD54 antibodies (injected i.v.) (Figure 7A). Pretreatment of mice with sodium cromoglycate or terfenadine for 30 min before peritoneal injection of eotaxin or RANTES completely abolished eotaxin‐ or RANTES‐induced mast cell accumulation in the peritoneum of mice (Figure 7B).
Figure 7.
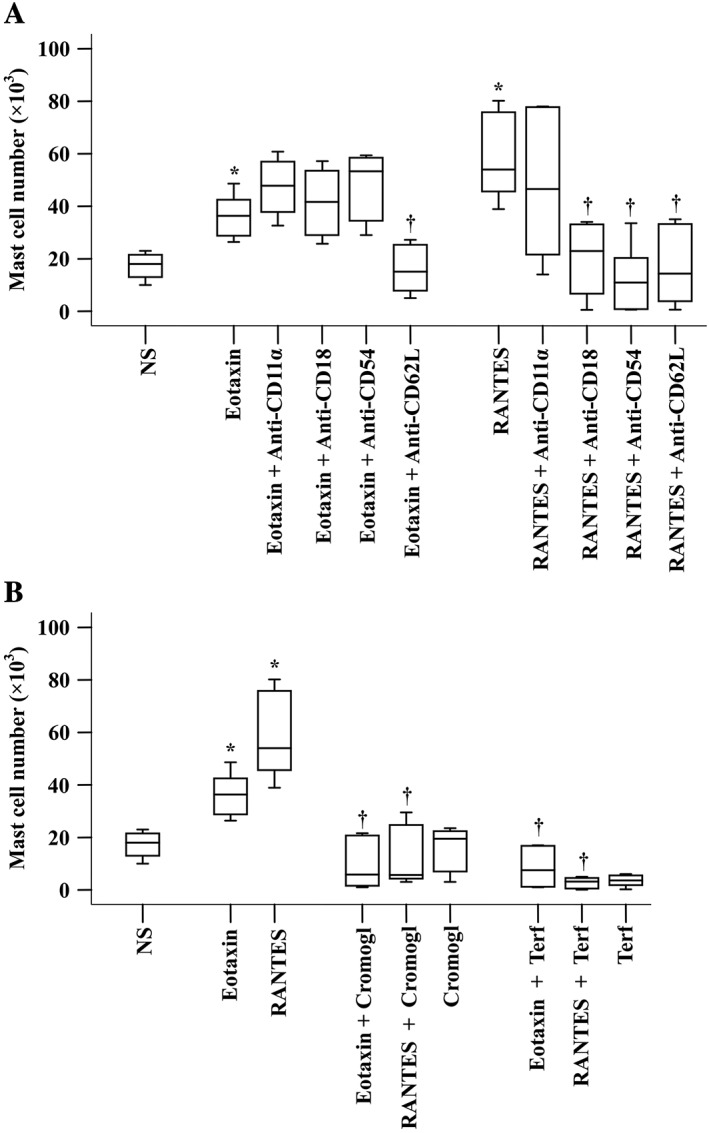
Inhibition of mast cell accumulation induced by antibodies against cell adhesion molecules and drugs. (A) Mice were pretreated with monoclonal antibody against L‐selectin (anti‐CD62L), CD11a (anti‐CD11a), CD18 (anti‐CD18) and ICAM‐1 (anti‐CD54), respectively (all at a dose of 1 mg·kg−1), for 30 min before i.p. injection of eotaxin (5 ng) or RANTES (5 ng) for 6 h. (B) Mice were pretreated with sodium cromoglycate (Cromogl, 20 mg·kg−1) and terfenadine (Terf, 2 mg·kg−1) for 30 min before i.p. injection of eotaxin (5 ng) or RANTES (5 ng) for 6 h. NS was employed as a vehicle. Data are displayed as a boxplot, which indicates the median, interquartile range, the largest and smallest values. Each data group represents results from six to seven animals. *P < 0.05 compared with the corresponding NS group. † P < 0.05 compared with the corresponding stimulus alone group.
Discussion
As for compound 48/80, CI is a potent mast cell degranulator. Thus, CI‐induced mast cell accumulation is most likely mediated by mast cell products released upon degranulation. Among mast cell granule products, tryptase (Liu et al., 2016) and histamine (Hofstra et al., 2003) have been found to provoke mast cell accumulation. Since both tryptase and histamine can activate mast cells and can be released from mast cells, a unique self‐amplification mechanism of mast cell infiltration and activation was previously proposed (He et al., 2012). As a major mast cell granule product, chymase may also have ability in induction of mast cell accumulation. Marked inhibition of CI‐induced mast cell accumulation by inhibitors of chymase and CI‐provoked production of mouse mast cell chymase 1 indicate that chymase is also likely to participate in mast cell accumulation in the peritoneum of mice. Indeed, the current study demonstrates for the first time that chymase is a potent chemoattractant for mast cells. Since chymase can induce mast cell degranulation (He and Walls, 1998b), the current finding that chymase provokes mast cell infiltration proposes a novel self‐amplification mechanism of mast cell accumulation.
Since airway higher responsiveness to mannitol in asthma is associated with chymase‐positive mast cells (Sverrild et al., 2016), chymase modulates IL‐33 levels and controls allergic sensitization in house‐dust‐mite‐induced airway inflammation (Waern et al., 2013), and chymase contributes to the pathogenesis of chronic dermatitis (Tomimori et al., 2002b), our current finding ought to be important in understanding the mechanisms of chymase‐related inflammatory diseases, including allergy.
It seems likely that chymase‐induced mast cell accumulation depends on its enzymatic activity as several inhibitors of chymase hydrolytic activity blocked chymase actions on mast cell migration. Chymase chemotactic activity appears to also rely on its intact catalytic site, as heat‐inactivation of the enzyme resulted in almost complete loss of its ability to induce mast cell recruitment. The enzymatic activity‐dependent function of chymase has been previously observed. For example, α1‐chymotrypsin, chymostatin, SBTI, SLPI and ZIGPFM suppressed chymase‐induced neutrophil and macrophage accumulation in the mouse peritoneum (He et al., 2004). Also, natural and synthetic chymase inhibitors eliminated histamine release from human mast cells ex vivo (Dietze et al., 1990). Another piece of evidence demonstrating that enzymatic activity is required for chymase‐induced mast cell migration is that α1‐chymotrypsin can also induce mast cell activation; they both belong to chymotrypsin family of proteases. Since α1‐chymotrypsin and chymase have a similar potency at inducing mast cell accumulation, we believe further that the action of chymase is dependent on its enzymatic activity.
It is likely that ICAM‐1 is a key adhesion molecule for chymase‐induced mast cell migration as it mediates the migration of these cells both in vivo and in vitro. The findings that ICAM‐1‐mediated mast cell migration has been observed in IL‐29‐induced mast cell accumulation (He et al., 2010) and that L‐selectin or ICAM‐1 deficiency reduces an immediate‐type hypersensitivity response by preventing mast cell recruitment (Shimada et al., 2003) supports the view that ICAM‐1 plays a key role in mast cell accumulation. L‐selectin also appears to be an important adhesion molecule for mast cell migration as it not only mediates chymase‐induced mast cell accumulation but also regulates mast cell recruitment in immediate‐type hypersensitivity response in a mouse model of contact atopic dermatitis (Shimada et al., 2003). Furthermore, the CD11a/CD18 complex seems to participate in chymase‐induced mast cell migration as anti‐CD11a and anti‐CD18 antibodies inhibited the action of chymase. Since anti‐CD11a and anti‐CD18 antibodies can suppress tryptase‐induced mast cell accumulation as well (Liu et al., 2016), it seems likely that the CD11a/CD18 complex plays an important role in protease‐induced mast cell accumulation.
The similar adhesion mechanisms involved in tryptase‐ and chymase‐provoked mast cell infiltration and the fact that trypase and chymase are released together from degranulated mast cells (Schwartz, 1990) indicate that there may be interactions between these two major mast cell granule products. Indeed, the additive or even synergistic interactions between tryptase and chymase were observed in the present study, which suggests that there must be some different adhesion and migration mechanisms involved in tryptase‐ or chymase‐induced mast cell accummulation. Obviously, more work is required to address this issue further.
It is reported that chymase induces release of stem cell factor (SCF) from human keratinocytes; the soluble SCF released then provokes mast cell accumulation (Nilsson et al., 1994). Similarly, chymase has been found to elicit IL‐8 release from eosinophils (Wong et al., 2009), and IL‐8 can recruit mast cells through CXCR1 (Lippert et al., 1998) and CXCR2 (Nilsson et al., 1999) receptors on the surface of HMCs. RANTES can be released from mast cells (Shakoory et al., 2004), and RANTES is chemotactic for mast cells (Nilsson et al., 1994; 1999). Previously, little was known of the influence of eotaxin on mast cell migration, but our current observations provide a clear message that eotaxin is probably a chemokine for mast cells. Similar to its effect on monocytes and neutrophils (Tani et al., 2000), chymase showed potent chemotactic activity for mast cells. The potency of chymase at inducing the trans‐endothelium migration of mast cells was comparable to that of fMLP.
A report that 5‐HT levels and the density of mast cells in the inflamed colon was higher than in controls suggests that the increase in 5‐HT levels in 2,4,6‐trinitrobenzene sulfonic acid (TNBS) ‐induced colitis may result from infiltrated mast cells (Magro et al., 2006). Sumatriptan, a 5‐HT receptor agonist, enhanced thalamic mast cell population, especially those containing 5‐HT (Dubayle et al., 2005), indicating that mast cells containing 5‐HT‐ and 5‐HT‐induced mast cell accumulation is mediated by its receptor. Taken together the fact that chymase induces 5‐HT release and mast cell accumulation and that both mouse and HMCs respond to 5‐HT through the 5‐HT1A receptor (Kushnir‐Sukhov et al., 2006), it is likely that 5‐HT contributes to chymase‐induced mast cell accumulation.
Inhibition of chymase‐induced mast cell accumulation by sodium cromoglycate may result from inhibition of the expression of ICAM‐1, as sodium cromoglycate is reported to be able to reduce mast cell numbers and expression of ICAM‐1 in bronchial mucosa of asthmatics (Hoshino and Nakamura, 1997). In contrast, inhibition of chymase‐induced mast cell accumulation by terfenadine could be due to the inhibitory activity of terfenadine on anti‐IgE‐induced mediator release from lung and skin mast cells (Massey et al., 1993) and inhibition of ICAM‐1 expression on epithelial cells (Ciprandi et al., 2003).
It should be noted that the chymase used in the mouse peritoneal experiments was human skin chymase. The amino acid identity between human chymase (UniProtKB – P23946) and murine chymase (UniProtKB – P21844) is 74.1%. In order to exclude the influence of foreign protein on mast cell accumulation, HSA was employed as a negative foreign protein control. The neglible effect of HSA on mast cell accumulation in mouse peritoneum supports that the postulate that the ability of chymase to induce mast cell accumulation is not related to it being a foreign protein.
In conclusion, chymase‐induced mast cell accumulation appears through a CD11a/CD18 complex, L‐selectin and ICAM‐1‐dependent mechanism and relies on its enzymatic activity. 5‐HT and eotaxin production from mast cells may also be involved in the event. The induction of mast cell accumulation by chymase implies further that chymase ought to be considered a major pro‐inflammatory mediator of mast cells. The provocation of mast cell accumulation by the major granule product of mast cells, chymase, suggests a novel self‐amplification mechanism for mast cell accumulation in allergic inflammation. Mast cell stabilizers as well as inhibitors of chymase may have potential as a treatment of allergic disorders.
Author contributions
H.Z. carried out most experiments and wrote a large part of the first draft of the manuscript. J.W., L.W. and M.Z. performed the elisa, analysed the data and wrote a part of the first draft of the manuscript. S.L., Z.F., C.X. and Y.Z. participated in animal experiment and cell counting. S.H. designed and conducted the study, analysed the data and wrote the second and final drafts of the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This project was sponsored by the grants from the ‘12th Five‐Year’ National Science and Technology Support Plan (2014BAI07B02); the National Natural Science Foundation of China (nos 81471592 and 81472016); Project of Scientific Research Special Fund for Public Industry in Forestry (201304103); Major Science and Technology Platform for Institution of Higher Education in Liaonng Province (2014168); ‘Twelfth Five‐Year’ Public Welfare Industry Special Scientific Research Project (2015SQ00136); Allergic Disease Translational Medicine Research Center of Liaoning Province (2015225016); Liaoning Provincial Engineering Research Center for Diagnosing and Treating Inflammatory Disease (20141093); Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC‐A06‐2014); and the National Natural Science Foundation of Liaoning Province (2014022027, 2014022019).
Zhang, H. , Wang, J. , Wang, L. , Zhan, M. , Li, S. , Fang, Z. , Xu, C. , Zheng, Y. , and He, S. (2018) Induction of mast cell accumulation by chymase via an enzymatic activity‐ and intercellular adhesion molecule‐1‐dependent mechanism. British Journal of Pharmacology, 175: 678–692. doi: 10.1111/bph.14117.
References
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciprandi G, Tosca MA, Cosentino C, Riccio AM, Passalacqua G, Canonica GW (2003). Effects of fexofenadine and other antihistamines on components of the allergic response: adhesion molecules. J Allergy Clin Immunol 112: S78–S82. [DOI] [PubMed] [Google Scholar]
- Conti P, Reale M, Barbacane RC, Felaco M, Grilli A, Theoharides TC (1998). Mast cell recruitment after subcutaneous injection of RANTES in the sole of the rat paw. Br J Haematol 103: 798–803. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze SC, Sommerhoff CP, Fritz H (1990). Inhibition of histamine release from human mast cells ex vivo by natural and synthetic chymase inhibitors. Biol Chem Hoppe Seyler 371 (Suppl): 75–79. [PubMed] [Google Scholar]
- Dubayle D, Serviere J, Menetrey D (2005). Evidence for serotonin influencing the thalamic infiltration of mast cells in rat. J Neuroimmunol 159: 20–30. [DOI] [PubMed] [Google Scholar]
- He S, Walls AF (1998a). Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo . Br J Pharmacol 125: 1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Walls AF (1998b). The induction of a prolonged increase in microvascular permeability by human mast cell chymase. Eur J Pharmacol 352: 91–98. [DOI] [PubMed] [Google Scholar]
- He S, Peng Q, Walls AF (1997). Potent induction of a neutrophil and eosinophil‐rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol 159: 6216–6225. [PubMed] [Google Scholar]
- He S, Gaca MD, Walls AF (1998). A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther 286: 289–297. [PubMed] [Google Scholar]
- He S, Gaca MD, McEuen AR, Walls AF (1999). Inhibitors of chymase as mast cell‐stabilizing agents: contribution of chymase in the activation of human mast cells. J Pharmacol Exp Ther 291: 517–523. [PubMed] [Google Scholar]
- He SH, Chen HQ, Zheng J (2004). Inhibition of tryptase and chymase induced nucleated cell infiltration by proteinase inhibitors. Acta Pharmacol Sin 25: 1677–1684. [PubMed] [Google Scholar]
- He S, Zhang H, Chen H, Yang H, Huang T, Chen Y et al (2010). Expression and release of IL‐29 by mast cells and modulation of mast cell behavior by IL‐29. Allergy 65: 1234–1241. [DOI] [PubMed] [Google Scholar]
- He S, Zhang H, Zeng X, Yang P (2012). Self‐amplification mechanisms of mast cell activation: a new look in allergy. Curr Mol Med 12: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Hofstra CL, Desai PJ, Thurmond RL, Fung‐Leung WP (2003). Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther 305: 1212–1221. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura Y (1997). The effect of inhaled sodium cromoglycate on cellular infiltration into the bronchial mucosa and the expression of adhesion molecules in asthmatics. Eur Respir J 10: 858–865. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop M, Ozunal ZG, Chai W, de Vries R, Fekkes D, Bouhuizen AM et al (2010). Mast cell degranulation mediates bronchoconstriction via serotonin and not via renin release. Eur J Pharmacol 640: 185–189. [DOI] [PubMed] [Google Scholar]
- Kushnir‐Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M et al (2006). 5‐Hydroxytryptamine induces mast cell adhesion and migration. J Immunol 177: 6422–6432. [DOI] [PubMed] [Google Scholar]
- Leza JC, Lizasoain I, Martin‐Clark OS, Lorenzo P (1992). Role of sodium cromoglycate on analgesia, locomotor activity and opiate withdrawal in mice. Psychopharmacology (Berl) 107: 595–600. [DOI] [PubMed] [Google Scholar]
- Limame R, Wouters A, Pauwels B, Fransen E, Peeters M, Lardon F et al (2012). Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real‐time technology and classic endpoint assays. PLoS One 7: e46536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert U, Artuc M, Grutzkau A, Moller A, Kenderessy‐Szabo A, Schadendorf D et al (1998). Expression and functional activity of the IL‐8 receptor type CXCR1 and CXCR2 on human mast cells. J Immunol 161: 2600–2608. [PubMed] [Google Scholar]
- Liu X, Wang J, Zhang H, Zhan M, Chen H, Fang Z et al (2016). Induction of mast cell accumulation by tryptase via a protease activated receptor‐2 and ICAM‐1 dependent mechanism. Mediators Inflamm 2016: 6431574: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro F, Fraga S, Azevedo I, Soares‐da‐Silva P (2006). Intestinal 5‐hydroxytryptamine and mast cell infiltration in rat experimental colitis. Dig Dis Sci 51: 495–501. [DOI] [PubMed] [Google Scholar]
- Massey WA, Charlesworth EN, Freidhoff L, Cooper P, Kagey‐Sobotka A, Lichtenstein LM (1993). Cutaneous IgE‐mediated inflammatory lesion size is inhibited by an H1 antagonist (terfenadine) while mediator release is unaffected in vivo and in vitro . Clin Exp Allergy 23: 399–405. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C (1964). Alpha‐chymotrypsin and the nature of enzyme catalysis. Science 143: 1287–1296. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Butterfield JH, Nilsson K, Siegbahn A (1994). Stem cell factor is a chemotactic factor for human mast cells. J Immunol 153: 3717–3723. [PubMed] [Google Scholar]
- Nilsson G, Mikovits JA, Metcalfe DD, Taub DD (1999). Mast cell migratory response to interleukin‐8 is mediated through interaction with chemokine receptor CXCR2/Interleukin‐8RB. Blood 93: 2791–2797. [PubMed] [Google Scholar]
- Schwartz LB (1990). Tryptase from human mast cells: biochemistry, biology and clinical utility. Monogr Allergy 27: 90–113. [PubMed] [Google Scholar]
- Schwartz LB (1994). Mast cells: function and contents. Curr Opin Immunol 6: 91–97. [DOI] [PubMed] [Google Scholar]
- Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM (1987). Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol 138: 2611–2615. [PubMed] [Google Scholar]
- Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G (2004). The role of human mast cell‐derived cytokines in eosinophil biology. J Interferon Cytokine Res 24: 271–281. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Hasegawa M, Kaburagi Y, Hamaguchi Y, Komura K, Saito E et al (2003). L‐selectin or ICAM‐1 deficiency reduces an immediate‐type hypersensitivity response by preventing mast cell recruitment in repeated elicitation of contact hypersensitivity. J Immunol 170: 4325–4334. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukenaga Y, Kido H, Neki A, Enomoto M, Ishida K, Takagi K et al (1993). Purification and molecular cloning of chymase from human tonsils. FEBS Lett 323: 119–122. [DOI] [PubMed] [Google Scholar]
- Sverrild A, Bergqvist A, Baines KJ, Porsbjerg C, Andersson CK, Thomsen SF et al (2016). Airway responsiveness to mannitol in asthma is associated with chymase‐positive mast cells and eosinophilic airway inflammation. Clin Exp Allergy 46: 288–297. [DOI] [PubMed] [Google Scholar]
- Tani K, Ogushi F, Kido H, Kawano T, Kunori Y, Kamimura T et al (2000). Chymase is a potent chemoattractant for human monocytes and neutrophils. J Leukoc Biol 67: 585–589. [DOI] [PubMed] [Google Scholar]
- Terakawa M, Tomimori Y, Goto M, Hayashi Y, Oikawa S, Fukuda Y (2005). Eosinophil migration induced by mast cell chymase is mediated by extracellular signal‐regulated kinase pathway. Biochem Biophys Res Commun 332: 969–975. [DOI] [PubMed] [Google Scholar]
- Terakawa M, Tomimori Y, Goto M, Fukuda Y (2006). Mast cell chymase induces expression of chemokines for neutrophils in eosinophilic EoL‐1 cells and mouse peritonitis eosinophils. Eur J Pharmacol 538: 175–181. [DOI] [PubMed] [Google Scholar]
- Tomimori Y, Muto T, Fukami H, Saito K, Horikawa C, Tsuruoka N et al (2002a). Chymase participates in chronic dermatitis by inducing eosinophil infiltration. Lab Invest 82: 789–794. [DOI] [PubMed] [Google Scholar]
- Tomimori Y, Muto T, Fukami H, Saito K, Horikawa C, Tsuruoka N et al (2002b). Mast cell chymase regulates dermal mast cell number in mice. Biochem Biophys Res Commun 290: 1478–1482. [DOI] [PubMed] [Google Scholar]
- Tryka E, Asano K, Ito J, Hisamitsu T, Suzaki H (1999). Influence of anti‐allergic agents on in vivo expression of co‐stimulatory molecules in normal mice. In Vivo 13: 415–420. [PubMed] [Google Scholar]
- Waern I, Lundequist A, Pejler G, Wernersson S (2013). Mast cell chymase modulates IL‐33 levels and controls allergic sensitization in dust‐mite induced airway inflammation. Mucosal Immunol 6: 911–920. [DOI] [PubMed] [Google Scholar]
- Wong MX, Roberts D, Bartley PA, Jackson DE (2002). Absence of platelet endothelial cell adhesion molecule‐1 (CD31) leads to increased severity of local and systemic IgE‐mediated anaphylaxis and modulation of mast cell activation. J Immunol 168: 6455–6462. [DOI] [PubMed] [Google Scholar]
- Wong CK, Ng SS, Lun SW, Cao J, Lam CW (2009). Signalling mechanisms regulating the activation of human eosinophils by mast‐cell‐derived chymase: implications for mast cell‐eosinophil interaction in allergic inflammation. Immunology 126: 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamuner SR, Zuliani JP, Fernandes CM, Gutierrez JM, de Fatima Pereira Teixeira C (2005). Inflammation induced by Bothrops asper venom: release of proinflammatory cytokines and eicosanoids, and role of adhesion molecules in leukocyte infiltration. Toxicon 46: 806–813. [DOI] [PubMed] [Google Scholar]


