Abstract
Native mass spectrometry (native-MS) of membrane proteins typically requires a detergent screening protocol, protein solubilization in the preferred detergent, followed by protein liberation from the micelle by collisional activation. Here, submicrometer nano-ESI emitter tips are used for native-MS of membrane proteins solubilized in both non-ionic and ionic detergent solutions. With the submicrometer nano-ESI emitter tips, resolved charge-state distributions of membrane protein ions are obtained from a 150 mM NaCl, 25 mM Tris-HCl with 1.1% octyl glucoside solution. The relative abundances of NaCl and detergent cluster ions at high m/z are significantly reduced with the submicrometer emitters compared to larger nano-ESI emitters that are commonly used. This technique is beneficial for significantly decreasing the abundances (by two to three orders of magnitude compared to the larger tip size: 1.6 μm) of detergent cluster ions formed from aqueous ammonium acetate solutions containing detergents that can overlap with the membrane protein ion signal. Resolved charge-state distributions of membrane protein ions from aqueous ammonium acetate solutions containing ionic detergents were obtained with the submicrometer nano-ESI emitters, which is the first report of native-MS of membrane proteins solubilized by ionic detergents.
Graphical Abstract
Introduction
Membrane proteins currently make up approximately 50% of therapeutic targets [1, 2] making their structural characterization a high priority. Native mass spectrometry (native-MS) has emerged as a powerful tool to characterize these difficult to analyze proteins [3–8]. As a result of their hydrophobicity, they are typically solubilized by encapsulation in either non-ionic or zwitterionic detergent micelles for analysis by native MS [4, 9]. However, these detergents can broaden the mass spectral peaks and can reduce the signal-to-noise ratios (S/N) of the membrane protein ions [4]. Upon collisional activation of protein-micelle complexes, which subsequently liberates the membrane proteins from the micelles, detergent related ions are frequently present in high abundance that can greatly suppress protein signal and increase spectral complexity [10]. During native-MS, it is essential to strike a balance between membrane protein ejection and dissociation of any membrane protein complexes, such as protein-protein or protein-ligand complexes, because preservation of these interactions while simultaneously disrupting the protein interactions with the detergent micelle can be difficult. Therefore, complete removal of detergent micelle signal from the spectrum may not be achieved.
Another challenge of native-MS is salt adduction. Nonvolatile salts can adduct to protein and protein complexes, broadening mass spectral peaks and decreasing mass measuring accuracy. To circumvent the adverse effects of nonvolatile salts in ESI solutions, protein solutions are typically exchanged into volatile ammonium salt solutions such as ammonium acetate or ammonium bicarbonate [11]. Specific salts in solution are often necessary to maintain the structures and functions of proteins, and buffer solutions containing ~150 mM KCl or NaCl are often used to mimic the cellular environment. Several methods for desalting protein ions, including adding reagents to ESI solutions [11–15] or reacting the protein ions with organic vapors [16] can be used but are only effective for solutions containing up to about 25 mM NaCl. Nano-ESI tip diameters are typically >1 μm, but emitter tips less than 1 μm in diameter can decrease salt adduction to protein and protein complex ions [17–20]. Recently, ESI mass spectra with resolved charge-state distributions of proteins and protein complexes were obtained from solutions containing ≥ 150 mM NaCl or KCl and a variety of commonly used buffers, such as Tris-HCl and HEPES [18, 19]. This effect was attributed to the formation of small nanodrops that limit the number of nonvolatile ions that can interact with the protein or protein complex and limit the size of clusters that can be formed [19, 20].
Here, the effectiveness of submicrometer nano-ESI emitter tips for desalting membrane protein ions from aqueous solutions containing detergents was investigated. Membrane protein ions, bacteriorhodopsin T47A (bR) and Aquaporin Z (AqpZ), were formed from aqueous ammonium acetate and a commonly used buffer that mimics the cellular environment (150 mM NaCl, 25 mM Tris-HCl) containing two times the critical micelle concentration (CMC) of non-ionic and ionic detergents with both conventionally sized and submicrometer nano-ESI tips.
Experimental
Mass spectral data were acquired using a Synapt G2Si mass spectrometer (Waters, Milford, MA) in the QB3/Chemistry Mass Spectrometry Facility at the University of California, Berkeley. Borosilicate capillary emitters (1.0 mm o.d./0.78 mm i.d., Sutter Instruments, Novato, CA) were pulled with a Flaming/Brown micropipette puller (Model P-87, Sutter Instruments, Novato, CA). Emitter tip diameters were measured with a scanning electron microscope (Hitachi TM-1000 SEM, Schaumburg, IL) at the Robert D. Ogg Electron Microscope Laboratory (University of California, Berkeley). Tip inner diameters were either 1.6 ± 0.1 μm or 0.57 ± 0.04 μm and replicate measurements were made with at least three different tips of each size (Supplemental Figure 1) [20].
Nano-electrospray was initiated by applying a potential of about +0.6 to 1.2 kV to a 0.127 mm diameter platinum wire inserted into the emitter and in contact with the solution. The sampling cone and source offset voltages were both 50 V, and the source temperature was 80 °C. A flow rate of argon collision gas of 6.0 mL/min was used in the trap. Ion activation at a collision voltage of 100–150 V in the trap to facilitate release the ions from the micelles.
The concentrations of bR and AqpZ were ~2 μM and ~30 μM, respectively. The bR stock solution containing octyl glucoside (OG) was diluted 100-fold with the buffer and detergent of interest. The AqpZ stock solution (150 mM NaCl 20 mM Tris-HCl 5% glycerol 1.1% OG) was diluted 5-fold. Two times the CMC of OG, sodium dodecyl sulfate (SDS), cetrimonium bromide (CTAB) or sarkosyl is 1.1, 0.55, 0.073, 0.85 % (w/v), respectively.
Results and Discussion
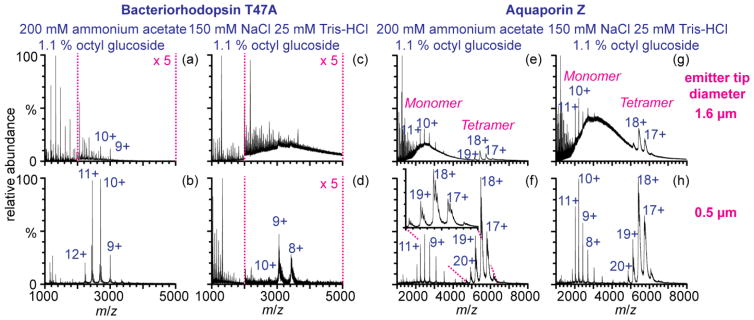
To determine the effects of nano-ESI emitter tip size on salt and detergent adduction to membrane protein ions, bR and AqpZ ions were formed from aqueous 200 mM ammonium acetate with 1.1% OG with two sizes of nano-ESI emitter tips (1.6 μm and 0.5 μm inner diameters, Figure 1a–d). Well-resolved charge-state distributions of bR and AqpZ ions are obtained from aqueous ammonium acetate and OG solutions with both size emitters (Figure 1). The charge-state distribution of bR ions formed with the submicrometer tips is slightly higher than from the larger tips consistent with previous results [21]. The charge state-distributions of AqpZ monomer and tetramer ions are similar with both tip sizes. Salt cluster ions are formed with both tip sizes, but the abundances of these clusters are lower by up to four orders of magnitude with the 0.5 μm emitter tips compared to the protein ion signal (Supplemental Figure 2a–c). For example, the abundance of the most intense cluster, (2OG + Na)+ (m/z 607), is one to three orders of magnitude lower with the submicrometer emitter tips than with the larger tips (Supplemental Figure 2a). Large cluster ions from m/z ~1500 to >4000 that overlap the charge-state distributions of bR and AqpZ ions are nearly eliminated with the 0.5 μm tips resulting in higher signal-to-noise ratios (S/N) for the protein ions with the small tips. For example, the S/N of the 10+ charge state of AqpZ monomer ions is seven times higher with the small tips. This demonstrates that submicrometer emitter tips are useful for decreasing the abundances of detergent cluster ions that can interfere with membrane protein ion signal. Several different peaks for each charge state of the AqpZ tetramer that are not resolved with the larger tips are resolved with the small tips. These differ in mass by ~760–1800 Da and may be due to adduction of sodiated OG dimers or due to the presence of phospholipids.
Figure 1.
(a–d) bR and AqpZ ions (e–h) formed from aqueous (a–b, e–f) 200 mM ammonium acetate with 1.1 % (w/v) OG or (c–d, g–h) 150 mM NaCl 25 mM Tris-HCl 1.1 % (w/v) OG with (a, c, e, g) 1.6 μm and (b, d, f, h) 0.5 μm emitter tips. The collision voltage was 100 V
To determine if the submicrometer emitter tips are effective at forming ions of membrane proteins from a commonly used buffer that mimics the cellular environment, bR and AqpZ ions were formed from 150 mM NaCl, 25 mM Tris-HCl and 1.1% OG with both emitter tip sizes (Figure 1c, d, g and h). The bR ions formed from this solution with the 1.6 μm tips are not resolved, but rather a broad distribution of NaCl and OG clusters ions from m/z ~2000 to 7000 is produced. A few broad peaks at m/z 3060 and 3410 may correspond to the 9+ and 8+ charge states of bR, which would result in a molecular weight (MW) of 27.5 kDa which is 3% greater than the un-adducted molecular weight of bR (MW = 26.75 kDa, calculated from elemental composition). AqpZ monomer and tetramer ions formed with the 1.6 μm tips from this solution are resolved (likely because the concentration of AqpZ is six times greater than that of bR) but high m/z cluster ions interfere with the protein ion signal. In contrast, AqpZ and bR ions formed with the submicrometer emitter tips from the same solution are clearly resolved with little background chemical noise. As previously demonstrated, high m/z cluster ions are nearly eliminated [19, 20]. The masses of bR and AqpZ ions formed from 150 mM NaCl, 25 mM Tris-HCl and 1.1 % OG with the submicrometer tips are 588 and 880 Da higher in mass than the calculated values, respectively, indicating that substantial adduction of salts and possibly detergent still occurs (Supplemental Table S-1). This adduction results in broad peaks that spread the tetramer signal in m/z and this contributes to the apparent high abundance of the monomer, which is significantly less adducted. Notably, there is no significant difference in the ratio of tetramer to monomer with the two tip sizes indicating that the small tips do not affect the stability of the tetramer. These results show that submicrometer emitter tips can be beneficial for native-MS of membrane protein complexes from solutions containing high ionic strengths of nonvolatile salts in addition to non-ionic detergents, which would be advantageous for membrane proteins that are only stable in specific buffers containing nonvolatile salts.
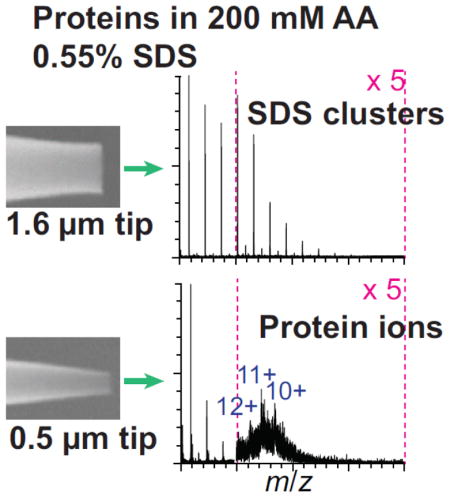
Ionic detergents are often added to membrane protein solutions because they are typically more effective at solubilizing membrane proteins than non-ionic detergents [22]. To determine if submicrometer nano-ESI tips are effective for native-MS from aqueous solutions containing ionic detergents, bR and AqpZ ions were formed with both size emitter tips from 200 mM ammonium acetate containing two times the CMC of SDS, a common ionic detergent (Figure 2). With the 1.6 μm emitter tips, no bR ions are observed (Figure 2a). AqpZ monomer ions are resolved from solution with the 1.6 μm tips, but there is a very broad peak of cluster ions from m/z ~2000–5000 (Figure 2c.) In contrast, charge-state distributions of the monomers of both proteins are clearly resolved with the 0.5 μm tips (Figure 2b,d). The charge states of the tetramer are not resolved, but the maxima in the distribution likely correspond to the 15+ and 14+ charge states. With the maximum possible collision energy, narrower peaks corresponding to the 16 to 18+ appear, consistent with removal of negatively charged SDS adducted to the protein ions. The high m/z cluster ions are nearly eliminated with the small tips (Supplemental Figure 2b). Resolved charge-state distributions of bR ions formed from 200 mM ammonium acetate containing two times the CMC of two other ionic detergents, sarkosyl and CTAB were obtained with submicrometer emitter tips (Supplemental Figure 3). Resolved charge-state distributions of AqpZ ions formed from 150 mM NaCl 25 mM Tris-HCl and 0.55% SDS were obtained with the submicrometer emitters, but not for bR ions (Supplemental Figure 4). This is the first report of native-MS of membrane protein from aqueous solutions containing ionic detergents. The use of submicrometer nano-ESI tips for native-MS of membrane proteins could be useful for membrane proteins that require ionic detergents for solubilization.
Figure 2.
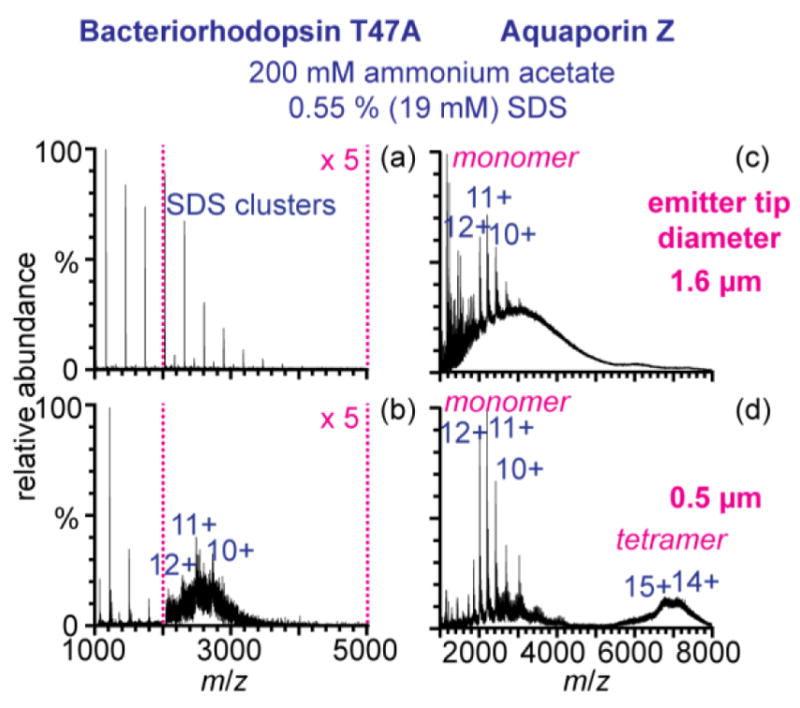
(a–b) bR and (c–d) AqpZ ions formed from aqueous 200 mM ammonium acetate containing 0.55 % (w/v) SDS with (a,c) 1.6 μm and (b, d) 0.5 μm emitter tips. The collision voltage was 150 V.
Conclusions
Submicrometer nano-ESI emitter tips are useful for decreasing the abundances of cluster ions of both ionic and non-ionic detergents for membrane protein ions formed from aqueous solutions containing two times the CMC of the detergent. The submicrometer emitter tips also are useful for obtaining resolved charge-state distributions of membrane protein ions from solutions containing high ionic strengths of nonvolatile salts (150 mM) with these detergents. This technique reduces the chemical noise over conventional nanoESI and makes possible native-MS of membrane proteins from solutions that are more conventionally used by biochemists to investigate the structures, dynamics and functions of these types of proteins. Importantly, this method may afford for the reduction in performing multiple detergent screens, which are currently used to find optimal conditions for native-MS analysis [4].
Supplementary Material
Supplemental Table 1. Mass increase from adduction for bR and AqpZ ions formed from 200 mM ammonium acetate or 150 mM NaCl 25 mM Tris-HCl and detergents with 0.5 μm and 1.6 μm emitter tips. The adduction to AqpZ tetramer was calculated with reference to the measured monomer mass of AqpZ (times four). The adduction to bR was calculated with reference to the elemental composition of bR.
Supplemental Figure 1. Four replicate mass spectra for AqpZ ions formed from 200 mM ammonium acetate with 1.1 % octyl glucoside using four different 0.5 μm emitter tips. These data show the reproducibility of mass spectra obtained with different nano-ESI emitters that are approximately the same size.
Supplemental Figure 2a. Mass spectra shown in Figure 1 in the m/z range from 500 to 1000 for (a–d) bR and AqpZ (e–h) from aqueous (a–b, e–f) 200 mM ammonium acetate with 1.1 % (w/v) OG or (c–d, g–h) 150 mM NaCl 25 mM Tris-HCl 1.1 % (w/v) OG with (a, c, e, g) 1.6 μm and (b, d, f, h) 0.5 μm emitter tips. The collision voltage was 100 V. These data show that the absolute abundance of m/z 607 corresponding to sodiated dimer of OG is lower by between >10 to ~1000 with the small tips compared to the larger tips.
Supplemental Figure 2b. Mass spectra shown in Figure 2 with m/z from 500 to 1000 for (a–b) bR and (c–d) AqpZ ions from aqueous 200 mM ammonium acetate containing 0.55 % (w/v) SDS with (a,c) 1.6 μm and (b, d) 0.5 μm emitter tips. These data show that the absolute abundance of m/z 599 corresponding to sodiated dimer of SDS is lower by ~1000 and m/z 607 corresponding to sodiated dimer of OG is lower by ~10,000 with the small tips compared to the larger tips.
Supplemental Figure 2c. AqpZ ions formed from 200 mM ammonium acetate with 1.1% octyl glucoside. The spectrum was acquired using >1 micron tips with a Synapt G2 mass spectrometer at Amgen. Nano-electrospray ionization was initiated by applying a potential of 0.6 kV. Sample cone was set at 40 V. Ion activation at 60 V and a backing pressure of 5 mbar were applied to facilitate release the ions from the micelles. These data show that a higher backing pressure can result in a lower abundance of clusters at higher m/z.
Supplemental Figure 3. bR formed from aqueous ammonium acetate with (a–b) 0.85 % sarkosyl or (c–d) 0.073 % CTAB with 0.5 μm and 1.6 μm emitter tips.
Supplemental Figure 4. (a–b) bR and (c–d) AqpZ ions formed from 150 mM NaCl 25 mM with 0.5 μm and 1.6 μm emitter tips.
Acknowledgments
The authors are grateful for financial support from the National Institutes of Health (R01GM097357 to E.R.W. and R01GM103479 to J.A.L.) and for funds to acquire the Synapt G2Si (S10OD020062) used in these experiments. The authors thank Drs. Ryan Leib and Anthony Iavarone for helpful discussions, Drs. Nicholas Woodall and James U. Bowie (UCLA) for the generous gift of the bacteriorhodopsin T47A mutant and Dr. Pascal Egea (UCLA) for preparation and gift of Aquaporin Z.
References
- 1.Hopkins AL, Groom CR. The druggable genome. Nature Reviews Drug Discovery. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Arinaminpathy Y, Khurana E, Engelman DM, Gerstein MB. Computational analysis of membrane proteins: the largest class of drug targets. Drug Discovery Today. 2009;14:1130–1135. doi: 10.1016/j.drudis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopper JTS, Yu YTC, Li D, Raymond A, Bostock M, Liko I, Mikhailov V, Laganowsky A, Benesch JLP, Caffrey M, Nietlispach D, Robinson CV. Detergent-free mass spectrometry of membrane protein complexes. Nat Methods. 2013;10:1206–1208. doi: 10.1038/nmeth.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laganowsky A, Reading E, Hopper JTS, Robinson CV. Mass spectrometry of intact membrane protein complexes. Nat Protoc. 2013;8:639–651. doi: 10.1038/nprot.2013.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey SR, Liu Y, Liu W, Wysocki VH, Laganowsky A. Surface induced dissociation as a tool to study membrane protein complexes. Chem Commun. 2017;53:3106–3109. doi: 10.1039/c6cc09606a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong X, Liu Y, Liu W, Liang X, Russell DH, Laganowsky A. Determining Membrane Protein-Lipid Binding Thermodynamics Using Native Mass Spectrometry. J Am Chem Soc. 2016;138:4346–4349. doi: 10.1021/jacs.6b01771. [DOI] [PubMed] [Google Scholar]
- 7.Campuzano IDG, Li H, Bagal D, Lippens JL, Svitel J, Kurzeja RJM, Xu H, Schnier PD, Loo JA. Native MS Analysis of Bacteriorhodopsin and an Empty Nanodisc by Orthogonal Acceleration Time-of-Flight, Orbitrap and Ion Cyclotron Resonance. Anal Chem. 2016;88:12427–12436. doi: 10.1021/acs.analchem.6b03762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kintzer AF, Sterling HJ, Tang II, Abdul-Gader A, Miles AJ, Wallace BA, Williams ER, Krantz BA. Role of the protective antigen octamer in the molecular mechanism of anthrax lethal toxin stabilization in plasma. J Mol Biol. 2010;399:741–758. doi: 10.1016/j.jmb.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reading E, Liko I, Allison TM, Benesch JLP, Laganowsky A, Robinson CV. The role of the detergent micelle in preserving the structure of membrane proteins in the gas phase. Angew Chem Int Ed Engl. 2015;54:4577–4581. doi: 10.1002/anie.201411622. [DOI] [PubMed] [Google Scholar]
- 10.Barrera NP, Robinson CV. Advances in the mass spectrometry of membrane proteins: from individual proteins to intact complexes. Annu Rev Biochem. 2011;80:247–271. doi: 10.1146/annurev-biochem-062309-093307. [DOI] [PubMed] [Google Scholar]
- 11.Hernández H, Robinson CV. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat Protoc. 2007;2:715–726. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- 12.Iavarone AT, Udekwu OA, Williams ER. Buffer loading for counteracting metal salt-induced signal suppression in electrospray ionization. Anal Chem. 2004;76:3944–3950. doi: 10.1021/ac049724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke DJ, Campopiano DJ. Desalting large protein complexes during native electrospray mass spectrometry by addition of amino acids to the working solution. Analyst. 2015;140:2679–2686. doi: 10.1039/c4an02334j. [DOI] [PubMed] [Google Scholar]
- 14.Cassou CA, Williams ER. Desalting protein ions in native mass spectrometry using supercharging reagents. Analyst. 2014;139:4810–4819. doi: 10.1039/c4an01085j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flick TG, Cassou CA, Chang TM, Williams ER. Solution additives that desalt protein ions in native mass spectrometry. Anal Chem. 2012;84:7511–7517. doi: 10.1021/ac301629s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeMuth JC, McLuckey SA. Electrospray droplet exposure to organic vapors: metal ion removal from proteins and protein complexes. Anal Chem. 2015;87:1210–1218. doi: 10.1021/ac503865v. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Guan QY, Wang J, Jiang XX, Wu ZQ, Xia XH, Xu JJ, Chen HY. Effect of Nanoemitters on Suppressing the Formation of Metal Adduct Ions in Electrospray Ionization Mass Spectrometry. Anal Chem. 2017;89:1838–1845. doi: 10.1021/acs.analchem.6b04218. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt A, Karas M, Dülcks T. Effect of different solution flow rates on analyte ion signals in nano-ESI MS, or: when does ESI turn into nano-ESI? J Am Soc Mass Spectrom. 2003;14:492–500. doi: 10.1016/S1044-0305(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 19.Susa AC, Xia Z, Williams ER. Native Mass Spectrometry from Common Buffers with Salts That Mimic the Extracellular Environment. Angew Chem Int Ed Engl. 2017;56:7912–7915. doi: 10.1002/anie.201702330. [DOI] [PubMed] [Google Scholar]
- 20.Susa AC, Xia Z, Williams ER. Small Emitter Tips for Native Mass Spectrometry of Proteins and Protein Complexes from Nonvolatile Buffers That Mimic the Intracellular Environment. Anal Chem. 2017;89:3116–3122. doi: 10.1021/acs.analchem.6b04897. [DOI] [PubMed] [Google Scholar]
- 21.Mortensen DN, Williams ER. Electrothermal supercharging of proteins in native MS: effects of protein isoelectric point, buffer, and nanoESI-emitter tip size. Analyst. 2016;141:5598–5606. doi: 10.1039/c6an01380e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Mass increase from adduction for bR and AqpZ ions formed from 200 mM ammonium acetate or 150 mM NaCl 25 mM Tris-HCl and detergents with 0.5 μm and 1.6 μm emitter tips. The adduction to AqpZ tetramer was calculated with reference to the measured monomer mass of AqpZ (times four). The adduction to bR was calculated with reference to the elemental composition of bR.
Supplemental Figure 1. Four replicate mass spectra for AqpZ ions formed from 200 mM ammonium acetate with 1.1 % octyl glucoside using four different 0.5 μm emitter tips. These data show the reproducibility of mass spectra obtained with different nano-ESI emitters that are approximately the same size.
Supplemental Figure 2a. Mass spectra shown in Figure 1 in the m/z range from 500 to 1000 for (a–d) bR and AqpZ (e–h) from aqueous (a–b, e–f) 200 mM ammonium acetate with 1.1 % (w/v) OG or (c–d, g–h) 150 mM NaCl 25 mM Tris-HCl 1.1 % (w/v) OG with (a, c, e, g) 1.6 μm and (b, d, f, h) 0.5 μm emitter tips. The collision voltage was 100 V. These data show that the absolute abundance of m/z 607 corresponding to sodiated dimer of OG is lower by between >10 to ~1000 with the small tips compared to the larger tips.
Supplemental Figure 2b. Mass spectra shown in Figure 2 with m/z from 500 to 1000 for (a–b) bR and (c–d) AqpZ ions from aqueous 200 mM ammonium acetate containing 0.55 % (w/v) SDS with (a,c) 1.6 μm and (b, d) 0.5 μm emitter tips. These data show that the absolute abundance of m/z 599 corresponding to sodiated dimer of SDS is lower by ~1000 and m/z 607 corresponding to sodiated dimer of OG is lower by ~10,000 with the small tips compared to the larger tips.
Supplemental Figure 2c. AqpZ ions formed from 200 mM ammonium acetate with 1.1% octyl glucoside. The spectrum was acquired using >1 micron tips with a Synapt G2 mass spectrometer at Amgen. Nano-electrospray ionization was initiated by applying a potential of 0.6 kV. Sample cone was set at 40 V. Ion activation at 60 V and a backing pressure of 5 mbar were applied to facilitate release the ions from the micelles. These data show that a higher backing pressure can result in a lower abundance of clusters at higher m/z.
Supplemental Figure 3. bR formed from aqueous ammonium acetate with (a–b) 0.85 % sarkosyl or (c–d) 0.073 % CTAB with 0.5 μm and 1.6 μm emitter tips.
Supplemental Figure 4. (a–b) bR and (c–d) AqpZ ions formed from 150 mM NaCl 25 mM with 0.5 μm and 1.6 μm emitter tips.