Dear Editor
Acute leukemias with ambiguous lineage encompass those leukemias that show no clear evidence of differentiation along a single lineage [1]. They are rare and account for less than 4% of all cases of acute leukemias. They include acute undifferentiated leukemia (AUL) and mixed phenotype acute leukemia (MPAL) with bilineage and biphenotypic blast subpopulations. Co-expression of myeloid and B-lymphoid antigens is most common in MPAL, followed by co-expression of myeloid and T-lymphoid antigens, accounting for 66–70% and 23–24% of MPALs, respectively [2]. Co-expression of B- and T-lineage-associated antigens is even rarer; overall there are too few cases to make any specific statements about clinical features, genetic lesions or prognosis of such patients. In assigning B lineage to a case of T cell leukaemia, CD79a and CD10 should not be considered as evidence of B-cell differentiation. Multiparameter flow cytometry is the method of choice for recognizing MPAL, with only few cases requiring immunohistochemical staining for characterization of blasts. Even when there are not two distinctly separable populations, most cases of MPAL show heterogeneity of expression of some antigens [3]. We present a challenging case of B/T type MPAL with equivocal expression of lineage-specific antigens on flowcytometry.
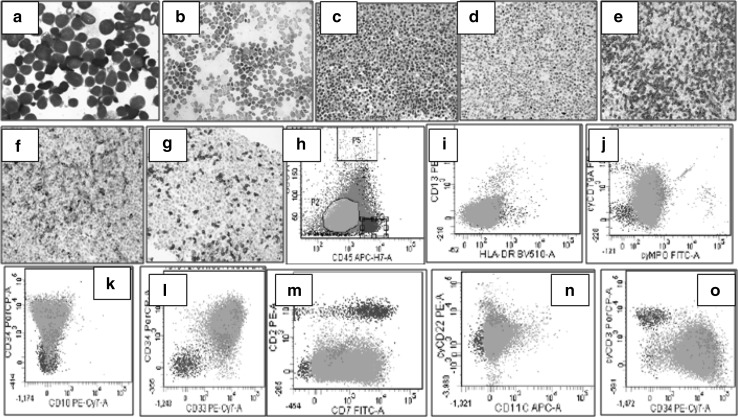
A previously healthy 42 year old male presented to the OPD with flu-like symptoms. A complete blood count (CBC) revealed pancytopenia with a white blood cell count of 1.9 × 109/L, hemoglobin of 8.3 g/dl, platelet count of 30 × 109/L and a differential count of N: 08, L: 31, M: 03, Blast: 58. Bone marrow aspiration and biopsy was performed and the samples were submitted for morphologic, flowcytometric, cytochemical, immunohistochemical, cytogenetic and molecular analysis. Bone marrow aspirate smears showed near-total replacement of normal hematopoietic components by a heterogenous population of small to medium sized blasts with round to slightly indented nuclei, condensed chromatin, inconspicuous nucleoli and scant cytoplasm. Blasts were negative for myeloperoxidase on cytochemical staining. Flowcytometry was performed on FACS CANTO-II (BD Biosciences) instrument using stain, lyse and wash technique. On flowcytometric analysis, blasts gated in the dim to intermediate CD45 region were positive for CD34, CD38, CD33, CD7 with heterogenous expression of CD19 (49%, moderate intensity; when compared to normal positive control in the sample), cytoCD79a (42.6%, moderate intensity) and cytoCD3 (14%, dim to moderate) [Fig. 1]. They were negative for all other markers including CD10, CD20, CD73, CD13, HLA-DR, CD14, CD117, CD64, CD5, CD2, CD4, CD8, CD3, CD56, cytoMPO, cytoCD22, CD15, CD11c, TDT, CD41a and CD1a. As clear-cut lineage determination was not possible due to absence of strong expression of lineage-specific antigens such as cytoCD3, CD19 and cytoCD79a, immunohistochemistry was done on bone marrow biopsy which showed diffuse replacement with two different population of immature cells, one showing positivity for PAX-5 and CD79a and the other with membranous positivity for CD3 along with down-regulation of CD2 expression (Fig. 1). Cytogenetic analysis showed a normal karyotype. Multiplex-PCR showed none of the ALL-specific translocations. Differentials mainly included B/T MPAL, Early T Precursor-ALL (ETP-ALL) with aberrant expression of CD19, cytoCD79a and PAX5 and AUL. However, a final decision was taken in favor of B/T mixed phenotype acute leukemia in accordance with the revised 2016 WHO criteria laid down by Arber et al. where it has been emphasized that cases in which it is possible to resolve 2 distinct blast populations, it is not necessary that the specific markers be present, but only that each individual population would meet a definition for either a B, T, or myeloid leukemia. Patient was started on UK-ALL induction protocol with vincristine and daunorubicin. However, Day +15 bone marrow showed 14% blasts. Patient later developed septic shock and thereafter left against medical advice.
Fig. 1.
a Bone marrow aspirate smears showed near-total replacement of normal hematopoietic components by a heterogenous population of small to medium sized blasts with round to slightly indented nuclei, condensed chromatin, inconspicuous nucleoli and scant cytoplasm. b Blasts were negative for Myeloperoxidase stain. c Bone Marrow Biopsy showing diffuse replacement with immature cells. d PAX5 nuclear positivity; of moderate intensity in one population of blasts. e Strong CD3 positivity in another population of blasts. f Down-regulated CD2 in CD3 positive blasts G) Areas with CD3 negative blasts. h–o Flowcytometric analysis of blasts showing positive expression of CD34, CD33, CD7 with heterogenous expression of CD19 (49%, moderate intensity), cytoCD79a (42.6%, moderate intensity)and cytoCD3(14%, dim to moderate)
This case brings out few important points. It stresses on the fact that despite extensive research, acute leukemias are heterogenous in terms of their antigenic expression where it is difficult to delineate all cases clearly in definitive WHO categories on the basis of existing set of markers. It also highlights on the role of upcoming markers like PAX5 (a transcription activator and a repressor of B-cell development) which are still not included in the definition of mixed lineage leukemias for lineage determination. Regarding the role of PAX5, it is suggested that any intervening mechanism that results in the loss of PAX-5 activity, may lead to de-differentiation and cause an outgrowth of cells with bipotential behavior [4]. Moreover, the diagnosis in this case could only be established by using both flowcytometry and immunohistochemistry in conjunction with each other, unlike most case reports published till now.
Compliance with Ethical Standards
Conflict of interest
The authors state that there is no conflict of interest present.
Ethical Approval
This case report does not contain any studies with human participants or animals performed by any of the authors. The identity of the patient is not disclosed here in this case.
Footnotes
Disclaimer: The identity of the patient is not disclosed here in this case.
References
- 1.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Kohla AS, Sabbagh AA, Omri HE, et al. Mixed phenotype acute leukemia with two immunophenotypically distinct B and T blasts populations, double Ph+ chromosome and complex karyotype: report of an unusual case. Clin Med Insights Blood Disord. 2015;8:25–31. doi: 10.4137/CMBD.S24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 4.Naghashpour M, Lancet J, Moscinski L, et al. Mixed phenotype acute leukemia with t(11;19)(q23;p13.3)/MLL-MLLT1(ENL), B/T-lymphoid type: A first case report. Am J Hematol. 2010;85(6):451–454. doi: 10.1002/ajh.21703. [DOI] [PubMed] [Google Scholar]