Abstract
Puberty is a process which integrates multiple inputs that ultimately cause an increase in gonadotrophin-releasing hormone (GnRH) secretion. While kisspeptin neurones play an integral role in GnRH secretion and puberty onset, other systems are also likely important. One potential component is nitric oxide (NO), a gaseous neurotransmitter synthesized by nitric oxide synthase (NOS). In this study, we sought to neuroanatomically characterise neuronal NOS (nNOS) in prepubertal female sheep and determine if oestradiol would exert effects on this system. Luteinising hormone secretion was reduced by oestradiol treatment in prepubertal ovariectomised ewes. Neurones immunoreactive for nNOS were identified in several areas with the greatest number present in the ventrolateral portion of the ventromedial hypothalamus followed by the ventromedial hypothalamus, preoptic area (POA) and arcuate nucleus (ARC). Next, we determined if nNOS neurones contained oestrogen receptor α (ERα) and could potentially communicate oestradiol (E2) feedback to GnRH neurones. Neuronal nitric oxide synthase neurones contained ERα with the percentage of coexpression (12 to 40%) depending upon the area analyzed. We next investigated if a neuroanatomical relationship existed between nNOS and kisspeptin or nNOS and GnRH neurones. A high percentage of kisspeptin neurones in the POA (79%) and ARC (99%) colocalised with nNOS. Kisspeptin close-contacts were also associated with nNOS neurones. A greater number of close-contacts were observed in the ARC than the POA. A high percentage of POA GnRH neurones (79%) also expressed nNOS, but no GnRH close-contacts were observed onto nNOS neurones. Neither the numbers of nNOS neurones in the POA or hypothalamus nor the percentage of nNOS coexpression with GnRH, kisspeptin or ERα were influenced by oestradiol. These experiments reveal that a neuroanatomical relationship exists between both nNOS and kisspeptin and nNOS and GnRH in prepubertal ewes. Therefore, nNOS may act both directly and indirectly to influence GnRH secretion in prepubertal sheep.
Keywords: sheep, puberty, nitric oxide, kisspeptin, GnRH
INTRODUCTION
Puberty is a complex process that has been investigated extensively in female mammals. In prepubertal female sheep, low concentrations of oestradiol (E2) potently inhibit the release of gonadotrophin-releasing hormone (GnRH) and thus luteinising hormone (LH). As female sheep approach puberty, the ability of E2 to inhibit GnRH and LH secretion decreases, thus allowing for an increase in the frequency of GnRH and LH release (1). While it is evident that E2 plays a critical role in the inhibition of GnRH and LH secretion prior to the initiation of puberty, the neural mechanisms by which this feedback is communicated to GnRH neurones is not well established. Oestradiol negative feedback cannot be directly communicated to GnRH neurones because these neurones do not express oestrogen receptor alpha (ERα), the oestrogen receptor known to mediate oestrogenic regulation of GnRH secretion (2, 3). Therefore, in sheep as well as in several other mammalian species, intermediary neurones that do express ERα must exist to transmit these effects to GnRH neurones.
In sheep, rodents, and primates, kisspeptin appears to play a critical role in puberty onset. The importance of kisspeptin in the initiation of puberty was first discovered when mutations in either kisspeptin or its receptor, Kiss1r, led to an absence of puberty and ultimately resulted in infertility in humans (4–6). Kisspeptin neurones are present in both the preoptic area (POA) and arcuate nucleus (ARC) of the hypothalamus, contain ERα (7), and potently stimulate GnRH/LH secretion (8, 9). However, kisspeptin is unlikely to be the sole communicator involved in puberty onset (10, 11).
Nitric oxide (NO) is another potential intermediary that may influence the initiation of puberty. Neuronal nitric oxide synthase (nNOS) is one of three forms of an enzyme that oxidizes L-arginine to L-citrulline and NO (12). In mice and rats, a high percentage of nNOS neurones contain ERα (13–16). Nitric oxide stimulates GnRH and LH release in rats (17) and deletion of nNOS results in hypogonadism and infertility in mice (18). Recent evidence also suggests NO release may be influenced directly by kisspeptin. Kisspeptin close-contacts have been observed onto nNOS neurones in the POA and ARC of adult female mice, but only nNOS neurones in the POA express Kiss1r (19).
Very few studies have examined the role of NO in the control of GnRH and LH secretion in species other than rodents and none have characterised the expression of nNOS in prepubertal sheep. Therefore, we aimed to evaluate the distribution of nNOS neurones in the POA and hypothalamus of prepubertal sheep, determine if nNOS neurones express ERα, and examine if a neuroanatomical relationship exists between nNOS and kisspeptin or nNOS and GnRH neurones. We also investigated whether the presence or absence of E2 would affect these relationships.
MATERIALS AND METHODS
Animals
All experiments were conducted at the West Virginia University Food Animal Research Facility in Morgantown, West Virginia. Procedures were approved by the West Virginia Animal Care and Use Committee and were performed in accordance with National Institutes of Health guidelines for use of animals in research. Twelve Suffolk ewe lambs born in late February to early March were purchased from a local producer and housed indoors where they received a commercial premium alfalfa-timothy cube food ration (crude protein ≥ 12%, crude fat ≥ 18%, crude fibre ≤ 32%; Triple Crown Nutrition, Inc., Wayzata, MN, USA) and had free access to water and a mineral block supplement. Experiments were performed in the fall (August) when lambs were 5–6 months of age and still prepubertal. Lambs were housed two per pen (2.06 × 2.06 metres) on raised flooring with a clear view of all other sheep. Indoor lighting simulated the natural changes in day length characteristic of the fall season. Ovariectomies (OVX) were performed under aseptic conditions. Animals were anesthetised by i.v. injection of ketamine (7 mg/kg) and midazolam (0.3 mg/kg) and then maintained on 2% isoflurane. Ovarian vasculature was ligated and ovaries removed via a midventral incision. Ewe lambs were selected randomly to receive either no implant (OVX; n=6) or a subcutaneous 1-cm long Silastic (inner diameter 0.34cm, outer diameter 0.46cm; Dow Corning Corp., Midland, MI, USA) implant containing crystalline oestradiol (Sigma-Aldrich, St. Louis, MO, USA) (OVX+E; n=6). Two weeks after OVX, blood samples were collected every 12 min for 4 h by jugular venipuncture, placed in heparinized tubes and plasma was stored at −20°C until assayed for LH. Immediately following the final blood sample, hypothalami were collected as described previously (20). Briefly, all sheep were heparinised (20,000 U) and euthanised using an intravenous overdose of sodium pentobarbital (Euthasol; Webster Veterinary, Devens, MA, USA). Heads were removed and perfused via carotid arteries with 4 L of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) containing 0.1% sodium nitrite. Blocks of tissue containing the POA and hypothalamus were removed and stored in a 4% paraformaldehyde solution for 24 h at 4°C and transferred to 20% sucrose until sectioned. Frozen coronal sections (30μm) were cut with a freezing microtome, collected in 5 series (150 μm apart) and stored in cryopreservative until the time of immunohistochemical staining.
Single-label immunohistochemistry for nNOS characterization
To initially characterise the distribution of nNOS neurones in the hypothalamus of prepubertal ewes, every fourth hemisection from one complete series of sections (600 μm) throughout the POA and hypothalamus was used from one OVX and one OVX+E ewe. On day 1 of the protocol, sections were washed 4×5 min in 0.1 M phosphate-buffered saline (PBS) to remove excess cryoprotectant and stored overnight at 4°C. The next day, sections were washed 4×5 min in PBS, placed in 1% H2O2 for 10 min, and subsequently washed 4×5 min in PBS. Tissue was then incubated for at least 1 h in a blocking solution containing PBS, 0.4% Triton X-100 (PBSTX; Sigma-Aldrich, St. Louis, MO, USA) and 4% normal goat serum (NGS; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) in PBS. Sections were incubated in a solution containing rabbit anti-nNOS antiserum (Cat.#24287; 1:15,000; ImmunoStar antibody, Hudson, WI, USA) in PBSTX and 4% NGS at room temperature for 16 h. After incubation with the primary antibody, sections were washed and then incubated in a solution containing biotinylated goat anti-rabbit IgG (1:400; Vector Laboratories, Burlingame, CA, USA), PBSTX, and 4% NGS for 1 h. The sections were washed and incubated in a solution containing avidin-biotin horseradish-peroxidase conjugate (Vectastain Elite ABC, 1:600; Vector Laboratories) for 1 h. Sections were then washed and incubated in a solution containing 3,3-diaminobenzidine (DAB; 0.2 mg/ml; Sigma-Aldrich) and hydrogen peroxide (0.012%; Sigma-Aldrich) in PBS for 10 min. The sections were washed, mounted on Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA, USA), and coverslipped using Eukitt Mounting Reagent (Fisher Scientific).
Dual-label immunohistochemistry for nNOS and ERα
To examine the relationship between nNOS and ERα, 4 hemisections containing the POA (with 2 being at the level of the organum vasculosum lamina terminalis (OVLT) and 2 in the medial POA (mPOA)), 3 sections containing the ventromedial hypothalamus (VMH), 4 sections containing the ventrolateral portion of the ventromedial hypothalamus (VL-VMH) and 2 sections in each the rostral, middle, and caudal arcuate from 6 OVX and 6 OVX+E ewes were selected for analysis. The protocol described above was replicated with the following changes: mouse anti-ERα antiserum (Cat.#M7047; Clone 1D5, 1:500; DAKO Corp.) was the first primary antibody used, biotinylated goat anti-mouse IgG (1:400; Vector Laboratories) was the secondary antibody, and nickel-sulfate (2%; Sigma-Aldrich) was added to DAB to produce a blue-black nuclear reaction product. The sections were washed and incubated in PBS containing 1% hydrogen peroxide for 10 min. After further washing, the sections were incubated in PBSTX and 4% NGS for at least 1 h. The sections were then incubated in a solution containing rabbit anti-nNOS antiserum (1:15,000) in PBSTX, and 4% NGS for 16 h. After incubation with the primary antibody, the sections were washed and then incubated in a solution containing biotinylated goat anti-rabbit IgG (1:400; Vector Laboratories), PBSTX, and 4% NGS for 1 h. The sections were washed and incubated in a solution containing avidin-biotin horseradish-peroxidase conjugate (Vectastain Elite ABC, Vector Laboratories; 1:600) for 1 h. Sections were then washed and incubated in a solution containing 3,3-diaminobenzidine (DAB; 0.2 mg/ml) with hydrogen peroxide (0.012%; Sigma) in PBS for 10 min which results in a brown cytoplasmic reaction product. The sections were washed, mounted on Superfrost microscope slides (Fisher Scientific,), and coverslipped using Eukitt Mounting Reagent (Fisher Scientific).
Dual-label immunofluorescent detection of nNOS and kisspeptin
In order to determine if an anatomical relationship existed between nNOS and kisspeptin, 4 hemisections containing the POA (2 at the level of the OVLT, 2 in the mPOA) and 4 sections containing the middle to caudal ARC were selected from 6 OVX and 6 OVX+E prepubertal ewes. The same steps as described above for the previous two experiments were used until tissue was placed in a blocking solution containing 20% NGS instead of 4% NGS. Tissue sections were placed in a solution containing rabbit anti-kisspeptin antiserum (Cat.#AB-9754; 1:10,000; Millipore, Darmstadt, Germany) in PBSTX and 4% NGS for 16 h. After incubation with the primary antibody, sections were incubated in a solution containing biotinylated goat anti-rabbit IgG and a solution containing avidin-biotin horseradish-peroxidase conjugate as stated in the protocols above. Sections were then washed and incubated for 10 min in biotinylated tyramine (TSA; 1:250; Perkin Elmer, Waltham, MA, USA) in PBS containing 3% H2O2 per 1 mL of solution. After washing, sections were incubated in a solution containing DyLight green 488-streptavidin (1:200, Fisher Scientific) for 1 h followed by washes and incubation in PBSTX and 4% NGS for at least 1 h. Sections were incubated in rabbit anti-nNOS antiserum (1:1,000) in PBSTX, and 4% NGS for 16 h. The following day, sections were incubated in Alexa555 goat anti-rabbit (1:200; Life Technologies, Carlsbad, CA, USA) for 1 h, washed, mounted on Superfrost slides (Fisher Scientific), coverslipped using Gelvatol and stored in the dark at 4°C until analysis.
Dual-label immunofluorescent detection of nNOS and GnRH
To detect nNOS and GnRH neurones in prepubertal ewes, the same protocol as above was used with minor changes. The rabbit anti-nNOS antiserum (Immunostar) was the TSA-amplified primary antibody and was therefore used at a concentration of 1:10,000. Rabbit anti-GnRH antiserum (Cat.#20075, Immunostar) was used at a concentration of 1:200. This protocol was used to stain 4 POA sections (2 at the level of the OVLT) each from 6 OVX and 6 OVX+E prepubertal ewes.
Immunohistochemistry Controls
Specificity of the nNOS antibody in sheep tissue was tested using recommended controls for immunostaining including peptide blocking controls and primary antibody omission controls (21). These controls abolished all nNOS staining (Supplemental Figure 1). In addition to testing the specificity of the nNOS antibody, we also ensured there was no cross-reactivity between any of the antibodies used in dual-label staining protocols. In these situations, controls in which antibodies were preadsorbed with the opposite peptide or one of the primary antibodies was omitted from the protocol were used (Supplemental Figures 2 and 3).
Data Analysis
Immunohistochemistry
The distribution of nNOS neurones in all tissue sections from 1 OVX and 1 OVX+E ewe was visualised and mapped using an AZ70 transmitted light microscope (Olympus, Center Valley, PA, USA).
The total number of nNOS neurones, as well as the number and percentage of nNOS neurones that contained ERα, was determined using an AZ70 transmitted light microscope (Olympus). For all experiments, cell counts were made by a single observer blinded to treatment groups.
The number of kisspeptin close-contacts onto nNOS neurones in prepubertal ewes was determined by capturing images of 10 nNOS neurones in the POA and 10 nNOS neurones in the ARC of each animal using a LSM 510 laser scanning confocal system (Zeiss, Hornwood, NY, USA) on a Zeiss Axio Image Z1 upright microscope with a Plan Apochromat × 63/1.4 oil objective. Confocal z-stacks of optical sections were taken at 1 μm intervals through each nNOS neurone. The number of close-contacts onto nNOS cell bodies was analysed using ZEN software (Zeiss). Because contacts were counted through the entire z-stack, markers were placed on each individual contact to ensure that no contacts were counted more than once. Orthogonal views were used to confirm that contacts were touching the cell in all planes. The same approach was used to identify the number of GnRH close-contacts onto nNOS neurones in the POA of prepubertal ewes.
To delineate the percentage of kisspeptin and nNOS neurones and GnRH and nNOS neurones that were colocalised in prepubertal ewes, sections were visualised using a fluorescent microscope (VS120, Olympus). For kisspeptin and nNOS analyses, the number of kisspeptin neurones, the number of nNOS neurones, and the percentage of each neuronal population that were colocalised with each other was recorded in 2 mPOA sections and 2 middle to caudal ARC sections for each animal. For GnRH and nNOS analyses, the number of GnRH neurones, the number of nNOS neurones, and the percentage of each neuronal population that were colocalised with each other was determined in the POA only.
Assays
Luteinising hormone concentrations were measured in duplicate by radioimmunoassay as described previously (22) using reagents provided by the National Hormone and Peptide Program (Torrance, CA, USA). Assays used 100 to 200 μL of plasma and sensitivity averaged 0.07 ng/tube (NIHS24) with intra- and interassay coefficients of variations being 12.7% and 18.2% respectively.
Statistical Analysis
Similar to previous work, three criteria were used to determine an LH pulse; (1) a peak must occur within two samples of the previous nadir; (2) the amplitude must be greater than the sensitivity of the LH assay; and (3) the LH concentration at the peak must exceed the 95% confidence limits (based on overall assay variability) of the concentration at both the preceding and subsequent nadir (23). Mean LH, LH pulse amplitude, and mean nNOS cell numbers were compared by t-test. The percentage of nNOS cells that contained ERα and the percent colocalisation between nNOS and kisspeptin and nNOS and GnRH cells were analysed via chi-squared analysis. A Wilcoxon Mann-Whitney test was used to compare LH pulse frequency. The number of kisspeptin and GnRH close-contacts were transformed to normalize variance using the square root and compared by t-test. P < 0.05 was considered statistically significant.
RESULTS
Circulating mean concentrations of LH were greater (P < 0.0001) in OVX (6.3 ± 0.9 ng/ml) than in OVX+E ewes (1.9 ± 0.1 ng/ml). A greater frequency of LH pulses (P < 0.05) was also observed in OVX (3.0 ± 0.4 pulses/4h) compared to OVX+E ewes (1.4 ± 0.7 pulses/4h). Amplitude of LH pulses between OVX and OVX+E ewes was not compared because the lack of pulses in 3 of the 6 OVX+E ewes hindered meaningful analysis.
Distribution of nNOS neurones in the POA and hypothalamus of prepubertal ewes
Abundant numbers of nNOS neurones were detected in the POA and hypothalamus of prepubertal ewes (Figure 1, 2). Both bipolar and multipolar neurones were observed throughout the continuum of sections, and the distribution of neurones was identical between the OVX and OVX+E ewes. In the diagonal band of Broca (DBB), most nNOS neurones were parvocellular (≤15 μm somal diameter) and very lightly stained. Lightly stained fibres were also seen. In these same sections, darkly stained magnocellular neurones (≥18 μm somal diameter) and fibres were present towards the ventrolateral edge of the section. In the medial septum, darkly stained and mostly magnocellular neurones were noted with relatively sparse fibre labeling. In contrast, densely packed fibres and cells of various sizes were observed in the lateral septum (Figure 1A). In the rostral POA, lightly stained parvocellular cells lined the outside edges of the OVLT. Similar to what was observed in sections where the DBB was present, a significant number of darkly stained magnocellular neurones and fibres were noted near the ventrolateral edge of the section (Figure 1B). A few lightly stained cells were also observed in the bed nucleus of the stria terminalis and subfornical organ in these rostral POA sections. In the medial POA, lightly stained parvocellular cells were observed lining the entire length of the third ventricle (Figure 1C, 2A). In some medial POA sections, nNOS neurones were located further away and more lateral to the ventricle (Figure 2A). Small, lightly stained neurones were also evident in the periventricular nucleus (PeV). In the paraventricular nucleus (PVN), both magnocellular and parvocellular neurones were lightly stained (Figure 1D, 1E, 2B). More darkly stained cells were seen on the outer edges of the PVN, as well as the areas immediately surrounding it (Figure 1D). Some lightly stained neurones and fibres were also present in the lateral hypothalamic area of these sections. In the dorsomedial hypothalamus, a small number of lightly stained neurones of various sizes, as well as lightly stained fibres, were noted. The greatest number of nNOS neurones was observed in the VL-VMH with a high number of neurones also present in the VMH (Figure 1F, 1G, 2C). Extremely dense fibre labeling was observed in both the VMH and VL-VMH. The larger neurones in these areas were more darkly stained compared to smaller neurones that were a much lighter brown color. At all levels of the ARC, lightly stained neurones and fibres were noted, and there were no observable differences in the number of neurones between the rARC, mARC, and cARC (Figure 1F–H, 2C). Fibres from nNOS neurones were also seen running from the ARC to the internal zone of the median eminence. In the ventral premammillary nucleus (PMv), a majority of the nNOS neurones identified had a bipolar orientation. Most of the neurones in this area were moderately stained with nNOS fibres also observed.
Figure 1.
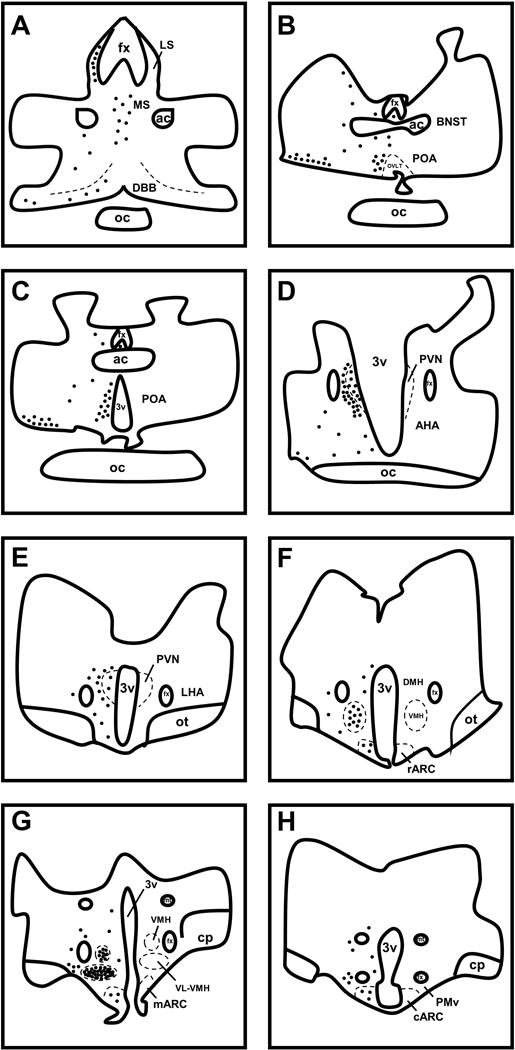
Drawings illustrating representative distributions of nNOS-IR neurones in the preoptic area and hypothalamus of prepubertal ewes. Each solid black circle represents 10 nNOS-IR neurones. The distribution of nNOS-IR neurones was identical on both sides of the POA and hypothalamus and is shown unilaterally to allow for visualization of labels. Ac, anterior commissure; AHA, anterior hypothalamic area; BNST, bed nucleus of the stria terminalis; cARC, caudal arcuate nucleus; mARC, middle arcuate nucleus; rARC, rostral arcuate nucleus; cp, cerebral peduncle; DBB, diagonal band of Broca; DMH, dorsomedial hypothalamus; fx, fornix; LHA, lateral hypothalamic area; LS, lateral septum; MS, medial septum; mt, mammillothalamic tract; oc, optic chiasm; ot, optic tract; OVLT, organum vasculosum of lamina terminalis; PMv, ventral premammillary nucleus; POA, preoptic area; PVN, paraventricular nucleus; VMH, ventromedial hypothalamus; VL-VMH, ventrolateral portion of the ventromedial hypothalamus; 3v, third ventricle.
Figure 2.
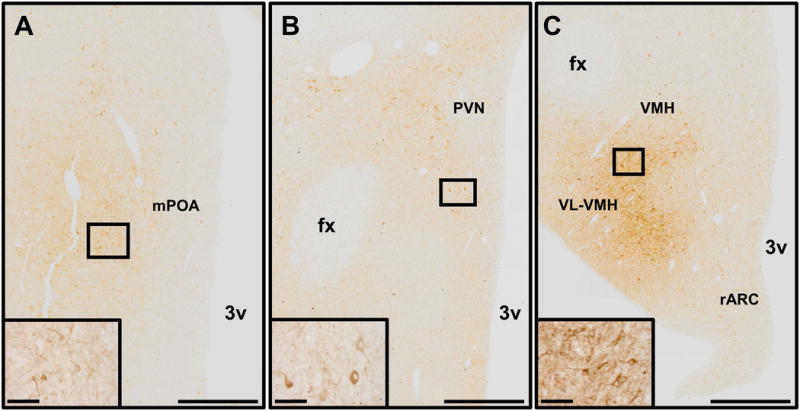
Images demonstrating the distribution of nNOS-immunoreactive neurones and fibres in the preoptic area (A), paraventricular nucleus (B), rostral arcuate nucleus, ventromedial hypothalamus, and ventrolateral portion of the ventromedial hypothalamus (C). Insets in each panel show higher magnification images of nNOS neurones contained in boxes present in each panel. Scale bars in lower magnification images = 500 μm. Scale bars in higher magnification insets = 50 μm fx, fornix; mPOA, medial preoptic area; PVN, paraventricular nucleus; rARC, rostral arcuate nucleus; VL-VMH, ventrolateral portion of the ventromedial hypothalamus; VMH, ventromedial hypothalamus; 3v, third ventricle.
Colocalisation of nNOS neurones with ERα
A portion of nNOS neurones in the POA and hypothalamus were found to contain ERα (Figure 3). Of the areas analysed, the highest percentage of nNOS neurones that contained ERα was found in the VL-VMH. However, no significant differences in the percentage of nNOS neurones that contained ERα were found between the OVX and OVX+E groups in the POA (19.8 ± 1.9% vs. 24.9 ± 2.1%), VMH (13.8 ± 1.6% vs. 10.9 ± 1.1%), VL-VMH (45.7 ± 4.9% vs. 37.0 ± 4.0%), or ARC (29.4 ± 2.9% vs. 29.5 ± 3.1%).
Figure 3.
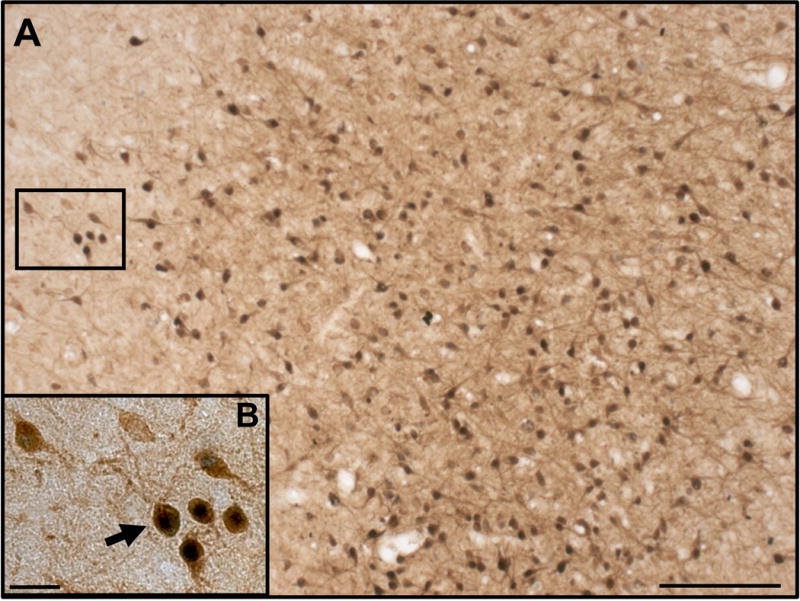
Dual-label detection of nNOS and ERα in the prepubertal ewe. Low magnification image depicting nNOS-immunoreactive neurones (brown) and ERα (black) in the VL-VMH of an OVX+E ewe (A). High magnification image of nNOS neurones shown in box in “A”. Black arrow indicates a nNOS neurone that contains ERα. White arrow indicates a nNOS neurone that does not contain ERα (B). Scale bar in A = 100 μm. Scale bar in B = 20 μm.
The effect of oestradiol on the number of nNOS neurones
The number of nNOS neurones present in the POA, VMH, VL-VMH, and ARC were compared between the OVX and OVX+E groups. No significant differences in the number of nNOS neurones were observed between OVX and OVX+E ewes in the POA (452 ± 36 vs. 476 ± 33), VMH (428 ± 26 vs. 406 ± 21), VL-VMH (1,507 ± 135 vs. 1,541 ± 130), or ARC (205 ± 10 vs. 216 ± 8).
Colocalisation of nNOS neurones with kisspeptin
Analysis of sections labeled for nNOS and kisspeptin revealed that a high percentage of kisspeptin neurones in both the POA and ARC colocalised with nNOS (Figure 4). In the ARC, 98.2 ± 0.7% of kisspeptin neurones colocalised with nNOS, and there were no differences between the OVX and OVX+E groups (Table 1). When comparing the OVX and OVX+E females, a greater percentage (P < 0.01) of nNOS neurones in OVX ewes (42.8 ± 5.2%) colocalised with kisspeptin compared to OVX+E ewes (21.6 ± 3.3%) in the ARC (Table 1). Few to no kisspeptin neurones were observed in the POA of the OVX group. Therefore, in the POA, the neuroanatomical relationship between nNOS and kisspeptin neurones was only characterised for the OVX+E group. Similar to the ARC, a high percentage (78.9 ± 4.9%) of kisspeptin neurones colocalised with nNOS (Table 1). There was a much higher number of nNOS neurones in the POA than kisspeptin neurones, and as such only 5.4 ± 1.1% of nNOS neurones were found to colocalise with kisspeptin.
Figure 4.
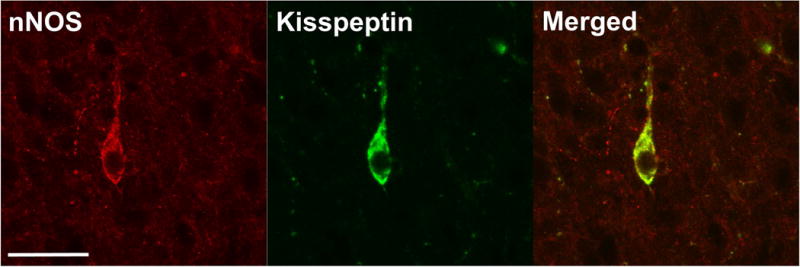
Representative images demonstrating kisspeptin colocalisation with nNOS in the prepubertal ewe. A neurone shown to be expressing nNOS (left), kisspeptin (middle) and the merged image (right) in the ARC of an OVX ewe. Scale bar = 50 μm.
Table 1.
Percent colocalisation of kisspeptin and nNOS in prepubertal ewes.
| Area | Treatment | % Kiss/nNOS | # Kiss neurones | % nNOS/Kiss | # nNOS neurones |
|---|---|---|---|---|---|
| ARC | OVX | 98.5 ± 1.1 | 134.3 ± 14.9a | 42.8 ± 5.2c | 250.2 ± 34.3 |
| ARC | OVX+E | 97.9 ± 1.1 | 45.3 ± 8.7b | 21.6 ± 3.3d | 208.3 ± 24.5 |
| POA | OVX+E | 78.9 ± 4.9 | 20.2 ± 5.3 | 5.4 ± 1.1 | 633.8 ± 100.6 |
Values are expressed at mean ± SEM. % Kiss/nNOS; percentage of kisspeptin neurones containing nNOS. # Kiss neurones; number of kisspeptin neurones per ewe. % nNOS/Kiss; percentage of nNOS neurones containing kisspeptin. # nNOS neurones; number of nNOS neurones per ewe. Within column, a, b; c, d differ (P < 0.01).
Colocalisation of nNOS neurones with GnRH
In the POA of prepubertal ewes, colocalisation between nNOS and GnRH neurones was observed (Figure 5). Overall, it was found that 78.5 ± 3.7% of GnRH neurones colocalised with nNOS. There was no difference when comparing OVX and OVX+E ewes (Table 2). Similar to the above data with nNOS and kisspeptin, there were many more nNOS neurones present in the POA than GnRH neurones, and therefore only 4.7 ± 1.1% of nNOS neurones colocalised with GnRH. There was no difference in the percentage of nNOS neurones that colocalised with GnRH between the OVX and OVX+E groups (Table 2).
Figure 5.
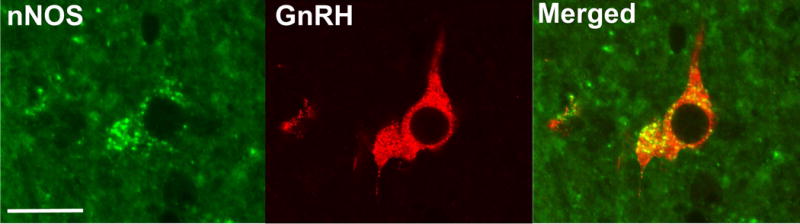
Representative images demonstrating GnRH colocalisation with nNOS in the POA of a prepubertal ewe. A neurone shown to be expressing nNOS (left), GnRH (middle) and the merged image (right) in the POA of an OVX ewe. Scale bar = 50 μm.
Table 2.
Percent colocalisation of GnRH and nNOS in prepubertal ewes.
| Area | Treatment | % GnRH/nNOS | # GnRH neurones | % nNOS/GnRH | # nNOS neurones |
|---|---|---|---|---|---|
| POA | OVX | 84.3 ± 4.6 | 35.3 ± 3.4 | 4.8 ± 0.7 | 645.0 ± 41.1 |
| OVX+E | 72.7 ± 4.9 | 31.0 ± 8.1 | 4.7 ± 2.3 | 664.7 ± 104.7 |
Values are expressed at mean ± SEM. % GnRH/nNOS; percentage of GnRH neurones containing nNOS. # GnRH neurones; number of GnRH neurones per ewe. % nNOS/GnRH; percentage of nNOS neurones containing GnRH. # nNOS neurones; number of nNOS neurones per ewe.
Inputs between kisspeptin and nNOS neurones
In addition to colocalisation of kisspeptin and nNOS, kisspeptin close-contacts onto nNOS neurones were also examined (Figure 6A). While nNOS close-contacts were seen apposed to kisspeptin neurones, they were not quantified because the punctate nature of the nNOS staining combined with the fact that a high percentage of kisspeptin neurones colocalised with nNOS made it difficult to correctly identify what was indeed a nNOS close-contact. Therefore, only kisspeptin close-contacts onto nNOS neurones were analysed (Figure 6A). When comparing the OVX and OVX+E groups, there was a strong trend (P = 0.067) for nNOS neurones in the ARC of OVX lambs to have a greater number of kisspeptin close-contacts compared to OVX+E lambs (3.2 ± 0.6 vs. 0.5 ± 0.2; Figure 5B). In contrast to ARC nNOS neurones that exhibited many kisspeptin close-contacts, very few kisspeptin close-contacts onto nNOS neurones were observed in the POA of OVX+E ewes (0.10 ± 0.03).
Figure 6.
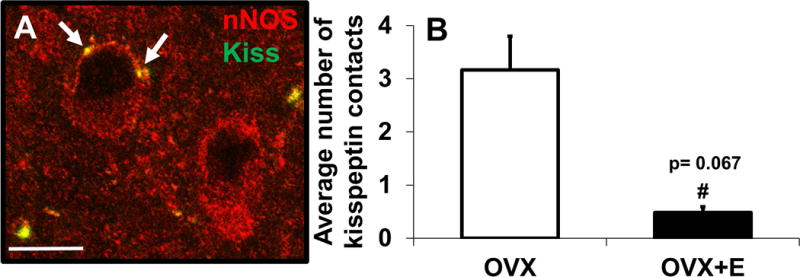
Kisspeptin close-contacts onto nNOS neurones in the prepubertal ewe. High magnification image showing nNOS (red) neurones with kisspeptin (green) close-contacts (arrows, top neurone) and without kisspeptin close-contacts (bottom neurone) (A). There was a trend for there to be an increased number of kisspeptin close-contacts onto nNOS neurones in OVX compared to OVX+E ewes (B). Scale bar in A = 10 μm.
Inputs between GnRH and nNOS neurones
Unlike the kisspeptin innervation that was observed onto nNOS neurones, no GnRH close-contacts were observed onto nNOS neurones in the POA of prepubertal ewes. There were nNOS close-contacts onto GnRH neurones, but because of the punctate nature of the nNOS staining and high percentage of colocalisation between nNOS and GnRH, nNOS contacts onto GnRH neurones are difficult to accurately identify and as such were not quantified.
DISCUSSION
In the present study, we characterised for the first time the distribution of nNOS neurones in the POA and hypothalamus of prepubertal ewes and found that a significant proportion of these expressed ERα. We also discovered that nNOS was coexpressed to a high degree in GnRH neurones in the POA and in both the ARC and POA population of kisspeptin neurones. As was expected, mean LH concentration and LH pulse frequency was decreased in OVX+E compared to OVX prepubertal ewes. However, we observed that neither numbers of nNOS neurones in the POA or various other hypothalamic areas nor the percentage of nNOS coexpression with GnRH, kisspeptin or ERα were influenced by oestradiol. In addition, kisspeptin close-contacts onto nNOS neurones were observed in the ARC, establishing a potentially functional connection between these two neuronal populations.
Because nitric oxide (NO) influences myriad physiological processes within the body (24), including reproduction (17, 18), it is not surprising that a large number of nNOS neurones are found in the POA and throughout the hypothalamus of prepubertal ewes. The distribution of nNOS neurones in this study was very similar to what has been previously reported in adult ewes using histochemistry for NADPH diaphorase (NADPHd)(25), which serves as an enzymatic marker for NOS neurones (26). The distribution of nNOS neurones in sheep also compares well to what has previously been described in rats (27), mice (13) nonhuman primates (28), and humans (29). Because NO is a gaseous neurotransmitter with a half-life of around 1 second, cells must be within approximately 100–200 μm of a NO-producing cell in order for NO to exert its effects (30). Thus, because nNOS neurones are so widely and densely distributed throughout the POA and hypothalamus, the potential exists for NO to influence the activity of many cells.
We observed a high degree of ERα expression in nNOS neurones. This is consistent with previous work in rodents, as ERα is found in a high percentage of nNOS/NADPHd neurones in the rat (14–16) and mouse (13, 16). Thus, we hypothesized that oestradiol treatment of OVX ewes would alter nNOS expression, either generally or in a region-specific manner. However, we did not observe an effect of oestradiol on the number of nNOS neurones present nor the percentage of nNOS neurones that contained ERα in any of the hypothalamic areas examined. In previous work conducted in rodents, treatment of ovariectomised adult rats with oestradiol benzoate using a positive feedback model increased the number of NADPHd-positive neurones in the VMH (15, 31) and medial POA (14). Relatively little work has been done in sheep, but in ovariectomised adult ewes, combinatorial insertion of oestradiol and progesterone implants increased the number of NADPHd-positive neurones in the VL-VMH, but not in the POA or ARC (25). It is possible that differences in species, age, or steroid environment of experimental animals may underlie the discrepancies between studies. In sheep, neurones located in the mediobasal hypothalamus, which includes the VMH and VL-VMH, are implicated in the control of estrus behavior (32) and the LH surge (33) both of which are stimulated by highly elevated levels of oestradiol. Therefore, it is possible that the chronic, low levels of oestradiol employed in our study may not have been an appropriate stimulus for eliciting a change in that subset of nNOS neurones.
Neuronal nitric oxide synthase may also act in concert with other neuronal inputs, such as kisspeptin, to influence the initiation of puberty. In adult female mice, kisspeptin fibres are associated with nNOS neurones in the POA and ARC, but only nNOS neurones in the POA express the receptor for kisspeptin, Kiss1r (19). In the current study, we also found that nNOS neurones were associated with kisspeptin fibres in the POA and ARC of prepubertal ewes. Notably, nNOS neurones in the ARC exhibited decidedly more kisspeptin close-contacts than nNOS neurones in the POA, and there was a strong trend for oestradiol to decrease the number of kisspeptin close-contacts onto nNOS neurones in the ARC. Further studies are needed to determine if these kisspeptin contacts originate from kisspeptin neurones in the POA or ARC. Regardless, these data may indicate a stronger neuroanatomical relationship between kisspeptin and nNOS in the ARC than in the POA of prepubertal ewes and as such may suggest the ARC is a more important area in which NO may act to influence GnRH secretion. Further studies are needed to determine areas within the hypothalamus that NO may act alone or through kisspeptin to regulate GnRH/LH secretion.
In contrast to what has previously been reported in mice, a high percentage of kisspeptin neurones in both the POA and ARC of prepubertal ewes also coexpressed nNOS. Further, the presence of oestradiol did not influence the percentage of kisspeptin neurones that expressed nNOS in the ARC of prepubertal ewes. However, we noted that oestradiol reduced the percentage of nNOS neurones that coexpressed kisspeptin in the ARC. Because nearly all kisspeptin neurones in the ARC colocalised with nNOS and oestradiol decreases the number of kisspeptin-immunopositive neurones in the ARC of prepubertal ewes (34, 35), the difference in the percentage of nNOS neurones coexpressing kisspeptin between OVX and OVX+E ewes is likely due to a decrease in the number of kisspeptin neurones. Thus, the relationship between nNOS and kisspeptin may be expressed in a species-dependent manner. In addition, we cannot rule out age-associated changes since our sheep were prepubertal, but mice from previous work were adults.
In addition to analysing the potential connections between nNOS and kisspeptin, we also examined the neuroanatomical relationship between GnRH and nNOS neurones. We found that a high percentage of GnRH neurones in the POA of prepubertal ewes coexpressed nNOS. This is in contrast to a previous study in rats where it was reported that there was no overlap between GnRH and nNOS expression (36). These results suggest that species variations may exist in nNOS and GnRH coexpression. In addition, we did not observe GnRH-containing contacts onto nNOS neurones. This is consistent with previous work in rodents (36, 37), where GnRH neurones are surrounded by nNOS neurones but, at least in mice, very few close-contacts between the two neuronal populations were observed. These data raise the possibility that nitric oxide synthesized within the POA of prepubertal ewes has the capacity to influence GnRH secretion in an autocrine or paracrine manner.
In summary, these results illustrate that nNOS-containing neurones are widely distributed throughout the POA and hypothalamus of prepubertal ewes, and these neurones colocalise to a high degree with ERα, kisspeptin and GnRH. Furthermore, a neuroanatomical relationship between nNOS and kisspeptin and nNOS and GnRH was established in prepubertal ewes, and the presence of oestradiol had minimal to no effect on the percentage of coexpression or numbers of close-contacts. Altogether, these results suggest the potential for nitric oxide to act both directly and indirectly to influence GnRH secretion in prepubertal ewes.
Supplementary Material
Acknowledgments
We thank Dr. Miroslav Valent and Gail Sager for their assistance with radioimmunoassays and animal surgeries. We would also like to thank Dr. Margaret Minch and Dr. Jennifer Fridley for veterinary care, and Dr. Al F. Parlow and the National Hormone and Peptide Program for reagents used to measure LH. Imaging experiments and analysis were performed in the West Virginia University Microscope Imaging Facility which is supported by the Mary Babb Randolph Cancer Center and National Institutes of Health Grants P20 RR016440 and P30 RR032138. This work was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture grant 2013-67015-20956 (SMH) and NIH grant P20GM103434 to the West Virginia IDeA Network for Biomedical Research Excellence.
Footnotes
MISS MICHELLE N. BEDENBAUGH (Orcid ID: 0000-0003-4844-3751)
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Foster DL, Ryan KD. Endocrine mechanisms governing transition into adulthood: a marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology. 1979;4:896–904. doi: 10.1210/endo-105-4-896. [DOI] [PubMed] [Google Scholar]
- 2.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;4:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 3.Lehman MN, Ebling FJ, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology. 1993;2:876–886. doi: 10.1210/endo.133.2.8344223. [DOI] [PubMed] [Google Scholar]
- 4.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;19:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;17:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 6.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;7:629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 7.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;3:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 8.d’Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, Colledge WH. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci. 2010;25:8581–8590. doi: 10.1523/JNEUROSCI.5486-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redmond JS, Macedo GG, Velez IC, Caraty A, Williams GL, Amstalden M. Kisspeptin activates the hypothalamic-adenohypophyseal-gonadal axis in prepubertal ewe lambs. Reproduction. 2011;4:541–548. doi: 10.1530/REP-10-0467. [DOI] [PubMed] [Google Scholar]
- 10.Terasawa E, Guerriero KA, Plant TM. Kisspeptin and puberty in mammals. Adv Exp Med Biol. 2013:253–273. doi: 10.1007/978-1-4614-6199-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;3:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forstermann U, Schmidt HH, Pollock JS, Sheng H, Mitchell JA, Warner TD, Nakane M, Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991;10:1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- 13.Chachlaki K, Malone SA, Qualls-Creekmore E, Hrabovszky E, Munzberg H, Giacobini P, Ango F, Prevot V. Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus. J Comp Neurol. 2017 doi: 10.1002/cne.24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura H, Yokosuka M, Hayashi S. Estrogenic induction of NADPH-diaphorase activity in the preoptic neurons containing estrogen receptor immunoreactivity in the female rat. J Neuroendocrinol. 1994;6:597–601. doi: 10.1111/j.1365-2826.1994.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 15.Okamura H, Yokosuka M, McEwen BS, Hayashi S. Colocalization of NADPH-diaphorase and estrogen receptor immunoreactivity in the rat ventromedial hypothalamic nucleus: stimulatory effect of estrogen on NADPH-diaphorase activity. Endocrinology. 1994;4:1705–1708. doi: 10.1210/endo.135.4.7925135. [DOI] [PubMed] [Google Scholar]
- 16.Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;4:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- 17.Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc Natl Acad Sci U S A. 1993;21:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;7:2767–2774. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- 19.Hanchate NK, Parkash J, Bellefontaine N, Mazur D, Colledge WH, d’Anglemont de Tassigny X, Prevot V. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J Neurosci. 2012;3:932–945. doi: 10.1523/JNEUROSCI.4765-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;11:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- 21.Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;4:477–478. doi: 10.1002/cne.20839. [DOI] [PubMed] [Google Scholar]
- 22.Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod. 1988;5:1032–1038. doi: 10.1095/biolreprod39.5.1032. [DOI] [PubMed] [Google Scholar]
- 23.Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;5:1286–1290. doi: 10.1210/endo-107-5-1286. [DOI] [PubMed] [Google Scholar]
- 24.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;11:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufourny L, Skinner DC. Influence of estradiol on NADPH diaphorase/neuronal nitric oxide synthase activity and colocalization with progesterone or type II glucocorticoid receptors in ovine hypothalamus. Biol Reprod. 2002;3:829–836. doi: 10.1095/biolreprod.102.004648. [DOI] [PubMed] [Google Scholar]
- 26.Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991;17:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada K, Emson P, Hokfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;3–4:295–316. doi: 10.1016/0891-0618(96)00133-0. [DOI] [PubMed] [Google Scholar]
- 28.Satoh K, Arai R, Ikemoto K, Narita M, Nagai T, Ohshima H, Kitahama K. Distribution of nitric oxide synthase in the central nervous system of Macaca fuscata: subcortical regions. Neuroscience. 1995;3:685–696. doi: 10.1016/0306-4522(95)00040-p. [DOI] [PubMed] [Google Scholar]
- 29.Sangruchi T, Kowall NW. NADPH diaphorase histochemistry of the human hypothalamus. Neuroscience. 1991;3:713–724. doi: 10.1016/0306-4522(91)90007-b. [DOI] [PubMed] [Google Scholar]
- 30.Thomas D, Flores-Santana W, Switzer C, Wink D, Ridnour L. Determinants of Nitric Oxide Chemistry: Impact of Cell Signaling Processes. In: Ignarro L, editor. Nitric Oxide Biology and Pathobiology. Amsterdam: Elsevier; 2010. pp. 3–18. [Google Scholar]
- 31.Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Regulation of neuronal nitric oxide synthase mRNA in lordosis-relevant neurons of the ventromedial hypothalamus following short-term estrogen treatment. Brain Res Mol Brain Res. 1998;1:105–108. doi: 10.1016/s0169-328x(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 32.Blache D, Fabre-Nys CJ, Venier G. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Res. 1991;2:241–249. doi: 10.1016/0006-8993(91)91488-m. [DOI] [PubMed] [Google Scholar]
- 33.Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;4:1752–1760. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- 34.Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;6:2756–2765. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez JA, Bedenbaugh MN, McCosh RB, Weems PW, Meadows LJ, Wisman B, Coolen LM, Goodman RL, Hileman SM. Does Dynorphin Play a Role in the Onset of Puberty in Female Sheep? J Neuroendocrinol. 2016;12 doi: 10.1111/jne.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;1:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 37.Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology. 2008;2:587–596. doi: 10.1210/en.2007-1260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


