Abstract
Objective
This study examined if methamphetamine use alone (METH + HIV−) and methamphetamine use in combination with HIV (METH + HIV+) were associated with hypothalamic-pituitary-adrenal (HPA) axis dysregulation as well as insulin resistance relative to a nonmethamphetamine-using, HIV-negative comparison group (METH-HIV−).
Methods
Using an intact groups design, serum levels of HPA axis hormones in 46 METH + HIV− and 127 METH + HIV+ men who have sex with men (MSM) were compared to 136 METH-HIV− men.
Results
There were no group differences in prevailing adrenocorticotropic hormone (ACTH) or cortisol levels, but the association between ACTH and cortisol was moderated by METH + HIV+ group (β= −0.19, p < .05). Compared to METH-HIV− men, METH + HIV+ MSM displayed 10% higher log10 cortisol levels per standard deviation lower ACTH. Both groups of methamphetamine-using MSM had lower insulin resistance and greater syndemic burden (i.e., sleep disturbance, severe depression, childhood trauma, and polysubstance use disorder) compared to METH-HIV− men. However, the disaggregated functional relationship between ACTH and cortisol in METH + HIV+ MSM was independent of these factors.
Conclusions
Further research is needed to characterize the bio-behavioral pathways that explain dysregulated HPA axis functioning in HIV-positive, methamphetamine-using MSM.
Keywords: adrenocorticotropic hormone, cortisol, HIV, insulin, methamphetamine, Syndemic
1 | INTRODUCTION
Although the acute effects of methamphetamine (meth) are attributable to sympathetic nervous system activation (Makisumi et al., 1998), the hypothalamic-pituitary-adrenal (HPA) axis response is thought to play an important role in the reinforcing effects of stimulants (Goeders, 2002). Consequently, meth has been shown to dysregulate HPA axis functioning in animal and human studies (Zuloaga, Jacobskind, & Raber, 2015). Acute meth exposure increases plasma cortisol in humans (Harris, Reus, Wolkowitz, Mendelson, & Jones, 2003; Sachar et al., 1981), and these meth-induced elevations in plasma cortisol may be partially independent of adrenocorticotropic hormone (ACTH) levels (Fehm, Holl, Steiner, Klein, & Voigt, 1984). To date, the ACTH-independent mechanism(s) for the acute effects of meth on elevated cortisol have not been clearly elucidated.
Chronic meth use may exert direct effects and indirect effects via altered stress responses on HPA axis dysregulation. Chronic meth users display lower basal levels of cortisol (Carson et al., 2012), which may be partially due to meth withdrawal. For example, increases in anxiety and depressive symptoms during meth withdrawal have been shown parallel elevations in serum ACTH and decreases in cortisol (Li et al., 2013; McGregor et al., 2005). There is also some evidence that younger meth users have greater increases in salivary cortisol during the Trier Social Stress Task (King, Alicata, Cloak, & Chang, 2010), but other research with stimulant users has not observed changes in salivary cortisol during this and another laboratory-based stressor (Harris, Reus, Wolkowitz, Mendelson, & Jones, 2005). In one study examining a naturalistic stressor, meth-dependent men displayed increases in plasma cortisol 2 days after inguinal hernia surgery, but intercurrent meth use was not measured during this period (Pirnia, Givi, Roshan, Pirnia, & Soleimani, 2016). More definitive research examining the mechanisms of HPA axis dysregulation in chronic meth users is clearly needed.
HPA axis dysregulation in chronic meth users is thought to be partially attributable to psychiatric comorbidities and metabolic changes. There is increasing recognition that some groups of meth users such as meth-using men who have sex with men (MSM) experience a syndemic of co-occurring psychosocial health problems including depression, childhood trauma, and polysubstance use that fuel risk for HIV/AIDS (Carrico et al., 2012; Leserman, 2008; Lopez-Patton et al., 2016; Parsons, Grov, & Golub, 2012). Syndemic factors such as depression could amplify HPA axis dysregulation among meth-using MSM. Although this is partially supported by laboratory-based research where depressed individuals display a paradoxical suppression of plasma cortisol in response to stimulants (Sachar et al., 1981), other studies with chronic meth users have not observed associations of psychiatric symptoms with cortisol (King et al., 2010; Li et al., 2013). Finally, some animal research demonstrates that acute meth administration stimulates the pancreas to secrete insulin (McMahon, Andersen, Feldman, & Schanberg, 1971), an effect that is augmented by a hyperthyroid state (McMahon, Feldman, & Schanberg, 1975). Although rising insulin levels lead to robust increases in plasma cortisol (Fehm et al., 1984), in one prior study chronic meth users displayed a lower blood glucose and body mass index (BMI) compared to nonusers (Lv et al., 2016). Taken together, syndemic burden and measures of glycemic metabolic functioning remain important possible confounders in examining HPA dysregulation among chronic meth users.
There are also well characterized neuroendocrine abnormalities in HIV (Brown, 2011). One study of patients who died from AIDS documented adrenal compromise such that almost all pathology results (99%) displayed inflammatory infiltrates and approximately half (48%) had evidence of cytomegalovirus infection in the adrenal glands (Rodrigues et al., 2002). HIV-associated dysregulation of the HPA axis is further supported by findings that HIV-positive men display elevated serum cortisol compared to HIV-negative men (Christeff, Gharakhanian, Thobie, Rozenbaum, & Nunez, 1992). Although these studies were conducted prior to the era of highly active antiretroviral therapy (ART), there is increasing recognition that even those on contemporary ART regimens who achieve virologic suppression display persistent immune activation and inflammation (Lederman, Funderburg, Sekaly, Klatt, & Hunt, 2013) that could be both a cause and consequence of HPA dysregulation.
The overarching goal of the present study was to examine the potential implications of meth use alone and in combination with HIV for HPA axis dysregulation. Using an intact groups design, HIV-negative and HIV-positive meth-using MSM (METH + HIV− and METH + HIV+) were compared to HIV-negative, nonmeth-using men (METH-HIV−). Consistent with prior research on meth withdrawal (Li et al., 2013), we hypothesized that there would be a main effect of meth such that both meth-using groups would display higher serum ACTH and lower serum cortisol (irrespective of HIV status). Because meth may increase cortisol through ACTH-independent pathways (Fehm et al., 1984), we also hypothesized that the magnitude of the association between serum ACTH and serum cortisol would be lower in both meth-using groups (irrespective of HIV status). We also explored whether METH + HIV+ MSM displayed greater HPA axis dys-regulation compared to METH + HIV− MSM.
2 | METHODS
2.1 | Participants and procedures
Approval for this project was obtained from the University of Miami Miller School of Medicine Institutional Review Board, and a Federal Certificate of Confidentiality was procured. Men were recruited from local clinics, hospitals, support groups, substance abuse treatment programs, and by word of mouth in Miami, Florida from November 2011 to February 2016. All participants completed a telephone screen. Meth users were required to be MSM who reported using meth in the past 3 months during the telephone screen. Men between the ages of 18 and 55 were enrolled to complete a one-time study visit that included the Structured Clinical Interview for DSM-IV (SCID) Substance Use Disorders module, administered by bachelor-level or master-level study personnel who were trained by a licensed psychologist (First, Spitzer, Gibbon, & Williams, 1997). All meth-using MSM were required to meet criteria for a DSM-IV meth use disorder. Participants also completed a battery of self-report psychosocial assessments and provided a fasting peripheral venous blood sample collected between 9 and 10 AM. Exclusion criteria, assessed via self-report, were a history of migraine, seizure, visual impairment, learning disorders, cardiovascular disease, diabetes mellitus, hypertension, current treatment for Hepatitis C or depression, and bereavement or loss of social support in the previous 3 months.
All METH + HIV− and METH + HIV+ MSM enrolled in this study met criteria for meth abuse or dependence. These groups of meth-using MSM were compared to METH-HIV− men who were not required to identify as MSM. The comparison group of METH-HIV− men were not required to be MSM because we are aware of no evidence to support perturbations in baseal levels of HPA axis hormones (our primary outcome) in heterosexual men versus MSM. Of the 346 men enrolled, 37 (11%) were excluded because their blood glucose levels were greater than 110 mg/dl indicating that these were not fasting samples or the presence of prediabetes. There were no differences between the METH + HIV− (n = 6; 12%), METH + HIV+ (n = 20; 14%), and METH-HIV− (n = 11; 8%) groups in the proportion of participants excluded for a blood glucose greater than 110 mg/dl (χ2 (2) = 2.93, p = .23). Among the 309 men who were included in the present study, 46 were METH + HIV− MSM, 127 were METH + HIV+ MSM, and 136 were METH-HIV− men. All participants were compensated $50 for their time and travel expenses.
2.2 | Measures
2.2.1 | Demographics and health status
Participants completed a demographic questionnaire assessing age, race/ethnicity, and health-related factors. All participants underwent a physical examination that included weight and height, which were used to calculate BMI.
2.2.2 | Syndemic conditions
Participants were administered the SCID and completed a battery of validated, self-report measures to screen for co-occurring sydnemic conditions. The Pittsburgh Sleep Quality index with a cutoff of >8 was administered to measure sleep disturbance (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Depression was assessed using the 20-item Centers for the Epidemiologic Study of Depression, using a cutoff of >27 to indicate severe depression (Reisner et al., 2009). Childhood trauma was assessed using a modified version of the Childhood Trauma Questionnaire, specifically the physical and sexual abuse subscales; a cutoff of >13 was used use for these subscales, representing severe to extreme childhood trauma (Bernstein et al., 2003). Polysubstance abuse or dependence was assessed using the SCID (First et al., 1997). A count variable was calculated to measure the presence of each of the four syndemic conditions (range = 0–4): sleep disturbance, severe depression, severe childhood abuse, and a polysubstance use disorder.
2.2.3 | Insulin resistance, ACTH, and cortisol
All participants provided a fasting blood sample to assess glucose and insulin. Insulin resistance was calculated on the basis of glucose and insulin using the Homeostasis Model Assessment Insulin Resistance (HOMA-IR) formula (Matthews et al., 1985), which has been reported to be superior in predicting cardiovascular risk relative to fasting glucose or fasting insulin alone (Gast, Tjeerdema, Stijnen, Smit, & Dekkers, 2012). Serum ACTH (IBL America ELISA Cat# BM7023; ENZO ELISA Cat# ENZ-KIT 138–0001) and cortisol (IBL ELISA America Cat# BM79135; ENZO ELISA Cat# ADI-900-071) were assessed by means of a radioimmunoassay of 10 ml blood samples collected in EDTA tubes that were centrifuged at 3500 rpm for 15 min at 4°C. The collected specimen was then distributed in a microcentrifuge tube and stored at −20°F until processed.
2.3 | Statistical analyses
Analyses of variance and chi-square tests were utilized to examine METH/HIV group differences in demographic factors, BMI, HOMA-IR scores, serum ACTH levels, and serum cortisol levels. Post hoc probing of a significant analyses of variance F test was conducted with the Bonferroni correction to reduce the family-wise error rate. Because of their skewed distribution, HOMA-IR, ACTH, and cortisol values were log10 transformed. The count of syndemic conditions violated the homogeneity of variance assumption, and as a result a chi-square test was used to examine METH group differences in the proportion of participants screening positive for three or more syndemic conditions. Subsequently, a multiple linear regression analysis tested whether the METH/HIV groups moderated the association of ACTH and cortisol after controlling for age, race/ethnicity, BMI, HOMA-IR score, and number of syndemic conditions. The interactions of the METH + HIV − and METH + HIV+ groups with ACTH were entered into a separate regression block and only significant interaction terms were retained in the final model. The significance of the incremental increase in explained variance (ΔR2) in log10 cortisol was tested to confirm moderation by the METH/HIV groups. The unadjusted parameter estimates for significant correlates were transformed using a simple formula (100*[10B-1]) to characterize the percent change in log10 cortisol per unit change in the independent variables that were statistically significant.
3 | RESULTS
Participant age ranged from 18 to 55 years, with a mean of 36.8 (SD = 9.8). Half of participants were Hispanic/Latino (51%), 26% were African American, 19% were Caucasian, and 4% were other racial or ethnic minorities or multiracial. Approximately half (47%) had a monthly personal income of less than USD $500. Most participants (61%) had a BMI indicating they were overweight or obese. Among the meth-using groups, one-third (37%) met criteria for meth abuse and approximately two-thirds met criteria for meth dependence (63%). Approximately 70% of meth-using participants met criteria for a polysubstance use disorder. The majority of METH + HIV+ participants (88%) were currently prescribed ART.
As detailed in Table 1, fewer METH-HIV− participants were Caucasian. More METH-HIV− participants were overweight or obese and had significantly higher HOMA-IR scores compared to the meth-using groups. METH + HIV+ participants were older and had a greater burden of co-occurring syndemic conditions compared to the METH-HIV− and METH + HIV− groups. METH + HIV− participants also had greater burden of co-occurring syndemic conditions compared to METH-HIV− participants. There were no METH group differences in prevailing levels of ACTH (F[2, 306] = 0.87, p = .42) or cortisol (F[2, 306] = 2.72, p = .07).
TABLE 1.
Group differences in sociodemographics, syndemic burden, and neuroendocrine functioning (N = 309)
| METH-HIV− (n = 136) | METH + HIV+ (n = 127) | METH + HIV− (n = 46) | ||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Race/ethnicity | 12 (9) | χ2 (6) = 18.24, p = .006 | ||
| Caucasian | 38 (28) | 34 (27) | 13 (28) | |
| African American | 79 (58) | 33 (26) | 9 (20) | |
| Hispanic/Latino | 7 (5) | 56 (44) | 21 (46) | |
| Body mass index (BMI) | χ2 (4) = 23.47, p < .001 | |||
| Normal | 34 (25) | 60 (48) | 25 (54) | |
| Overweight | 58 (43) | 47 (37) | 14 (31) | |
| Obese | 44 (32) | 19 (15) | 7 (15) | |
| >3 Syndemic conditions | 1 (1)a,b | 79 (62)a,c | 20 (44)b,c | χ2 (2) = 116.42, p < .001 |
| M (SD) | M (SD) | M (SD) | ||
| Age | 35.26 (10.45)a | 39.85 (8.39)a,c | 33.22 (9.50)c | F(2, 306) = 11.59, p < .001 |
| Log10 HOMA-IR | 0.51 (0.30)a,b | 0.34 (0.30)a | 0.28 (0.35)b | F(2, 306) = 13.96, p < .001 |
| Log10 ACTH | 1.30 (0.29) | 1.31 (0.37) | 1.37 (0.21) | F(2, 306) = 0.87, p = .42 |
| Log10 cortisol | 1.99 (0.15) | 2.03 (0.19) | 2.04 (0.19) | F(2, 306) = 2.72, p = .07 |
METH-HIV− versus METH + HIV+, p < .05.
METH-HIV− vs METH + HIV−, p < .05.
METH + HIV+ versus METH + HIV−, p < .05.
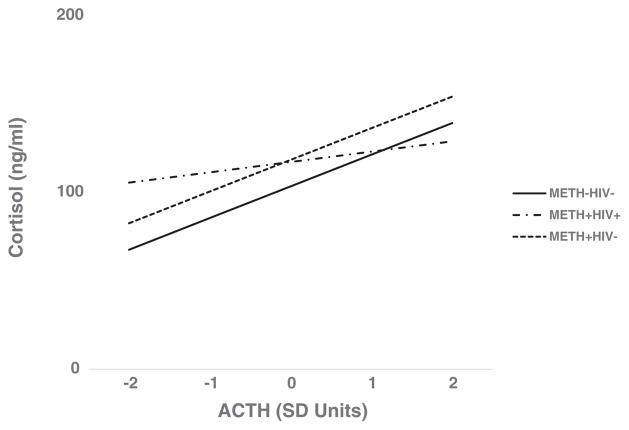
A multiple linear regression analysis examined if the METH/HIV groups moderated the association of ACTH with log10 cortisol. As shown in Table 2, the addition of the METH + HIV+ X log10 ACTH term significantly increased the explained variance in log10 cortisol (ΔR2 for the addition of the interaction term = 0.016, p = .020). The model accounted for 15.5% of the variance in log10 cortisol. METH + HIV+ group significantly moderated the association of ACTH and log10 cortisol (β= −0.19; t = −2.34, p = .020). For each one standard deviation lower ACTH, log10 cortisol was approximately 10% higher in METH + HIV+ participants. The slope of the association between ACTH and cortisol was substantially flatter in METH + HIV+ participants compared to METH-HIV− participants (see Figure 1). Because the interaction of METH + HIV− X log10 ACTH was not statistically significant (β= 0.07; t = 1.17, p = .245), it was excluded from the final model.
TABLE 2.
Correlates of log10 plasma cortisol (N = 308)
| Unstandardized B | Beta | 100(10B - 1) | t | p | |
|---|---|---|---|---|---|
| METH/HIV group | |||||
| METH-HIV− (reference) | |||||
| METH + HIV+ | 0.033 | 0.094 | 0.99 | .323 | |
| METH + HIV− | 0.017 | 0.036 | 0.48 | .631 | |
| Age (decade) | −0.023 | −0.132 | −5.16 | −2.26 | .025 |
| Race/ethnicity | |||||
| Caucasian (reference) | |||||
| African American | −0.038 | −0.096 | −1.32 | .189 | |
| Hispanic/Latino | −0.003 | −0.009 | −0.12 | .906 | |
| Other ethnic minority | 0.050 | 0.061 | 1.03 | .304 | |
| Body mass index (BMI) | |||||
| Normal (reference) | |||||
| Overweight | −0.010 | −0.029 | −0.47 | .640 | |
| Obese | −0.036 | −0.088 | −1.35 | .177 | |
| HOMA-IR score | −0.006 | −0.011 | −0.19 | .851 | |
| Count of Syndemic conditions | 0.002 | 0.019 | 0.22 | .827 | |
| Log10 ACTH | 0.237 | 0.438 | 72.58 | 5.23 | <.001 |
| Meth + HIV+ × ACTH | −0.044 | −0.193 | −9.64 | −2.34 | .020 |
Note. R2 (with interactions) = 0.155; ΔR2 for the addition of the interaction term = 0.016, p = 0.019.
FIGURE 1.
METH/HIV group moderates the association of serum ACTH with serum cortisol (N = 308)
4 | DISCUSSION
Findings from this study highlight that METH + HIV+ MSM display disaggregation of the functional relationship between serum ACTH and cortisol. Despite the fact that there were no prevailing group differences in the absolute levels of ACTH and cortisol, METH + HIV+ MSM had 10% higher log10 cortisol per standard deviation lower ACTH compared to METH-HIV− men. Interestingly, METH + HIV− MSM did not show a similar disaggregated association between ACTH and cortisol levels, in contrast to prior studies in which meth users displayed higher basal ACTH and lower cortisol (Carson et al., 2012; Li et al., 2013).
It is plausible that the combined effects of meth and HIV are most detrimental for HPA axis functioning. Prior laboratory and in vitro research observed that meth, inflammation, and microbial translocation may increase cortisol output through ACTH-independent pathways (Dinan & Cryan, 2012; Fehm et al., 1984; Path, Bornstein, Ehrhart-Bornstein, & Scherbaum, 1997). Although results of the present study highlight the potential combined effects of meth and HIV for HPA dysregulation, we were unable to examine the association of HIV alone without a METH-HIV+ group in this study. There is increasing recognition of persistent inflammation in treated HIV infection (Lederman et al., 2013), which is thought to be due in part to intestinal damage by HIV and could be augmented by alcohol or other substance use (Justice & Falutz, 2014). HIV-induced increases in pro-inflammatory cytokines such as IL-6 could bind to adrenal inflammatory receptors and amplify cortisol output independent of prevailing ACTH levels (Path et al., 1997). It is also plausible that HIV-associated adrenal pathology such as inflammatory infiltrates increase responsivity to ACTH. Further research is needed to examine the extent to which observed HPA axis dysregulation in METH + HIV+ MSM reflect the effects of meth, HIV, or both.
Both meth-using MSM groups displayed lower levels of insulin resistance and greater syndemic burden compared to METH-HIV− men. However, these did not account for observed HPA axis dysregulation among METH + HIV+ MSM. One previous study observed that meth users display lower blood glucose levels (Lv et al., 2016), but it did not measure insulin or account for HIV status. Results of the present study build upon these findings by demonstrating the meth-using MSM display lower HOMA-IR scores irrespective of HIV status compared to METH-HIV− men. Consistent with prior research, results also highlight that METH + HIV+ MSM displayed greater burden of co-occurring syndemic conditions compared to METH + HIV− MSM (Carrico et al., 2012; Lopez-Patton et al., 2016; Parsons et al., 2012). Despite the fact that greater syndemic burden was not associated with higher cortisol, the implications of syndemic conditions for HPA axis functioning remains an important area for further research with MSM.
Results of the present study should be interpreted in context of some important limitations. First, serum measures cannot capture important aspects of diurnal variation in HPA axis functioning. Future research should include repeated measures of salivary cortisol to better understand whether meth users display dysregulation of diurnal cortisol patterns. Salivary and urinary specimen collection methods should also be employed to examine meth-related changes in output of sympathetic-adrenal-medulla (SAM) axis hormones such as norepinephrine. The SAM axis may be more responsive to meth-induced changes being that its acute effects are mediated by the sympathetic nervous system and increases in norepinephrine could modulate HPA axis functioning (Dinan & Cryan, 2012). It is also noteworthy that this study examined basal levels of ACTH and cortisol. Future studies should consider including functional measures of positive (e.g., ACTH challenge) and negative (e.g., dexamethasone suppression) feedback to better understand the underlying source(s) of HPA dysregulation. Studies should also examine dose–response associations of meth use with HPA axis dysregulation by administering self-report measures as well as collecting specimens (e.g., urine and hair) for toxicology testing. Finally, another possible limitation of this study is that both meth-using MSM groups were compared to METH-HIV− men who were not required to be MSM. Although findings remained significant after controlling for group differences in syndemic burden, future research should examine whether results generalize to meth-using heterosexual men.
5 | CONCLUSIONS
This study demonstrates that HIV-positive, meth-using MSM display disaggregation of the functional relationship between ACTH and cortisol compared to HIV-negative, non-meth-using men. These findings will inform the scientific premise for future research examining the bio-behavioral processes whereby HIV alone or in combination with meth contribute to HPA axis dysregulation in MSM. Further research should examine the extent to which HIV alone or in combination with meth potentiate microbial translocation and inflammatory processes that could account for greater HPA axis dysregulation.
Acknowledgments
Funding information
National Institute of Allergy and Infectious Diseases, Grant/Award Number: P30 AI073961; Miami Center for AIDS Research (CFAR); National Institute on Drug Abuse, Grant/Award Number: R01-DA031201
This research was supported by the National Institute on Drug Abuse (R01-DA031201; Kumar, PI) and additional support was provided by the Miami Center for AIDS Research (CFAR), funded by a grant from the National Institute of Allergy and Infectious Diseases (P30 AI073961; Pahwa, PI). We would like to express our gratitude for the efforts of the participants who completed these study procedures.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
References
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, … Zule W. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Brown TT. The effects of HIV-1 infection on endocrine organs. Best Practice & Research. Clinical Endocrinology & Metabolism. 2011;25(3):403–413. doi: 10.1016/j.beem.2011.04.005. https://doi.org/10.1016/j.beem.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Pollack LM, Stall RD, Shade SB, Neilands TB, Rice TM, … Moskowitz JT. Psychological processes and stimulant use among men who have sex with men. Drug and Alcohol Dependence. 2012;123(1–3):79–83. doi: 10.1016/j.drugalcdep.2011.10.020. https://doi.org/10.1016/j.drugalcdep.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Bosanquet DP, Carter CS, Pournajafi-Nazarloo H, Blaszczynski A, McGregor IS. Preliminary evidence for lowered basal cortisol in a naturalistic sample of methamphetamine polydrug users. Experimental and Clinical Psychopharmacology. 2012;20(6):497–503. doi: 10.1037/a0029976. https://doi.org/10.1037/a0029976. [DOI] [PubMed] [Google Scholar]
- Christeff N, Gharakhanian S, Thobie N, Rozenbaum W, Nunez EA. Evidence for changes in adrenal and testicular steroids during HIV infection. Journal of Acquired Immune Deficiency Syndromes. 1992;5(8):841–846. [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37(9):1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. https://doi.org/10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Fehm HL, Holl R, Steiner K, Klein E, Voigt KH. Evidence for ACTH-unrelated mechanisms in the regulation of cortisol secretion in man. Klinische Wochenschrift. 1984;62(1):19–24. doi: 10.1007/BF01725188. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders, clinician version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: Meta-analysis. PLoS One. 2012;7(12):e52036. doi: 10.1371/journal.pone.0052036. https://doi.org/10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27(1–2):13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Harris DS, Reus VI, Wolkowitz OM, Mendelson JE, Jones RT. Altering cortisol level does not change the pleasurable effects of methamphetamine in humans. Neuropsychopharmacology. 2003;28(9):1677–1684. doi: 10.1038/sj.npp.1300223. https://doi.org/10.1038/sj.npp.1300223. [DOI] [PubMed] [Google Scholar]
- Harris DS, Reus VI, Wolkowitz OM, Mendelson JE, Jones RT. Repeated psychological stress testing in stimulant-dependent patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29(5):669–677. doi: 10.1016/j.pnpbp.2005.04.012. https://doi.org/10.1016/j.pnpbp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Justice A, Falutz J. Aging and HIV: An evolving understanding. Current Opinion in HIV and AIDS. 2014;9(4):291–293. doi: 10.1097/COH.0000000000000081. https://doi.org/10.1097/COH.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G, Alicata D, Cloak C, Chang L. Psychiatric symptoms and HPA axis function in adolescent methamphetamine users. Journal of Neuroimmune Pharmacology. 2010;5(4):582–591. doi: 10.1007/s11481-010-9206-y. https://doi.org/10.1007/s11481-010-9206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Advances in Immunology. 2013;119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. https://doi.org/10.1016/B978-0-12-407707-2.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine. 2008;70(5):539–545. doi: 10.1097/PSY.0b013e3181777a5f. https://doi.org/10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Li SX, Yan SY, Bao YP, Lian Z, Qu Z, Wu YP, Liu ZM. Depression and alterations in hypothalamic-pituitary-adrenal and hypothalamic-pituitary-thyroid axis function in male abstinent methamphetamine abusers. Human Psychopharmacology. 2013;28(5):477–483. doi: 10.1002/hup.2335. https://doi.org/10.1002/hup.2335. [DOI] [PubMed] [Google Scholar]
- Lopez-Patton M, Kumar M, Jones D, Fonseca M, Kumar AM, Nemeroff CB. Childhood trauma and METH abuse among men who have sex with men: Implications for intervention. Journal of Psychiatric Research. 2016;72:1–5. doi: 10.1016/j.jpsychires.2015.09.009. https://doi.org/10.1016/j.jpsychires.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv D, Zhang M, Jin X, Zhao J, Han B, Su H, … He J. The body mass index, blood pressure, and fasting blood glucose in patients with methamphetamine dependence. Medicine (Baltimore) 2016;95(12):e3152. doi: 10.1097/MD.0000000000003152. https://doi.org/10.1097/MD.0000000000003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makisumi T, Yoshida K, Watanabe T, Tan N, Murakami N, Morimoto A. Sympatho-adrenal involvement in methamphetamine-induced hyperthermia through skeletal muscle hypermetabolism. European Journal of Pharmacology. 1998;363(2–3):107–112. doi: 10.1016/s0014-2999(98)00758-4. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of meth-amphetamine withdrawal. Addiction. 2005;100(9):1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. https://doi.org/10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McMahon EM, Andersen DK, Feldman JM, Schanberg SM. Methamphetamine-induced insulin release. Science. 1971;174(4004):66–68. doi: 10.1126/science.174.4004.66. [DOI] [PubMed] [Google Scholar]
- McMahon EM, Feldman JM, Schanberg SM. Further studies of methamphetamine-induced insulin release. Toxicology and Applied Pharmacology. 1975;32(1):62–72. doi: 10.1016/0041-008x(75)90195-7. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Grov C, Golub SA. Sexual compulsivity, co-occurring psychosocial health problems, and HIV risk among gay and bisexual men: Further evidence of a syndemic. American Journal of Public Health. 2012;102(1):156–162. doi: 10.2105/AJPH.2011.300284. https://doi.org/10.2105/AJPH.2011.300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Path G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: Expression and effects on steroidogenesis. The Journal of Clinical Endocrinology and Metabolism. 1997;82(7):2343–2349. doi: 10.1210/jcem.82.7.4072. https://doi.org/10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- Pirnia B, Givi F, Roshan R, Pirnia K, Soleimani AA. The cortisol level and its relationship with depression, stress and anxiety indices in chronic methamphetamine-dependent patients and normal individuals undergoing inguinal hernia surgery. Medical Journal of the Islamic Republic of Iran. 2016;30:395. [PMC free article] [PubMed] [Google Scholar]
- Reisner SL, Mimiaga MJ, Skeer M, Bright D, Cranston K, Isenberg D, … Mayer KH. Clinically significant depressive symptoms as a risk factor for HIV infection among black MSM in Massachusetts. AIDS and Behavior. 2009;13(4):798–810. doi: 10.1007/s10461-009-9571-9. https://doi.org/10.1007/s10461-009-9571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues D, Reis M, Teixeira V, Silva-Vergara M, Filho DC, Adad S, Lazo J. Pathologic findings in the adrenal glands of autopsied patients with acquired immunodeficiency syndrome. Pathology, Research and Practice. 2002;198(1):25–30. doi: 10.1078/0344-0338-00180. https://doi.org/10.1078/0344-0338-00180. [DOI] [PubMed] [Google Scholar]
- Sachar EJ, Halbreich U, Asnis GM, Nathan RS, Halpern FS, Ostrow L. Paradoxical cortisol responses to dextroamphetamine in endogenous depression. Archives of General Psychiatry. 1981;38(10):1113–1117. doi: 10.1001/archpsyc.1981.01780350047005. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Jacobskind JS, Raber J. Methamphetamine and the hypothalamic-pituitary-adrenal axis. Frontiers in Neuroscience. 2015;9:178. doi: 10.3389/fnins.2015.00178. https://doi.org/10.3389/fnins.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]