Abstract
Background
The treatment of postoperative pain is a challenge after posterior spinal fusions. Pain management using predominantly opioids is often associated with multiple adverse effects, while multimodal postoperative analgesia may provide adequate pain relief with fewer opioid side effects.
Questions/Purposes
The purpose of this review is to determine whether addition of 150 mg pregabalin daily would reduce narcotic requirements and improve outcomes after posterior lumbar fusion (PLF).
Methods
The method used is a randomized, controlled trial of elective PLF patients who received pregabalin or placebo. With institutional review board (IRB) approval, 86 patients undergoing elective posterior lumbar fusion, ASA I–III, were randomized to receive either a placebo or pregabalin after obtaining written informed consent. Both arms, i.e., placebo and pregabalin, consisted of 43 patients each.
The 86 patients for elective PLF were randomly assigned to receive 150 mg of pregabalin 1 h before surgery and then 150 mg daily, or a placebo tablet. All patients received a similar general anesthetic and in the post-anesthesia care unit (PACU), started on intravenous (IV) patient-controlled analgesia (PCA) of hydromorphone (0.2 mg/ml). Postoperative pain was assessed daily until discharge using a Numerical Rating Scale (NRS) at rest and with physical therapy (PT). Patients were also assessed twice daily for level of sedation and nausea and/or vomiting and expected PT milestones. All narcotics (IV, oral) were documented.
Results
Demographics and operative time between groups were similar. PCA hydromorphone administration and oral narcotic intake were not statistically different between the two groups. However, an increased incidence of nausea and vomiting in the placebo group reached statistical significance (p < 0.05). In addition, there was no statistical difference between groups with respect to achieving PT milestones and hospital discharge day.
Conclusion
After PLF, patients receiving pregabalin 150 mg/day did not have reduced IV narcotic usage, improved PT milestones, or reduced length of hospital stay. We were unable to demonstrate an analgesic advantage to prescribing pregabalin to patients undergoing lumbar spinal fusions.
Electronic supplementary material
The online version of this article (10.1007/s11420-017-9584-2) contains supplementary material, which is available to authorized users.
Keywords: posterior lumbar fusions, pregabalin, multimodal analgesia, postoperative analgesia, pain scores after spinal fusions
Introduction
The treatment of postoperative pain continues to be a challenge, particularly after posterior spinal fusions. Many of these patients have been treated with analgesics or other modalities for prolonged periods before choosing the surgical alternative. In addition, the narcotic-based anesthetic required for the procedure may induce postoperative hyper-analgesia [3]. Inadequate treatment of this pain can result in prolonged hospitalization, cardiopulmonary complications, and poor surgical outcome [1]. However, the narcotic treatment of pain is often associated with multiple adverse effects. Multimodal postoperative analgesia has been instituted to reduce pain while limiting the adverse side effects of opioids [11, 16–18]. Pregabalin has been shown to be efficacious in the management of chronic pain syndromes with limited adverse side effects [4, 5]. Hence, multiple studies have attempted to demonstrate the benefits of including pregabalin in multimodal postoperative pain management [13, 14, 20]. These studies have yielded conflicting results with regard to reduced pain, opioid consumption, and improved outcome [2, 6, 8, 9, 12–14, 19, 20].
We propose that the addition of pregabalin to acute pain regimen after posterior spinal fusions should reduce narcotic requirements and hence improve outcome by reducing narcotic-induced side effects. Although recent studies have also examined the administration of pregabalin after spinal fusions [6, 8, 9], this study was conducted with a uniform anesthetic regimen and similar procedure performed by two spine surgeons at one institution. Pain scores were controlled as well as physical therapy milestones to assess whether changes in the pain regimen would affect narcotic consumption, narcotic induced side effects, and length of hospitalization.
Patients and Methods
With IRB approval, patients scheduled for elective posterior 1–4 level lumbar spinal fusion with segmental instrumentation were randomly assigned (random number generator, sealed envelope) and consented to receive pregabalin or placebo. Patients in the pregabalin group received 150 mg of pregabalin with a sip of water 1 h prior to surgery and then 150 mg daily (75 mg BID) for a total of 2 weeks. Patients in the placebo group received a placebo tablet with a sip of water prior to surgery and a placebo tablet twice a day for a total of 2 weeks. All clinicians involved in the care of these patients were blinded to group assignment. Patients taking pregabalin or gabapentin preoperatively were required to stop these medications for 2 weeks prior to entering the study. Patients with renal insufficiency (Cr > 1.5), major psychiatric disorders, or receiving methadone or buprenorphine treatment were considered not eligible for the study. The original study design included patients being followed after discharge from the hospital with 14 days of either pregabalin or placebo. This report includes only the hospital experience.
All patients in the study received a general anesthetic which consisted of midazolam 5 mg, nitrous oxide (50%), isoflurane 0.4%, fentanyl 1–2 μcg/kg/h, ketamine 2 μg/kg/min, and propofol 25 μcg/kg/h. All patients received intravenous ondansetron 4 mg and dexamethasone 8 mg prior to extubation. Patients were extubated in the operating room and within 15 min of arriving in the post-anesthesia care unit (PACU), started on intravenous hydromorphone in a patient-controlled analgesia (PCA) modality, without a basal rate and bolus dosing which was adjusted by the acute pain team to achieve a Numerical Rating Scale (NRS) for pain ≤ 5. When tolerated on postoperative day (POD) 1 or 2, the analgesic regimen was switched to oral oxycodone or a narcotic equivalent, if the oxycodone was not tolerated, and acetaminophen 650 mg every 6 h. All narcotics (oral and intravenous) were recorded and oral narcotics were converted to milligram morphine equivalents.
Postoperative pain was assessed daily until discharge using NRS pain scores at rest and during physical therapy; a NRS of 0 is defined as no pain and a 10 defined as the worst pain ever experienced. Pain was assessed in the PACU on the day of surgery 1 h after the PCA infusion was started and in the morning of each postoperative day. A physical therapist asked each patient to rate their highest NRS for pain during physical therapy. In addition, patients were assessed by the physical therapist twice daily and the first time they were able to ambulate approximately 20 ft was recorded. It is expected that in order to be discharged from the hospital, patients are able to sit up and touch the floor with their feet on the morning of POD 1, stand and walk to a chair by the afternoon of POD 1 (~ 20 ft), and by PODs 4–5, walk around the hospital floor and climb four stairs in preparation for discharge. Patients were also assessed twice a day, starting on POD 1 for their level of sedation using the following subjective scale: 1: awake and alert, 2: minimally sedated, 3: moderately sedated, 4: deeply sedated, and 5: unarousable (University of Michigan Sedation Scale). The presence of nausea and/or vomiting was also recorded. Other adverse effects such as dizziness and blurred vision were recorded. A subset of narcotic-tolerant patients (oxycodone utilization > 60 mg/day) was analyzed separately for total IV narcotic utilization.
Physical therapy milestones were recorded using Research Electronic Data Capture (REDCap) tools hosted as Hospital for Special Surgery [7]. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.
For the study, we assumed that pregabalin therapy would reduce by 30%; the PCA hydromorphone consumption of our postoperative spine patients using data from our pain service over the 6 months prior to initiating the study (53 ± 20 ml for the first 24 h) with a power of 80 and a significance of p ≤ 0.05, we would require 25 patients per group. In an attempt to avoid drop-outs during the study and a wider variability in opioid consumption, we enrolled more patients per group. Continuous data is presented as mean ± standard deviation and analyzed by the Student t test, p < 0.05 significant. Categorical data, level of sedation, nausea and vomiting, and physical therapy milestones were compared using chi-squared test. We also conducted a multivariant analysis comparing narcotic utilization between the two groups adjusted for sex, number of levels fused, and estimated blood loss (EBL), and accounting for multiple time points using the generalized estimating equations approach.
Results
There were 205 patients who were approached to enter the study; 90 were consented for the study, and 86 were still enrolled in the study on POD 1, 43 in each group (Fig. 1). There were no significant differences in the demographic profile of the patients or the operative details between groups (Table 1).
Figure 1.
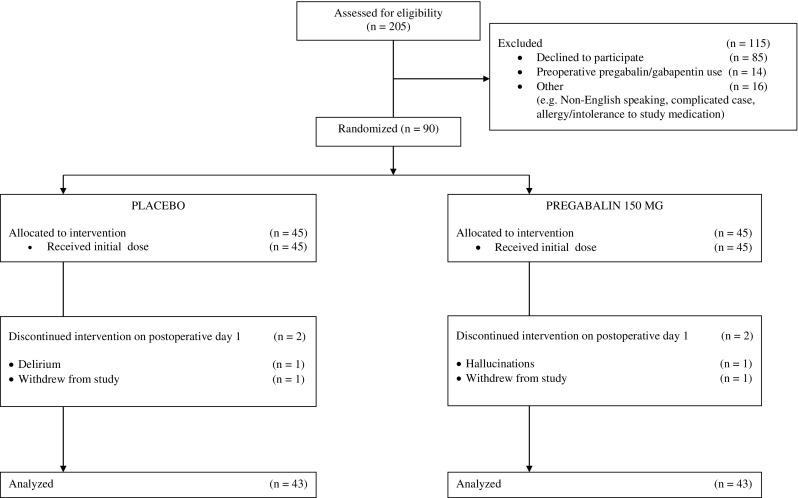
CONSORT diagram. Patient flow through the study.
Table 1.
Demographics and operative details
| Placebo (C) | Pregabalin (P) | |
|---|---|---|
| Patients (n) | 43 | 43 |
| Age (years) | 56 ± 13 | 57 ± 13 |
| Weight (kg) | 88 ± 20 | 81 ± 19 |
| Height (cm) | 170 ± 10 | 168 ± 9 |
| Gender (female/male) | 21/22 | 24/19 |
| Levels fused | ||
| 1 | 26 | 26 |
| 2–4 | 17 | 17 |
| Estimated blood loss (ml) | 788 ± 489 | 1028 ± 678 |
| Length of surgery (min) | 298 ± 56 | 312 ± 66 |
| ASA (n) | ||
| I | 2 | 4 |
| II | 37 | 33 |
| III | 4 | 6 |
| Preoperative narcotic dependence | 12 | 16 |
ASA American Society of Anesthesiologists physical status classification
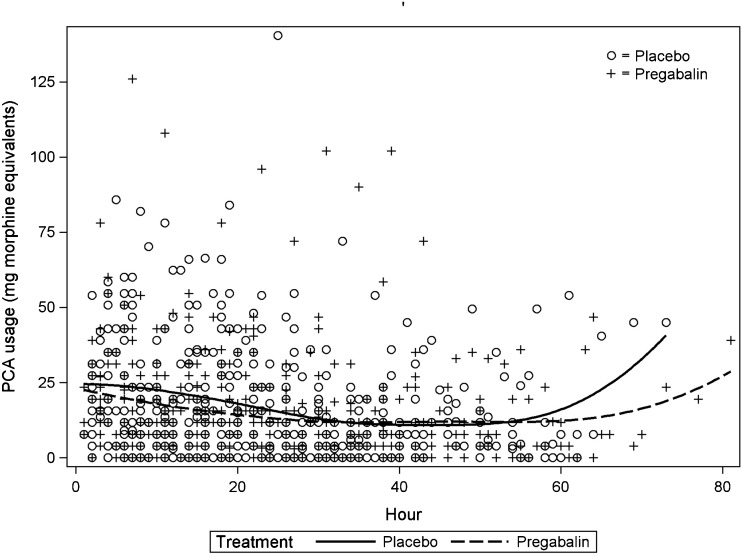
PCA hydromorphone usage was not statistically different between groups (Table 2) and is reflected in a similar rate of PCA demand with time (Fig. 2). All of the patients in the study completed 24 h of PCA, but by 48 h, only 23% of the patients (9 control and 11 pregabalin) continued to utilize the PCA. Oral analgesics were initiated on the day of surgery, but only 58% (25/43) of the placebo patients and 49% (21/43) of the pregabalin patients required oral analgesic supplementation on the day of surgery. Oral narcotic utilization was not statistically different for both groups. In addition, there were no statistically significant differences in pain scores between the groups at rest or with physical therapy. Both groups were also not statistically different with respect to levels of sedation, achievement of physical therapy milestones, and length of hospitalization. Only treated nausea and vomiting was significantly reduced in the pregabalin group on PODs 1 and 2 (Table 3). A multivariable analysis comparing PCA consumption, oral opioid intake, NRS pain scores, and length of hospitalization between the two groups with adjustment for sex, number of levels fused, and EBL did not identify any statistically significant differences between the placebo and pregabalin group (p = 0.819).
Table 2.
Postoperative narcotic utilization
| Placebo (C) | Pregabalin (P) | p value | |
|---|---|---|---|
| PCA (ml) | |||
| 0–6 h | 15 ± 10 | 12 ± 8 | 0.08 |
| 7–12 h | 11 ± 11 | 9 ± 13 | 0.08 |
| 13–18 h | 10 ± 11 | 8 ± 9 | 0.51 |
| 19–24 h | 8 ± 9 | 7 ± 8 | 0.11 |
| 25–48 h | 15 ± 5 | 15 ± 6 | 0.89 |
| Oral opioids* | |||
| POD 0 | 16 ± 22 | 18 ± 50 | 0.83 |
| POD 1 | 92 ± 114 | 63 ± 121 | 0.26 |
| POD 2 | 87 ± 93 | 86 ± 150 | 0.96 |
| POD 3 | 80 ± 87 | 73 ± 95 | 0.69 |
*Morphine equivalents in milligram
PCA patient-controlled analgesia, POD postoperative day
Figure 2.
Patient-controlled analgesia (PCA) administration after surgery. The amounts were converted to milligram morphine equivalents. The solid line represents the placebo group. The dotted line represents the pregabalin group.
Table 3.
Postoperative course
| Placebo (C) | Pregabalin (P) | |
|---|---|---|
| NRS | ||
| Day of surgery | 3.8 ± 2.6 | 2.9 ± 2.2 |
| POD 1 rest | 3.7 ± 2.1 | 3.3 ± 2.4 |
| POD 1 PT | 4.3 ± 3.0 | 4.5 ± 3.4 |
| POD 2 rest | 3.3 ± 2.0 | 3.4 ± 2.2 |
| POD 2 PT | 4.6 ± 2.7 | 3.9 ± 2.4 |
| POD 3 rest | 3.1 ± 1.8 | 3.2 ± 1.8 |
| POD 3 PT | 3.1 ± 2.5 | 3.2 ± 2.5 |
| Treated N/V (n) | ||
| Day of surgery | 6 | 5 |
| POD 1 | 9 | 2a |
| POD 2 | 10 | 3a |
| POD 3 | 4 | 2 |
| Sedation ≥ 3 (n) | ||
| POD 1 | 3 | 4 |
| POD 2 | 1 | 1 |
| POD 3 | 0 | 0 |
| % pts amb on POD 2b | 93 | 91 |
| Length of hospitalization (days) | 4.8 ± 2.1 | 4.7 ± 2.0 |
POD postoperative day, NRS Numerical Rating Scale for pain (0 = no pain, 10 = worst pain), Treated N/V nausea and vomiting which required intervention
ap < 0.05
bThe percentage of patients who were able to ambulate unassisted down a hospital floor
However, for a subset of preoperative narcotic-tolerant patients (n = 13 [pregabalin group], n = 11 [placebo group]), total PCA hydromorphone usage was significantly higher in the placebo group (101 ml) than that in the pregabalin group (59 ml, p = 0.03).
Discussion
Pain after posterior spinal fusions can be intense and limit postoperative physical therapy and rehabilitation. Excessive use of opioids to treat this pain results in nausea, vomiting, excessive sedation, ileus, and pruritus; all of which also limit postoperative positive progression. Hence, multiple attempts have been made to develop multimodal postoperative analgesia which limits opioid utilization but still provides adequate analgesia [11, 17, 18]. Pregabalin has been included in some acute postoperative pain protocols with limited success [2, 6, 8, 9, 12–14, 19, 20].
A few studies in the spine literature have reported that the addition of pregabalin to acute postoperative analgesia has reduced pain, narcotic consumption, and improved quality of life after spinal fusions [6, 9, 19]. Kim et al. [9] reported that a 150 mg but not 75-mg dose of pregabalin administered before and 12 h after surgery reduced intravenous fentanyl PCA/ketorolac consumption and the incidence of nausea for 48 h after spinal fusion, while maintaining similar pain scores in both groups. In another study, pregabalin 300 mg prior to surgery and 150 mg a day for 48 postoperative hours reduced intravenous morphine/ketorolac consumption and pain scores at rest and with movement for the first 12 h [6]. There was the suggestion for improved quality of life in the pregabalin group at 3 months but not 1 year after surgery. After lumbar discectomies, pregabalin 75 mg preoperatively and for 7 days after surgery reduced the number of intravenous doses of tramadol and static and dynamic pain intensity during the first 24 postoperative hours. Although for both the pregabalin group and the control group, the pain scores were low (VAS < 1) [8].
The limitations of this study include the relatively small number of patients studied, which may have impacted on our ability to demonstrate significance in a population with variable postoperative analgesic requirements. However, based on our previous experience with narcotic utilization by the same patient population, if there was an advantage to the addition of pregabalin to the pain regimen, it should have been revealed with this number of patients. In addition, except for oral analgesic utilization on POD 1, actual narcotic consumption was very similar between the two groups. The strength of this study is founded in a randomized controlled trial of pregabalin during the entire hospital stay in our typical (preoperative use of opioids) spine fusion patient comparing pain scores, narcotic requirements, side effects of analgesics, and physical therapy milestones. In conclusion, we were unable to demonstrate an analgesic advantage to the addition of pregabalin at 150 mg a day to our postoperative pain protocol.
In this study, we assessed whether the addition of pregabalin to an opioid pain regimen after posterior lumbar fusions would reduce narcotic consumption and improve outcomes. We chose a dose of pregabalin (150 mg/day) which in previous studies had limited adverse side effects, but was an effective analgesic. The anesthetic included ketamine, which has been shown to reduce postoperative opioid requirements [15]. NRS pain scores were kept at or below 5 at rest and with physical therapy using either intravenous PCA or oral opioids; hence, for the study, we were only comparing opioid consumption, adverse effects of opioids, and physical therapy milestones. Ketorolac was not included in the postoperative pain regimen, as two of the previous studies, due to its possible negative role in spinal fusions [10]. However, all of the patients in this study received continuous acetaminophen. The previous studies also administered a different intravenous narcotic, this report employing hydromorphone. We were unable to demonstrate a significant advantage for pregabalin compared to controls with regard to opioid consumption, physical therapy milestones, or length of hospital stay. Over 90% of the patients in both groups achieved their physical therapy milestones by POD 2 and were discharged within the expected number of days. Only nausea and vomiting was significantly reduced in patients in the pregabalin group on PODs 1 and 2, possibly related to the increased oral narcotic utilization on POD 1 in the placebo group which did not reach significance.
In contrast to the studies of Kim et al. [9] and Gianesello et al. [6], we included patients preoperatively treated with opioids. Hence, our analgesic requirements maybe less homogeneous than a population of patients who were naïve to narcotics. In our study, there was some indication that a subset of narcotic-tolerant patients may have benefited from pregabalin treatment, with decreased intravenous PCA usage.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Acknowledgements
We would like to thank Kara Fields, MS, for assistance with data analysis and creating Fig. 2.
Funding
This study was funded by Hospital for Special Surgery Anesthesiology Department Research and Education Fund, New York, NY. REDCap, used to record physical therapy milestone data, was funded by the CTSC grant (grant number UL1 TR000457-06) from the National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD. Pfizer (New York, NY) provided the placebo and pregabalin medications.
Compliance with Ethical Standards
Conflicts of Interest
Michael K. Urban, MD, PhD; Kristy M. Labib, MD; Shane C. Reid, MBS, BA; Amanda K. Goon, BA; and Valeria Rotundo have declared that they have no conflict of interest. Frank P. Cammisa Jr., MD reports royalties for patent with NuVasive. Federico P. Girardi, MD reports royalties paid for patents with DePuy Spine, LANX, Inc., NuVasive, and Ortho Development Corp., outside the work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level II: Randomized Controlled Trial.
Work performed at Hospital for Special Surgery, New York, NY.
Electronic supplementary material
The online version of this article (10.1007/s11420-017-9584-2) contains supplementary material, which is available to authorized users.
References
- 1.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 2.Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010; 110:199–207. 10.1213/ANE.0b013e3181c4273a. [DOI] [PubMed]
- 3.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: a systematic review and a meta-analysis. Br J Anaesth. 2014; 112:991–1004. 10.1093/bja/aeu137. [DOI] [PubMed]
- 4.Gajraj NM. Pregabalin for pain management. Pain Pract. 2005;5:95–102. doi: 10.1111/j.1533-2500.2005.05205.x. [DOI] [PubMed] [Google Scholar]
- 5.Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–15. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 6.Gianesello L, Pavoni V, Barboni E, Galeotti I, Nella A. Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol. 2012;24:121–6. doi: 10.1097/ANA.0b013e31823a885b. [DOI] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed]
- 8.Khurana G, Jindal P, Sharma JP, Bansal KK. Postoperative pain and long-term functional outcome after administration of gabapentin and pregabalin in patients undergoing spinal surgery. Spine (Phila Pa 1976). 2014; 39:E363–8. 10.1097/BRS.0000000000000185. [DOI] [PubMed]
- 9.Kim JC, Choi YS, Kim KN, Shim JK, Lee JY, Kwak YL. Effective dose of peri-operative oral pregabalin as an adjunct to multimodal analgesic regimen in lumbar spinal fusion surgery. Spine (Phila Pa 1976). 2011; 36:428–33. 10.1097/BRS.0b013e3181d26708. [DOI] [PubMed]
- 10.Li Q, Zhang Z, Cai Z. High-dose ketorolac affects adult spinal fusion: a meta-analysis of the effect of perioperative nonsteroidal anti-inflammatory drugs on spinal fusion. Spine (Phila Pa 1976). 2011; 36:E461–8. 10.1097/BRS.0b013e3181dfd163. [DOI] [PubMed]
- 11.Mathiesen O, Dahl B, Thomsen BA, Kitter B, Sonne N, Dahl JB et al. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J. 2013; 22:2089–96. 10.1007/s00586-013-2826-1. [DOI] [PMC free article] [PubMed]
- 12.Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth. 2008; 101:535–41. 10.1093/bja/aen215. [DOI] [PubMed]
- 13.Singla NK, Chelly JE, Lionberger DR, Gimbel J, Sanin L, Sporn J et al. Pregabalin for the treatment of postoperative pain: results from three controlled trials using different surgical models. J Pain Res. 2015; 8:9–20. 10.2147/JPR.S67841. [DOI] [PMC free article] [PubMed]
- 14.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007; 104:1545–56, table of contents. 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed]
- 15.Urban MK, Yadeau JT, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: A prospecitve randomized trial. HSS J 2008;4(1)62–65. 10.1007/s11420-007-9069-9. [DOI] [PMC free article] [PubMed]
- 16.White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101:S5–22. doi: 10.1213/01.ANE.0000177099.28914.A7. [DOI] [PubMed] [Google Scholar]
- 17.White PF. Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs. 2008;9:76–82. [PubMed] [Google Scholar]
- 18.White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology. 2010; 112:220–5. 10.1097/ALN.0b013e3181c6316e. [DOI] [PubMed]
- 19.Yu L, Ran B, Li M, Shi Z. Gabapentin and pregabalin in the management of postoperative pain after lumbar spinal surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2013; 38:1947–52. 10.1097/BRS.0b013e3182a69b90. [DOI] [PubMed]
- 20.Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011; 106:454–62. 10.1093/bja/aer027. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)